
The effect of gamma irradiation on mechanical, and thermal
properties of recycling polyethylene terephthalate and low density
polyethylene (R-PET/LDPE) blend compatibilized by ethylene
vinyl acetate (EVA)
K. Abdel Tawab
•
Sayeda M. Ibrahim
•
M. M. Magida
Received: 6 June 2012 / Published online: 30 August 2012
Ó Akade´miai Kiado´, Budapest, Hungary 2012
Abstract
A study has been made on the compatibility of
recycled polyethylene terephthalate (R-PET) and low den-
sity polyethylene (LDPE) blend in the presence of ethylene
vinyl acetate (EVA) as a compatibilizing agent prepared by
extrusion hot stretching process. EVA content in the blend as
a compatibilizing agent was an enhancement effect on
radiation crosslinking of R-PET/EVA/LDPE blends and the
highest radiation crosslinking was obtained when the EVA
content was reached at 10 % EVA when irradiated by
gamma irradiation. Blends containing different (EVA) ratios
were irradiated to different doses of gamma irradiation 25,
50 and 100 kGy. The effect of the compatibilizer and radi-
ation on mechanical, thermal properties of R-PET together
with LDPE and morphology has been investigated. It was
found that gamma irradiation together with the presence of
compatibilizing agent (EVA) has positive effect on the
mechanical and thermal properties of R-PET/LDPE blend.
The structural properties of R-PET/LDPE modified by
gamma irradiation and EVA as compatibilizing agent was
examined by SEM. Also, it was found that the optimum
concentration of EVA and gamma irradiation dose was
found to be 10 % EVA and 100 kGy, respectively.
Keywords
Irradiation
EVA LDPE Thermal
Introduction
Blending and alloying have long been demonstrated to be an
effective way to improve properties of an existing polymer
[
]. However, most polymer blends are thermodynamically
immiscible and also technologically non-compatible, hence
form multi-phase systems during processing [
]. Conse-
quently, the resulting blend exhibit weak interfacial adhe-
sion and thus poor mechanical properties. One of the
classical methods to ensure adhesion between the phases
(reduction of the interfacial tension) is the use of third
component, a compatibility which results in a fine and more
stable morphology, better adhesion between the phases and
consequently better mechanical properties of the final
product [
Polyethylene terephthalate (PET) is widely used in
packaging materials especially in beverage package, owing
to its good mechanical properties and excellent barrier
properties. Most of these beverage bottles are used only
once, which inevitably creates serious resource waste and
white pollution. Therefore, with the increased awareness of
the environmental protection, governments pay more and
more attention to the issue of the recycling waste recycled
polyethylene terephthalate (R-PET). Compared to other
post-consumed plastics, the source of recycled R-PET is
more stable, and R-PET bottles are easier to separate and
purified [
]. Low density polyethylene (LDPE) and ethyl-
ene vinyl acetate (EVA) have shown to be good modifiers
for improving permanent deformation and thermal crack-
ing properties [
EVA is copolymer of ethylene and vinyl acetate. It is an
extremely elastic material that can be sintered to form a
porous material similar to rubber, yet with excellent
toughness. It is three times as flexible as LDPE, showing
tensile elongation 750 % with a peak melting of 96
°C
K. Abdel Tawab
S. M. Ibrahim (
&) M. M. Magida
Department of Radiation Chemistry, National
Center for Radiation Research and Technology, P.O. Box 29,
Nasr City, Cairo, Egypt
e-mail: sayda.ibrahim@yahoo.com
123
J Radioanal Nucl Chem (2013) 295:1313–1319
DOI 10.1007/s10967-012-2163-6

(205
°F). EVA can attain same degree of crosslinking as
LDPE at lower dose 100–150 kGy. Therefore, it is possible
by blending certain composition of EVA with LDPE to
improve the irradiation crosslinking performance LDPE [
EVAs are leading polymers for hot-melt manufacturing.
EVA based hot-melts are able to fulfill various requirements
in applications such as packaging, bookbinding or labels
sticking. They are highly flexible products compatible with
many other polymers and additives, and are easy to process.
Most of researchers improved the compatibility and
mechanical properties with addition of compatibilizers
consisting of a graft or block copolymer. Usually, one side
of the copolymer is miscible with PE; the other reacts with
the functional groups of PET during reaction extrusion,
such as PE-g-GMA [
], PE-g-MA [
,
], PE-g-BHI
[
], and SEBS-g-MA [
], EVA based graft-copoly-
mers [
,
Kang et al. [
] investigated the effect of EVA
copolymer and maleic anhydride-grafted ethylene vinyl
acetate copolymer (EVA-g-MA) on poly(butylenes tere-
phthalate)/linear low density polyethylene (PBT/LLDPE)
blends. They found EVA-g-MA is better than EVA to
improve structure and mechanical properties of blends.
In this research, blends of R-PET, that comes from PET
beverage bottles and LDPE. R-PET/LDPE blend modified
by EVA was systematically changed from 0 to 10 % of the
total weight. The effect of the compatibilizer and radiation
on mechanical, thermal properties of R-PET together with
LDPE and morphology has been investigated.
