
Addition of citrus pulp and apple pomace in diets for dogs: influence
on fermentation kinetics, digestion, faecal characteristics and bacterial
populations
Sebastián Brambillasca
, Carolina Deluca
, Martín Fraga
and
Cecilia Cajarville
a
Departamento de Nutrición Animal, Facultad de Veterinaria, Universidad de la
República, Montevideo, Uruguay;
b
Departamento de Microbiología, Instituto de Investigaciones
Biológicas Clemente Estable, Montevideo, Uruguay
(Received 26 July 2013, accepted 4 October 2013)
Fermentation kinetics, digestibility, faecal characteristics and bacterial populations
(aerobes, anaerobes, lactobacilli, lactic acid bacteria, enterococci, coliforms and clos-
tridia) of dog food mixed with citrus pulp and apple pomace were evaluated. The in
vitro gas production of a pre-digested dog food mixed with 0, 30, 50 and 70 g/kg dry
matter (DM) of citrus pulp or apple pomace was measured, and also an experiment
with dogs fed the same dog food with or without the addition of 70 g/kg of either fresh
citrus pulp or apple pomace was conducted. Gas production increased linearly
(p < 0.001) and quadratically (p < 0.001) as fibre levels augmented. The inclusion
of fibre sources in the diets resulted in higher faecal output (p = 0.005) and defecation
frequency (p < 0.001), and lower faecal pH (p < 0.001) and digestibility values
(p < 0.01). Faecal consistencies and microbial populations did not differ among
treatments. The addition of fresh citrus and apple was effective to stimulate the hindgut
fermentation, but slightly depressed the digestion.
Keywords: apple pomace; bacteria; citrus pulp; digestibility; dogs; feeding;
fermentation; fibre
1.
Introduction
The inclusion of fermentable fibre in the diets of monogastric animals is an interesting
strategy to improve the gastrointestinal health and microbiota ecosystem in the gut. Fibre
sources from dehydrated vegetables and fruits are being used by the pet food industry to
reduce the incidence of constipation and plasma glucose concentrations in diabetic
animals (Biagi et al.
). Citrus pulp and apple pomace contain fermentable fibre that
result in compounds associated with positive impacts on animal gut physiology (Bosch
et al.
), and their beneficial effects on intestinal microbiota and microbial metabolites
have been reported previously through in vitro and in vivo approaches (Sunvold, Fahey,
Merchen, and Reinhart 1995; Sunvold, Fahey, Merchen, Titgemeyer, et al. 1995; Swanson
et al.
; Middelbos et al.
; Biagi et al.
). The aforementioned studies used the
additives in a purified (i.e. pectins) or in a dehydrated form. The dehydration process
involves heating the raw material and the addition of desiccant substances (i.e. calcium
hydroxide), that increase ash and fibre contents of the material (Martínez
–Pascual and
Fernández
–Carmona
) which can affect digestive utilisation. However, little is known
*Corresponding author. Email:
Archives of Animal Nutrition, 2013
Vol. 67, No. 6, 492
–502, http://dx.doi.org/10.1080/1745039X.2013.857079
© 2013 Taylor & Francis
about animal responses when those fibre sources are used fresh (or just oven dried,
without addition of other substances) as supplements of dog food, which can be useful
in formulation of home-made diets and supplements.
We tested the hypothesis that the inclusion of fresh citrus and apple by-products in the
diet of dogs may improve the hindgut fermentation and may have a positive impact on
microbial ecology as was demonstrated for dehydrated by-products. Therefore, our
purpose was to evaluate fermentation characteristics of two fibre sources (citrus pulp
and apple pomace) added in different levels to a pre-digested dog food, and to determine
whether the supplementation of a dog food with fresh citrus pulp and apple pomace
affects the digestibility of nutrients, stool characteristics and faecal microbial populations.
2.
Materials and methods
Two experiments were conducted: an in vitro gas production trial and an in vivo feeding
trial. All procedures were approved by the Bioethics Committee of the Veterinary Faculty
(Facultad de Veterinaria, UdelaR, Uruguay).
2.1.
Experiment 1: in vitro gas production
In this trial mixtures of a pre-digested dog food (PRED) with either apple pomace (APP) or
citrus pulp (CIT) were prepared in order to test four different inclusion levels: 0, 30, 50, and
70 g/kg of each fibre source on a dry matter (DM) basis. Mixtures and the PRED were used as
substrates in an in vitro gas production experiment (a total of seven substrates). Citrus pulp
and apple pomace were obtained from a juice processing industry (Frigorífico Uruguayo S.A.,
Damaso A. Larrañaga 3551, Montevideo, Uruguay). The material collected was the pulp and
peel remaining after the mechanical fruit squeezing. In order to assure the freshness and
hygienic quality of the material, it was collected immediately after the extraction of juice,
chopped with a blender in the laboratory and stored at
–20°C throughout the study. PRED was
obtained by a pepsin
–pancreatin hydrolysis of a commercial dog food (Purina Excellent
®
based on chicken by-product meal, cereals, meat and bone meal, vegetable protein meal,
animal fat, minerals and vitamins; Nestlé Argentina, Buenos Aires, Argentina) as described by
Cone et al. (
) and filtered with nylon gauze with pores of 43
μm. Prior to the mixing,
PRED, APP and CIT were oven-dried at 55°C for 48 h and ground to pass a 1-mm screen.
