
WHAT’S NEW IN?
MR imaging of the neonatal brain at 3 Tesla
Mary Rutherford*, Christina Malamateniou, Julie Zeka, Serena Counsell
Imaging Sciences Department, Clinical Sciences Centre, Imperial College London, Hammersmith Hospital,
Du Cane Road, London W12 ONN, UK
Received 29 July 2004; accepted 9 August 2004
KEYWORDS
Neonate;
brain;
magnetic resonance
imaging;
3 Telsa
Summary
3 Telsa MR scanners are now becoming more widely available and 3 Telsa
is likely to become the filed strength of choice for clinical imaging of the brain. The
neonatal brain can be safely and successfully imaged at 3 Telsa. The improved signal
to noise afforded by a higher field strength may be used to improve image quality or
shorten acquisition times. This may be exploited for conventional T1 and T2 weighted
imaging and also for advanced techniques such as diffusion tensor imaging,
angiography and functional magnetic resonance studies.
Q
2004 Published by Elsevier Ltd on behalf of European Paediatric Neurology Society.
Introduction
Magnetic resonance (MR) imaging has revolutio-
nised neuropediatrics. The worldwide availability
of MR facilities has resulted in detailed images of
the brain in a wide range of neurological disorders.
These more specific phenotypes have resulted in
the identification of new syndromes and the
realisation that a specific genetic defect may
manifest in a variety of phenotypes. Most clinical
MR imaging is performed at 0.5–1.5 T. More recently
a generation of 3 T MR scanners has become
available and current opinion is that 3 T MR imaging
will become the clinical standard, initially in
neuroimaging, and eventually throughout the body.
The greater signal-to-noise (SNR) afforded with
higher field strengths may be exploited to
improve image quality or to shorten acquisition
times. There are now several reviews and studies
comparing 1.5–3 T imaging of the brain in
adults.
Systems with 3 T have been exploited
to increase detection of multiple sclerosis
lesions,
to improve the blood oxygenation level
dependent (BOLD) effect for functional magnetic
resonance
imaging
(fMRI)
and
to
improve
enhancement following contrast administration.
There is as yet little information about the role
and use of 3 T imaging in the paediatric
population.
Increased field strength provides not only increased
SNR, but increased susceptibility caused by paramag-
netic effects due to local heterogeneity in magnetic
field from, for instance, the frontal sinuses. Highfield
imaging also results in increased chemical shift and
increased heat deposition [specific absorption rates
(SARs)]. Radiofrequency (RF) power varies with the
square of the field strength, therefore imaging at 3 T,
produces four times more RF power and sequences may
have to be adjusted to operate within radiological
safety guidelines. These limits are usually preset into
the scanner software. The SAR at 3 T is not prohibitive
for neonatal and infant scanning, as sequences can be
European Journal of Paediatric Neurology (2004) 8, 281–289
1090-3798/$ - see front matter Q 2004 Published by Elsevier Ltd on behalf of European Paediatric Neurology Society.
doi:10.1016/j.ejpn.2004.08.003
* Corresponding author.
E-mail address: m.rutherford@imperial.ac.uk (M. Rutherford).
adjusted and still produce good quality images, but it is
a problem for fetal imaging, making it unlikely that fetal
imaging will be performed at 3 T for the foreseeable
future. Studies modelling heat deposition within the
pregnant uterus during imaging at 3 T are required.
Increased susceptibility is not a major problem in
neonatal imaging, as neonatal and infant sinuses are not
formed and aerated until later in childhood.
The increased SNR afforded by imaging at 3 T can
be used to obtain information in a shorter time. This
is very valuable when imaging unsedated children
who may have difficulties lying still or for sedated
sick neonates: in each circumstance time is of the
essence. The combination of parallel imaging with
phased array coils at 3 T may improve SNR further
but perhaps at the expense of signal homogeneity,
which is a potential disadvantage for quantification
studies.
Conventional imaging
Imaging at 3 T provides superb detail of the
immature brain.
show examples of
images obtained at 3 T and a comparison of images
obtained at 1.5 and 3 T in the same examination.
The increased SNR allows high-resolution images to
be obtained in acceptable acquisition times such as
a multisliced T2 weighted sequences suitable for
reformatting (
). Increased acquisition times
are acceptable when imaging postmortem and
excellent quality images can be produced (
The field of postmortem imaging is in its infancy but
the advantage of imaging at high field when SAR is
not an issue is evident. T1 relaxation times lengthen
with increased field strength and parameters have
to be optimised.
Indeed image contrast optimis-
ation of conventional spin echo T1 weighted
imaging is difficult, and an inversion recovery
sequence is preferable to achieve T1 weighting at
3 T. Representative values for parameters at 1.5
and at 3 T are shown in
At 3 T, T1 weighted images are noticeable for
the increased conspicuity of vessels, even when a
rest slab is used (
). Prospective studies are
required to assess the significance of these vessels.
