
Christina Baldonado: Analysis of the Different Pedicellariae in Sea Urchins
p. 1 of 9.
Analysis of the Different Pedicellariae in Sea Urchins
by
Christina Baldonado
(Biol 515 at SDSU, Fall 2001)
Copyright © 2002 by Christina Baldonado and Brian T. Hentschel (hentsche@sunstroke.sdsu.edu)
INTRODUCTION TO ECHINOIDEA
Echinoidea is one of the six classes within the phylum echinodermata. Echinoderms are also
known as “spiny-skinned” animals. The adults are very different than those of other phyla (Pearse
et al 1987). Although sea urchins are very different from other echinoderms they still show the
same features such as tube feet, madreporite, and the penta - radial symmetry that are
characteristic of all echinoderms.
These features allow them to live in many places. Sea urchins may congregate in the colder
water of shore but travel to shallower water in search of food. They are usually found on hard
surfaces in crevices or holes that they dug with their spines and teeth. The mouth is located on the
lower surface and the anus opens on the top surface. They feed on algae, plankton, kelp, and
sometimes barnacles and mussels. Their predators include sea otters, starfishes, crabs, and wolf
eels (Parker and Kalvass 1992).
The oldest echinoids are approximately 450 million years old (Smith 2001). The diversity of
echinoids increased in the Paleozoic and Devonian periods. This was also when the
Archaeocidarids appeared, which are the precursors of the modern echinoids. The
archaeocidarids had spines and a more effective lantern than previous representatives. Then in the
Jurassic period there is a differentiation in the major line of echinoids into two groups: regular and
irregular. The irregular echionoids, sand dollars and sea biscuits, are specialized for deposit
feeding, have secondary bilateral symmetry superimposed on the primary penta-radial pattern,
and burrow in the substrate. The regular echinoids are almost perfectly spherically symmetrical
and live on the surface of a substrate (Smith 2001). The Sea urchin is a representative regular
echinoid (Pearse et al. 1987).
Although the echinoids seem very different from other representative echinoderms, they still
have the main echinoderm characters plus characters that are unique to this class (Pearse et al
1987). All sea urchins have a hard calcareous shell called a test. This test is covered with a thin
epithelium and is armed with spines. The spines are used mainly in locomotion and protection.
Between the spines, are the tube feet (characteristic of echinoderms) which are used in food
capture and holding on to a substrate.
A feature that is unique to echinoidea and asteroidea are the pedicellariae (Chenoweth 1994).
Pedicellariae, in general, are multifunctional appendages involved in defense, feeding, and
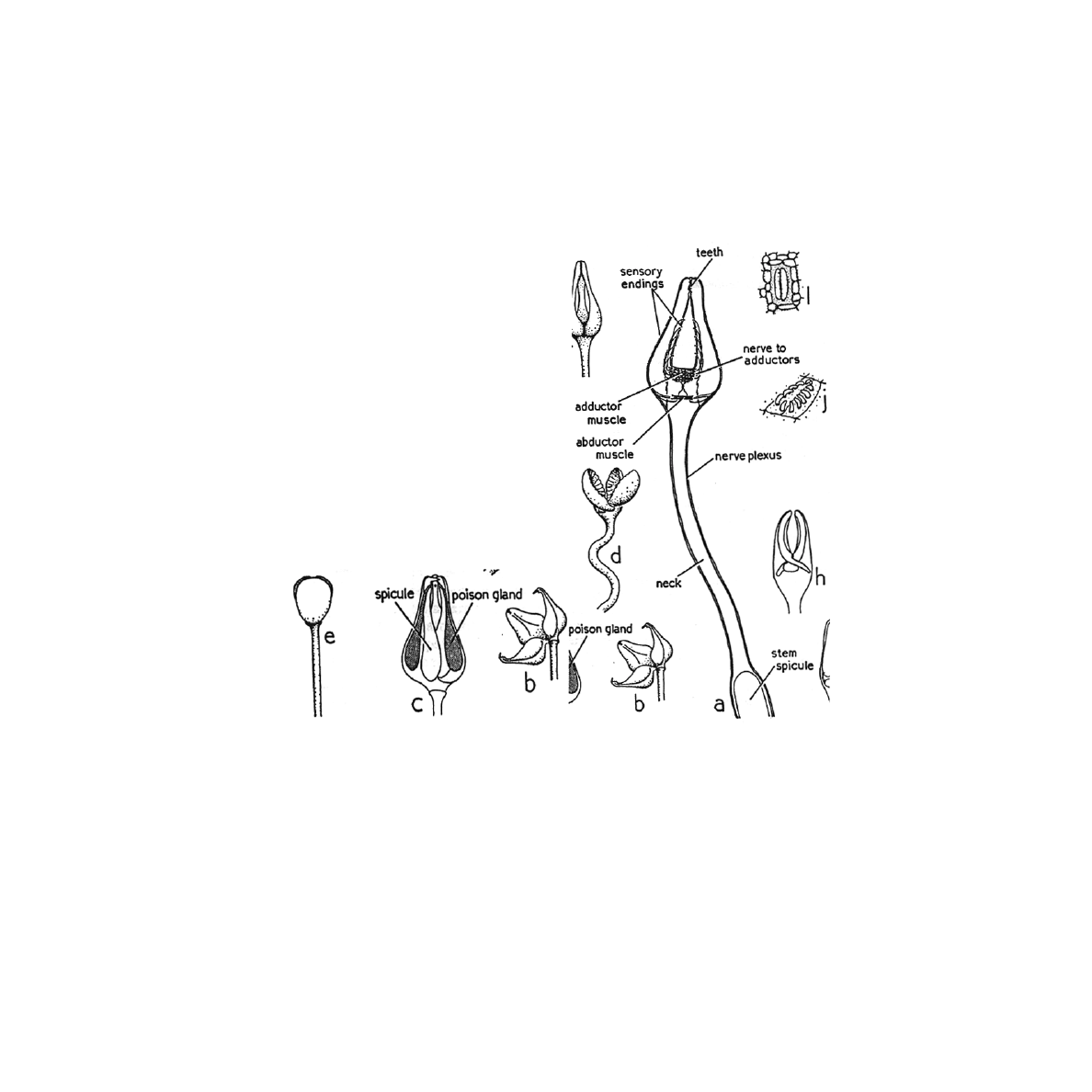
cleaning. The basic structure of pedicellariae consists of a head, neck, and stalk (figure1a). The
head usually has three jaws and, in some pedicellariae, contains the poison glands (Harrison
1994). These pedicellariae differ between these two classes. Because the pedicellariae are so
different it is probable that they may have evolved twice (Nichols 1966). The asteroidea have
three recognized types and the echinoidea have four recognized types (Campbell 1983). This
