
Chemical Engineering Science 62 (2007) 3197 – 3217
Synthesis of esters: Development of the rate expression for the Dowex 50
Wx8-400 catalyzed esterification of propionic acid with 1-propanol
Sami H. Ali
∗
, Alia Tarakmah, Sabiha Q. Merchant, Taher Al-Sahhaf
Chemical Engineering Department, Kuwait University, PO Box 5969, Safat 13060, Kuwait
Received 20 July 2006; received in revised form 5 March 2007; accepted 13 March 2007
Available online 19 March 2007
Abstract
The kinetics of the esterification reaction of propionic acid with 1-propanol over the ion-exchange resin Dowex 50Wx8-400 has been studied
in this investigation. Kinetic experiments were conducted using a 1 L Lab-Max system at a stirrer speed of 900 rpm over a temperature range
of 303.15 –333.15 K. The catalyst loading was varied from 10 to 60 g dry cat/L and acid to alcohol molar ratios of 1:1, 1:2, 1:4, 2:1 and 4:1
were employed. The equilibrium constants for this reaction were determined in separate experiments at 303.15, 313.15 and 323.15 K. The
values were equal to 33.18, 30.62 and 28.37, respectively, with a standard enthalpy change of reaction of 6.4 kJ/mol. These values show the
reaction to be mildly exothermic. It was found that both external and internal diffusion limitations did not affect the overall reaction rate. The
conversion of propionic acid increased with increasing temperature and catalyst loading and decreased with increasing initial mole fraction of
acid. The increase in chain length of acid or alcohol or branching had a retarding effect on the conversion. Several kinetic models were tested
to correlate the kinetic data, the pseudo-homogeneous (P-H) model, the Eley–Rideal (E–R) model, the Langmuir–Hinshelwood (L–H) model,
the modified Eley–Rideal (M-E–R) model and the modified Langmuir–Hinshelwood (M-L–H) model. In all models, the activity coefficients
were estimated using UNIFAC to account for the non-ideal thermodynamic behavior of reactants and products. A correction factor for the resin
affinity for water (
) was used in both M-E–R and M-L–H models. The above models predicted the kinetic behavior of the studied system
with an overall error ranging from 1.65% to 13.32%. Water was found to be more strongly adsorbed than other species present in the system.
The M-E–R model between adsorbed 1-propanol and non-adsorbed propionic acid which assumes surface reaction as the rate controlling step,
with
equal to 2, was found to be the best model with the least overall error (1.65%). The activation energy for the esterification was estimated
to be 67.3 kJ/mol by this model.
䉷 2007 Elsevier Ltd. All rights reserved.
Keywords: Batch reactor; Catalysis; Esterification; Kinetics; 1-Propyl propionate; Reaction engineering
1. Introduction
Esterification is an important reaction in the chemical engi-
neering industry. The production of esters plays a major role
in the production of flavors and fragrances, solvents, plasti-
cizers, pesticides and herbicides, medicinal and surface-active
agents. Because of the wide use of esters in the chemical
industry (
), it is believed that the
number of commercial esters exceeds 500, with over 100
manufacturers (
). Esterification of alcohols with
∗
Corresponding author. Fax: +965 4839498.
E-mail address:
(S.H. Ali).
0009-2509/$ - see front matter
䉷
2007 Elsevier Ltd. All rights reserved.
doi:10.1016/j.ces.2007.03.017
mono-carboxylic acids has been studied extensively by earlier
workers (
Rao et al., 1978; Chang and Yeh, 1984; Dakshina-
murty et al., 1984; Namba et al., 1985; Xu and Chuang, 1996;
Zhang et al., 1998
). These studies involve either homogeneous
or heterogeneous catalyzed esterification reactions.
The most commonly used mineral acid is probably sulfuric
acid because of its effectiveness (
). But, close
control is required since even modest increases in concentra-
tion or temperature can cause dehydration of alcohols to ethers
or olefins (
). Besides alternative acidic catalysts
like para-toluene sulfonic acid and ion-exchange resins con-
taining sulfonic acids, the tri-chloride and sulfate of aluminum
can also be employed (
). The sulfonic
acids have good catalytic activity and generally cause fewer

3198
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
side reactions than sulfuric acid. A somewhat higher molar
concentration of the sulfonic acid may be required in order
to achieve the same reaction rate that can be obtained with a
given quantity of sulfuric acid (
). In industrial
applications, the use of mineral acids as catalysts is limited
because it suffers from several drawbacks. The acid catalyst
recovery is uneconomical (
) and high acid
concentration will increase the corrosion rate, which increases
the cost of the operation (
). Furthermore,
dealing with homogeneous catalysts waste is very hard because
they have to be neutralized for product separation (
), which is considered to be a costly process (
). The use of solid catalyst such as ion-exchange resins
has received great attention. Application of these catalysts has
several advantages; recovery of the catalyst is easily achieved
by filtration (
); continuous operation
in columns is possible (
); the
purity of the products is higher compared with homogeneous
catalyst since solid acid catalysts are selective and the formation
of by-products is less significant (
); waste
or disposal problems are eliminated (
); isolation of reaction intermediates is possible (
studied the effect of acid structure on
the reaction rate. This study was carried out by the esterifica-
tion reaction of methanol with a homologous series of aliphatic
organic acids in the presence of Amberlite IR-120 as the cat-
alyst. Also,
studied the effect of alcohol
structure and molecular weight on the reaction rate constant
for the esterification reaction of propionic acid with different
alcohols over styrene–butadiene–phenol formaldehyde (SBPF)
as a catalyst. Esterification of acetic, propionic and pentanoic
acids with different alcohols using a polymer fiber-supported
sulfonic acid, Smopex-101, as a catalyst was studied by
. Since there is a lack of information on the influ-
ence of acid and/or alcohol structure on esterification reactions
catalyzed by Dowex 50Wx8-400 it was decided to be one of
the aims of this investigation.
studied the esterification of
1-propanol with propionic acid using Dowex-50W. The exper-
iments were carried out in a batch reactor. The influence of
different variables on the conversion of the reactants was stud-
ied. An empirical model correlating the specific reaction rate
constant in terms of the studied variables was established. It was
proposed that the surface reaction is the rate controlling step
between adsorbed propionic acid and non-adsorbed 1-propanol.
However,
did not account for the
system non-ideality or for differences in the adsorption of the
reactants and products. Therefore, the lack of systematic kinetic
studies for the esterification of propionic acid with 1-propanol
with the aim of establishing the influences of reaction parame-
ters on the reaction kinetics along with elucidation of the most
probable reaction mechanism by systematic testing of estab-
lished mathematical models is the main impetus for the current
study.
Several kinetic models have been adopted to describe the ki-
netics of heterogeneous catalytic esterification reactions. The
pseudo-homogeneous (P-H) model is similar to the power-
law model for homogeneous reactions (
Pöpken et al., 2000; Lee et al., 2002; Gangadwala et al., 2003
The P-H model assumes that surface reaction is the controlling
step and adsorption is negligible for all components. Whenever
the adsorption of the molecules taking part in the reaction oc-
curs, the L–H model is applicable for correlating the kinetic
data (
Bhatia et al., 1973; Lee et al., 2000; Pöpken et al., 2000;
). On the other hand, the Eley–Rideal
(E–R) model can be applied when the reaction between one
adsorbed species and one non-adsorbed reactant from the bulk
liquid phase is assumed to occur (
Tan, 2001; Gangadwala et al., 2003
Our research group (
) studied the
esterification reaction of acetic acid with 2-propanol using
different ion-exchange resins (Dowex 50Wx8-400, Amber-
lite IR-120 and Amberlyst 15). Under the studied conditions,
the highest conversion was obtained for the system catalyzed
by Dowex 50Wx8-400 at 4 h and 343 K, 1:1 acid to alcohol
molar ratio and 40 g dry cat/L catalyst loading. It was also
found that the systems catalyzed by gel-type resins (Dowex
50Wx8-400 and Amberlite IR-120) exhibited some similari-
ties in their reaction kinetics. The data were fitted to different
models such as the P-H, the E–R, the L–H and the M-L–H
models.
The purpose of our investigation is to study the reaction of 1-
propanol with propionic acid catalyzed by the cation-exchange
resin Dowex 50Wx8-400. The impact of different variables
such as catalyst loading, temperature and acid to alcohol ra-
tio was investigated. Other factors investigated include the im-
pact of catalyst moisture content on the esterification reaction,
the effect of using sulfuric acid rather than Dowex 50Wx8-
400, and the effect of ion-exchange resin catalyst type and
the impact of the presence of water on the reaction. The reac-
tion of acetic or butyric versus propionic acid with 1-propanol
was compared using Dowex 50 Wx8-400. Also, a comparison
was made between five different alcohols, methanol, ethanol,
1-propanol, 2-propanol and 1-butanol, reacting with propionic
acid in the esterification reaction using Dowex 50 Wx8-400.
On the other hand, the significance of both external and inter-
nal diffusion limitations on the esterification system was stud-
ied. Several kinetic models were tested to correlate the kinetic
data, namely the P-H model, the E–R model, the L–H model,
the modified Eley–Rideal (M-E–R) model and the modified
Langmuir–Hinshelwood (M-L–H) model. The non-ideality of
the system behavior was accounted for by universal functional
activity coefficient (UNIFAC).
2. Theory
2.1. Reaction and reaction mechanism
Esters can be formed by the reaction of a carboxylic acid
with an alcohol forming the ester and water molecules. This es-
terification (reversible) reaction, also called the intermolecular
dehydration reaction, is a very important and a common type
of reaction in the chemical industry. The general esterification
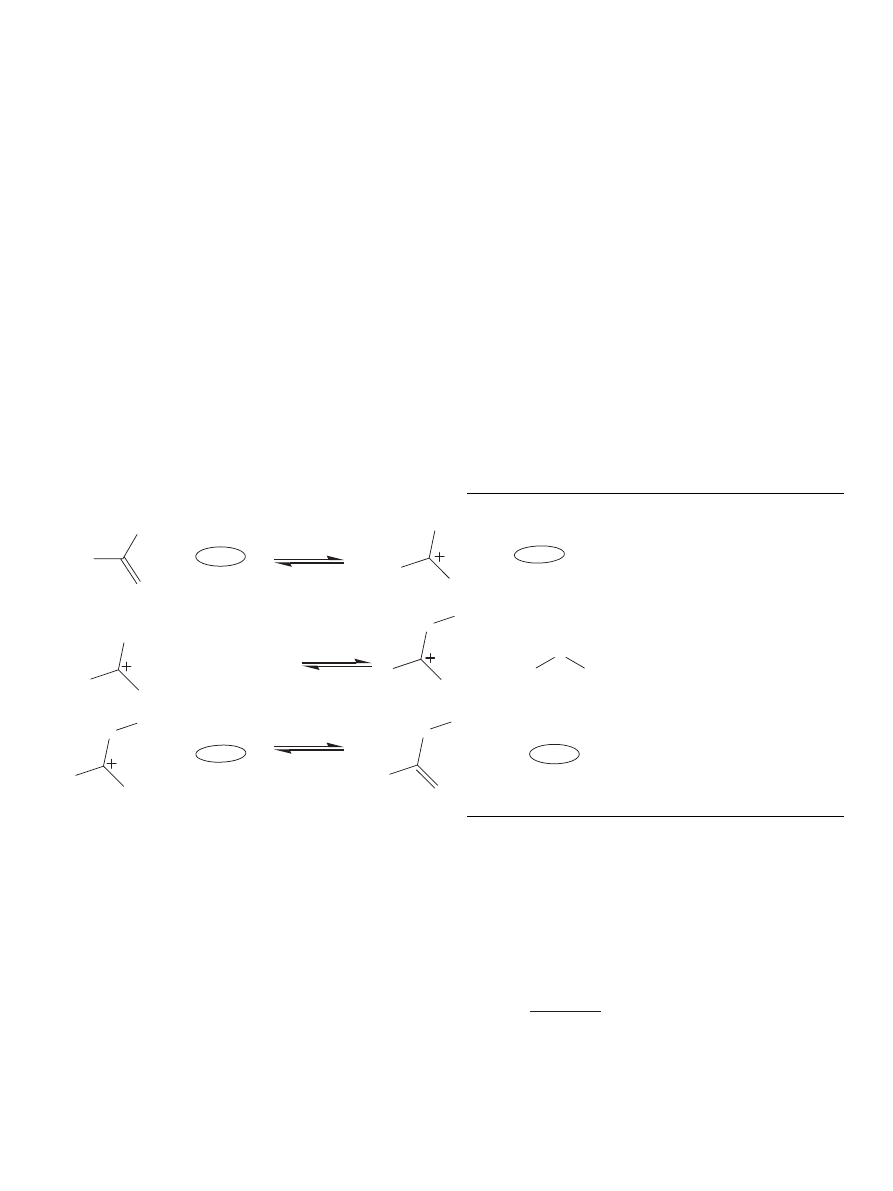
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
3199
reaction is shown below:
acid
+ alcohol ↔ ester + water.
(1)
The catalytic esterification reaction of a propionic acid and 1-
propanol to form 1-propyl propionate and water is given by
CH
3
CH
2
COOH
+ CH
3
CH
2
CH
2
OH
k
f
←→
k
b
CH
3
CH
2
COOCH
2
CH
2
CH
3
+ H
2
O.
(2)
The reaction mechanism for the formation of esters from car-
boxylic acids and alcohols in the presence of acidic catalyst
was presented by
. The reaction is initiated
by the transfer of a proton from the catalyst to the carboxylic
acid. The proton becomes attached to one of the lone pairs on
the oxygen which is double-bonded to the carbon. The trans-
fer of the proton to the oxygen gives it a positive charge. This
results in a fair amount of positive charge on the carbon atom.
Then, the positive charge on the carbon atom is attacked by the
hydroxyl group of the alcohol molecule. After that, a molecule
of water is lost from the ion. Finally, the catalyst is recovered
by the transfer of proton from the ion to the catalyst surface.
This mechanism is represented by the following scheme:
R
O
OH
H- CAT
+
R
OH
OH
+
CAT
R
OH
OH
+
HO-R'
R
OH
O
+
H
O
H
+
CAT
R'
R
OH
O
R'
R
O
O
R'
H- CAT
+
The donation of a proton is commonly assumed to be a fast
step, while the nucleophilic substitution is usually assumed to
be slow followed by fast steps resulting in the formation of
ester and water and the recovery of the catalyst.
2.2. Diffusion
To have a purely kinetic study, it is necessary to eliminate
both external and internal diffusion limitations. For our case
study, where the reaction of propionic acid with 1-propanol over
solid catalyst was carried out in a batch reactor, the external
mass transfer resistance to the esterification reaction is directly
related to the stirrer speed. The effect of external diffusion lim-
itation on the esterification reaction rate was studied by earlier
workers (
Krishnaiah and Rao, 1984; Yadav and Kulkarni, 2000;
Yadav and Thathagar, 2002; Ali and Merchant, 2006
). To study
the external diffusion effect on the reaction rate, different stirrer
speeds should be applied to the reaction system. If the produc-
tion of the ester is independent of stirrer speed, this indicates
that external diffusion is not the rate controlling step. Thus, to
ensure that the reaction rate is not influenced by external dif-
fusion, the experiments should be run at a high enough stirrer
speed. In general, external diffusion controls the overall rate
in catalytic processes if the viscosity of the reactant mixture is
very high or the stirrer speed is very low (
The effect of internal diffusion on the rate of the reaction cat-
alyzed by a solid catalyst (ion-exchange resin) is dependent on
many parameters such as catalyst composition, particle size, re-
action medium and temperature. The effect of internal diffusion
on the catalytic reaction can be studied by screening catalyst
into different particle sizes or by calculating certain dimension-
less parameters such as the well-known Weisz–Prater criterion.
Earlier workers studied the significance of internal diffusion on
the esterification reactions (
Bhatia et al., 1973; Krishnaiah and
). Some of these
studies tested the reaction for internal diffusion limitation by
using different particle sizes (
Bhatia et al., 1973; Gangadwala
et al., 2003; Pääkkönen and Krause, 2003
), while other studies
used certain criterion for such a purpose (
1984; Bart et al., 1996; Ali and Merchant, 2006
). The mea-
sured values of the rate of the reaction (
−r
A(obs)
) are used to
calculate the Weisz–Prater criterion in order to determine the
possibility of internal diffusion limiting the reaction. The di-
mensionless Weisz–Prater parameter (C
W P
) can be defined as
follows (
C
W P
= −
r
A(obs)
c
R
2
c
D
e
C
li
,
(3)
where
−r
A(obs)
is the rate of the reaction at a given time in
mol/g of catalyst/s,
c
is the catalyst density in g/cm
3
, R
c
is
the effective radius of the catalyst is the ratio of catalyst pellet
volume to catalyst pellet external surface area in cm, D
e
is the

3200
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
effective diffusivity in cm
2
/s and C
li
is the limiting reactant
concentration in the mixture at a given time in mol/cm
3
.
The definition of D
e
is given below:
D
e
=
2
v
D
lm
,
(4)
where
v
is the void fraction and D
lm
is the diffusivity of limiting
reactant in the mixture in (cm
2
/s).
The multi-component diffusivity was calculated using Perkin
and Geankoplis method (
) as shown below:
D
lm
0.8
m
=
n
i=1
i=l
x
i
D
li
0.8
i
,
(5)
where x
i
is the mole fraction of the component i, D
li
is the
binary diffusivity of limiting reactant in component i (calculated
by Tyn and Calus method), and
i
and
m
are the viscosity of
component i and the mixture in cp, respectively. The viscosity of
the pure components and the mixture is obtained from HYSYS
program version 3.1.
All the terms in Eq. (3) are either measured or known. If
C
W P
>1 there are no internal diffusion limitations and no con-
centration gradient exists within the pellet, but if C
W P
?1 in-
ternal diffusion limits the reaction (
2.3. Kinetic models
Different models were tested in our investigation. These
models are based on different assumptions regarding the re-
action mechanism and the rate controlling step. The precise
reaction mechanism needs to be defined. For instance, for a
situation where adsorption is the rate controlling step, there are
possibly three different reaction mechanisms. Adsorbed pro-
pionic acid is reacting with 1-propanol in the fluid, adsorbed
1-propanol is reacting with propionic acid in the fluid or ad-
sorbed propionic acid is reacting with adsorbed 1-propanol.