The common sites to detect vessels are in the
basal ganglia, the cerebellum and the brainstem
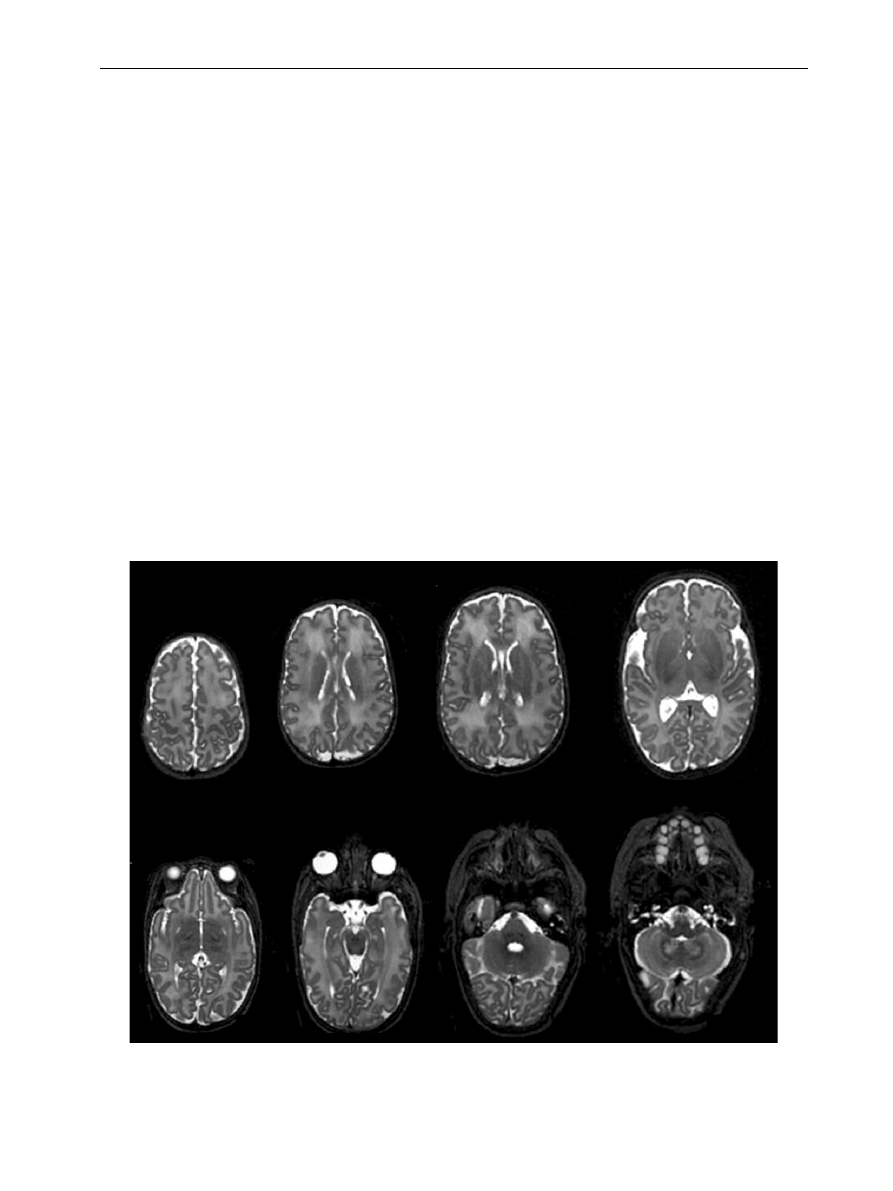
Figure 1
T2 weighted fast spin echo of a neonate at 3 Tesla. Transverse plane showing normal appearences to the
neonatal brain.
M. Rutherford et al.
282
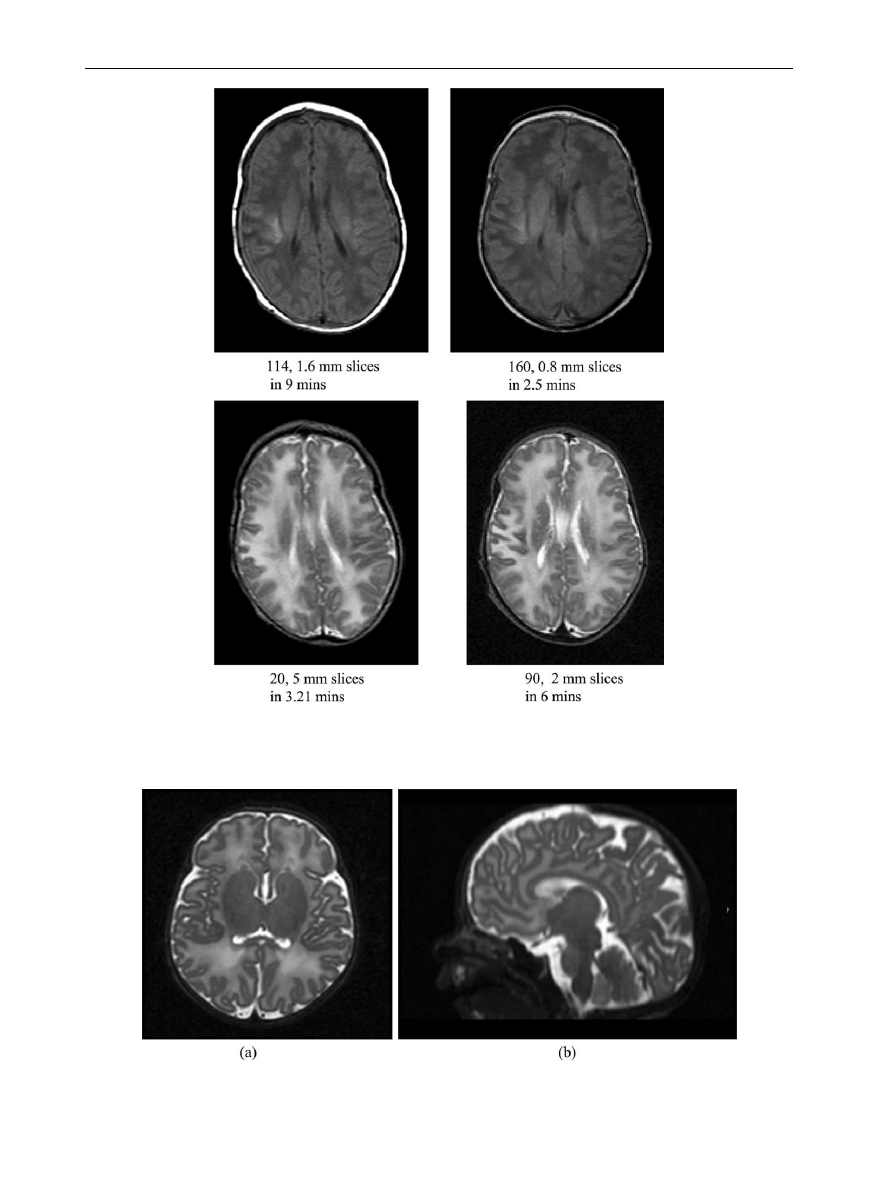
Figure 2
Images obtained at 1.5 (on left) and 3 Tesla (on right) in an infant with a neonatal encephalopathy. The
number of slices, slice thickness and sequence acquisition times are shown beneath each image.