paper focuses on the pedicellariae of the sea urchins because they are more complex than those of
asteroids.

Christina Baldonado: Analysis of the Different Pedicellariae in Sea Urchins
p. 2 of 9.
INTRODUCTION TO ECHINOID PEDICELLARIAE
The pedicellariae of echinoids can be classified into four types: trifoliate or triphyllous,
tridentate, and globiferous or gemmiform (Harrison 1994). Distribution varies according to
pedicellariae type (Peters and Campbell 1987). Campbell (1983) noted the distribution of the
various pedicellariae. The triphyllous pedicellariae are evenly distributed on the test except there
are fewer around the madreporite region. The tridentate and ophiocephalous pedicellariae are all
around the urchin with the tridentate showing variation in height. The globiferous pedicellariae
usually are more numerous in the madreporite region. Further information on the role of the
madreporite is needed to further understand distribution (Campbell 1983). I think these
distributions may probably reflect the functions of the different pedicellariae.
The tridentate pedicellariae are the largest and most common. A calcite rod supports the first
two thirds of the stem. The head is on a flexible neck capable of considerable movement. The
blades touch at the tips, where there are two or three tooth-like projections (figure 1a) (Nichols
1966).
The ophiocephalous pedicellariae have serrations present down the whole length of the distal
side of each jaw. Each jaw has an inwardly directed process possibly to give better holding power
(figure 1d) (Nichols 1966). The skeleton of the ophiocephalous pedicellariae is designed to retain
struggling organisms for longer periods of time as long as contact between the jaw blades and the
organism is maintained (Campbell 1974).
Tridentate and ophiocephalous pedicellariae are held closed and lowered on the test
(Campbell 1983). The Opening of these two types can be elicited by stimulation of the outside of
the valves, which are the moveable jaws. The jaw movements have been revealed to have simple
receptor cells in the jaw epithelium, which are connected to nerve tracts, which coordinate the jaw
movement (Peters and Campbell 1987). The closing of the valves is then elicited by the touching
of the inner surfaces. There is a brief delay between the movement of the stimulus and the
initiation of closure response. This delay may reduce the efficiency of these types of pedicellariae
in trapping moving objects, which repeatedly collide with them (Campbell 1983).
The triphyllous pedicellariae have three broad leaf-like blades that meet along their lateral
edges (figure 1e) (Nichols 1966). The sensory region of the triphyllous pedicellariae has yet to be
mapped (Peters and Campbell 1987). These pedicellariae are considered to have spontaneous
activity. The spontaneous activity requires that some kind of impulse- generating mechanism
resides in the head (Peters and Campbell 1987). Campbell (1983) described the triphyllous
pedicellariae as spending most of their time browsing the test epithelium. He went on to explain
that they are able to move one valve separately from the other three and that these types are not
especially sensitive to touch, but can hold objects for brief periods.
