
Assessment of the fertiliser potential of digestates from farm
and agroindustrial residues
Jose´ Antonio Alburquerque
, Carlos de la Fuente
, Alicia Ferrer-Costa
, Lucı´a Carrasco
Juan Cegarra
, Manuel Abad
, Marı´a Pilar Bernal
a
Department of Soil and Water Conservation and Organic Waste Management, Centro de Edafologı´a y Biologı´a Aplicada del Segura,
CSIC, P.O. Box 164, 30100 Murcia, Spain
b
Instituto Agroforestal Mediterra´neo, Universidad Polite´cnica de Valencia, P.O. Box 22012, 46071 Valencia, Spain
a r t i c l e i n f o
Article history:
Received 22 February 2011
Received in revised form
11 February 2012
Accepted 22 February 2012
Available online 14 March 2012
Keywords:
Anaerobic co-digestion
Digestate composition
Agroindustrial residues
Biodegradability
Phytotoxicity
a b s t r a c t
The sustainability of biogas production systems depends greatly on the appropriate
disposal of the digestates produced. The main agrochemical characteristics of 12 digestates
from the anaerobic co-digestion of farm and agroindustrial residues were determined and
compared with quality standards to assess their potential use as fertilisers. The digestates
have a high fertilising potential, associated mainly with their contents of NH
4
-N; however,
their recycling in agriculture might be restricted by their Cu and Zn contents, salinity,
biodegradability, phytotoxicity and hygiene characteristics, which must be addressed to
obtain the maximum benefits. Such characteristics determine the need for applying pre- or
post-treatments to increase digestate quality until acceptable levels. Therefore, digestate
quality must be taken into account when managing the co-digestion process, including
substrate selection, in order to use digestates as fertilisers without the additional cost of
post-digestion conditioning treatments.
ª 2012 Elsevier Ltd. All rights reserved.
1.
Introduction
The non-renewable nature of fossil energy sources and their
contribution to global warming have caused a great, world-
wide interest in the development and implementation of
renewable energy programmes. In this context, technologies
such as anaerobic digestion - for which the share of the
renewable energy market is increasing - play a major role,
leading to benefits for residue management, energy supply
and the environment
.
The vast amounts of biodegradable residues and by-
products produced by the livestock and agroindustrial
sectors have a large potential for biogas production through
co-digestion. This constitutes a great incentive for the
implementation of the anaerobic co-digestion of animal
manures and slurries with other residues, as put forward in
the Spanish Slurry Biodigestion Plan
.
Anaerobic digestion produces biogas and a very-wet
residue called digestate which is a mixture of partially-
degraded organic matter (OM), microbial biomass and inor-
ganic compounds. The direct application of digestates to soil
is currently considered an inexpensive means for their
disposal and for recovery of their mineral and organic
constituents for agricultural systems. During anaerobic
digestion, labile organic constituents are mostly degraded,
leading to an increase in the stability of the remaining OM
contained in the digestate
. However, the prevalence of
efficiency criteria for energy production (biogas) at an
* Corresponding author. Tel.:
þ34 968 396313; fax: þ34 968 396213.
E-mail addresses:
(J.A. Alburquerque).
Available online at
http://www.elsevier.com/locate/biombioe
b i o m a s s a n d b i o e n e r g y 4 0 ( 2 0 1 2 ) 1 8 1
0961-9534/$
e see front matter ª 2012 Elsevier Ltd. All rights reserved.
industrial scale can lead to a limited residence time of the
material in the digester (after which energy efficiency starts to
decline); this can produce a digestate that is not completely
exhausted in terms of easily-degradable organic compounds.
This may generate problems during storage (odour emission,
production of toxic compounds, pathogen re-growth and
phytotoxicity) and cause unfavourable impacts on the soil-
plant system, thus limiting the potential fertilising value of
digestate when unstable materials are added to soil
.
These can cause an extensive range of deleterious effects on
crops, such as prevention or delay of seed germination, plant
death or marked reductions in growth, which must be
addressed. Bioassays incorporating plant material are simple,
reproducible, rapid and economic tests that identify phyto-
toxic materials.
On the other hand, the agricultural use of digestates should
be considered as a recovery process instead of a simple
disposal method. But, the market demand for digestates as
soil conditioners or fertilisers depends on the compliance with
quality standards, as regulated by European and national
guidelines
as well as by quality protocols for digestate
application in Germany
and the United Kingdom
. In
Spain, no specific, applicable standards for digestates, which
would favour their use in agriculture, have been developed -
which represents a major barrier for the development of
anaerobic digestion.
In this study, the main agrochemical characteristics of
a number of digestates, including phytotoxicity, have been
determined, by analysing twelve samples from several,
representative co-digestion processes carried out in Spain,
and then compared to the quality criteria established in order
to assess their potential use as fertilisers in agricultural
systems.
2.
Materials and methods
2.1.
Digestate origin and sampling
After the anaerobic co-digestion of twelve mixtures incorpo-
rating pig (PS) or cattle (CS) slurries as major components, the
corresponding,
representative
digestate
samples
were
collected for analysis. According to the origin of the digestate,
the samples were classified into the following four groups
(on a fresh mass basis):
- From PS plus energy crop residues (PS-EC):
þ9.6% rape
residue (PS-EC1),
þ4.5% sunflower residue (PS-EC2) and
þ5.4% corn residue (PS-EC3).
- From PS plus animal by-products (PS-AB):
þ0.6% pasteurised
slaughterhouse
residues
(PS-AB1),
þ3.8% pasteurised
slaughterhouse residues (PS-AB2) and
þ1.0% sludge from
a slaughterhouse wastewater treatment plant
þ6.5% bio-
diesel wastewaters (PS-AB3).
- From CS plus glycerine (CS-G):
þ4% glycerine (CS-G1) and
þ6% glycerine (CS-G2 and CS-G3).
- From CS plus agroindustrial residues (CS-AW):
þ5% orange
peel residue (CS-AW1),
þ10% orange peel residue (CS-AW2)
and
þ4.3% cattle manure þ11.6% maize-oat silage
(CS-AW3).
As shown in
, these samples included two diges-
tates derived from industrial processes (PS-AB3 and CS-AW3),
while the rest came from laboratory-scale experiments run in
order to optimise biogas production by using co-substrates
such as glycerine or orange peel residues (for CS) and
slaughterhouse or energy crop residues (for PS). The diges-
tates were sampled directly after anaerobic digestion
processes (without post-treatments), stored at a temper-
ature
< 4
C and processed quickly to prevent chemical or
biological alterations.
2.2.
