
Biomaterials 23 (2002) 313–317
Apatite formation on zirconium metal treated with aqueous NaOH
Masaki Uchida
a,
*, Hyun-Min Kim
a
, Fumiaki Miyaji
b
, Tadashi Kokubo
a
,
Takashi Nakamura
c
a
Department of Material Chemistry, Faculty of Engineering, Graduate School of Engineering, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan
b
Department of Material Science, Faculty of Science and Engineering, Shimane University, Matsue 690-8504, Japan
c
Department of Orthopaedic Surgery, Graduate School of Medicine, Kyoto University, Sakyo-ku, Kyoto 606-8507, Japan
Received 21 July 2000; accepted 7 March 2001
Abstract
Previous studies by the authors have shown that titanium metal, titanium alloys and tantalum metal which were subjected to
aqueous NaOHsolution and subsequent heat treatments form an apatite surface layer upon immersion in a simulated body fluid
(SBF) with ion concentrations nearly equal to those in human blood plasma. These metals form the apatite surface layer even in
living body, and bond to living bone through the apatite layer. In the present study, the apatite-forming ability of NaOH-treated
zirconium metal in SBF has been investigated. A hydrated zirconia gel layer was formed on the surface of the zirconium metal
on exposure to 1–15 m NaOHaqueous solutions at 958C for 24 h. It was observed that the metals treated in NaOHaqueous
solutions with concentrations above 5 m form an apatite layer on their surface in SBF. This indicates that the Zr–OHgroup of the
zirconia gel induces apatite nucleation. The present study points to the possibility of obtaining bioactive zirconium after treatment
by NaOH. # 2001 Elsevier Science Ltd. All rights reserved.
Keywords:
Zirconium; Alkali treatment; Bioactivity; Simulated body fluid (SBF); Apatite; Zirconia gel
1. Introduction
An essential requirement for an artificial material to
bond to living bone is the formation of a bonelike
apatite layer on its surface in body environment [1,2]. In
earlier works, we reported that titanium metal [3–9],
titanium alloys [10,11] and tantalum metal [12–14]
which were exposed to NaOHaqueous solution and
subjected to subsequent heat treatments form a bonelike
apatite layer on their surfaces in a simulated body fluid
(SBF) with ion concentrations nearly equal to those
found in human blood plasma. As in SBF, these NaOH-
and heat-treated metals form the apatite in the living
body, and bond to bone through the apatite layer. They
are now expected to be useful as bone substitutes even
under highly loaded conditions such as is found in
femoral and tibial bones since they exhibit high fracture
toughness as well as high bone-bonding ability [15].
It is scientifically and technically interesting to know
to what other metals the NaOHtreatment can be
applied to induce apatite-forming ability. In this view,
zirconium metal is worthy of note, since it shows high
mechanical strength and good biocompatibility, thereby
being a material of interest as surgical implant [16–18].
More practically, this metal is considered as an
important alloying component for titanium metal to
improve its mechanical properties [19,20].
In this present study, the apatite-forming ability of
zirconium metal treated with aqueous NaOHhas been
investigated in SBF. It is known that materials that form
a bonelike apatite layer on their surfaces in SBF form
the same apatite layer even in the living body, and they
can chemically bond to living bone thorough the apatite
layer [21–23].
2. Materials and methods
2.1. NaOH treatment of zirconium metal
Plates of commercially pure zirconium metal (Kobe
Steel Ltd., Japan) with dimensions 10 10 1 mm
3
were
abraded using #400 diamond plate, and washed with
*Corresponding author. Tel.: +81-75-753-5537; fax: +81-75-753-
4824.
E-mail address:
uchida@sung7.kuic.kyoto-u.ac.jp (M. Uchida).
0142-9612/02/$ - see front matter # 2001 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 1 ) 0 0 1 1 0 - 7
acetone and distilled water in an ultrasonic cleaner.
They were then immersed for 24 h in 5 ml of 1–15 m
NaOHaqueous solutions at 958C. The metal plates
were then gently washed with distilled water, and dried
at 408C for 24 h.
