Appetite 48 (2007) 12–19
Research report
From motivation to behaviour: A model of reward sensitivity,
overeating, and food preferences in the risk profile for obesity
Caroline Davis
a,b,c,
, Karen Patte
a
, Robert Levitan
c
, Caroline Reid
a
,
Stacey Tweed
b
, Claire Curtis
a
a
Department of Kinesiology & Health Sciences, York University, Toronto, Canada
b
Graduate Programme in Psychology, York University, Toronto, Canada
c
Centre for Addiction and Mental Health, Toronto, Canada
Received 30 March 2006; received in revised form 18 May 2006; accepted 23 May 2006
Abstract
The reinforcing effects of addictive drugs and palatable foods are regulated, at least in part, by a common biological mechanism. The
reactivity or sensitivity of these brain reward regions have been found to correlate significantly with the risk for a variety of drug
addictions. Sensitivity to Reward (STR) is conceptualised as a psycho-biological personality trait rooted firmly in the availability of
dopamine in the mesocorticolimbic (‘common reward’) pathways, and as such is a good candidate for studying motivational factors and
eating behaviours. The purpose of the present study was to examine whether STR was related to behaviours that contribute to excess
body weight. Structural equation modelling procedures were used with a sample of healthy adult women (n ¼ 151). We hypothesised that
STR would positively predict overeating and a preference for foods high in fat and sugar; and that these two behaviour would, in turn,
predict a higher Body Mass Index. Results provided an excellent fit of the model to our data confirming our view that a personality trait
like STR can only influence a physical condition like body weight indirectly by the way it co-varies with behaviours that contribute
directly to variation in the outcome variable.
r
2006 Elsevier Ltd. All rights reserved.
Keywords: Sensitivity to reward; Overeating; Food preferences; BMI
Introduction
The reinforcing effects of addictive drugs and palatable
foods are regulated, at least in part, by a common brain
mechanism depending crucially on the level of dopamine
(DA) activation in mesocorticolimbic regions (e.g.
Risinger, Freeman, Rubinstein, Low,
). Indeed, the sub-cortical brain does not
seem to differentiate among rewards provoked by natural
reinforcers like food, illicit drugs like cocaine, or beha-
viours like gambling (
Kelley, Schiltz, & Landry, 2005
). For
example, two recent studies found that obese women had
lower rates of alcohol (
) and marijuana
use (
Warren, Frost-Pineda, & Gold, 2005
) than their
normal weight, age-matched counterparts, and that, in
both cases, an inverse relationship existed between body
mass index (BMI) and drug use. The authors concluded
that overeating competes with pharmacologic agents for
brain reward sites, and thereby may serve as a buffer for
the use and abuse of other addictive behaviours. Com-
plementary to this viewpoint is evidence that appetite
suppression is a major pharmacological effect of chronic
drug use (see
Cochrane, Malcolm, & Brewerton, 1998
Clearly many factors influence the kinds of pleasure we
pursue in our daily lives. The context—through classical
and operant conditioning processes—is particularly im-
portant in determining the choices we make and the
frequency of their use (
). In most
Western societies, the convenience, the availability, and the
ARTICLE IN PRESS
0195-6663/$ - see front matter r 2006 Elsevier Ltd. All rights reserved.
doi:
Corresponding author. 343 Bethune College, 4700 Keele Street,
Toronto, Ontario, Canada, M3J 1P3. Tel.: +1 416 736 2100;
fax: +1 416 736 5774.
E-mail address:
relatively low cost, make tasty foods a highly salient reward
for many people. Aggressive marketing by the fast-food
industry further enhances the temptation to overindulge.
In view of the many commonalities between food and
drug reward, some have argued that chronic overeating can
be modelled as an addictive behaviour similar to other
substance-dependent disorders (e.g.
;
;
Wang, Volkow, Thanos, & Fowler, 2004
). Consequently,
there is a growing interest in examining the role of DA
neurotransmission in the risk profile for obesity—especially
how its variation in the population affects individual
differences in vulnerability to overeating.
Undoubtedly there are many routes to obesity so it is
reasonable to assume that weight-gaining individuals
possess a variable set of biological and behavioural
susceptibility factors (
). On the one
hand, there is evidence that hypo-dopaminergic function-
ing—what has been called a Reward Deficiency Syndrome
(RDS)
1
—underlies a range of addictions including alco-
holism, cocaine abuse, and pathological gambling (see
). Some have recently
argued that RDS is also a factor in the development of
obesity (e.g.