Also, the rate controlling step can be the surface reaction step
or the step involving desorption of any of the products. Com-
bining the reaction mechanism with the rate controlling step in
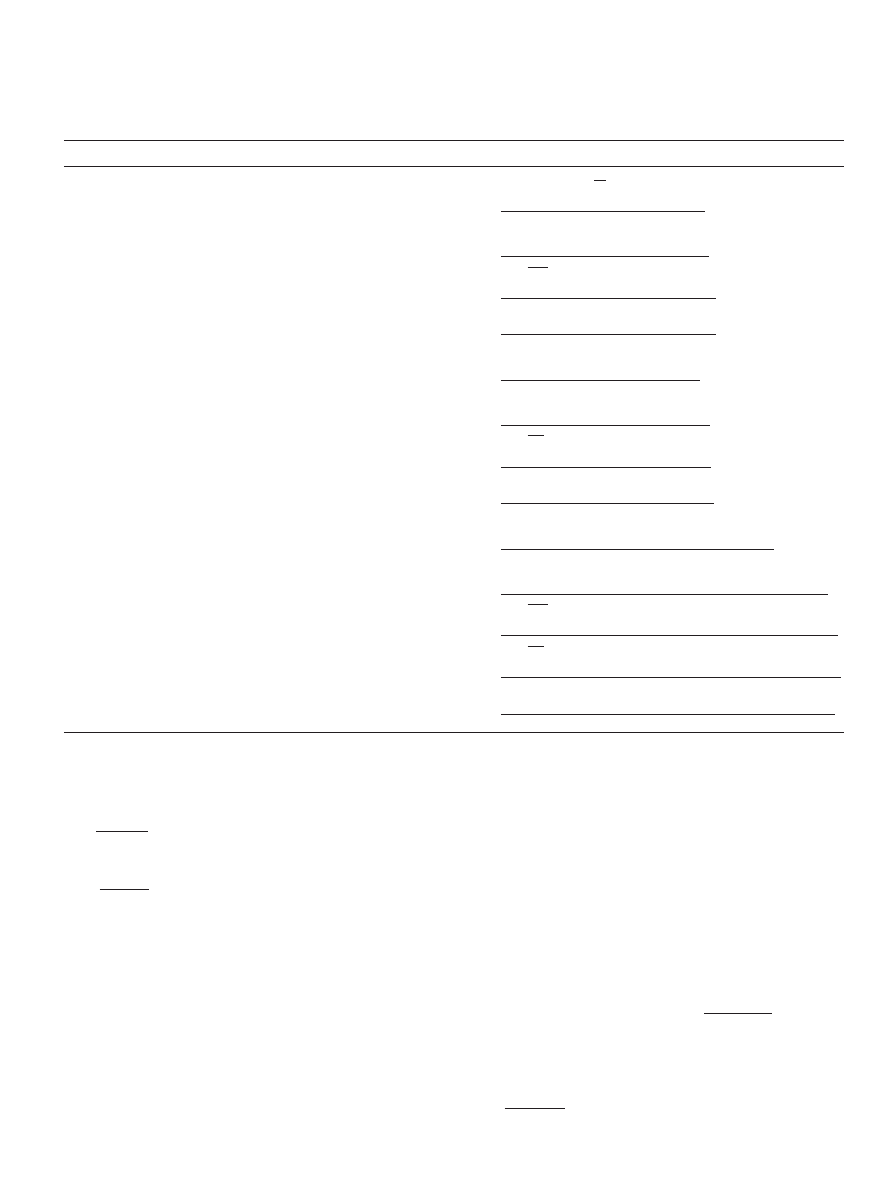
our case (for the reaction of propionic acid and 1-propanol) 13
models are possible as shown in
. By taking into ac-
count the strong water affinity for the cation-exchange resins
(
Lee et al., 2000, 2002; Gangadwala et al., 2003; Ali and
) a correction term (
) was added to the activity
term for water in these equations (with
values equal to 1, 2
and 3) resulting in 39 different rate expressions. The values of
the correction term for the affinity of resin for water (
) were
chosen according to previous workers results (
2002; Gangadwala et al., 2003; Ali and Merchant, 2006
). In
addition to these 39 possibilities, the P-H model was tested,
resulting in a total of 40 different rate expressions (see Table
1). Therefore, the main differences between these models are
the assumed reaction mechanism and the rate controlling step
in addition to the value of the correction term for water affin-
ity. The P-H is the simplest model, where the adsorption and
desorption of reactants and products are neglected. The second
type of models, the L–H, can describe the kinetic data based
on the assumption that both reactants are adsorbed. However,
if a model derived on the basis of the assumption that one of
the adsorbed reactants is reacting with another in the fluid, then
it can be described by the E–R model. Because the affinity of
the resin for water is strong, the activity of water in the rate
expression is raised to a power
, which can take values of 2
and 3 (
> 1) in the M-L–H and the M-E–R models.
In
, M
cat
is the mass of the catalyst in gram, n is the
total number of moles, a
i
is the activity of species i(a
i
=
i
x
i
),
i
is the activity coefficient of species i, x
i
is the mole fraction
of species i, k
f
is the forward reaction rate constant in mol/g
catalyst/s, K
a
is the overall reaction equilibrium constant, K
i
is the adsorption constant for species i, and
is the correction
term for strong water affinity for the cation-exchange resins.
The general expression for the overall reaction equilibrium
constant is given by
K
a
= K
s
K
acid
K
alc
K
ester
K
water
,
(6)
where K
s
is the experimentally measured (surface reaction)
equilibrium constant, K
acid
, K
alc
, K
ester
and K
water
are the ad-
sorption equilibrium constants for acid, alcohol, ester and wa-
ter, respectively. For the P-H model, K
a
= K
s
, while for the
L–H model, K
a
is as in Eq. (6). However, in the case of the
E–R model, only two species (one reactant and one product)
are being adsorbed. So, there are four possibilities of K
a
ex-
pressions with one adsorption equilibrium constant term of one
of the reactants in the numerator of Eq. (6) and another one for
one of the products in the denominator.
2.4. Estimation of activity coefficients
Some correlations for determining the activity coefficient
uses the contributions of interactions between functional
groups, such as –OH,
&O, –CH
3
, etc. rather than the interac-
tion between the molecules in the mixture. The determination
of the activity coefficient can be done without the need for ac-
tual data, by assembling the pure component from individual
groups and assessing the contributions of their interactions.
One of these methods is the UNIFAC (universal functional
activity coefficient) model. UNIFAC was used to account for
system non-ideality. We have found that UNIFAC accurately
predicted the system non-linearity (
The activity coefficient consists of two parts, combinatorial
and residual parts, and it is expressed as
ln
i
= ln
C
i
+ ln
R
i
.
(7)
The combinatorial part of the activity coefficients (
) is given by
ln
C
i
= ln
i
x
i
+
z
2
q
i
ln
i
i
+ l
i
−
i
x
i
j
x
j
l
j
,
(8)
where the coordination number z
= 10, x
i
is the mole fraction
of species i and l
i
is given by
l
i
=
z
2
(r
i
− q
i
) − (r
i
− 1).
(9)
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
3201
Table 1
Rate expressions for different rate controlling mechanisms
Adsorption status of reactants
Limiting step
Rate expression
a
Non
Surface reaction
r
i
= M
cat
k
f
(a
acid
a
alc
−
1
K
a
a
ester
a
water
)
Adsorbed propionic acid reacting
with 1-propanol in the fluid
Surface reaction
r
i
=
M
cat
k
f
K
acid
(a
acid
a
alc
− (a
ester
a
water
/K
a
))
(1 + K
acid
a
acid
+ K
water
a
water
)
Adsorption of acid
r
i
=
M
cat
k
acid
(a
acid
− (a
ester
a
water
/(K
a
a
alc
)))
(1 +
K
acid
K
a
(a
ester
a
water
/a
alc
) + K
water
a
water
)
Desorption of ester
r
i
=
M
cat
K
a
k
ester
(a
alc
a
acid
/a
water
− (a
ester
/K
a
))
(1 + K
acid
a
acid
+ K
a
K
ester
(a
alc
a
acid
/a
water
))
Desorption of water
r
i
=
M
cat
K
a
k
water
(a
alc
a
acid
/a
ester
− (a
water
/K
a
))
(1 + K
acid
a
acid
+ K
a
K
water
(a
alc
a
acid
/a
ester
))
Adsorbed 1-propanol reacting with
propionic acid in the fluid
Surface reaction
r
i
=
M
cat
k
f
K
alc
(a
acid
a
alc
− (a
ester
a
water
/K
a
))
(1 + K
alc
a
alc
+ K
water
a
water
)
Adsorption of alcohol
r
i
=
M
cat
k
alc
(a
alc
− (a
ester
a
water
/(K
a
a
acid
)))
(1 +
K
alc
K
a
(a
ester
a
water
/a
acid
) + K
water
a
water
)
Desorption of ester
r
i
=
M
cat
K
a
k
ester
(a
alc
a
acid
/a
water
− (a
ester
/K
a
))
(1 + K
alc
a
alc
+ K
a
K
ester
(a
alc
a
acid
/a
water
))
Desorption of water
r
i
=
M
cat
K
a
k
water
(a
alc
a
acid
/a
ester
− (a
water
/K
a
))
(1 + K
alc
a
alc
+ K
a
K
water
(a
alc
a
acid
/a
ester
))
Adsorbed propionic acid reacting
with adsorbed 1-propanol
Surface reaction
r
i
=
M
cat
k
f
K
acid
K
alc
(a
acid
a
alc
− (a
ester
a
water
/K
a
))
(1 + K
acid
a
acid
+ K
alc
a
alc
+ K
ester
a
ester
+ K
water
a
water
)
2
Adsorption of acid
r
i
=
M
cat
k
acid
(a
acid
− (a
ester
a
water
/(K
a
a
alc
)))
(1 +
K
acid
K
a
(a
ester
a
water
/a
alc
) + K
alc
a
alc
+ K
ester
a
ester
+ K
water
a
water
)
Adsorption of alcohol
r
i
=
M
cat
k
alc
(a
alc
− (a
ester
a
water
/(K
a
a
acid
)))
(1 +
K
alc
K
a
(a
ester
a
water
/a
acid
) + K
acid
a
acid
+ K
ester
a
ester
+ K
water
a
water
)
Desorption of ester
r
i
=
M
cat
K
a
k
ester
(a
alc
a
acid
/a
water
− (a
ester
/K
a
))
(1 + K
acid
a
acid
+ K
alc
a
alc
+ K
a
K
ester
(a
alc
a
acid
/a
water
) + K
water
a
water
)
Desorption of water
r
i
=
M
cat
K
a
k
water
(a
alc
a
acid
/a
ester
− (a
water
/K
a
))
(1 + K
acid
a
acid
+ K
alc
a
alc
+ K
ester
a
ester
+ K
a
K
water
(a
alc
a
acid
/a
ester
))
a
a
water
= (x
water
water
)
.
Surface area fraction and volume fraction are as follows:
i
=
q
i
x
i
j
q
j
x
j
,
(10)
i
=
r
i
x
i
j
r
j
x
j
.
(11)
Parameters r
i
and q
i
are calculated as the sum of the individ-
ual group volume and surface area parameters R
k
and Q
k
as
follows:
r
i
=
k
v
(i)
k
R
k
,
(12)
q
i
=
k
v
(i)
k
Q
k
,
(13)
where v
(i)
k
is the number of k groups in molecule i.
The residual part of the activity coefficient (
) is given by
ln
R
i
=
k
v
(i)
k
(ln
k
− ln
(i)
k
),
(14)
where
k
is the group residual activity coefficient in the mixture
and
(i)
k
is the residual activity coefficient of group k in a
reference solution containing only molecules of types i (pure
component). The
k
or
(i)
k
is calculated by
ln
k
(or
(i)
k
)
= Q
k
1
− ln
m
m
mk
−
m
m
km
n
n
nm
.
(15)
Here
m
is the area fraction of group m and it is calculated
according to the following equation:
m
=
Q
m
X
m
n
Q
n
X
n
,
(16)
3202
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
where Q
m
is a group parameter and X
m
is the mole fraction of
group m in the mixture:
X
m
=
i
v
(i)
m
x
i
i
n
v
i
n
x
i
.
(17)
The group interaction coefficient
mn
) is
given by
mn
= exp
−
a
mn
+ b
mn
T
T
.
(18)
3. Experimental
3.1. Catalysts
Three ion-exchange catalysts were used; Dowex 50Wx8-400
(Aldrich catalog # 217514), Amberlyst 15 (Aldrich catalog #
216380) and Amberlite IR-120 (Aldrich catalog # 216534).
These catalysts were used after they have been vacuum-dried
at a temperature of 343 K for 48 h. Drying at much higher
temperatures (temperature > 373 K) could lead to the loss of
active sulfonic acid sites of the catalysts. The properties of the
ion-exchange resins are shown in
. The homogeneous
catalyst used, sulfuric acid (product # UN1830), was supplied
by BDH and had a purity of greater than 99% and a specific
gravity of 1.835.
3.2. Chemicals
1-Propanol with a purity of 99.5% was supplied by Schar-
lau (catalog # AI0437). 2-Propanol with a purity of 99.5%
was supplied by Fluka (catalog # 59300). Methanol analytical
grade (99.8% pure) was supplied by AJAX chemicals (UN #
1230). Ethanol reagent grade with 99.8% purity was supplied
by Scharlau (catalog # ET0016). 1-Butanol reagent grade of
99.5% purity was supplied by Fluka (catalog # 19420). Propi-
onic acid having a product # 81912 and Butyric acid with a cat-
alog # 19215 both have a purity of 99% and were supplied by
Fluka, while acetic acid with a purity of 99.8% was supplied by
Riedel-de Haen (product # 27225). The purity of all alcohols
Table 2
Properties
a
of the cation exchange resins used
Catalyst
Dowex 50Wx8-400
Amberlite IR-120
Amberlyst 15
Manufacturer
Dow Chemical Co.
Rohm & Haas
Rohm & Haas
Supplier
Aldrich
Aldrich
Aldrich
Catalog #
217514
216534
216380
Polymer type
Gel-type
Gel-type
Macro reticular
Matrix type
Styrene–divinyl benzene (DVB)
Styrene–divinyl benzene (DVB)
Styrene–divinyl benzene (DVB)
Functional group
Sulfonic acid
Sulfonic acid
Sulfonic acid
Standard ionic form
H
+
H
+
H
+
Total exchange capacity (meq/mL)
1.7
1.9
1.8
Cross-linking (% DVB)
8
8
20
Moisture content (% mass)
54
45
< 1.6
Particle size range (mm)
0.04–0.07
0.30–1.20
0.30–1.20
a
As reported by the manufacturer.
and acids was checked by gas chromatographic analysis and
found to be comparable to the listed values mentioned above.
For titration purposes, the alkali used was a standard solution
of sodium hydroxide (NaOH) with 0.1024 N in water. This so-
lution was supplied by Aldrich (product # 31,948-1). The con-
centration of the alkali solution was confirmed by back titrating
with a freshly prepared solution of potassium hydrogen phtha-
late of known concentration. Potassium hydrogen phthalate was
supplied by Aldrich (catalog # 17992-2) and had purity greater
than 99.9%.
3.3. Kinetic runs
All the kinetic runs were carried out in a Lab-Max reactor
system. The equipment used in this experiment (
) con-
sisted mainly of a 1 L glass batch reactor system. The reac-
tor was continuously stirred using a four blade glass impeller
driven by an electrical motor. The shell of the reactor vessel
was filled with oil to either heat or cool the reaction mixture. A
temperature probe was inserted into the reactor to measure the
mixture’s temperature with an accuracy of
±0.1 K. The reactor
temperature was automatically controlled. The Lab-Max had a
stirrer speed ranging from 0 to 1500 rpm. A measured amount
of acid and catalyst (liquid or vacuum-dried solid catalyst) was
added to the reactor, and the temperature of the reactor was
raised to the required reaction temperature. A certain amount
of alcohol was separately preheated to the reaction temperature
in a heating bath. The preheated alcohol was added to the re-
actor and the time of initiation of the reaction was noted (zero
time). A sample of the reaction mixture was immediately with-
drawn, filtered and quenched to stop the reaction. The sample
was titrated with a standard NaOH solution. The reproducibil-
ity of the titration results was found to be within
±1.5%. This
was done by repeating the titration procedure three times. Other
samples were withdrawn and titrated at different time intervals
and the reaction was followed for 240 min.