Materials and experimental techniques
Materials
R-PET used in this work was based on clear bottles used for
mineral water. The bottles were first crushed into small
pieces and then granulated. The R-PET granules were then
washed with acetone and dried in vacuum oven. LDPE
pellets were produced in Lortrene, CDF Company, France,
and supplied by El-Sewedy Company for plastic industry
(Sedplast), 10th of Ramadan City, Cairo, Egypt. The density
of the LDPE is 935 kg/m
3
, melt flow index (MFI) *3.5 g/
10 min and had a crystallinity ratio of about 45 %. EVA
with MFI 135 g/10 min, melting point 67
°C contains 18 %
vinyl acetate, from Arkema Inc., North America.
Sample preparation
R-PET, LDPE and EVA were blended together with dif-
ferent ratios by using elaboratory plasticorder PL 2100,
mixer. The mixing process was carried out by setting the
temperature at 250
°C for 5 min. Thin sheets of *1.0 mm
in thickness of the blends were prepared by compression
molding in a hot press at 250
°C for 5 min.
Sample irradiation
Irradiation was carried out in air using Co-60 source at
NCRRT, Cairo, Egypt, at a dose rate of 5 kGy/h. The
integral radiation dose was selected within the range
25–100 kGy.
Measurements
Tensile strength and elongation at break
The measurements of tensile strength (T
b
) and percentage
of elongation at break (E
b
) were carried out using an
Instron model 1195, UK, at a crosshead speed of 50 mm/
min.
Differential scanning calorimetry (DSC)
Differential scanning calorimetry (DSC) studies of R-PET/
LDPE blends were performed by using (Perkin-Elmer
DSC-7 station). A heating rate of 10
°C/min was utilized
under nitrogen atmosphere over the temperature range
from 0 to 300
°C.
Scanning electron microscope (SEM)
The surface morphology indicated by Scanning electron
microscope (SEM) technique, Jeol (Japan) took the
micrographs with a JSA-5400 instrument. A sputter coater
was used to precoat conductive gold onto the fracture
surfaces before observing the micrographs at 30 kV.
Thermogravimetric analysis (TGA)
The thermogravimetric analysis (TGA) studies were car-
ried out on a Shimadzu-30 (TGA-30) at a heating rate of
10
°C/min in air over a temperature range from room
temperature up to 600
°C. The weight loss recorded for
blends upon heating from room temperature up to 600
°C
in the presence of flowing nitrogen gas and employing a
constant rate of heating.
Results and discussions
Due to the problem of polyethylene terephthalate to be
recycled as in its original behavior as a thermoplastic
polymer, where the melting process gives hard flakes like
thermosetting polymers as a result of thermal degradation
1314
K. Abdel Tawab et al.
123
in its polyethylene series giving a low molecular weight of
PET. Therefore, to avoid this problem we add LDPE to
PET through its melting process to compensate and keep-
ing the long chain series of its molecular weight. Also,
EVA should be adding to LDPE as a compatibilizing agent
to improve the compatibility with R-PET. Gamma irradi-
ation is being applied to recovered LDPE samples in effort
to improve properties of the recycled material [
]. Like
wise, gamma irradiation has been applied in an effort to
improve the properties of a blend of LDPE and HDPE
mixed waste material through crosslinking; a competing
process of oxidative chain scission has been problematic
with this approach [
Mechanical properties
Radiation influences the properties of polymer materials.