The last type, the globiferous pedicellariae, have two or more teeth distally to pierce prey
(figure 1b and c) (Nichols 1966). The three venom glands, one on each jaw, make up the head.
Each gland is bifurcated into two compartments uniting into a single terminal duct (Chia 1970).
Around the poison glands there may be a muscle sheath, to ensure discharge of the poison at the
proper time (Nichols 1966). These pedicellariae close and release venom only when an object
containing the appropriate chemical touches the sensory hillock. The sensory hillock is an
aggregation of chemreceptor cells positioned near the valves so when an object is detected it is in
Christina Baldonado: Analysis of the Different Pedicellariae in Sea Urchins
p. 3 of 9.
the grasp of the jaws and certain to be caught (Campbell 1983). Tactile stimulation of the valves
causes them to open further exposing the sensory hillock to its fullest (Campbell & Laverack
1968). These pedicellariae may be able to make numerous responses prior to receiving the
chemostimulation that initiates closure and venom release. Globiferous pedicellariae have a very
interesting behavior of reacting differently to different stimuli. Muscles also effect the
globiferous pedicellariae reactions.
Figure 1. The different pedicellariae types in echinoidea a) Tridentate, showing head,
neck, and stem. b and c) globiferous pedicellariae opened and closed, respectively. d)
ophiocephalous pedicellariae, open. e) Triphyllous pedicellariae, closed (adapted
from Nichols 1966).
Campbell (1983) described the skeleton as having modifications for insertion of adductor,
abductor, and flexor muscles. The existence of these muscles allows for opening and closing of
the jaws. The gripping powers of the pedicellariae are not limited to the muscles. Collagen fibers
link the stem with the valves. These transmit any increase in tension in the stem to the valves
making them close harder (Campbell 1983). Each of the above pedicellariae is specialized for
specific functions, which will be reviewed later.
Christina Baldonado: Analysis of the Different Pedicellariae in Sea Urchins
p. 4 of 9.
EVOLUTION OF PEDICELLARIAE
Early in the discovery of pedicellariae they were described as being parasites on the sea urchin
test. Later they were recognized as parts of the urchin test (Campbell 1983). Most recently,
Haude (1998) suggested that the structural resemblance between the pedicellariae and spines