Figure 3
Multi sliced T2 sequence acquired in the transverse plane (a) reformatted into the sagittal plane (b).
MR imaging of the neonatal brain at 3 Tesla
283
a and b). We have found an inversion
recovery sequence to be a good alternative for
T1 weighted images without vessel contamination.
This increased vessel conspicuity, however, may
be exploited and MR angiography and venography at
3 T gives superb definition of the vascular tree.
MR angiography is a very well established non-
invasive technique for imaging the intra-cranial
vasculature at 1.5 T and lower magnetic field
strengths in adult studies, in cohorts of healthy
volunteers and patients. The same technique in
neonatal imaging has been underused and there are
very few reports in the literature. Neonatal brain
vessels are rather small, with lower blood flow
velocities in comparison to adult cerebral vessels
and frequently presenting with turbulent flow. This
makes MR angiography of the neonatal brain a
highly demanding and technically challenging area
of MR imaging not only for the anatomic depiction
of the vasculature, but also for performing quanti-
tative diameter and flow measurements. Currently
time-of-flight and phase contrast angiography are
the two imaging techniques regularly used for
imaging neonatal brain vessels.
Vascular theories for the regional susceptibility
of the neonatal brain to ischaemic injury
have
been discussed for years but there has been little
study of the morphology and physiology of the brain
vessels in the normal brain, in the high-risk neonate
or following a significant brain lesion. Middle
cerebral artery infarction is the most frequent
form of neonatal stroke, the left side being far more
frequently involved than on the right particularly in
those infarcts with a posterior distribution.
However, there have been no angiography studies in
these infants to try and explain this predilection.
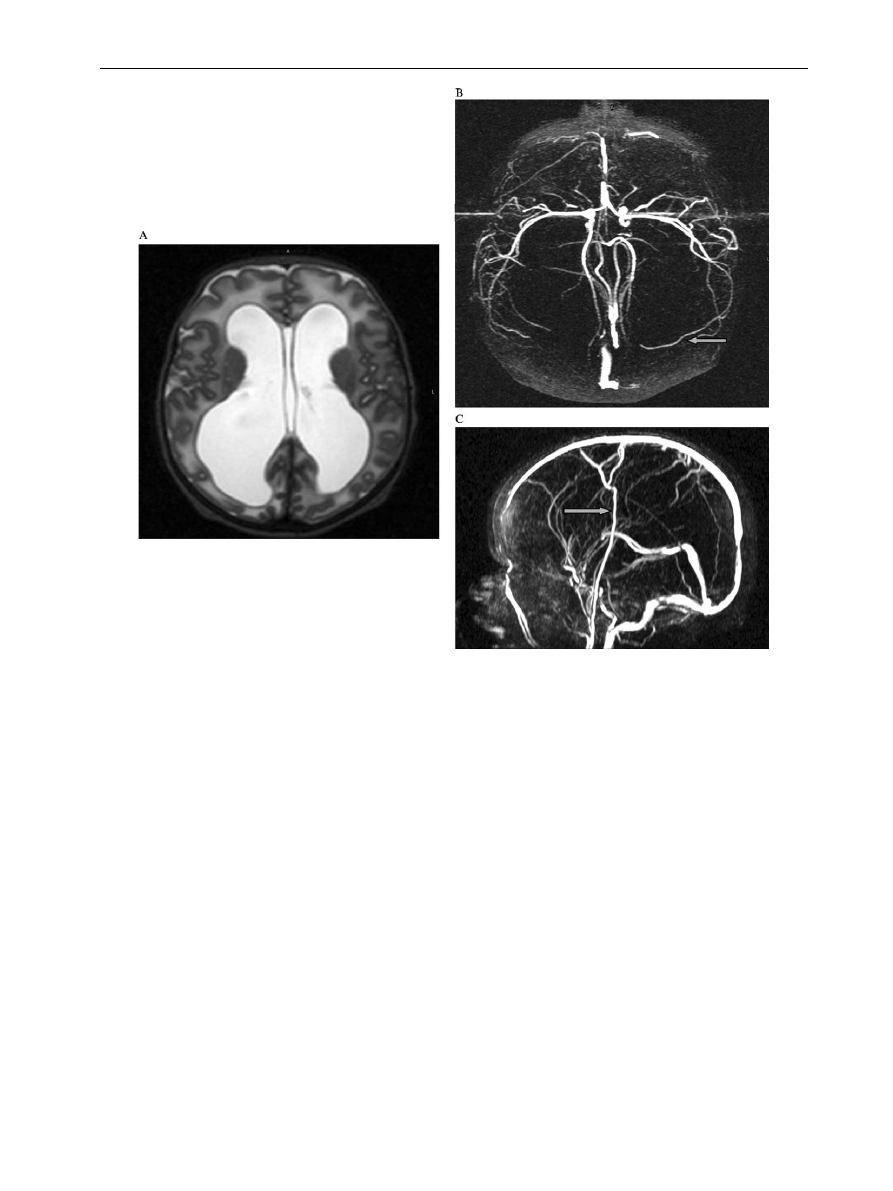
Arteriovenous malformations are relatively rare but
angiograms of the circle of Willis and carotid
arteries and venograms of the dural sinuses would
be extremely useful for describing anatomic vari-
ations (
), and whether these predispose to
brain injury in a given clinical situation.