). On the other hand,
enhanced DA functioning, which is characterized by a
heightened hedonic capacity and greater behavioural acti-
vation (e.g.
Cohen, Young, Baek, Kessler, & Ranganath,
), fosters strong appetitive responses to the natural
pleasures in life. For instance, amplification of the DA
signal in human participants via a small dose of oral
methylphenidate
2
increased their desire to eat in response
to a palatable food cue (
). There is also
evidence that obese individuals have enhanced sensitivity in
brain areas associated with the sensory (e.g. lips, tongue,
mouth) processing of food (
Regrettably, our current obesigenic environment can
exploit those with a high sensitivity to reward (STR) by
promoting consumption beyond caloric need. Non-homeo-
static eating can take several forms including eating that is
driven by emotional states (‘comfort’ eating) or by
environmental cues such as the sight and smell of food
stimuli. It is also characterised by frequent snacking and
episodes of binge eating. In addition, hedonic processes—
commonly regarded as the pleasure that is associated with
food—are of central relevance to other aspects of eating. A
primary target of such influence is an enhanced preference
for foods that are fat and sweet since they typically provide
us with a greater reinforcement value than bland food
(
). In a recent study of high and low-
fat phenotypes—those habitually consuming a diet con-
taining 443% or
o32% fat respectively—the former
group comprised a significantly greater number of obese
individuals (
). However, among the
high-fat group there was considerable variability in BMI,
and results indicated that those who were prone to weight
gain reported higher hedonic responsiveness to eating, and
a greater intensity of pleasurable sensations from the taste
of food. We predicted that those who are highly sensitive to
reward would therefore also be more responsive (than their
anhedonic counterparts) to the perceived palatability of
sweet and fatty foods.
To date, only a handful of studies has investigated the
relationship between reward sensitivity, eating behaviours,
and body weight, and are all supportive of positive links
(
;
Loxton &
). For example,
found that young women who were more sensitive to
reward reported stronger food cravings and had a higher
BMI. Likewise,
found that anhedonic
women were less likely to overeat, after controlling for
depression in their regression model. However, as a body
of work these studies are limited by the general use of small
samples of primarily young, normal-weight women, by self-
report instead of objective measures of body weight, and by
single markers of overeating.
The present study expands on this research by using a
more comprehensive set of measures to reflect the
constructs of interest, and by testing a large sample of
women who are more representative of the adult popula-
tion in terms of BMI and (pre-menopausal) age. We used
structural equation modelling (SEM) to test the prediction
that sensitivity to reward is a phenotypic positive influence
