Postępy Biochemii 59 (4) 2013
365
Patrycja Sroczynska
*
Biotech Research and Innovation Centre
(BRIC), University of Copenhagen, Copenha-
gen, Denmark
*
Biotech Research and Innovation Centre (BRIC),
University of Copenhagen, Ole Maaløes Vej 5,
2200 Copenhagen, Denmark; e-mail: patrycja.
sroczynska@bric.ku.dk
Received: September 25, 2013
Accepted: October 14, 2013
Key words: hematopoiesis, hemogenic endo-
thelium, hemangioblast, embryo
Abbreviation: HSCs — hematopoietic stem
cells; ES cells — embryonic stem cells; YS —
yolk sac; P-Sp — paraaortic splanchnopleura;
AGM — aorta-gonad-mesonephros; E — em-
bryonic day; BL-CFC — blast colony-forming
cell; Bry — Brachyury; Flk-1 — fetal liver ki-
nase 1; AcLDL — acetylated low-density lipo-
protein; EHT — endothelial to hematopoietic
transition; Ncx1 — sodium-calcium exchan-
ger-1; Scl — stem cell leukemia
Acknowledgements: I would like to thank
Georges Lacaud and Valerie Kouskoff, who
introduced me to the field of embryonic hema-
topoiesis.
Hemogenic endothelium — ontogenesis and role in blood production
ABSTRACT
E
ndothelial and hematopoietic lineages have long been thought to develop from a com-
mon ancestor, the hemangioblast. Alternatively, clusters of hematopoietic cells in the
dorsal aorta were observed to form in a close association with endothelial wall of the aorta,
leading to the hypothesis that a special subset of endothelial cells, called the hemogenic en-
dothelium, generates hematopoietic cells. Recent advances in time-lapse imaging, condition-
al labeling of cells in vivo and embryonic stem cell differentiation provided new evidence for
the existence of both, the hemangioblast and hemogenic endothelium. Importantly, these
seemingly contradictory theories can be merged into one model of hematopoietic differentia-
tion from mesoderm.
INTRODUCTION
Human hematopoietic system produces between 10
11
and 10
12
new blood cells
per day during steady state and even more during infection or after injury. This
can be achieved thanks to the life-long presence of hematopoietic stem cells
(HSCs), which are defined by two properties: the potential to differentiate into
all types of blood cells (multipotency) and the ability to generate progeny with
the same potential (self-renewal). This initial pool of HSCs, that supports blood
production throughout the whole life, is generated during embryonic develop-
ment. It is therefore of outmost importance to understand the process of hemat-
opoietic cell commitment from mesoderm in the embryo.
Experiments leading to the current view of hematopoietic development star-
ted at the beginning of the 20
th
century and were performed over decades in se-
veral different vertebrate models, among others: zebrafish, chicken, mouse and
human embryos. In addition to these in vivo studies, the in vitro differentiation
of embryonic stem (ES) cells provided an excellent experimental system to study
the earliest steps of hematopoiesis. Data described in this review refer to mouse
studies, unless otherwise stated.
EMBRYONIC HEMATOPOIESIS
The initial steps of hematopoietic commitment in the adult take place in the
bone marrow, which provides a niche for the HSCs. In the growing embryo the
need for oxygen supply and specialized blood cell production precedes the for-
mation of the bone marrow. Embryonic blood production occurs therefore in
several waves and utilizes distinct anatomical sites: yolk sac (YS), paraaortic
splanchnopleura (P-Sp), aorta-gonad-mesonephros (AGM) that forms in the pla-
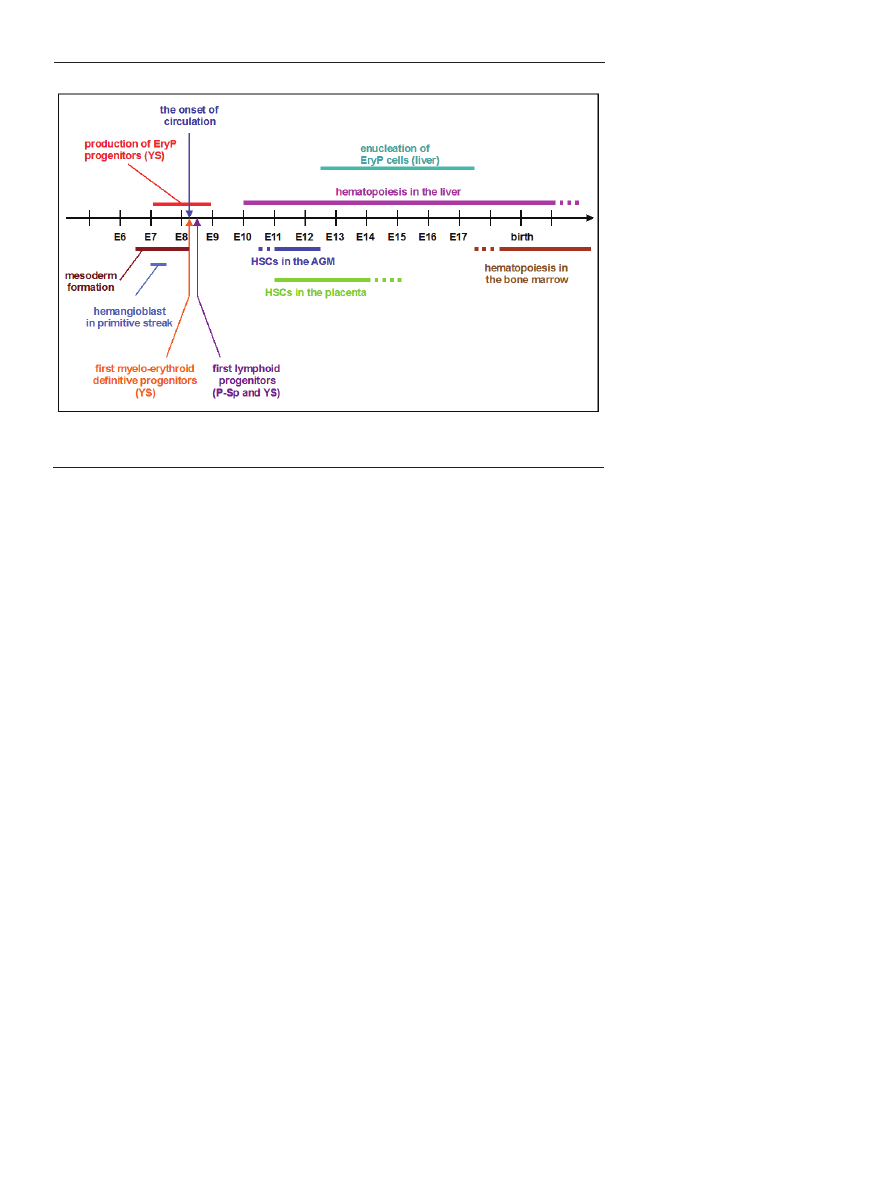
ce of P-Sp, placenta, allantois, chorion and fetal liver (Fig. 1).