Furthermore, the use of high magnetic field
strength at 3 T appears to be a very promising area,
due to the inherently high SNR, which may help in
improving vessel conspicuity of the neonatal intra-
cranial vessels or further reduce scanning time.
MRA protocols must be tailored to the needs and
adapted to the specific features of the neonatal
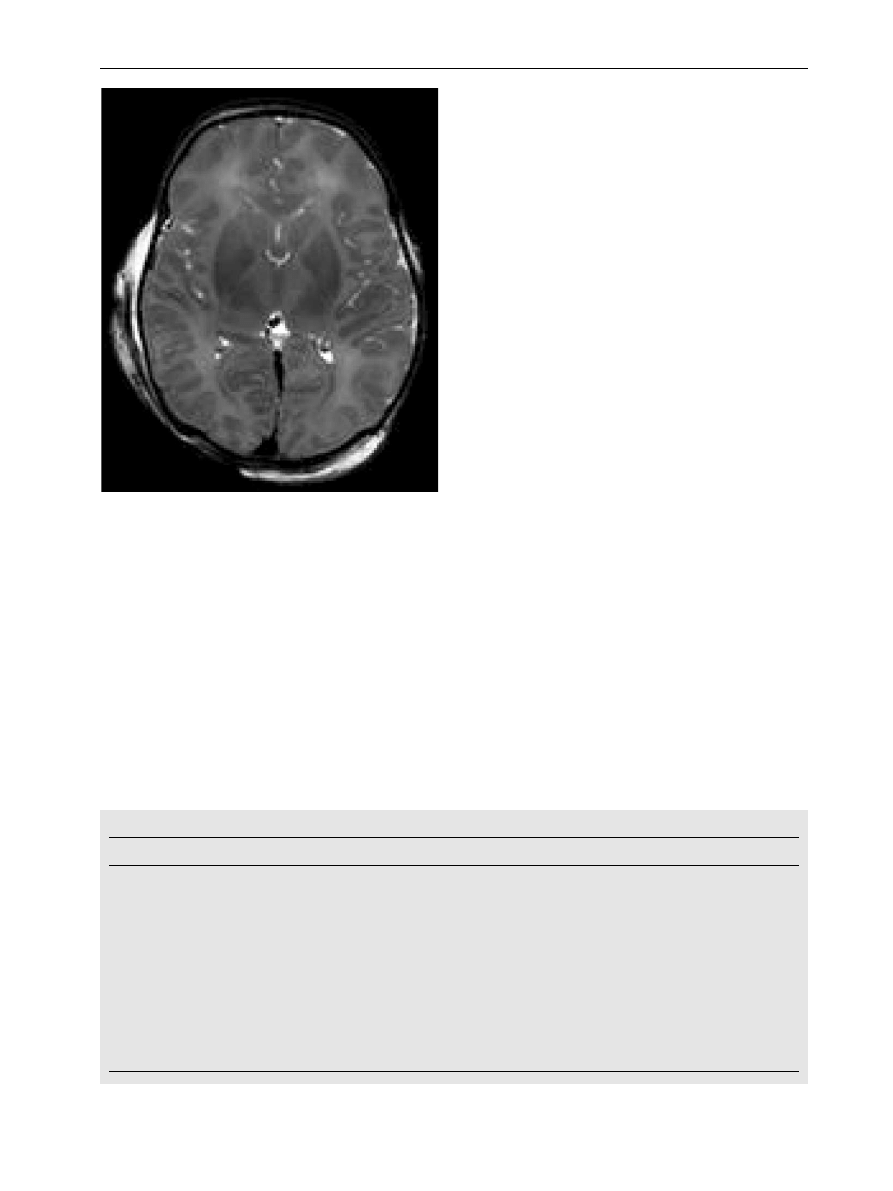
Figure 4
Postmortem image at 3 Tesla in an infant with
neonatal encephalopathy taken one day after death.
Acquisition time was 13 minutes. There is bilateral
abnormal low signal intensity within the basal ganglia
and thalami.
Table 1
Image parameters for T1 weighted volume acquisitions at 1.5 and 3 T.
1.5 T
3 T
1.5 T
3 T
Sequence
RF spoiled gradient
echo
MP rage
T2 weighted TSE
T2 weighted TSE
Echo time (ms)
6
4.6
210
160
Repetition time (ms)
27
17
4200
z10,000
Flip angle (degrees)
30
13
20
90
Matrix
192!256
256!256
192!56
224!256
Slices
114
160
20
90
Slice thickness (mm)
1.6
0.8
5
2
Gap
–
–
–
–
Scan duration (min)
9
2.5
3:21
6
Field of view
240
200
240
220
Number signal averages
1
1
2
1
M. Rutherford et al.
284
brain, such as implementation of short scan times
to prevent motion artefacts, use of low flip angles
and out of phase imaging to better saturate the
subcutaneous fat that obscures in some cases the
visibility of the vessels in the three-dimensional
maximum intensity projection, implementation of
ramped RF pulse and multiple thin volume strat-
egies to maintain intra-vascular signal at the distal
cortical branches. In this way, more subtle response
of intra-cranial vasculature to acquire pathology in
the immature brain can be studied (
).