3.4. Equilibrium runs
The equilibrium runs were carried out in 80 cm
3
glass cells
having outer glass jackets facilitating water flow to control the
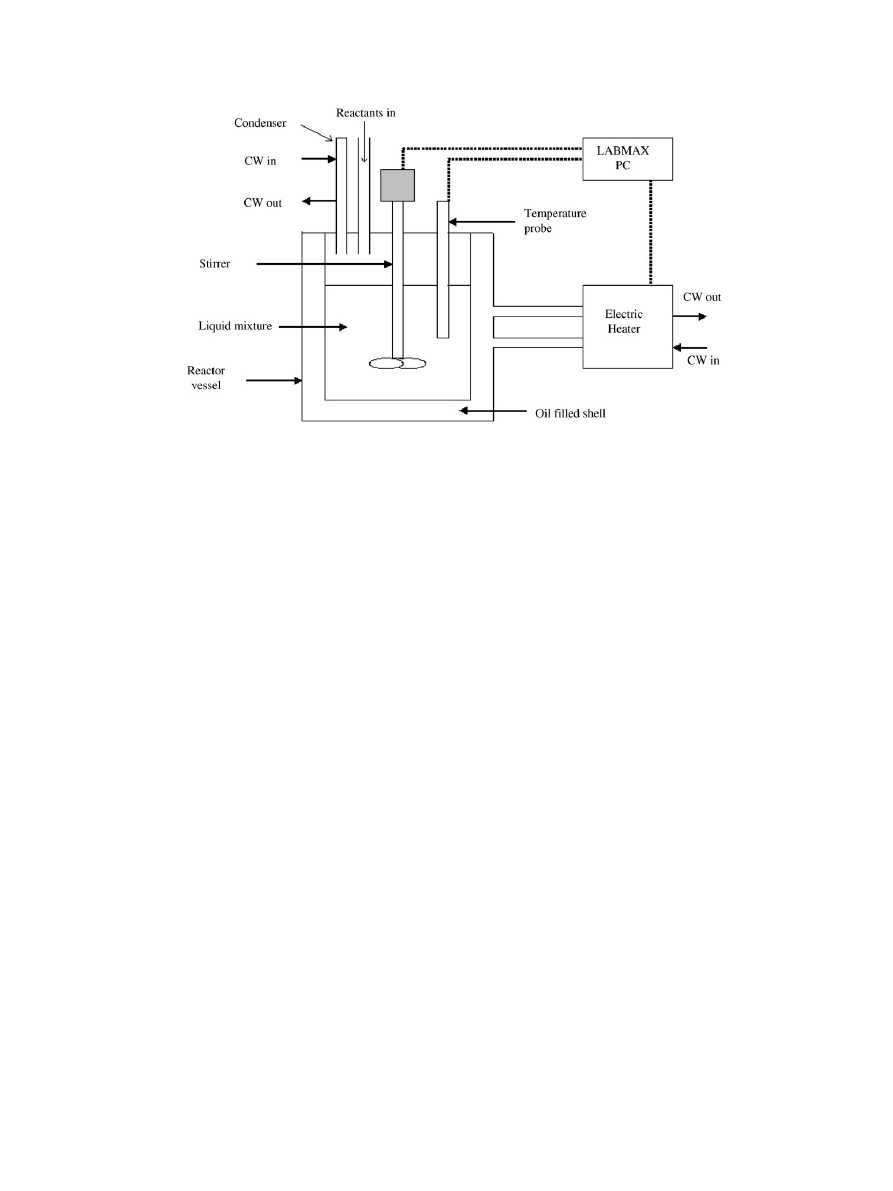
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
3203
Fig. 1. Experimental setup of the Lab-Max apparatus.
temperature from a circulating water bath. The glass stoppers
for the cells were fitted with smaller diameter stoppers at the
top to facilitate the removal of the reaction sample by means
of a thin graduated pipette. This involved removing only the
small upper stopper. Equimolar amounts of propionic acid and
1-propanol (around 0.2 mol of each) along with 2% by volume
of concentrated sulfuric acid were allowed to react in the cells
until equilibrium was reached (as evidenced by the absence of
any change in the amount of alkali required to neutralize the
acid present in 0.1 ml of the withdrawn sample). The reaction
volume was not allowed to exceed half the volume of the cells.
The reaction temperatures studied were 303.15, 313.15 and
323.15 K.
4. Results and discussion
The reaction conditions were systematically altered to study
the effect of rpm, nature of catalyst, catalyst loading, temper-
ature, acid to alcohol molar ratio, presence of water, changing
acid and alcohol types on the reaction kinetics. In addition,
three runs were carried out using sulfuric acid as the catalyst to
determine the reaction equilibrium. The details of the reaction
conditions of these runs are given in
4.1. External and internal diffusion significance
The esterification reaction in this investigation is a
liquid–solid catalytic reaction where different processes are
taking place: external and internal diffusions, adsorption of at
least one of the reactants, surface reaction and desorption of
products followed by back diffusion of the products into the liq-
uid bulk. To study the kinetics of the esterification reaction, the
effect of external and internal diffusion limitations should be
eliminated. The external mass transfer resistance is affected by
the speed of agitation in our case as has been stated previously.
Therefore, five experiments were carried out at different stirrer
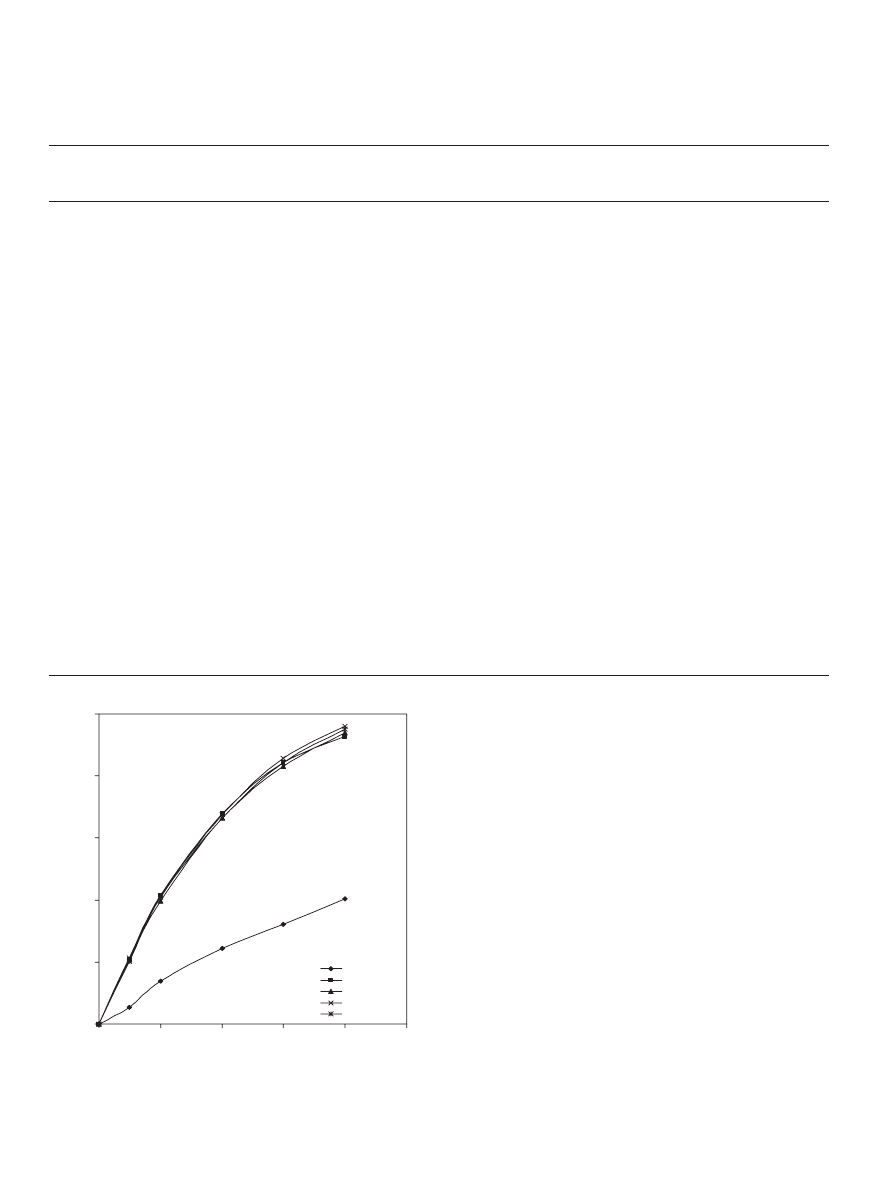
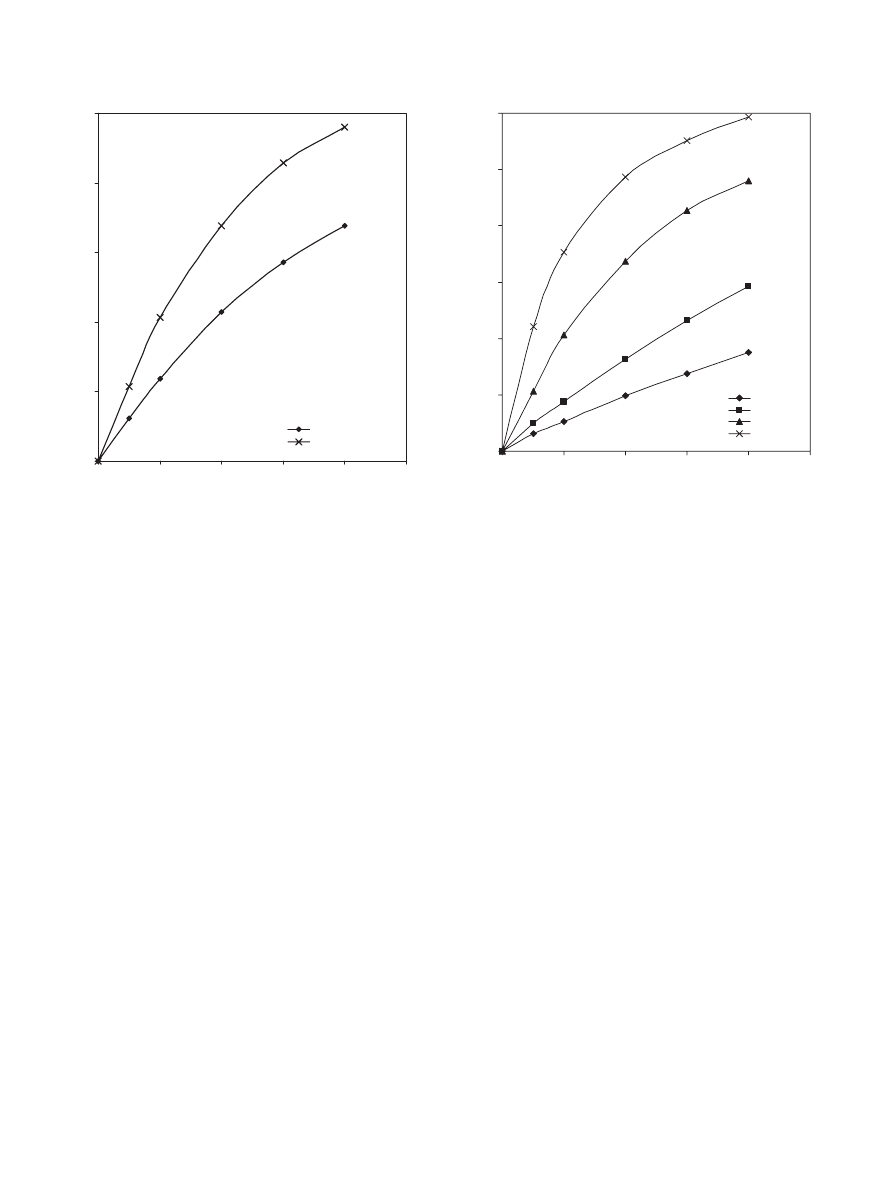
speeds (100, 200, 600, 900 and 1000 rpm). It was found that
the conversion of acid was independent of stirrer speed except
at 100 rpm as shown in
, because at 100 rpm the catalyst
was not well distributed inside the reactor. This means that for
the setup used in this study (stirrer of 5 cm diameter
× 1 cm
bore diameter and reactor of 10 cm internal diameter), the
external diffusion limitation is negligible at stirrer speeds of
200 rpm and above. Therefore, a stirrer speed of 900 rpm was
maintained during all experiments to ensure that the measured
reaction rate was free from external diffusion effects.
studied the methyl acetate synthesis using Am-
berlyst 15 and they found that there was no external diffusion
effect for stirrer speed ranging from 160 to 760 rpm. In the
study undertaken by
, low rpm values
were found to have a negligible effect on reaction rate because
of the low viscosity of the studied system (reaction of methanol
with dilute acetic acid solution). Furthermore,
studied the effect of stirrer speed (ranged from 100
to 560 rpm) for methyl acetate synthesis using Amberlyst 15,
and found that at stirrer speeds of 170 rpm and above, external
diffusion did not control the overall rate in the ion-exchange
resin catalyzed process. This result is in line with the work
of
found that
a stirrer speed of 500 rpm was sufficient to eliminate external
diffusion limitations for the esterification reaction of benzyl
alcohol with acetic acid with Amberlyst 15 catalyst.
found that in the reaction of 2-propanol with
acetic acid, external mass transfer limitation was negligible
at a stirrer speed above or equal to 500 rpm (the stirrer speed
studied range was from 50 to 900 rpm). A higher range of
stirrer speeds (700–1200) rpm was studied by
for the esterification of 1-propanol with acetic acid
over Dowex-50W. The absence of a stirrer speed effect was
observed at 1100 and 1200 rpm, which is much higher than
3204
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
Table 3
Summary of experimental conditions
Run #
Temperature (K)
Acid
to
alcohol
rounded molar
ratio
Catalyst
loading
(g dry cat/L)
Stirrer speed
(rpm)
Acid
Alcohol
Water
Catalyst
1
323.15
1:1
40
100
Propionic
1-Propanol
–
Dowex 50Wx8-400
2
323.15
1:1
40
200
Propionic
1-Propanol
–
Dowex 50Wx8-400
3
323.15
1:1
40
600
Propionic
1-Propanol
–
Dowex 50Wx8-400
4
323.15
1:1
40
900
Propionic
1-Propanol
–
Dowex 50Wx8-400
5
323.15
1:1
40
1000
Propionic
1-Propanol
–
Dowex 50Wx8-400
6
323.15
1:1
60
900
Propionic
1-Propanol
–
Amberlyst 15
7
323.15
1:1
60
900
Propionic
1-Propanol
–
Amberlite IR-120
8
323.15
1:1
60
900
Propionic
1-Propanol
–
Dowex 50Wx8-400
9
323.15
1:1
10
900
Propionic
1-Propanol
–
Dowex 50Wx8-400
10
323.15
1:1
20
900
Propionic
1-Propanol
–
Dowex 50Wx8-400
11
323.15
1:1
60
900
Propionic
1-Propanol
–
Dowex 50Wx8-400
12
323.15
1:1
40
900
Propionic
1-Propanol
–
Wet Dowex 50Wx8-400
13
323.15
1:1
2% Volume
900
Propionic
1-Propanol
–
H
2
SO
4
14
303.15
1:1
2% Volume
900
Propionic
1-Propanol
–
H
2
SO
4
15
303.15
1:1
40
900
Propionic
1-Propanol
–
Dowex 50Wx8-400
16
313.15
1:1
40
900
Propionic
1-Propanol
–
Dowex 50Wx8-400
17
333.15
1:1
40
900
Propionic
1-Propanol
–
Dowex 50Wx8-400
18
323.15
2:1
40
900
Propionic
1-Propanol
–
Dowex 50Wx8-400
19
323.15
1:2
40
900
Propionic
1-Propanol
–
Dowex 50Wx8-400
20
323.15
1:4
40
900
Propionic
1-Propanol
–
Dowex 50Wx8-400
21
323.15
4:1
40
900
Propionic
1-Propanol
–
Dowex 50Wx8-400
22
323.15
1:1
40
900
Propionic
1-Propanol
Water added
Dowex 50Wx8-400
23
323.15
1:1
40
900
Acetic
1-Propanol
–
Dowex 50Wx8-400
24
323.15
1:1
40
900
Butyric
1-Propanol
–
Dowex 50Wx8-400
25
323.15
1:1
40
900
Propionic
Methanol
–
Dowex 50Wx8-400
26
323.15
1:1
40
900
Propionic
Ethanol
–
Dowex 50Wx8-400
27
323.15
1:1
40
900
Propionic
1-Butanol
–
Dowex 50Wx8-400
28
323.15
1:1
40
900
Propionic
2-Propanol
–
Dowex 50Wx8-400
29
303.15
1:1
2% Volume
Equilibrium runs
Propionic
1-Propanol
–
H
2
SO
4
30
313.15
1:1
2% Volume
Propionic
1-Propanol
–
H
2
SO
4
31
323.15
1:1
2% Volume
Propionic
1-Propanol
–
H
2
SO
4
0
0.1
0.2
0.3
0.4
0.5
0
3600
7200
10800
14400
18000
Time (sec)
Conversion of propionic acid
100 rpm
200 rpm
600 rpm
900 rpm
1000 rpm
Fig. 2. Effect of stirrer speed (rpm) on the conversion of propionic acid at
323 K, 1:1 propionic acid to 1-propanol molar ratio and 40 g dry cat/L catalyst
loading of Dowex 50Wx8-400.
that found by
and
Therefore, the significance of external mass transfer limitation
which is directly related to stirrer speed in batch systems de-
pends on several factors such as the viscosity of the system,
reactions conditions, type of species used and the presence of
diluents, in addition to the type and properties of catalyst used.
To investigate the internal diffusion effect on the reaction
rate, analysis based on the Weisz–Prater criterion was under-
taken. Data from 10 experiments (4, 10, 11 and 15–21) were
fitted to the Weisz–Prater equation where Dowex 50Wx8-400
was the catalyst. The Weisz–Prater criterion was determined
for the initial stages (1800 s) of runs 4, 10, 11 and 15–21 (these
runs were used for modeling purpose). The results are shown
in
. It was found that values of the internal diffusion
parameter are significantly less than one (C
W P
>1). These
results indicate that internal diffusion does not limit the reac-
tion of propionic acid with 1-propanol over Dowex 50Wx8-
400 for the reaction conditions implemented in this study. The
C
W P
parameter values ranges between 3.63E
−4 to 1.22E −3.
These values are of the same order as those obtained by
and
, which varied
from 1.3E
− 4 to 1.0E − 2 and from 6.0E − 4 to 1.7E − 3, re-
spectively. Both studies were undertaken for the esterification
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
3205
Table 4
Significance of internal diffusion for different runs
Run #
Experimental parameters
a
C
li
at 30 min (mol/cm
3
)
r
A
(obs)
at 30 min (mol/g
cat
/s)
D
e
(cm
2
/s)
C
W P
4
40/323.15/1
0.0055
8.78E
−06
1.22E
−06
8.73E
−04
10
20/323.15/1
0.0058
1.02E
−05
1.16E
−06
1.02E
−03
11
60/323.15/1
0.0052
7.84E
−06
1.31E
−06
7.73E
−04
15
40/303.15/1
0.0060
2.35E
−06
7.23E
−07
3.63E
−04
16
40/313.15/1
0.0059
3.80E
−06
9.21E
−07
4.70E
−04
17
40/333.15/1
0.0048
1.48E
−05
1.69E
−06
1.22E
−03
18
40/323.15/2
0.0077
7.51E
−06
9.00E
−07
7.29E
−04
19
40/323.15/0.5
0.0036
6.22E
−06
1.44E
−06
8.03E
−04
20
40/323.15/0.25
0.0021
4.01E
−06
1.58E
−06
7.97E
−04
21
40/323.15/4
0.0096
6.39E
−06
5.83E
−07
7.71E
−04
a
First number: catalyst loading in g dry cat/L; second number: temperature in K; third number: acid to alcohol molar ratio.