on weight gain—especially in an environment that pro-
liferates with tempting and available foods—via its
influence on overeating and food preferences (see
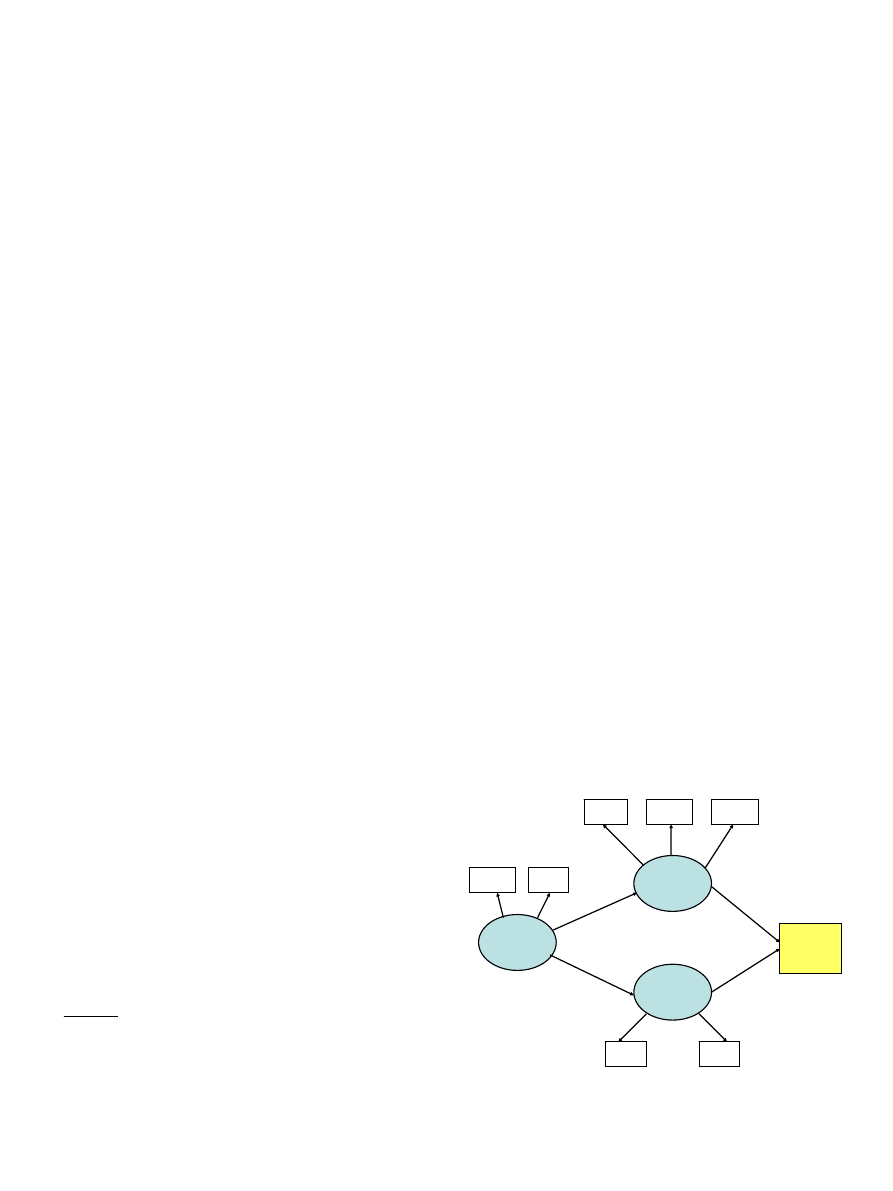
The first three constructs in the path diagram were
modelled as multi-factorial latent variables. Sensitivity to
ARTICLE IN PRESS
Sensitivity
to Reward
BMI
Reward
Sensitivity
BAS
Scale
Overeating
Food
Preferences
High
Fat
High
Sugar
Binge
Eating
External
Eating
Emotional
Eating
Fig. 1. Structural equation model testing relationships among Sensitivity
to Reward, Overeating, Food Preferences, and Body Mass Index in a
sample of adult women.
1
When there is sub-optimal functioning of the brain reward cascade,
which could be caused by certain genetic variants, especially in the DA
system, the brain of that person is likely to need a DA ‘fix’ to feel good and
to improve the DA deficit (
).
2
Methylphenidate (a.k.a.Ritalin) is a psychomotor stimulant that has a
mechanism of action similar to cocaine. It binds to the DA transporter
increasing neurotransmitter availability in the synapse.
C. Davis et al. / Appetite 48 (2007) 12–19
13

Reward had two commonly used measures of this
personality trait. The measured observations for Over-
eating comprised binge eating, emotionally-driven eating,
and eating prompted by external stimuli rather than
hunger. All these aspects of overeating have been
implicated in the risk profile for obesity (e.g.
Meigs, Hayden, Williamson, & Nathan, 2002
Herbeth, Mejean, & Siest, 2000
). Finally, Food Preferences had two observed
measures: a preference for high fat food and a preference
for sweet food.
Methods
Subjects
One hundred and fifty-one healthy pre-menopausal
women between the ages of 25 and 50 years (mean ¼ 33.5
years; SD ¼ 7.1) took part in the study. They were solicited
from posters placed at two university campuses, and at
various hospital and community centres in the urban core
of a large Canadian city, asking for volunteers to
participate in a ‘‘health psychology study’’. Participants
were screened initially during a structured telephone
interview and excluded if they had any serious medical
condition, were underweight (based on their reported
height and weight), were pregnant or had recently given
birth, or were currently being treated for any psychiatric
disorder including an eating disorder. They were also
required to be fluent in English. Eighty-four percent of the
participants were born in Canada, or an English speaking
Western country like the United States or Britain. Their
mean BMI was 27.6 (SD ¼ 5.9). Based on accepted BMI
weight-status categories (
),
38.5%
of
the
sample
were
normal
weight
(BMI418.5 and
o25); 27% were overweight (BMI425
and
o30); and 34% were obese (BMI430). One subject
had a BMI of 18.4.
Measures
1. Sensitivity to Reward was measured by 2 self-report
questionnaires, both designed to assess individuals’
capacity for pleasure and reward—i.e. their degree of
appetitive motivation and response to signals or
reinforcement and/or non-punishment.