PRIMITIVE HEMATOPOIESIS
All hematopoietic cells are derived from mesoderm, which forms in the pro-
cess known as gastrulation starting in the mouse embryo from embryonic day
(E) 6.5. The first restricted hematopoietic cells are of erythroid lineage and are
called primitive erythrocytes, due to several unique features distinguishing
them from adult-type, definitive erythrocytes. These primitive erythrocytes de-
velop in the blood islands, which are clusters of primitive erythroid cells surro-
unded by endothelial cells in the YS. In the mouse embryo blood islands emerge
around E8.25 [1].
Progenitors for the primitive erythrocytes can be detected by colony-forming
assay already at the primitive streak stage (E7.0) and disappear by E9.0 [2]. These
transient erythroid cells, unlike definitive erythrocytes, enter circulation while
still containing nuclei. They mature in the bloodstream and in the fetal liver,
366
www.postepybiochemii.pl
enucleate between E12.5 and E17.5, and continue to circu-
late until 5 days after birth [3,4]. Primitive erythrocytes are
also bigger than definitive erythrocytes, contain higher le-
vels of hemoglobin and express unique globin genes: βH1,
εy and ζ [5].
DEFINITIVE HEMATOPOIESIS
In this review term “definitive hematopoiesis” refers to
all hematopoietic lineages other than primitive erythroid
cells, i.e. myeloid, lymphoid and definitive erythroid. Ho-
wever, different definitions of definitive hematopoiesis can
be met in other reviews, e.g. hematopoiesis restricted to the
HSCs able to reconstitute adult recipient and the progeny of
such HSCs [6]. Alternatively, definitive hematopoietic cells
can be divided into several classes, based on the develop-
mental potential of the cells [7].
The short-time window of the generation of primitive
erythroid progenitors (E7.0-E9.0) is overlapped and follo-
wed by the generation of macrophage and definitive ery-
throid progenitors starting from E8.25 [2]. This first wave
of definitive hematopoiesis initiates in the YS and coincides
with the onset of circulation (Fig. 1). Since the circulation is
not fully functional for the first two days, the first defini-
tive progenitors are thought to be of YS origin [8]. Howe-
ver, other parts of the pre-circulation embryo, intraembry-
onic P-Sp and extraembryonic allantois and chorion, when
isolated and pre-cultured in vitro, are also able to generate
myeloid and definitive erythroid cells [9-11]. The emerging
hematopoietic progenitors, of both primitive and definitive
lineages, express the αIIb integrin (also known as CD41),
which is the earliest hematopoietic extracellular marker [12-
14].
In the second wave of definitive hematopoiesis, lympho-
id progenitors are formed in parallel with the progenitors
of erythro-myeloid potential. The origin of the first lym-
phoid progenitors was a matter
of a long-standing controversy.
Pre-circulation P-Sp, but not YS,
when isolated from the embryo
and cultured in vitro, gave rise to
lymphoid progenitors [15]. Ho-
wever, more recent studies with
the sodium-calcium exchanger-1
(Ncx1), deficient mouse embry-
os that lack cardiac contractions
and circulation, provided evi-
dence that not only P-Sp but also
YS and placenta autonomously
generate lymphoid progenitors
[16,17].
HSCS
In the final wave of definitive
hematopoiesis, the embryo ge-
nerates HSCs, which, unlike he-
matopoietic progenitors, possess
long-term multilineage reconsti-
tution potential of adult hemato-
poietic system upon transplanta-
tion. The first HSCs can be isola-
ted from the AGM region of the embryo at E10.5 [18,19].
Within AGM, the emerging HSCs are specifically associated
with the endothelial wall of the dorsal aorta [20]. HSCs can
be also found at E10.5 in the major arteries of the embryo:
the vitelline artery, that connects embryo proper and YS,
and the umbilical artery, that connects the embryo proper
and placenta [20], and at E11.0 in the placenta [21].
The hematopoietic progenitors and HSCs colonize fetal
liver starting from E10.0 and E11.5, respectively. Thereafter
liver becomes the major hematopoietic organ of the embryo,
where HSCs expand reaching their highest number of about
1600 HSCs per liver at E16 [22]. Subsequently HSCs migrate
to the developing bone marrow, which becomes the hema-
topoietic center of the organism.
HEMANGIOBLAST
Based on the observation that primitive erythroid cells
and endothelial cells develop in close association to each
other in the blood islands, it was suggested that the hema-
topoietic and endothelial lineages derive from a common
precursor, named the hemangioblast [23,24]. The first direct
evidence for the existence of the hemangioblast was provi-
ded by studies of hematopoietic commitment using mouse
ES cell differentiation. They led to identification of an in vi-
tro equivalent of the hemangioblast, called the blast colony-
-forming cell (BL-CFC). Single BL-CFC contains endothelial,
hematopoietic (definitive and primitive) and smooth musc-
le potential [25,26]. It is defined by co-expression of the me-
sodermal marker Brachyury (Bry) and fetal liver kinase 1
(Flk-1, known also as vascular endothelial growth factor
receptor type 2, VEGFR-2) [27].
The existence of the hemangioblast in vivo was first do-
cumented in 2004, when the group of Gordon Keller iden-
Figure 1. Milestones of hematopoietic development in the mouse embryo.
