sc insideatoms bmp
ArOMS AND RADPOACT!VITY
1
~\
i
Charge essentials
There are two types of eieciric charge: positive {+) and negatri/e (-). Like charges repet; unlike charges attract.
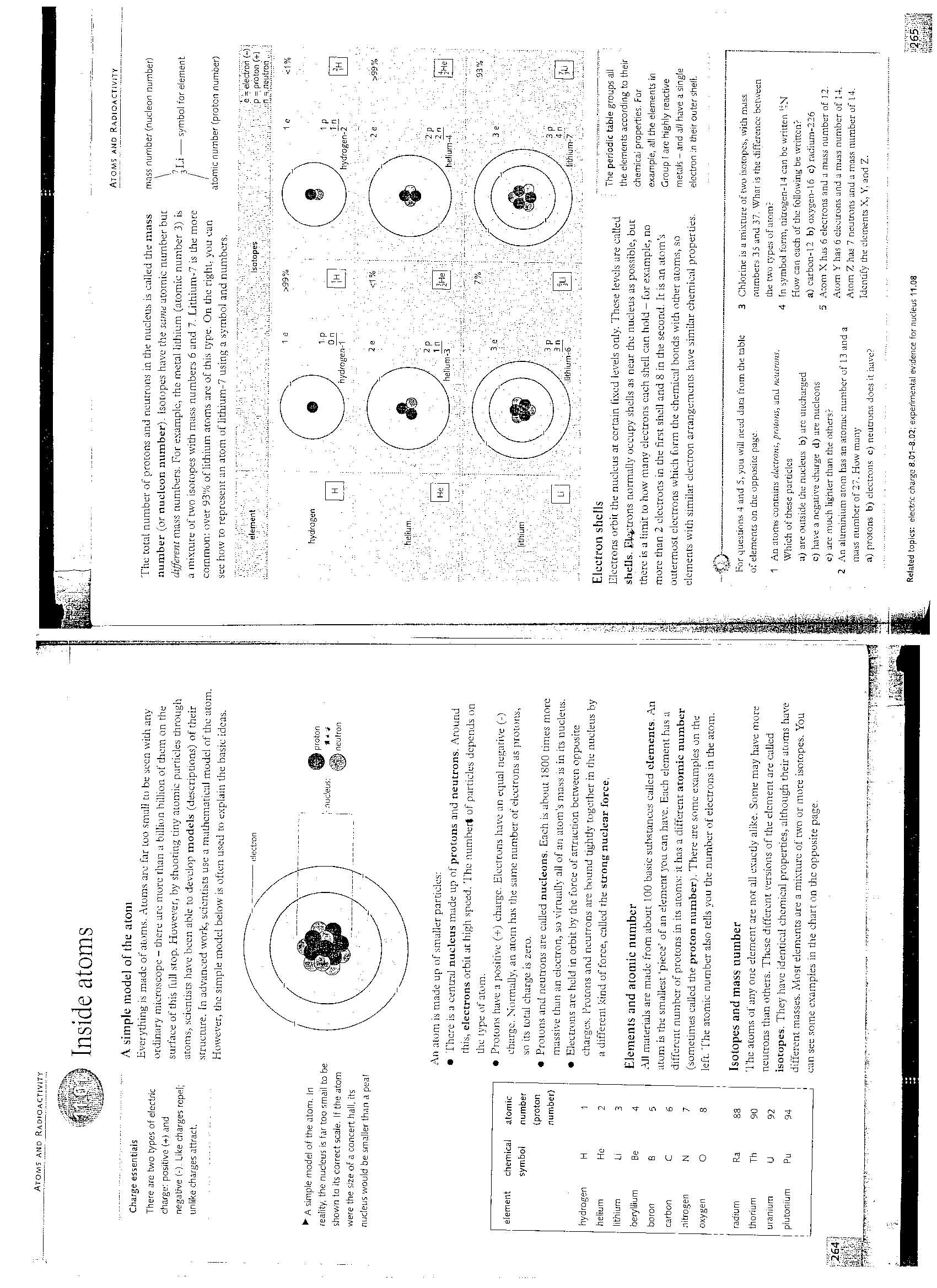
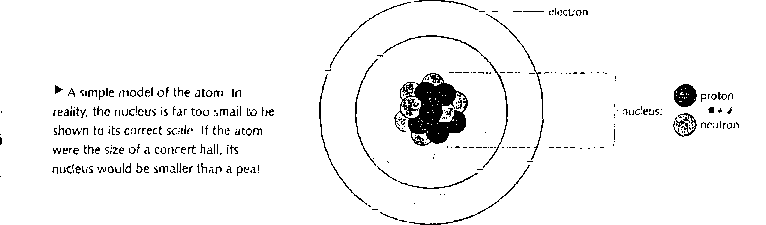
A aimplt; model of the atom
Everything iii madę of atoms. Atoms are far Loo smali tu be scen wilii any ordinary microscope - there ure morę than a billion billion of them on the surface oftliis tuli stop. Howerer, by shooting tiny aminie partieles through atoms, scientists have been able to develop models (descriptions) of their struć turę. [n advanced work, scientists use a maLhcmatieul model of the atom. However, the simple modei below is often used to explain the basie idcas.
|
! element |
Chemical symbol |
atomie number (proton number) |
|
hydrogen |
H |
1 |
|
helium |
He |
2 |
|
lithium |
Li |
3 |
|
beryllium |
Be |
4 |
|
borort |
B |
5 |
|
carbon |
C |
6 |
|
n itrogen |
N |
7 |
|
oxygen |
O |
8 |
|
radium |
Ra |
88 |
|
thorium |
Th |
90 |
|
u rani u m |
U |
92 |
|
piutonium |
Pu |
94 |
An atom is madę up of smailer partieles:
• There is a central nucleus madę up of protons and neutrons. Around rhis, electrons orbit at high speed. The numbert of partieles depends on
the iype of atom.