(i) The STR subscale of the SPSR Questionnaire
Torrubia, Avila, Molto, & Caseras, 2001
) comprises
24 forced-choice items which assess the respondent’s
approach responses under various conditions of
reward such as physical sensations (‘‘Is it easy
for you to associate tastes and smells to very
pleasant events?’’). This scale has shown good
internal
consistency,
temporal
stability,
and
concurrent validity (e.g.
). The alpha coefficient in the present study
was 0.77.
(ii) The Behavioural Activation (BAS) subscale of the
BIS/BAS questionnaire (
)
comprises 13 items which assess one’s persistent
pursuit of desired goals, the desire for new and
pleasing stimuli, and the positive anticipation of
rewarding events in the future. In this study, we used
the total score instead of the three subscales to
decrease the number of parameters to be estimated
(relative to the sample size) in the SEM, and because
there was high internal consistency of the total score.
The a coefficient was 0.88.
2. Overeating was assessed by three separate scales, each
reflecting the tendency to overeat in response to certain
triggers:
(i) The Dutch Eating behaviour Questionnaire [DEBQ]
(
Van Strien, Frijters, Bergers, & Defares, 1986
)
assesses three aspects of eating behaviour. In this
study only the Emotional Eating subscale (e.g. the
degree to which eating is prompted by emotional
states like tension and worry rather than by hunger)
and the External subscale (e.g. the degree to which
one tends to overeat if food looks and smells good)
were used. The Dietary Restraint subscale was not
deemed a useful index of overeating given its focus
on dieting and calorie restriction.
(ii) The Binge Eating Questionnaire [BEQ] (
) assesses the frequency and
severity of symptoms associated with binge eating
(such as loss of control over eating, and negative
affect following a binge) and with purging (e.g. self-
induced vomiting). Binge eating was quantified by
summing the responses to 5 (yes–no) questions
tapping aspects of the behaviour (e.g. ‘‘Are there
times when you feel you cannot voluntarily stop
eating?’’).
3. Food Preferences were assessed by The Food Preference
Questionnaire (
). This scale was
designed as a 2 [FAT: high vs. low] 3 [CARBOHY-
DRATE: high simple, high complex, low carbohydrate/
high protein] measure of preference for various kinds of
macronutrients. It contains 72 common foods in each of
the six cells listed in random order. Respondents
indicate their preference of each on a 9-point Likert
scale. A high-fat preference score is obtained by
summing the 36 high-fat items and calculating the
mean. The high-sugar preference score comprises the 24
high-sugar items. The authors report good reliability
and validity of these measures, and the a coefficients for
our sample were 0.96 and 0.87, respectively.
4. BMI (weight[kg]/height[m
2
]) was calculated from height
and weight measured with the participant wearing
indoor clothing and standing in stocking feet.
Procedure
For those who passed the telephone screening interview
a testing appointment was arranged at the closest of two
ARTICLE IN PRESS
C. Davis et al. / Appetite 48 (2007) 12–19
14
available research facilities. After giving signed informed
consent, a more detailed screening interview took place to
confirm eligibility criteria. The questionnaire package was
then completed, and height and weight were measured. At
the end of the study, each participant was paid a small
stipend for her time and out-of-pocket expenses.
Results
The proposed model (see
) was tested using SEM
and Amos 6.0 software. SEM is a useful statistical
procedure for researchers who want to test a theory
involving causal processes, and therefore is well suited to
the management of cross-sectional data for inferential
purposes (
). The kurtosis and skew of the eight
variables in the model were between 1.04 and 0.50 and
0.53 and 0.96, respectively—values which are well within
the acceptable range to proceed with SEM according to
West, Finch, and Curran (1995)
In this study, STR was modelled as a latent variable with
two measured variables. Overeating and Food Preferences
were also latent variables comprising three and two
measured variables, respectively. Prior to analysing the
structural model, the measurement model was tested using
exploratory factor analysis with Promax rotation. As
expected, a clear three-factor solution emerged, and the
correlations among factors were modest (0.27–0.39). It is
noteworthy, however, that although the external eating
variable had a strong loading on the Overeating factor, as
predicted, it also had a modest relationship with the STR
factor (0.27). This cross-loading is understandable since
external eating reflects an appetitive response to food cues
in one’s environment and STR describes one’s general
capacity for pleasure, as well as the motivation to seek out
reinforcing stimuli. A list of the factor loadings is shown in
.