Analytical methods for digestate characterisation
The following parameters were determined in the fresh
digestate samples: electrical conductivity (EC) and pH; dry
matter content (DM) after drying the digestate sample at
105
C for 24 h; the volatile solids, which reflect the OM
content, by loss on ignition at 500
C for 24 h. The total organic
carbon (TOC) and total nitrogen (TN) were measured in freeze-
dried samples, by automatic microanalysis (EuroVector
elemental analyser). The dissolved organic carbon (DOC) was
measured after filtration of the fresh digestate (through
a synthetic filter with a pore diameter of 0.45
mm), using an
automatic analyser for liquid samples (TOC-V CSN Analyzer,
Shimadzu). Ammonium was determined by steam-distillation
from alkalised fresh samples with MgO, and chloride (Cl) by
potentiometry with silver nitrate. After HNO
3
/HClO
4
(2:1 v/v)
digestion, the following elements were determined by induc-
tively coupled plasma-optical emission spectrometry (ICP-
OES, Thermo Elemental Co. Iris Intrepid II XDL): P, K, S, Na, Ca,
Mg, Fe, Cu, Mn, Zn, B, Pb, Cd, Cr and Ni. The 5 d biochemical
oxygen demand (BOD
5
) was determined, with respirometric
Oxitop
IS 6 equipment (WTW, Germany), based on pressure
measurement, which is automatically computed as an oxygen
value with units of mg L
1
. In the Oxitop
equipment, the
cumulative oxygen consumption was recorded each day
during a period of 5 d. Salmonella spp. and Escherichia coli were
determined according to the modified method of the USEPA
Table 1
e The main technical aspects of the anaerobic co-
digestion processes which produced the 12 digestates
studied.
Digestate
Operation
Scale
Temperature (
C)
HRT (d)
PS-EC1
CONT
LS
35
30
PS-EC2
CONT
LS
35
30
PS-EC3
CONT
LS
35
30
PS-AB1
CONT
LS
35
20
PS-AB2
CONT
LS
35
30
PS-AB3
CONT
IS
37
21
CS-G1
DISCONT
LS
35
40
CS-G2
DISCONT
LS
35
40
CS-G3
CONT
LS
55
22
CS-AW1
DISCONT
LS
38
28
CS-AW2
DISCONT
LS
38
40
CS-AW3
CONT
IS
38.5
25
CONT: continuous operation, DISCONT: discontinuous operation,
LS: laboratory-scale (2 L to 6 L digester), IS: industrial-scale (3000 m
3
digester), and HRT: hydraulic residence time.
b i o m a s s a n d b i o e n e r g y 4 0 ( 2 0 1 2 ) 1 8 1
182
and the most probable number (MPN) method
,
respectively.
2.3.
Potential phytotoxicity
To obtain homogeneous samples, the 12 digestate samples
were sieved (0.25 mm mesh-size) and then centrifuged. The
supernatants thus obtained were shaken and tested in
bioassays involving seed germination and seedling growth,
with both cress (Lepidium sativum ‘Alenois’) and lettuce (Lac-
tuca sativa ‘Bionda degli Ortelani’).
2.3.1.
Seed germination bioassays
In these bioassays, the Petri dishes used (8.5 cm diameter)
each contained 2 filter papers (base and cover), which were
moistened with 1 mL of the digestate. Digestate samples were
tested at concentrations of 100% (pure), 20%, 10%, 1% and 0.1%
(volume fraction), which were prepared with distilled water.
Ten seeds per dish were sown and each experimental treat-
ment was replicated 5 times. The dishes were transferred to
a germination chamber under controlled conditions of
temperature (17
C and 23
C for lettuce and cress, respec-
tively) and darkness for 3 d (cress) or 5 d (lettuce). After this
period, the number of germinated seeds was counted, the
radicle lengths of these seeds measured and the germination
index (GI) calculated as a percentage of the control (distilled
water), according to Zucconi et al.
2.3.2.
Plant growth bioassays
Plastic cell trays (10 cells/tray and 33 mL/cell), with a drainage
hole in the bottom of each cell, were filled with horticultural
perlite (1 mm
e2 mm in diameter) and used for these bioas-
says. Trays (in triplicate for each treatment tested) were
placed in a holder vessel (18 cm
6.5 cm 5 cm) - containing
400 mL of distilled water - for 24 h, until the perlite was
saturated by capillarity. Then, 2 seeds of cress or lettuce per
cell were sown and maintained under the same wetting
conditions for 9 d or 13 d, respectively, until seed germination
and seedling emergence occurred (and only 1 seedling/cell
was left). After this period, the water in the holder vessel was
replaced completely by 200 mL (equivalent to the perlite
container volume) of the digestate concentrations to be
examined: 20%, 10%, 1% and 0.1%, keeping trays without
digestate addition as a control. Pure digestate was not tested
for plant growth bioassays since GI was 0% for all the digestate
samples. In these bioassays, and due to the limited availability
of adequate volumes of the samples obtained from the raw
digestates, only 7 co-digestion mixtures were studied: PS-EC1,
PS-EC2, PS-EC3, PS-AB2, PS-AB3, CS-G3 and CS-AW3. Imme-
diately following the sowing, the trays and their holder vessels
were transferred to a growth chamber with controlled condi-
tions: 17
C (lettuce) and 23
C (cress) during the first
week, followed by 19
C (lettuce) and 21
C (cress)
during the remaining 3 weeks. During the bioassay, the daily
photoperiod was 16 h at a photosynthetic irradiance of
250
mmol m
2
s
1
provided by fluorescent tubes (Sylvania Cool
White VHO). Solution losses from the holder vessels (by plant
uptake
þ evaporation) were restored periodically using
distilled water. From the second week, a liquid, mineral
compound fertiliser (Hesi Coco
, The Netherlands; N-P
2
O
5
-
K
2
O 24:21:27, at 3 or 1.5 mL per L distilled water for cress and
lettuce, respectively) was added (in 3 successive applications)
to the digestate solutions to maintain seedling growth. At the
end of the experiment (4 weeks from sowing), 5 seedlings per
tray were harvested and their total dry mass (shoot
þ roots)
determined after drying at 105
C.
2.4.
Statistical analyses
Basic statistical analyses of data, correlation coefficients and
regression models were produced using the SPSS 18.0 program
for Windows. The normal distribution of the data was checked
by the Shapiro-Wilk’s test, and failed data were adjusted to
a normal distribution through logarithmic transformation.
3.
Results and discussion
3.1.