Suitable crosslinking of polymer materials is favorable to
improving the properties of polymer materials, while
excessive crosslinking made polymer materials brittle and
lost the value of industrial application. We characterized the
mechanical properties of unirradiated and irradiated R-PET/
LDPE blends with and without EVA concentrations at dif-
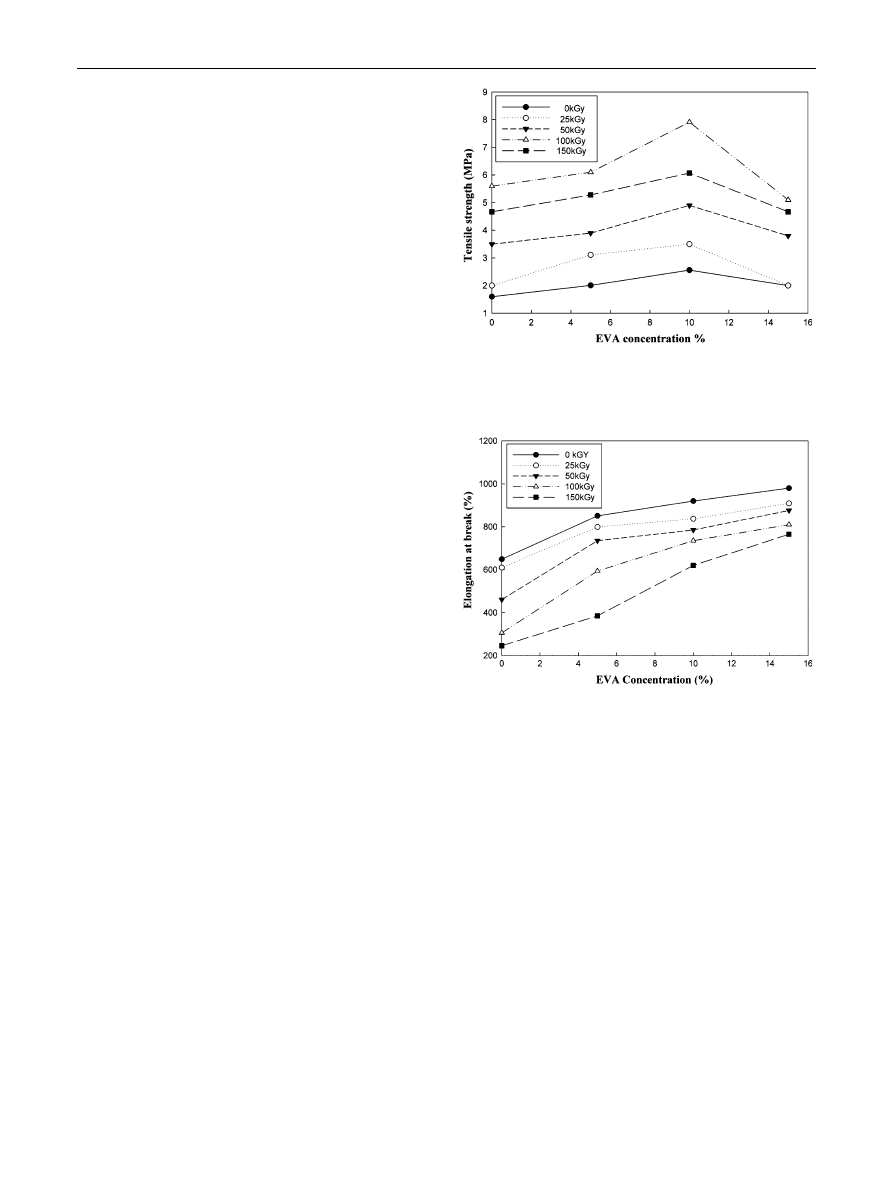
ferent irradiation doses are shown in Figs.
and
. Figure
illustrates the T
b
as a function of different EVA concentra-
tions. The results obtained from this figure indicate that the
Ts of the blend increased with increasing EVA concentration
up to 10 %. It is obvious that T
b
values are correlated to
irradiation dose. As the irradiation dose increases up to
100 kGy, the T
b
increases as shown in Fig.
. These results
identify that the radiation-resistant properties of EVA and
the impact of the progressive chain crosslinking, taking
place by radiation in the EVA content, on the overall blend
T
b
values [
]. The latter value exhibited declination with
the higher EVA concentration than 10 % accompanied with
higher doses than 100 kGy. The reduction is expectedly
caused by chain scission brought about by excessive doses.
Figure
demonstrates variation in E
b
with different EVA
concentrations at different irradiation doses. Increasing EVA
concentration results in increase in E
b
of the blends. Gener-
ally, increasing radiation dose results in reduction in E
b
of
blends. As the radiation dose increases more crosslinking is
produced in the sample matrix preventing the structural
reorganization during drawing [
]. This ever increasing
three dimensional gel-like structures brings about a decrease
in internal chain mobility and elongation [
]. Thus, the
radiation cross linking that occurs in the EVA content is
affirmed by the E
b
dependence on the blend composition.
Thermal behavior by DSC
The glass transition temperature measured by DSC is most
widely used for determining the compatibility of polymer
blends. It has usually been associated with the onset of
segmental mobility in the amorphous phase of an amor-
phous or semicrystalline polymer. Most polymers are either
completely amorphous or have an amorphous like com-
ponent even if they are crystalline. Such materials are hard
and rigid glassy below a fairly defined temperature known
as glass transition temperature (Tg). At temperatures above
the Tg, the amorphous polymers are soft and flexible.
DSC technique was used to investigate the compatibility
of R-PET/LDPE blends. Figures
and
show the DSC
thermograms of unirradiated and irradiated at 100 kGy for
R-PET/LDPE (50/50) blend compatibilized with different
ratios of EVA as a compatibilizing agent. LDPE as a
semicrystalline polymer has T
g
of -130
°C, while PET has
T
g
of 64
°C. Based on the thermodynamic theory of glass
transition temperature, the familiar Fox equation (shown
below) derived to predict the T
g
of binary mixtures of
Fig. 1
Effect of EVA concentration % on the T
b
of R-PET/LDPE
blend at different irradiation dose
Fig. 2
Effect of EVA concentration % on the E
b
% R-PET/LDPE
blend at different irradiation doses
The effect of gamma irradiation
1315
123
miscible polymers was applied to the present blend as
follow:
Fox equation:
1=T
g
¼ M
1
=T
g
1
þ M
2
=T
g
2
where Tg, Tg1 and Tg2 are the glass transition temperatures
of the final blend and the individual polymers, respectively.
M
1
and M
2
is the mass fractions of the individual polymers.