Chemical composition of the substrates is presented in
Table 1.
Chemical composition of the substrates used in Experiment 1 and experimental diets in
Experiment 2 [g/kg, DM basis].
Experiment 1
Experiment 2
PRED*
CIT
#
APP
†
CON
‡
CON + CIT
§
CON + APP
Dry matter
929.7
145.0
210.0
928.5
679.7
746.2
Organic matter
945.8
964.8
982.5
929.7
930.3
931.3
Ash
54.2
35.2
17.5
70.3
69.7
68.7
Crude protein
140.3
61.0
26.5
243.6
229.0
223.9
Neutral detergent fibre
318.3
220.0
340.2
105.7
113.7
122.2
Acid detergent fibre
80.5
162.7
237.7
47.5
56.3
61.5
Note: *PRED, Pre-digested control dry dog food;
#
CIT, Citrus pulp;
†
APP, Apple pomace;
‡
CON, Commercial
dog food;
§
CON + CIT, Commercial dog food supplemented with 70 g/kg DM of citrus pulp;
¶
CON + APP,
Commercial dog food supplemented with 70 g/kg DM of apple pomace.
Archives of Animal Nutrition
493

The in vitro gas production procedure was performed using 125 ml fermentation bottles.
Each substrate was incubated in triplicate, and three bottles with no substrate were also
included as inoculum blank (a total of 24 bottles). Substrates were weighed (0.5 g DM) and
placed in the bottles. Then, three solutions as described by Williams et al. (
) were added
separately to each fermentation bottle under continuous CO
2
stream. Briefly, these solutions
were 38 ml of a basal solution (macro- and micro-minerals, short-chain fatty acids and
haemin), 0.5 ml of a vitamins/buffer phosphate solution (vitamins and KH
2
PO
4
) and 0.5 ml
of a reducing solution (Na
2
S
∙ 9H
2
O and cysteine HCl). Afterward, bottles were sealed with
butyl rubber stoppers and stored at 4°C for 8 h to hydrate substrates. Prior to inoculation,
bottles were pre-warmed in a water bath at 39°C for 2 h, and 2 ml of a bicarbonate buffer
was added. Then, each bottle was inoculated with 10 ml of diluted dog faeces (1:5 w/v), and
butyl stoppers were fastened with aluminium crimp seals and remained in the water bath
throughout the measurement period. All manipulations were performed under continuous
CO
2
stream. The whole procedure was conducted in one run.
Faecal inoculum was prepared using faeces obtained from three adult Cocker Spaniel
dogs (two females, one male; BW: 12.6 ± 0.4 kg). Animals were housed in 2.0 m × 2.0 m
individual cages and received 27 g DM/kg BW
0.75
of Purina Excellent
®
for 12 days prior
faecal collection. Faeces were collected immediately after defecation, sealed in plastic
bags under anaerobic conditions, transported in a pre-warmed container to the laboratory
(<1 h between collection and inoculation) and pooled quantitatively to provide a unique
representative inoculum. Faeces were diluted with a saline sterile solution (9 g NaCl/l) in
a 1:5 ratio (w/v), homogenised using a hand-mixer and strained through four layers of
cheesecloth. The fluid obtained was then continually flushed with CO
2
and stirred until
inoculation.
Gas production was measured in the bottles at 2, 4, 6, 8, 10, 12, 18, 24, 48 and 67 h
after inoculation with a transducer fixed to a pressure meter (840065, Sper Cientific,
Scottsdale, AZ, USA) and registered in psi units. Gas volume in millilitre was predicted
from psi values. For this purpose in a parallel trial, gas volume was measured with a
syringe and simultaneously gas pressure was recorded in psi from each bottle following
the procedure described by Theodorou et al. (
). Then, gas volume in millilitres was
calculated from pressure using the equation
Volume ml
½ ¼ 4:0289 psi þ 0:1687 psi
2
n
¼ 34; R
2
¼ 0:9822
:
Cumulative gas volume recorded during the fermentation was related to the incubated OM
(organic matter cumulative volume, OMCV). The data for cumulative gas production
were fitted to the model (Groot et al.
):
G
¼ A= 1þ B=t
ð
Þ
C
h
i
where G is the total gas produced [ml/g]; A is the asymptotic gas production [ml/g]; B is
the switching characteristic of the curve; C is the time at which one-half of the asymptote
has been reached (t
½
, [h]) and t is the time [h]. Maximum fermentation rate (R
max
, [ml/h])
and time at which it occurs (t
max
[h]) were also calculated (Bauer et al.
):
R
max
¼ ðA C
B
Þ B t
max
ðB1Þ
= 1 þ C
B
t
B
max
2
t
max
¼ C B 1
ð
Þ= B þ 1
ð
Þ
ð1=BÞ
h
i
494
S. Brambillasca et al.

2.2.
Experiment 2: in vivo feeding trial
This trial was performed to test the influence of fresh citrus pulp and apple pomace included
in the diet of dogs on digestion of nutrients, faecal characteristics and bacterial populations.