2.2. Immersion of the specimens in SBF
The zirconium plates untreated and treated with
NaOHaqueous solutions were immersed in 30 ml of
SBF with ion concentrations nearly equal to those in
human blood plasma (Table 1) at 36.58C for various
periods. The SBF was prepared by dissolving reagent-
grade NaCl, KCl, K
2
HPO
4
3H
2
O, MgCl 6H
2
O, CaCl
2
,
and Na
2
SO
4
in distilled water, and buffering the solution
to
pH7.4
with
tris-hydroxymethyl-aminomethane
((CH
2
OH)
3
CNH
3
) and hydrochloric acid at 36.58C.
After a set time period up to 28 days, the zirconium
plates were removed from the SBF, washed with distilled
water, and dried in a clean bench.
2.3. Surface analysis of the specimens
The structural changes occurring on the surface
of the zirconium metal due to the NaOHtreatment
and subsequent immersion in the SBF were investigated
using thin-film X-ray diffractometry (TF-XRD; RINT-
1400, Rigaku Co., Japan) and scanning electron
microscopy (SEM, S2500CX; Hitachi Co., Japan). The
TF-XRD was performed on the metals using a step rate
of 2y=28/min, with a 18 glancing angle against the
incident beam. Some TF-XRD were performed on the
metal samples using a scanning rate of 2y=0.028 step,
followed by 10 s hold time, with a 0.18 glancing angle
against the incident beam. X-ray photoelectron spectro-
