
Contents
lists
available
at
Process
Biochemistry
j o
u
r n
a l
h o m
e p a g e :
w w w . e l s e v i e r . c o m / l o c a t e / p r o c b i o
Production
of
xylooligosaccharides
using
immobilized
endo-xylanase
of
Bacillus
halodurans
Yu-Sheng
Lin
,
Min-Jen
Tseng
,
Wen-Chien
Lee
a
Department
of
Chemical
Engineering,
National
Chung
Cheng
University,
Chiayi,
Taiwan
b
Department
of
Life
Science,
National
Chung
Cheng
University,
Chiayi,
Taiwan
a
r
t
i
c
l
e
i
n
f
o
Article
history:
Received
10
June
2011
Received
in
revised
form
10
August
2011
Accepted
12
August
2011
Available
online
19
August
2011
Keywords:
Immobilized
enzyme
Endo-xylanase
Xylooligosaccharides
Bacillus
halodurans
Anionic
exchanger
XOS
Corncob
a
b
s
t
r
a
c
t
Endo-xylanase
secreted
by
the
alkaliphilic
Bacillus
halodurans
was
immobilized
onto
an
anionic
exchange
resin
via
the
ionic
linkage
and
the
highest
immobilized
amount
was
achieved
at
pH
8.
Approximately
0.4
mg
of
enzyme
could
be
coupled
onto
1
g
of
anionic
exchanger.
Time
courses
of
the
xylooligosaccharides
(XOS)
produced
from
corncob
xylan
indicated
that
the
immobilized
enzyme
tends
to
use
shorter
xylan
chains
as
the
substrate
and
to
produce
more
xylobiose
and
xylotriose
initially.
On
the
contrary,
when
free
enzyme
was
employed,
products
at
initial
stage
of
the
reaction
exhibited
higher
level
of
oligomers
with
degree
of
polymerization
greater
than
4,
suggesting
that
free
xylanase
worked
on
both
longer
(insoluble)
and
shorter
(soluble)
xylan
chains.
At
the
end
of
24
h
reaction,
XOS
mixture
contained
a
total
of
25.2%
and
32.5%
(w/w)
of
xylobiose
and
xylotriose
with
immobilized
xylanase
and
free
xylanase,
respectively.
The
conversions
for
converting
substrate
xylan
to
soluble
XOS
with
immobilized
xylanase
was
determined
to
be
80.9%,
which
was
lower
than
the
use
of
free
xylanase
(99.8%).
The
combination
of
free
and
immobilized
xylanase
can
be
employed
to
further
improve
the
conversion
of
XOS.
©
2011
Elsevier
Ltd.
All
rights
reserved.
1.
Introduction
Xylooligosaccharides
(XOS)
are
typically
produced
by
enzy-
matic
approach
using
plant
source
of
xylan
as
raw
material.
The
xylanase-catalyzed
hydrolysis
yields
XOS
products
composed
of
mainly
xylobiose,
xylotriose,
and
a
small
fraction
of
oligosaccha-
rides
with
a
higher
degree
of
polymerization
(DP).
Xylobiose
and
xylotriose
are
the
most
important
components
of
human
probiotic.
Agro-wastes
such
as
corncob
(also
known
as
maize
cores)
have
been
frequently
used
as
the
xylan
source
for
the
production
of
XOS.
Zhu
et
al.
ammonia-pretreated
corncob
as
the
raw
material.
The
enzymatic
reaction
at
pH
5
and
50
◦
C
using
a
dosage
of
0.04
g/g
solids
endoxylanase
X2753
resulted
in
15.74
g/l
XOS
in
72
h.
In
addi-
tion
to
corncob,
Yang
et
al.
bagasse,
wheat
bran,
and
peanut
shell
as
raw
materials.
After
extraction
by
NaOH,
these
materials
were
converted
to
XOS
by
the
action
of
xylanase
from
Thermobi-
fida
fusca
at
pH
7
and
60
◦
C.
The
XOS
yields
after
24
h
reaction
were
29.5%,
23.7%,
7.6%,
and
10.1%,
respectively
from
the
four
raw
corn-
cob,
bagasse,
wheat
bran,
and
peanut
shell.
After
extraction
with
24%
KOH,
cotton
stalk
was
used
as
the
raw
material
to
produce
XOS
by
the
action
of
an
Aspergillus
niger
secreted
enzyme,
Veron
∗ Corresponding
author.
Fax:
+886
5
2721206.
address:
(W.-C.
Lee).
191
(AB
Enzymes
Co.,
Germany),
at
pH
5.4
and
40
◦
C.
An
XOS
yield
of
53%
was
obtained
after
enzymatic
reaction
for
24
h
In
the
present
work,
endo-xylanases
purified
from
alkaliphilic
Bacillus
halodurans
and
immobilized
with
anionic
exchange
resin
were
employed
for
the
production
of
XOS
from
corncob.
This
strain,
which
was
previously
named
as
Bacillus
firmus
rei-
dentified
by
16S
rDNA
gene
sequencing
as
a
B.
halodurans.