suggests that pedicellariae originated from spines. He found that the shafts of early pedicellariae
are similar to echinoid spines. The evidence he found was that fossil echinoid pedicellariae had
two or three blades. All lower Paleozoic pedicellariae have blades, which are similar to spines.
Haude (1998) then speculated that spines could have developed gripping actions leading to
tridentate pedicellariae.
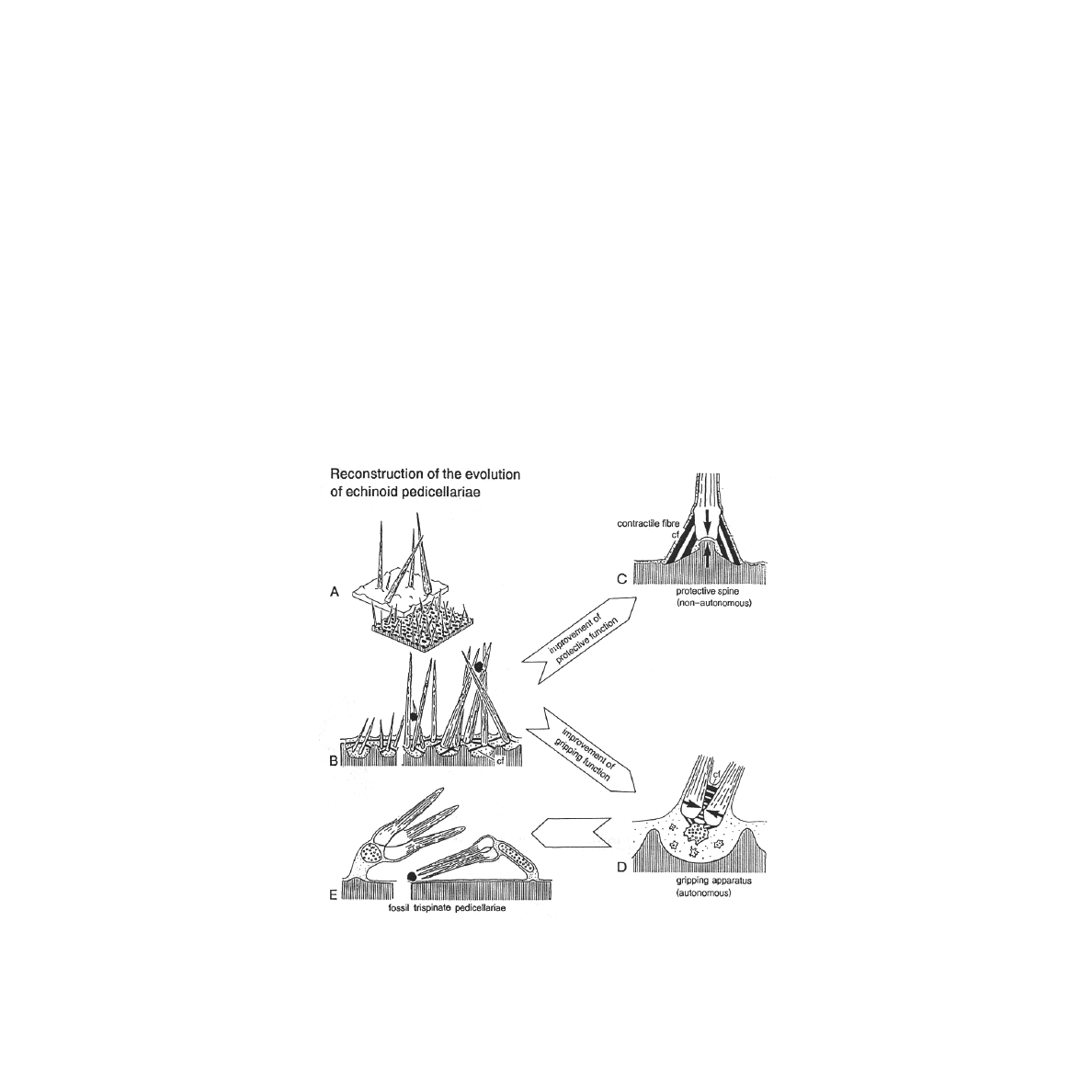
By constructional morphological deduction Haude (1998) tried to explain the evolution of
pedicellariae as a series of four developmental stages: (1) the spine-like stereomic structure and
growth of blades, (2) the points of pivoting contact, (3) the direction of force of contractile fibers,
and (4) the radius of the lateral action of the head (Figure 2). The stereomic structure of the
echinoderm calcite is an architectural device for lightweight construction for a mechanical
purpose. A longitudinally growing and originally fragile structure would be stabilized by lateral
expansion of the longitudinal bars. The structure would converge to a distal point.
Figure 2. Diagram of reconstruction of pedicellariae from spines. a) spines ball and
socket joint. b) connective tissue. c) protective spine as non-autonomous. d) gripping
autonomous. e) tridentate pedicellariae fossil (Adapted from Haude 1998).
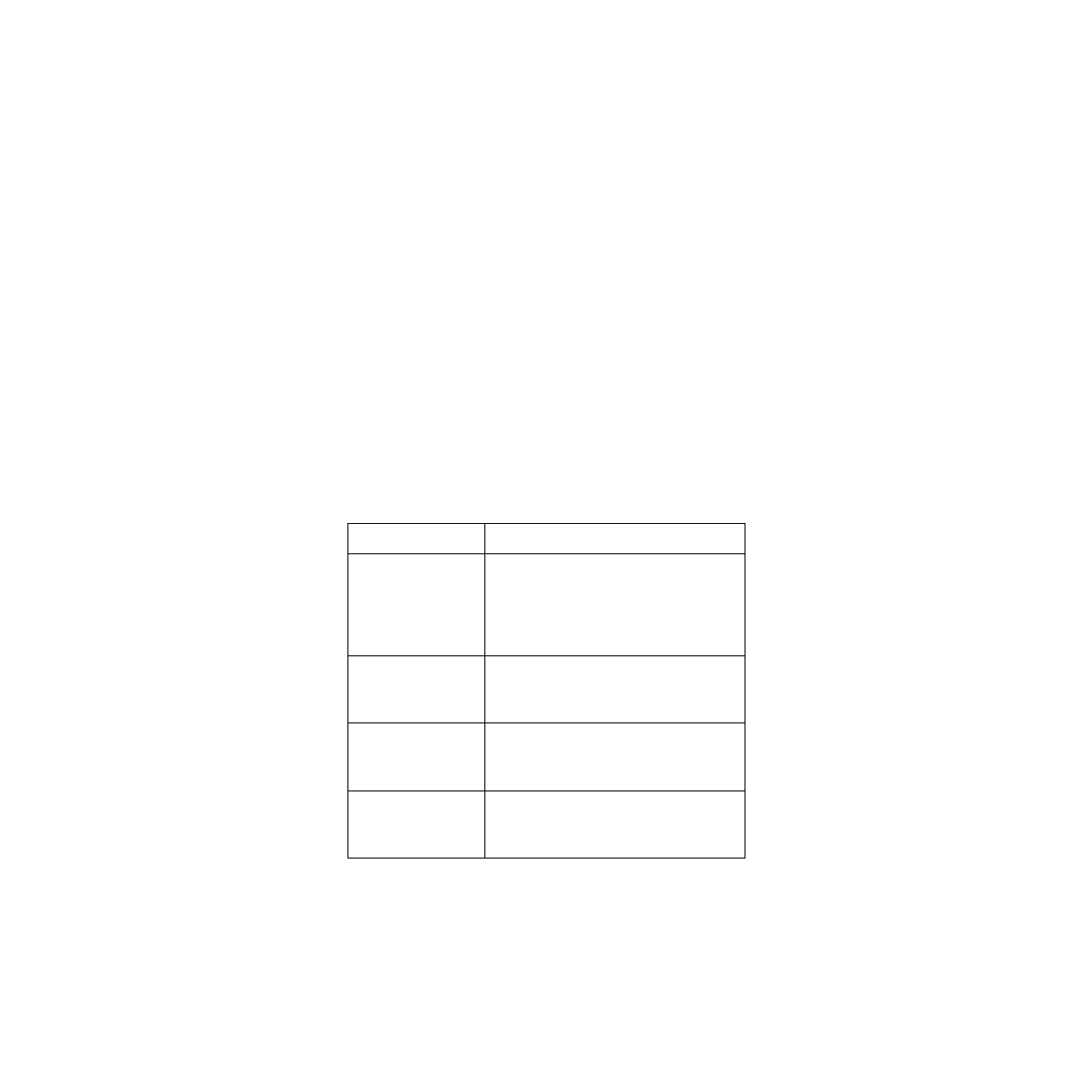
Christina Baldonado: Analysis of the Different Pedicellariae in Sea Urchins
p. 5 of 9.
Haude (1998) discovered that the points of pivoting between the blades of recent pedicellariae
show hinge structures. The Ordovician blades already clearly had identifiable hinge-like crests.
A protective spine has a mechanical non-autonomous apparatus, with force connection to the
substrate by contractile fibers and a ball-and-socket articulation. Later a mechanically
autonomous apparatus with lateral articulation of contractile fibers above and below and a
possible skeletal ossicle below the base acted as potential mechanical support. Then the three
blades in the head of the fossil tridentate pedicellariae develop.
It may be that ophiocephalous pedicellariae which were lacking from the cidaroids have
evolved in the more advanced echinoids from the tridentate form of their ancestors (Haude 1998).
It is evident that the triphyllous pedicellariae represent another level of pedicelliarial evolution
characterized by their spontaneous and independent jaw movements (Campbell 1974).