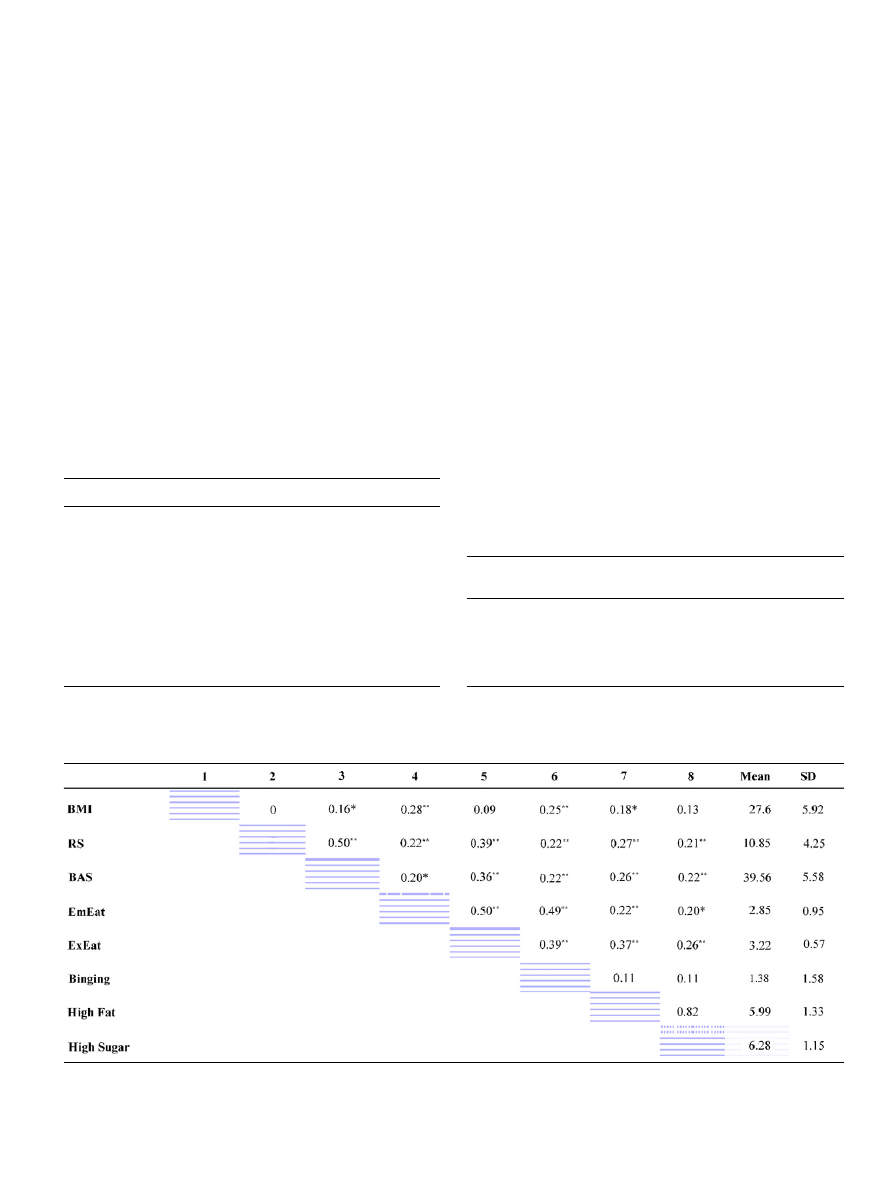
presents a bivariate correlation matrix
of all the measured variables in the study, including BMI,
and the sample means and standard deviations for these
variables.
ARTICLE IN PRESS
Table 1
Factor loadings for the measurement model
Factor 3
Factor 2
Factor 1
Sensitivity to reward
Reward Sensitivity Scale (SPSRQ)
0.87
0.01
0.02
BAS scale (BIS/BAS)
0.88
0.04
0.02
Overeating
Binge eating
0.02
0.85
0.14
Emotional eating
0.12
0.89
0.04
External eating
0.27
0.58
0.15
Food preferences
High fat preference
0.02
0.01
0.95
High sugar preference
0.05
0.03
0.97
Table 2
Intercorrelations, means, and standard deviations for all measured variables
BMI ¼ Body Mass Index. RS ¼ Sensitivity to Reward subscale of the SPSR questionnaire. BAS ¼ Behavioral Activation Scale of the BIS/BAS
questionnaire. EmEat ¼ Emotional Eating subscale of the DEBQ questionnaire. ExEat ¼ External Eating subscale of the DEBQ questionnaire.