Two
endo-xylanases
(endo-1,4-
-xylanase,
EC
3.2.1.8)
purified
from
this
B.
halodurans
have
molecular
weights
of
45
kDa
and
23
kDa,
respectively,
and
both
showed
enzymatic
activity
over
the
pH
range
of
5.0–11.0
at
37
◦
C
the
calculated
pI
value
for
the
dom-
inant
secreted
proteins
in
45
kDa
xylanase
is
4.5,
we
therefore
tried
to
immobilize
the
enzyme
from
B.
halodurans
onto
anionic
exchange
resin
(Lewatit
MonoPlus
MP64)
by
the
ionic
binding
in
this
work.
Like
other
industrial
enzymes,
xylanase
can
be
theo-
retically
immobilized
by
different
methods
with
various
carriers.
Several
conventional
enzyme
immobilization
methods
including
ionic
binding
on
DEAE-Sephadex
resin,
inclusion
in
polyacrylamide,
and
covalent
binding
by
glutaraldehyde
to
chitosan,
chitin,
Amber-
lite,
Duolite,
florisil,
and
gelatin
have
been
examined
for
the
immobilization
of
xylanase
from
Talaromyces
thermophilus
2.
Materials
and
methods
2.1.
Preparation
of
enzyme
B.
halodurans
Thonburi
(deposited
to
Bioresource
Collection
and
Research
Cen-
ter
of
Taiwan
with
a
deposited
number
of
BCRC
910501)
was
cultivated
at
37
◦
C
1359-5113/$
–
see
front
matter
©
2011
Elsevier
Ltd.
All
rights
reserved.
doi:
2118
Y.-S.
Lin
et
al.
/
Process
Biochemistry
46
(2011)
2117–2121
for
5
days
in
Emerson
medium
containing
0.55%
yeast
extract
(Bacto),
0.5%
peptone
(Bacto),
0.02%
MgSO
4
(Merck),
0.1%
K
2
HPO
4
(Merck),
and
2%
corncob
(obtained
from
local
market).
The
initial
pH
was
adjusted
to
10
with
1
M
NaOH
(Showa).
The
5
d
cul-
ture
was
centrifuged
and
the
supernatant
was
precipitated
with
ammonia
sulfate
on
ice.
The
resultant
precipitate
was
dissolved
using
100
mM
Tris–HCl
(J.T.
Baker)
buffer
and
then
dialyzed
against
the
100
mM
Tris–HCl
buffer
containing
10%
glyc-
erol.
The
activity
of
xylanase
was
measured
by
calculating
the
release
of
reducing
sugars
from
birchwood
xylan
via
modified
dinitrosalicylic
acid
approach
l
of
sample
was
mixed
with
160
l
Tris–HCl
buffer
(100
mM,
pH
8.0)
containing
1%
brichwood
xylan
(Sigma).
The
reaction
was
incubated
at
37
◦
C
for
5
min
and
0.4
ml
of
DNS
reagent
composed
of
1%
dinitrosalicylic
acid
(RDH),
0.2%
phenol
(Riedel-de
Haën),
0.05%
sodium
sulfite,
and
1%
sodium
hydroxide
(J.T.
Baker)
was
added
to
stop
the
reaction,
the
solution
was
then
boiled
for
5
min
absorbance
at
500
nm
was
measured
after
adding
2.5
ml
of
water.
One
unit
of
xylanase
was
defined
as
the
amount
of
enzyme
required
to
release
1
mol
of
xylose
from
birchwood
xylan
in
1
min.
2.2.
Enzyme
immobilization
Ion-exchange
resin
Lewatit
MonoPlus
MP64
was
used
for
the
immobilization
of
xylanase
by
ionic
binding.
Macroporous
styrene-divinylbenzene
copolymer
hav-
ing
quaternary
amine
functionality
was
introduced
as
weak
basic
anion
exchange
resin.
As
supplied
by
the
vendor,
this
highly
monodispersive
resin
exhibits
an
aver-
age
particle
size
of
0.59
mm,
and
its
beads
are
highly
chemically
and
osmotically
stable.
Thus,
this
anionic
exchanger
was
used
for
the
immobilization
of
enzyme
at
pH
greater
than
its
pI
value.
The
ion
exchanger
(100
mg)
was
equilibrated
with
1
ml
of
Tris–HCl
buffer
(100
mM,
pH
8.0)
and
incubated
with
various
doses
(1,
2,
3,
5,
9,
and
11
l)
of
concentrated
xylanase
(127.8
U/ml)
under
4
◦
C
for
24
h.
The
unbound
enzyme
was
removed
by
washing
with
the
same
buffer
until
no
activity
or
soluble
protein
was
detected.
The
enzyme
activity
obtained
in
buffer
after
the
washing
was
taken
as
unbound
enzyme.
To
study
the
effect
of
pH
on
enzyme
immobilization,
ion
exchanger
(100
mg)
was
equilibrated
in
buffers
of
different
pH
(5.0–8.0)
and
incubated
with
2
l
of
concentrated
xylanase
(127.8
U/ml)
under
4
◦
C
for
24
h.