Diffusion weighted imaging is able to measure
the random molecular motion of water within
tissues as an apparent diffusion coefficient. DWI
requires the use of diffusion sensitive gradients,
measured by a b value. The higher the b value the
more sensitive the DWI sequence to water motion.
In acute ischaemia there is a reduction in ADC
values, corresponding to reduced water motion as
in perinatal stroke. This reduction in measurable
water motion results from a relative decrease of
mobile extracellular water and an increase in more
restricted intra-cellular water that occurs with
cellular swelling.
The directional dependence of water motion or
anisotropy may be measured with diffusion tensor
imaging. Anisotropy results from restricted motion
in one direction, e.g. across a white matter tract as
opposed to along white matter tract. Relative
anisotropy (RA) or fractional anisotropy (FA) may
be used to assess both tissue maturity with
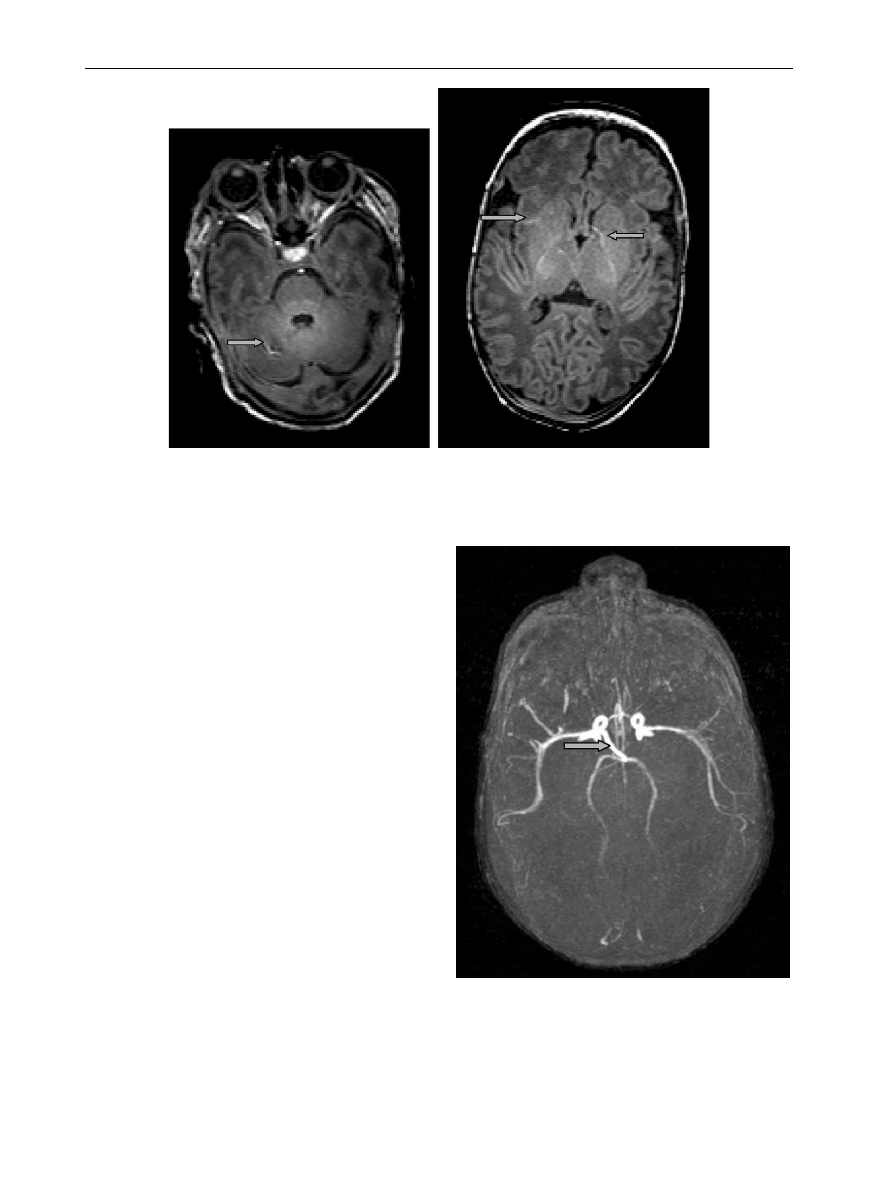
Figure 5
T1 weighted MP RAGE images showing high signal intensity from normal vessels within the cerebellum and
within the basal ganglia (arrows).
Figure 6
MR angiogram in a neonate with periventri-
cular leucomalacia. There is a rare variation of the right
posterior communicating artery (arrow) which is arising
directly off the internal carotid. The left posterior
communicating artery is absent and the Circle of Willis
therefore incomplete.
MR imaging of the neonatal brain at 3 Tesla
285
increases in FA and RA with myelination and tissue
integrity with decreases in FA and RA with some
pathological processes.
Diffusion imaging at 3 T opens up possibilities for
improving SNR in neonatal imaging. This may be
used to shorten examination times but the increase
in SNR can be used to reduce partial-volume
effects, which is beneficial for fibre tracking. This
increased SNR allows diffusion weighted imaging at
high b values with adequate resolution of the
images. For diffusion sequences imaging at 3 T,
allows sufficient SNR to increase b values to over
1000 mm
2
/s.