Postępy Biochemii 59 (4) 2013
367
tified a clonal precursor to hematopoietic, endothelial and
vascular smooth muscle cells in the posterior primitive
streak of gastrulating mouse embryos [28]. A cell with he-
matopoietic and endothelial potential was also identified in
zebrafish [29], fruit fly [30] and chicken [31]. In each case the
hemangioblast was found to be a rare precursor cell (fre-
quency lower than 1% within Bry
+
Flk-1
+
cell population
of E7.5 mouse embryo), and present only during a narrow
window of development (in the mouse embryo between
E7.0 and E7.5). In addition to its infrequency and transience,
the hemangioblast has no single specific marker, making its
tracking in vivo a very challenging task. It remains therefore
unknown whether all blood and endothelial cells, or only a
special subset of them, are generated through this common
precursor. It has been however shown by lineage tracing
experiments that essentially all embryonic and adult hema-
topoietic cells are progeny of Flk-1-expressing cells [32]. In
addition, Flk-1-deficient mouse embryos die between E8.5
and E9.5 lacking both endothelial and hematopoietic types
of cells [33].
Paradoxically, the blood islands, that originally triggered
the hemangiobast hypothesis, were found to be of a non-clo-
nal origin and hematopoietic and endothelial cells in sin-
gle blood islands were shown to be derived from different
precursors [34]. This observation does not however invali-
date the hemangioblast theory, but rather suggests that the
hemangioblast’s progeny reach the blood islands already
after their commitment to endothelial and hematopoietic
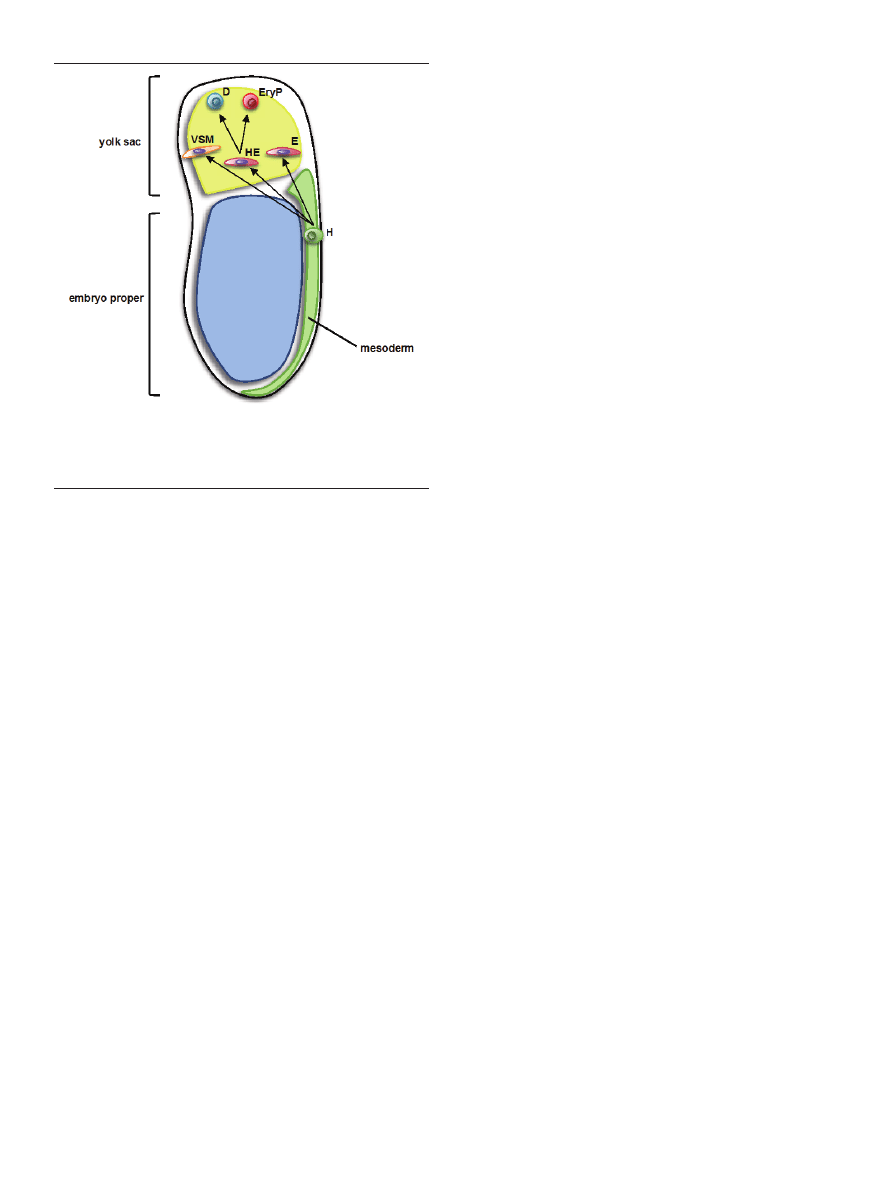
lineages [35] (Fig. 2).
HEMOGENIC ENDOTHELIUM
In parallel to the hemangioblast hypothesis, a seemingly
contradictory theory developed, according to which hema-
topoietic cells are generated by a special subset of endothe-
lial cells, called the hemogenic endothelium. This theory
originated from the observations that hematopoietic cells
form characteristic clusters that are closely associated with
endothelial cells in the wall of the dorsal aorta [36-39]. When
endothelial cells were isolated from mouse embryo at dif-
ferent stages between E8.5 and E10.5, and cultured in vitro,
they differentiated into definitive erythroid, myeloid and
lymphoid progenitors [40-42]. Importantly, long-term adult
repopulating HSCs were also shown to emerge in the endo-
thelial layer of the main vessels of the embryo: the aorta and
the vitelline and umbilical arteries [20,43]. These early HSCs
express a whole panel of endothelial cell surface markers,
such as VE-cadherin, Tie2, CD31 and CD34 [43-46] together
with hematopoietic markers, CD41 (early hematopoietic
marker) and CD45 (late hematopoietic marker).