scopy (XPS; MT5500 ULVAC-PHI Co. Ltd., Japan)
measurements were carried out on the metals that had
been subjected to the NaOHtreatment. The photoelec-
tron take-off angle was set at 458, and MgK
a
radiation
was used as the source. In order to remove potential
contamination of the metal surfaces, Xe-ion sputtering
was carried out at 3 keV for 1 min before a measurement
was taken. As a standard, the observed binding energies
were corrected from comparison with the binding energy
of C
1s
in CH
2
(284.6 eV).
3. Results
SEM observations showed no structural changes on
the surface of the zirconium metal after immersion in
1–15 m NaOHaqueous solutions at 958C for 24 h. Fig. 1
shows the TF-XRD patterns of the surfaces of
zirconium metals before and after 1–15 m-NaOHtreat-
Table 1
Ion concentrations of human blood plasma and SBF
Ion
Concentration/mm
Blood plasma
SBF
Na
+
142.0
142.0
K
+
5.0
5.0
Mg
2+
1.5
1.5
Ca
2+
2.5
2.5
Cl
103.0
147.8
HCO
3
27.0
4.2
HPO
4
2
1.0
1.0
SO
4
2
0.5
0.5
pH7.20–7.40
7.40
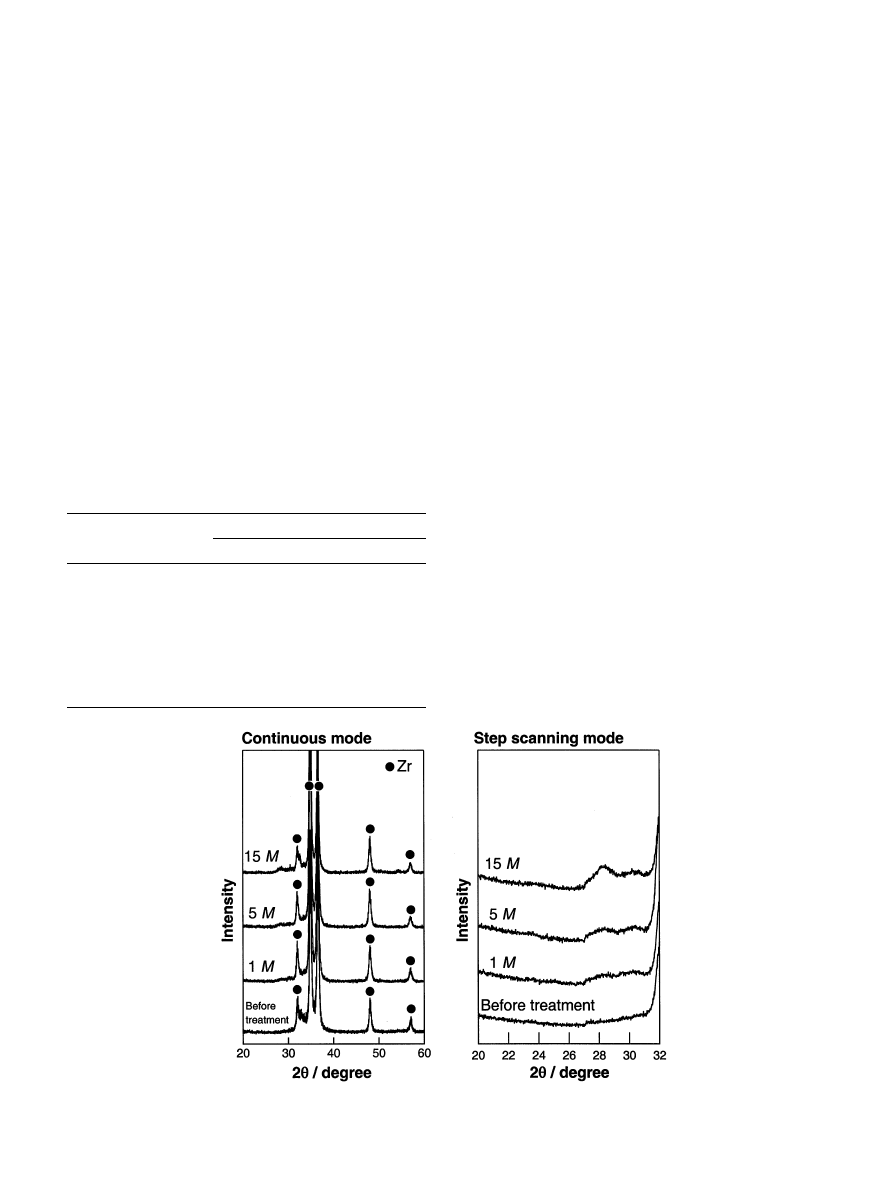
Fig. 1. TF-XRD patterns of the surfaces of zirconium metals treated with NaOHsolutions with various concentrations at 958C for 24 h.
M. Uchida et al. / Biomaterials 23 (2002) 313–317
314
ment at 958C for 24 h. In all the NaOH-treated samples,