Physico-chemical characterisation
All digestate samples had low dry matter contents (
these
being
classified
as
liquid
products
(DM
mass
fraction
15%
). They were characterised by slightly-
alkaline pH values (
> 7.5), except in the case of CS-G1 and
CS-G3. The trend is for the pH to increase as anaerobic
digestion progresses, due to volatile fatty acid degradation
and ammonia production
. In addition, the pH is also
conditioned by the addition of strong bases or carbonates to
control pH and the buffer capacity of the system during
anaerobic digestion
. Generally, the pH of digestates derived
from animal slurries is in the alkaline range, around 8
.
The highest EC values were found in the PS digestates and
the CS-AW3 sample (
> 20 dS m
1
,
), which also had the
highest concentrations of Cl, while CS-G1, CS-G2 and PS-AB2
exhibited the highest Na concentration (
). Therefore,
special care must be taken since excessive doses or continued
applications of digestates rich in Cl and Na to soils could lead
to an increase in soil salinity and inhibit plant growth.
Regarding the plant nutrient content and hence the fertil-
iser value, the most-significant property of the digestates was
that a large proportion of the N occurred as inorganic forms
(
), representing NH
4
-N more than 70% of the TN (on
a mass basis) in the PS mixtures and 39%
e61% in mixtures
with CS. This form of N can be easily lost by ammonia vola-
tilisation during storage and land spreading due to the alka-
line pH of the digestates
. In addition, NH
4
-N is nitrified
rapidly in soil under favourable conditions, this form being
highly available to crops but also subjected to leaching
through the soil profile, which may result in groundwater
pollution. Therefore, storage and land spreading operations
with digestates must be carefully controlled to avoid negative
environmental impacts.
The TOC/TN ratio was highest for CS-AW3, CS-G1, CS-G2
and CS-G3 (
> 8), clearly higher than the mean value of 4
reported by Siebert et al.
for digestates from different
sources. Unbalanced C/N ratios in digestates can limit their
use in agriculture, as an excess of degradable organic
substrate for microorganisms with respect to N leads to
immobilisation of this nutrient in the microbial biomass and
b i o m a s s a n d b i o e n e r g y 4 0 ( 2 0 1 2 ) 1 8 1
183
to crop deficiency, while excess N causes losses through
ammonia volatilisation, nitrate leaching, etc.
.
The nutrient concentrations of the digested materials were
within the range found by other authors
. As a whole, the
major elements were N and K, followed by P and Ca (
). PS-EC1, PS-EC2 and PS-EC3 together with PS-AB2 and
CS-AW3 showed the highest concentrations of P, K, Ca and
Mg, whereas the S concentration was generally higher in PS
samples than in those from CS mixtures (
). From the
standpoint of quality criteria for digested materials
, no
limits have been established for their nutrient contents,
although a number of parameters should be declared,
Table 3
e The concentrations (mg L
L1
) of elements in the 12 digestate samples (mean value, expressed on a fresh mass
basis). CV: coefficient of variation.
Digestate
S
Ca
Mg
Na
Cl
Fe
Mn
Zn
Cu
B
Pig slurry
PS-EC1
401
1993
633
666
1574
155
22.9
49.2
8.4
3.2
PS-EC2
367
1970
721
699
1495
143
23.4
45.9
7.0
3.2
PS-EC3
417
1863
698
697
1613
224
31.0
62.5
7.8
2.7
PS-AB1
219
799
324
696
1598
51
11.4
84.4
14.3
2.2
PS-AB2
302
828
365
995
2120
63
15.4
140.2
15.1
3.1
PS-AB3
680
218
67
726
1993
22
2.9
34.7
4.0
2.3
Median
384
1345
499
698
1606
103
19.1
55.8
8.1
2.9
(CV)
(39.3)
(59.4)
(55.4)
(16.5)
(14.9)
(69.9)
(56.1)
(55.6)
(46.2)
(16.5)
Cattle slurry
CS-G1
180
1550
267
1164
685
117
13.7
18.1
10.8
1.8
CS-G2
265
1753
333
1842
665
165
17.1
28.3
13.0
4.8
CS-G3
48
192
79
66
448
95
3.2
10.6
1.4
1.3
CS-AW1
113
1008
257
276
366
30
6.0
7.7
2.8
1.7
CS-AW2
131
1035
314
303
452
39
6.9
8.0
3.1
3.5
CS-AW3
457
4026
698
746
1418
301
27.5
27.7
10.8
3.4
Median
155
1293
290
525
558
106
10.3
14.4
6.9
2.6
(CV)
(73.2)
(82.1)
(62.8)
(91.6)
(57.6)
(80.4)
(72.8)
(56.8)
(72.8)
(49.4)
ANOVA
a
NS
NS
NS
NS
**
NS
NS
*
NS
NS
NS: not significant. * and **: significant at probability level P
< 0.05 and P < 0.01, respectively.
a considering the following grouped mixtures: pig slurry
þ energy crop residues (PS-EC1, -2 and -3), pig slurry þ animal by-product (PS-AB1, -2
and -3), cattle slurry
þ glyceryne (CS-G1, -2 and -3) and cattle slurry þ agroindustrial residues (CS-AW1, -2 and -3).
Table 2
e The main characteristics and composition of the 12 digestate samples (mean value, expressed on a fresh mass
basis). EC: electrical conductivity, DM: dry matter, TOC: total organic carbon, DOC: dissolved organic carbon, BOD
5
: 5
d biochemical oxygen demand, TN: total nitrogen and CV: coefficient of variation.