Based on the above findings it was suggested that the
definitive hematopoietic cells derive directly from hemo-
genic endothelial cells. This theory had been however lac-
king unequivocal evidence. It was also speculated that the
hematopoietic cells of the intra-aortic clusters might in fact
derive from the mesenchyme underlying the endothelium
[47]. The first direct evidence of a bona fide endothelial ori-
gin of hematopoietic cells came from studies performed in
chicken embryos. Jaffredo et al. injected acetylated low-den-
sity lipoprotein (AcLDL), a marker specific for endothelial
cells and macrophages, labeled with fluorescent dye, into
chicken embryos at the stage of development when macro-
phages are not yet detected. Newly generated CD45
+
hema-
topoietic cells in the aorta contained AcLDL, proving their
endothelial origin [48]. The same question was addressed
in a mouse study using an inducible reporter system allo-
wing a timed labeling of VE-cadherin-expressing cells [49].
Endothelial (VE-cadherin
+
) cells were labeled in vivo in mo-
use embryos for about 48 h starting from E9.5. The proge-
ny of labeled cells contributed to a pool of hematopoietic
cells both in the fetal liver and in the bone marrow. Since
only a portion of adult hematopoietic cells in this study was
derived from the labeled endothelial precursors, it remains
uncertain whether all blood cells arise from the hemogenic
endothelium.
It is now widely accepted that hematopoietic cells have
an endothelial ancestor. Little is known however about the
mechanism through which the endothelial cells lose their
endothelial-specific properties and become blood cells. Re-
cent live imaging studies provided an opportunity to follow
endothelial to hematopoietic transition (EHT) in a real time.
Zebra fish embryos, due to their transparency, provided an
ideal model to follow the emergence of hematopoietic cells
[50-52]. It was observed that the hematopoietic cells form
specifically at the ventral wall of the dorsal aorta. Impor-
tantly, the hematopoietic cells do not emerge from endothe-
lial cells as a result of cell division. Instead, endothelial cells
bend in a characteristic stretched manner, round up and fi-
nally detach from neighboring cells [52].
A direct observation of the emergence of hematopoietic
cells in the aorta inside mouse embryo is hindered by the
opaque nature of the embryo. Biosset et al. used slices of mo-
use embryos to overcome this problem and visualize aorta
[53]. They observed cells expressing CD31 (endothelial mar-
Figure 2. Schematic view of hemangioblast commitment to endothelial, blood
and vascular smooth muscle lineages in gastrulating mouse embryo. H — he-
mangioblast; E — endothelium; HE — hemogenic endothelium; VSM — vascular
smooth muscle; D — definitive hematopoietic progenitor; EryP — primitive ery-
throid progenitor.
368
www.postepybiochemii.pl
ker) turning on the expression of CD41 (early hematopoietic
marker) concurrently with budding of these cells from the
ventral wall into the lumen of the aorta. It is still unclear
if hematopoietic cells in the mouse embryo are formed in
an EHT similar to the one described in the zebrafish. Ano-
ther intriguing question is whether the CD41
+
cells budding
from the aortal wall are HSCs or rather an intermediate sta-
ge between endothelium and HSCs, called pre-HSCs. It is
evident that within intra-aortic clusters the majority of cells
are not HSCs, since e.g. at E11.5 there are more than 400 he-
matopoietic cells but only 2 HSCs in the aorta [54].
HEMANGIOBLAST
VS. HEMOGENIC ENDOTHELIUM
Are hemangioblast and hemogenic endothelium just two
different names for the same cell population? Or could they
be two distinct blood precursors, each giving rise to a spe-
cific subset of hematopoietic cells? Two studies, in which
cells falling into hemangioblast or hemogenic endothelium
category were distinguished and analyzed in the same set
of experiments, shed light on this puzzle [55,56]. Time-lapse
imaging and single cell sorting led to the conclusion that a
single mesodermal Flk1
+
cell without an endothelial pheno-
type can give rise to an endothelial cell, which can subse-
quently generate hematopoietic cells. Thus, the hemangio-
blast does not generate hematopoietic and endothelial cells
simultaneously, but gives rise to blood cells through an en-
dothelial intermediate stage. Both primitive and definitive