According to Fox equation:
For R-PET=LDPE 50=50
ð
Þ
1=T
g
¼ 0:5=337 þ 0:5=143; which gives
T
g
¼ 72:2
C
:
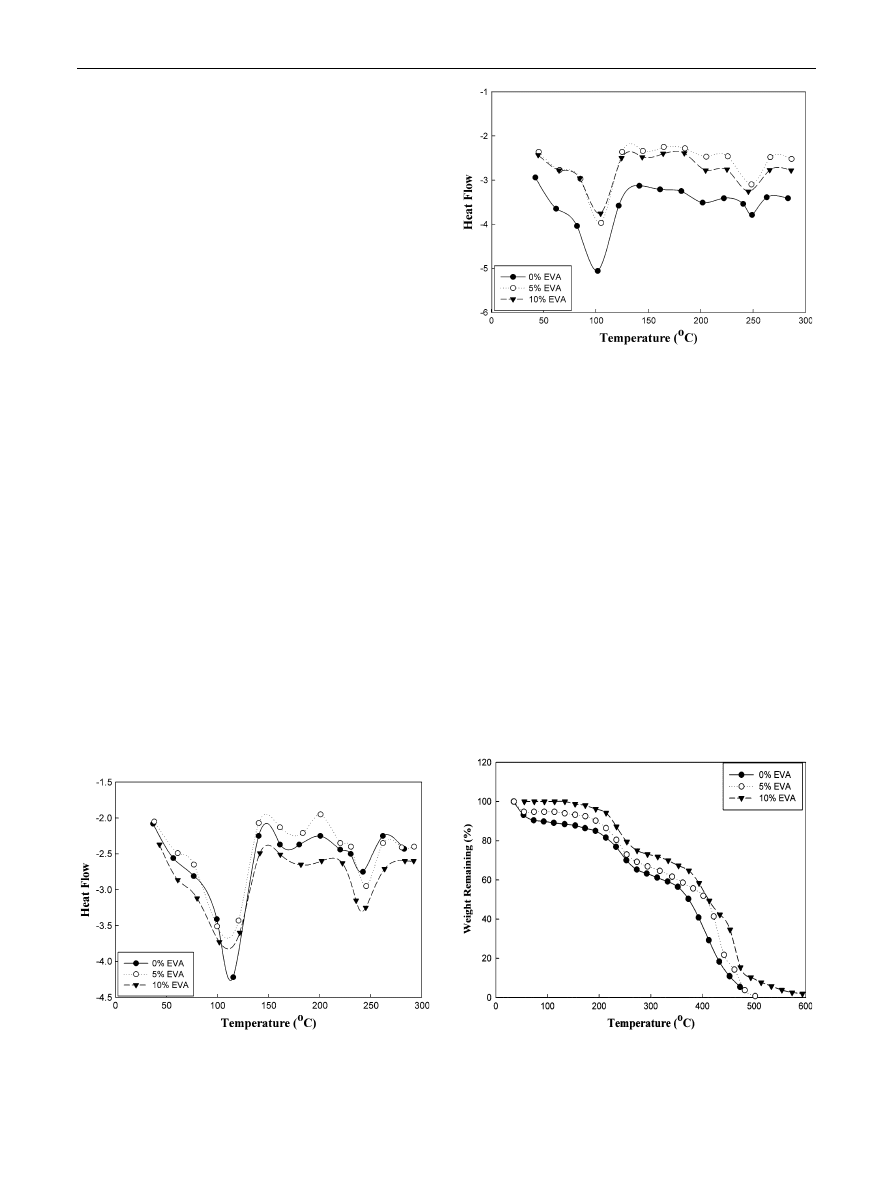
As shown in Fig.
, the DSC scans of unirradiated
R-PET/LDPE blend showed an endothermic peaks at 112
and 245
°C which are due to the T
m
of LDPE and R-PET
polymers, respectively. Also, an exothermic peak can be
seen at 200
°C, which is due to crystalline melting tem-
perature (T
c
), not that for LDPE or R-PET polymers. The
appearance of this peak is a result of non-compatibility.
The existence of two melting peaks for each polymer in the
blend which coincide exactly with peaks corresponding to
two pure components indicates no probability of the
compatibility of the crystalline phases of the two polymers.
The polymers may be intimately mixed in the molten state
but as the blends are cooled from the melt, the crystalli-
zation of different components occurs separately, leading
to two distinctly different crystalline phases. [
On the other hand, addition of EVA as a compatibilizing
agent to LDPE improves the compatibility with R-PET.
This is due to that crystallinity of EVA is lower than that of
LDPE, that is to say, the amorphous region’s content of
EVA is higher than that of LDPE. It is shown that R-PET/
LDPE blend in the presence of 10 % EVA content is
compatible in the amorphous region for all the R-PET,
LDPE and EVA as shown in Fig.
.
The disappearance of the crystallization peak is related
to the change of morphology that is caused by the addition
of 10 % EVA. This proves that EVA can be used as a
successful interfacial compatibilizer for R-PET/LDPE. It is
well known that the compatible effect results from the
miscibility between the polyethylene chains of EVA and
the polyolefins, and the compatibility of the ester groups of
EVA and R-PET. Additionally, the generation of the
transesterification reaction between EVA and R-PET can
greatly improve the interfacial interactions of the blends.