Binging ¼ scores on the BEQ. High Fat ¼ High fat preference subscale of the Food Preference questionnaire. High Sugar ¼ High sugar preference
subscale of the Food Preference questionnaire. * ¼
o0.05. ** ¼ o 0.01.
Table 3
Descriptive measures of the model fit
Fit Index
Acceptable
model fit
Obtained
value
Comparative Fit Index (CFI)
X
0.95
0.99
Std Root Mean Sq Residual (SRMR)
p0.08
0.04
Root Mean Sq Error Approx (RMSEA)
p0.06
0.04
Goodness-of-Fit Index (GFI)
X
0.95
0.97
Adj Goodness-of-Fit Index (AGFI)
X
0.90
0.93
C. Davis et al. / Appetite 48 (2007) 12–19
15
w
2
analysis tested the hypothesis that the relationships
proposed in the model are a reasonable explanation of
those existing in the data. We obtained a significant
(w
2
¼
33:91, df ¼ 17, p ¼ 0:009). However, a non-signifi-
cant w
2
—indicating a good absolute or overall model fit—is
frequently not obtained. This may occur either because the
model is slightly mis-specified or does not account for all
the measurement error. Therefore, it is important to
examine other indices that have been developed to assess
the fit of the model. These values (e.g. the root-mean-
square error [RMSEA] was 0.081 and should be
o0.06;
and the goodness-of-fit index [GFI] was 0.947 and should
be 40.95), also suggested that the model was not a
particularly good fit to the data.
Model modification
Each postulated modification to a model should
be defensible primarily from a theoretical perspective
(
;
). In other words, purely
data-driven decisions are inappropriate in SEM. Given the
theoretical association (as discussed above) and the modest
cross-loading of the external eating indicator and the
sensitivity to reward factor, a path between sensitivity to
reward and external eating was freed. This simple
modification reduced the w
2
by 13.81 and produced a
non-significant value (w
2
¼
20:11, df ¼ 16, p ¼ 0:215)
indicating a good absolute or overall model fit. We also
confirmed the goodness-of-fit of the model by examining
other indices developed for this purpose (see
;
).
lists five commonly reported fit
indices and the values considered representative of a good
fit. The Comparative Fit Index evaluates the fit of the
estimated model relative to the fit of the independent model
(where no relationships are estimated between variables).
The Standardized Root Mean Squared Residual is an index
of the average differences between the sample variances
and covariances and the estimated (model) variances and
covariances. The Root Mean Square Error of Approxima-
tion is a popular measure that also takes into consideration
the complexity of the model (i.e. the degrees of freedom).
The Goodness of Fit Index is a measure of the proportion of
variance and covariance that the proposed model is able to
explain (similar to R
2
in regression). And finally, the
Adjusted Goodness of Fit Index is the GFI adjusted for
the degree of parsimony in the model. It can be seen from
the values we obtained in the present analysis that in every
case the observed value indicates a good fitting model.
Path coefficients assess the magnitude of the relationships
among the latent and measured variables in the model. An
examination of critical ratios
3
(CR) for each coefficient
indicated that all paths were statistically significant and the
CR values ranged between 2.10 (p ¼ 0:036) and 5.59
(p
o0.0001).
shows the standardized regression
weights for each path tested in the revised model.