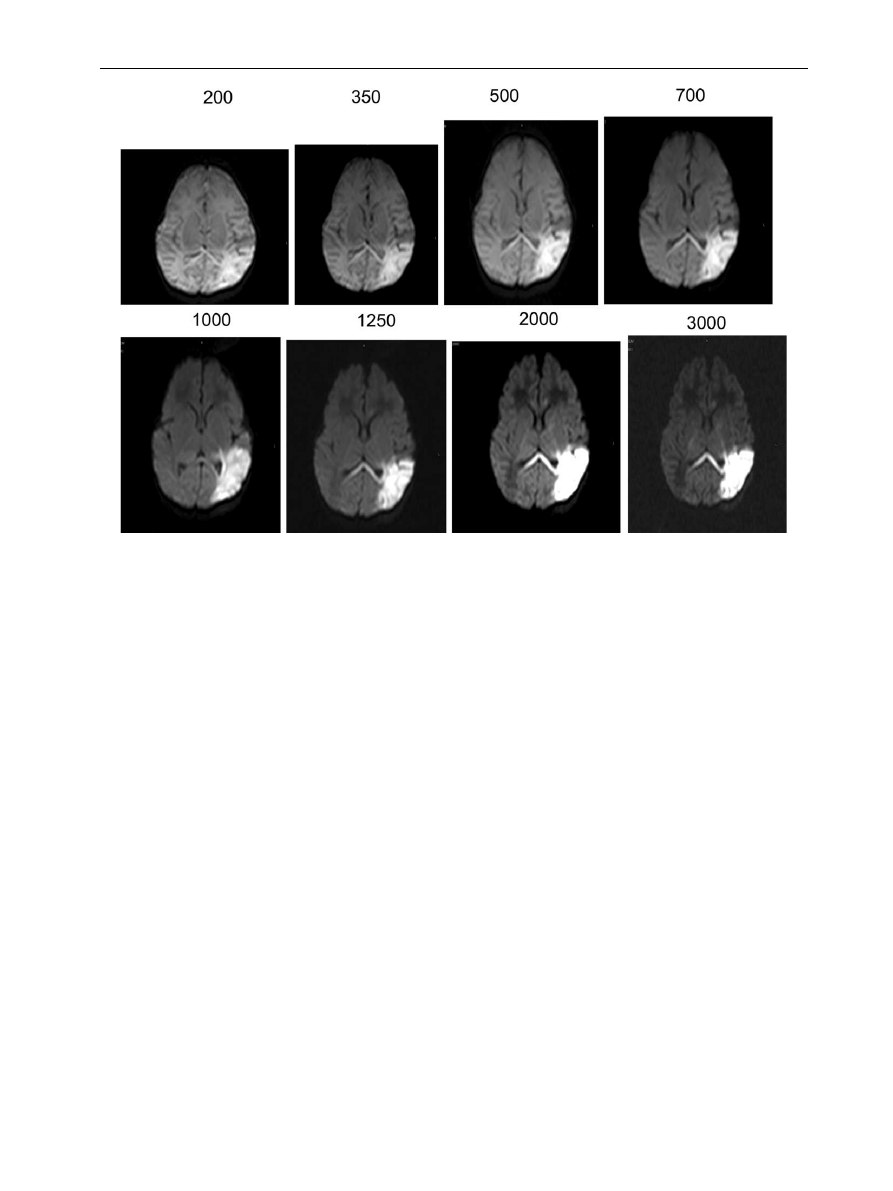
shows a series of diffusion
images of the neonatal brain obtained at different b
values. It can be seen that the tissue contrast within
the images changes with increasing b values
(
). In addition, we have shown that high b
value imaging increases conspicuity of perinatally
acquired lesions and in some children identified
lesions that were not visible at lower values.
Increased lesion conspicuity has also been reported
with higher b values in adult studies.
Further
improvements in diffusion imaging may also be
made by using phased array coils, which allow
parallel imaging techniques such as SENSE to be
used.
This technique can be used to reduce
imaging time, however, it is probably more ben-
eficial to reduce the EPI factor and hence reduce
image distortions.