Buffers
used
were
citrate–phosphate,
pH
5.0–6.0,
and
Tris–HCl,
pH
7.0–8.0.
2.3.
Operational
stability
of
the
immobilized
xylanase
The
immobilized
enzyme
was
assayed
for
5
cycles,
each
with
a
duration
of
5
min.
For
each
cycle,
500
l
of
2.0%
(w/v)
xylan
in
100
mM
Tris–HCl
buffer
(pH
8.0)
was
added
to
the
immobilized
enzyme
(0.05
g;
0.255
U)
and
incubated
for
5
min
under
continuous
shaking
at
37
◦
C.
At
the
end
of
reaction,
the
immobilized
enzyme
was
collected
by
centrifugation
at
8000
×
g
for
30
s.
The
supernatant
was
assayed
for
reducing
sugars.
For
running
the
succeeding
cycle,
the
immobilized
enzyme
was
redissolved
in
500
l
of
2.0%
(w/v)
xylan
in
100
mM
Tris–HCl
buffer
(pH
8.0)
and
processed
with
the
abovementioned
procedures.
2.4.
Production
of
xylooligosaccharides
Xylan
was
obtained
from
the
alkali
extraction
of
corncob.
Corncob,
which
was
obtained
locally,
was
chopped
and
milled
into
powders.
Powders
of
corncob
were
treated
with
15%
NaOH
with
a
solid–liquid
ratio
of
1:20
(w/v)
at
90
◦
C
for
90
min.
The
resultant
soluble
fraction
was
neutralized
with
acetic
acid
to
a
final
pH
of
5.0.
Three-time
volume
of
95%
ethanol
was
then
added
and
the
resultant
mixture
was
incubated
for
60
min
at
room
temperature.
Xylan,
the
substrate
for
XOS
production,
was
obtained
from
the
recovered
precipitate.
Free,
immobilized
xylanase
and
a
combination
of
free
and
immobilized
xylanase
with
a
total
activity
of
0.255
U
were
added
separately
to
1
ml
of
2.0%
(w/v)
xylan
in
100
mM
Tris–HCl
buffer
(pH
8.0).
The
mixture
was
incubated
at
50
◦
C
with
mild
agi-
tation
(30
rpm).
A
sample
of
100
l
was
taken
at
different
time
intervals
and
diluted
with
D.I.
water
to
10%
prior
to
filtration.
The
filtrate
was
analyzed
by
HPLC
using
a
Biorad
Aminex
HPX-87H
column
(300
×
7.8
mm
i.d.)
to
examine
the
concentrations
of
xylobiose,
xylotriose,
and
xylose.
The
mobile
phase
was
5.0
mM
sulfuric
acid
at
a
flow-rate
of
0.6
ml/min.
The
retention
times
of
hydrolytic
products
(xylose,
xylo-
biose,
and
xylotriose)
were
compared
with
known
standards
(Megazyme)
with
a
refractive
index
detector
at
65
◦
C
column
temperature.
3.
Results
and
discussion
3.1.
Immobilization
of