Digestate
pH
EC
(dS m
1
)
DM
(g L
1
)
TOC
(g L
1
)
DOC
(g L
1
)
BOD
5
(g L
1
)
TN
(g L
1
)
NH
4
-N
(g L
1
)
P
(g L
1
)
K
(g L
1
)
TOC/TN
Pig slurry
PS-EC1
7.82
26.0
43.9
14.7
4.3
6.5
3.6
2.9
1.1
3.1
4.1
PS-EC2
7.92
24.1
38.3
12.2
3.7
4.0
3.5
2.6
1.1
3.1
3.5
PS-EC3
7.90
23.3
28.3
8.3
3.7
4.7
3.4
2.7
1.2
2.7
2.4
PS-AB1
7.95
21.1
21.0
5.8
1.2
2.3
2.9
2.2
0.5
2.2
2.0
PS-AB2
7.86
30.8
29.5
8.4
3.5
6.2
4.9
3.4
0.8
3.1
1.7
PS-AB3
8.20
30.3
19.5
5.9
2.4
2.2
4.0
3.5
0.2
2.0
1.5
Median
7.91
25.0
28.9
8.4
3.6
4.4
3.6
2.8
0.9
2.9
2.2
(CV)
(1.7)
(15.2)
(31.8)
(38.6)
(35.6)
(43.7)
(18.2)
(16.9)
(50.9)
(18.2)
(40.9)
Cattle slurry
CS-G1
5.64
14.5
38.3
17.8
10.6
37.5
1.9
1.0
0.5
1.8
9.5
CS-G2
7.35
11.7
72.9
42.8
27.6
52.5
2.3
0.9
0.4
1.6
18.5
CS-G3
6.35
5.2
17.6
8.3
8.2
10.6
0.6
0.4
0.1
0.8
13.6
CS-AW1
7.86
8.7
24.4
9.4
1.2
1.3
1.4
0.8
0.2
1.1
6.6
CS-AW2
7.90
10.0
17.6
5.8
1.0
1.2
1.5
0.9
0.2
1.2
3.8
CS-AW3
7.50
25.7
90.1
33.7
5.4
5.9
4.0
2.4
0.8
3.1
8.5
Median
7.42
10.9
31.4
13.6
6.8
8.3
1.7
0.9
0.3
1.4
9.0
(CV)
(12.8)
(56.2)
(71.0)
(77.5)
(109.6)
(118.9)
(58.0)
(67.1)
(71.9)
(49.8)
(52.2)
ANOVA
a
*
*
NS
NS
*
**
NS
**
*
NS
***
NS: not significant. *, ** and ***: significant at probability level P
< 0.05, P < 0.01 and P < 0.001, respectively.
a considering the following grouped mixtures: pig slurry
þ energy crop residues (PS-EC1, -2 and -3), pig slurry þ animal by-product (PS-AB1, -2
and -3), cattle slurry
þ glyceryne (CS-G1, -2 and -3) and cattle slurry þ agroindustrial residues (CS-AW1, -2 and -3).
b i o m a s s a n d b i o e n e r g y 4 0 ( 2 0 1 2 ) 1 8 1
184

including: bulk density, DM, OM, pH, salt content, TN, P
2
O
5
,
K
2
O, CaO, MgO, S, NH
4
-N, NO
3
-N, micronutrients, Cl and Na.
In the current Spanish legislation for fertilisers
,
minimum limits for N, P and K contents are specified for
marketable
liquid
organo-mineral
fertilisers.
However,
according to the legal requirements, digestates cannot be
considered balanced fertiliser products and they must be
complemented with other inorganic fertilisers. Another
significant limitation is the digestate TOC content, generally
lower than the required concentration (
> 4% on a mass basis).
Therefore, the inclusion of a new fertiliser category in the
current Spanish legislation, specifically considering digestate
composition, may be advisable.
The most-abundant micronutrient in the digestates was Fe
(
), CS-AW3 having the highest value due to the use of Fe
salts during its anaerobic digestion. High concentrations of Cu
and Zn were found in the digestates due to the use of pig and
cattle slurry as the major co-digestion substrates, since these
two elements are frequently used as additives - to prevent pig
and cattle diseases and to stimulate livestock growth. The Cu
and Zn concentrations were especially high in digestates
arising from PS mixtures (on a dry mass basis): (76
e682)
mg kg
1
, with a median value of 173 mg kg
1
for Cu, and
(222
e4757) mg kg
1
with a median value of 446 mg kg
1
for Zn.
Some values were higher than the established limits
, so
special care must be taken with respect to Cu and Zn
concentrations, especially for digestate produced from PS.
With respect to heavy metals, the concentrations on a dry
mass basis in some digestate samples for Ni (n
¼ 7, 6 mg kg
1
to 36 mg kg
1
), Pb (n
¼ 7, 11 mg kg
1
to 46 mg kg
1
), Cr (n
¼ 2,
3 mg kg
1
and 55 mg kg
1
) and Cd (n
¼ 6, 0.1 mg kg
1
to
1.0 mg kg
1
) were lower than both the limits established by the
cited Spanish legislation and the quality protocols for the
production and use of digestates
. The concentrations of
heavy metals found in this study were similar to those
reported previously for digested materials from substrates of
different origin
.
Our results reveal the influence of the raw materials on the
digestate characteristics. The coefficients of variation (
) of most of the parameters analysed reveal, in general,
a high degree of variability. Thus, digestates should be ana-
lysed fully before use. However, highly-significant correla-
tions between the DM and parameters such as TOC, Ca and Fe
were found, while TN, NH
4
-N, K, S, Zn and Cl were correlated
significantly with EC (
), indicating their preferential
association with the solid or liquid digestate fraction,
respectively.
The use of regression equations based on highly-
significant correlations between EC and ionic species (NH
4
þ
,
K
þ
, etc.) resulted in a reliable tool for providing a quick esti-
mation of nutrient contents in animal slurries
. As
a result, the EC and DM of digested materials can be used
successfully to estimate other valuable and useful parameters
which are much-more difficult and time-consuming to
analyse (
) and which, together with BOD or DOC data,
characterise the quality of digested materials, as discussed in
the following section.
3.2.
Digestate biodegradability
The digestate samples showed a great variability in their
degree of stability, according to the BOD
5
values (
These results indicate the great influence on digestate
stability not only of the raw materials used as co-digestion
substrates but also of the co-digestion process, as shown by
the clear differences in the organic load and its biodegrad-
ability among digestate samples from cattle slurry
þ glycerine
mixtures (CS-G). The CS-G digestates showed BOD
5
values
that were clearly higher than those of the other digestates
tested (
), indicating that they had the lowest degree of
microbial stability, associated with the presence of easily-
degradable compounds. The industrial digestate sample
(CS-AW3) had the highest DM content of all the digestates
produced from CS mixtures, but only 16% of the TOC occurred
as DOC, which led to a higher degree of stability (less biode-
gradable material) than for the digestates from CS-G mixtures.
This is related to the use of cattle manure and, especially,
silage (solid lignocellulosic materials) as co-substrates for
Table 4
e Correlation matrix between selected parameters related to the digestate composition (n [ 12).
Parameters
DM
EC
TOC
TN
NH
4
-N
P
K
Ca
Mg
Fe
Mn
Zn
Cl
DM
1
EC
NS
1
TOC
0.946***
NS
1
TN
NS
0.973***
NS
1
NH
4
-N
NS
0.984***
NS
0.951***
1
P
NS
0.607*
NS
0.644*
0.599*
1
K
NS
0.875***
NS
0.900***
0.837**
0.878***
1
Ca
0.856***
NS
0.725**
NS
NS
0.612*
0.577*
1
Mg
0.584*
NS
NS
NS
NS
0.923***
0.780**
0.800**
1
Fe
0.789**
NS
0.705*
NS
NS
0.655*
NS
0.886***
0.752**
1
Mn
0.684*
NS
NS
NS
NS
0.932***
0.787**
0.806**
0.941***
0.859***
1
Zn
NS
0.814**
NS
0.836**
0.813**
0.649*
0.793**
NS
NS
NS
NS
1
Cl
NS
0.968***
NS
0.936***
0.979***
NS
0.814**
NS
NS
NS
NS
0.888***
1
NS: not significant. *, ** and ***: significant at probability level P
< 0.05, 0.01 and 0.001, respectively.
