Pseudomonas syringae pv. phaseolicola can be
separated into two genetic lineages distinguished
by the possession of the phaseolotoxin
biosynthetic cluster
Jose´ A. Oguiza,
1
Arantza Rico,
1
Luis A. Rivas,
1
Laurent Sutra,
2
3
Alan Vivian
3
and Jesu´s Murillo
1
Correspondence
Jesu´s Murillo
jesus@unavarra.es
1
Instituto de Agrobiotecnologı´a y Recursos Naturales, CSIC-UPNA, and Laboratorio de
Patologı´a Vegetal, Departamento de Produccio´n Agraria, Universidad Pu´blica de Navarra,
31006 Pamplona, Spain
2
UMR de Pathologie Ve´ge´tale INRA-INH-Universite´, Beaucouze´, 49071 France
3
Centre for Research in Plant Science, University of the West of England, Coldharbour Lane,
Bristol BS16 1QY, UK
Received 10 July 2003
Revised
30 October 2003
Accepted 31 October 2003
The bean (Phaseolus spp.) plant pathogen Pseudomonas syringae pv. phaseolicola is
characterized by the ability to produce phaseolotoxin (Tox
+
). We recently reported that the
majority of the Spanish P. syringae pv. phaseolicola population is unable to synthesize this
toxin (Tox
”
). These Tox
”
isolates appear to lack the entire DNA region for the biosynthesis of
phaseolotoxin (argK-tox gene cluster), as shown by PCR amplification and DNA hybridization
using DNA sequences specific for separated genes of this cluster. Tox
+
and Tox
”
isolates also
showed genomic divergence that included differences in ERIC-PCR and arbitrarily primed-PCR
profiles. Tox
+
isolates showed distinct patterns of IS801 genomic insertions and contained a
chromosomal IS801 insertion that was absent from Tox
”
isolates. Using a heteroduplex mobility
assay, sequence differences were observed only among the intergenic transcribed spacer of
the five rDNA operons of the Tox
”
isolates. The techniques used allowed the unequivocal
differentiation of isolates of P. syringae pv. phaseolicola from the closely related soybean (Glycine
max) pathogen, P. syringae pv. glycinea. Finally, a pathogenicity island that is essential for the
pathogenicity of P. syringae pv. phaseolicola on beans appears to be conserved among Tox
+
, but
not among Tox
”
isolates, which also lacked the characteristic large plasmid that carries this
pathogenicity island. It is proposed that the results presented here justify the separation of the
Tox
+
and Tox
”
P. syringae pv. phaseolicola isolates into two distinct genetic lineages, designated
Pph1 and Pph2, respectively, that show relevant genomic differences that include the
pathogenicity gene complement.
INTRODUCTION
Pseudomonas syringae pv. phaseolicola is a seed-borne
pathogen of bean (Phaseolus vulgaris) worldwide that causes
the halo blight disease. Disease symptoms are typically
watersoaked lesions that eventually develop a surrounding
yellow halo produced by the release of the non-specific
toxin, phaseolotoxin (Mitchell, 1978). Based on their
virulence to a range of bean cultivars, nine races of
P. syringae pv. phaseolicola have been distinguished (Taylor
et al., 1996). Recently, the ability of this pathogen to pro-
duce disease in bean has been shown to be based on the
possession of a pathogenicity island (PAI), localized to a
150 kb plasmid, that includes genes that are either essential
for pathogenicity on bean and soybean or that contribute
to aggressiveness in an additive fashion (Jackson et al., 1999;
Tsiamis et al., 2000). In addition to the PAI, P. syringae
pv. phaseolicola strains are defined by possession of
the argK-tox gene cluster, which directs phaseolotoxin
3Deceased (d. 16 December 2002); this paper is dedicated to his
memory.
Abbreviations: AP-PCR, arbitrarily primed PCR; ERIC-PCR, extragenic
repetitive consensus PCR; REP-PCR, repetitive extragenic palindromic
PCR; EEL, exchangeable effector locus; HMA, heteroduplex mobility
assay; ITS, internal transcribed spacer; PAI, pathogenicity island.
The EMBL accession numbers for the sequences reported in this paper
are AJ568000 (IS50, 734 bp), AJ568001 (IS50, 295 bp), AJ568002
(ERIC, 1289 bp), AJ550186 (EEL-Pph1), AJ550187 (EEL-Pph2) and
AJ550188 (EEL-Pseudomonas syringae pv. glycinea).
0002-6635
G
2004 SGM
Printed in Great Britain
473
Microbiology (2004), 150, 473–482
DOI 10.1099/mic.0.26635-0

biosynthesis and appears to increase virulence (Patil et al.,
1974; Mitchell, 1978; de la Fuente-Martı´nez et al., 1992).
Additionally, phaseolotoxin has been considered a useful
determinative character unique to P. syringae pv. phaseoli-
cola among the bacterial bean pathogens. It is generally
believed that only isolates able to synthesize phaseolotoxin
(Tox
+
isolates) are of epidemiological significance and,
hence, this DNA region is commonly used as a target for
PCR detection and identification of P. syringae pv. phaseoli-
cola (Schaad et al., 1995).
P. syringae pv. phaseolicola can readily be distinguished
from other pathovars of P. syringae pathogenic to beans,
such as pathovars syringae and glycinea, by nutritional
characteristics and because only P. syringae pv. phaseolicola
isolates produce water-soaked lesions on bean pods
(Palleroni, 1984; Vo¨lksch & Weingart, 1997; Marques
et al., 2000). In general, P. syringae pv. phaseolicola appears
to be a more or less homogeneous pathovar, although it
displays a degree of genetic and phenotypic variation that
overlaps with isolates from P. syringae pv. glycinea (Marques
et al., 2000). On the basis of phenotypic characteristics
and ERIC-PCR-generated profiles, strains of P. syringae
pv. glycinea, P. syringae pv. phaseolicola isolated from
bean and P. syringae pv. phaseolicola isolated from kudzu
(Pueraria lobata), can be divided into three distinct groups
(Vo¨lksch & Weingart, 1997). Additionally, intrapathovar
variation in P. syringae pv. phaseolicola can be linked, in
some cases, to the host plant species of isolation (Marques
et al., 2000). Isolates that produce natural infections on
kudzu vine are distinguished, among other characters, for
carrying a plasmid-borne efe gene (Nagahama et al., 1994)
and, similar to isolates from Vigna radiata, by their REP-
PCR profile with ERIC primers (Vo¨lksch & Weingart, 1997;
Marques et al., 2000).
Most isolates of P. syringae pv. phaseolicola are reported to
be Tox
+
and naturally occurring isolates unable to syn-
thesize phaseolotoxin (Tox
2
isolates), which usually possess
the corresponding argK-tox gene cluster region, are rare
(Rudolph, 1995; Schaad et al., 1995). We reported recently,
however, that over 60 % of the Spanish field isolates of
P. syringae pv. phaseolicola were Tox
2
and did not produce
the expected PCR amplification using a primer pair specific
for ORF6 (Rico et al., 2003), which is essential for phaseo-
lotoxin biosynthesis and is routinely used as a target for the
detection of this pathogen (Schaad et al., 1995). Addition-
ally, Tox
2
isolates did not show hybridization to an ORF6-
specific DNA probe (Rico et al., 2003), suggesting the
absence of part or of the entire argK-tox gene cluster. This
raised the possibility that the Spanish Tox
2
isolates were
genetically separable from the more common isolates that
synthesize phaseolotoxin. In this study, we analyse the
genetic variability within the Spanish P. syringae pv. phaseo-
licola population, in comparison with P. syringae pv.
phaseolicola and P. syringae pv. glycinea isolates from
international collections. Collectively, our results allowed
the differentiation of two genetic lineages in P. syringae
pv. phaseolicola and suggest the separate evolution of
their pathogenicity gene complement.
METHODS
Bacterial strains and growth conditions.
Escherichia coli DH5a
was used for cloning purposes and was propagated in LB at 37
uC
(Sambrook et al., 1989). The type races of P. syringae pv. phaseoli-
cola 1281A (race 1), 1301A (race 3), 1302A (race 4), 1449B (race 7),
2656A (race 8) and 2709A (race 9) have been described elsewhere
(Taylor et al., 1996). Strains Hb-1b and M2/1 of P. syringae pv.
phaseolicola were isolated from beans in an unknown place and
Germany, respectively, and do not produce phaseolotoxin (Vo¨lksch
& Weingart, 1997). Another 13 Tox
+
and 24 Tox
2
P. syringae
pv. phaseolicola isolated in Spain were characterized previously
(Rico et al., 2003). P. syringae pv. phaseolicola CFBP1390 and P.
syringae pv. glycinea CFBP2214 are the pathotype strains and were
obtained from C. Manceau (INRA, Angers, France). P. syringae
pv. glycinea strains PG4180 and 49a/90 (both race 4) were obtained
from M. Ullrich (Bremen University, Bremen, Germany). P. syringae
strains were routinely grown on King’s medium B (KMB) (King
et al., 1954) at 25–28
uC.
PCR analysis.