Table 5
Equilibrium mole fractions, conversions, activity coefficients and constants
T (K)
Acetic acid
1-Propanol
Propyl propionate
Water
Equilibrium
constant K
s
Equilibrium
Equilibrium
Activity
Equilibrium
Equilibrium
Activity
Equilibrium
Activity
Equilibrium
Activity
mole
conversion
coefficient
mole
conversion
coefficient
mole
coefficient
mole
coefficient
fraction
fraction
fraction
fraction
303.15
0.1638
0.6724
0.6842
0.1638
0.6724
1.0698
0.3362
1.9273
0.3362
2.9911
33.1789
313.15
0.1654
0.6692
0.7101
0.1654
0.6692
1.0505
0.3346
1.9083
0.3346
2.9248
30.6202
323.15
0.1678
0.6644
0.7239
0.1678
0.6644
1.0376
0.3322
1.8815
0.3322
2.8897
28.3703
of 1-propanol with acetic acid;
used Dowex
monospheres, while
used Dowex-
50W as the catalyst. In addition, in a previous work, our group
found C
W P
to range from 9.3E
− 5 to 5.0E − 4 for the esterifi-
cation of 2-propanol with acetic acid over Dowex 50Wx8-400
(
4.2. Effect of temperature on reaction equilibrium
Three experimental runs were carried out at 303.15, 313.15
and 323.15 K to obtain the equilibrium constant values. The
alcohol to acid molar ratio of 1:1 and sulfuric acid with a
concentration of 2% by volume were used. The experiments
were undertaken to determine the equilibrium mole fractions of
propionic acid, 1-propanol, propyl propionate and water. The
results for the equilibrium runs are shown in
The following equation was used to determine the equilib-
rium constant K
s
:
K
s
=
(x
ester
)
eq
(x
water
)
eq
(x
acid
)
eq
(x
alc
)
eq
ester
water
acid
alc
,
(19)
where (x
i
)
eq
is the equilibrium mole fraction of component i
and
i
is the activity coefficient of component i determined
by the UNIFAC model. The values of the activity coefficients
are reported in
. From
it is obvious that the
activity coefficients of 1-propanol, 1-propyl propionate and
water decreased and the activity coefficient of propionic acid
increased as the reaction temperature increased. Increasing the
3.32
3.34
3.36
3.38
3.4
3.42
3.44
3.46
3.48
3.5
3.52
0.00305
0.0031
0.00315
0.0032
0.00325
0.0033
0.00335
1/T (1/K)
ln Ks
Experimental Run
Linear fit
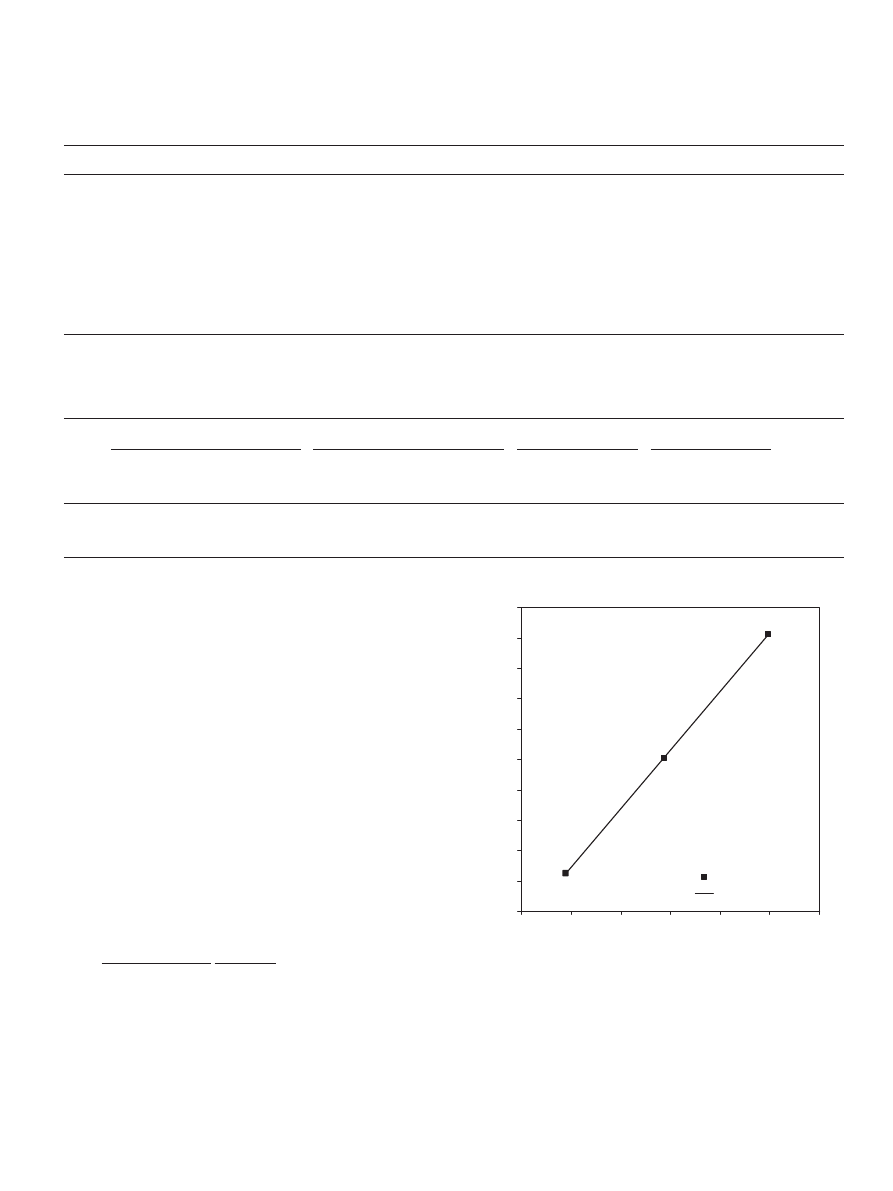
Fig. 3. The natural logarithm of equilibrium constant versus the reciprocal
of the absolute temperature.
temperature from 303.15 to 323.15 K, the equilibrium conver-
sion of the reactants decreased from 0.6724 to 0.6644.
The plot of (ln K
s
) versus (1/T ) with a linear regression of
R
2
fit
= 0.99 (
) gives the following equation:
K
s
= 2.6445 Exp(6376/RT ).
(20)
3206
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
This equation is consistent with the Van’t Hoff equation for
the effect of temperature on the chemical reaction equilibrium
constant:
ln
K
S
K
SR
= −
H
0
R
R
1
T −
1
T
R
.
(21)
Accordingly, the standard enthalpy change of reaction
H
0
R
is
−6.4 kJ/mol. Therefore, the reaction of propionic acid with
1-propanol is exothermic, and the equilibrium constant has a
weak dependence on temperature. The same trend was ob-
tained by
for the methyl acetate synthe-
sis with
H
0
R
equal to
−6.5 kJ/mol. The equilibrium constant
was found to have values of 30.2, 27.4 and 24.0 at 313.15,
318.15 and 323.15 K, respectively. Also, in a previous work
(
) we have found that
H
0
R
was equal
to
−5.4 kJ/mol for the esterification reaction between acetic
acid and 2-propanol, and the equilibrium constant to be 29.4,
26.0 and 23.0 at 303, 323 and 343 K, respectively. These re-
sults indicate that the reaction is mildly exothermic. Different
results were obtained by
for the esterification
of acetic acid with 1-propanol and
for the
esterification of propionic acid with 1-butanol. They found that
the equilibrium constant increased with increasing temperature,
which indicates that these esterification reactions are endother-
mic.
found that the equilibrium constants at
303.15, 323.15 and 343.15 K were 10.3, 21.7 and 32.3, respec-
tively, while
found that the corresponding
values at 363, 373 and 383 K are 29.1, 29.4 and 30.1, respec-
tively. It has to be mentioned that the equilibrium constant is
found to be independent of temperature for many esterification
reactions, because the heat of reaction for these reactions were
almost equal to zero or quite small in value (
In this study the performance of several models (in which
different components are assumed to be adsorbed to different
extents on the catalyst surface) were compared. The P-H model,
which is the simplest model used, assumes reaction homogene-
ity and its rate equation calls for a homogeneous reaction equi-
librium constant (K
s
) as given by Eq. (20). In all other models
the reaction is heterogeneously catalyzed by Dowex 50Wx8-
400. Therefore, the equilibrium constant (K
a
) is a combination
of adsorption equilibrium constants (K
i
) for different species
i in addition to the surface reaction equilibrium constant (K
s
)
as expressed in Eq. (6). Since the functional group in the solid
catalyst (Dowex 50Wx8-400) used is sulfonic acid, it was
considered useful to obtain values of the surface reaction
equilibrium constant (K
s
) from a reaction system catalyzed
homogeneously by sulfuric acid at the same catalyst load-
ing. The homogeneous reaction equilibrium constant (K
s
)
was maintained for all other models in order to be able to
have a clear direct comparison of different species adsorption
equilibrium constants (K
i
) calculated by these models.
4.3. Effect of reaction parameters
By eliminating both external and internal diffusion limita-
tions, the esterification reaction is purely kinetically controlled.
0
0.1
0.2
0.3
0.4
0.5
0.6
0
3600
7200
10800
14400
18000
Time (sec)
Convesion of propionic acid
Amberlyst 15
Amberlite IR-120
Dowex 50Wx8-400
Fig. 4. Effect of catalyst type on the conversion of propionic acid at 323 K,
900 rpm, 1:1 propionic acid to 1-propanol molar ratio and 60 g dry cat/L
catalyst loading.
4.3.1. Effect of type of ion-exchange resin catalyst
Ion-exchange resins have been found to be suitable catalysts
for esterification reactions (
Sharma et al., 1973; Dakshina-
murty et al., 1984; Xu and Chuang, 1996; Yadav and Thathagar,
2002
). In recent studies, sulfuric acid is being replaced
by ion-exchange resins due to environmental regulations.
Moreover, using ion-exchange resins has several advantages as
discussed previously. In this investigation, three types of ion-
exchange resins (Dowex 50Wx8-400, Amberlite IR-120 and
Amberlyst 15) were chosen for this purpose. Among these
catalysts, Dowex 50Wx8-400 was found to be the best, yield-
ing the highest conversion of propionic acid as shown in
Fig.
4
. The performance of different ion-exchange resin catalysts
for this kind of reactions is actually attributed to the type of
reactants and products involved, in addition to the structure
and characterization of the ion-exchange resins.
4.3.2. Effect of using sulfuric acid
(H
2
SO
4
) compared with
Dowex 50Wx8-400 as a catalyst
A comparison of the effect of using sulfuric acid rather than
Dowex 50Wx8-400 on the conversion of propionic acid as a
function of time was made at two different temperatures (303.15
and 323.15 K) with identical experimental conditions (
In these experiments, equivalent catalyst loadings were used
for both Dowex (40 g dry cat/L) and sulfuric acid (almost 2%
of total liquid volume so as to equal 40 g dry cat/L). Higher
conversion of propionic acid over a period of time of 4 h was
obtained with sulfuric acid rather than Dowex 50Wx8-400 at
the two temperatures studied. Since sulfuric acid is in the same
phase as the reactants (homogeneous liquid phase), the avail-
ability of free protons in this liquid–liquid catalytic reaction
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
3207
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
0
3600
7200
10800
14400
18000
Time (sec)
Conversionof propionic acid
H2SO4 at 323.15 K
Dowex 50Wx8-400 at323.15 K
H2SO4 at 303.15 K
Dowex 50Wx8-400 at303.15 K
Fig. 5. Effect of H
2
SO
4
compared with Dowex 50Wx8-400 on the conversion
of propionic acid at different temperatures, 900 rpm, 1:1 acid to alcohol molar
ratio and 40 g dry cat/L catalyst loading.
mixture results in having faster rates of reaction compared to
the case where ion-exchange resins are used (liquid–solid re-
action mixture). However, taking into consideration the advan-
tages of ion-exchange resin, Dowex 50Wx8-400 is the preferred
catalyst.
compared the behavior of the
ion-exchange resin Amberlyst 15 with the behavior of sulfuric
acid as catalyst for the conversion of acetic acid as a function
of time. The concentration of catalyst and the reaction condi-
tions were maintained the same. They (
found that sulfuric acid resulted in a higher conversion than
Amberlyst 15. However, by taking into consideration that us-
ing sulfuric acid as a catalyst suffers from several drawbacks
in industrial application, Amberlyst 15 was found to be more
effective as a catalyst for the synthesis of benzyl alcohol with
acetic acid. The effect of using a homogeneous catalyst versus
heterogeneous catalyst for the esterification of propionic acid
with 1-butanol was investigated by
. Sulfuric
acid was compared with Amberlyst 35 among other solid cat-
alysts at the same catalyst concentration of 1 wt% in solution.
They found that using a concentration of 1 wt% Amberlyst 35
in solution gave less conversion than a concentration of 1 wt%
of sulfuric acid in solution. However, Amberlyst 35 was se-
lected as the best choice for this synthesis because it withstands
higher temperatures than the other tested solid catalysts.
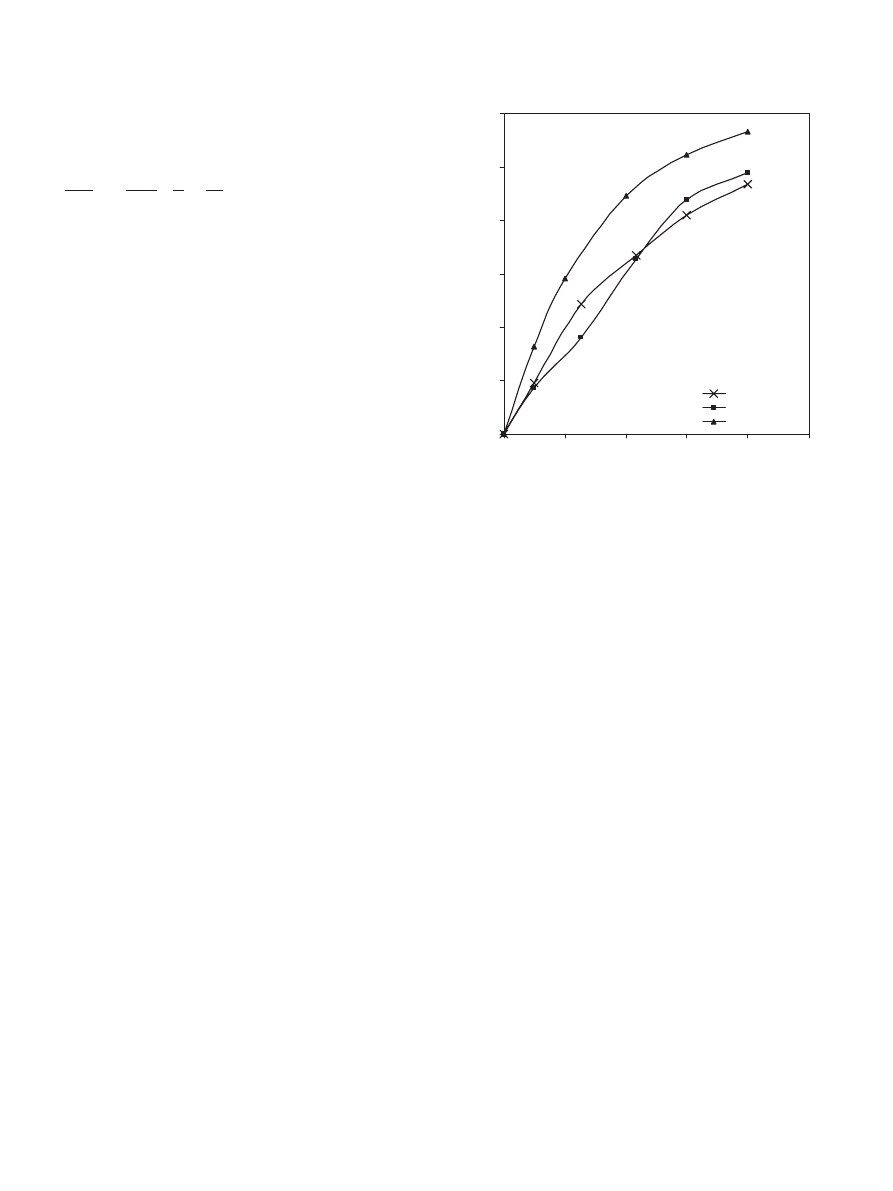
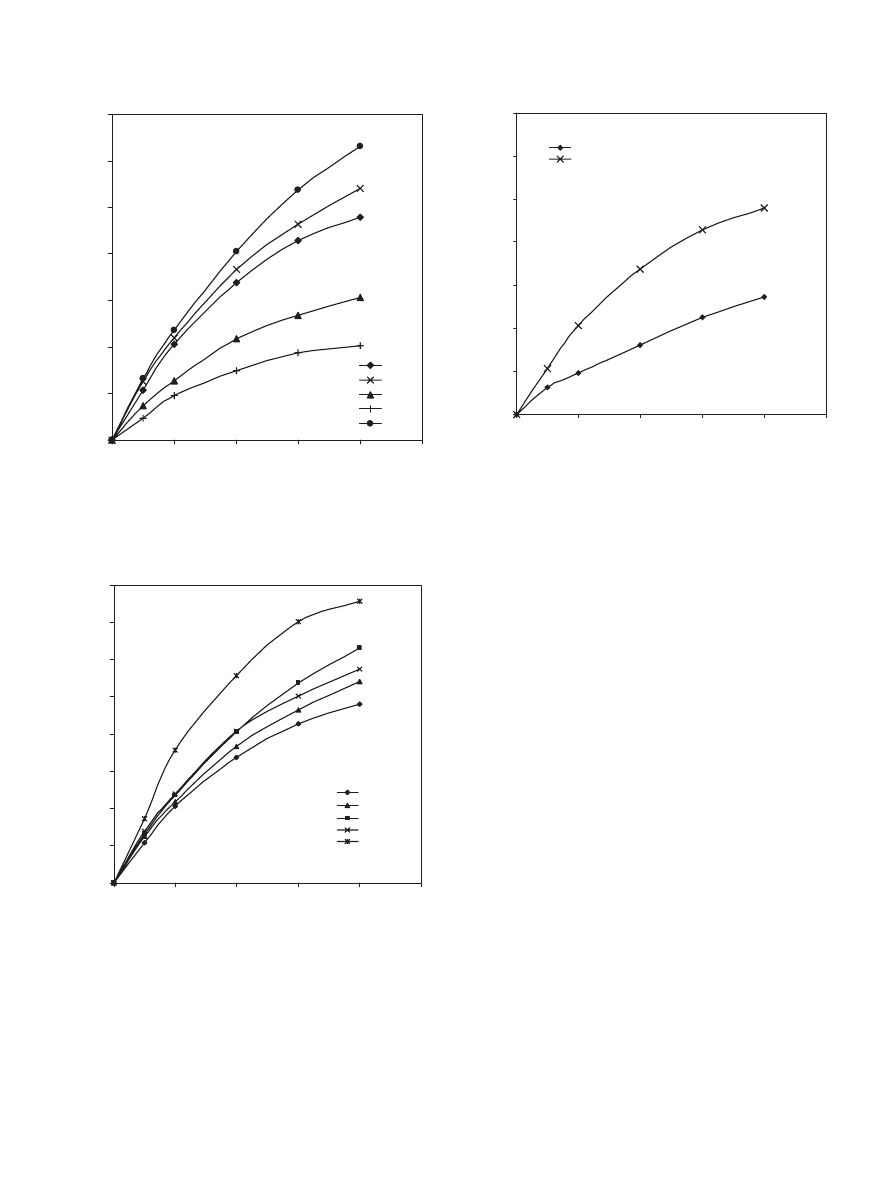
4.3.3. Effect of catalyst loading
The effect of catalyst loading of Dowex 50Wx8-400 on the
conversion of propionic acid was investigated by setting the
catalyst loadings at 10, 20, 40 and 60 g dry cat/L. It is observed
that the conversion of propionic acid increases proportionally
with catalyst loading (see
). As expected, increasing the
0
0.1
0.2
0.3
0.4
0.5
0.6
0
3600
7200
10800
14400
18000
Time (sec)
Conversion of propionicacid
10 g dry cat/L
20 g dry cat/L
40 g dry cat/L
60 g dry cat/L
Fig. 6. Effect of Dowex 50Wx8-400 loading on the conversion of propionic
acid at 323 K, 900 rpm and 1:1 propionic acid to 1-propanol molar ratio.