Six healthy adult Cocker Spaniel dogs (three males, three females; 12.7 ± 0.7 kg BW) were
randomly assigned to three diets according to a 3 × 3 Latin square design. The feedstuffs
used in this experiment were the same as used in Experiment 1, but the dry food was used as
is (without pre-digestion) and the fibrous sources were used fresh. Diets consisted in Purina
Excellent
®
(CON), 930 g/kg CON plus 70 g/kg citrus pulp on a DM basis (CON + CIT) or
930 g/kg CON plus 70 g/kg apple pomace on a DM basis (CON + APP). Portions of citrus
and apple were daily thawed and mixed with CON immediately prior to feeding. Chemical
composition of the diets is presented in
. Each dog was fed daily 27 g DM/kg
BW
0.75
of each diet offered in equal meals at 09:00 h and 16:00 h. Citrus and apple were
included at the highest level without compromising the fixed intake. For this purpose, a pre-
experimental period was performed beginning with 120 g/kg of fibre sources on DM basis
(following Fahey, Merchen, Corbin, Hamilton, Serbe, Lewis, et al. 1990) and controlling
intake. The level of citrus an apple was reduced until no refusals were observed. Therefore,
the level of inclusion of both fibre sources was equaled at 70 g/kg in a DM basis. On as fed
basis it represented on average 208, 277 and 254 g/d, for CON, CON + CIT and
CON + APP respectively. Animals had free access to fresh water throughout the experiment.
Each experimental period of the Latin square consisted of a 5-day diet adaptation phase,
followed by 3 day for collection of faeces, and 1 day of faecal sampling for bacterial
enumeration.
During the collection phase dogs were checked hourly for defecation from 08:00 to
18:00 h. After defecation, faecal consistency and pH were determined individually and
immediately. Faecal consistency was scored using a scale of 1 (indicating liquid consis-
tency) to 5 (indicating firm consistency) as described by Strickling et al. (
). Faecal pH
was measured with a digital pH-meter (eChem Instruments Pte. Ltd., Oakton, Singapore)
diluting 1 g of faeces in 10 ml of distilled water as described by Hesta, Janssens, et al.
(
). Total individual faeces were weighed, placed in plastic bags and immediately
frozen at
−20°C. Faecal samples were later thawed and mixed so that pooled samples were
representative of each dog and period. Apparent digestibility of nutrients [DM, OM, crude
protein (CP), neutral detergent fibre (NDF), acid detergent fibre (ADF)] were calculated as:
Apparent digestibility
%
½
¼ Nutrient intake g
½ Faecal nutrient output g
½ Þ=Nutrient intake g
½ 100%:
ð
For bacterial culture a unique faecal sample per dog was collected the last day of each
experimental period. One g of fresh faeces was mixed with 10 ml of sterile phosphate
saline solution containing 0.5 g/l of cysteine and processed immediately after excretion in
a Stomacher blender (Seward, UK). Serial dilutions were made (10
−2
to 10
−9
) and
triplicates were spread onto different culture media for enumeration of colony forming
units (CFU). Total aerobes were grown in Trypticase Soy Agar (TSA; Difco, Inc., Detroit,
USA), and total anaerobes were cultured in TSA with 0.5 g cysteine per litre. Rogosa agar
was used for lactobacilli counts (Merck, Darmstadt, Germany) following Swanson et al.
(
), deMan
– Rogosa – Sharpe (MRS) agar (Merck, Darmstadt, Germany) for lactic
acid bacteria, mEnterococcus agar (Becton Dickinson, Massachusetts, USA) for entero-
cocci, McConkey agar (Merck, Darmstadt, Germany) for coliforms and Sulfite Polymyxin
Suphadiazine (SPS) agar (Difco, Inc., Detroit, MI, USA) for Clostridium perfringens. All
Archives of Animal Nutrition
495

culture media were incubated 37°C for 48 h, TSA/cysteine and SPS plates were incubated
in anaerobic jars (Oxoid, UK), MRS and Rogosa plates under microaerophilic atmosphere
in a candle jar, whereas the rest of the plates were incubated under aerobic conditions.
Bacterial counts were expressed as log
10
CFU per gram of fresh faeces.
2.3.
Chemical analysis
Feeds, substrates and faeces were analysed using AOAC (
) methods for DM (ID 967.03),
ash (ID 942.05) and CP (ID 984.13). OM was calculated by difference (OM = 1000
− Ash).
NDF and ADF fractions were determined sequentially using an ANKOM
220
fibre
analyser (Ankom Technology Corp., Fairport, NY, USA) using nylon bags and heat stable
α-amylase and expressed inclusive of residual ash. Individual faecal samples were thawed
and analysed for ammonia content by steam distillation (Hesta, Roosen, et al.
2.4.