Genetic variability among P. syringae strains was
examined by PCR fingerprinting of repetitive DNA sequences using
primers for extragenic repetitive consensus (ERIC), repetitive extra-
genic palindromic (REP) and the arbitrarily primed PCR (AP-PCR)
techniques. For ERIC and REP analyses, primers and reaction condi-
tions were as described by McManus & Jones (1995). AP-PCR was
carried out using the universal M13 reverse primer (59-AGCGGA-
TAACAATTTCACAGG-39) or a single 20 bp oligonucleotide primer
(59-GGTTCCGTTCAGGACGCTAC-39) complementary to the IS50
portion of Tn5, as described by Sundin & Murillo (1999). For the
amplification of phaseolotoxin biosynthetic genes, we assayed two
different primer pairs which are specific for DNA regions separated
in the genome that are essential for phaseolotoxin biosynthesis.
Primers PHA19 and PHA95 amplify a 480 bp internal fragment
from the amidinotransferase gene amtA (Marques et al., 2000;
Herna´ndez-Guzma´n & Alvarez-Morales, 2001) and primers OCTF-
03 and OCT-R amplify a 632 bp DNA fragment of the ornithine
carbamoyltransferase gene argK (Sawada et al., 2002), which confers
resistance to phaseolotoxin. Amplification of genes included in the
pathogenicity island was performed with primers DL-04523 (59-GT-
AATCGAGTCGCCGCTCTG-39) and DR-05216 (59-GAAAGTGAA-
GCGAACGCAAG-39) for avrD, and primers CL-19541 (59-GATCG-
TAAGAACGGGCGATT-39) and CR-20852 (59-CGTGCATGGTAG-
CATGTATGAA-39) for avrPphC. The exchangeable effector locus
(EEL) region of the hrp pathogenicity island (Alfano et al., 2000)
was amplified using primers avrPphE-FOR (Stevens et al., 1998) and
queA-2 (59-AATCAGGGAATCGGGGAGTT-39) within the coding
regions of the hrpK and queA genes, respectively. A 627 bp fragment
from the insertion sequence element IS801 (Romantschuk et al., 1991)
was amplified from P. syringae pv. phaseolicola strain 1449B using
primers IS801F (59-AGTCCTGCCTACACACCTCGA-39) and IS801R1
(59-GCCTCTTTGTGGAACGACAG-39). The occurrence of a chromo-
somal insertion of IS801 in P. syringae pv. phaseolicola was tested by
amplification with primers RP-1 and RP-2 (Gonza´lez et al., 1998).
For amplifications, bacterial cell suspensions of isolates grown on
KMB were prepared in 500 ml sterile distilled water and subjected to
freeze–thaw lysis. Standard PCR reactions were performed in a final
volume of 25 ml containing as template 50 ng total genomic DNA or
5 ml bacterial lysates, using either Taq DNA polymerase (Biotaq;
Bioline) or Ready To Go PCR Beads (Amersham Pharmacia Biotech).
General molecular techniques.
Total DNA was extracted using a
Puregene DNA isolation kit (Gentra Systems), according to the
474
Microbiology 150
J. A. Oguiza and others
manufacturer’s instructions. Plasmids were isolated by a modified
alkaline lysis procedure (Zhou et al., 1990) and intact native plasmids
were separated by electrophoresis on 0?6 % agarose gels in 16 TAE
as described previously (Murillo et al., 1994). PCR products were
purified using the GFX PCR DNA purification kit (Amersham
Pharmacia Biotech). DNA sequencing was performed by MWG-
Biotech AG. Nucleotide sequences were aligned using
CLUSTALW
(Thompson et al., 1997) and database comparisons were made via
the
BLASTN
,
BLASTP
and
TBLASTX
algorithms (Altschul et al., 1997).
Preliminary sequence data from P. syringae pv. tomato DC3000
and pv. syringae B728a genome projects were obtained from The
Institute for Genomic Research (http://www.tigr.org) and the DOE
Joint Genome Institute (http://www.jgi.doe.gov) websites, respectively.
For Southern blots, chromosomal DNA was routinely digested with
appropriate restriction enzymes, and DNA fragments separated by
electrophoresis in 1 % agarose gels were transferred to a nylon
membrane (Roche Diagnostics). For the preparation of DNA probes,
specific DNA fragments were gel-extracted and cloned into the
pGEM-T Easy vector (Promega). After restriction digestion, the inserts
were separated by electrophoresis, excised from the gels and used as
probes. Preparation of labelled probes with digoxigenin, Southern
hybridization and detection of the hybridized DNA were carried out
with the DIG DNA labelling and detection kit (Roche Diagnostics).
Heteroduplex mobility assay (HMA).
The sequence polymorph-
ism of the internal transcribed spacer (ITS) region between 16S and
23S rRNA genes was analysed using a DNA HMA (Delwart et al.,
1993). The ITS region was amplified using primers D21 and D22
(Manceau & Horvais, 1997) and PCR products were migrated in
5 % polyacrylamide gels (Delwart et al., 1993).
RESULTS
A group of P. syringae pv. phaseolicola isolates
lack the phaseolotoxin biosynthetic gene
cluster
In a previous study (Rico et al., 2003), 94 Spanish Tox
2
isolates lacked ORF6, which is contained in the argK-tox
gene cluster and used for detection purposes (Schaad et al.,
1995; Zhang & Patil, 1997). By PCR amplification and
DNA hybridization we tested the conservation of the
argK-tox gene cluster among a collection of six P. syringae
pv. phaseolicola type races, 13 Tox
+
Spanish isolates, 24
Spanish Tox
2
isolates and the two Tox
2
strains Hb-1b and
M2/1, isolated elsewhere. We focused on genes argK and
amtA, which currently define the ends of the cluster, for
their importance in the detection of this pathogen (Schaad
et al., 1995; Herna´ndez-Guzma´n & Alvarez-Morales, 2001).
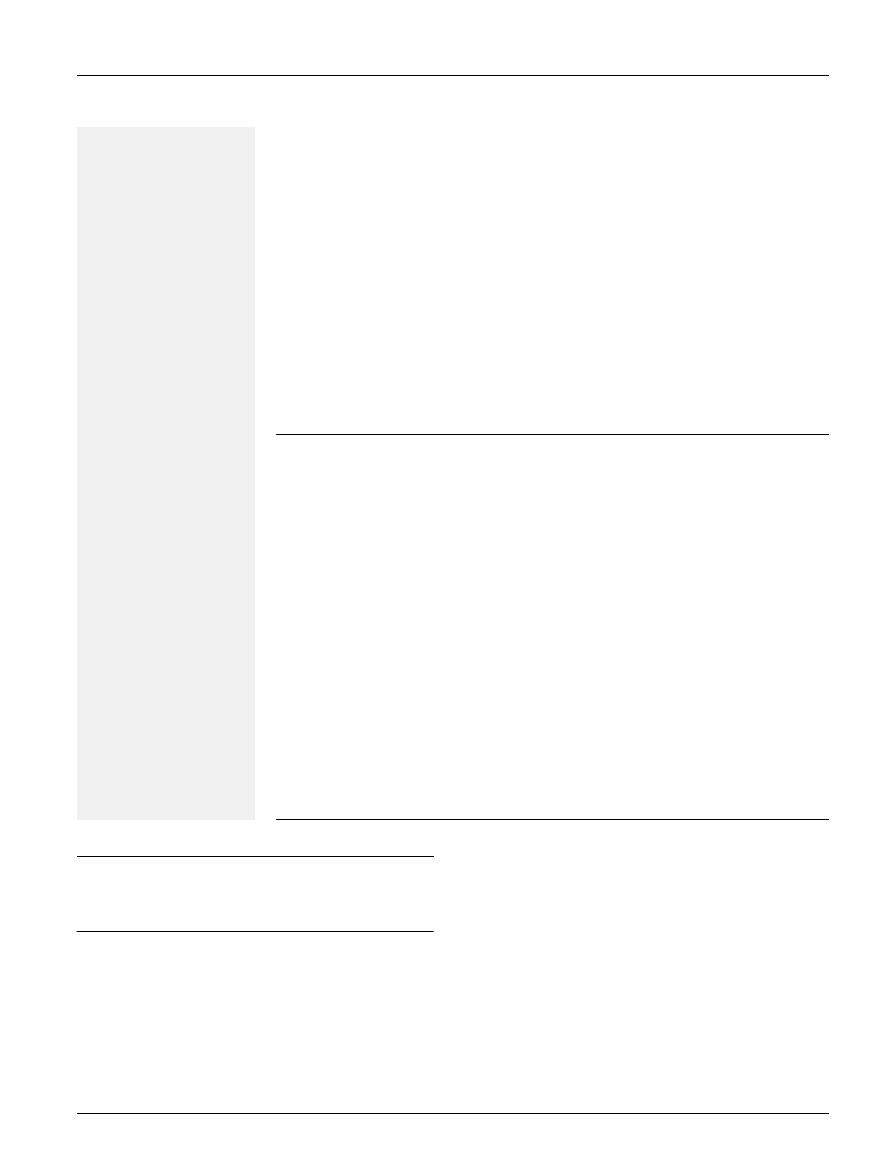
PCR amplification using primers internal to amtA (Fig. 1a)
and argK (not shown) yielded the expected 480 and 632 bp
amplification products, respectively, for all the Tox
+
iso-
lates tested, as well as for the Tox
2
isolates Hb-1b and
M2/1. Conversely, no strong specific amplicons were
observed for any of the Spanish Tox
2
isolates or for P.
syringae pv. glycinea strains PG4180 and 49a/90 (Fig. 1a).