EC: electrical conductivity (dS m
1
). DM: dry matter, TOC: total organic carbon, TN: total nitrogen. NH
4
-N, P, K, Ca, and Mg in g L
1
on a fresh
mass basis. Fe, Mn, Zn and Cl in mg L
1
on a fresh mass basis. The DM, TOC and Zn data were adjusted to a normal distribution (Shapiro-Wilk’s
test) through logarithmic transformation.
b i o m a s s a n d b i o e n e r g y 4 0 ( 2 0 1 2 ) 1 8 1
185

anaerobic digestion, which enriched the digestate in OM of
low biodegradability.
The CS-AW1 and CS-AW2 samples had the lowest BOD
5
values and percentages of DOC with respect to TOC (
<16%),
indicating a high stability degree. By contrast, digested
materials where PS was the main substrate showed an inter-
mediate behaviour, with BOD
5
values between 2 g L
1
and
7 g L
1
and DOC values between 1 g L
1
and 4 g L
1
, repre-
senting from 20% to 40% of the TOC in the digestates.
Therefore, differences in stability degree between diges-
tates were related directly to the concentration of DOC, readily
available for microorganisms. The BOD
5
was correlated
significantly with both TOC (r
¼ 0.722; P < 0.01) and, especially,
DOC (r
¼ 0.960; P < 0.001), which demonstrates the strong
influence of DOC on digestate biodegradability characteristics.
A significant regression equation was obtained for BOD
5
and
DOC (g L
1
fresh digestate mass): lgBOD
5
¼ 1.172lgDOC þ 0.057,
which allows cautious prediction of BOD
5
from DOC data.
Considering the BOD data after 24 h, which corresponds to
the period of maximum biological activity, and expressing
them as the average oxygen uptake rate (on an organic
matter basis), only the CS-AW3 sample satisfied the respira-
tion index (
<1 mg g
1
h
1
) proposed for stabilised biowastes
. On the other hand, using the criterion established by
Ponsa´ et al.
, the digestates can be classified as follows
(on a dry mass basis): 1) low biodegradability (
<2 mg g
1
h
1
):
PS-AB3, CS-AW1, CS-AW2 and CS-AW3 (1.4, 0.9, 1.0
and 0.8 mg g
1
h
1
, respectively); 2) moderate biodegradability
(2 mg g
1
h
1
e5 mg g
1
h
1
): PS-AB1, PS-AB2, PS-EC1, PS-EC2
and PS-EC3 (2.1, 3.2, 2.4, 2.2 and 2.6 mg g
1
h
1
, respectively);
and 3) high biodegradability (
> 5 mg g
1
h
1
): digestates from
CS-G mixtures (8.2, 20.0 and 11.8 mg g
1
h
1
for CS-G1, CS-G2
and CS-G3, respectively). The procedure for certifying the
quality of digestates in the United Kingdom
establishes
a dissolved chemical oxygen demand (COD) lower than
0.43 g g
1
OM as a stability requirement. In our study, the CS-G
digestates did not meet this requirement, assuming that DOC
contributes greatly to COD, thus underlining the high biode-
gradability of the CS-G digestates. In such cases, a longer
residence time during anaerobic co-digestion, further pro-
cessing or post-treatment stabilisation is recommendable
before digestate application to soil, in order to obtain the
maximum agricultural and environmental benefits.
Among the quality criteria defined for digestates, biode-
gradability or the degree of stability is the parameter for
which there is least agreement. As a result, different
threshold values have been established as quality criteria,
based on respiration indices
, volatile fatty acid contents
or chemical oxygen demand
. In our previous work
, digestate composition parameters such as DOC, BOD
and DOC/TN were related to the C and N dynamics in a soil
amended with digestates. In this case, a digestate suitable
for use as a fertiliser was defined based on DOC
< 1.5 g L
1
,
BOD
5
< 2.5 g L
1
and DOC/TN
< 1, while digestates showing
characteristics such as DOC
< 5.5 g L
1
, BOD
5
< 6.0 g L
1
and
DOC/TN
< 1.5 were established as less-appropriate for fer-
tilisation purposes, with a curing or maturation period being
required in order to increase digestate stability. Highly-
biodegradable
digested
materials,
such
as
the
CS-G
samples, were not suitable for agricultural use as they
caused a high CO
2
-C production and led to N-immobilisation
in soil, thus greatly limiting their N-fertilising potential in
the short-term.
3.3.
Evaluation of the potential phytotoxicity of digestates
and their hygiene
The effects of the 12 digestates and 5 digestate concentrations
studied (100% -pure-, 20%, 10%, 1% and 0.1%) on the GI,
expressed as a percentage of the control (distilled water), of
cress and lettuce seeds (germination bioassays) are shown in
(a) and (b), respectively. Notable and highly-significant
effects of both digestates and concentrations were found.
Among the 12 digestates examined, the average GI ranged
from 34% (CS-G3) to 75% of the control (CS-AW1) for cress and
from 34% (CS-G3) to 82% (CS-AW2) for lettuce and, with regard
to the concentration tested, from 0% (100% digestate concen-
tration, both cress and lettuce) to 102% (1% digestate
concentration, cress) or 110% (1% digestate concentration,
lettuce). At a digestate concentration of 1%, various digestates,
particularly PS-AB1 and CS-AW3, exceeded the threshold
value for GI of 125% (of the control), in both the cress and
lettuce bioassays; thus, these two digestate solutions could be
considered to have plant nutrient or plant growth stimulant
attributes, as suggested by Emino and Warman
and
Moldes et al.
The GI of both cress and lettuce at the digestate concen-
trations of 20% and 10% were inversely correlated with the EC
values (P
< 0.01) of the digestates (an indicator of their salt
content) and also with the concentrations of TN (P
< 0.01),
NH
4
-N (P
< 0.05), K (P < 0.01), S (P < 0.05), Zn (P < 0.01) and Cl
(P
< 0.01). McLachlan et al.
obtained similar results when
they studied the potential phytotoxicity of digestates
produced from municipal solid wastes, high soluble salt
concentrations being the major reason for phytotoxicity. Tam
and Tiquia
related the phytotoxic effects of spent pig litter
to its salt, NH
4
-N, Cu and Zn contents.