a broad peak was observed between 288 and 308 in 2y, in
addition to the sharp peaks ascribed to zirconium. The
intensity of this broad peak increased with increasing
NaOHconcentration. This broad peak is ascribed to an
amorphous phase formed on the metal by the NaOH
treatment.
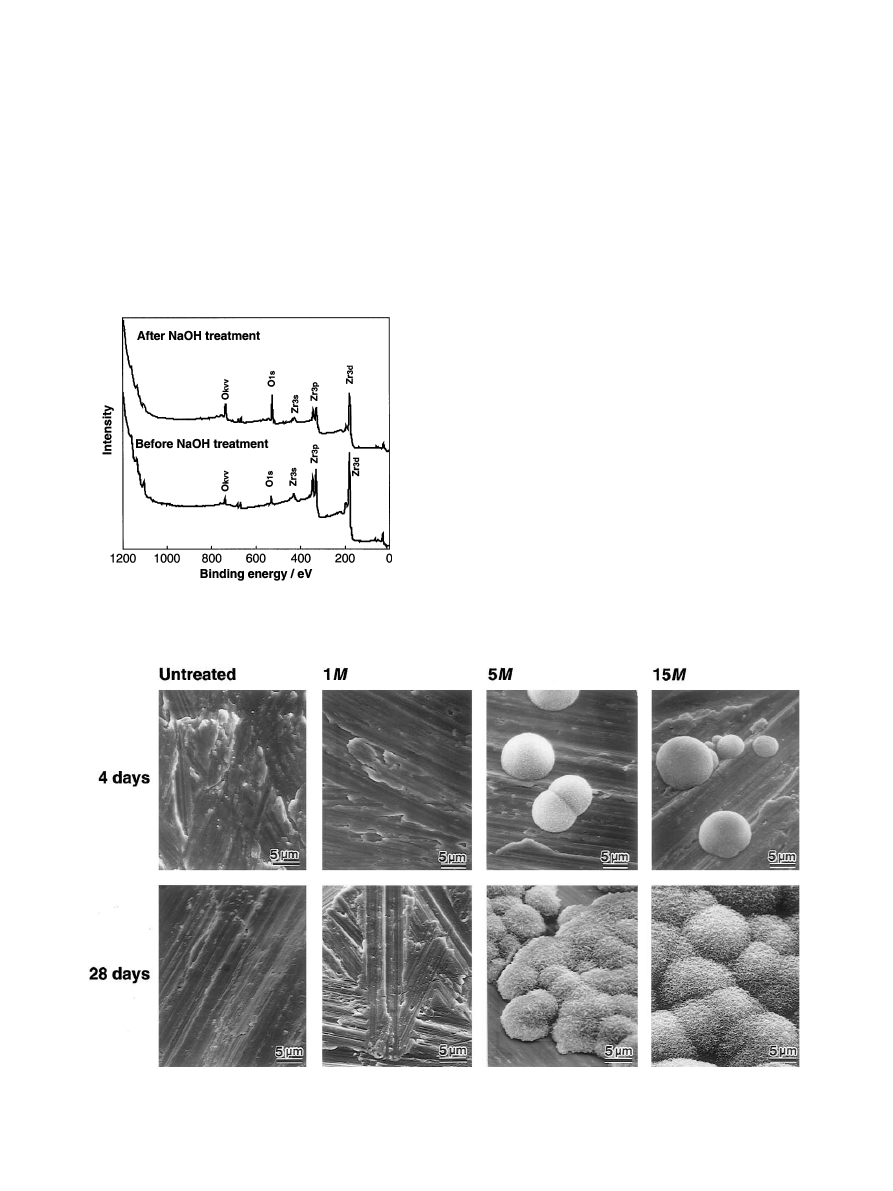
Fig. 2 shows the XPS survey spectra of the surfaces of
zirconium metals before and after 15 m-NaOHtreat-
ment at 958C for 24 h. After the NaOHtreatment, the
intensity of the O
1s
peaks increased, while that of the
Zr
3d
and Zr
3p
peaks decreased. No peak assigned to Na
was detected after the NaOHtreatment.
Fig. 3 shows SEM photographs of the surfaces of
zirconium metals which were untreated and treated with
the NaOHsolutions and then immersed in the SBF for 4
and 28 days, respectively. There were no precipitations
on the surface of zirconium substrates which were
untreated or treated with 1 m NaOHsolution. On the
other hand, island-like spherulites were observed on
parts of the zirconium metals treated with a NaOH
solution greater than 5 m after immersion for 4 days.
The spherulites were observed on the lower surface of
the zirconium substrate, where the metal surface was
faced to the bottom of the vessel, but not on the upper
surface of the zirconium substrate.