endo-xylanase
According
to
previous
findings,
the
alkaliphilic
bacterium
B.
halodurans
Thonburi
could
secret
two
xylan-degrading
enzymes
with
molecular
weights
of
45
and
23
kDa,
respectively
The
protein
extracts
of
these
two
enzymes
in
different
ratios
can
be
obtained
by
culturing
B.
halodurans
in
Emerson
medium
supple-
mented
with
different
sources
of
xylan
(corncob,
wheat
bran,
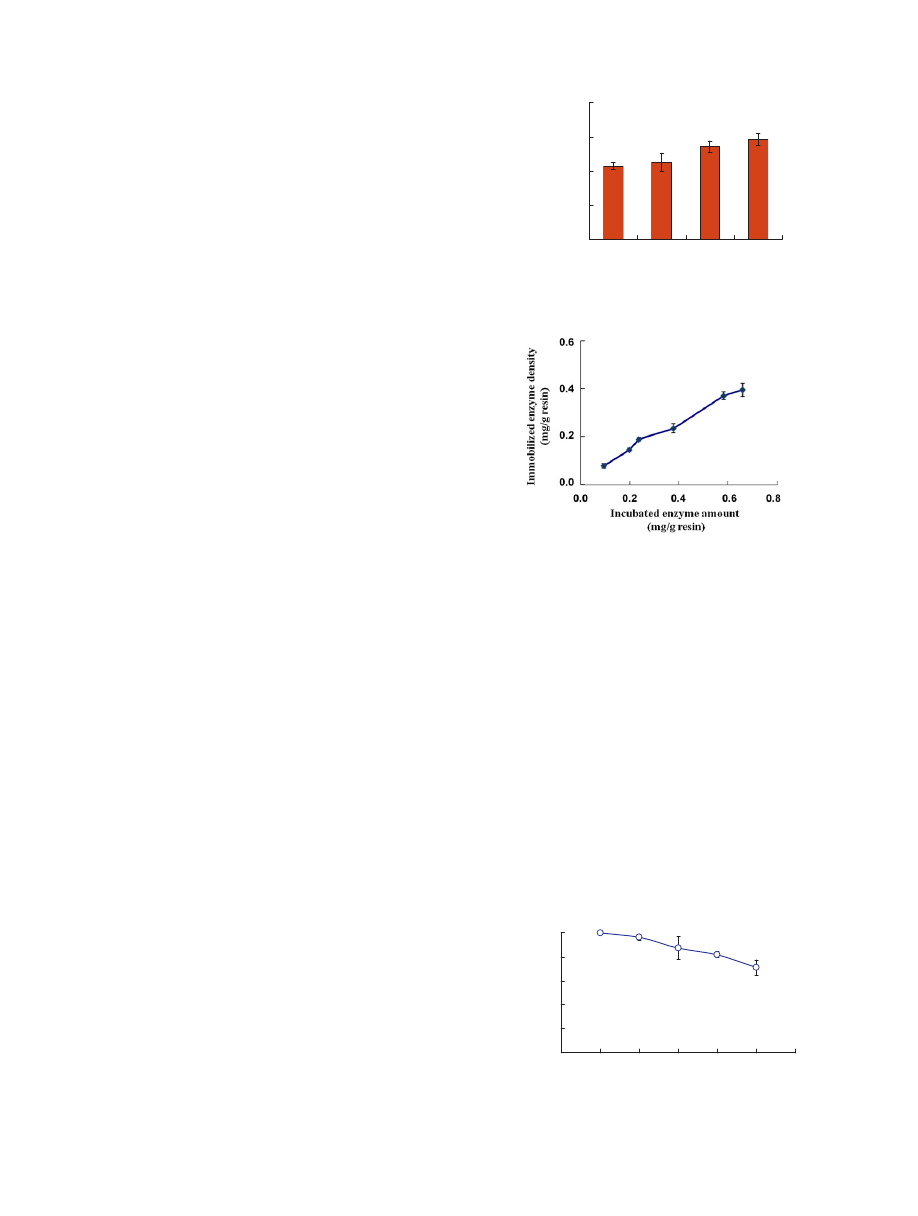
and
0.00
0.05
0.10
0.15
0.20
8
7
6
5
pH value
Immobilized protein
(mg/g resin)
Fig.
1.
Effect
of
pH
on
the
immobilization
of
B.
halodurans
xylanase
onto
anionic
exchange
resin
(Lewatit
MonoPlus
MP64).
Fig.
2.
Effect
of
the
amount
of
added
enzyme
protein
on
the
immobilization
of
xylanase
from
B.
halodurans
on
anionic
exchange
resin.
birchwood
xylan)
as
the
inducer.
As
the
corncob
(2%)
was
used
as
the
inducer
in
the
present
work,
only
one
prominent
band
at
45
kDa
xylanase
was
observed
in
the
SDS-PAGE
(data
not
shown).
The
anionic
exchange
resin
was
thus
used
for
the
immobilization
of
the
endo-xylanase
via
the
ionic
linkage.
The
amount
of
xylanase
bound
onto
anionic
exchanger
varied
with
the
pH
value
of
the
enzyme
solution
in
the
range
between
5
and
8.
The
higher
the
pH
value,
the
stronger
the
anionic
groups
on
the
enzyme.
This
was
attributed
to
the
deprotonization
of
func-
tion
groups
on
enzyme
protein
in
the
conditions
with
pH
value
greater
then
its
pI
value.
As
shown
in
the
highest
immo-
bilized
amount
was
achieved
at
pH
8.
Since
the
45
kDa
xylanase
showed
the
pH
optimum
at
6–8
for
its
enzymatic
activity
immobilization
of
this
enzyme
was
thus
carried
out
at
pH
8
for
further
study.
When
pH
was
set
to
8,
the
immobilized
amount
of
enzyme
increased
with
the
amount
of
enzyme
in
the
solution
applied
for
immobilization.
About
0.4
mg
of
enzyme
could
be
cou-
pled
onto
1
g
of
anionic
exchanger,
as
shown
in
the
work
of
Kapoor
and
Kuhad
series
of
ion
exchangers
(DEAE-sepharose,
Q-S,
CM-sepharose,
Amberlite
IR-120,
and
Amberlite
IR-440)
was
0
20
40
60
80
100
6
5
4
3
2
1
0
Cycle number
Retention of xylanase
activity (%)
Fig.
3.
Reusability
of
the
B.
halodurans
xylanase
immobilized
on
anionic
exchange
resin.
Y.-S.
Lin
et
al.