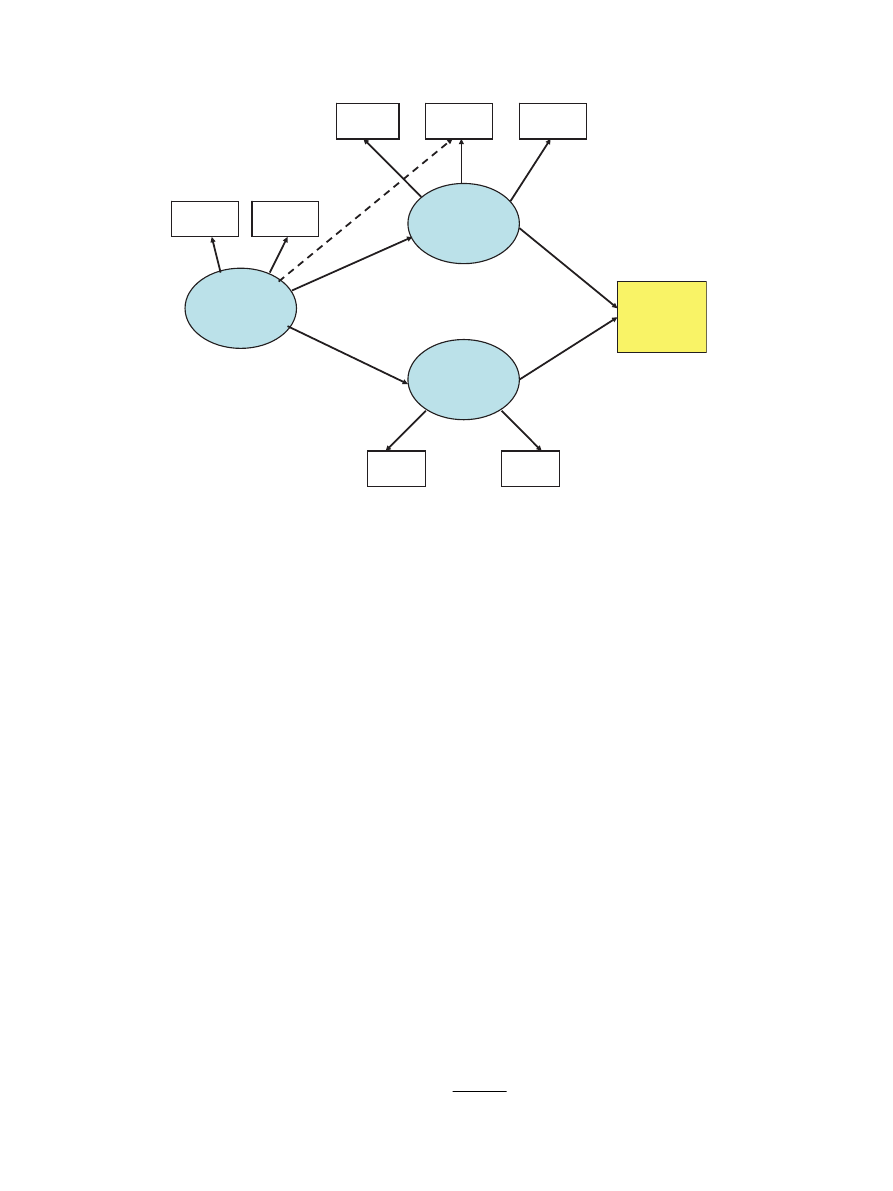
Discussion
In a large sample of healthy adult women, we tested the
theory that STR would predict (i) the tendency to eat
beyond caloric need and in the absence of hunger and,
ARTICLE IN PRESS
Sensitivity
to Reward
BMI
Reward
Sensitivity
BAS
Scale
Overeating
Food
Preferences
High
Fat
High
Sugar
Binge
Eating
External
Eating
Emotional
Eating
0.57
**
0.41
**
0.71**
0.68**
0.71**
0.72**
0.60**
0.32
**
0.18
*
1.0**
0.73**
0.39**
Fig. 2. Modified structural equation model showing standardized path coefficients for all statistical significant associations.
3
The critical ratio is calculated by dividing the unstandardized estimate
by its standard error.
C. Davis et al. / Appetite 48 (2007) 12–19
16

(ii) a heightened preference for sweet and fatty foods—two
factors which consistently co-vary with body weight in the
positive direction. SEM procedures are advantageous in
behavioural sciences research because they allow us to
study constructs that are difficult to observe directly or
which have complex manifestations—like overeating.
There are also no other widely and easily applied statistical
methods for modelling multivariate relationships (
). In the present study, the structural model (see
)
provided an excellent fit to the data after we accounted for
the moderate relationship between external eating and
STR, which emerged from testing the measurement model.
While SEM cannot establish the causal direction of
specified pathways, a good fitting model can confirm a
theoretically sound set of relationships.
Overeating
The specified path between STR and overeating was
strongly positive, supporting our earlier work (
) and that of other researchers (e.g.
)—albeit in the present study, using a more compre-
hensive set of markers for each construct. It is generally
agreed that variation in reward sensitivity (and related
constructs like behavioural activation and novelty/sensa-
tion seeking) is regulated by DA and influenced by the level
of activation in reward-sensitive regions of the midbrain
(
;
). Therefore, appetitive responses to food should be
enhanced in more reactive individuals—as we found—and
contribute to a greater level of consumption and the
tendency to overeat.
Our findings are, however, conceptually at variance with
the RDS view of overeating and obesity. For example,
found that obese individuals had a
significant reduction in DA D2 receptor availability.