catalyst loading means more available active sites for this reac-
tion, which results in higher reaction rate. A similar trend was
obtained by earlier workers (
Rao et al., 1979; Xu and Chuang,
1996; Bart et al., 1996; Yadav and Kulkarni, 2000
). This re-
sult agrees with the model rate expression where the term M
cat
is explicitly added. This clearly indicates that M
cat
is directly
proportional to the reaction rate.
4.3.4. Effect of the catalyst moisture content (wet versus dry
Dowex 50Wx8-400)
To study the effect of moisture content of the ion-exchange
resin on the reaction, two experiments were conducted at the
same reaction conditions for a time period of 4 h as shown
in
. The first experiment was undertaken using a wet
catalyst (stock sample) where the weight of water was taken
into account, while for the second experiment the catalyst was
vacuum-dried at a temperature of 343 K for 48 h. The two ex-
periments were carried out using the same dry mass of cata-
lyst. As shown in
, the conversion of acid was higher
with dry catalyst compared with wet catalyst. Earlier work-
ers used different dried ion-exchange resins (Dowex 50W-x8
and x2, Amberlyst 15 and 35, Amberlite IR-120) for studying
the kinetics of the esterification synthesis (
Krishnaiah and Rao, 1984; Pöpken et al., 2000; Gangadwala
et al., 2003; Ali and Merchant, 2006
oven-dried Dowex 50w-x8 at 353.15–358.15 K for the ester-
ification of ethanol with propionic acid. Amberlyst 15 was
vacuum-dried at 363.15 K for 48 h by
for the methyl acetate synthesis. Furthermore,
3208
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
0
0.1
0.2
0.3
0.4
0.5
0
3600
7200
10800
14400
18000
Time (sec)
Conversion of propionic acid
Wet Dowex 50Wx8-400
Dry Dowex 50Wx8-400
Fig. 7. Effect of moisture content (wet versus dry Dowex) on the conversion
of propionic acid at 323 K, 900 rpm, 1:1 propionic acid to 1-propanol molar
ratio and 40 g dry cat/L catalyst loading.
, who studied the esterification of 1-propanol with
acetic acid, preheated Dowex 50W-x2 and x8 in an air oven at
338.15 K for 20 min before use. They found that the moisture
content of the solid ion-exchange resins had a retarding effect
on the conversion of acid. Moreover, a recent study on homo-
geneously catalyzed esterification (
) has estab-
lished that besides promoting ester hydrolysis, the presence of
water significantly decreases the activity of the catalytic pro-
tons. The presence of water can be expected to have a simi-
lar effect for the studied heterogeneous systems also, since the
catalyst used is acidic in nature. Therefore, it is important to
dry the catalyst before running an experiment since the pres-
ence of water can promote ester hydrolysis and adversely effect
catalytic activity. Both these factors can cause considerable de-
crease in the acid conversions. The role of water in heteroge-
neous esterification is further discussed in the section “Effect
of adding water to the reaction mixture”.
4.3.5. Effect of temperature
The effect of temperature was studied by varying the reaction
temperature from 303.15 to 333.15 K at constant conditions of
acid to alcohol molar ratio, catalyst concentration and stirrer
speed.
shows that increasing the temperature brings more
collisions and therefore more successful collisions. These suc-
cessful collisions have sufficient energy (activation energy) to
break the bonds and form products and thus result in higher
values of conversion of propionic acid, which agrees with pre-
vious esterification studies (
Venkateswarlu et al., 1976; Rao
et al., 1976; Sai, 1988; Lee et al., 1999; Awad et al., 1997;
0
0.1
0.2
0.3
0.4
0.5
0.6
0
3600
7200
10800
14400
18000
Time (sec)
Conversion of propionic acid
303.15 K
313.15 K
323.15 K
333.15 K
Fig. 8. Effect of temperature on the conversion of propionic acid at 900 rpm,
1:1 propionic acid to 1-propanol molar ratio and 40 g dry cat/L catalyst loading
of Dowex 50Wx8-400.
). The temperature 323.15 K was se-
lected as the standard temperature for subsequent runs because
it is high enough to yield a high conversion, while gas-bubble
formation is insignificant.
4.3.6. Effect of acid to alcohol molar ratio
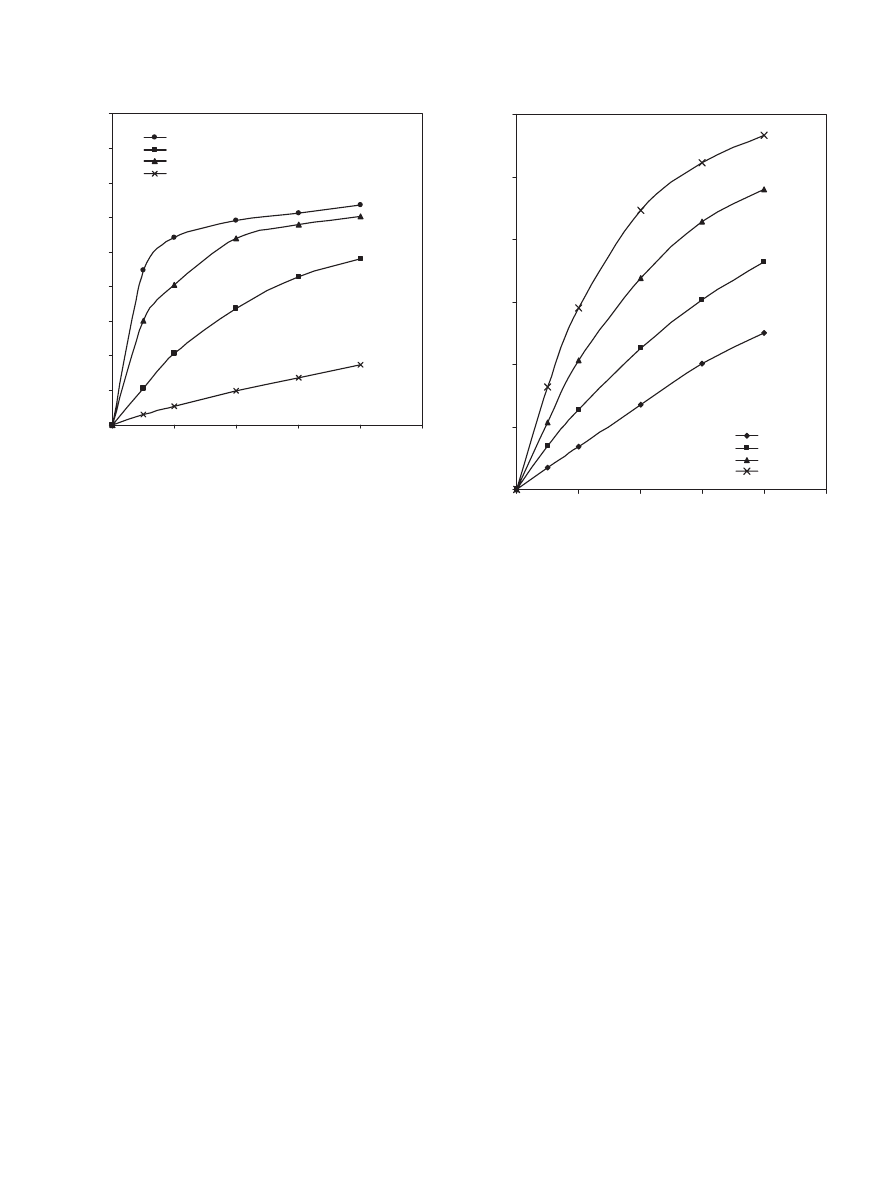
The effect of acid to alcohol molar ratio was investigated
by varying the acid to alcohol molar ratio (ranging from 1:4
to 4:1). From
it is obvious that increasing the amount
of alcohol initially increases the conversion of propionic acid.
After 4 h of reaction time the conversion of propionic acid for
the run with acid to alcohol molar ratio of 1:4 is approximately
17% more than that for the run with a ratio of 1:2 and 32%
more than that for the run with a ratio of 1:1.
, who studied the esterification reaction of 1-propa-
nol with propionic acid over Dowex-50W, found that the con-
version of acid increased with increasing initial alcohol to acid
molar ratio. Also, some other workers (
2000; Yadav and Thathagar, 2002
) found that the conversion
of acid increases with decreasing the acid to alcohol molar ra-
tio.
is a re-plot of
but in terms of conversion
of the limiting reactant rather than propionic acid. As shown
in
, changing the molar ratio from 1:1 to 1:2 increases
the conversion of the limiting reactant. A further increase in
the amount of alcohol initially leads to a higher conversion
of the limiting reactant. Also, increasing the molar ratio from
1:1 to 2:1 increases the conversion of the limiting reactant
and a further increase in the acid to alcohol initial molar ra-
tio leads to a more significant increase in the conversion of the
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
3209
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0
3600
7200
10800
14400
18000
Time (sec)
Conversion of propionic acid
1 : 1
1 : 2
2 : 1
4 : 1
1 : 4
Fig. 9. Effect of propionic acid to 1-propanol molar ratio on the conversion
of propionic acid at 323 K, 900 rpm and 40 g dry cat/L catalyst loading of
Dowex 50Wx8-400.
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0
3600
7200
10800
14400
18000
Time (sec)
Conversion of the limiting reactant
1 : 1
1 : 2
1 : 4
2 : 1
4 : 1
Fig. 10. Effect of propionic acid to 1-propanol molar ratio on the conversion
of the limiting reactant at 323 K, 900 rpm and 40 g dry cat/L catalyst loading
of Dowex 50Wx8-400.
limiting reactant.
shows that acid to alcohol molar ratio
of 2:1 gives a higher conversion of the limiting reactant than
1:2. Also, a more significant increase in conversion was ob-
tained at a ratio of 4:1 rather than 1:4. As a result, conversion
of the limiting reactant is higher in cases different than 1:1 and
a more significant increase in conversion of the limiting reac-
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0
3600
7200
10800
14400
18000
Time (sec)
Conversion of propionic acid
run with water (acid : alcohol : water = 1 : 1 : 1)
run without water (acid : alcohol = 1 : 1)
Fig. 11. Effect of adding water initially on the conversion of propionic
acid at 323 K, 900 rpm, 1:1 propionic acid to 1-propanol molar ratio and
40 g dry cat/L catalyst loading of Dowex 50Wx8-400.
tant is obtained by increasing the initial amount of acid than
increasing the initial amount of alcohol.
4.3.7. Effect of adding water to the reactants mixture
The effect of adding water to the reactant mixture on the
conversion of propionic acid is shown in
. A compari-
son between two experiments was made over a time period of
4 h, under the same conditions. However, one experiment was
done in the presence of initial amounts of water in the reaction
mixture (acid: alcohol: water equals 1:1:1). It is obvious from
that adding water decreased the conversion of propionic
acid. This observed decrease is due to several reasons. Water
is one of the products for this esterification reaction, so adding
water initially will promote the reverse reaction. In addition,
water is found to have a very strong affinity for cation-exchange
resins such as Dowex 50Wx8-400 (
). Also,
adsorption of water is most likely higher than the other species
present in the system.
studied methyl acetate
esterification over Amberlyst-15 and measured the adsorption
of the different species present in their system relative to the
adsorption of methyl acetate. They found that water is adsorbed
more strongly than the other reactants and products, which fur-
ther support our results. Therefore, the initial presence of water
in the system will lead to lower conversions.
4.3.8. Effect of using different acids
The study of the esterification of 1-propanol with different
acids (acetic, butyric and propionic) is represented in
This figure shows that the conversion of propionic acid de-
creased with increasing the acid chain length due to steric hin-
drance. It was found that at 4 h of reaction, the conversion of
acetic acid was 62%, propionic acid 48% and butyric acid 31%.
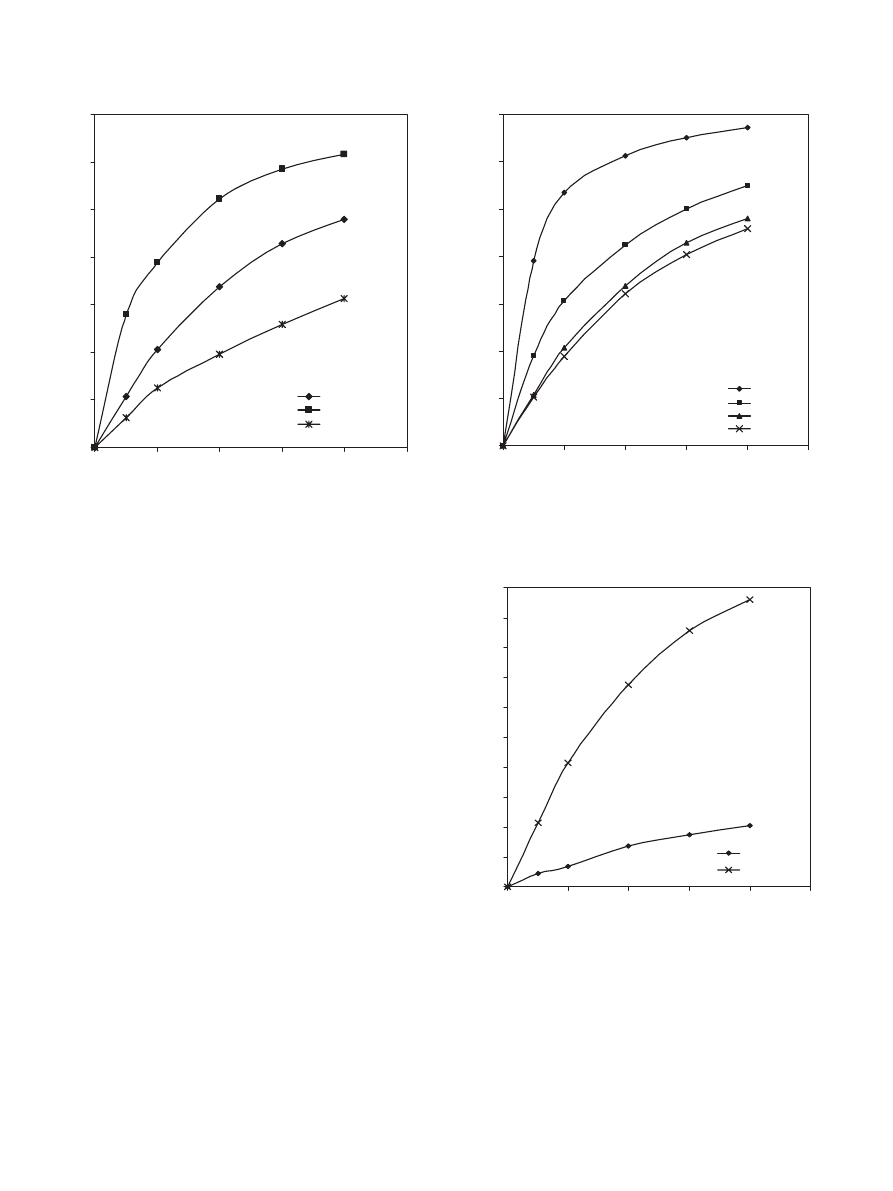
3210
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0
3600
7200
10800
14400
18000
Time (sec)
Conversion of acid
Propionic acid
Acetic acid
Butyric acid
Fig. 12. Effect of using different acids on the conversion of the acid at 323 K,
900 rpm, 1:1 acid to 1-propanol molar ratio and 40 g dry cat/L catalyst loading
of Dowex 50Wx8-400.