Statistical analysis
Data were analysed using the MIXED procedure of SAS software (version 8.2; SAS
Institute, Cary, NC, USA). In Experiment 1, the effects of the fibre source included and
the inclusion level were tested using the model:
Y
i
¼ μþF
i
þL
j
þ ðF LÞ
ij
þ e
ij
;
where Y is the variable to be tested; µ is the mean; F
i
is the fixed effect of the fibre
source (i = CIT, APP); L
j
is the fixed effect of the inclusion level (j = 0, 30, 50 and
70 g/kg); (F
∙ L)
ij
is the interaction between fibre source and inclusion level and e
ij
the
error term. Linear and quadratic effects for increasing inclusion levels of fibre sources
were also tested. Additionally, the fermentation parameters of citrus pulp and apple
pomace incubated solely were compared. For Experiment 2, the model used was
Y
ijk
¼ μ þ D
i
þ P
j
þ T
k
þ e
ijk
where Y is the variable to be tested; µ is the mean; D
i
is the random effect of the dog
(n = 6); P
j
is the fixed effect of period (n = 3); T
k
is the fixed effect of treatment (k = CON,
CON + CIT, CON + APP) and e
ij
is the error term. Means were separated by pre-planned
orthogonal contrasts (independent linear comparisons between groups, Doncaster and
Davey
) in order to study the effects of fibre inclusion in the diet (CON vs.
CON + CIT + CON + APP) and the fibre source used (CON + CIT vs. CON + APP).
Faecal microbial populations were compared among treatments after logarithmic trans-
formation of microbial counts, by the NPAR1WAY procedure of SAS. For values below
the detection limit, 1 · 10
2
was used as count value. Significance was declared at p < 0.05,
and tendencies at p < 0.10.
3.
Results
The fermentation characteristics of citrus pulp and apple pomace incubated solely are
shown in
. Both fibre sources presented similar values for gas production, never-
theless APP presented a lower t
½
value (p = 0.006) and a higher R
max
(p = 0.048).
The effects of each fibre source added to PRED and the inclusion levels used (0, 30, 50
and 70 g/kg DM) on the in vitro fermentation parameters are presented in
. No
significant interactions between fibre source and inclusion level were detected for any of
496
S. Brambillasca et al.
the parameters studied, so the responses to the different inclusion levels were analysed for
CIT and APP altogether. Increasing the inclusion level produced similar responses for
both fibre sources. Gas production measured (OMCV) increased similarly for both fibre
sources at an increasing rate of inclusion (linear p < 0.001, quadratic p < 0.001). Half-time
Table 2.
In vitro fermentation parameters of fibre sources incubated solely.
Fibre source
CIT*
APP
#
SEM
†
p-values
Fermentation parameter
OMCV
‡
[ml/g OM]
120.95
129.75
6.09
0.365
t
1/2
§
[h]
8.21
5.46
0.36
0.006
R
max
¶
[ml/h]
10.84
14.08
1.04
0.048
t
max
◊
[h]
0.78
1.33
0.31
0.276
Notes: *CIT, Citrus pulp;
#
APP, Apple pomace;
†
SEM, Standard error of means; measurements were based on
three replicates per substrate;
‡
OMCV, OM cumulative volume;
§
t
½
, Half-time of the asymptotic gas production;
¶
R
max
, Maximal rate of gas production;
◊
t
max
, Time of occurrence of R
max
.
40
50
60
70
80
90
100
110
0
10
20
30
40
50
60
70
OMCV [ml gas/g OM]
Fibre source level [g/kg DM]
CIT
APP
Source: p = 0.437
Level: p < 0.001
Source × Level: p = 0.637
A
4
6
8
10
0
10
20
30
40
50
60
70
R
max
[ml/h]
Fibre source level [g/kg DM]
CIT
APP
Source: p = 0.144
Level: p = 0.690
Source × Level: p = 0.881
B
4
6
8
10
12
0
10
20
30
40
50
60
70
Fibre source level [g/kg DM]
CIT
APP
Source: p= 0.078
Level: p = 0.004
Source × Level: p = 0.552
C
0
0.2
0.4
0.6
0.8
1.0
1.2
0
10
20
30
40
50
60
70
Fibre source level [g/kg DM]
CIT
APP
Source: p = 0.751
Level: p = 0.073
Source × Level: p = 0.953
D
t
1/2
[h]
t
max
[h]
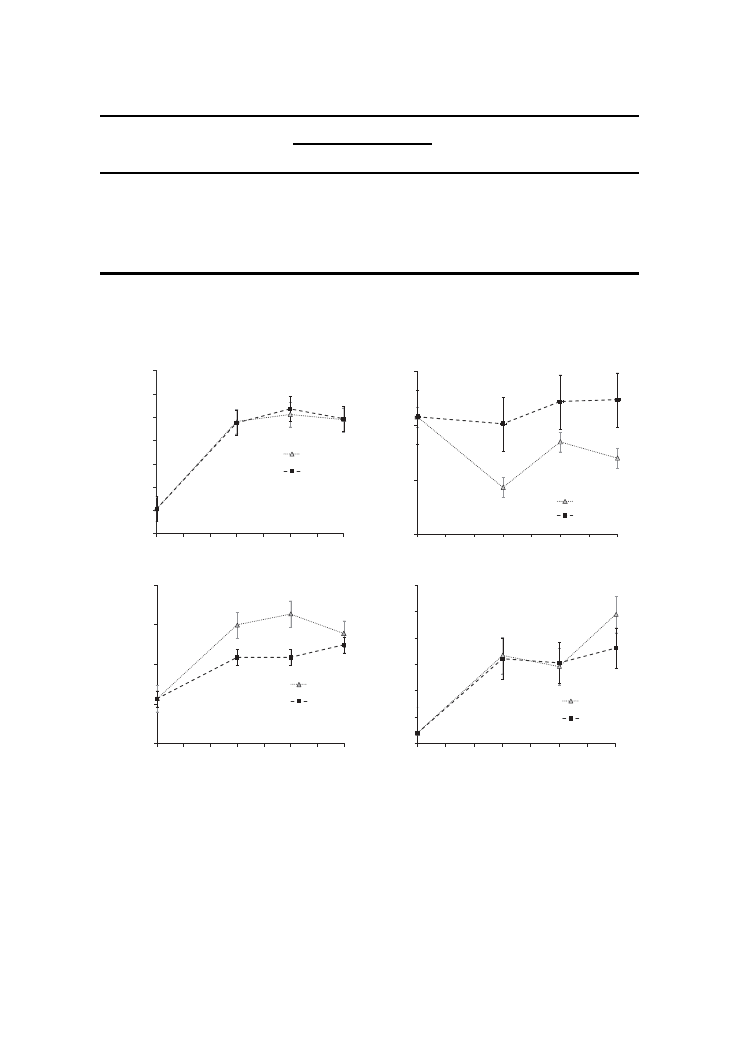
Figure 1.