hematopoietic lineages were generated through this sequ-
ence of events. It is however impossible to say if HSCs are
also formed in the hemangioblast–hemogenic endothelium–
hematopoietic progenitor sequence of transitions, as HSCs
cannot be efficiently generated in vitro using current ES cell
differentiation protocols. It is likely that in the embryo the
short-lived hemangioblast from the E7.0-E7.5 primitive stre-
ak [28] gives rise to hemogenic endothelium (along with
non-hemogenic endothelium and vascular smooth muscle
cells), which can be found in the yolk sac starting from E7.5
[40,41,55] and later in the AGM, the vitelline and umbilical
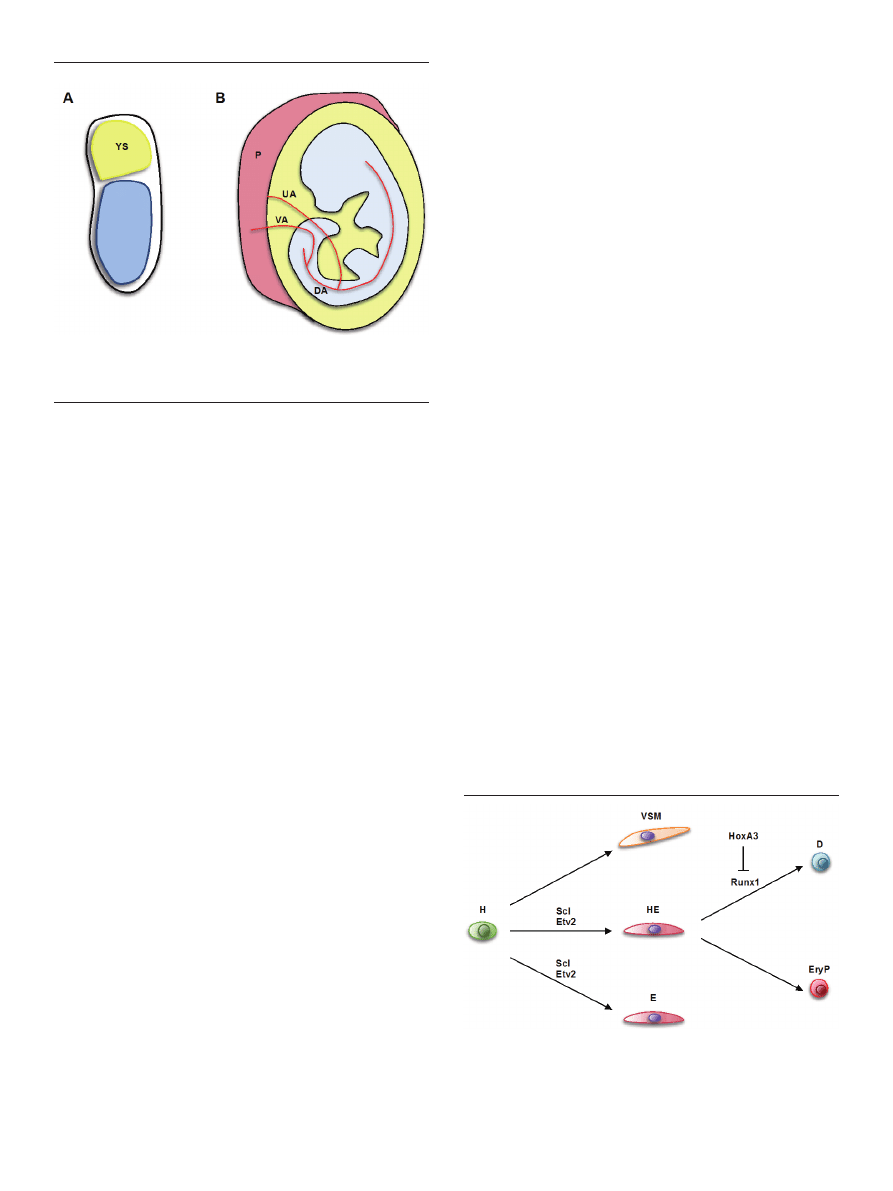
arteries and the placenta [49]. Anatomical sites with docu-
mented presence of hemogenic endothelium are schemati-
cally shown in figure 3.
TRANSCRIPTIONAL REGULATION OF
HEMOGENIC ENDOTHELIUM
Evidence for role of specific transcription factors in the
development of hemogenic endothelium and hematopoie-
tic progenitors was obtained mostly through observation of
developmental defects in knockout mice and during in vitro
differentiation of knockout ES cells.
HEMANGIOBLAST — HEMOGENIC
ENDOTHELIUM TRANSITION
The activity of the transcription factor stem cell leukemia
(Scl), known also as T-cell acute lymphoblastic leukemia 1
(Tal1), is critical for the formation of both endothelial and
hematopoietic types of cells. Scl
-/-
mouse embryos die by
E9.5 lacking any type of blood cells [57]. However, deletion
of Scl specifically in cells expressing Tie2 (endothelial mar-
ker) did not have a major impact of hematopoiesis [58], in-
dicating that Scl is critically needed for blood development
before the hemogenic endothelium stage. Indeed, dissecting
hematopietic commitment in vitro using differentiation of
ES cells showed that Scl is necessary for the generation of
hemogenic and non-hemogenic endothelial cells from the
hemangioblast, but does not appear to be necessary for the
development of hemangioblast from mesoderm or its diffe-
rentiation into smooth muscle cells [55,59] (Fig. 4).
The expression of Scl was shown to be controlled by Etv2
[60]. Etv2
-/-
mouse embryos lack vasculature and blood cells
and die around E9.5 [61]. Detailed analysis of hematopoie-
tic commitment in Etv2
-/-
embryos and ES cells showed that
Etv2 is not necessary for the development of Flk1
+
meso-
derm, but is absolutely critical for the formation of endothe-
lial cells, including hemogenic endothelium [62,63].
HEMOGENIC ENDOTHELIUM — HEMATOPOIETIC
PROGENITORS TRANSITION
Runx1 (known also as acute myeloid leukemia 1, Aml1)
has long been known as a master regulator of hematopo-
iesis. Runx1
-/-
mouse embryos develop hemorrhages in the
Figure 3. Anatomical sites with reported hemogenic endothelium activity. (A)
Gastrulating mouse embryo. YS — Yolk sac. (B) Midgestation mouse embryo.
DA — dorsal aorta; UA — umbilical artery; VA — vitelline artery; P — placenta.
Drawings are not to scale.
Figure 4. Transcriptional control of hemogenic endothelium development and
differentiation into hematopoietic progenitors. H — hemangioblast; E — endo-
thelium; HE — hemogenic endothelium; VSM — vascular smooth muscle; D —
definitive hematopoietic progenitor; EryP — primitive erythroid progenitor.

Postępy Biochemii 59 (4) 2013
369
central nervous system and die by E12.5 [64,65]. The acti-
vity of Runx1 is necessary for the generation of definitive
hematopoietic progenitors both in vivo and in vitro [65,66].
Primitive erythropoietic progenitors still develop in the ab-
sence of Runx1 with only minor defects [67]. Importantly,
Runx1 activity is specifically required in endothelial cells, as
shown in mice with Runx1 deletion in Tie2
+
or VE-cadherin
+
endothelial cells [68,69]. Moreover, re-expression of Runx1
in Runx1
-/-
endothelial cells rescues definitive hematopoiesis
[55,70]. Together, these data define the window of Runx1
function in the hematopoietic development specifically at
the transition from hemogenic endothelium to definitive he-
matopoietic progenitors (Fig. 4).
A recent study showed that Runx1 and Hoxa3 are expres-
sed in a mutually exclusive manner and act antagonistical-
ly. High Hoxa3 levels maintain endothelial character of the
cells and repress hematopoiesis. In contrast, Runx1 expres-
sion overrides endothelial gene expression program establi-
shed by Hoxa3 and promotes hematopoiesis [71].