/
Process
Biochemistry
46
(2011)
2117–2121
2119
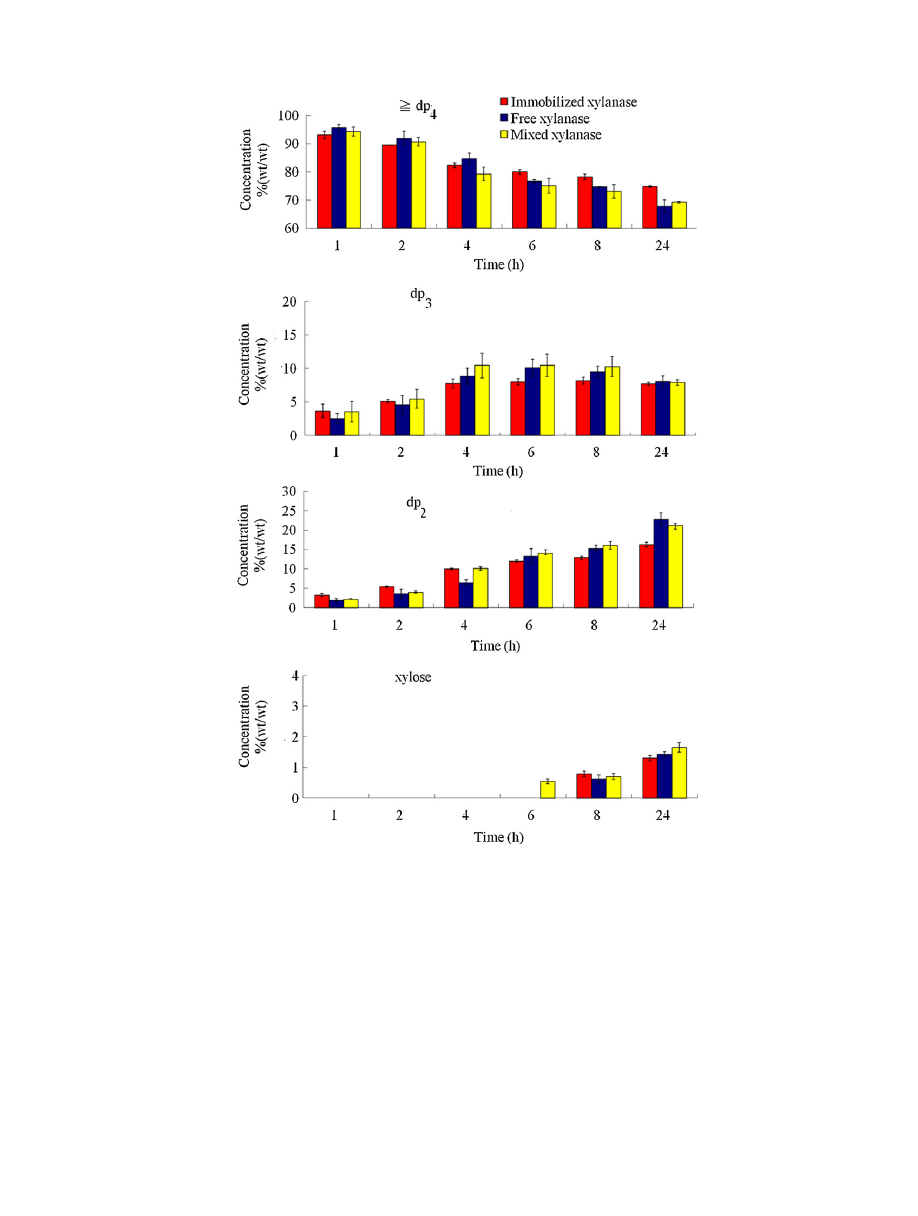
Fig.
4.
Time
courses
of
the
xylooligosacchride
production
from
corncob
xylan
by
free
and
immobilized
xylanase
from
B.
halodurans.
A
combined
use
of
free
and
immobilized
xylanase
was
also
presented.
Each
batch
of
reaction
was
carried
out
with
0.255
U/ml
xylanase
using
2%
corncob
substrate
at
pH
8
and
50
◦
C.
For
each
data
bar,
the
number
of
repeated
runs
ranged
from
3
to
6.
The
concentration
of
each
product
was
expressed
in
term
of
gram
per
100
g
of
total
products,
%
(w/w).
incubated
with
20
mg
lyophilized
xylanase
per
unit
gram
of
ion
exchanger
for
the
immobilization
of
xylanase
by
ionic
binding.
Based
on
the
detection
of
unbound
enzyme
in
washing
buffer,
the
bound
amounts
of
enzyme
was
found
to
range
from
1.2
to
3.8
mg/g-ion
exchange.
Bound
efficiency
was
low
in
the
range
from
6.2
to
19.2%.
For
the
sake
of
reducing
unbound
enzyme,
the
present
work
carried
out
xylanase
immobilization
with
added
enzyme
of
0.7
mg/g-ion
exchanger,
which
resulted
in
the
maximum
bound
enzyme
0.4
mg/g-ion
exchanger.
The
bound
efficiency
was
much
higher
(60.2%).
Anionic
exchanger-bound
xylanase
was
capable
to
be
reused
for
several
times
without
losing
too
much
of
its
enzymatic
activity.
As
shown
in
the
activity
of
immobilized
xylanase
on
anionic
exchange
resin
decreased
gradually
with
number
of
repeated
uses.
The
immobilized
enzyme
retained
about
71%
of
its
original
activ-
ity
after
reuse
for
5
cycles.