FUNCTIONS OF PEDICELLARIAE
Each of the four types of pedicellariae are believed to have a variety of functions (Campbell
1983). It has been shown that pedicellariae in sea urchins display some ordered responses to
specific stimuli. These are summarized in table 1 (Campbell and Rainbow 1977). Different types
of pedicellariae react differently to varying stimuli. The ophiocephalous and the globiferous are
the most varied.
Table 1. Summary of Pedicellariae functions. (adapted from Campbell and Rainbow, 1977).
Campbell (1973) described the reactions of the different pedicellariae to varying stimuli such
as, light, parts of asteroid ray with tube feet, and swimming nauplii of
Artemia salina
in three
different species,
E.esculentus, P.miliaris
, and
T.gratilla
. Campbell (1973) described the
quiescent state of tridentate, ophiocephalous, and globiferous pedicellariae as being non
stimulated with jaws closed and stems flat against the surface of the test. In the experiment, it was
found that the triphyllous pedicellariae have continued spontaneous jaw movement even when the
Type
Function
Tridentate
Picking less active objects from
surface, possibly capture small
swimming organisms
Ophiocephalous
Detain objects which move over
the test surface
Globiferous
Repel larger animals with poi-
son glands for defense
Triphyllous
Scrape surface (cleaning) and
food gathering

Christina Baldonado: Analysis of the Different Pedicellariae in Sea Urchins
p. 6 of 9.
other pedicellariae are in the quiescent state. The other three types display more specific
responses to particular locally applied stimuli. The alert response is made up of two factors,
selection and direction. The type of pedicellariae that responds depends on the nature of the
stimulus and the point of application. The same types of pedicellariae act in similar manners
where neighboring pedicellariae adopt the same stance. The types of stimuli that trigger a
response are related to those that trigger jaw reflexes. Consequently tactile stimuli are effective in
alerting tridentate and ophiocephalous pedicellariae, which are specialized in picking up objects
and detaining organisms respectively. The qualities of the tridentate pedicellariae indicate that
they probably do not deal with moving objects like the ophiocephalous, but that their larger
forcep-shaped jaws are suited more towards picking less active objects from the test surface
(Campbell 1974).