For concentrations of 1% and, especially, 0.1%, TN, NH
4
-N,
K, S, Zn and Cl were not correlated significantly with the GI for
cress, but the latter was correlated positively with EC, TN,
Table 5
e The linear regression equations (y [ Ax D B)
calculated for selected parameters determined in the
digestates (
n [ 12).
y
x
A
B
r
lgTOC
lgDM
1.176***
0.708**
0.946***
Ca
3.807***
4.286**
0.856***
Fe
289.6**
318.4*
0.789**
TN
EC
0.141***
0.117
NS
0.973***
NH
4
-N
0.124***
0.431*
0.984***
K
0.084***
0.543
NS
0.875***
lgZn
0.037**
0.764**
0.814**
Cl
0.070***
0.144
NS
0.968***
NS: not significant, *, ** and ***: significant at P
< 0.05, 0.01 and 0.001,
respectively.
TOC: total organic carbon, TN: total nitrogen, DM: dry matter, NH
4
-
N, K, Ca, and Cl in g L
1
(fresh mass basis). Fe and Zn in mg L
1
(fresh mass basis). EC: electrical conductivity in dS m
1
.
b i o m a s s a n d b i o e n e r g y 4 0 ( 2 0 1 2 ) 1 8 1
186
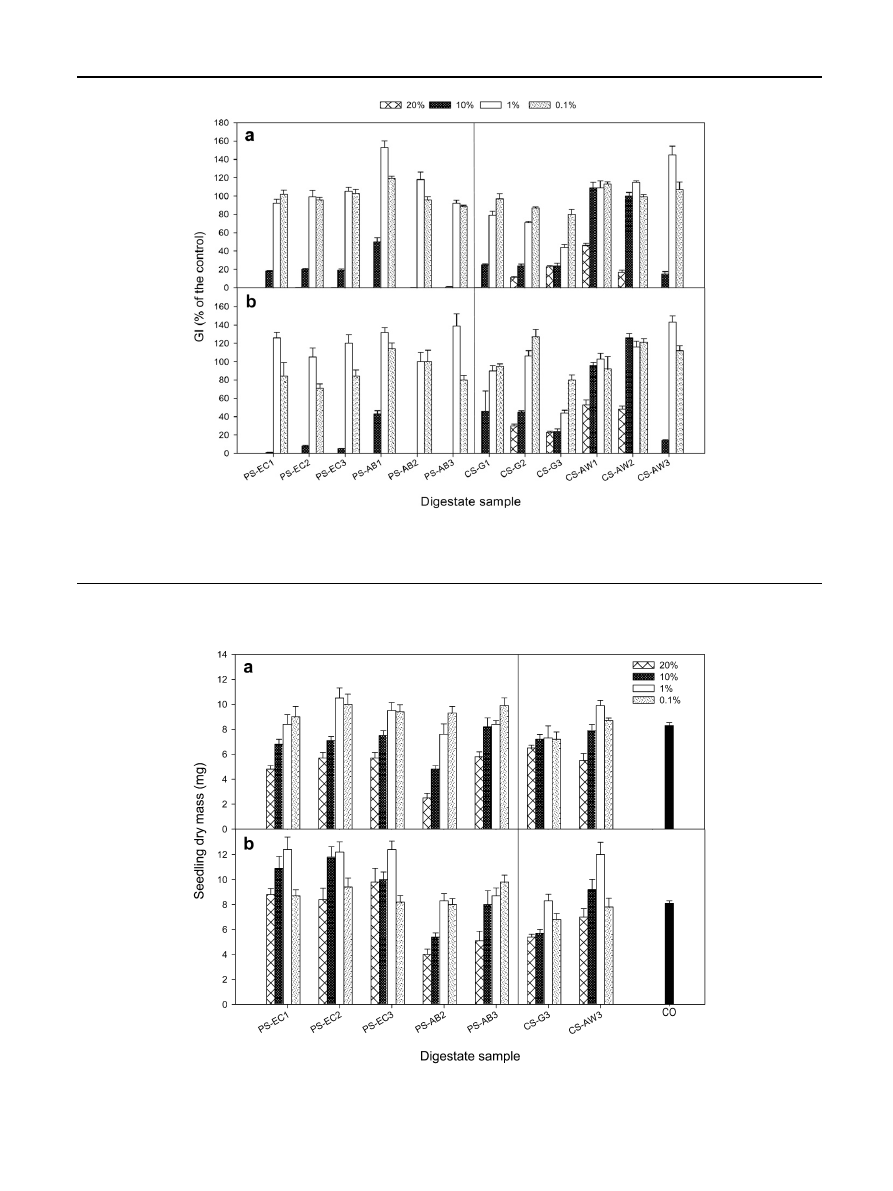
Fig. 1
e The effects of digestates and concentrations on the germination index (GI, expressed as percentage of the control) of
cress (a) and lettuce (b) seeds. Each value is the mean of five replications ± the standard error of the mean. GI was 0% for all
the pure (100%) digestate samples studied.
Fig. 2
e The effects of digestates and concentrations on the biomass accumulation (expressed as total dry mass, shoot D roots,
in mg) of cress (a) and lettuce (b) seedlings. Each value is the mean of three replications ± the standard error of the mean. Control
(without digestate addition): CO.
b i o m a s s a n d b i o e n e r g y 4 0 ( 2 0 1 2 ) 1 8 1
187
NH
4
-N and S for lettuce at 1% concentration (P
< 0.05). Indeed,
parameters related to digestate stability (DOC, DOC/TN and
BOD) correlated negatively with GI, especially for cress at both
concentrations of 1% and 0.1% (P
< 0.05). Several authors
related decreases in the phytotoxic effects of digestates to
reductions in the amount of easily-biodegradable organic
compounds.
The influence of digestates PS-EC1, PS-EC2, PS-EC3, PS-AB2,
PS-AB3, CS-G3 and CS-AW3, at the concentrations of 20%, 10%,
1% and 0.1%, and of the control (without digestate addition), on
biomass accumulation in cress and lettuce plants - expressed
as total dry mass (shoot
þ roots) per seedling - is presented in
(a) and (b), respectively. Significant differences in seedling
dry mass among both digestates and concentrations were
found, the best results (on average) being obtained with PS-
AB3, PS-EC1, PS-EC2, PS-EC3 and CS-AW3 in cress and with
PS-EC1, PS-EC2 and PS-EC3 in lettuce; the concentrations of 1%
(cress and lettuce) or 0.1% (cress) yielded the greatest seedling
biomass. In addition, most of the best treatments indicated
above were as efficient as the untreated controls, or even more
so, with regard to seedling biomass accumulation. This indi-
cates - as do the seed germination results - plant nutrient,
growth stimulant or even phytohormone-like effects of these
digestate solutions.