Possible
causes
for
the
gradual
decrease
in
enzyme
activity
over
cycling
could
be
owing
to
the
elution
of
enzyme
from
the
carrier
at
high
reaction
temperature
(50
◦
C)
and
clogging
of
carrier
by
macromolecular
xylan
fragments.
However,
this
xylanase-immobilized
preparation
was
found
to
be
superior
or
comparable
to
those
reported
in
the
literature.
According
to
Kapoor
and
Kuhad
in
case
of
xylanase
(from
Bacillus
pumilus
Strain
MK001)
immobilized
on
gelatin
by
entrapment
and
chitin
by
phys-
ical
adsorption,
more
than
half
of
the
activity
was
lost
after
four
cycles.
Xylanase
immobilized
on
Q-sepharose
with
ionic
binding
and
HP-20
beads
with
covalent
binding
showed
better
retention

2120
Y.-S.
Lin
et
al.
/
Process
Biochemistry
46
(2011)
2117–2121
Table
1
Calculation
of
the
conversion
of
xylan
to
soluble
oligomers
by
enzymatic
reaction
for
24
h.
Total
soluble
oligomers
initially
presented
(g/l)
Total
soluble
oligomers
in
the
final
product
(g/l)
Total
fraction
of
xylobiose
and
xylotriose
in
the
product
%(w/w)
Immobilized
xylanase
9.79
16.17
22.5
80.9
62.5
Free
xylanase
9.96
19.96
33.8
99.8
99.6
Combined
preparation
9.60
18.95
27.6
94.8
89.9
a
Based
on
the
total
soluble
oligomers
produced
with
respect
to
the
initial
substrate
concentration
(20
g/l).
b
Based
on
the
soluble
oligomers
produced
from
insoluble
xylan
initially
presented
in
the
substrate.
rate,
with
up
to
70.0%
of
its
original
activity
retained
after
seven
cycles.
3.2.
Production
of
XOS
Xylooligosaccharides
are
usually
produced
from
xylan
by
enzy-
matic
hydrolysis.
Corncob
is
an
ideal
raw
material
for
producing
XOS
due
to
its
relatively
high
hemi-cellulose
(xylan)
content.
The
time
courses
of
XOS
production
by
0.255
U/ml
xylanase
(immobi-
lized,
free,
or
a
combined
use
of
immobilized
and
free
enzyme)
using
2%
corncob
substrate
at
pH
8
and
50
◦
C
are
given
in
When
20
g/l
xylan
was
employed
for
XOS
production,
the
ini-
tial
amount
of
soluble
fraction
with
DP
4
was
9.0–10.0
g/l
in
the
reaction
mixture.
No
XOS
with
DP
less
than
4
was
observed
at
ini-
tial
stage
of
the
reaction.
This
soluble
fraction
was
xylooligomers
produced
due
to
the
autolysis
in
alkaline
conditions.
When
immo-
bilized
xylanase
was
applied
to
the
xylan
solution,
the
soluble
xylooligomers
were
hydrolyzed
into
oligomers
with
low
DP.
Also,
insoluble
xylan
was
digested
to
become
soluble
high
DP
xylooligomers
and
subsequently
degraded
into
oligomers
with
lower
DP.
As
shown
in
the
fraction
of
xylooligomers
with
DP
4
decreased
significantly
in
the
first
hour
and
decreased
to
81.5%,
w/w
at
the
fourth
hour,
followed
by
a
gradual
decrease
to
73.5%
at
the
end
of
24
h
reaction.
On
the
other
hand,
the
fraction
of
xylotriose
increased
gradually
from
3.6
to
8.4%
from
the
first
hour
to
the
eighth
hour,
and
then
decreased
slightly
to
7.9%
at
24
h.
At
the
end
of
24
h
reaction,
the
XOS
mixture
contained
a
total
of
25.2%
(w/w)
of
xylobiose
and
xylotriose
by
using
immobilized
xylanase.
When
free
xylanase
was
used
instead
of
immobilized
enzyme,
the
concentrations
of
xylobiose
and
xylotriose
were
ini-
tially
lower.
Although
the
time
courses
for
xylotriose
and
xylobiose
were
similar,
the
use
of
free
enzymes,
when
compared
with
the
use
of
immobilized
enzyme,
exhibited
higher
concentrations
of
xylotriose
and
xylobiose
after
fourth
and
sixth
hour,
respectively.
At
the
end
of
24
h
reaction,
the
XOS
mixture
contained
a
total
of
32.5%
(w/w)
of
xylobiose
and
xylotriose
when
free
xylanase
was
used.
The
higher
xylotriose
and
xylobiose
concentrations
at
the
initial
stage
of
the
reaction
observed
with
the
immobilized
enzyme
suggest
that
the
immobilized
enzyme
tends
to
act
on
the
termini
of
xylan
chains.
On
the
contrary,
enzyme
molecules
attacked
everywhere
in
the
chains
of
xylooligomers
when
free
enzyme
was
used.
In
addi-
tion
to
the
production
of
xylobiose
and
xylotriose,
xylooligomers
with
lower
DP
were
also
produced
from
the
high
DP
xylooligomers
during
the
enzymatic
reaction
of
free
enzyme.