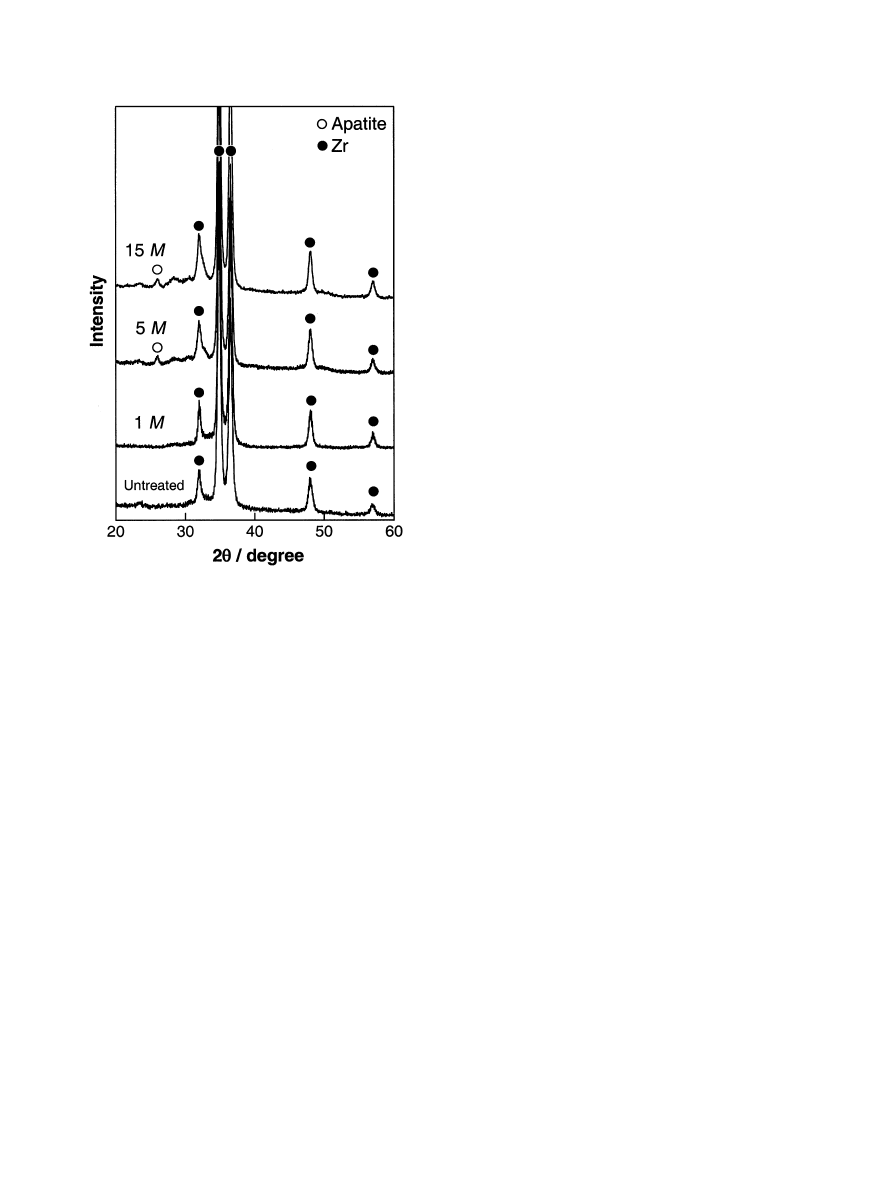
Fig. 4 shows the TF-XRD patterns of the lower
surfaces of the zirconium metals in contact with the
bottom of the vessel, which were untreated and treated
with NaOHsolutions and then immersed in the SBF for
28 days. It can be seen from Figs. 3 and 4 that the
spherulites observed by SEM and TF-XRD are crystal-
line apatite.
4. Discussion
It is clear from the above results that zirconium metal
can form an apatite layer on its surface in SBF when it
Fig. 2. XPS survey spectra of the surfaces of zirconium metals before
and after 15 m-NaOHtreatment at 958C for 24 h.
Fig. 3. SEM photographs of the surfaces of zirconium metals which were untreated and treated with NaOHsolutions, and then immersed in SBF for
4 days and 28 days.
M. Uchida et al. / Biomaterials 23 (2002) 313–317
315
has been previously treated with NaOHsolution with a
concentration greater than 5 m, at 958C for 24 h. This
can be explained as follows.
It is well known that zirconium metal is usually
covered with a passive zirconium oxide layer, and this
passive layer can react with the NaOHsolution. It has
been reported that, when zirconium metal is immersed
in aqueous NaOHsolutions with concentrations higher
than 1 m, hydration of the passive layer occurs [24]. Our
observations, as presented in Figs. 1 and 2, are
consistent with this previous report. Figs. 1 and 2 show
that the hydrated zirconium oxide layer exists as an
amorphous structure, with its thickness increasing with
increasing NaOHconcentration. This amorphous layer
is considered to be a zirconia hydrogel layer. In the case
of NaOH-treated titanium and tantalum metals, Na
+
ions are incorporated into the gel layers, which are
formed by the NaOHtreatment [3,4,12]. In contrast to
these metals, Fig. 2 shows that zirconium metal forms a
Na-free zirconia hydrogel layer.