The movements of the stems bring the pedicellariae jaws into an area where they can operate
in a more effective manner or remove them from an area where they are not useful, making room
for others that are useful (Campbell 1974). This mobility increases their ability to react to
different stimuli and effect settling barnacle cyprids.
Campbell and Rainbow (1977) studied the mechanism by which
Echinus esculentus
prevents
barnacle larvae from settling. They conducted three experiments (1) comparing the settlement of
larvae on live and dead test preparations, (2) determining if the physical nature of the test deters
settlement, and (3) to determine whether or not the test epithelium or any of the pedicellariae play
a role in repelling settlement by virtue of their chemical characteristics. The first experiment
showed that the cyprids would settle on dead sea urchins, but not on live urchins. The Second
experiment determined that the larvae will settle on the urchin, so it isn’t repellent itself. When
barnacle extract was added it enhanced the settlement of the larvae. The final experiment found
that the globiferous pedicellaria are not chemically repulsive to the barnacle larvae. The
conclusions of the study were that the barnacle larvae are prevented from settling on live and fit
urchins, and there is no chemical repulsion or physical unsuitability of the skeleton. They also
found that the mobile pedicellariae prevent settlement by actively removing the larvae. It is
thought that mobile pedicellariae, such as triphyllous and ophiocephalous prevent settlement by
removing the larvae.
The globiferous type is much more complicated than the other three pedicellariae types. The
main function of the globiferous pedicellariae is in defense from large predators, such as asteroids
(Campbell 1974). They contain a venomous apparatus consisting of a valve, venom gland, and a
muscular envelope (Ghyoot 1994).
There have been some conflicting theories behind how the venom is released into the predator.
O’Connel et al. (1974) believes that the gland cells release their vesicle complement when the
venom sac muscles contract. It was once thought that the terminal tooth functioned as a needle
injecting poison directly into a wound, much like a spider (Chia 1970). Ghyoot (1994) discovered
that the above may not be the case. His study on the
Sphaerechinus granularis
found that the
function of the tooth is to puncture the predator and allow the pedicellaria head or complete
pedicellariae, depending on the species, to remain in the puncture site. This allows for increased
sting time and increased amount of venom released. Consequently, they are only able to function
once. In addition, this may avoid damage to the urchin if the pedicellariae were to be torn off by
the predator. The venom has been known to have paralyzing effects on various marine
invertebrates including gastropods and crustaceans (Campbell 1983). In a study by Cannone

Christina Baldonado: Analysis of the Different Pedicellariae in Sea Urchins
p. 7 of 9.
(1970) two urchins and a starfish were placed away from each other in the same container to see
what effect this would have on the globiferous pedicellariae. It was found that this had no effect
on the globiferous pedicellariae. This was the expected result because these two both can be found
in similar environments. When a starfish arm was placed in contact with the urchin this resulted
in excitation of all globiferous pedicellariae over the whole test.
REGENERATION IN PEDICELLARIAE
Because pedicellariae are important in the survival of sea urchins they must be able to
regenerate after damage or loss. The globiferous pedicellariae are the most studied of the four
types.
After a globiferous pedicellariae discharges its venom it is important for the sea urchin to
replace it rapidly to maintain an effective defense. In experiments where only the globiferous
pedicellariae were removed it took 25 days to regenerate a functional pedicellariae (Chia 1970).
At this stage the head has reached maturity but the stalk is only half the length of a full-grown
stalk. Consequently, later stages of the regeneration process are concerned only with elongation
of the stalk (Chia 1970). It was later determined that most of the secretory activity of the head and
stalk glands of globiferous pedicellariae takes place during a limited period in the later stages of
regeneration. The new globiferous pedicellariae regenerates at the same place as the removed
pedicellariae (Holland and Holland 1975). This regeneration pattern does not seem to be
synchronized because pedicellariae of various stages have been observed. It has been found that
the order of pedicellariae regeneration is also important. The triphyllous type are the first to
regenerate then the globiferous, ophiocephalous, and last the tridentate type (Chia 1970). Those
pedicellariae on the aboral half seem to grow more rapidly than those on the oral side (Chia 1970).
Another study by Campbell and Savill (1998) based on the
Psammechinus miliaris
was done
to show the effects of various conditions on the regeneration pattern. The results of the
experiment showed that the triphyllous type are the most numerous followed by the globiferous
type, tridentate, and the fewest being the ophiocephalous. When all of the pedicellariae were
removed in the presence of
Asterias rubens
the relative composition of pedicellariae was similar
before and after regeneration. There was no net increase of globiferous pedicellariae, but a
greater proportion of globiferous organs differentiated and became functional synchronously.
When only the triphyllous type were left and all other types were removed in presence of
A.rubens
the other three regenerated similarly to the above.