Factors involved in the regulation of primitive hemato-
poiesis are largely unknown and it remains unclear how
and at which stage of hematopoietic commitment the choice
between definitive and primitive developmental programs
is made. It has been however suggested that this choice is
determined by the interplay between Wnt and Notch signa-
ling pathways [72].
FINAL REMARKS
•
Given their indispensable role in the production of
HSCs and hematopoietic progenitors, still relatively little is
known about hemogenic endothelial cells:
•
What markers distinguish hemogenic endothelium
from other endothelial cells?
•
Do all endothelial cells have the potential to beco-
me hemogenic?
•
What extracellular signals determine hemogenic
vs. non-hemogenic status of endothelial cells?
•
Does hemogenic endothelium generate both endo-
thelial and hematopoietic cells, or is its potential restricted
to hematopoietic lineage?
•
Is hemogenic endothelium specific only for embry-
onic development or is it present also in adults?
Several studies suggest that adult HSCs posses endothe-
lial potential (reviewed in [35,73]), however hemogenic en-
dothelial activity in the steady-state adult hematopoiesis is
yet to be shown. Further studies are needed to fully under-
stand the transition from endothelium to blood cells. In vitro
ES cell differentiation has proven to be a powerful tool in
dissecting the earliest stages of hematopoietic commitment.
Not only it faithfully resembles hematopoietic development
in the embryo, but also it gives access to rare cell popula-
tions that are otherwise extremely difficult, if not impossi-
ble, to isolate or track in vivo.
REFERENCES
1. Ferkowicz MJ, Yoder MC (2005) Blood island formation: longstanding
observations and modern interpretations. Exp Hematol 33: 1041-1047
2. Palis J, Robertson S, Kennedy M, Wall C, Keller G (1999) Development
of erythroid and myeloid progenitors in the yolk sac and embryo pro-
per of the mouse. Development 126: 5073-5084
3. Fraser ST, Isern J, Baron MH (2007) Maturation and enucleation of pri-
mitive erythroblasts during mouse embryogenesis is accompanied by
changes in cell-surface antigen expression. Blood 109: 343-352
4. Isern J, Fraser ST, He Z, Baron MH (2008) The fetal liver is a niche for
maturation of primitive erythroid cells. Proc Natl Acad Sci USA 105:
6662-6667
5. McGrath K, Palis J (2005) Hematopoiesis in the yolk sac: more than
meets the eye. Exp Hematol 33: 1021-1028
6. Medvinsky A, Rybtsov S, Taoudi S (2011) Embryonic origin of the
adult hematopoietic system: advances and questions. Development
138: 1017-1031
7. Dzierzak E, Speck NA (2008) Of lineage and legacy: the development
of mammalian hematopoietic stem cells. Nat Immunol 9: 129-136
8. McGrath KE, Koniski AD, Malik J, Palis J (2002) Circulation is esta-
blished in a stepwise pattern in the mammalian embryo. Blood 101:
1669-1675
9. Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I (2001) Intraem-
bryonic, but not yolk sac hematopoietic precursors, isolated before cir-
culation, provide long-term multilineage reconstitution. Immunity 15:
477-485
10. Zeigler BM, Sugiyama D, Chen M, Guo Y, Downs KM, Speck NA
(2006) The allantois and chorion, when isolated before circulation or
chorio-allantoic fusion, have hematopoietic potential. Development
133: 4183-4192
11. Corbel C, Salaün J, Belo-Diabangouaya P, Dieterlen-Lièvre F (2007)
Hematopoietic potential of the pre-fusion allantois. Develop Biol 301:
478-488
12. Ferkowicz MJ (2003) CD41 expression defines the onset of primitive
and definitive hematopoiesis in the murine embryo. Development 130:
4393-4403
13. Mikkola HKA (2002) Expression of CD41 marks the initiation of defi-
nitive hematopoiesis in the mouse embryo. Blood 101: 508-516
14. Corbel C, Salaün J (2002) αIIb integrin expression during development
of the murine hemopoietic system. Dev Biol 243: 301-311
15. Cumano A, Dieterlen-Lièvre F, Godin I (1996) Lymphoid potential,
probed before circulation in mouse, is restricted to caudal intraembry-
onic splanchnopleura. Cell 86: 907-916
16. Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC
(2008) All primitive and definitive hematopoietic progenitor cells
emerging before E10 in the mouse embryo are products of the yolk
sac. Blood 111: 3435-3438
17. Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Con-
way S, Orkin SH, Yoder MC, Mikkola HK (2008) The Emergence of
hematopoietic stem cells is initiated in the placental vasculature in the
absence of circulation. Cell Stem Cell 2: 252-263
18. Medvinsky A, Dzierzak E (1996) Definitive hematopoiesis is autono-
mously initiated by the AGM region. Cell 86: 897-906
19. Müller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzakt E
(1994) Development of hematopoietic stem cell activity in the mouse
embryo. Immunity 1: 291-301
20. de Bruijn MF, Speck NA, Peeters MC, Dzierzak E (2000) Definitive he-
matopoietic stem cells first develop within the major arterial regions of
the mouse embryo. EMBO J 19: 2465-2474
21. Gekas C, Dieterlen-Lièvre F, Orkin SH, Mikkola HKA (2005) The pla-
centa is a Niche for hematopoietic stem cells. Develop Cell 8: 365-375
22. Ema H, Nakauchi H (2000) Expansion of hematopoietic stem cells in
the developing liver of a mouse embryo. Blood 95: 2284-2288
23. Sabin FR (1920) Studies on the origin of blood vessels and of red corpu-
scules as seen in the living blastoderm of the chick during the second

370
www.postepybiochemii.pl
day of incubation: contributions to embryology. Contrib Embryol 9:
213-262
24. Murray PDF (1932) The development in vitro of the blood of the early
chick embryo. Proc R Soc Lond 11: 497-521
25. Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G (1998) A
common precursor for hematopoietic and endothelial cells. Develop-
ment 125: 725-732
26. Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller
G (1997) A common precursor for primitive erythropoiesis and defini-
tive haematopoiesis. Nature 386: 488-493
27. Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G,
Kouskoff V (2003) Tracking mesoderm induction and its specification