From
this
point
of
view,
it
seems
that
there
was
a
steric
hindrance
for
immobilized
xylanases
to
access
the
intermediate
portion
of
the
xylan
chain.
A
combined
use
of
half
free
and
half
immobilized
enzyme
led
to
time
courses
with
trend
similar
to
having
trade-off
from
either
free
or
immobilized
xylanase.
Based
on
the
total
amount
of
sub-
strate,
the
amount
of
total
soluble
fraction
in
the
products
from
24
h
reaction
using
immobilized
enzyme
made
of
a
conversion
of
80.9%.
However,
when
free
enzyme
with
the
same
enzymatic
activity
was
used,
the
conversion
could
approach
as
high
as
99.8%.
This
indicates
that
the
final
products
were
almost
soluble.
Since
no
insoluble
mat-
ter
left
after
the
reaction,
we
believe
that
the
xylan
obtained
from
NaOH
extraction
and
ethanol
precipitation
was
very
pure.
A
con-
version
of
94.8%
can
be
achieved
with
the
combinative
approach.
If
the
initial
soluble
fractions
were
exclusive
from
the
calculation,
the
conversions
for
the
insoluble
substrate
to
soluble
XOS
were
deter-
mined
to
be
62.5,
99.6,
and
89.9%
for
the
use
of
immobilized,
free,
and
combined
preparations
of
xylanase,
respectively
(
The
efficiency
for
converting
corncob
xylan
to
XOS
under
the
catalysis
of
endo-xylanase
from
B.
halodurans
was
higher.
If
only
xylobiose
and
xylotriose
were
taken
into
account,
the
use
of
immo-
bilized
xylanase
for
24
h
reaction
led
to
a
product
composing
of
4.5
g
of
xylobiose
and
xylotriose
from
20
g
xylan.
When
free
xylanase
was
used,
product
composed
of
6.8
g
of
xylobiose
and
xylotriose
from
20
g
xylan
was
obtained
after
24
h
reaction.
Recently,
a
two-
stage
process
based
on
a
steam
explosion
pretreatment
followed
by
enzymatic
hydrolysis
was
used
for
XOS
production,
and
a
max-
imum
yield
of
28.6
g
XOS/100
g
xylan
in
corncobs
was
achieved,
in
which
more
than
90%
of
xylobiose
and
xylotriose
were
contained
in
XOS
syrup
The
product
from
the
enzymatic
reaction
was
composed
of
xylo-
biose,
xylotriose,
oligomers
(DP
4),
and
small
amounts
of
xylose.
After
reaction
for
24
h,
the
xylose
content
was
less
than
1.8%
(w/w)
in
the
product
mixture,
regardless
of
whichever
xylanase
was
used
(free
or
immobilized).
In
the
present
work,
alkaline-extracted
corn-
cob
xylan
was
used
as
substrate
for
XOS
production.
When
xylan
with
a
soluble
fraction
(DP
4)
in
the
range
of
9.0–10.0
g/l,
conver-
sions
of
xylan
to
soluble
XOS
with
immobilized
and
free
xylanase
in
24
h
reaction
were
80.9%
and
99.8%,
respectively.
On
the
other
hand,
the
conversion
was
lower
for
xylan
with
lower
soluble
frac-
tion
under
the
same
reaction
time.
For
example,
when
the
initial
concentration
of
soluble
xylan
was
6.9
g/l,
the
XOS
conversion
by
using
immobilized
and
free
xylanase
would
be
50.9
and
65.9%,
respectively
under
the
same
enzyme
dosage.
The
higher
the
ini-
tial
percentage
of
insoluble
fraction
presented
in
the
substrate,
the
harder
the
accession
of
enzyme
molecule
to
longer
xylan.
Thus,
the
conversions
became
lower
due
to
the
slower
degradation
rate.
Although
extraction
of
xylan
with
steam
or
acid
has
been
proven
capable
of
increasing
the
XOS
yield,
large
amounts
of
undesired
products,
such
as
monosaccharides
and
their
dehydration
products
were
produced
4.
Conclusion
Endo-xylanase
secreted
by
B.
halodurans
can
be
well
immo-
bilized
with
0.59
mm
anionic
exchange
resin
(Lewatit
MonoPlus
MP64)
for
repeated
uses
in
the
production
of
XOS
from
alkaline-
extracted
corncob.
Interestingly,
time
courses
associated
with
XOS
production
from
corncob
xylan
by
the
catalysis
of
immobilized
enzyme
differ
slightly
from
those
using
free
forms
of
xylanase.
Xylobiose
and
xylotriose
were
produced
mainly
from
the
solu-
ble
xylan
(xylooligomers)
in
the
first
few
hours
under
the
action
of
immobilized
enzyme.
While
free
enzyme
can
effectively
cat-
alyze
the
hydrolysis
of
glucosidic
bonds
at
every
positions
in
the
xylan
chains;
xylobiose,
xylotriose,
and
xylooligomers
with
lower
DP
were
all
produced
simultaneously
during
the
time
course
of
enzyme
reaction.