In vitro fermentation parameters of fibre sources added to a pre-digested dog food at 0,
30, 50 and 70 g/kg DM.
Note: CIT, citrus pulp; APP, apple pomace; Panel A, organic matter cumulative volume; Panel B,
maximal rate of gas production; Panel C, half-time of the asymptotic gas production; Panel D, time
of occurrence of maximal rate of gas production; (means ± SEM; measurements were based on three
replicates per fibre source and level).
Archives of Animal Nutrition
497

of the asymptotic gas production was higher (p = 0.004) and the time needed to reach the
maximal tended to be higher (p = 0.073) with the inclusion of both fibre sources.
In the second experiment, all dogs remained healthy and diets were totally consumed
throughout the study. Faecal parameters and apparent nutrients digestibility for the
different treatments are presented in
. The addition of fibre sources in the diets
led to a higher wet faecal output (p = 0.005) and defecation frequency (p < 0.001) with a
lower DM content of faeces (p = 0.004), without varying faecal consistency. A lower
faecal pH (p < 0.001) was observed with the addition of fibre, but ammonia concentration
in faeces was similar among treatments. Apparent digestibility of nutrients decreased with
the addition of both fibre sources, and microbial groups quantified were neither influenced
by the addition of citrus pulp nor apple pomace (
4.
Discussion
According to other studies, and considering the rates of gas production obtained in our
experiment (R
max
), citrus pulp and apple pomace can be characterised as slowly fermen-
table fibre sources (Bosch et al.
). This implies that these sources of fibre are
expected to be fermented in the hindgut, and to produce moderate amounts of organic
acids through the GIT. Both fibrous added to the PRED enhanced the extent of fermenta-
tion by the microbiota, evidenced by the increase in the gas produced and consistent with
the increase in fermentable substrates. By contrast, the inclusion of citrus and apple
slowed down the fermentation (higher t
1/2
and a tendency in the t
max
to be greater in
the fibre containing substrates) compared to PRED incubated solely. This can be related to
the origin of the inoculum, as it was provided by dogs fed without fibre added. This fact
suggests that the number of microbes capable of degrading the substrates was not high
enough, and/or the microbiota needed to adapt to the fibrous substrates. The fermentative
profiles of the mixtures were similar for both fibre sources at the levels tested, although
Table 3.
Effect of fibre inclusion on characteristics of faeces and apparent digestibility of nutrients.
Treatment
Contrast p-value
§
CON* CON + CIT
#
CON + APP
†
SEM
‡
Fibre
Source
Faecal parameters
Wet faeces [g/d]
95.6
148.0
146.0
20.92
0.005
0.912
Dry faeces [g/d]
35.7
46.3
45.7
7.22
0.035
0.909
Faecal DM [%]
38.1
31.2
31.7
0.16
0.004
0.814
Defecation frequency [d
−1
]
1.17
1.83
1.78
0.11
<0.001
0.735
Consistency [1: liquid; 5: firm]
4.41
4.32
4.24
0.22
0.147
0.777
Faecal pH
6.99
6.58
6.51
0.05
<0.001
0.245
Ammonia [mg N-NH
3
/g DM]
2.23
1.92
2.14
0.18
0.375
0.391
Apparent digestibility [%]
Dry matter
84.90
81.07
80.42
1.171
0.004
0.645
Organic matter
88.67
84.53
84.12
0.937
<0.001
0.713
Crude protein
86.15
83.41
81.68
1.243
0.007
0.202
Neutral detergent fibre
50.72
36.05
32.85
3.282
<0.001
0.466
Acid detergent fibre
45.10
26.37
23.25
3.583
<0.001
0.548
Notes: *CON, commercial dog food;
#
CON + CIT, commercial dog food supplemented with 70 g/kg DM of
citrus pulp;
†
CON + APP: commercial dog food supplemented with 70 g/kg DM of apple pomace;
‡
SEM,
standard
error
of
means;
§
Contrast
probability,
Fibre,
effect
of
fibre
inclusion
(CON
vs.
CON + CIT + CON + APP); Source, effect of fibre source (CON + CIT vs. CON + APP).