We determined that the published sequence of primer
PHA19 (Marques et al., 2000) showed two mismatches in
its 59 end with the sequence of the amtA gene deposited in
the databases (accession no. AF186235; Herna´ndez-Guzma´n
& Alvarez-Morales, 2001). Although the argK gene was
shown to be highly conserved (Sawada et al., 1999), these
results suggest that the observed lack of amplification
observed for some of the Tox
2
isolates might be due to
possible sequence variations in their argK-tox gene cluster
with respect to the primers used. We therefore examined the
conservation of this cluster by DNA hybridization.
Internal fragments of amtA and argK were amplified as
above from the Tox
+
strain 1449B, labelled with digoxi-
genin and used as probes in Southern analysis of the selected
45 isolates detailed above. As expected, all the strains that
produced specific PCR bands with the two primer pairs
also showed hybridization to the amtA and argK probes
(Fig. 1b and not shown). In all cases, the homologous DNA
was located to a 0?8 kb EcoRI fragment for the amtA probe
(Fig. 1b) and to an 8 kb HindIII fragment for the argK
probe (not shown). On the other hand, the strains that did
not produce specific PCR amplification products did not
Fig. 1. Detection of the argK-tox gene cluster. (a) PCR amplifi-
cation of a 480 bp fragment from the amtA gene using primers
PHA19/PHA95 (Marques et al., 2000; Herna´ndez-Guzma´n &
Alvarez-Morales, 2001). Lanes: 1, P. syringae pv. phaseolicola
(Pph) isolate 1281A; 2, 2709A; 3, 1449B; 4, CYL215;
5, CYL281; 6, CYL285; 7, CYL207; 8, CYL283; 9, CYL286;
10, CYL233; 11, CYL275; 12, CYL325; 13, CYL352; 14,
CYL309; 15, CYL314; P. syringae pv. glycinea (Pgy) isolates
16, 49a/90; 17, PG4180. Pph1 and Pph2 correspond to the
two genetic lineages of P. syringae pv. phaseolicola. (b)
Southern hybridization of EcoRI-digested total DNA. An internal
fragment of the amtA gene was amplified from P. syringae pv.
phaseolicola strain 1449B with primers PHA19/PHA95, labelled
with digoxigenin and used as probe. Lanes are as described
above. Sizes are indicated to the left in kb.
http://mic.sgmjournals.org
475
Genetic lineages of P. syringae pv. phaseolicola
hybridize with either of the two probes, suggesting that they
may lack the entire argK-tox gene cluster. We propose to
designate the group of strains containing the argK-tox
gene cluster Pph1, and the group of strains lacking this
cluster Pph2.
Isolates containing or lacking the argK-tox
gene cluster can be differentiated into two
groups by REP-PCR
The phaseolotoxin biosynthetic cluster appears to have
been acquired by horizontal gene transfer (Sawada et al.,
1997, 1999) and, as a consequence it is possible that the
P. syringae pv. phaseolicola isolates containing this DNA
and those lacking it might represent distinct genetic
lineages. We used PCR fingerprinting of repetitive DNA
sequences (REP-PCR) to assess the genetic diversity among
the above 21 Pph1 and 24 Pph2 isolates. We also analysed
two strains of P. syringae pv. glycinea, because strains of
this pathovar also lack the argK-tox gene cluster and are
closely related phylogenetically to P. syringae pv. phaseoli-
cola (Gardan et al., 1999; Marques et al., 2000; Yamamoto
et al., 2000).
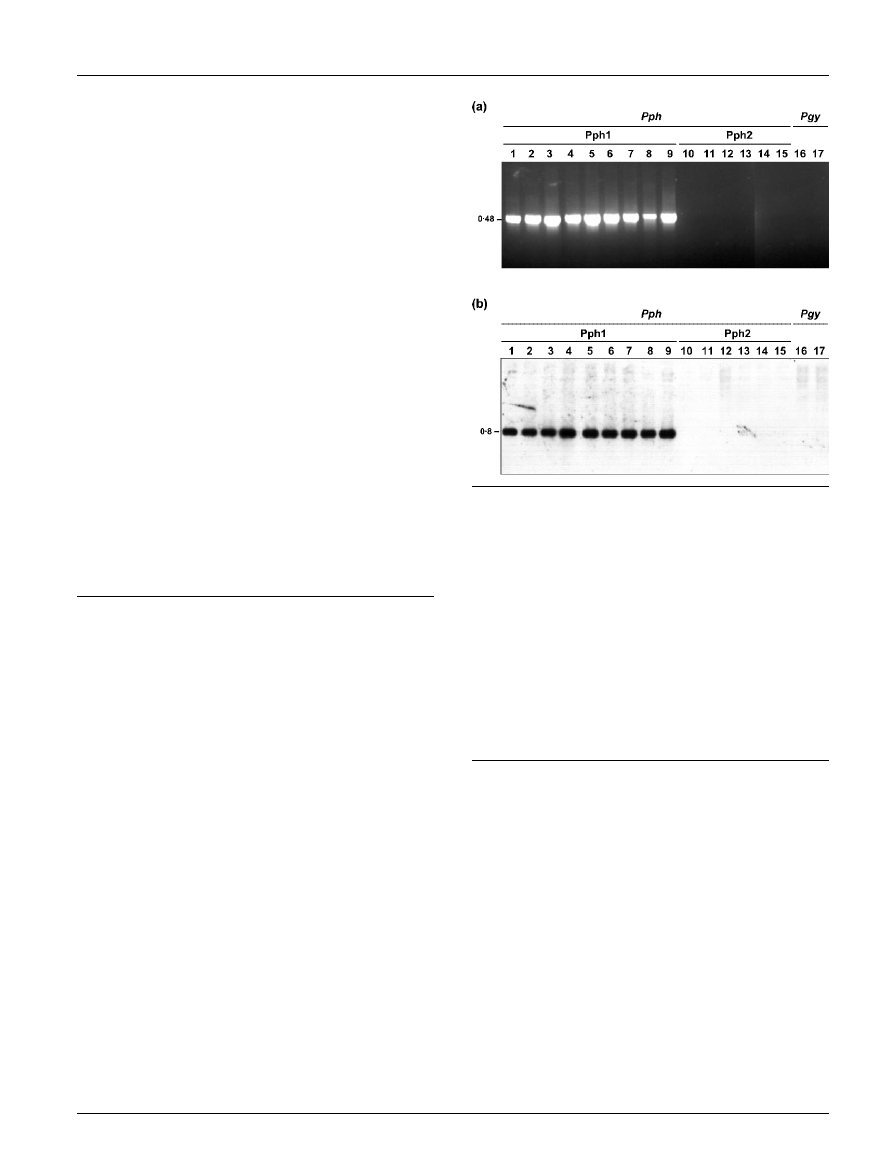
The REP-PCR amplification profiles were similar among all
isolates examined (Fig. 2), although strains of P. syringae
pv. phaseolicola showed several strong differential bands
that allowed their distinction from the P. syringae pv.
glycinea isolates. One of these was a 1700 bp band present
in the ERIC profile (Fig. 2). Additionally, strains belonging
to Pph1 and Pph2 could be distinguished on the basis of
significant differences in their REP-PCR banding profiles
(Fig. 2). Besides several minor differential bands, a strong
734 bp band was present in the IS50 profile of all the Pph1
strains (Fig. 2), independently of their place of isolation.
Hybridization experiments showed that the 45 P. syringae
pv. phaseolicola isolates examined contained several frag-
ments with homology to the sequences included in the
734 bp fragment (not shown). However, the pattern of
hybridization to the probe showed significant differences
between Pph1 and Pph2 isolates (not shown), indicating
the existence of more dissimilarities than those revealed by
REP-PCR. The analysis of the nucleotide sequence of the
734 bp band, obtained in this work, indicated that it is a
mosaic (Table 1) that probably resulted from a reorganiza-
tion event. Comparison with the databases showed that
parts of this sequence are also repeated and scattered in
different positions of the P. syringae pv. tomato DC3000
genome and plasmid pDC3000A (Table 1).
All the Pph2 isolates showed a characteristic REP-PCR
profile that included two strong differential bands: a
1289 bp band present in the ERIC profile and a 295 bp
band amplified by the IS50 primer (Fig. 2). The nucleotide
sequences of the 1289 and 295 bp bands were also deter-
mined and analysed. The 1289 bp band appears to be well
conserved, since its nucleotide sequence was highly con-
served in the genomes of P. syringae pv. tomato DC3000 and
pv. syringae B728a (Table 1) and because the P. syringae
pv. glycinea strains contained a co-migrating band (Fig. 2).
All Pph1 and Pph2 isolates showed a unique 10 kb EcoRI
hybridization band in Southern experiments using the
1289 bp fragment as a probe (not shown). In contrast, the
295 bp band showed strong hybridization only to genomic
DNA from Pph2 isolates, and the homologous DNA was
localized to a native plasmid of 40–50 kb (not shown). The
comparison of the nucleotide sequence of the 295 bp band
with the databases suggests that it is a chimera of sequences
that are separated in other P. syringae strains (Table 1).