Overall
conversions
from
insoluble
substrate
to

Y.-S.
Lin
et
al.
/
Process
Biochemistry
46
(2011)
2117–2121
2121
soluble
XOS
were
80.9
and
99.8%
with
immobilized
and
free
forms
of
xylanase
in
a
24
h
reaction,
respectively.
However,
as
the
immo-
bilized
enzyme
was
used,
the
lower
conversion
for
yielding
XOS
could
be
improved
by
introducing
the
combination
of
free
and
immobilized
xylanase
to
the
reaction.
Acknowledgement
This
study
was
supported
by
the
Ministry
of
Economic
Affairs
(Taiwan)
under
grant
no.
98-EC-17-A-13-S1-116.
References
[1] Zhu
Y,
Kim
TH,
Lee
YY,
Chen
R,
Elander
RT.
Enzymatic
production
of
xylooligsac-
charides
from
corn
stover
and
corncobs
treated
with
aqueous
ammonia.
Appl
Biochem
Biotechnol
2006;129–132:586–98.
[2] Yang
CH,
Yang
SF,
Liu
WH.
Production
of
xylooligosaccharides
from
xylans
by
extracellular
xylanases
from
Thermobifida
fusca.
J
Agric
Food
Chem
2007;55:3955–9.
[3]
Akpinar
O,
Ak
O,
Kavas
A,
Bakir
U,
Yilmaz
L.
Enzymatic
production
of
xylooligosaccharides
from
cotton
stalks.
J
Agric
Food
Chem
2007;55:5544–51.
[4]
Chang
P,
Tsai
WS,
Tsai
CL,
Tseng
MJ.
Cloning
and
characterization
of
two
ther-
mostable
xylanases
from
an
alkaliphilic
Bacillus
firmus.
Biochem
Biophys
Res
Commun
2004;319:1017–25.
[5]
Tseng
MJ,
Yap
MN,
Ratanakhanokchai
K,
Kyu
KL,
Chen
ST.
Purification
and
char-
acterization
of
two
cellulase
free
xylanases
from
an
alkaliphilic
Bacillus
firmus.
Enzyme
Microb
Technol
2002;30:590–5.
[6]
Maalej-Achouri
I,
Guerfali
M,
Gargouri
A,
Belghith
H.
Production
of
xylo-oligosaccharides
from
agro-industrial
residues
using
immobilized
Talaromyces
thermophilus
xylanase.
J
Mol
Catal
B:
Enzymatic
2009;59:
145–52.
[7]
Bailey
MJ,
Beiley
P,
Poutanen
K.
Interlaboratory
testing
of
methods
for
assay
of
xylanase
activity.
J
Biotechnol
1992;23:257–70.
[8]
Kapoor
M,
Kuhad
RC.
Immobilization
of
xylanase
from
Bacillus
pumilus
strain
MK001
and
its
application
in
production
of
xylo-oligosaccharides.
Appl
Biochem
Biotechnol
2007;142:125–38.
[9] Teng
C,
Yan
Q,
Jiang
Z,
Fan
G,
Shi
B.
Production
of
xylooligosaccha-
rides
from
the
steam
explosion
liquor
of
corncobs
coupled
with
enzymatic
hydrolysis
using
a
thermostable
xylanase.
Bioresour
Technol
2010;101:
7679–82.
[10] Yuan
QP,
Zhang
H,
Qian
ZM,
Yang
XJ.
Pilot-plant
production
of
xylo-
oligosaccharides
from
corncob
by
steaming,
enzymatic
hydrolysis
and
nanofiltration.
J
Chem
Technol
Biotechnol
2004;79:1073–9.
[11]
Yang
R,
Xu
S,
Wang
Z,
Yang
W.
Aqueous
extraction
of
corncob
xylan
and
production
of
xylooligosaccharides.
LWT
Food
Sci
Technol
2005;38:
677–82.
Document Outline
- Production of xylooligosaccharides using immobilized endo-xylanase of Bacillus halodurans
Wyszukiwarka
Podobne podstrony:
78 1101 1109 Industrial Production of Tool Steels Using Spray Forming Technology
Production of b xylanase and b xylosidase
~$Production Of Speech Part 2
GB1008594A process for the production of amines tryptophan tryptamine
pair production of black holes on cosmic strings
BoyerTiCS Religious Thought as a By Product of Brain Function
Production Of Speech Part 1
Production Of Speech Part 2
Production of Energy from Biomass Residues 020bm 496 1993
Machine Production of Screen Subtitles for Large Scale Production
~$Production Of Speech Part 2
the production of urban sprawl lepizig
Techniques to extract bioactive compounds from food by products of plant origin
Production of recombinant proteins in E coli
GB1008594A process for the production of amines tryptophan tryptamine
Chiodelli&Tzfadia The Multifaceted Relation between Formal Institutions and the Production of Infor
SMeyer WO8901464A3 Controlled Process for the Production of Thermal Energy from Gases and Apparatus
więcej podobnych podstron