498
S. Brambillasca et al.

apple incubated solely was fermented faster than citrus. Altogether, we found an improve-
ment in the fermentation activity which can be of interest for intestinal health according to
other studies (Bosch et al.
; Biagi et al.
).
In the in vivo trial, feeding regime was adjusted for suitable maintenance of the dogs.
Despite the fibre sources were used fresh and this caused an enlargement of the volume of
the diets, the levels of fibre in the diets (NDF and ADF) were not larger than 12% and 6%,
respectively, for the fibre supplemented groups. These levels of fibre were similar or
above levels used in other experiments (Fahey, Merchen, Corbin, Hamilton, Serbe, Lewis,
et al. 1990; de-Oliveira et al.
). Interestingly, diets were completely eaten, even
though the volume of the diets was enlarged with fibre addition. As suggested by others
(Diez et al.
), adding fibre may have a dilution effect on energy density but this effect
may not be important when animals receive the amount of feed based on individual
energy requirements, at least at the fibre levels used in our study.
The inclusion of both citrus and apple in the diet led to higher faecal outputs and
defecation frequencies, in agreement with previous reports that communicated the
decrease in the transit time through the GIT (Fahey, Merchen, Corbin, Hamilton, Serbe,
Lewis, et al. 1990), higher output and moisture content of stools, and higher defecation
frequency (Fahey, Merchen, Corbin, Hamilton, Serbe, and Hirakawa 1990; Wakshlag
et al.
) as a consequence of the increase in the fibre levels of the diet. The higher
amounts of faeces excreted in the fibrous diets were as a consequence of an increase on
DM and water output, and can be related to less digestible components and the high
water-binding capacity of fibre sources (Sunvold, Fahey, Merchen, Titgemeyer, et al.
; Swanson et al.
). An interesting observation in the present study is that faecal
scores were not affected by treatments, and that the inclusion of fresh citrus and apple at
this level led to well-formed faeces. This was not expected as DM content of faeces and
digestibility of nutrients were depressed with the inclusion of fibre, and according to other
Table 4.
Effect of fibre inclusion on faecal bacterial populations [CFU log
10
/g fresh faeces]
§
.
Treatment
p-Values
‡
CON*
CON + CIT#
CON + APP
†
Total aerobes
8.31
7.95
7.92
0.834
(6.48
–8.93)
(6.66
–9.48)
(6.89
–8.88)
Total anaerobes
8.60
8.50
7.71
0.623
(6.00
–9.51)
(6.95
–9.89)
(6.38
–8.93)
Lactic acid bacteria
8.25
8.24
7.65
0.823
(5.45
–9.41)
(6.45
–9.28)
(6.08
–9.11)
Lactobacilli
6.18
7.59
6.23
0.692
(5.30
–8.98)
(4.00
–9.00)
(4.48
–8.63)
Enterococci
¶
6.10
5.39
6.81
0.672
(<2.00
–7.48)
(5.00
–8.30)
(4.77
–7.62)
Coliforms
8.41
8.15
7.91
0.895
(6.46
–9.34)
(6.639.10)
(6.70
–9.15)
Clostridium perfringens
¶
7.53
7.00
6.74
0.490
(<2.00
–8.23)
(<2.00
–7.85)
(<2.00
–8.93)
Notes:
§
Median values for each treatment are reported; the minimum and maximum values are shown
in parenthesis; *CON, commercial dog food;
#
CON + CIT, commercial dog food supplemented with 70 g/kg
DM of citrus pulp;
†
CON + APP, commercial dog food supplemented with 70 g/kg DM of apple pomace;
‡
p, probability of diet effect as determined by Kruskal
–Wallis test;
¶
There were four values above the limit of
detection.
Archives of Animal Nutrition
499

authors, low faecal DM content is associated with soft faeces and low digestibility values
(Twomey et al.
; Carciofi et al.
).
A positive response derived from the addition of fibre was the lower faecal pH values
observed, consistent with the higher gas volume obtained in the in vitro experiment,
suggesting a higher fermentation activity and organic acid production in the hindgut
(Flickinger et al.
; Twomey et al.
). Indeed, the low pH values in the intestine
can be protective against pathogenic bacteria in the gut (Seifert and Waltz
).
However, faecal ammonia was not reduced with the addition of fibre, and bacterial
populations were similar among treatments. Therefore we could not confirm that this
addition increased bacterial mass and/or ammonia incorporation into bacterial mass, as
reported by others (Flickinger et al.
; Biagi et al.
).
Apparent digestibilities were diminished by the inclusion of both fibre sources, as
reported by others (Fahey, Merchen, Corbin, Hamilton, Serbe, and Hirakawa 1990;
Lewis et al.
; Burkhalter et al.
). The decrease in apparent CP digestibility
could be due to a lower intestinal digestion of proteins itself, but not as a conse-
quence of higher faecal CP from bacterial mass, as no differences were noticed in
aerobes and anaerobes total counts among treatments. This fact may be considered
specially when using these by-products in diets for growing dogs. The reduction in
apparent digestibility was more evident in the fibre fractions analysed, NDF and
ADF, which suffered a reduction of about 30 and 40% respectively. Despite both
citrus and apple caused a higher fermentation activity in the hindgut, this was not
reflected in higher digestibility values for the fibre fractions.