Titanium and tantalum metals form HTiO
3
nH
2
O or
HTa
2
O
6
H
2
O when they exposed to NaOHsolution
[4,12]. These negatively charged hydrates react with
positively charged Na
+
ions to form sodium titanate or
sodium tantalate hydrogel. In contrast, zirconium metal
is assumed to rarely form such anion species, because of
its higher corrosion durability than titanium and
tantalum metals in NaOHsolution, thereby forming
merely a thin Na-free zirconia hydrogel layer on its
surface [24]. With an aid of this gel layer, the NaOH-
treated zirconium metal forms an apatite surface layer in
SBF (see Figs. 3 and 4). This is attributed to a catalytic
effect of the Zr–OHgroups, abundant on the surface of
the zirconia gel, for apatite nucleation, similar to that
which has been shown in Si–OH[25], Ti–OH[26] and
Ta–OHgroups [27]. The present authors have already
shown that zirconia hydrogel, prepared by hydrolysis
and polycondensation of zirconium-alkoxide, forms an
apatite on its surface in SBF [28]. This indicates that the
surface Zr–OHgroups abundant on the gel can be
effective centres for apatite nucleation.
In the case of NaOH-treated titanium and tantalum
metals in SBF, their surfaces were fully covered with an
apatite layer within 28 days [3,4,12]. In contrast to these
metals, the NaOH-treated zirconium metal formed a
small amount of apatite on its surface even after
immersion in SBF for 28 days. Phenomenally, the
apatite was formed on the bottom surface of the
substrate. Similar apatite formation was observed on
simply heat-treated titanium metal immersed in SBF by
Wang et al. [29]. They assumed that the surface
surrounded with a small closed space is favorable for
apatite formation.
Low apatite-forming ability of NaOH-treated zirco-
nium metal is attributed to the lack of Na
+
ions in the
surface gel layer on the zirconium metal. Titanium and
tantalum metals form sodium titanate hydrogel, and
sodium tantalate hydrogel layers, respectively, on their
surfaces after NaOHtreatment. These metals release
Na
+
ions from the surface gel layers into the SBF, via
an ion-exchange with H
3
O
+
ions present in the SBF. As
a result, the ionic activity product of the apatite in the
surrounding SBF is increased by the increase in
concentration of OH ions. The increased ionic activity
product accelerate apatite nucleation on the Ti–OHor
Ta–OHgroups. In the case of NaOH-treated zirconium,
apatite nucleation, catalysed by the Zr–OHgroups,
cannot be enhanced by the release of the Na
+
ion from
its surface.
Once the apatite nuclei are formed, they can
spontaneously grow by consuming the calcium and
phosphate ions in the SBF, as the SBF is already
supersaturated with respect to the apatite even before
immersion of the metal [30].
5. Conclusions
Zirconium metal forms an apatite on its surface in
SBF after previous treatment with NaOHsolutions with
concentrations above 5 m. The apatite nucleation is
Fig. 4. TF-XRD patterns of the surfaces of zirconium metals which
were untreated and treated with NaOHsolutions, and then immersed
in SBF for 28 days.
M. Uchida et al. / Biomaterials 23 (2002) 313–317
316

induced by Zr–OHgroups in a zirconia hydrogel layer,
which forms on the metal on exposure to NaOH
solution. The result indicates the possibility of obtaining
bioactive zirconium metal by a simple chemical treat-
ment. The apatite-forming ability of NaOH-treated
zirconium metal is, however, lower than those of
NaOH-treated titanium and tantalum metals.
Acknowledgements
This work was supported by Grant-in-Aid for
Scientific Research, the Ministry of Education, Science,
Sports and Culture, Japan.
References
[1] Kokubo T. Biocative glass ceramics: properties and application.