Conservation of the exchangeable effector loci
The hrp cluster encodes a type III secretion system that
injects specialized proteins, or effectors, into the plant host
cell; these effectors appear to be the main host range deter-
minants, promoting pathogenicity or defence reactions of
the plant. In P. syringae, the hrp cluster is bordered by two
DNA regions containing diverse effector genes (Alfano et al.,
Fig. 2. Repetitive PCR fingerprinting (ERIC, IS50, Reverse and
REP) patterns of P. syringae pv. phaseolicola (Pph) and P.
syringae pv. glycinea (Pgy) isolates. M, 1 kb DNA ladder
(Promega). Lanes 1–17 are as described in the legend to
Fig. 1. The size (in bp) of the differential bands observed in the
ERIC and IS50 profiles are indicated.
476
Microbiology 150
J. A. Oguiza and others

2000). One of them, the exchangeable effector locus (EEL),
begins 3 nt downstream of the stop codon of the hrp gene
hrpK and ends near tRNA
Leu
, queA and tgt sequences, which
are highly conserved among different Pseudomonas species.
The size and gene sequence of the EEL are highly diverse
among different isolates of P. syringae (Charity et al., 2003;
Deng et al., 2003).
The EEL region from different isolates belonging to both
groups of P. syringae pv. phaseolicola and from P. syringae
pv. glycinea strains PG4180 and 49a/90 was amplified by
PCR using primers located within the coding regions of
genes hrpK and queA. Identical 2?4 kb PCR amplification
products were observed for all the isolates examined (not
shown), suggesting that the EEL region is conserved among
Pph1, Pph2 and P. syringae pv. glycinea. The EEL sequence
(1083 bp) between gene queA and the effector gene avrPphE,
located immediately downstream of hrpK, was determined
for one representative isolate each of Pph1 (strain 1449B),
Pph2 (strain CYL325) and P. syringae pv. glycinea (strain
49a/90). Pairwise comparison showed from one to a maxi-
mum of three nucleotide differences, indicating a high
degree of conservation. The analysis of the 1083 bp EEL
sequence showed the presence of an ORF homologous
(85 % identity) to ORF3 (eelF1) located in the EEL region
of P. syringae pv. tomato DC3000 (Alfano et al., 2000;
Charity et al., 2003).
The 150 kb virulence plasmid of Pph1 is not
present in Pph2
Strains of P. syringae pv. phaseolicola usually contain a large
native plasmid of around 150 kb that, in the race 7 strain
1449B, was shown to carry the PAI (Jackson et al., 1999). We
therefore decided to evaluate the conservation and physical
location of the PAI between groups Pph1 and Pph2 by
examination of the plasmid profiles and by Southern hybri-
dization with probes specific for effector genes avrD and
avrPphC, which are located in the leftmost border and in
the centre of the PAI, respectively (Yucel et al., 1994; Jackson
et al., 1999). avrD is widely distributed in P. syringae and
restricts infection on certain soybean cultivars by triggering
a defence response, as does avrPphC. Additionally, avrPphC
also behaves as a virulence gene on bean cultivar Canadian
Wonder (Tsiamis et al., 2000).
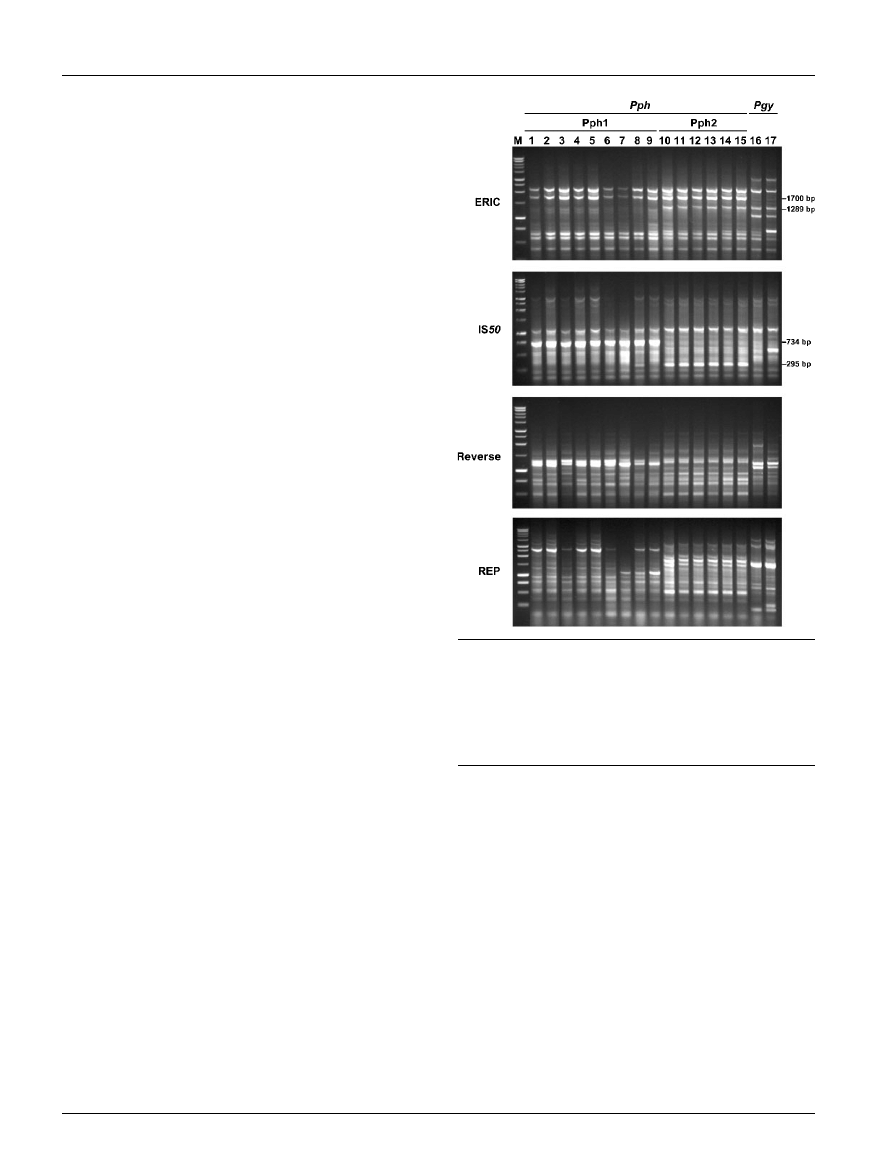
The profiles of Pph1 isolates showed diverse native plas-
mids and all of them contained a large plasmid similar to the
150 kb virulence plasmid present in strain 1449B (Fig. 3a).
In contrast, the Pph2 isolates contained one or two native
plasmids of 30–50 kb, with absence of the typical 150 kb
plasmid present in Pph1 (Fig. 3a). DNA probes specific for
genes avrD and avrPphC showed hybridization with the
large plasmid present in strain 1449B and in all the other
Pph1 isolates (Fig. 3b), indicating that the physical location
of the PAI is conserved in Pph1. Conversely, avrD did not
show hybridization with any of the plasmids of the Pph2
isolates (Fig. 3b), although it hybridized to a 5?6 kb HindIII
fragment when digested total genomic DNA was used
instead of intact native plasmids (not shown). The avrPphC
probe, however, hybridized to a single plasmid of 40–50 kb
in each Pph2 isolate (Fig. 3b). These results suggest a dif-
ferent organization of the pathogenicity genes included in
the PAI among Pph1 and Pph2 isolates.
IS801 insertion patterns are different for Pph1
and Pph2
The 1512 nt insertion sequence element IS801 has a limited
distribution among P. syringae (Romantschuk et al., 1990,
1991) and is thought to produce permanent insertions
due to its putative replicative transposition mechanism
(Mendiola et al., 1994; Richter et al., 1998). Therefore, we
examined the profile of IS801 insertions as a potential
method of fingerprinting strains of P. syringae pv. phaseoli-
cola. Genomic and plasmid DNA of selected P. syringae
Table 1. Features of the ERIC and IS50 profile bands that differentiated strains of Pph1 and Pph2
Band specificity/
size (bp)*
Primer
Position
Relevant nucleotide homologies (nucleotide position/accession no.)
Identity
(%)
Pph1/734
IS50
321–466
P. syringae pv. tomato DC3000 genome (5 346 878–5 347 023)
92
P. syringae pv. syringae B728a genome Psyr_6 (NZ_AABP020 00006)
92
365–466
P. syringae pv. tomato DC3000 plasmid pDC3000A (30 047–30 148)
96
466–734
P. syringae pv. tomato DC3000 genome (908 368–908 100)
95
Pph2/295
IS50
19–276
Plasmid pIAA1, DNA region downstream IAA lysine synthetase gene
P. syringae pv. savastanoi (M35373)
97
124–249
DNA region upstream type III effector HopPmaD gene; P. syringae
pv. maculicola (AF458043)
96
251–277
P. syringae pv. tomato DC3000 genome (16 683–16 709)
100
DNA IS801 insertion sequence element; P. syringae (X57269)
100
Pph2 and Pgy/1289
ERIC
1–1289
P. syringae pv. tomato DC3000 genome (3 101 476–3 100 187)
82
P. syringae pv. syringae B728a genome Psyr_7 (NZ_AABP020 00007)
84
*Pgy, P. syringae pv. glycinea.