To conclude, citrus pulp and apple pomace included in a dog diet enhanced the
fermentation activity in the hindgut, and led to well-formed faeces with small reductions
in nutrient digestion. These by-products can be considered for being included fresh in
home-made diets, but it would be necessary increasing the concentration of nutrients to
compensate the reductions on digestibility. Furthermore, additional studies concerning the
most suitable levels of addition of these feedstuffs are needed.
Acknowledgements
The authors thank CIDEC
– Facultad de Veterinaria, Lucía Reyes for her contribution in the gas in
vitro trial and Nestlé, Uruguay for kindly providing the pet food.
Funding
The authors thank CIDEC
– Facultad de Veterinaria for the financial support of this project.
References
[AOAC] Association of Official Analytical Chemist. 1990. Official methods of analysis. 15th ed.
Arlington (VA): AOAC.
Bauer E, Williams BA, Voigt C, Mosenthin R, Verstegen MWA. 2001. Microbial activities of faeces
from unweaned and adult pigs, in relation to selected fermentable carbohydrates. Anim Sci.
73:313
–322.
Biagi G, Cipollini I, Grandi M, Zaghini G. 2010. Influence of some potential prebiotics and fibre-
rich foodstuffs on composition and activity of canine intestinal microbiota. Anim Feed Sci
Technol. 159:50
–58.
Bosch G, Pellikaan WF, Rutten PGP, van der Poel AFB, Verstegen MWA, Hendriks WH. 2008.
Comparative in vitro fermentation activity in the canine distal gastrointestinal tract and fermen-
tation kinetics of fiber sources. J Anim Sci. 86:2979
–2989.
500
S. Brambillasca et al.

Burkhalter TM, Merchen NR, Bauer LL, Murray SM, Patil AR, Brent JL, Fahey Jr GC. 2001. The
ratio of insoluble to soluble fiber components in soybean hulls affects ileal and total-tract
nutrient digestibilities and fecal characteristics of dogs. J Nutr. 131:1978
–1985.
Carciofi AC, Pontieri R, Ferreira CF, Prada F. 2006. Avaliação de dietas com diferentes fontes
protéicas para cães adultos. R Bras Zootec. 35:754
–760.
Cone JW, Jongbloed AW, Van Gelder AH, de Lange L. 2005. Estimation of protein fermentation
in the large intestine of pigs using a gas production technique. Anim Feed Sci Technol.
123
–124:463–472.
de Oliveira LD, Takakura FS, Kienzle E, Brunetto MA, Teshima E, Pereira GT, Vasconcellos RS,
Carciofi AC. 2012. Fibre analysis and fibre digestibility in pet foods
– a comparison of
total dietary fibre, neutral and acid detergent fibre and crude fibre. J Anim Phys Anim Nutr.
96:895
–906.
Diez M, Hornick JL, Baldwin P, Van Eenaeme C, Istasse L. 1998. The influence of sugar-beet fibre,
guar gum and inulin on nutrient digestibility, water consumption and plasma metabolites in
healthy Beagle dogs. Res Vet Sci. 64:91
–96.
Doncaster CP, Davey AJH. 2007. Analysis of variance and covariance: how to choose and construct
models for the life sciences. Cambridge (UK): Cambridge University of Press.
Fahey Jr GC, Merchen NR, Corbin JE, Hamilton AK, Serbe KA, Hirakawa DA. 1990. Dietary fiber
for dogs: II. Iso-total dietary fiber (TDF) additions of divergent fiber sources to dog diets and
their effects on nutrient intake, digestibility, metabolizable energy and digesta mean retention
time. J Anim Sci. 68:4229
–4235.
Fahey Jr GC, Merchen NR, Corbin JE, Hamilton AK, Serbe KA, Lewis SM, Hirakawa DA.
1990. Dietary fiber for dogs: I. Effects of graded levels of dietary beet pulp on nutrient
intake, digestibility, metabolizable energy and digesta mean retention time. J Anim Sci.
68:4221
–4228.
Flickinger EA, Scheinjen EMWC, Patil AR, Hussein HS, Grieshop CM, Merchen NR, Fahey Jr GC.
2003. Nutrient digestibilities, microbial populations, and protein catabolites as affected by
fructan supplementation of dog diets. J Anim Sci. 81:2008
–2018.
Groot JCJ, Cone JW, Williams BA, Debersaques FMA, Lantinga EA. 1996. Multiphasic analysis of
gas production kinetics for in vitro fermentation of ruminant feeds. Anim Feed Sci Technol.
64:77
–89.
Hesta M, Janssens GPJ, Debraekeleer J, Millet S, De Wilde R. 2003. Fecal odor components in
dogs: nondigestible oligosaccharides and resistant starch do not decrease fecal H
2
S emission.
J Appl Res Vet Med. 1:225
–232.
Hesta M, Roosen W, Janssens GPJ, Millet S, De Wilde R. 2003. Prebiotics affect nutrient digest-
ibility but not faecal ammonia in dogs fed increased dietary protein levels. Brit J Nutr. 90:1007
–
1014.