Biomaterials 1991;12:155–63.
[2] Hench LL. Bioceramics: from concept to clinic. J Am Ceram Soc
1991;74:1487–510.
[3] Kokubo T, Miyaji F, Kim HM, Nakamura T. Spontaneous
formation of bone like apatite layer on chemically treated
titanium metals. J Am Ceram Soc 1996;79:1127–9.
[4] Kim HM, Miyaji F, Kokubo T, Nakamura T. Apatite-forming
ability of alkali-treated Ti metal in body environment. J Ceram
Soc Jpn 1997;105:111–6.
[5] Kim HM, Miyaji F, Kokubo T, Nakamura T. Effect of heat
treatment on apatite-forming ability induced by alkali treatment.
J Mater Sci Mater Med 1997;8:341–7.
[6] Kim HM, Miyaji F, Kokubo T, Nakamura T. Bonding strength
of bonelike apatite layer to Ti metal substrate. J Biomed Mater
Res (Appl Biomater) 1997;38:121–7.
[7] Yan WQ, Nakamura T, Kobayashi M, Kim HM, Miyaji F,
Kokubo T. Bonding of chemically treated titanium implants to
bone. J Biomed Mater Res 1997;37:267–75.
[8] Yan WQ, Nakamura T, Kawanabe K, Nishiguchi S, Oka M,
Kokubo T. Apatite layer-coated titanium for use as bone bonding
implants. Biomaterials 1997;18:1185–90.
[9] Nishiguchi S, Nakamura T, Kobayashi M, Kim HM, Miyaji F,
Kokubo T. The effect of heat treatment on bone-bonding ability
of alkali-treated titanium. Biomater 1999;20:491–500.
[10] Kim HM, Miyaji F, Kokubo T, Nakamura T. Preparation of
bioactive Ti and its alloys via simple chemical surface treatment.
J Biomed Mater Res 1996;32:409–17.
[11] Nishiguchi S, Nakamura T, Kato H, Fujita H, Kim HM, Miyaji
F, Kokubo T. Enhancement of bone bonding strength of titanium
alloys by alkali and heat treatment. In: LeGeros RZ, LeGeros JP,
editors. Bioceramics 11. Singapore: World Scientific Publishing
Co. Pte. Ltd., 1998. p. 675–8.
[12] Miyazaki T, Kim HM, Miyaji F, Kokubo T, Kato H, Nakamura
T. Bioactive tantalum metal prepared by NaOHtreatment.
J Biomed Mater Res 2000;50:35–42.
[13] Miyazaki T, Kim HM, Kokubo T, Miyaji F, Kato H, Nakamura
T. Effect of thermal treatment on apatite-forming ability of
NaOH-treated tantalum metal. J Mater Sci Mater Med,
submitted.
[14] Kato H, Nakamura T, Nishiguchi S, Fujita H, Miyazaki T,
Miyaji F, Kim HM, Kokubo T. Bonding of alkali- and heat-
treated tantalum implants to bone. J Biomed Mater Res (Appl
Biomater) 2000;53:28–35.
[15] Long M, Rack HJ. Titanium alloys in total joint replacement}a
materials science perspective. Biomaterials 1998;19:1621–39.
[16] Hanawa T, Masuhara E, Ohkawa S, Sugawara T, Kondou S, Ota
M. Change in surface layer of zirconium in electrolyte solution. J J
Dent Mater 1992;11:469–74.
[17] Jonansson CB, Wennerberg A, Albrektsson T. Quantitative
comparison of screw-shaped commercially pure titanium and
zirconium implants in rabbit tibia. J Mater Sci Mater Med
1994;5:340–4.
[18] Thomsen P, Larsson C, Ericson LE, Sennerby L, Lausmaa J,
Kasemo B. Structure of the interface between rabbit cortical bone
and implants of gold, zirconium and titanium. J Mater Sci Mater
Med 1997;8:653–65.
[19] Kobayashi E, Matsumoto S, Doi H, Yoneyema T, Hamanaka H.