This implies that the conversion and hence the reaction rate in-
creases as the chain length decreases. A similar conclusion was
made by
4.3.9. Effect of using different alcohols
The effect of different alcohols on the conversion of pro-
pionic acid was investigated by running multiple experiments
using different alcohols (methanol, ethanol, 1-propanol and 1-
butanol) and holding other parameters constant. Increasing the
chain length of the alcohol decreased the conversion of propi-
onic acid, as indicated in
. The propionic acid conversion
at 4 h of reaction was 67%, 55%, 48%, 46% when reacting with
methanol, ethanol, 1-propanol and 1-butanol, respectively. The
hindrance effect is the main reason for the decrease in the con-
version of propionic acid as the alcohol chain length increases.
and
results agree with our
results. These authors concluded that alcohol chain length had
a retarding effect on the overall reaction rate. The esterification
of propionic acid with 1-propanol and 2-propanol over Dowex
50Wx8-400 was investigated and the results are shown in
. As shown in
, the reaction of propionic acid with
1-propanol had a higher conversion than the reaction of the
propionic acid with 2-propanol. At 4 h of reaction, it was found
that the conversion of propionic acid reacting with 1-propanol
is 48% while the conversion of propionic acid reacting with
2-propanol is 10%. This indicates that branching of the alcohol
had a retarding effect on the conversion and hence the reaction
rate due to steric hindrance.
found that
the rate of esterification reaction of propionic acid with linear
alcohols was higher than that of branched ones.
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0
3600
7200
10800
14400
18000
Time (sec)
Conversion of propionic acid
methanol
ethanol
1-propanol
1-butanol
Fig. 13. Effect of using different alcohols on the conversion of propionic acid
at 323 K, 900 rpm, 1:1 propionic acid to alcohol molar ratio and 40 g dry cat/L
catalyst loading of Dowex 50Wx8-400.
0
0.05
0.1
0.15
0.2
0.25
0.3
0.35
0.4
0.45
0.5
0
3600
7200
10800
14400
18000
Time (sec)
Conversion of propionic acid
2-propanol
1-propanol
Fig. 14. Effect of using 2-propanol versus 1-propanol on the conversion of
propionic acid at 323 K, 900 rpm, 1:1 propionic acid to alcohol ratio and
40 g dry cat/L catalyst loading of Dowex 50Wx8-400.
, who studied the esterification of acetic and propionic
acids with different alcohols over Smopex-101, as a catalyst,
demonstrated that branching of the alcohol chain decreased
the reaction rate. In contrast,
found
that branching in the hydrocarbon chain length of an aliphatic
primary alcohol had insignificant effect on the esterification

S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
3211
Table 6
The UNIFAC groups present in the different components and their R and Q
values
Group
CH
3
CH
2
COOH
OH
H
2
O
CH
2
COO
Propionic acid
1
1
1
0
0
0
1-Propanol
1
2
0
1
0
0
Propyl propionate
2
2
0
0
0
1
Water
0
0
0
0
1
0
R
0.9011
0.6744
1.3013
1
0.92
1.6764
Q
0.848
0.54
1.224
1.2
1.4
1.42
reaction of acetic acid with monohydric alcohols (1-butanol,
2-butanol, 1-hexanol, cyclo-hexanol and benzyl alcohol) using
Amberlite IR-120 as a catalyst.
4.4. Modeling
From the different kinetic runs involved in this investiga-
tion, 10 runs were selected for modeling. These runs (4, 10,
11 and 15–21) represented the reaction of propionic acid with
1-propanol catalyzed by Dowex 50Wx8-400 at different re-
action conditions. Kinetic data from these selected runs are
fitted to 40 rate expressions as discussed previously. The activ-
ity coefficients of the reactants and the products used in these
equations were determined using the UNIFAC model. In this
investigation, UNIFAC was incorporated into the used Mathe-
matica software. To calculate the UNIFAC activity coefficients
in our system, the four components present in the system were
divided into the following sub-groups as reported in
propionic acid (1 CH
3
, 1 CH
2
and 1 COOH); 1-propanol (1
CH
3
, 2 CH
2
and 1 OH); 1-propyl propionate (2 CH
3
, 2 CH
2
and 1 CH
2
COO); water (1 H
2
O).
Experimental data fitted to 40 rate expressions were tested
using Mathematica software (Statistics Nonlinear fit function).
The aim of this fitting is to minimize the mean-square differ-
ences between calculated values of rate (r
calc
obtained from
Mathematica) with values obtained from experimental data
(r
exp
) as shown below:
min
=
all data samples
(r
calc
− r
exp
)
2
,
(22)
where the experimental data of mole fraction of limiting com-
ponent were fitted as a function of time (x
exp
=f (t)). Then this
function was differentiated in order to obtain the experimental
rate (r
exp
) as follows:
r
exp
= −n
dx
exp
dt
.
(23)
This data-fitting was done to obtain the model parameters. Some
of these models gave negative values for the adsorption equi-
librium constants (K
acid
, K
alc
, K
ester
and/or K
water
) and/or the
pre-exponential factor (A
f
). As a result, out of 40 models only
17 models might be suitable for describing the reaction kinet-
ics.
list the number and five parts name assigned
for these 40 models. The first part is for the number of sites
involved, either dual (Dl) or single (Sg). The second part is for
T
able
7
Kinetic
parameters
for
the
P-H
and
the
dif
ferent
dual-site
models
Model
#
Model
A
f
E
f
k
a
(mol/g/s)
K
acid
K
alc
K
ester
K
w
ater
T.
Av
g
.
(mol/g/s)
(J/mol)
Error
303.15
K
313.15
K
323.15
K
333.15
K
1P
H
2
.61E
+
06
66,342
9.7E
−
06
2.2E
−
05
4.9E
−
05
1.0E
−
04
–
–
–
–
2.26
2
Dl-Ac
Alc-Ads-Ac-
13
.16E
+
08
83,557
1.3E
−
06
3.6E
−
06
9.8E
−
06
2.5E
−
05
2.00
−
0.99
0.00
0.41
–
3
Dl-Ac
Alc-Ads-Ac-
28
.60
E
+
08
86,279
1.2E
−
06
3.5E
−
06
9.7E
−
06
2.5E
−
05
4.00
−
0.98
0.01
1.09
–
4
Dl-Ac
Alc-Ads-Ac-
31
.09E
+
09
86,955
1.1E
−
06
3.4E
−
06
9.6E
−
06
2.5E
−
05
3.00
−
0.99
0.10
1.91
–
5
Dl-Ac
Alc-Ads–Alc-
14
.93E
+
09
91,422
8.7E
−
07
2.8E
−
06
8.2E
−
06
2.3E
−
05
−
1.05
−
5.30
−
14.93
10
–
6
Dl-Ac
Alc-Ads-Alc-
28
.71E
+
20
159,373
3.0E
−
07
2.3E
−
06
1.5E
−
05
8.9E
−
05
2
−
6
.59E
+
22
1.00
10.04
–
7
Dl-Ac
Alc-Ads–Alc-
38
.50
E
+
10
99,342
6.5E
−
07
2.3E
−
06
7.4E
−
06
2.3E
−
05
−
1.09
−
1.67
−
0.99
9.80
–
8
Dl-Ac
Alc-Rxn-Ac
Alc-
17
.12E
+
06
65,720
3.4E
−
05
7.8E
−
05
1.7E
−
04
3.5E
−
04
1.00
1.57
0.90
2.00
4.33
9
Dl-Ac
Alc-Rxn-Ac
Alc-
21
.05E
+
07
68,613
1.6E
−
05
3.8E
−
05
8.5E
−
05
1.8E
−
04
2.60
3.00
1.50
6.00
2.63
10
Dl-Ac
Alc-Rxn-Ac
Alc-
31
.15E
+
07
68
549
1.8E
−
05
4.2E
−
05
9.5E
−
05
2.0E
−
04
1.80
2.48
0.70
6.28
1.83
11
Dl-Ac
Alc-Des-Wt-
13
.42E
+
05
74,676
4.6E
−
08
1.2E
−
07
2.9E
−
07
6.7E
−
07
−
0.13
0.37
−
0.76
0.03
–
12
Dl-Ac
Alc-Des-Wt-
25
.57E
+
04
57,390
7.2E
−
06
1.5E
−
05
2.9E
−
05
5.6E
−
05
2.00
3.00
1.30
6.00
5.78
13
Dl-Ac
Alc-Des-Wt-
33
.01E
+
05
70,064
2.5E
−
07
6.2E
−
07
1.4E
−
06
3.1E
−
06
−
0.25
0.25
−
1.372
0.13
–
14
Dl-Ac
Alc-Des-Es-
13
.42E
+
05
70,000
3.0E
−
07
7.2E
−
07
1.7E
−
06
3.6E
−
06
−
0.21
0.46
0.17
−
1.08
–
15
Dl-Ac
Alc-Des-Es-
2
−
v
e
82,915
––––
−
0.04
0.88
−
5.58E
−
12
−
0.38
–
16
Dl-Ac
Alc-Des-Es-
3
2.32E
−
01
50,606
4.4E
−
10
8.4E
−
10
1.5E
−
09
2.7E
−
09
0.37
−
0.13
0.00
−
1.30
–
a
Reaction,
adsorption
or
desorption.
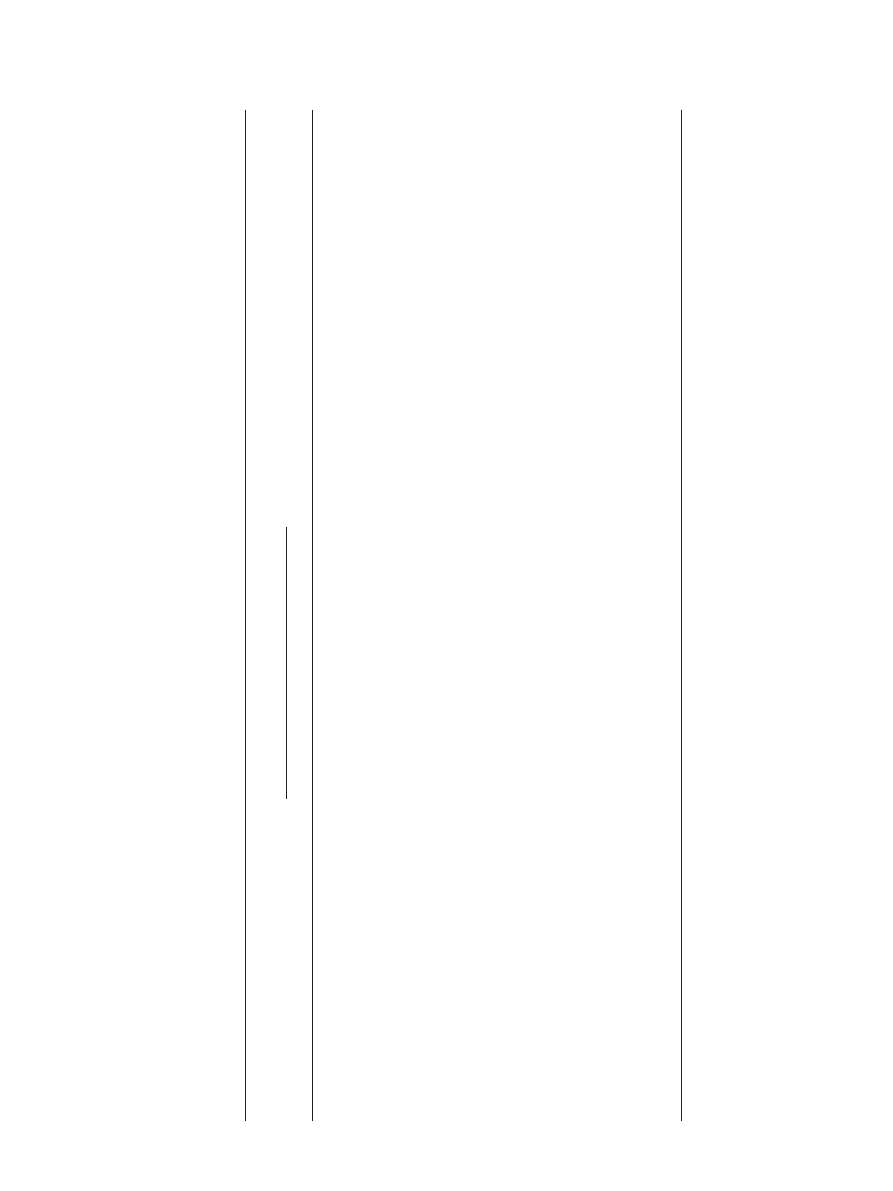
3212
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
T
able
8
Kinematic
parameters
for
the
dif
ferent
single-site
models
Model
Model
A
f
E
f
k
a
(mol/g/sec)
K
acid
K
alc
K
ester
K
w
ater
T.
Av
g
.
#
(mol/g/s)
(J/mol)
Error
303.15
K
313.15
K
323.15
K
333.15
K
17
Sg-Ac-Ads-Ac-
17
.33E
+
11
103,119
1.2E
−
06
4.6E
−
06
1.6E
−
05
5.0E
−
05
−
8
.04E
+
13
–
–
1.94
–
18
Sg-Ac-Ads-Ac-
29
.44E
+
12
110,217
9.6E
−
07
3.9E
−
06
1.4E
−
05
4.9E
−
05
2.33
–
–
3.82
11.37
19
Sg-Ac-Ads-Ac-
31
.42E
+
13
111,388
9.1E
−
07
3.7E
−
06
1.4E
−
05
4.9E
−
05
−
2
–
–
14.04
–
20
Sg-Ac-Rxn-Ac-
14
.22E
+
07
75,089
4.9E
−
06
1.3E
−
05
3.1E
−
05
7.1E
−
05
5.11
–
–
6.00
6.33
21
Sg-Ac-Rxn-Ac-
2
−
v
e
66,223
––––
−
0.23
–
–
−
0.57
–
22
Sg-Ac-Rxn-Ac-
3
−
v
e
65,441
––––
−
0.21
–
–
−
0.64
–
23
Sg-Ac-Des-Wt-
12
.72E
+
07
82,208
1.9E
−
07
5.3E
−
07
1.4E
−
06
3.5E
−
06
0.11
–
–
0.16
4.63
24
Sg-Ac-Des-Wt-
27
.94E
+
06
69,154
9.64E
−
06
2.31E
−
05
5.27E
−
05
1.14E
−
04
3.09
–
–
10.00
6.66
25
Sg-Ac-Des-Wt-
3
−
v
e
42,844
––––
−
1
.87E
+
10
–
–
−
7
.31E
+
10
–
26
Sg-Ac-Des–Es–
17
.33E
+
05
72,264
2.59E
−
07
6.47E
−
07
1.53E
−
06
3.42E
−
06
0.3
–
0.22
–
5.64
27
Sg-Ac-Des-Es-
22
.09E
+
06
82,760
1.15E
−
08
3.27E
−
08
8.74E
−
08
2.20E
−
07
0.076
–
0.013
–
5.71
28
Sg-Ac-Des-Es-
3
−
v
e
82,761
––––
0.03
–
−
0.15
–
–
29
Sg-Alc-Ads-Alc-
11
.85E
+
14
118,640
6.7E
−
07
3.0E
−
06
1.2E
−
05
4.6E
−
05
–
0.62
–
1.25
12.61
30
Sg-Alc-Ads-Alc-
21
.53E
+
15
124,541
5.3E
−
07
2.6E
−
06
1.1E
−
05
4.5E
−
05
–
2.00
–
3.59
13.32
31
Sg-Alc-Ads-Alc-
33
.29E
+
15
126,723
4.8E
−
07
2.4E
−
06
1.1E
−
05
4.4E
−
05
–
−
7
.00
E
+
17
–
9.17
–
32
Sg-Alc-Rxn-Alc-
17
.77E
+
06
66,939
2.3E
−
05
5.3E
−
05
1.2E
−
04
2.5E
−
04
–
0.55
–
0.70
2.05
33
Sg-Alc-Rxn-Alc-
28
.00
E
+
06
67,322
2.0E
−
05
4.7E
−
05
1.0E
−
04
2.2E
−
04
–
0.60
–
0.80
1.65
34
Sg-Alc-Rxn-Alc-
35
.95E
+
06
67,607
1.3E
−
05
3.1E
−
05
7.0E
−
05
1.5E
−
04
–
1.00
–
1.35
1.77
35
Sg-Alc-Des-Wt-
1
−
v
e
59,723
––––
–
−
1
.52E
+
08
–
−
3
.82E
+
08
–
36
Sg-Alc-Des-Wt-
2
−
v
e
55,561
––––
–
−
2
.32E
+
08
–
−
7
.24E
+
08
–
37
Sg-Alc-Des-Wt-
3
−
v
e
54,002
––––
–
−
6
.22E
+
08
–
−
2
.44E
+
09
–
38
Sg-Alc-Des-Es-
1
−
v
e
57,298
––––
–
−
2
.24E
+
08
−
5
.65E
+
08
–
–
39
Sg-Alc-Des-Es-
22
.20
E
+
06
82,715
1.2E
−
08
3.5E
−
08
9.4E
−
08
2.4E
−
07
–
0.08
0.01
–
4.71
40
Sg-Alc-Des-Es-
3
−
v
e
82,757
––––
–
0.03
−
0.15
–
–
a
Reaction,
adsorption
or
desorption.