Therefore, agricultural uses of stable digestates can be
conditioned by their phytotoxic effects during early growth
(germination), due mainly to salinity. Thus, digestate appli-
cation rates must consider the concentrations of Na and Cl,
and also heavy metals (especially Cu and Zn), in order to
avoid any risk of metal accumulation in soil as well as sali-
nisation or phytotoxic effects, already detected following
excessive application of animal manure and slurries
.
In order to avoid phytotoxicity, digestate application to soil
should be done well in advance of sowing, avoiding direct
contact with young plants or germinating seed. The
maximum benefit from the nutrients provided can be
obtained if digestate is applied together with the irrigation
water, acting as a dilutor.
Another significant biological aspect of digestate quality
is hygiene. In Spain, the RD 824/2005
states the
requirements for fertilisers prepared from certain organic
residues, which must not exceed the following maximum
levels of micro-organisms: Salmonella spp., absent in 25 g of
product, and E. coli,
< 1000 MPN g
1
fresh product. In the
present study, E. coli was detected in most digestate samples
at levels lower than 1000 MPN g
1
fresh digestate, but
Salmonella spp. was present in 25 g fresh digestate in some
samples (data not shown). This must be related to the fact
that most digestate samples were derived from mesophilic
processes (with the exception of CS-G3) without post-
treatment, where hygiene is not guaranteed. So, further
treatment for inactivation of pathogens is required, to avoid
health risks. Both the European legislation
and
quality standards for certification of digestates
are
severe, focusing on three aspects in the case of anaerobic
digestion: process management (thermophilic temperature,
retention time, etc.), pre- or post-treatments (pasteurisation,
steaming, sterilisation, etc.) and end-product requirements
that ensure the sanitation of the digested materials accord-
ing to the source of the feedstocks.
4.
Conclusions
The digestates had a high potential fertiliser value due to their
contents of N, P, K and micronutrients. However, their high
variability and unbalanced contents necessitate their analysis
before they can be integrated into fertilisation programmes.
Together with the sanitary quality of the digestates,
a minimum degree of stability of their OM is required to obtain
the maximum benefits of digestate recycling in agriculture,
which must be harmonised at an international level. There-
fore, digestate characterisation is an unavoidable task, to
determine the application rates, phytotoxicity risk and need
for a safety period, before sowing, or a stabilisation treatment,
before soil application.
Acknowledgements
This research was funded by the “Ministerio de Ciencia e
Innovacio´n, Plan Nacional I
þDþI 2008-2011” and EU through
FEDER Funds “Fondo Europeo de Desarrollo Regional, una
manera de hacer Europa”, in the framework of the project
“singular estrate´gico PROBIOGAS” (Refs.: PSS-120000-2008-58;
PSS-120000-2008-62). The authors thank all the research
groups involved in the project PROBIOGAS (
), especially the GIRO and AINIA Technological
Centres, the University of Oviedo, San Ramo´n Group and
Treatments of Juneda Society (Tracjusa), for providing the
digested materials used in this work. The authors also thank
Dr. D.J. Walker for the English revision.
r e f e r e n c e s
[1] Al Seadi T. Good practice in quality management of AD
residues from biogas production. Esberg, DK. University of
Southern Denmark. 2002, 32 p. Report for IEA Bioenergy task
24: energy from biological Conversion of organic waste.
[2] Ward AJ, Hobbs PJ, Holliman PJ, Jones DL. Optimisation of the
anaerobic digestion of agricultural resources. Bioresour
Technol 2008;99(17):7928
e40.
[3] Real Decreto 949/2009, de 5 de junio, por el que se establecen
las bases reguladoras de las subvenciones estatales para
fomentar la aplicacio´n de los procesos te´cnicos del Plan de
biodigestio´n de purines. BOE 2009; 151:52291
e301.
[4] Grigatti M, Di Girolamo G, Chincarini R, Ciavatta C. Potential
nitrogen mineralization, plant utilization efficiency and soil
CO
2
emissions following the addition of anaerobic digested
slurries. Biomass Bioenerg 2011;35(11):4619
e29.
[5] Chadwick DR. Digestate as a fertiliser and environmental
concerns. In: Anaerobic digestion stakeholder workshop.
Session IV: building the market for digestate. UK: Exeter
University; September 3-4, 2007.
[6] Schievano A, Adani F, Tambone F, D’Imporzano G, Scaglia B,
Genevini PL. What is digestate? In: Adani F, Schievano A,
Boccasile G, editors. Anaerobic digestion: opportunities for
agriculture and environment. Milano Italy: Regione
Lombardia Publisher; 2009. p. 7
e18.
[7] Abdullahi YA, Akunna JC, White NA, Hallett PD, Wheatley R.
Investigating the effects of anaerobic and aerobic post-
treatment on quality and stability of organic fraction of
b i o m a s s a n d b i o e n e r g y 4 0 ( 2 0 1 2 ) 1 8 1
188

municipal solid waste as soil amendment. Bioresour Technol
2008;99(18):8631
e6.
[8] Salminen E, Rintala J, Ha¨rko¨nen J, Kuitunen M,
Ho¨gmander H, Oikari A. Anaerobically digested poultry
slaughterhouse wastes as fertiliser in agriculture. Bioresour
Technol 2001;78(1):81
e8.
[9] European Commission. Working document: biological
treatment of biowaste (2nd draft). Directorate-General
Environment A.2-Sustainable Resources. Brussels; 2001. 22 p.
[10] Real Decreto 824/2005 de 8 de julio sobre productos
fertilizantes. BOE 2005; 171: 25592
e669.
[11] Siebert S, Thelen-Ju¨ngling M, Kehres B. Development of
quality assurance and quality characteristics of composts
and digestates in Germany. In: Rodic-Wiersma L, Barth J,
Bidlingmaier W, de Bertoldi M, Diaz LF, editors. 6th
International conference ORBIT 2008-Moving Organic Waste
Recycling Towards Resource Management and Biobased
Economy. Wageningen, The Netherlands; October 13-15,
2008, p. 1
e12.
[12] BSI. PAS 110:2010. Specification for whole digestate,
separated liquor and separated fibre derived from the
anaerobic digestion of source-segregated biodegradable
materials. 60p. British Standards Institution Publications
[accessed 27.01.12] Available at:
downloads/PAS110_vis_10.af954016.8536.pdf
; 2010.