Mechanical properties of the binary titanium–zirconium alloys
and their potential for biomedical materials. J Biomed Mater Res
1995;29:943–50.
[20] Kobayashi E, Doi H, Yoneyama T, Hamanaka H, Gibson IR,
Best SM, Shelton JC, Bonfield W. Influence of aging heat
treatment on mechanical properties of biomedical Ti–Zr based
ternary alloys containing niobium. J Mater Sci Mater Med
1998;9:625–30.
[21] Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T.
Solution able to reproduce in vivo surface-structure change in
bioactive glass-ceramic A-W. J Biomed Mater Res 1990;24:
723–34.
[22] Ohtsuki C, Kokubo T, Takatsuka K, Yamamuro T. Composi-
tional dependence on bioactivity of glassed in the system CaO–
SiO
2
–P
2
O
5
: its in vitro evaluation. J Ceram Soc Jpn 1991;99:1–6.
[23] Ohtsuki C, Kushitani H, Kokubo T, Kotani S, Yamamuro T.
Apatite formation on the surface of ceravital-type glass-ceramic in
the body. J Biomed Mater Res 1991;25:1363–70.
[24] Gadallah AG, Mazhar AA, El-Taib Heakal F, Ameer MA.
Formation and dissolution of anodic oxide films on zirconium in
NaOH: kinetic studies. J Appl Electrochem 1989;19:213–8.
[25] Li P, Ohtsuki C, Kokubo T, Nakanishi K, Soga N, Nakamura T,
Yamamuro T. Apatite formation induced by silica gel in a
simulated body fluid. J Am Ceram Soc 1992;75:2094–7.
[26] Li P, Ohtsuki C, Kokubo T, Nakanishi K, Soga N, Nakamura T,
Yamamuro T. A role of hydrated silica, titania and alumina in
forming biologically active apatite on implant. Berlin, Germany:
Transactions of Fourth World Biomaterials Congress, 1992.
p. 5.
[27] Miyazaki T, Kim HM, Kokubo T, Miyaji F, Kato H, Nakamura
T. Induction and acceleration of bonelike apatite formation of
tantalum oxide gel in simulated body fluid. J Sol-Gel Sci Tech,
submitted.
[28] Uchida M, Kim HM, Miyaji F, Kokubo T, Nakamura T.
Apatite-forming ability of zirconia gel in modified SBF solutions.
In: LeGeros RZ, LeGeros JP, editors. Bioceramics 11. Singapore:
World Scientific Publishing Co. Pte. Ltd., 1998. p. 77–80.
[29] Wang XX, Hayakawa S, Tsuru K, Osaka A. A comparative study
of in vitro apatite deposition on heat-, H
2
O
2
-, and NaOH-treated
titanium surfaces. J Biomed Mater Res 2001;54:172–8.
[30] Ohtsuki C, Kokubo T, Yamamuro T. Mechanism of apatite
formation on CaO–SiO
2
–P
2
O
5
glasses in simulated body fluid. J
Non-Cryst Solids 1992;143:84–92.
M. Uchida et al. / Biomaterials 23 (2002) 313–317
317
Wyszukiwarka
Podobne podstrony:
Biomimetic apatite formation on chemically treated titanium
Prezentacja formatka
Formaty plików dźwiękowych
Przekroje Format A2
h 1 formatka 2012 budowa hv
P3 PLAN KONSERWATORSKI (FORMAT 2000x2500)
Formatki do zaj z OC Cwiczenie Nieznany
Automatyczne formatowanie dokumentu, informatyka, grafika
Formatowanie(1), Edukacja, Informatyka
2013.09.17 FORMATKA RYSUNKOWA A4
format[1], Szkoła, Systemy Operacyjnie i sieci komputerowe, systemy, semestr I
Przenoszenie formatu z komórki na komórkę, excel
sciaga format normalny
Formatka!!!
WORD FORMATION PRACTICE N 1
Format scenariusza(1)
word formation od EXTEND
4 Formatowanie zaawansowane punktury i numeratory Cwiczenie 4
17 Money Making Candle Formations
więcej podobnych podstron