S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
3213
Table 9
Errors obtained while fitting experimental runs with different models
Model #
Run no.
4
10
11
15
16
17
18
19
20
21
T.Avg.Error
Catalyst loading (g dry cat/L)
40
20
60
40
40
40
40
40
40
40
Temperature (K)
323.15
323.15
323.15
303.15
313.15
333.15
323.15
323.15
323.15
323.15
Acid:alcohol rounded molar ratio
1:1
1:1
1:1
1:1
1:1
1:1
2:1
1:2
1:4
4:1
Models tested
Individual error of each experimental run
1
PH
1.07
1.74
2.17
1.21
1.06
2.39
1.05
5.54
5.10
1.28
2.26
8
Dl-Ac Alc-Rxn-Ac Alc-
1
6.19
3.62
10.62
0.98
1.15
9.52
0.91
3.06
6.01
1.27
4.33
9
Dl-Ac Alc-Rxn-Ac Alc-
2
3.44
1.76
7.30
1.12
0.99
5.54
0.81
2.17
2.32
0.86
2.63
10
Dl-Ac Alc-Rxn-Ac Alc-
3
1.13
1.03
4.14
1.39
1.35
1.93
2.43
3.37
1.18
0.37
1.83
12
Dl-Ac Alc-Des-Wt-
2
4.61
6.16
4.37
2.38
2.80
5.45
1.70
5.99
22.67
1.64
5.78
18
Sg-Ac-Ads-Ac-
2
12.35
8.78
17.62
7.49
9.32
3.72
1.72
17.75
29.79
5.14
11.37
20
Sg-Ac-Rxn-Ac-
1
6.96
4.71
11.09
3.17
2.36
6.96
4.32
4.77
14.22
4.77
6.33
23
Sg-Ac-Des-Wt-
1
4.23
3.47
5.80
3.49
2.63
0.74
1.03
6.05
18.09
0.78
4.63
24
Sg-Ac-Des-Wt-
2
4.42
6.45
2.57
3.86
4.16
3.11
1.98
8.87
29.43
1.77
6.66
26
Sg-Ac-Des-Es-
1
3.59
4.35
3.04
3.14
2.50
4.66
1.30
8.45
24.26
1.12
5.64
27
Sg-Ac-Des-Es-
2
5.02
4.09
7.14
4.14
3.54
0.43
0.72
6.64
24.17
1.22
5.71
29
Sg-Alc-Ads-Alc-
1
14.37
10.54
18.97
8.06
11.00
1.49
10.30
6.96
36.12
8.26
12.61
30
Sg-Alc-Ads-Alc-
2
14.65
10.71
19.78
8.33
11.64
0.72
10.41
6.59
42.01
8.38
13.32
32
Sg-Alc-Rxn-Alc-
1
2.68
2.20
5.00
1.22
0.75
2.53
1.26
1.81
2.36
0.67
2.05
33
Sg-Alc-Rxn-Alc-
2
1.49
1.72
2.99
1.35
0.93
1.18
2.08
3.00
1.36
0.40
1.65
34
Sg-Alc-Rxn-Alc-
3
0.91
1.74
2.27
1.54
0.80
2.17
3.00
2.15
2.63
0.53
1.77
39
Sg-Alc-Des-Es-
2
4.18
3.40
5.94
3.44
2.94
0.35
0.67
5.37
19.68
1.09
4.71
the reactants being adsorbed; acid (Ac) and/or alcohol (Alc).
The third part is for the controlling step; adsorption (Ads), sur-
face reaction (Rxn) or desorption (Des). The fourth part is for
the controlling component. The fifth part is for the correction
factor for the resin affinity for water (
= 1, 2 or 3). The total
average error (T.Avg.Error) between the predicted and experi-
mental mole fractions of acid was calculated as follows:
T.Avg.Error
=
all data samples
|x
exp
− x
pred
|/x
exp
n
samples
× 100.
(24)
show the total average error obtained for these 17
models which ranged between 1.65% and 13.32%. For all of
these models (except the P-H model), the same trend of adsorp-
tion equilibrium constant exists K
water
> K
alc
> K
acid
> K
ester
(see
). The trend of our calculated adsorption
equilibrium constants agrees with the trend in solubility pa-
rameter of the components as has been suggested in our earlier
work (
). The solubility parameter val-
ues obtained from the AICHE DIPPER
䉸
at 25
◦
C are 47.81,
24.45, 19.49 and 17.57 (J/cm
3
)
0.5
for water, 1-propanol, propi-
onic acid and 1-propyl propionate, respectively.
shows
that there are only four models (models # 10, 32, 33 and 34)
which perform better than the simplest possible model namely
the P-H model (model # 1 with T. Avg. Error of 2.26%). These
four models have total average error values below 2.26%. All
these models are surface reaction controlling models with dif-
ferent reaction mechanisms. The mechanism of three of these
models is a single site (E–R and M-E–R) where 1-propanol is
being adsorbed reacting with non-adsorbed propionic acid re-
sulting in adsorbed water and non-adsorbed ester with different
water adsorption affinities (
= 1, 2 or 3), and the mechanism
for the other model is a dual site (M-L–H) where both propi-
onic acid and 1-propanol are being adsorbed with a water ad-
sorption affinity of 3 (
= 3). The total average errors of these
four models are 2.05%, 1.65%, 1.77% and 1.83%, respectively.
The activation energy (E
f
) for the five potential models varied
between 66.3 and 68.5 kJ/mol while the pre-exponential factor
(A
f
) varied between 2.61E + 06 and 1.15E + 07 mol/g/s.
To select the best model representing the data, the individual
errors (as reported in
) for each model were compared
in addition to the accuracy of prediction of equilibrium mole
fractions by each model. The equilibrium mole fractions were
obtained from the kinetic models by using the fitted parameters
to predict the mole fractions at large enough times where the
system is considered to be in an equilibrium status. As shown
in
, the P-H model had an error around 5% in two
runs (runs # 19 and 20). The M-L–H (model # 10), where the
surface reaction is rate controlling and the water adsorption
affinity is 3 (
= 3), gave a bad prediction of the equilibrium
mole fractions. The three remaining models (models # 32, 33
and 34) are single-site reaction mechanism models where 1-
propanol is being adsorbed with the surface reaction being the
rate controlling step. The difference between these models is
the correction term representing the water adsorption affinity
(
= 1, 2 or 3). All three models give a good prediction for
the equilibrium mole fraction at different reaction temperatures
with errors less than 1.5%.
Since one of the aims of this work is to arrive at the reac-
tion mechanism which best describes the kinetics of this het-
erogeneous system over the wide range of conditions studied
(this includes different temperatures, acid to alcohol molar ra-
tios and catalyst loadings), the standard deviation of the indi-
vidual error of each experimental run around the total average
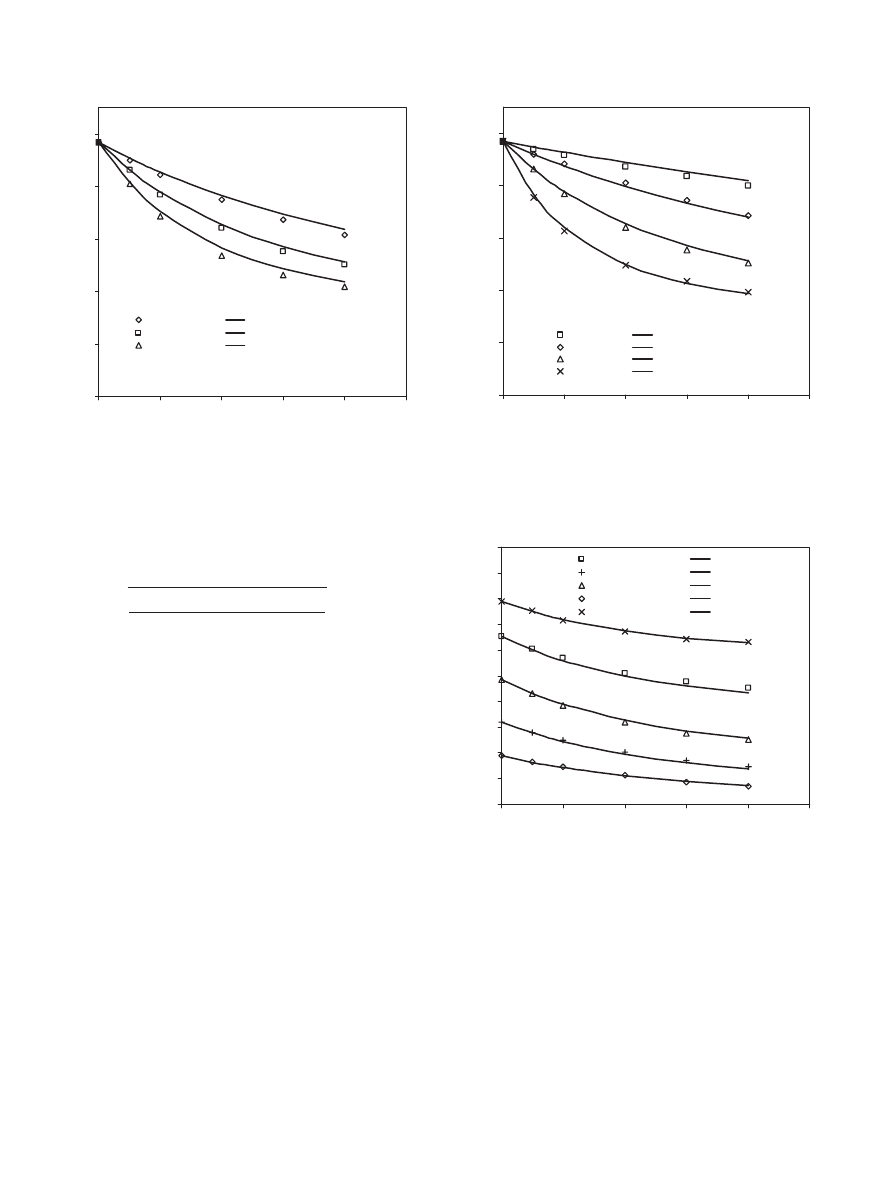
3214
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
0
0.1
0.2
0.3
0.4
0.5
0
3600
7200
10800
14400
18000
Time (sec)
Mole fraction of propionic acid
Exp.; 20 g dry cat/L
Sg-Al-Rxn-Al-a2; 20g dry cat/L
Exp.; 40 g dry cat/L
Sg-Al-Rxn-Al-a2; 40g dry cat/L
Exp.; 60 g dry cat/L
Sg-Al-Rxn-Al-a2; 60g dry cat/L
Fig. 15. Experimental versus predicted (by the best model; model # 33) mole
fraction of propionic acid as a function of time at different catalyst loadings
of Dowex 50Wx8-400 at 323 K, 900 rpm, 1:1 propionic acid to 1-propanol
molar ratio.
error (STDEV) were performed for models # 32, 33 and 34
according to the following equation:
STDEV
=
(Individ.Error − T.Avg.Error)
2
(N − 1)
,
(25)
where Individ.Error is the individual error of each experimental
run, T.Avg.Error is the total average error, and N is the number
of experimental runs considered in the modeling process.
STDEV for models # 32, 33 and 34 were found to be equal
to 1.3, 0.8 and 0.8, respectively. This indicates that models #
33 and 34 are better than model # 32 in predicting reaction
kinetics. Though the standard deviation values of models # 33
and 34 are comparable to one another, the total average error
of model # 33 is lower than that of model # 34, 1.65% versus
1.77%, respectively. Hence model # 33, a single-site M-E–R
model wherein adsorbed 1-propanol reacts with non-adsorbed
propionic acid resulting in non-adsorbed ester and adsorbed
water (with
= 2), is selected as the model best capable of
describing the studied reaction kinetics.
Interestingly, earlier workers (
) have also proposed single-site mechanisms
for acidic ion-exchange resin catalyzed esterifications involv-
ing propionic acid.
, who studied
the propyl propionate esterification reaction over Dowex-50W,
proposed that the rate controlling step was the surface reac-
tion (involving a single site) between adsorbed propionic acid
reacting with 1-propanol in the bulk. However, no such mathe-
matical expression was presented; but rather, an empirical rela-
tionship correlating the specific reaction rate constant in terms
of the studied variables was reported. Furthermore,
found that their heterogeneously catalyzed esterification
reaction over Amberlyst 35 follows the E–R theory in which
0
0.1
0.2
0.3
0.4
0.5
0
3600
7200
10800
14400
18000
Time (sec)
Mole fraction of propionic acid
Exp.; 303.15 K
Sg-Al-Rxn-Al-a2; 303.15K
Exp.; 313.15 K
Sg-Al-Rxn-Al-a2; 313.15K
Exp.; 323.15 K
Sg-Al-Rxn-Al-a2; 323.15K
Exp.; 333.15 K
Sg-Al-Rxn-Al-a2; 333.15K
Fig. 16. Experimental versus predicted (by the best model; model # 33) mole
fraction of propionic acid as a function of time at different temperatures, cat-
alyst loading 40 g dry cat/L of Dowex 50Wx8-400, 900 rpm and 1:1 propionic
acid to 1-propanol molar ratio.
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0
3600
7200
10800
14400
18000
Time (sec)
Mole fraction of propionic acid
Exp. Ratio; 2 : 1
Sg-Al-Rxn-Al-a2; 2 : 1
Exp. Ratio; 1 : 2
Sg-Al-Rxn-Al-a2; 1 : 2
Exp Ratio; 1 : 1
Sg-Al-Rxn-Al-a2; 1 : 1
Exp. Ratio; 1 : 4
Sg-Al-Rxn-Al-a2; 1 : 4
Exp. Ratio; 4 :1
Sg-Al-Rxn-Al-a2; 4 : 1
Fig. 17. Experimental versus predicted (by the best model; model # 33) mole
fraction of propionic acid as a function of time at different propionic acid to
1-propanol molar ratios at 323 K, catalyst loading of 40 g dry cat/L of Dowex
50Wx8-400 and 900 rpm.
adsorbed propionic acid reacts with 1-butanol in the bulk. They
(
) assumed that the rate controlling step is the
adsorption step involving the acid species. However, there are
some objections regarding the values of the reported adsorption
equilibrium constants for propionic acid and water, which were
fitted as a function of temperature. One of these objections re-
garding the zero values of the adsorption equilibrium constants
for propionic acid and water at 353 K, which are not reasonable.
Another one is related to the un-explained and non-systematic
variations and changes in the values of the propionic acid
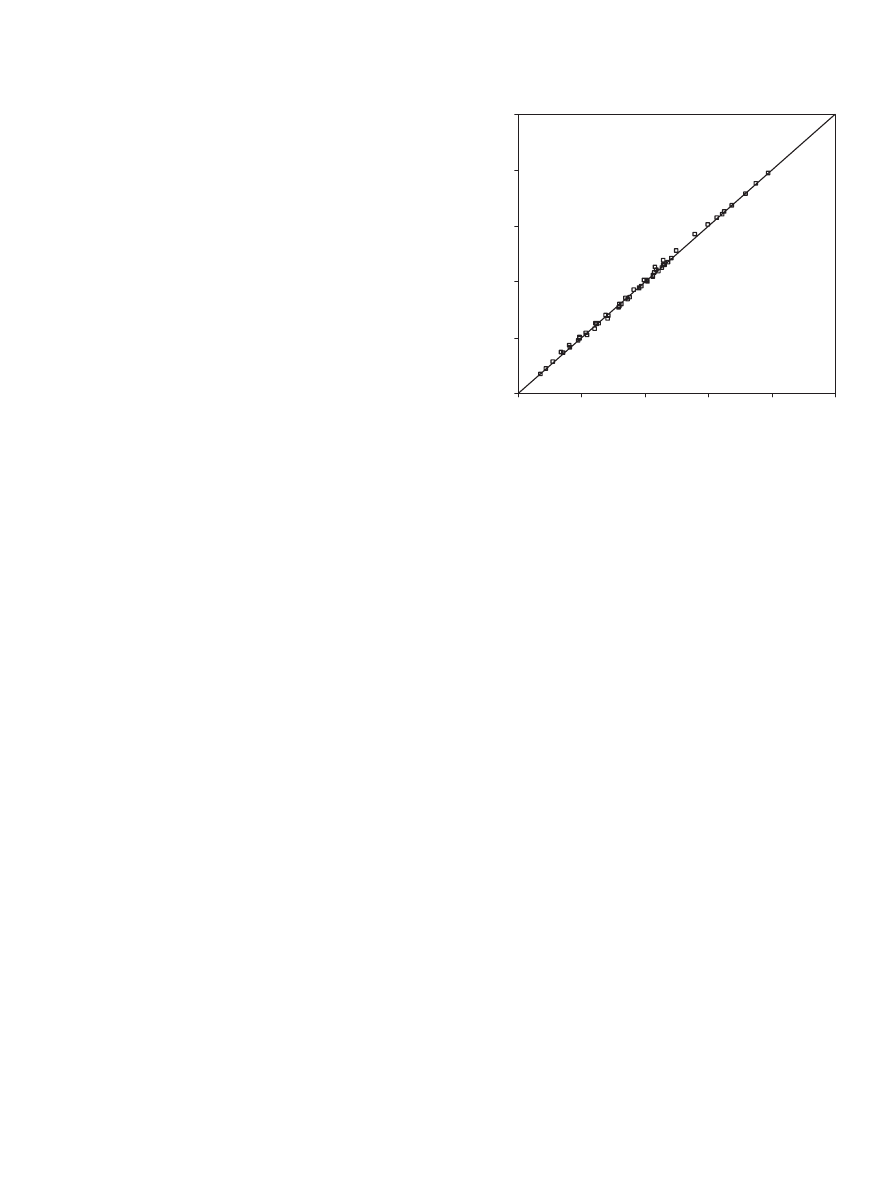
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
3215
adsorption equilibrium constants as a function of temperature.
In addition, for the temperature range from 363 to 383 K, they
(
) reported that the adsorption equilibrium
constants for propionic acid are higher than that for water,
which is opposite to what is expected.
ex-
perimentally determined the adsorption equilibrium constants
for the species involved in methyl acetate synthesis using Am-
berlyst 15. They (
) found that the adsorp-
tion equilibrium constant for water was higher than acetic acid.