[13] Method 1682 USEPA. Salmonella in sewage sludge (biosolids)
by modified semisolid Rappaport-Vassiliadis (MSRV) medium.
U.S: EPA Office of Water; 2005. 41p. EPA/821/R-04/028.
[14] International Organisation for Standardisation. Detection
and enumeration of presumptive Escherichia coli - most
probable number technique. ISO 7251:2005; 2005. 13p.
[15] Zucconi F, Pera A, Forte M, de Bertoldi M. Evaluating toxicity
of immature compost. Biocycle 1981;22(2):54
e7.
[16] Tambone F, Genevini P, D’Imporzano G, Adani F. Assessing
amendment properties of digestate by studying the organic
matter composition and the degree of biological stability
during the anaerobic digestion of the organic fraction of
MSW. Bioresour Technol 2009;100(12):3140
e2.
[17] Smith KA, Metcalfe P, Grylls J, Jeffrey W, Sinclair A. Nutrient
value of digestate from farm-based biogas plants in Scotland.
ADAS UK Ltd and SAC Commercial Ltd; 2007. 44 p. Report for
Scottish Executive Environment and Rural Affairs
Department-ADA/009/06.
[18] Sommer SG, Husted S. The chemical buffer system in raw
and digested animal slurry. J Agr Sci 1995;124:45
e53.
[19] Bernal MP, Alburquerque JA, Moral R. Composting of animal
manures and chemical criteria for compost maturity
assessment. A Review Bioresour Technol 2009;100(22):
5444
e53.
[20] PRE/630/2011 Orden de 23 de marzo, por la que se modifican
los anexos I, II, III, IV, V, y VI del Real Decreto 824/2005, de 8
de junio, sobre productos fertilizantes. BOE 2011;
72:31871
e31910.
[21] Edelmann W, Baier U, Engeli H. Environmental aspects of the
anaerobic digestion of the organic fraction of municipal solid
wastes and of agricultural wastes. 8p [accessed 27.01.12]
Available at:
http://www.arbi.ch/LCA%2520AD%
; 2004.
[22] Moral R, Perez-Murcia MD, Perez-Espinosa A, Moreno-
Caselles J, Paredes C. Estimation of nutrient values of pig
slurries in southeast Spain using easily determined
properties. Waste Manage 2005;25(7):719
e25.
[23] Suresh A, Choi HL, Oh DI, Moon OK. Prediction of the
nutrients value and biochemical characteristics of swine
slurry by measurement of EC-Electrical conductivity.
Bioresour Technol 2009;100(20):4683
e9.
[24] Ponsa´ S, Gea T, Sa´nchez A. Different indices to express
biodegradability in organic solid wastes. J Environ Qual 2010;
39:706
e12.
[25] Alburquerque JA, de la Fuente C, Bernal MP. Chemical
properties of anaerobic digestates affecting C and N
dynamics in amended soils. Agr Ecosyst Environ in press,
doi:10.1016/j.agee.2011.03.007
).
[26] Emino ER, Warman PR. Biological assay for compost quality.
Compost Sci Util 2004;12(4):342
e8.
[27] Moldes A, Cendo´n Y, Lo´pez G, Barral MT. Biological quality of
potting media based on MSW composts: a comparative
study. Compost Sci Util 2006;14(4):296
e302.
[28] McLachlan KL, Chong C, Voroney RP, Liu HW, Holbein BE.
Assessing the potential phytotoxicity of digestates during
processing of municipal solid waste by anaerobic digestion:
comparison to aerobic composts. Acta Hortic 2004;638:
225
e30.
[29] Tam NFY, Tiquia S. Assessing toxicity of spent pig litter using
a seed germination technique. Resour Conserv Recy 1994;
11(1
e4):261e74.
[30] Drennan MF, DiStefano TD. Characterization of the curing
process from high-solids anaerobic digestion. Bioresour
Technol 2010;101(2):537
e44.
[31] L’Herroux L, Le Roux S, Appriou P, Martinez J. Behaviour of
metals following intensive pig slurry applications to
a natural field treatment process in Brittany (France).
Environ Pollut 1997;97(1
e2):119e30.
[32] Moral R, Perez-Murcia MD, Perez-Espinosa A, Moreno-
Caselles J, Paredes C, Rufete B. Salinity, organic content,
micronutrients and heavy metals in pig slurries from south-
eastern Spain. Waste Manage 2008;28(2):367
e71.
[33] Regulation (EC) No 1774/2002 of the European Parliament and
the Council of 3 October 2002 laying down health rules
concerning animal by-products not indented for human
consumption. Off J Eur Commun 2002;L273:1
e95.
[34] Regulation (EC) No 1069/2009 of the European Parliament and
of the Council of 21 October 2009 laying down health rules as
regards animal by-products and derived products not
intended for human consumption and repealing Regulation
(EC) No 1774/2002 (Animal by-products Regulation). Off J Eur
Commun 2009;L300:1
e33.
[35] Regulation (EC) No 208/2006 of 7 February 2006 amending
Annexes VI and VIII to regulation (EC) No 1774/2002 of the
European parliament and of the council as regards
processing standards for biogas and composting plants and
requirements for manure. Off J Eur Commun 2006;L36:25
e31.
b i o m a s s a n d b i o e n e r g y 4 0 ( 2 0 1 2 ) 1 8 1
189
Document Outline
- Assessment of the fertiliser potential of digestates from farm and agroindustrial residues
Wyszukiwarka
Podobne podstrony:
Właściwości chemiczne pofermentu i wpływ na dynamikę C i N w glebie Hiszpania 2012
Koszty wytworzenia i wartość nawozowa pofermentu z różnych instalacji Belgia 2014
Wartość nawozowa pofermentu i gnojowicy Włochy 2016
Przestępczość, Przestępczość nieletnich jako problem społeczny zawiera w sobie znacz¬ny potencjał ni
Biokataliza – potencjalne zastosowania w przemyśle chemicznym
Laborki surowce i procesy przemysłu nieorg Skład chemiczny i właściwości nawozów mineralnych nasze
Właściwości nawozowe i skład chemiczny różnych rodzajów pofermentu i kompostu Włochy 2010
Rozwiązania technologiczno aparaturowe do odzyskiwania fosforanów(V) ze ścieku z przemysłu nawozoweg
06 Kwestia potencjalności Aid 6191 ppt
potencjal spoczynkowy i jego pochodzenie
Przemyśl to
3 Prawo własności przemysłowej
Zanieczyszczenia powstające w przemyśle metalurgii żelaza prezentacja
ryzyko zawodowe w przemys
więcej podobnych podstron