The EVA content in the blend R-PET/LDPE has an
enhancement effect on irradiation crosslinking of the
blends irradiatiated by c-ray, and the highest radiation
crosslinking of the blend is observed when EVA content is
Fig. 3
DSC scans of unirradiated R-PET/LDPE (50/50) blend
compatibilized by different concentrations of EVA content
Fig. 4
DSC scans of irradiated R-PET/LDPE (50/50) blend at
100 kGy compatibilized by different concentrations of EVA content
Fig. 5
TGA thermograms of unirradiated R-PET/LDPE (50/50)
blend compatibilized by different concentrations of EVA content
1316
K. Abdel Tawab et al.
123
10 % at a dose 100 kGy as shown in Fig.
. These blends
are compatible in the amorphous region, and the compat-
ibility and higher amorphous region’s content of the blends
are favorable to the enhancement effect of EVA on the
radiation crosslinking of R-PET/LDPE blends.
Thermal decomposition behavior
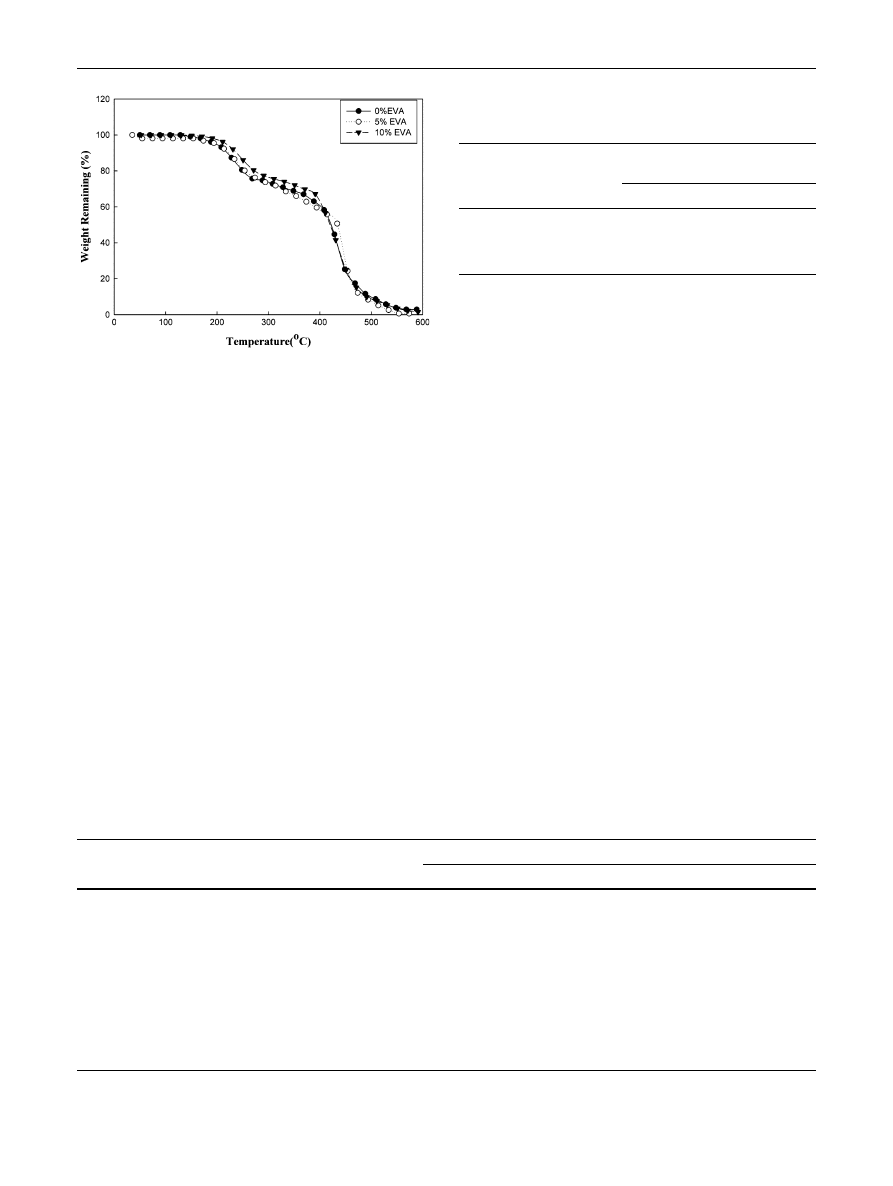
TGA is the most favored technique for comparing and
ranking the thermal stability of the polymers. Figures
and
show the TGA thermograms of R-PET/LDPE polymer
blends with and without EVA as a compatibilizing agent
with different ratios before and after they had been exposed
to different doses of gamma irradiation 50 and 100 kGy.
Table
summarizes the percentage weight loss at decom-
position temperature for the unirradiated and irradiated
polymer blends. During thermal decomposition, the TGA
curves display a two-stage decomposition process. The first
step is roughly from 200 to 450
°C, corresponding to the
decomposition of EVA i.e. PVA chain in the EVA where,
EVA is a block copolymer consisting of the chains of PE
and PVA. The second step is about from 450 to 500
°C, it
may be assigned to the decomposition of PE chains in the
LDPE, the backbone of EVA and R-PET. From Figs.