Also,
found the same to be true for the
same heterogeneously catalyzed system. In addition, in our pre-
vious work (
) we have found that the
adsorption equilibrium constants for the different species are
proportional to their solubility parameters (as found in this cur-
rent study and mentioned previously), which implies that the
adsorption constant for water is expected to be higher in value
than for propionic acid. Furthermore, both
and
suggest mechanisms wherein
the acid is selectively adsorbed on the catalyst and the alcohol
remains in the bulk phase. This scenario is not plausible con-
sidering the fact that the catalysts involved are sulfonic acid
ion-exchange resins and alcohols are known to be apprecia-
bly sorbed on such catalysts (
Song et al., 1998; Pöpken et al., 2000
). Moreover, the model
proposed by
which is represented
by model # 20 (Sg-Ac-Rxn-Ac-
1; single-site acid adsorbed-
surface reaction controlled, with
equal to 1) in this investiga-
tion showed a total average error of 6.33%. This error is signifi-
cantly higher than for model # 33 for propyl propionate synthe-
sis over Dowex 50Wx8-400 suggesting that the latter model is
better in predicting the reaction kinetics at different conditions.
The activation energy for the forward reaction was estimated
to be 67.3 kJ/mol. This value seems reasonable since it is quite
comparable (even though it is for a different catalyst) to the
value of 63.7 kJ/mol reported by
for the es-
terification of propionic acid and 1-butanol over Amberlyst 35,
which was best represented by L–H model. On the other hand,
the value of the activation energy of 39.1 kJ/mol reported by
for the esterification reaction of 1-
propanol and propionic acid over Dowex-50W is significantly
lower than our value. In fact, this value (39.1 kJ/mol) is sig-
nificantly smaller than the reported value (activation energy of
54.3 kJ/mol) for even less hindered esterification system stud-
ied by
for propyl acetate synthesis
by Dowex-50W.
The best model results were compared with the experimental
values for different reaction conditions in
. These
figures show comparisons between the experimental and the
predicted (by the best model; model # 33) mole fraction of
propionic acid at different catalyst loading, temperature and
acid to alcohol molar ratio. The M-E–R model (model # 33) was
effective in predicting the mole fraction of propionic acid for the
reaction system under different reaction conditions as shown
in
. In addition, the mole fraction of propionic acid
was accurately predicted by model # 33 as can be seen in
. This parity plot clearly goes along with our finding
(low total average error of 1.65% with STDEV of 0.8).
0
0.2
0.4
0.6
0.8
1
0
0.2
0.4
0.6
0.8
1
Calculated propionic acid mole fraction
Experimental propionic acid mole fraction
Fig. 18. Parity plot for the experimental versus calculated (using the best
model; model # 33) propionic acid mole fraction.
An attempt was made to further improve the prediction ca-
pability of the models by considering the correction factor
as
a variable rather than a constant value. However, it was found
that the model prediction by M-E–R model gave the best pre-
diction at
= 2 and gave slightly less accurate predictions for
other values of
ranging between 1 and 3. Therefore, it seems
that the optimum value of
is 2 as presented by model # 33.
5. Conclusions
The esterification reaction of propionic acid with 1-propanol
was successfully carried out over Dowex 50Wx8-400. The
experimental results indicated that, under the implemented
reaction conditions, Dowex 50Wx8-400 was a more effective
catalyst for this reaction compared with the other ion-exchange
resins tested, namely Amberlite IR-120 and Amberlyst 15.
A stirrer speed of 200 rpm or higher, in the used 1 L reactor,
was found to be effective for eliminating external diffusion ef-
fect. The significance of internal mass transfer limitation was
quantified by the use of the Weisz–Prater criterion for exper-
iments carried at different catalyst concentration, temperature
and acid to alcohol molar ratio. It was found that the internal
diffusion effect was negligible (C
W P
>1) under the employed
esterification reaction conditions. The conversion of acid in-
creased with increasing temperature and catalyst loading and
decreased with increasing initial amount of acid. The increase
in the acid chain length had a retarding effect on the conver-
sion of acid. Similarly, an increase in alcohol chain length or
branching decreased conversion. Adding water initially to acid
and alcohol mixture also decreased the conversion. The stan-
dard enthalpy change of reaction was found to be 6.4 kJ/mol.
This esterification reaction was found to be mildly exothermic.
Among various kinetic models employed in this investigation,

3216
S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
the reaction of propionic acid with 1-propanol catalyzed by
Dowex 50Wx8-400 was best presented by the modified M-E–R
model with a total average error of 1.65%. This model is based
on a controlling step of surface reaction between adsorbed 1-
propanol with non-adsorbed propionic acid forming 1-propyl
propionate and water (with a correction factor for the resin
affinity for water equal to 2). The activation energy for the for-
ward reaction was estimated to be 67.3 kJ/mol. UNIFAC was
used successfully to account for the non-ideal thermodynamic
behavior of the reactants and the products.
Notation
a
i
activity of component i in the liquid phase
a
mn
a parameter of the interaction coefficient be-
tween groups m and n
A
f
pre-exponential factor for the forward reac-
tion leading to ester formation, mol/g/s
b
mn
a parameter of the interaction coefficient be-
tween groups m and n
C
li
limiting reactant concentration in the mixture
at a given time, mol/cm
3
C
W P
Weisz–Prater parameter
D
e
effective diffusivity, cm
2
/s
D
li
diffusivity of limiting reactant in component
i, cm
2
/s
D
lm
diffusivity of limiting reactant in the mixture,
cm
2
/s
E–R
Eley–Rideal model
E
f
activation energy for the forward reaction
leading to ester formation, kJ/mol
H
0
R
standard enthalpy change of reaction, kJ/mol
Individ.Error
individual error of each experimental run
k
f
forward reaction rate constant for esterifica-
tion, mol/g/s
k
∗
forward reaction rate constant (adsorption,
desorption or surface reaction), mol/g/s
K
a
esterification reaction equilibrium constant
for the overall reaction
K
i
adsorption equilibrium constant for species i
present in the system
K
s
experimentally measured esterification reac-
tion equilibrium constant
K
SR
surface reaction equilibrium constant at a ref-
erence temperature
L–H
Langmuir–Hinshelwood model
M-E–R
modified Eley–Rideal model
M-L–H
modified Langmuir–Hinshelwood model
M
cat
mass of the catalyst, g
M
i
molar mass of component i, g/mol
n
total number of moles in the system, mol
n
samples
number of samples
N
total number of runs considered for modeling
P-H
pseudo-homogeneous model
Q
UNIFAC group area parameter
r
catalyst pellet radius, cm
r
A(obs)
observed reaction rate at a given time, mol/g
of catalyst/s
r
calc
calculated reaction rate, mol/s
r
exp
reaction rate determined from experimental
data, mol/s
R
UNIFAC group volume parameter
R
c
ratio of catalyst pellet volume to catalyst pel-
let external surface area, cm
R
2
fit
correlation coefficient for a fit
R
g
ideal gas law constant, 8.314 J/mol/K
STDEV
standard deviation
t
time, s
T
temperature, K
T.Avg.Error
total average error
T
R
reference temperature, K
V
i
molar volume of component i, cm
3
/mol
x
exp
experimental mole fraction
(x
i
)
eq
equilibrium mole fraction of component i
x
pred
predicted mole fraction
z
coordination number
Greek letters
exponential term accounting for water affin-
ity for the resin
i
activity coefficient of component i
C
i
combinatorial part of the activity coefficient
of component i
R
i
residual part of the activity coefficient of
component i
i
viscosity of component i, cp
m
viscosity of the mixture, cp
surface area fraction
v
void fraction of the catalyst
c
catalyst density, g/cm
3
mn
energy interaction parameter between com-
ponents m and n
k
activity coefficient of group k at mixture com-
position
i
k
activity coefficient of group k of pure com-
ponent i
volume fraction
References
Ali, S.H., Merchant, S.Q., 2006. Kinetics of the esterification of acetic
acid with 2-propanol: impact of different acidic cation exchange resins
on reaction mechanism. International Journal of Chemical Kinetics 38,
593–612.
Awad, M.M., Salem, A.M., Swelam, A.A., 1997. Kinetics of catalyzed
esterification of propionic acid with various alcohols using synthetic cation
exchange resin. Journal of the Indian Chemical Society 74, 467–469.
Bart, H.J., Kaltenbrunner, W., Landschutzer, H., 1996. Kinetics of
esterification of acetic acid with propyl alcohol by heterogeneous catalysis.
International Journal of Chemical Kinetics 28, 649–656.
Bhatia, S., Rajamani, K., Rajkhowa, P., Rao, M.G., 1973. Ion exchange resin
catalysis. Ion Exchange and Membrane 1, 127–133.
Chang, J.R., Yeh, M.Y., 1984. Kinetics of phase transfer catalytic preparation
of benzyl benzoate. Journal of the Chinese Chemical Society 31, 185–190.

S.H. Ali et al. / Chemical Engineering Science 62 (2007) 3197 – 3217
3217
Chiplunkar, M., Hong, M., Malone, M.F., Doherty, M.F., 2005. Experimental
study of feasibility in kinetically-controlled reactive distillation. A.I.Ch.E.
Journal 51 (2), 464–479.
Dakshinamurty, P., Ramarao, M.V.S., Ramachandramurty, Ch.V., 1984.
Kinetics of catalytic esterification of propan-1-ol with propanoic acid using
cation exchange resin. Journal of Chemical Technology and Biotechnology
34A, 257–261.
El Ewady, Y.A., El Wakil, A.M., El Nahas, M.R., Moussa, M.N.H., 1984.
Esterification of methanol with a homologous series of acids using
Amberlite IR-20. Journal of the Indian Chemical Society LXI, 517–519.
El-Noamany, H.M., Shaheen, F.A., El-Kenawy, O.S., Zaher, F.A., 1994.
Studies on some factors affecting the rate of esterification of acetic acid
with monohydric alcohols. Modeling Measurements and Control 43 (1),
25–43.
Fogler, H.S., 1992. Elements of Chemical Reaction Engineering. second ed..
Prentice-Hall, New York. pp. 607–647.
Gangadwala, J., Manker, S., Mahajani, S., 2003. Esterification of acetic acid
with butanol in presence of ion exchange resins as catalysts. Industrial
and Engineering Chemistry Research 42, 2146–2155.
Hansen, H.K., Coto, B., Kuhlmann, B., 1992. UNIFAC with linearly
temperature-dependant group-interactional parameters. Technical Report
(SEP 9212), IVC-SEP Research Engineering Center, Institute for
Kemiteknik, The Technical University of Denmark, Lyngby.
Krishnaiah, D., Rao, M.B., 1984. Kinetics of esterification of n-propyl alcohol
with acetic acid catalyzed by Dowex-50W. Indian Journal of Technology
22, 268–271.
Lee, M.J., Wu, H.T., Kang, C.H., Lin, H.M., 1999. Kinetic behavior of amyl
acetate synthesis catalyzed by acidic cation exchange resin. Journal of the
Chinese Institute of Chemical Engineers 30 (2), 117–122.
Lee, M.J., Wu, H.T., Lin, H.M., 2000. Kinetics of catalytic esterification
of acetic acid and amyl alcohol over Dowex. Industrial and Engineering
Chemistry Research 39, 4094–4099.
Lee, M.J., Chiu, J.Y., Lin, H.M., 2002. Kinetics of catalytic esterification of
propionic acid and n-butanol over Amberlyst 35. Industrial and Engineering
Chemistry Research 41, 2882–2887.
Lilja, J., Murzin, D.Yu., Salmi, T., Aumo, J., Mäki-Arvela, P., Sundell, M.,
2002. Esterification of different acids over heterogeneous and homogeneous
catalysts and correlation with the Taft equation. Journal of Molecular
Catalysis 182–183, 555–563.
Liu, W.T., Tan, C.S., 2001. Liquid-phase esterification of propionic acid with
n-butanol. Industrial and Engineering Chemistry Research 40, 3281–3286.
Liu, Y., Lotero, E., Goodwin Jr., J.G., 2006. Effect of water on sulfuric acid
catalyzed esterification. Journal of Molecular Catalysis A: Chemical 245,
132–140.
McKetta, J.J., 1983. Encyclopedia of Chemical Processing and Design, vol.
19. Marcel Dekker, New York, pp. 381–402.
Nagaraju, N., Mehboob, P., 1996. A comparison of catalytic activity of
zeolites with some Lewis acids in esterification reaction. Industrial Journal
of Chemical Technology 3, 253–255.
Namba, S., Wakushima, Y., Shimizu, T., Masumoto, H., Yashima, T., 1985.
Catalytic application of hydrophobic properties of high-silica zeolites.
Catalysis by Acids and Bases, 205–211.
Othmer, K., 1994. Encyclopedia of Chemical Technology, fourth ed. vol. 9.
Wiley, New York, pp. 781–811.
Pääkkönen, P.K., Krause, A.O.I., 2003. Diffusion and chemical reaction in iso-
amylene etherification within a cation exchange resin. Journal of Applied
Catalysis 245, 289–301.
Poling, B.E., Prausnitz, J.M., O’Connell, J.P., 2001. The Properties of Gases
and Liquids. fifth ed. McGraw-Hill, New York. pp. 8.75–8.111.
Pöpken, T., Götze, L., Gmehling, J., 2000. Reaction kinetics and chemical
equilibrium of homogeneously and heterogeneously catalyzed acetic acid
esterification with methanol and methyl acetate hydrolysis. Industrial and
Engineering Chemistry Research 39, 2601–2611.
Rao, B.A., Raghavaiah, C.V., Reddy, M.S., Chiranjivi, C., 1979. Esterification
of 1-butanol with propionic acid in a stirred tank batch reactor. Chemical
and Petrochemicals Journal, 7–12.
Rao, S.K.D.R., Sinha, R., Rao, R.J., 1978. Liquid phase catalytic esterification
of isopropanol with propionic acid. Chemical and Petrochemicals Journal,
11–14.
Rao, Y.V., Reddy, M.S., Rao, C.V., 1976. A study on the reaction kinetics of
n-octyl alcohol esterification with acetic acid in the presence of aluminum
sulphate catalyst. Chemical and Petrochemicals Journal, 27–35.
Rehfinger, A., Hoffmann, U., 1990. Kinetics of methyl tertiary butyl ether
liquid phase synthesis catalyzed by ion exchange resin-I. Intrinsic rate
expression in liquid phase activities. Chemical Engineering Science 45,
1605–1617.
Roy, R., Bhatia, S., 1987. Kinetics of esterification of benzyl alcohol with
acetic acid catalyzed by cation exchange resin (Amberlyst-15). Journal of
Chemical Technology and Biotechnology 37, 1–10.
Sai, P.S.T., 1988. Esterification kinetics of acetic acid with 1-butanol. Journal
of Energy Heat Mass Transfer 10 (2), 181–189.
Sharma, O.N., Nageshwar, G.D., Mene, P.S., 1973. Esterification of ethanol
with propionic acid catalysed by a cation exchange resin. Indian Journal
of Technology 11, 360–362.
Song, W., Venimadhavan, G., Manning, J.M., Malone, M.F., Doherty, M.F.,
1998. Measurements of residue curve maps and heterogeneous kinetics in
methyl acetate synthesis. Industrial and Engineering Chemistry Research
37, 1917–1928.
Venkateswarlu, K., Sinha, R., Rao, R.J., 1976. Kinetics of esterification of
n-butanol with propionic acid in a stirred tank batch reactor. Chemical
and Petrochemical Journal, 3–10.
Xu, Z.P., Chuang, K.T., 1996. Kinetics of acetic acid esterification over ion
exchange catalysts. Canadian Journal Chemical Engineering 74, 493–500.
Yadav, G.D., Kulkarni, H.B., 2000. Ion-exchange resin catalysis in the
synthesis of isopropyl lactate. Reactive and Functional Polymers 44,
153–165.
Yadav, G.D., Thathagar, M.B., 2002. Esterification of maleic acid with ethanol
over cation exchange resin catalysts. Reactive and Functional Polymers
52, 99–110.
Zhang, H.B., Zhang, K., Yuan, Z.Y., Zhao, W., Li, H.X., 1998. Esterification
reaction of acetic acid with n-amyl alcohol over zeolite beta. Journal of
Natural Gas Chemistry 7 (4), 336–345.
Document Outline
- Synthesis of esters: Development of the rate expression for the Dowex 50 Wx8-400 catalyzed esterification of propionic acid with 1-propanol
- Introduction
- Theory
- Experimental
- Results and discussion
- External and internal diffusion significance
- Effect of temperature on reaction equilibrium
- Effect of reaction parameters
- Effect of type of ion-exchange resin catalyst
- Effect of using sulfuric acid (H2SO4) compared with Dowex 50Wx8-400 as a catalyst
- Effect of catalyst loading
- Effect of the catalyst moisture content (wet versus dry Dowex 50Wx8-400)
- Effect of temperature
- Effect of acid to alcohol molar ratio
- Effect of adding water to the reactants mixture
- Effect of using different acids
- Effect of using different alcohols
- Modeling
- Conclusions
- References
Wyszukiwarka
Podobne podstrony:
1 s2 0 S000925099800520X main
1 s2 0 S0009279709005614 main
1 s2 0 S0009254115002983 mainid Nieznany
1 s2 0 S0020025512001946 main
1 s2 0 S0378382002000085 main
1 s2 0 S0304397599001000 main
1 s2 0 S0006291X05021595 main
1 s2 0 S0040603111000104 main 2
1 s2 0 S0944501312001358 main
1 s2 0 S0166218X96000583 main
1 s2 0 S0005273614000303 main
1 s2 0 S0304397502001342 main
1 s2 0 S0377221798003622 main (1)
1 s2 0 S0022169496031423 main
1 s2 0 S1046592814002101 main
1 s2 0 0166218X93E0153P main
1 s2 0 S0022000006001474 main
więcej podobnych podstron