Contrast administration
Administration of a gadolinium-based contrast
agent produces higher contrast between tumour
and normal brain at 3 T than at 1.5 T, helps to
detect more cerebral metastases at 3 versus 1.5 T
in single and cumulative triple dose, improves the
evaluation of macroadenomas of the hypophysis,
and makes MR venography at 3 T clinically
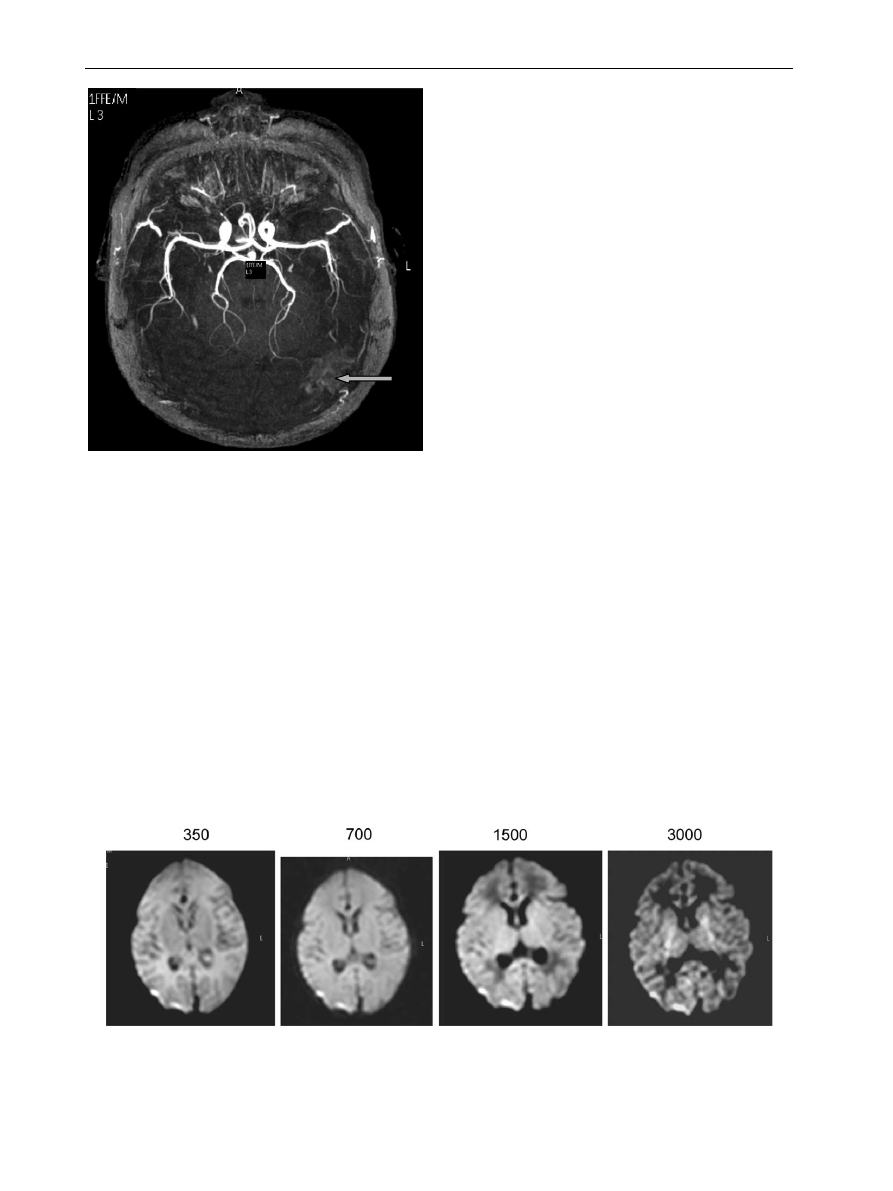
Figure 7
(A) T2 weighted image. Neonate with marked ventricular dilation causing compression of the surrounding
brain. (B) MR phase contrast angiogram show displacement of the middle cerebral arteries by the ventricles and slightly
excessive peripheral branching (arrow). (C) MR venogram shows excessive peripheral veins (arrow).
M. Rutherford et al.
286
attractive with increase in spatial resolution within
the same measurement time, thus providing more
detailed information.
(Fig. 8). It’s role in studying
more specific pathologies within the neonatal and
infant brain has yet to be established.
Spectroscopy at 3 T
With high magnetic fields improved SNR can allow
greater accuracy in quantitative measurements and
can be used to reduce voxel size, and thus minimise
partial-volume effects in heterogenous structures
such as the brain. Increased chemical shift
dispersion reduces the overlap of resonances
obtained in spectroscopy and thus increases the
number of metabolites that can be identified and
accurately quantified.
This would allow depiction
of resonances from for instance glutamate.
Functional magnetic resonance imaging
(fMRI)
Functional magnetic resonance imaging (fMRI) uses
BOLD contrast. BOLD fMRI at 1.5 T can achieve a
spatial resolution of up to 3–5 mm. Studies at 3 T
will increase SNR and thereby enhance spatial
resolution and specificity of fMRI.
This will
provide improved resolution of cortical anatomy.
In addition as field strength increases, the field
gradient around the capillaries becomes larger and
extends further into the parenchyma, thus increas-
ing the participation of the brain tissue in the
functional signal. There are no reports as yet of
functional imaging in neonates and infants at 3 T.
Summary
The neonatal brain can be imaged safely at 3 T.
Problems with increased relaxation times and
increased heat deposition can be overcome but
altering sequence parameters. Increased suscepti-
bility is not a problem because of the immature
sinuses. The increased SNR afforded at higher field
strength allows fast or more detailed images. Specific
improvements in anatomical definition and in lesion
detection and conspicuity may also be obtained in
more advanced techniques such as diffusion weighted
imaging, angiography and venography.
Figure 9
Diffusion imaging of a normal brain of a term born infant imaged at 3 Tesla. Single shot EPI (TR 2500/TE 100)
Slice thickness 4 mm. Signal averages 2–8. Variation in tissue signal intensity with increasing b value (range
350–3000 mm
2
/s).
Figure 8
MR angiogram of a neonate with a posterior
perinatal infarct imaged at 23 days of age. There is a
“blush” of high signal intensity (arrow) in the region of
the infarct that may represent neovascularisation.
MR imaging of the neonatal brain at 3 Tesla
287
Acknowledgements
Staff at the Robert Steiner MR Unit and the
Department of Paediatrics, Hammersmith Hospital,
The Medical Research Council, The Academy of
Medical Sciences, The Health Foundation, Philips
Medical Systems are acknowledged.
References
1. Frayne R, Goodyear BG, Dickhoff P, Lauzon ML, Sevick RJ.
Magnetic resonance imaging at 3.0 Tesla: challenges and
advantages in clinical neurological imaging. Invest Radiol
2003;38:385–402.
2. Kim DS, Garwood M. High-field magnetic resonance tech-
niques for brain research. Curr Opin Neurobiol 2003;13:
612–9.