What broader conclusions can be drawn from these experiments? The order of regeneration
shows that there are priorities in this process. This could be due to either physiological or
ecological conditions. Although the globiferous type does regenerate quickly they need more
time to differentiate. Since, in the presence of
A.rubens
the globiferous organs differentiated and
became functional at the same time suggests that there is an increase in the synchrony of complete
regeneration in the presence of danger. The tridentate and ophiocephalous types regenerating last
and in significantly lower numbers than originally may be a reflection of lower priority for their
replacement in laboratory environment. This decrease could also be due to the fact that they have
a more massive CaC0
3
skeleton hindering regeneration rates. It has also determined that
tridentate, trifoliate, and ophiocephalous types do not regenerate at the point of removal. When
regeneration does take place at the same point the pedicellariae is not always the same type as the
previous one (Emson and Tanti, 1998).

Christina Baldonado: Analysis of the Different Pedicellariae in Sea Urchins
p. 8 of 9.
CONCLUSIONS AND FUTURE RESERCH IDEAS
The pedicellariae are very important for survival of sea urchins. This structure has brought
about much interest to biologists. It is interesting to see something that looks so simple can be so
complicated.
Although there is much known about this structure there are still some avenues that need to be
explored further. There are many studies on the globiferous pedicellariae but little is known
about the triphyllous pedicellariae. These pedicellariae may represent another level of
pedicellarial evolution characterized by their spontaneous independent jaw movements (Campbell
1974). I feel that there is still a lot that needs to be understood about the evolution of the different
pedicellariae for further understanding in their function and behavior. They seem to be much
smaller and therefore harder to study (Campbell 1974).
The idea that diatoms and ciliates might live on the triphyllous pedicellariae jaws is a topic
that needs further research (Campbell and Newman 1994). I think it would be interesting to find
out if this is a mutualistic, parasitic, or commensal relationship. I would test this by first
observing the pedicellariae and then investigating more about their evolution to see how they may
have possibly come to contain these organisms. I think that the evolution of these pedicellariae is
a major key in their understanding. I think this relationship is either mutualist or commensal
because these pedicellariae function in cleaning the urchin test. If it is parasitic, how can these
pedicellariae function in cleaning when they are unable to rid themselves of parasites? What
makes this type more suitable for these organisms over the other three types? I would test this by
maybe removing the organisms from the triphyllous pedicellariae to see if they can survive on
another pedicellariae type.
There is still some confusion about the head glands and the secretory products. There are two
theories. One theory proposed by O’Connel (1974) says that there is a continuos activity in
secretory cells of fully developed pedicellariae. The second theory proposed by Holland and
Holland (1975), which has more supporters, says that they are inactive and already differentiated
and the reason behind why globiferous pedicellariae are only able to function once before they are
regenerated. I feel that this could be possibly studied further by the use of scanning electron
microscopy or transmission electron microscopy to further understand the internal properties of
the globiferous pedicellariae. These are some reasons to continue studying this fascinating
structure that these strange animals possess.
LITERATURE CITED
Campbell, A.C.1983. Form and Function of Pedicellariae. Echinoderm Studies.
1
:139-167.
Campbell, A.C. 1974. Observations on the Activity of Echinoid Pedicellariae: II Jaw Responses
of tridentate and ophiocephalous pedicellariae. Mar. Behav. Physiol.
3
: 17-34.
Campbell, A.C. 1973. Observations on the Activity of Echinoid Pedicellariae: I.Stem responses
and their Significance. Mar. Behav.Physiol.
2
: 33-61.
Campbell, A.C., and B.L. Savill. 1998. Regeneration of Pedicellariae in
Psammechinus miliaris
(Gmelin). Pages 601-606
in
Echinoderms. Mooi & Telford, editors. London, UK.
Campbell, A.C., and J.P. Newman. 1994.Echinoid triphyllous Pedicellariae as a Platform for

Christina Baldonado: Analysis of the Different Pedicellariae in Sea Urchins
p. 9 of 9.
Epizoic Organisms. Pages 595-600.
in
Echinoderms Through Time. David, Guille, Feral, &
Roux, editors. Balkema, Rotterdam, UK.
Campbell, A.C. and P.S. Rainbow. 1977. The Role of Pedicellariae in Preventing Barnacle
Settlement on the Sea-Urchin test. Mar. Behav.Physiol.
4
: 253-260.
Campbell, A.C., and M.S. Laverarck. 1968. The response of Pedicellariae From Echinus
esculentus (L). Jour. Exp.Mar.Biol.Ecol.
2
: 191-214.
Cannone, A.J. 1970. The Anatomy and Venom-Emitting mechanism of the Globiferous
pedicellariae of the Urchin
Psrechinus angulosus
(Leske) With notes on their behavior. Zool.