http://mic.sgmjournals.org
477
Genetic lineages of P. syringae pv. phaseolicola
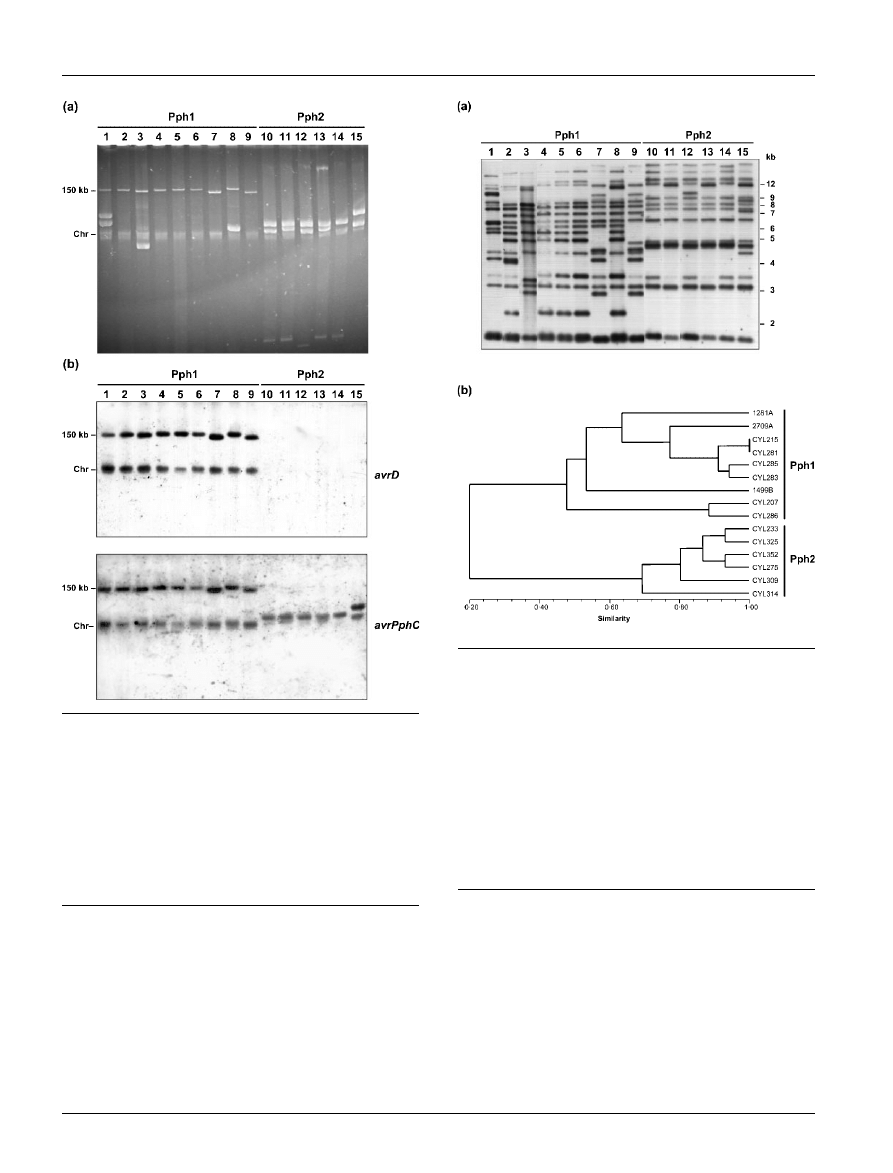
pv. phaseolicola strains was digested with PstI and subjected
to Southern hybridization using the leftmost 627 bp frag-
ment of IS801 as probe. Strains of Pph1 and Pph2 could be
unequivocally differentiated by their IS801 hybridization
fingerprint (Fig. 4a), although there was some variation in
the number and size of bands within each group. IS801
hybridization patterns were used to calculate genetic dis-
tances and to construct a tree (Fig. 4b) that clearly clustered
together isolates of each group. The hybridization pattern
of P. syringae pv. phaseolicola strain RW60, which lacks the
150 kb virulence plasmid (Jackson et al., 1999), and of PstI-
digested total plasmid DNA from several P. syringae pv.
phaseolicola strains (not shown), indicate that four to seven
hybridizing bands per strain could correspond to chromo-
somal DNA. Since most of these chromosomal insertions
were present in both Pph1 and Pph2 isolates, we explored
the conservation of previously described IS801 insertions.
Race 2 isolates of P. syringae pv. phaseolicola, but not race 1
isolates, were reported to harbour an IS801 insertion in a
putative avirulence gene, and primers RP-1 and RP-2 were
Fig. 3. Conservation of the plasmid-borne PAI among P.
syringae pv. phaseolicola isolates. Native plasmids were iso-
lated by alkaline lysis and separated uncut by electrophoresis
on a 0?6 % agarose gel in 16 TAE (a); this gel was blotted
simultaneously onto two nylon membranes and hybridized to
probes consisting of the full-length genes avrD and avrPphC
(b). Lanes 1–15 are as described in the legend to Fig. 1.
Chr, Chromosomal DNA and linearized plasmids. The size and
location of the plasmid containing the PAI in P. syringae pv.
phaseolicola strain 1449B is indicated to the left.
Fig. 4. Distribution of IS801 elements in P. syringae pv.
phaseolicola isolates. (a) Hybridization profiles with a probe
corresponding
to
the
left
region
of
the
IS801
element
(Romantschuk et al., 1991). Lanes 1–15 are as described in
the legend to Fig. 1. Sizes are indicated to the right in kb. (b)
Dendrogram obtained from cluster analysis of the IS801 hybri-
dization patterns of P. syringae pv. phaseolicola isolates. Hybri-
dization bands were scored either as present (1) or absent (0).
A similarity matrix was constructed using Jaccard’s coefficient
with the software
NTSYS
-
PC
(Rohlf, 1993). Cluster analysis was
performed with the UPGMA method using the SAHN proce-
dure and a dendrogram was constructed and plotted with the
TREE
option. The calculated cophenetic value (r) was 0?98.
478
Microbiology 150
J. A. Oguiza and others
designed to amplify a 2?7 kb-specific fragment from the
strains containing this insertion and a 1?2 kb-specific frag-
ment from the strains lacking it (Gonza´lez et al., 1998). In
our hands, however, amplification with RP-1 and RP-2
produced a 2?7 kb band from each of the 21 Pph1 strains
utilized in this study (not shown), as well as with another
111 Tox
+
P. syringae pv. phaseolicola strains from our
collection, irrespective of their race assignment. In contrast,
94 P. syringae pv. phaseolicola isolates that lacked tox-
specific DNA (Rico et al., 2003), including the 24 examined
here and P. syringae pv. glycinea strain PG4180, produced
a 1?2 kb band after PCR amplification with primers RP-1
and RP-2 (not shown). No amplification was observed for
strains P. syringae pv. glycinea 49a/90, pv. tomato DC3000
or pv. syringae B728a. However, the genome sequences
of DC3000 and B728a show high homology to the DNA
amplified from P. syringae pv. phaseolicola with RP-1 and
RP-2 (accession no. Y09452), although they do not con-
tain an IS801 insertion in this fragment, suggesting the
occurrence of primer mismatches that prevented PCR
amplification.
Pph1 and Pph2 can be differentiated by HMA
analysis of the ITS sequences
The ITS is a non-coding sequence located between the 16S
and 23S rRNA genes that is frequently used for taxonomic
studies (Gurtler & Stanisich, 1996). HMA is a PCR-based
technique (Delwart et al., 1993) that facilitates the analysis
of even minor sequence differences among the five rDNA
operons present in P. syringae and it has been successfully
used for the establishment of phylogenetic relationships
among P. syringae pathovars and other species of bacteria
(Sutra et al., 2001; Catara et al., 2002).