,
and Table
, it was shown that the thermal stability of
R-PET/LDPE increases with increasing the ratio of EVA
up to 10 % for the unirradiated and irradiated blends. Also,
the thermal stability of irradiated R-PET/LDPE polymer
blend with all its compositions are thermally more stable
than those of unirradiated, this is due to crosslinking. These
results illustrated that the higher thermal stability of irra-
diated blends because the radiation crosslinking increased
with increasing radiation dose and also with increasing
EVA content in the blend up to 10 % (i.e. EVA content is
an enhancement effect on the radiation crosslinking).
The rate of reaction (dw/dt) was plotted against the
heating temperatures for R-PET/LDPE blend with and
without EVA as a compatibilizing agent before and after
c-radiation with a dose 100 kGy, which is shown in Figs.
and
as an example. It can be seen that these types of
curves generally display similar trends. Also, note that all
blends, whether before or after c-radiation, showed more
than one maximum with increasing the temperature. This
Fig. 6
TGA thermograms of irradiated R-PET/LDPE (50/50) blend
at 100 kGy compatibilized by different concentrations of EVA
content
Table 1
Weight loss (%) at different decomposition temperatures for unirradiated and irradiated R-PET/LDPE (50/50) blend at different
irradiation doses compatibilized by different concentrations of EVA content
Polymer blend composition (%)
Radiation dose (kGy)
Weight loss (%)
200
°C
300
°C
350
°C
400
°C
450
°C
500
°C
R-PET/LDPE (50/50)
0
29.94
36.74
43.54
59.19
89.12
–
50
19.34
25.63
36
40
79.01
96.69
100
19.42
27.19
31.07
41.75
74.76
91.27
R-PET/EVA/LDPE (50/5/45)
0
27.07
33.09
38.35
48.13
78.2
99.25
50
10.66
24.32
27.6
40.17
75.41
93.17
100
19.88
26.29
33.98
40.39
75.65
91.67
R-PET/EVA/LDPE (50/10/40)
0
24.1
31.93
40.37
46.39
73.5
90.37
50
20.52
26.93
32.7
41.67
65.39
89.75
100
13.97
22.65
27.93
32.84
75.1
90.19
Table 2
Temperatures of maximum rate of reaction for R-PET/
LDPE with and without EVA at different ratios before and after
gamma irradiation
Polymer blend composition
Temperature of maximum rate of
reaction (
o
C)
unirradiated
100 kGy
R-PET/LDPE 50/50
392
228 and 408
R-PET/EVA/LDPE 50/5/45
421
245 and 425
R-PET/EVA/LDPE 50/10/45
230 and 430
233 and 453
The effect of gamma irradiation
1317
123
behavior indicates that the thermal decomposition of these
blends passes through two stages. The temperatures of the
maximum value of the rate of reaction (T
max
) differ from
one blend to another. The (T
max
) values of unirradiated and
irradiated R-PET/LDPE, R-PET/5 % EVA/LDPE and
R-PET/10 % EVA/LDPE are shown in Table
. Table
shows that unirradiated and irradiated R-PET/10 % EVA/
LDPE possess higher thermal stability than unmodified
Fig. 7
The rate of reaction (dw/dt) versus the temperature for
unirradiated R-PET/LDPE (50/50) blend compatibilized by different
concentrations of EVA content
Fig. 8
The rate of reaction (dw/dt) versus the temperature for
irradiated R-PET/LDPE (50/50) blend at 100 kGy compatibilized by
different concentrations of EVA content
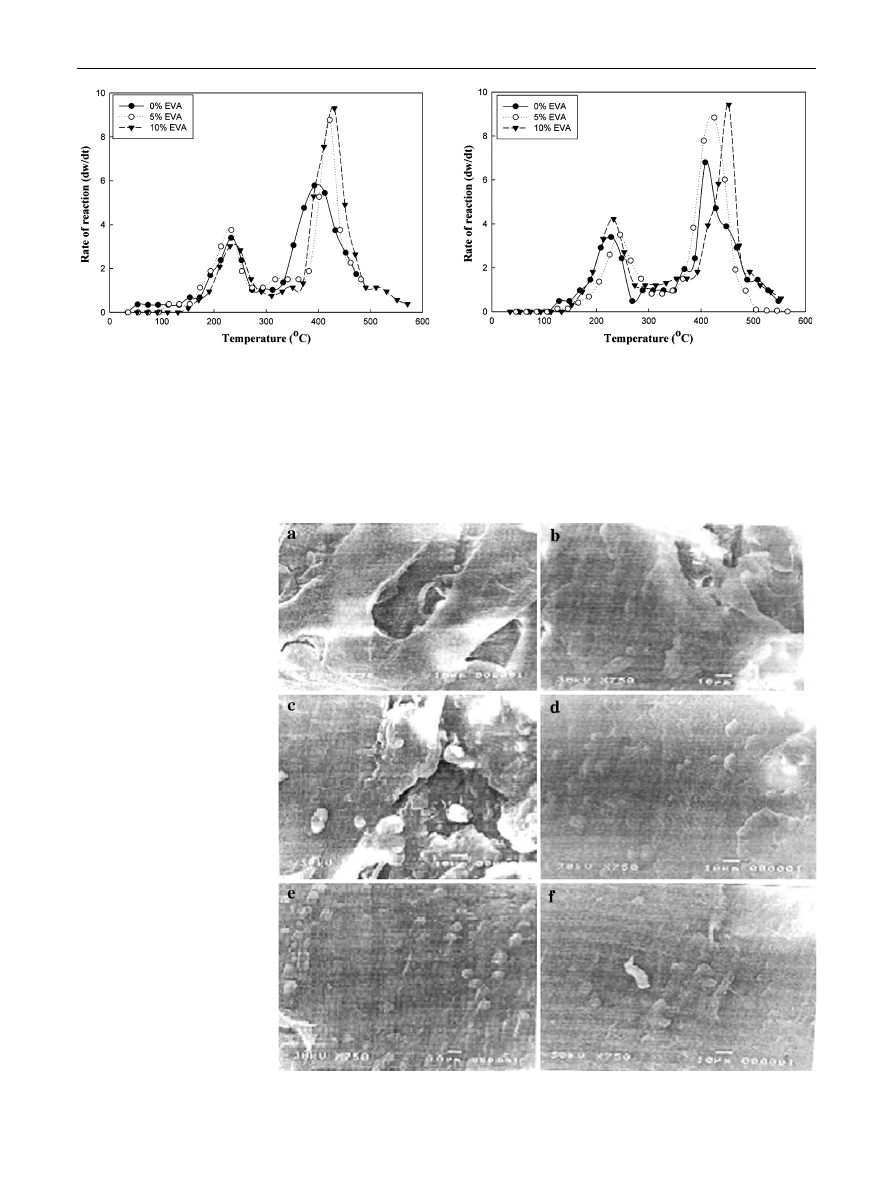
Fig. 9
SEM of the fracture
surfaces of unirradiated and
irradiated R-PET/LDPE (50/50)
blend at 100 kGy
compatibilized by different