to the hemangioblast during embryonic stem cell differentiation. De-
velopment 130: 4217-4227
28. Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G (2004) Haeman-
gioblast commitment is initiated in the primitive streak of the mouse
embryo. Nature 432: 625-630
29. Vogeli KM, Jin S-W, Martin GR, Stainier DYR (2006) A common pro-
genitor for haematopoietic and endothelial lineages in the zebrafish
gastrula. Nature 443: 337-339
30. Mandal L, Banerjee U, Hartenstein V (2004) Evidence for a fruit fly he-
mangioblast and similarities between lymph-gland hematopoiesis in
fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nature
Genet 36: 1019-1023
31. Weng W, Sukowati EW, Sheng G (2007) On hemangioblasts in chic-
ken. PLoS ONE 2: e1228
32. Lugus JJ, Park C, Ma YD, Choi K (2009) Both primitive and definitive
blood cells are derived from Flk-1+ mesoderm. Blood 113: 563-566
33. Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman
ML, Schuh AC (1995) Failure of blood-island formation and vasculo-
genesis in Flk-1-deficient mice. Nature 376: 62-66
34. Ueno H, Weissman IL (2006) Clonal analysis of mouse development
reveals a polyclonal origin for yolk Sac blood islands. Develop Cell 11:
519-533
35. Lancrin C, Sroczynska P, Serrano AG, Gandillet A, Ferreras C, Ko-
uskoff V, Lacaud G (2009) Blood cell generation from the hemangio-
blast. J Mol Med 88: 167-172
36. Dieterlen-Lièvre F, Martin C (1981) Diffuse intraembryonic hemopoie-
sis in normal and chimeric avian development. Dev Biol 88: 180-191
37. Garcia-Porrero JA, Godin IE, Dieterlen-Lièvre F (1995) Potential in-
traembryonic hemogenic sites at pre-liver stages in the mouse. Anat
Embryol 192: 425-435
38. Tavian M, Coulombel L, Luton D, Clemente HS, Dieterlen-Lièvre F,
Péault B (1996) Aorta-associated CD34+ hematopoietic cells in the ear-
ly human embryo. Blood 87: 67-72
39. Jordan HE (1917) Aortic cell clusters in vertebrate embryos. Proc Natl
Acad Sci USA 3: 149-156
40. Nishikawa SI, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M,
Kataoka H, Katsura Y (1998) In vitro generation of lymphohematopo-
ietic cells from endothelial cells purified from murine embryos. Immu-
nity 8: 761-769
41. Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H
(1998) Progressive lineage analysis by cell sorting and culture identi-
fies FLK1+VE-cadherin+ cells at a diverging point of endothelial and
hemopoietic lineages. Development 125: 1747-1757
42. Yokomizo T, Ogawa M, Osato M, Kanno T, Yoshida H, Fujimoto T,
Fraser S, Nishikawa S, Okada H, Satake M, Noda T, Nishikawa S, Ito Y
(2001) Requirement of Runx1/AML1/PEBP2αB for the generation of
haematopoietic cells from endothelial cells. Genes Cells 6: 13-23
43. de Bruijn MF, Ma X, Robin C, Ottersbach K, Sanchez MJ, Dzierzak E
(2002) Hematopoietic stem cells localize to the endothelial cell layer in
the midgestation mouse aorta. Immunity 16: 673-683
44. Taoudi S, Medvinsky A (2007) Functional identification of the hema-
topoietic stem cell niche in the ventral domain of the embryonic dorsal
aorta. Proc Natl Acad Sci USA 104: 9399-9403
45. Taoudi S, Gonneau C, Moore K, Sheridan JM, Blackburn CC, Taylor E,
Medvinsky A (2008) Extensive hematopoietic stem cell generation in
the AGM region via maturation of VE-cadherin+CD45+ pre-definitive
HSCs. Cell Stem Cell 3: 99-108
46. Matsubara A, Iwama A, Yamazaki S, Furuta C, Hirasawa R, Morita Y,
Osawa M, Motohashi T, Eto K, Ema H, Kitamura T, Vestweber D, Na-
kauchi H (2005) Endomucin, a CD34-like sialomucin, marks hemato-
poietic stem cells throughout development. J Exp Med 202: 1483-1492
47. Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, Godin
I, Cumano A (2005) Characterization of purified intraembryonic he-
matopoietic stem cells as a tool to define their site of origin. Proc Natl
Acad Sci USA 102: 134-139
48. Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lièvre F (1998) Intraaor-
tic hemopoietic cells are derived from endothelial cells during ontoge-
ny. Development 125: 4575-4583
49. Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Bec-
ker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe
ML (2008) Fate tracing reveals the endothelial origin of hematopoietic
stem cells. Cell Stem Cell 3: 625-636
50. Lam EYN, Hall CJ, Crosier PS, Crosier KE, Flores MV (2010) Live ima-
ging of Runx1 expression in the dorsal aorta tracks the emergence of
blood progenitors from endothelial cells. Blood 116: 909-914
51. Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D (2010)
Haematopoietic stem cells derive directly from aortic endothelium du-
ring development. Nature 464: 108-111
52. Kissa K, Herbomel P (2010) Blood stem cells emerge from aortic endo-
thelium by a novel type of cell transition. Nature 464: 112-115
53. Boisset J-C, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E,
Robin C (2010) In vivo imaging of haematopoietic cells emerging from
the mouse aortic endothelium. Nature 464: 116-120
54. Boisset J-C, Robin C (2012) On the origin of hematopoietic stem cells:
progress and controversy. Stem Cell Res 8: 1-13
55. Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud
G (2009) The haemangioblast generates haematopoietic cells through a
haemogenic endothelium stage. Nature 457: 892-895
56. Eilken HM, Nishikawa S-I, Schroeder T (2009) Continuous single-cell
imaging of blood generation from haemogenic endothelium. Nature
457: 896-900
57. Shivdasani RA, Mayer EL, Orkin SH (1995) Absence of blood forma-
tion in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Natu-
re 373: 432-434
58. Schlaeger TM, Mikkola HKA, Gekas C, Helgadottir HB, Orkin SH
(2005) Tie2Cre-mediated gene ablation defines the stem-cell leukemia
gene (SCL/tal1)-dependent window during hematopoietic stem-cell
development. Blood 105: 3871-3874
59. D’Souza SL, Elefanty AG, Keller G (2005) SCL/Tal-1 is essential for
hematopoietic commitment of the hemangioblast but not for its deve-
lopment. Blood 105: 3862-3870
60. Wareing S, Mazan A, Pearson S, Göttgens B, Lacaud G, Kouskoff V
(2012) The Flk1-Cre-mediated deletion of ETV2 defines its narrow
temporal requirement during embryonic hematopoietic development.