• Protons have a positive (+) charge. Electrons haee an equal negative {-) eharge. Normally, an atom has the same number of electrons as protons, so lis to tal charge is zero.
• Protons and neutrons are called nucleons. Each is about 1800 times morę massive than an electron, so virrually all of an aiom's mass is in its nucleus.
• Electrons are held in orbit by the furce of attracrion between opposite charges, Protons and neutrons are bound tightly togerher in rlie nucleus by a different kind of force, called the strong nuelear force,
Elements and atomie number
AJl materials are madę from about 1.00 basie substances called elements. An atom is rbe smallest ‘piece’ of an element you can have. Each element has a different number of protons in its atoms: k has a different atomie number (sometim.es called the proton number), There are some esamples on the left. The atomie number also tells you tłie number of electrons in the atom.
Isotopes and mass uurnber
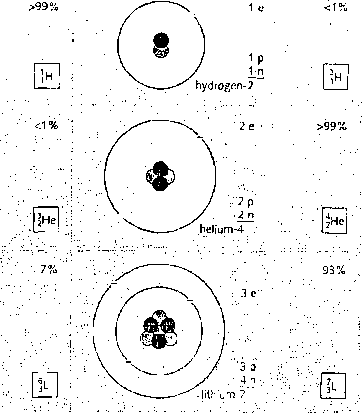
The atoms oi any one element are not all esuetly alike. Some may have morę neutrons than others. Thcse different cersions of the element are called isotopes. They havc identical Chemical properties, although their atoms have different masses. Most elements are a mixture of two or morę isotopes. You can see some exampies in the chart on drc opposite page.
i
!
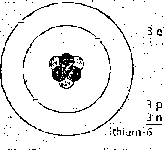
The co tal number of protons and neutrons in the nucleus is calkd the mass mass number (nudeon number) number (or nu cl eon number). Isotopes hcwc the same atomie number but \
different mass numbers. For exumple, the metal lithium (atomie number 3) is ' U — symbol for element
a mimire of two isotopes with mass numbers 6 and 7. Lithium-7 is Lhe morę j
cornmon: over 93% of lichium atoms are of his type. On the right, you can at'm|c number (proton number)
see how to represent an atom uf lithium-7 using a symbol and numbers,
, j u.:X .'.7,, f-f-f ' _e= eiedrori {-) i
■ . . element • f - . : i • ' • fi, i; • ' . 'ć • " .' . , .V.ć. • ; . Isotopes .0.,:. yy;1;. 7' '■ l; '.'.fp = proton _fm) 1
fiydrogen
helium.

. [ithium
The periodic table groups ai) the elements according to their Chemical properties, For exampier all the elements in Group I are highly reactiye metals - and aII have a single electron :n their outer shefl.
Electron shclls
Electrons orbit the nucleus at certain lixcd levels only. These levcls are called shells. Ją^trons normally occupy shdls as near the nucleus as possible, but there is a limit to how many electrons each Shell can hołd - for example, no morę than 2 electrons tn dte first shell and 8 in the second. It is an atom’s outermost electrons whieh form the Chemical bonds with otber atoms, so elements with similar electron arrangements have similar Chemical properlies.
For ąuestions 4 and 3, you will need data frum che table of elements on the opposite page.
3 Chlorine is a mixture of two isotopes, with mass numbers 33 and 37. Whar is the difference between the two cypes of atom?
4 In symbol form, nitrogen-14 can be written 1 2iN How can each of the folio wing be written?
a) carbon-12 b)oxygen-l6 c) radium-226 3 Atom X has 6 electrons and a mass number of 12. Atom Y has 6 elecLrons and a mass number of 14. Atom Z has 7 neutrons and a mass number of 14. Idcntify the elements X, Y, and Z.
Related topics: electńc charge 8,01-8,02; experimerstal ev;,dence for nucleus 11.08

An atoms eoncains electrons, prosura, and nemrons. Whieh of these partieles
a) are outside the nucleus b) are uucharged
c) have a negative charge d) are nucleons e) are much lighter than the othersr
An aluminium atom has an atomie number of 13 and a mass number of 27. How many
a) protons b) electrons c) neutrons does it have?
Wyszukiwarka
Podobne podstrony:
2.2.3 Primary and secondary services There are two classes of allocation shown in the following Tabl
Smiiing elevates mood and creates a sense of well-being. There are two kinds of smiles. The first, c
Reported speech angiekski 2 _____7 Reported Speech There are three types of Reported Speech: stateme
system 56 THE BEST HOURS FOR DOING THE EXERCISES and their proper seąuence when learnt There are two
s&h 007 PBEFACH There are two ways of writiug and ar-l-auging a succt.-s.sfnl hook. One is to siiupl
s&h 007 PBEFACH There are two ways of writiug and ar-l-auging a succt.-s.sfnl hook. One is to siiupl
46407 Picture 6 Flying Parts and Wings There are two types °t Wings: Those that are vertical to the
htdctmw 025 As usual, we ll study the pix on the page opposite. And this time there are two new word
111 HENDERSON B, 1976, Two types of film theory, in Movies and methods, Los Angeles, U.C.L.A. Bill N
As is shown in Figurę A.5, there are two major łdnds of diagram types: structure diagrams and behavi
Subscription warrants Basically, there are two main types of warrants, subscription warrants and der
page 5 Count, say and write. Grammar There is one cap. ł There are two caps. There is one watch. Th
więcej podobnych podstron