concentrations of EVA content.
a
unirradiated 0 % EVA,
b
irradiated 0 % EVA,
c
unirradiated 5 % EVA,
d
irradiated 5 % EVA,
e
unirradiated 10 % EVA,
f
irradiated 10 % EVA
1318
K. Abdel Tawab et al.
123

R-PET/LDPE blend. This indicates that the thermal sta-
bility of R-PET/LDPE increases with increasing the ratio
of EVA up to 10 % for the unirradiated and irradiated
blends. Also, the thermal stability of irradiated R-PET/
LDPE polymer blend with all its compositions are ther-
mally more stable than those of unirradiated, this is due to
crosslinking.
Scanning electron microscope
Figure
represents the SEM micrographs of the fracture
surface of unirradiated and irradiated R-PET/LDPE blend
with and without EVA. As shown in Fig.
a it is clear that,
LDPE dispersed as the island phase in R-PET matrix. So, the
unirradiated R-PET/LDPE blend exhibited two-phase mor-
phology. A continuous phase was noticed of irradiated
R-PET/LDPE blend at 50 kGy as shown in Fig.
b. The
compatibility of the unirradiated blend is improved by adding
different concentration of EVA (5 and 10 %) as shown in
Fig.
c, e respectively. On the other hand, a smooth surface
with uniform distribution of the two polymers emphasizing
the established compatibility by irradiation as shown in
Fig.
d, f. This may be accounted for crosslinking and dis-
appearance of separation due to phase contraction. Finally,
the compatibility of the blend is improved greatly as the
content of EVA increased up to 10 % at 100 kGy.
Conclusion
This study has confirmed that Gamma-radiation has posi-
tive effects on the properties of the compatibilized blend
R-PET/EVA/LDPE. It was found that:
1.
Crosslinking of the compatibilized blend increase as
the dose increase up to100 kGy. The amount of
crosslinking in the blend increases as the composition
of EVA increase.
2.
The T
b
of the blend increased when irradiated up to
100 kGy.
And decreased with further increase in dose. Increasing
the radiation dose than 100 kGy result in reduction in E
b
of
the blend. DSC show the disappearance of the crystallization
peak, which is related to the change of morphology that is
caused by the addition of 10 % EVA. DSC scans show that
R-PET/LDPE blends compatibilized with EVA as a com-
patibilizing agent are compatible in the amorphous region,
and the compatibility and higher amorphous region’s con-
tent of the blends are favorable to the enhancement effect of
EVA on the radiation crosslinking of R-PET/EVA/LDPE
blends. This proves that EVA can be used as a successful
interfacial compatibilizer for R-PET/LDPE. Also, TGA
show that the thermal stability of R-PET/LDPE increases
with increasing the ratio of EVA up to 10 % for the unir-
radiated and irradiated blends. Similarly, crosslinking may
also suggest the irradiated R-PET/EVA/LDPE blends are
thermally more stable than the unirradiated blends. SEM
shows that the unirradiated R-PET/LDPE blend exhibited
two-phase morphology. The compatibility of the unirradi-
ated blend is improved by adding different concentration of
EVA up to 10 %. On the other hand, a smooth surface with
uniform distribution of the two polymers emphasizing the
established compatibility by irradiation as the compatibility
of the blend is improved greatly as the content of EVA
increased up to 10 % at 100 kGy.