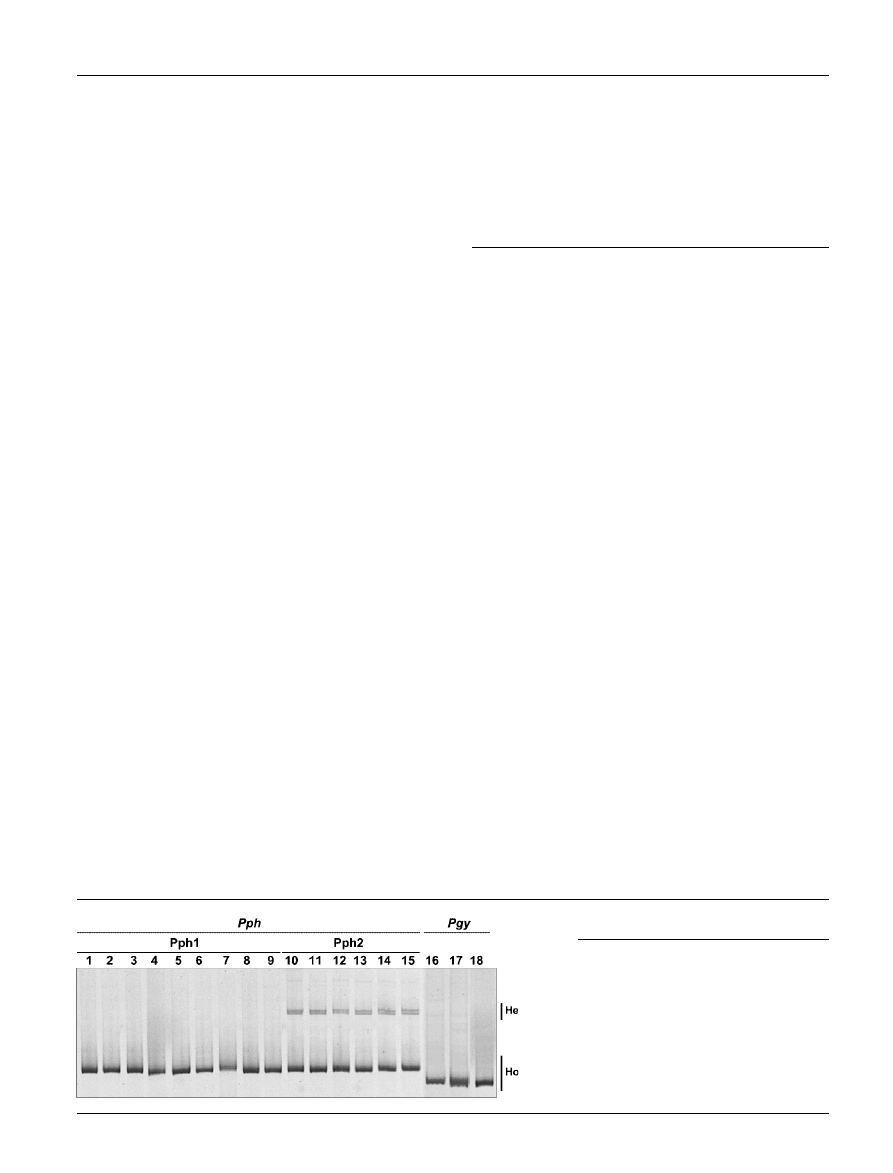
Different electrophoretic HMA profiles obtained by direct
migration of PCR-amplified ITS, indicated a clear diversity
between P. syringae pv. glycinea and P. syringae pv. phaseo-
licola and allowed the differentiation of groups Pph1 and
Pph2 (Fig. 5). The HMA profiles of all the Pph1 isolates
presented a unique homoduplex band, indicating that the
ITS copies in the different rDNA operons were identical
within each strain. Conversely, the Pph2 isolates showed a
homoduplex band that co-migrated with that observed for
Pph1 isolates, but also showed two supplementary bands
with reduced mobility that correspond to heteroduplexes,
indicating sequence differences between the ITS copies in
the different rDNA operons within each strain. For the three
P. syringae pv. glycinea strains analysed, the ITS sequences
were identical within each strain and shorter than the ITS
sequences of P. syringae pv. phaseolicola (Fig. 5).
DISCUSSION
Our results show that P. syringae pv. phaseolicola is com-
posed of at least two genetic lineages which present impor-
tant differences in their virulence gene complement and
other genetic determinants. We propose to designate these
lineages as two genomic groups, Pph1 and Pph2. The groups
differ in possession of the phaseolotoxin biosynthetic gene
cluster, REP-PCR profiles, plasmid content, the conserva-
tion of a PAI, the insertion pattern of IS801 and the HMA
profile of ITS sequences. Rico et al. (2003) previously
demonstrated that Pph2 isolates did not cross-react with a
commercial polyclonal antibody raised against the more
common Pph1 isolates, suggesting the existence of other
significant differences between these groups. All Pph2
isolates can utilize mannitol as sole carbon source (Rico
et al., 2003) while the majority of Pph1 isolates cannot
(Palleroni, 1984). Pph1, Pph2 and P. syringae pv. glycinea
strains share identical gyrB and rpoD gene sequences
(Yamamoto et al., 2000; Rico et al., 2003), although
P. syringae pv. phaseolicola and P. syringae pv. glycinea
can be readily distinguished and each pathovar has a
characteristic, although overlapping, host range (Palleroni,
1984; Vo¨lksch & Weingart, 1997; Marques et al., 2000).
Additionally, all the techniques used in this study revealed
genomic differences that allowed the clear separation of
Pph1 and Pph2, and confirmed the separation between
P. syringae pv. phaseolicola and P. syringae pv. glycinea.
ERIC- and AP-PCR have been used successfully with
P. syringae for intrapathovar strain differentiation (Louws
et al., 1994; Sundin et al., 1994; Little et al., 1998), including
P. syringae pv. phaseolicola (Vo¨lksch & Weingart, 1997;
Marques et al., 2000). AP-PCR was very discriminating in
this study and produced two strong bands that were specific
for each of the groups. Although these bands are composed
of highly repetitive DNA (see Table 1), they could be used
as potential markers for the rapid identification of Pph1
and Pph2 strains by PCR.
Fig. 5. HMA profiles of P. syringae pv. pha-
seolicola (Pph) and P. syringae pv. glycinea
(Pgy) isolates obtained by direct migration in
a 5 % polyacrylamide gel of PCR-amplified
ITS. Lanes: 1, P. syringae pv. phaseolicola
CFBP1390;
2–17, as
described
in
the
legend to Fig. 1; 18, P. syringae pv. glycinea
CFBP2214. The positions of heteroduplex
(He) and homoduplex (Ho) bands are marked.
http://mic.sgmjournals.org
479
Genetic lineages of P. syringae pv. phaseolicola

The hybridization patterns of genomic DNA to IS801
clearly distinguished Pph1 and Pph2, but the phylogenetic
significance of this observation is uncertain because most
of the hybridizing bands corresponded to plasmid DNA.
However, a chromosomal insertion of IS801 that was pres-
ent in all Pph1 strains, but absent from Pph2 and strains of
other P. syringae pathovars, could be a reliable marker for
identification. This is because IS801 belongs to a family of
insertion elements that follow a replicative rolling-circle
transposition (Mendiola et al., 1994; Richter et al., 1998),
making it likely that IS801 insertions would be perma-
nent. Also, the relatively relaxed target specificity of IS801
(Richter et al., 1998) makes the independent occurrence of
two insertions in the fragment amplified by RP-1 and RP-2
rather improbable, even more so if we take into account
the limited occurrence of IS801 chromosomal insertions in
P. syringae pv. phaseolicola. In contrast to a previous report
(Gonza´lez et al., 1998), our results show that this IS801
insertion is not race-specific.
Additional evidence for the separation of groups Pph1 and
Pph2 is provided by the different HMA patterns of the ITS
sequences, indicating the existence of sequence differences
among the ITS copies only in the Pph2 genomes. This is
significant because sequence differences in the ITS among
pathovars of P. syringae are strongly correlated with signi-
ficant genomic differences (Manceau & Horvais, 1997;
Sawada et al., 1997). By using DNA hybridization, several
restriction fragment length polymorphisms have been des-
cribed among different P. syringae pv. phaseolicola strains
(Gonza´lez et al., 2000), suggesting further variation asso-
ciated to the rDNA operons of this bacterium. We do not
know, however, if there are similar restriction site variations
that could distinguish Pph1 and Pph2.
Our results concerning genes involved in pathogenicity
also suggest the separate evolution of at least part of the
pathogenicity gene complement for Pph1 and Pph2. The
differential capacity to synthesize phaseolotoxin, which is
a putative virulence factor, is accompanied by differences
in the genomic organization of the effector genes avrD
and avrPphC. However, the EEL sequences adjacent to the
hrp cluster are highly conserved among Pph1, Pph2 and
P. syringae pv. glycinea, indicating that the genes responsible
for host range have a different genomic location.
Among many other plant-pathogenic bacteria, including
several pathovars of P. syringae, only strains of P. syringae
pv. phaseolicola and P. syringae pv. actinidiae, as well as
a single isolate of P. syringae pv. syringae, were found to
produce phaseolotoxin and contain DNA specific for this
biosynthetic gene cluster (Tourte & Manceau, 1995; Sawada
et al., 1997; Tamura et al., 2002). The complete conservation
of the argK coding sequence, as compared to the phylo-
geny of the chromosomal genes gyrB and rpoD, and the
pathogenicity-related genes hrpL and hrpS, suggests that
the argK-tox gene cluster was horizontally transferred after
the divergence of the ancestor of P. syringae into the
modern pathovars (Sawada et al., 1999). In support of this,
we showed that the internal organization of the argK-tox
gene cluster was highly conserved among diverse Pph1
strains. Moreover, all the Pph2 isolates appear to lack the
entire argK-tox gene cluster, because they failed to hybri-
dize to two specific probes that correspond to well separated
genes (amtA and argK) within this cluster. Therefore, it
seems likely that the capacity to infect beans was acquired
by P. syringae pv. phaseolicola earlier than the capacity to
synthesize phaseolotoxin. The role of this toxin in patho-
genicity is not clear, although there is some evidence that it
might increase the virulence of the infection (de la Fuente-
Martı´nez et al., 1992) or allow it to become systemic on
bean plants (Patil et al., 1974). However, the production
of phaseolotoxin is considered a defining characteristic of
P. syringae pv. phaseolicola and strains unable to synthesize
it are only rarely reported (Rudolph, 1995; Schaad et al.,
1995; Vo¨lksch & Weingart, 1998; Marques et al., 2000),
suggesting that the production of phaseolotoxin, or the
activity of other gene(s) that might have been co-transferred
with the argK-tox gene cluster, could confer an important
selective advantage.
The PAI in P. syringae pv. phaseolicola strain 1449B spans
around 30 kb of contiguous DNA located in the 150 kb
native plasmid and includes several effector genes, some
of which are involved in pathogenicity and virulence
(Jackson et al., 1999; Tsiamis et al., 2000). Other pathovars
of P. syringae contain homologues of one or more of the
genes included in this PAI, although the PAI itself is not
conserved among them and the number of genes and their
physical location (plasmid versus chromosome) is highly
variable (Jackson et al., 2002). However, the PAI would
appear to be conserved among the Pph1 group of strains
since all of them contained a large plasmid that hybridized
to both avrD- and avrPphC-specific probes. By contrast, in
all the Pph2 isolates the DNA homologous to avrD was
located in the chromosome while a plasmid smaller than
50 kb contained the avrPphC homologue.