Africana.
5
: 179-190.
Chenoweth, S. 1994. The Green Sea Urchin in Maine. Page 8
in
Fishery & Biology. Maine Dept.
of Marine Resources, West Boothbay Harbor, Me.
Chia, Fu-Shiang. 1970. Histology of the Globiferous Pedicellariae of
Psammechinus miliaris
(Echinodermata: Echinoidea). Journal Zoology. London.
160
:9-16.
Emson, R.H., and K.T. Tanti. 1998. Pedicelliarial Regeneration Pattern in
Psammechinus
miliaris
: Randon or Predictable. Page 645 i
n
Echinoderms. Mooi & Telford, editors.
Balkema, Rotterdam, London, UK.
Ghyoot, M., Ph Dubois, and M. Jangoux. 1994. The venom Apparatus of The Globiferous
Pedicellariae of the Toxopneustid
Sphaerechinus granularis
(Echinodermata, Echinoida):
Fine Structure and Mechanism of venom Discharge. Zoomorphology
114
: 73-82.
Harrison. F.W., and Chia, Fu-shiang. 1994. Microscopic Anatomy of Invertebrates. Wiley-Liss,
New York, CA, USA.
Haude, R. 1998. Evolutionary Reconstruction of Primitive (spinate) Echinoid Pedicellariae.
Pages 675-679
in
Echinoderms. Mooi & Telford, editors. Balkema, Rotterdam, London, UK.
Holland, L.A., and N.D. Holland. 1975. The Fine Structure of Epidermal Glands of regenerating
and mature Globiferous Pedicellariae of a sea Urchin (
Lytechinus pictus
) Tissue and Cell.
7(4)
: 723-737.
Nichols, D. 1966. Echinoderms. Hutchinson & Co. London. UK.
O’Connell, M.G., C.B. Alender, and E.M. Wood. 1974. A Fine Structure Study of Venom Gland
Cells in Globiferous Pedicellariae From
Strongylocentrotus purpuratus
(Stimpson). J.
Morphology.
142
:411-432.
Parker, D and P.Kalvass, 1992. Sea Urchins in California’s Living Marine Resources & their
Utilization, (ed) W.L. Leet, C.M. Dewees, and C.W. Haugen. www.Seaurchin.org/Sea-Grant-
Urchins.
Pearse, V., J. Pearse, M. Buchsbaum, and R. Buchsbaum.1987. Living Invertebrates. The
Boxwood press, Pacific Grove, California, USA.
Peters, B.H. and A.C. Campbell. 1987. Morphology of the Nervous and Muscular Systems in the
Heads of Pedicellariae from the Sea Urchin
Echinus esculentus.
Journal of Morphology.
193
:
35-51.
Smith, Andrew B. 2001. Natural History Museum. Dept. Palaentology. www.nhm.ac.uk.
Document Outline
- INTRODUCTION TO ECHINOIDEA
- INTRODUCTION TO ECHINOID PEDICELLARIAE
- Figure 1. The different pedicellariae types in echinoidea a) Tridentate, showing head, neck, and ...
- EVOLUTION OF PEDICELLARIAE
- Figure 2. Diagram of reconstruction of pedicellariae from spines. a) spines ball and socket joint...
- FUNCTIONS OF PEDICELLARIAE
- Table 1. Summary of Pedicellariae functions. (adapted from Campbell and Rainbow, 1977).
- REGENERATION IN PEDICELLARIAE
- CONCLUSIONS AND FUTURE RESERCH IDEAS
- LITERATURE CITED
Wyszukiwarka
Podobne podstrony:
A syntactic contrastive analysis of the relative clauses in Arabic and English
Analysis of Police Corruption In Depth Analysis of the Pro
The algorithm of solving differential equations in continuous model of tall buildings subjected to c
Freedom in the United States Analysis of the First Amendme
Homosexuals in the Military Analysis of the Issue
Parametric Analysis of the Ignition Conditions of Composite Polymeric Materials in Gas Flows
Walterowicz, Łukasz A comparative analysis of the effects of teaching writing in a foreign language
Tsitsika i in (2009) Internet use and misuse a multivariate regresion analysis of the predictice fa
interactive art vs social interactions analysis of interactive art strategies in the light of erving
An%20Analysis%20of%20the%20Data%20Obtained%20from%20Ventilat
The?uses of the Showa Restoration in Japan
Analysis of the Persian Gulf War
Analysis of the Holocaust
Analysis of the Infamous Watergate Scandal
Road Not Taken, The Extensive Analysis of the Poem
Analysis of the End of World War I
Night Analysis of the Novel
Preliminary Analysis of the Botany, Zoology, and Mineralogy of the Voynich Manuscript
Victory, The Analysis of the Poem
więcej podobnych podstron