Stem Cells 30: 1521-1531
61. Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Go-
mez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K (2008) ER71 Acts
Downstream of BMP, Notch, and Wnt Signaling in Blood and Vessel
Progenitor Specification. Cell Stem Cell 2: 497-507
62. Wareing S, Eliades A, Lacaud G, Kouskoff V (2012) ETV2 expression
marks blood and endothelium precursors, including hemogenic endo-
thelium, at the onset of blood development. Dev Dyn 241: 1454-1464
63. Kataoka H, Hayashi M, Nakagawa R, Tanaka Y, Izumi N, Nishikawa
S, Jakt ML, Tarui H, Nishikawa S (2011) Etv2/ER71 induces vascular
mesoderm from Flk1+PDGFR+ primitive mesoderm. Blood 118: 6975-
6986
64. Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA
(1996) Disruption of the Cbfa2 gene causes necrosis and hemorrhaging
in the central nervous system and blocks definitive hematopoiesis.
Proc Natl Acad Sci USA 93: 3444-3449
65. Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR (1996)
AML1, the target of multiple chromosomal translocations in human
Postępy Biochemii 59 (4) 2013
371
Śródbłonek krwiotwórczy — ontogeneza i rola w hematopoezie
Patrycja Sroczyńska
*
Biotech Research and Innovation Centre (BRIC), University of Copenhagen, Ole Maaløes Vej 5, 2200 Kopenhaga, Dania
*
e-mail: patrycja.sroczynska@bric.ku.dk
Słowa kluczowe: hematopoeza, śródbłonek, hemangioblast, zarodek
STRESZCZENIE
Przez długi czas uważano, że linie komórek śródbłonkowych i hematopoetyczych rozwijają się ze wspólnej komórki prekursorowej, heman-
gioblastu. Z drugiej strony, obserwowano skupiska komórek hematopoetycznych w aorcie grzbietowej zarodka tworzące się w bezpośrednim
sąsiedztwie komórek śródbłonka ściany aorty. Doprowadziło to do sformułowania hipotezy, że subpopulacja komórek śródbłonkowych,
zwana śródbłonkiem krwiotwórczym, jest źródłem komórek hematopoetycznych. Ostatnie badania z wykorzystaniem obrazowania poklat-
kowego, warunkowego znakowania komórek in vivo oraz różnicowania zarodkowych komórek macierzystych dostarczyły nowych dowodów
na istnienie zarówno hemangioblastów jak i śródbłonka krwiotwórczego. Co ważne, te pozornie sprzeczne teorie mogą zostać połączone w
jeden model różnicowania komórek linii hematopoetycznej i śródbłonkowej z mezodermy.
leukemia, is essential for normal fetal liver hematopoiesis. Cell 84: 321-
330
66. Lacaud G, Gore L, Kennedy M, Kouskoff V, Kingsley P, Hogan C,
Carlsson L, Speck N, Palis J, Keller G (2002) Runx1 is essential for he-
matopoietic commitment at the hemangioblast stage of development
in vitro. Blood 100: 458-466
67. Yokomizo T, Hasegawa K, Ishitobi H, Osato M, Ema M, Ito Y, Yama-
moto M, Takahashi S (2008) Runx1 is involved in primitive erythropo-
iesis in the mouse. Blood 111: 4075-4080
68. Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA (2009)
Runx1 is required for the endothelial to haematopoietic cell transition
but not thereafter. Nature 457: 887-891
69. Li Z, Chen MJ, Stacy T, Speck NA (2006) Runx1 function in hematopo-
iesis is required in cells that express Tek. Blood 107: 106-110
70. Liakhovitskaia A, Gribi R, Stamateris E, Villain G, Jaffredo T, Wilkie
R, Gilchrist D, Yang J, Ure J, Medvinsky A (2009) Restoration of Runx1
expression in the Tie2 cell compartment rescues definitive hematopo-
ietic stem cells and extends life of Runx1 knockout animals until birth.
Stem Cells 27: 1616-1624
71. Iacovino M, Chong D, Szatmari I, Hartweck L, Rux D, Caprioli A, Cle-
aver O, Kyba M (2010) HoxA3 is an apical regulator of haemogenic
endothelium. Nat Cell Biol 13: 72-78
72. Cheng X, Huber TL, Chen VC, Gadue P, Keller GM (2008) Numb me-
diates the interaction between Wnt and Notch to modulate primitive
erythropoietic specification from the hemangioblast. Development
135: 3447-3458
73. Hirschi KK (2012) Hemogenic endothelium during development and
beyond. Blood 119: 4823-4827
Wyszukiwarka
Podobne podstrony:
365 Revision 2 id 501036 Nieznany (2)
365 Revision 1 id 501035 Nieznany (2)
365 Level3 wordlist id 457589 Nieznany (2)
II CR 371 70 id 209812 Nieznany
365 Level2 wordlist id 457588 Nieznany (2)
365 Level1 wordlist id 457587 Nieznany (2)
365 dni do sukcesu id 36191 Nieznany
Abolicja podatkowa id 50334 Nieznany (2)
4 LIDER MENEDZER id 37733 Nieznany (2)
katechezy MB id 233498 Nieznany
metro sciaga id 296943 Nieznany
perf id 354744 Nieznany
interbase id 92028 Nieznany
Mbaku id 289860 Nieznany
Probiotyki antybiotyki id 66316 Nieznany
miedziowanie cz 2 id 113259 Nieznany
LTC1729 id 273494 Nieznany
D11B7AOver0400 id 130434 Nieznany
więcej podobnych podstron