It was suggested that kudzu strains could represent a
different group because they can also be differentiated from
other P. syringae pv. phaseolicola strains by their REP- and
ERIC-PCR fingerprints, esterase zymotypes, O-serogroup,
capacity to utilize mannitol, ethylene production and
infection of kudzu plants (Goto & Hyodo, 1987; Vo¨lksch
& Weingart, 1997; Marques et al., 2000). In our opinion, it
seems more likely that the kudzu strains could represent a
subdivision of the Pph1 group, because they also harbour
the argK-tox gene cluster and it is unlikely that this group
of genes has been independently acquired by P. syringae
pv. phaseolicola twice during evolution. In addition, strains
isolated from Vigna spp., which also possess the argK-tox
gene cluster, can also be differentiated by their ERIC-PCR
pattern and their O-serogroup (Marques et al., 2000).
Nevertheless, given the existence of several independent
characters that separate the currently delineated Pph1 and
Pph2, there is a likelihood that other possible genomic
groups may exist within P. syringae pv. phaseolicola, showing
480
Microbiology 150
J. A. Oguiza and others

characteristics intermediate between the different groups.
We have clearly demonstrated the existence of two
P. syringae pv. phaseolicola genetic lineages and provided
a basis for a clearer understanding of the mechanisms
behind the acquisition of virulence genes and their cluster-
ing in pathogenicity islands.
ACKNOWLEDGEMENTS
We wish to thank Sophie Bonneau for technical assistance with HMAs
and C. Manceau, M. Ullrich and B. Vo¨lksch for kindly supplying us
with bacterial strains. We are grateful to Trevor Williams for critical
reading of the manuscript and helpful suggestions. J. A. O. was
supported by the Ramo´n y Cajal Programme of the Spanish Ministerio
de Ciencia y Tecnologı´a (MCyT). This work was supported by grants
AGL2001-1948-CO2-01, HI2001-0081 (MCyT) and a grant from the
Departamento de Educacio´n y Cultura, Gobierno de Navarra.
REFERENCES
Alfano, J. R., Charkowski, A. O., Deng, W.-L., Badel, J. L., Petnicki-
Ocwieja, T., van Dijk, K. & Collmer, A. (2000).
The Pseudomonas
syringae Hrp pathogenicity island has a tripartite mosaic structure
composed of a cluster of type III secretion genes bounded by
exchangeable effector and conserved effector loci that contribute to
parasitic fitness and pathogenicity in plants. Proc Natl Acad Sci U S A
97, 4856–4861.
Altschul, S. F., Madden, T. L., Scha¨ffer, A. A., Zhang, J., Zhang, Z.,
Miller, W. & Lipman, D. J. (1997).
Gapped
BLAST
and
PSI
-
BLAST
: a new
generation of protein database search programs. Nucleic Acids Res 25,
3389–3402.
Catara, V., Sutra, L., Morineau, A., Achouak, W., Christen, R. &
Gardan, L. (2002).
Phenotypic and genomic evidence for the revision
of Pseudomonas corrugata and proposal of Pseudomonas mediterranea
sp. nov. Int J Syst Evol Microbiol 52, 1749–1758.
Charity, J. C., Pak, K., Delwiche, C. F. & Hutcheson, S. W. (2003).
Novel exchangeable effector loci associated with the Pseudomonas
syringae hrp pathogenicity island: evidence for integron-like assembly
from transposed gene cassettes. Mol Plant–Microbe Interact 16,
495–507.
de la Fuente-Martı´nez, J. M., Mosqueda-Cano, G., Alvarez-
Morales, A. & Herrera-Estrella, L. (1992).
Expression of a bacterial
phaseolotoxin-resistant ornithyl transcarbamylase in transgenic
tobacco confers resistance to Pseudomonas syringae pv. phaseolicola.
Biotechnology 10, 905–909.
Delwart, E. L., Shpaer, E. G., Louwagie, J., McCutchan, F. E.,
Grez, M., Rubsamen-Waigmann, H. & Mullins, J. I. (1993).
Genetic
relationships determined by a DNA heteroduplex mobility assay:
analysis of HIV-1 env genes. Science 262, 1257–1261.
Deng, W.-L., Rehm, A. H., Charkowski, A. O., Rojas, C. M. &
Collmer, A. (2003).
Pseudomonas syringae exchangeable effector loci:
sequence diversity in representative pathovars and virulence function
in P. syringae pv. syringae B728a. J Bacteriol 185, 2592–2602.
Gardan, L., Shafik, H., Belouin, S., Broch, R., Grimont, F. & Grimont,
P. A. D. (1999).
DNA relatedness among the pathovars of Pseudo-
monas syringae and description of Pseudomonas tremae sp. nov. and
Pseudomonas cannabina sp. nov. (ex Sutic and Dowson 1959). Int
J Syst Bacteriol 49, 469–478.
Gonza´lez, A. I., Ruiz, M. L. & Polanco, C. (1998).
A race-specific
insertion of transposable element IS801 in Pseudomonas syringae
pv. phaseolicola. Mol Plant–Microbe Interact 11, 423–428.
Gonza´lez, A. J., Landeras, E. & Mendoza, M. C. (2000).
Pathovars of
Pseudomonas syringae causing bacterial brown spot and halo blight in
Phaseolus vulgaris L. are distinguishable by ribotyping. Appl Environ
Microbiol 66, 850–854.
Goto, M. & Hyodo, H. (1987).
Ethylene production by cell-free
extract of the kudzu strains of Pseudomonas syringae pv. phaseolicola.
Plant Cell Physiol 28, 405–414.
Gurtler, V. & Stanisich, V. A. (1996).
New approaches to typing and
identification of bacteria using the 16S–23S rDNA spacer region.
Microbiology 142, 3–16.
Herna´ndez-Guzma´n, G. & Alvarez-Morales, A. (2001).
Isolation
and characterization of the gene coding for the amidinotransferase
involved in the biosynthesis of phaseolotoxin in Pseudomonas
syringae pv. phaseolicola. Mol Plant–Microbe Interact 14, 545–554.
Jackson, R. W., Athanassopoulos, E., Tsiamis, G. & 7 other authors
(1999).
Identification of a pathogenicity island, which contains genes
for virulence and avirulence, on a large native plasmid in the bean
pathogen Pseudomonas syringae pathovar phaseolicola. Proc Natl
Acad Sci U S A 96, 10875–10880.
Jackson, R. W., Mansfield, J. W., Ammouneh, H. & 13 other authors
(2002).
Location and activity of members of a family of virPphA
homologues in pathovars of Pseudomonas syringae and P. savastanoi.
Mol Plant Pathol 3, 205–216.
King, E. O., Ward, N. K. & Raney, D. E. (1954).
Two simple media for
the demonstration of pyocyanin and fluorescein. J Lab Clin Med 44,
301–307.
Little, E. L., Bostock, R. M. & Kirkpatrick, B. C. (1998).
Genetic
characterization of Pseudomonas syringae pv. syringae strains from
stone fruits in California. Appl Environ Microbiol 64, 3818–3823.
Louws, F. J., Fullbright, D. W., Stephens, C. T. & de Bruijn,
F. J. (1994).
Specific genomic fingerprints of phytopathogenic
Xanthomonas and Pseudomonas pathovars and strains generated
with repetitive sequences and PCR. Appl Environ Microbiol 60,
2286–2295.
Manceau, C. & Horvais, A. (1997).
Assessment of genetic diversity
among strains of Pseudomonas syringae by PCR-restriction fragment
length polymorphism analysis of rRNA operons with special
emphasis on P. syringae pv. tomato. Appl Environ Microbiol 63,
498–505.
Marques, A. S. dos A., Corbie`re, R., Gardan, L., Tourte, C.,
Manceau, C., Taylor, J. D. & Samson, R. (2000).
Multiphasic
approach for the identification of the different classification levels of
Pseudomonas savastanoi pv. phaseolicola. Eur J Plant Pathol 106,
715–734.
McManus, P. S. & Jones, A. L. (1995).
Genetic fingerprinting of
Erwinia amylovora strains isolated from tree-fruit crops and Rubus
spp. Phytopathology 85, 1547–1553.
Mendiola, M. V., Bernales, I. & de la Cruz, F. (1994).
Differential
roles of the transposon termini in IS91 transposition. Proc Natl Acad
Sci U S A 91, 1922–1926.
Mitchell, R. E. (1978).
Halo blight of beans: toxin production by
several Pseudomonas phaseolicola isolates. Physiol Plant Pathol 13,
37–49.
Murillo, J., Shen, H., Gerhold, D., Sharma, A. K., Cooksey, D. A. &
Keen, N. T. (1994).
Characterization of pPT23B, the plasmid
involved in syringolide production by Pseudomonas syringae pv.
tomato PT23. Plasmid 31, 275–287.
Nagahama, K., Yoshino, K., Matsuloa, M., Sato, M., Tanase, S.,
Ogawa, T. & Fukuda, H. (1994).