3. Baudendistel KT, Heverhagen JT, Knopp MV. Clinical MR at
3 Tesla: current status. Radiologe 2004;44:11–18.
4. Sicotte NL, Voskuhl RR, Bouvier S, Klutch R, Cohen MS,
Mazziotta JC. Comparison of multiple sclerosis lesions at 1.5
and 3.0 Tesla. Invest Radiol 2003;38:423–7.
5. Trattnig S, Ba-Ssalamah A, Noebauer-Huhmann IM,
Barth M, Wolfsberger S, Pinker K, Knosp E. MR contrast
agent at high-field MRI (3 Tesla). Top Magn Reson Imaging
2003;14:365–75.
6. Ethofer T, Mader I, Seeger U, Helms G, Erb M, Grodd W,
Ludolph
A,
Klose
U.
Comparison
of
longitudinal
metabolite relaxation times in different regions of the
human brain at 1.5 and 3 Tesla. Magn Reson Med 2003;
50(6):1296–301.
7. Al-Kwifi O, Emery DJ, Wilman AH. Vessel contrast at three
Tesla in time-of-flight magnetic resonance angiography of
the intracranial and carotid arteries. Magn Reson Imaging
2002;20:181–7.
8. Volpe JJ, Herscovitch P, Perlman JM, Kreusser KL,
Raichle ME. Positron emission tomography in the
asphyxiated term newborn: parasagittal impairment of
cerebral blood flow. Ann Neurol 1985;17:287–96.
9. Lou HC. The ‘lost autoregulation hypothesis’ and brain
lesions in the newborn—an update. Brain Dev 1988;10:
143–6.
10. Dean LM, Taylor GA. The intracranial venous system in
infants: normal and abnormal findings on duplex and color
Doppler sonography. AJR Am J Roentgenol 1995;164:
151–6.
11. Govaert P, Matthys E, Zecic A, Roelens F, Oostra A,
Vanzieleghem B. Perinatal cortical infarction within middle
cerebral artery trunks. Arch Dis Child Fetal Neonatal Ed
2000;82:59–63.
12. Mercuri E, Cowan F, Gupte G, Manning R, Laffan M,
Rutherford M, Edwards AD, Dubowitz L, Roberts I. Pro-
thrombotic disorders and abnormal neurodevelopmental
outcome in infants with neonatal cerebral infarction.
Pediatrics 2001;107:1400–4.
13. Yoshiura T, Mihara F, Tanaka A, Ogomori K, Ohyagi Y,
Taniwaki T, Yamada T, Yamasaki T, Ichimiya A, Kinukawa N,
Kuwabara Y, Honda H. High b value diffusion-weighted
imaging is more sensitive to white matter degeneration in
Alzheimer’s disease. Neuroimage 2003;20:413–9.
Figure 10
3 Tesla diffusion imaging. Single shot EPI (TR 2500/TE 100). Slice thickness 4 mm. Signal averages 2–8.
Middle cerebral artery infarct at different b values (range 300–3000 mm
2
/s) in a term infant aged 5 days.
M. Rutherford et al.
288

14. Jaermann T, Crelier G, Pruessmann KP, Golay X, Netsch T, van
Muiswinkel AM, Mori S, van Zijl PC, Valavanis A, Kollias S,
Boesiger P. SENSE-DTI at 3 T. Magn Reson Med 2004;51:230–6.
15. Tibbo P, Hanstock C, Valiakalayil A, Allen P. 3-T proton MRS
investigation of glutamate and glutamine in adolescents at
high genetic risk for schizophrenia. Am J Psychiatry 2004;
161:1116–8.
16. Yang TT, Menon V, Reid AJ, Gotlib IH, Reiss AL. Amygdala
activation associated with happy facial expressions in
adolescents: a 3-T functional MRI study. J Am Acad Child
Adolesc Psychiatry 2003;42(8):979–85.
17. Heinke W, Fiebach CJ, Schwarzbauer C, Meyer M,
Olthoff D, Alter K. Sequential effects of propofol on
functional brain activation induced by auditory language
processing: an event-related functional magnetic reson-
ance imaging study. Br J Anaesth 2004;92:641–50. Epub
2004 Apr 02.
18. Barbier EL, Marrett S, Danek A, Vortmeyer A, van
Gelderen P, Duyn J, Bandettini P, Grafman J, Koretsky AP.
Imaging cortical anatomy by high-resolution MR at 3.0 T:
detection of the stripe of Gennari in visual area 17. Magn
Reson Med 2002;48:735–8.
MR imaging of the neonatal brain at 3 Tesla
289
Document Outline
Wyszukiwarka
Podobne podstrony:
Eric Racine Pragmatic Neuroethics Improving Treatment and Understanding of the Mind Brain Basic Bioe
Nazi Euthanasia of the Mentally Ill at Hadamar
Dynamic gadolinium enhanced subtraction MR imaging – a simple technique for the early diagnosis of L
MR spectroscopic studies of the brain in psychiatric disorders
The Challenge of Reinstating Hepatitis B Vaccination at Birth
Childhood Trauma, the Neurobiology of Adaptation, and Use dependent of the Brain
Cigarettes and Their?struction of the Brain
Pros and Cons of the?ath Penalty
Jo Walton At The Bottom Of The Garden
Collagens building blocks at the end of the
The Masque Of The Red?ath (2)
Chairman of the board at 14
Ralph Abraham, Terence McKenna, Rupert Sheldrake Trialogues at the Edge of the West Chaos, Creativi
At The Dark End Of The Street (6 Horn)
Stephen King Hotel At The End Of The Road
Jack L Chalker Watchers at the Well 02 Shadows of the Well of Souls
Dead zones of the imagination On violence, bureaucracy, and interpretive labor David GRAEBER
więcej podobnych podstron