Finally, the study offers a new type of blend with high
yield of mechanical properties which could be used in high
loaded packing processes. Also, blends can be used for
stretch packaging, medical packaging, and heavy duty bags.
References
1. Utrac LA (1995) Polymer alloys and blends. Hanser, New York
2. Favis BD (2000) In: Paul DR, Bucknall CB (eds) Polymer blends:
formulation, vol 1. Wiley, New York
3. Elias L, Fenouillot F, Majeste JC, Cassagnau Ph (2009) Aout,
19e`me congre`s Franc¸ais de me´canique. Marseille, p 24–28
4. Zhang Y, Guo W, Zhang H, Wu C (2009) Polym Degrad Stab
94:1135–1141
5. Lepe AP, Martinez FJ, Gallegos C, Gonzalez O, Munoz ME,
Santamaria A (2003) Fuel 82:1339–1348
6. Karlsson R, Isacsson U (2006) J Mater Sci 42:101–108
7. Navarro FJ, Partal P, Garcia-Morales M, Martin-Alfonso
MJ, Martinez-Boza F, Gallegos C, Bordado JCM, Diogo AC
(2009) J Ind Eng Chem 15:458–464
8. Sharif J, Abdul Aziz HS, Hashim K (2000) Radiat Phys Chem
58:191–197
9. Martinez JG, Benavides R, Guerrero C (2007) J Appl Polym Sci
104:560–565
10. Boutevin B, Lusinchi JM, Pietrasanta Y, Robin J (1996) J Polym
Eng Sci 36:879–884
11. Kim DH, Park KY, Kim JY, Suh KD (2000) J Appl Polym Sci
78:1017–1024
12. Kim DH, Park KY, Suh KD, Kim JY (2000) Pure Appl Chem A
37:1141–1152
13. Dimitrva TL, La Mantia FP, Pilati F, Toselli M, Valenza A, Visco
A (2000) Polymer 41:4817–4824
14. Kalfoglou NK, Skafidas DS, Sotiropoulou DD (1994) Polymer
35:3624–3630
15. Retolaza A, Eguiazabal JL, Nazabal J (2002) J Polym Eng Sci
42:2072–2083
16. Kang TK, Kim Y, Lee WK, Park HD, Cho WJ, Ha CS (1999)
J Appl Polym Sci 72:989–997
17. Adem E, Avalos-Barja M, Carrillo D, Vazquez M, Sanchez E,
Carreon MP, Burillo G (1998) Radiat Phys Chem 52:171–176
18. Suarez JCM, Mano EB, Bonelli CM (1999) Polym Eng Sci
39:1398–1403
19. Thomas S, Gupta BR, De SK (1987) Polym Degrad Stab
18:189–212
20. De Boer J, Pennings L (1983) Colloid Polym Sci 261:750–756
21. Gad YH, Magida MM, El-Nahas HH (2010) Ind Eng Chem
16:1019–1042
22. Ray I, Khastgir D (1993) Polymer 34:2030–2035
The effect of gamma irradiation
1319
123

Copyright of Journal of Radioanalytical & Nuclear Chemistry is the property of Springer Science & Business
Media B.V. and its content may not be copied or emailed to multiple sites or posted to a listserv without the
copyright holder's express written permission. However, users may print, download, or email articles for
individual use.
Wyszukiwarka
Podobne podstrony:
wpływ dodatku nanowypełniacza na wlaściwości mechaniczne i tribiologiczne kompozytów do zastosowań s
Negatywne skutki działania promieniowania UV na organizm
Wyznaczanie współczynników osłabiania promieniowania gamma 1, Pracownia Zak˙adu Fizyki Technicznej P
Analiza budowy i działania robotów przemysłowych na przykładzie robota PRO 30 ( Politechnika Krakows
sciaga na egzamin z mechaniki old word, Politechnika Lubelska, Studia, Studia
Wyznacznie współczynnika osłabiania promieniowania gamma, Pracownia Zak˙adu Fizyki Technicznej Polit
Mechanizm wyzwalania reakcji biologicznych pod wpływem działania promieni UV
Wojna, Mechanizmy i skutki działania systemu totalitarnego, Mechanizmy i skutki działania systemu to
Wojna, Mechanizmy i skutki działania systemu totalitarnego, Mechanizmy i skutki działania systemu to
Pomiar właściwości mechanicznych błon glutenowych podczas obróbki termicznej
Mechanizm wyzwalania reakcji biologicznych pod wpływem działania promieni UV
mechanizmy i skutki działania systemu totalitarnego (na wybr
Badanie właściwości mechanicznych brykietu na maszynie wytrzymalościowej
Mechanizm wyzwalania reakcji biologicznych pod wpływem działania promieni UV
Działanie promieniowania na poz molekularnym
Badanie właściwości mechanicznych brykietu na maszynie wytrzymalościowej
6 Wpływ recyklingu na reologicznych i mechanicznych właściwości poli (kwasu mlekowego) mieszaniny po
Mechanizm dzialania czynnikow fizykalnych na organizmczlowieka
więcej podobnych podstron