Ethylene production by strains
of the plant-pathogenic bacterium Pseudomonas syringae depends
upon the presence of indigenous plasmids carrying homologous
genes for the ethylene-forming enzyme. Microbiology 140, 2309–2313.
http://mic.sgmjournals.org
481
Genetic lineages of P. syringae pv. phaseolicola

Palleroni, N. J. (1984).
Genus I. Pseudomonas. In Bergey’s Manual of
Systematic Bacteriology, pp. 141–199. Edited by N. R. Krieg & J. G.
Holt. Baltimore, MD: Williams & Wilkins.
Patil, S. S., Hayward, A. C. & Emmons, R. (1974).
An ultraviolet-
induced non-toxigenic mutant of Pseudomonas phaseolicola of altered
pathogenicity. Phytopathology 64, 590–595.
Richter, G. Y., Bjo¨rklo¨f, K., Romantschuk, M. & Mills, D. (1998).
Insertion specificity and trans-activation of IS801. Mol Gen Genet
260, 381–387.
Rico,
A.,
Lo´pez,
R.,
Asensio,
C.,
Aizpu´n,
M.,
Asensio-
S.-Manzanera, C. & Murillo, J. (2003).
Nontoxigenic strains of
P. syringae pv. phaseolicola are a main cause of halo blight of beans
in Spain and escape current detection methods. Phytopathology 93,
1553–1559.
Rohlf,
F.
J.
(1993).
NTSYS-PC
Numerical
Taxonomy
and
Multivariate Analysis System. Version 1.8. Setauket, NY: Exeter
Publishing Ltd.
Romantschuk, M., Zhao, Y., McCluskey, K., Williams, J. & Mills, D.
(1990).
Repeated sequences in Pseudomonas syringae pv. phaseoli-
cola; distribution and possible function as insertion sequences.
Symbiosis 8, 21–31.
Romantschuk, M., Richter, G. Y., Mukhopadhyay, P. & Mills, D.
(1991).
IS801, an insertion sequence element isolated from Pseudo-
monas syringae pathovar phaseolicola. Mol Microbiol 5, 617–622.
Rudolph, K. W. E. (1995).
Pseudomonas syringae pathovars. In
Pathogenesis and Host Specificity in Plant Diseases, pp. 47–138. Edited
by U. S. Singh, R. P. Singh & K. Kohmoto. Oxford: Elsevier.
Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989).
Molecular Cloning:
a Laboratory Manual, 2nd edn. Cold Spring Harbor, NY: Cold
Spring Harbor Laboratory.
Sawada, H., Takeuchi, T. & Matsuda, I. (1997).
Comparative analysis
of Pseudomonas syringae pv. actinidiae and pv. phaseolicola based on
phaseolotoxin-resistant ornithine carbamoyltransferase gene (argK)
and 16S–23S rRNA intergenic spacer sequences. Appl Environ
Microbiol 63, 282–288.
Sawada, H., Suzuki, F., Matsuda, I. & Saitou, N. (1999).
Phylo-
genetic analysis of Pseudomonas syringae pathovars suggests the
horizontal gene transfer of argK and the evolutionary stability of hrp
gene cluster. J Mol Evol 49, 627–644.
Sawada, H., Kanaya, S., Tsuda, M., Suzuki, F., Azegami, K. &
Saitou, N. (2002).
A phylogenomic study of the OCTase genes in
Pseudomonas syringae pathovars: the horizontal transfer of the argK-
tox cluster and the evolutionary history of OCTase genes on their
genomes. J Mol Evol 54, 437–457.
Schaad, N. W., Cheong, S. S., Tamaki, S., Hatziloukas, E. &
Panopoulos, N. J. (1995).
A combined biological and enzymatic
amplification (BIO-PCR) technique to detect Pseudomonas syringae
pv. phaseolicola in bean seed extracts. Phytopathology 85, 243–248.
Stevens, C., Bennett, M. A., Athanassopoulos, E., Tsiamis, G.,
Taylor, J. D. & Mansfield, J. W. (1998).
Sequence variations in alleles
of the avirulence gene avrPphE.R2 from Pseudomonas syringae
pv. phaseolicola lead to loss of recognition of the AvrPphE protein
within bean cells and a gain in cultivar-specific virulence. Mol
Microbiol 29, 165–177.
Sundin, G. W. & Murillo, J. (1999).
Functional analysis of the
Pseudomonas syringae rulAB determinant in tolerance to ultraviolet
B (290–320 nm) radiation and distribution of rulAB among P.
syringae pathovars. Environ Microbiol 1, 75–88.
Sundin, G. W., Demezas, D. H. & Bender, C. L. (1994).
Genetic and
plasmid diversity within natural populations of Pseudomonas
syringae with various exposures to copper and streptomycin
bactericides. Appl Environ Microbiol 60, 4421–4431.
Sutra, L., Bonneau, S., Hardy, S. & Gardan, L. (2001).
Assessment of
the genetic diversity of Pseudomonas syringae group using a DNA
heteroduplex mobility assay performed on the internal transcribed
spacer (ITS). In 11th Congress of the Mediterranean Phytopathological
Union, pp. 13–15. Evora, Portugal.
Tamura, K., Imamura, M., Yoneyama, K., Kohno, Y., Takikawa, Y.,
Yamaguchi, I. & Takahashi, H. (2002).
Role of phaseolotoxin
production by Pseudomonas syringae pv. actinidiae in the formation
of halo lesions of kiwifruit canker disease. Physiol Mol Plant Pathol
60, 207–214.
Taylor, J. D., Teverson, D. M., Allen, D. J. & Pastor-Corrales, M. A.
(1996).
Identification and origin of races of Pseudomonas syringae pv.
phaseolicola from Africa and other bean growing areas. Plant Pathol
45, 469–478.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F. &
Higgins, D. G. (1997).
The
CLUSTALX
windows interface: flexible
strategies for multiple sequence alignment aided by quality analysis
tools. Nucleic Acids Res 25, 4876–4882.
Tourte, C. & Manceau, C. (1995).
A strain of Pseudomonas syringae
which does not belong to pathovar phaseolicola produces phaseolo-
toxin. Eur J Plant Pathol 101, 483–490.
Tsiamis, G., Mansfield, J. W., Hockenhull, R. & 8 other authors
(2000).
Cultivar specific avirulence and virulence functions assigned
to avrPphF in Pseudomonas syringae pv. phaseolicola, the cause of
bean halo-blight disease. EMBO J 19, 3204–3214.
Vo¨lksch, B. & Weingart, H. (1997).
Comparison of ethylene-
producing Pseudomonas syringae strains isolated from kudzu
(Pueraria lobata) with Pseudomonas syringae pv. phaseolicola and
Pseudomonas syringae pv. glycinea. Eur J Plant Pathol 103, 795–802.
Vo¨lksch, B. & Weingart, H. (1998).
Toxin production by pathovars of
Pseudomonas syringae and their antagonistic activities against
epiphytic microorganisms. J Basic Microbiol 38, 135–145.
Yamamoto, S., Kasai, H., Arnold, D. L., Jackson, R. W., Vivian, A. &
Harayama, S. (2000).
Phylogeny of the genus Pseudomonas:
intrageneric structure reconstructed from the nucleotide sequences
of gyrB and rpoD genes. Microbiology 146, 2385–2394.
Yucel, I., Slaymaker, D., Boyd, C., Murillo, J., Buzzell, R. I. & Keen,
N. T. (1994).
Avirulence gene avrPphC from Pseudomonas syringae
pv. phaseolicola 3121: a plasmid-borne homologue of avrC closely
linked to an avrD allele. Mol Plant–Microbe Interact 7, 677–679.
Zhang, Y. X. & Patil, S. S. (1997).
The phtE locus in the
phaseolotoxin gene cluster has ORFs with homologies to genes
encoding amino acid transferases, the AraC family of transcriptional
factors, and fatty acid desaturases. Mol Plant–Microbe Interact 10,
947–960.
Zhou, C., Yang, Y. & Jong, A. Y. (1990).
Miniprep in ten minutes.
Biotechniques 8, 172–173.
482
Microbiology 150
J. A. Oguiza and others
Wyszukiwarka
Podobne podstrony:
Pseudomonas bez fasolotoksyny
Pseudomonas
Ausgewählte polnische Germanismen (darunter auch Pseudogermanismen und Regionalismen) Deutsch als F
Pseudokibice piłkarscy, TG, ściagii, ŚCIĄGI, Ściągi itp, WOS,WOK,Przedsiębiorczość, Referaty i Ściąg
Zupa Fasolowa, przepisy
Algorytmy krokowe, blokowe i pseudokod
Zupa fasolow1, PRZEPISY
5 Pseudowychowanie, Pedagogika
Pseudo Longinos — O górności. teoria, Studia - polonistyka, egzamin z estetyki
zupa fasolowa
10. Pseudowychowanie, Psychologia, Teoretyczne podstawy wychowania
Fasolówka pikantna
Co wiesz na temat czynników chorobotwórczości i diagnostyki zakażeń Pseudomonas?ruginosa
Haemophilus, Bordatella, Pseudomonas, Brucella, Francisella, Pasteurella, Legionella
Leki zawierające efedrynę i pseudoefedrynę jako źródło metkatynonu
lichtenstein, struktury?nych i złożoność obliczeniowa,Badanie?ektywności algorytmów pseudowielomiano
356 , Pseudokibice są w każdym mieście, nawet jeśli nie ma w nim klubu sportowego
PSEUDOMONAS
więcej podobnych podstron