Lewis LD, Magerkurth JH, Roudebush P, Morris ML, Mitchell Jr EE, Teeter SM. 1994. Stool
characteristics, gastrointestinal transit time and nutrient digestibility in dogs fed different fiber
sources. J Nutr. 124:2716S
–2718S.
Martínez
–Pascual J, Fernández–Carmona J. 1980. Composition of citrus pulp. Anim Feed Sci
Technol. 5:1
–10.
Middelbos IS, Fastinger ND, Fahey Jr GC. 2007. Evaluation of fermentable oligosaccharides in
diets fed to dogs in comparison to fiber standards. J Anim Sci. 85:3033
–3044.
Seifert S, Waltz B. 2007. Inulin and oligofructose: review of experimental data on immune
modulation. J Nutr. 137:2563S
–2567S.
Strickling JA, Harmon DL, Dawson KA, Gross KL. 2000. Evaluation of oligosaccharide addition to
dog diets: influences on nutrient digestion and microbial populations. Anim Feed Sci Technol.
85:205
–219.
Sunvold GD, Fahey Jr GC, Merchen NR, Reinhart GA. 1995. In vitro fermentation of selected
fibrous substrates by dog and cat fecal inoculum: influence of diet composition on substrate
organic matter disappearance and short-chain fatty acid production. J Anim Sci. 73:1110
–1122.
Sunvold GD, Fahey Jr GC, Merchen NR, Titgemeyer EC, Bourquin LD, Bauer LL, Reinhart GA.
1995. Dietary fiber for dogs: IV. In vitro fermentation of selected fiber sources by dog fecal
inoculum and in vivo digestion and metabolism of fiber-supplemented diets. J Anim Sci.
73:1099
–1109.
Archives of Animal Nutrition
501

Swanson KS, Grieshop CM, Clapper GM, Shields Jr RG, Belay T, Merchen NR, Fahey Jr GC.
2001. Fruit and vegetable fiber fermentation by gut microflora from canines. J Anim Sci.
79:919
–926.
Swanson KS, Grieshop CM, Flickinger EA, Bauer LL, Healy HP, Dawson KA, Merchen NR, Fahey
Jr GC. 2002. Supplemental fructooligosaccharides and mannanoligosaccharides influence
immune function, ileal and total tract nutrient digestibilities, microbial populations and con-
centrations of protein catabolites in the large bowel of dogs. J Nutr. 132:980
–989.
Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France. J. 1994. A simple gas production
method using a pressure transducer to determine the fermentation kinetics of ruminant feeds.
Anim Feed Sci Technol. 48:185
–197.
Twomey LN, Pluske JR, Rowe JB, Choet M, Brown W, McConnell MF, Pethick DW. 2003. The
effects of increasing levels of soluble non-starch polisaccharides and inclusion of feed enzymes
in dog diets on faecal quality and digestibility. Anim Feed Sci Technol. 108:71
–82.
Wakshlag JJ, Simpson KW, Struble AM, Dowd SE. 2011. Negative fecal characteristics are
associated with pH and fecal flora alterations during dietary changes in dogs. Intern J Appl
Res Vet Med. 9:278
–283.
Williams BA, Bosch MW, Boer H, Verstegen MWA, Tamminga S. 2005. An in vitro batch culture
method to assess potential fermentability of feed ingredients for monogastric diets. Anim Feed
Sci Technol. 123
–124:445–462.
502
S. Brambillasca et al.

Copyright of Archives of Animal Nutrition is the property of Taylor & Francis Ltd and its
content may not be copied or emailed to multiple sites or posted to a listserv without the
copyright holder's express written permission. However, users may print, download, or email
articles for individual use.
Document Outline
- Abstract
- 1. Introduction
- 2. Materials and methods
- 3. Results
- 4. Discussion
- Acknowledgements
- References
Wyszukiwarka
Podobne podstrony:
Signs of the Zodiac and the Planets in their exaltations
[13]Role of oxidative stress and protein oxidation in the aging process
Unsolved Mysteries An Exhibition of Unsolved Mysteries and Enigmatic Findings in the History of Hum
(IV)Relative therapeutic efficacy of the Williams and McKenzie protocols in back pain management
The Effects of Probiotic Supplementation on Markers of Blood Lipids, and Blood Pressure in Patients
The Wannsee Conference, the Fate of German Jews, and Hitler s Decision in Principle to Exterminate A
Edward Epstein Agency of fear Opiates and politican power in America
Labyrinth13 True Tales of Occult Crime and Conspiracy by Curt Rowlett 3rd edn (2013)
Ecumeny and Law 2013 No 1 Marriage covenant paradigm of encounter of the de matrimonio thought of th
Isaacs 2013 Journal of Paediatrics and Child Health
Insensitive Semantics~ A Defense of Semantic Minimalism and Speech Act Pluralism
Estimation of Dietary Pb and Cd Intake from Pb and Cd in blood and urine
Development of Carbon Nanotubes and Polymer Composites Therefrom
Functional Origins of Religious Concepts Ontological and Strategic Selection in Evolved Minds
Leaf and Apple Calendar Numbers
Analysis of soil fertility and its anomalies using an objective model
Modeling of Polymer Processing and Properties
DICTIONARY OF AUSTRALIAN WORDS AND TERMS
więcej podobnych podstron