Others have also found a higher prevalence of the Taq1A
allele (thought to be linked with lower receptor levels) in
obese individuals (e.g.
;
). One reason for this apparent contradiction may be
the sample differences in BMI. The RDS studies have
generally used morbidly obese subjects, typically recruited
from obesity treatment clinics. For example, in the study
by
, the obese adults all had a BMI440
(Class III obesity). By contrast, in the study by
, which showed a positive link between
STR, food cravings, and body weight, 83% of the
sample were normal weight young women. Since none
of the participants in the present study had a BMI440,
it is important to emphasis that our results cannot
extrapolate to women at the very high end of the weight
distribution.
Traditionally, much of human obesity research in the
behavioural sciences has treated excess adiposity as a
homogeneous condition—at least statistically—by includ-
ing all participants with a BMI 430 in a single category.
Nowadays BMI values among the obese typically span
a broad range from 30 to over 60, and the prevalence of
morbid obesity is rising at twice the rate of milder forms
(
Freedman, Khan, Serdula, Galuska, & Dietz, 2002
Clearly it will behove future researchers to consider obesity
as a heterogeneous set of conditions (defined largely by
degree of severity) which are likely to differ in their clinical
symptoms, their profile of risk factors, and their response
to treatment strategies. Indeed, from the perspective of the
present study, it may be that the relationships we found
among STR, overeating, food preferences, and BMI are
only positive in the range from normal weight to mildly
obese, and that a different set (and direction) of relation-
ships may exist in those with morbid obesity. For example,
food may serve as a positive reinforcer for some individuals
and as a negative reinforcer (i.e. a ‘self-medicating’
behaviour) for others, thereby influencing the frequency
and magnitude of their overeating, and subsequent weight
gain.
Food preferences
We also found that STR was positively related to a
preference for sweet and fatty food. At this point, we can
only speculate on the mechanisms underlying this associa-
tion. A combination of both physiological and psycholo-
gical motivations are clearly at the heart of our selection
and preference for certain foods (
;
). For
example, when palatable foods are consumed trace
amounts of endogenous opiates are released in the brain
(e.g.
Drewnowski, Krahn, Demitrack, Nairn, & Gosnell,
), which in turn, increase DA transmission in brain
reward regions. The ‘pleasure’ experienced from this
behaviour should therefore be enhanced in those with a
greater sensitivity in brain reward regions. On the other
hand, the social and psychological context is also of great
importance in forming our food preferences. Since highly
palatable foods are often associated with pleasurable social
interactions and celebrations, hedonic individuals may be
more likely to partake in, and enjoy, social gatherings, and
therefore to form more positive conditioned responses to
tasty food cues in their environment.
Finally, it is important to acknowledge that although the
association between Food Preferences and BMI was
statistically significant, it was weaker than the other
relationships in the model. One explanation could be a
relatively restricted range on the measured markers of this
latent variable (viz. high fat and high sugar preference)
because fat and sweet foods have a great universal appeal.
The high means and small standard errors for these two
scales confirm this possibility.
Conclusions
Personality can only influence a physical condition like
body weight indirectly by the way it co-varies with
behaviours that contribute directly to its variation in the
ARTICLE IN PRESS
C. Davis et al. / Appetite 48 (2007) 12–19
17

population. STR is a biologically-based trait rooted firmly
in the reactivity of the mesocorticolimbic (‘common
reward’) DA pathways and as such is a good candidate
for study in the area of ingestive behaviours. As predicted,
a high STR was related to overeating as well as an
heightened preference for sweet and fatty foods; and these
two factors were positively correlated with BMI. Future
studies
should
extend
this
research
to
those
with
severe obesity, and should also test the model in a sample
of adult men.
References
Blum, K., & Braverman, E. R. (2001). ‘‘Reward deficiency syndrome’’: An
emerging concept. Molecular Psychiatry, 6(Suppl. 1), S2–S5.
Blum, K., Braverman, E. R., Wood, R. C., Gill, J., Li, C., Chen, T. J. H.,
et al. (1996). Increased prevalence of Taq 1 A(1) allele of the DA
receptor gene (DRD(2)) in obesisty with comorbid substance use
disorder: A preliminary report. Pharmacogenetics, 6, 297–305.
Blundell, J. E., Stubbs, R. J., Golding, C., Croden, F., Alam, R.,
Whybrow, S., et al. (2005). Resistance and susceptibility to weight
gain: Individual variability in response to high-fat diet. Physiology &
Behavior, 86, 614–622.
Boomsma, A. (2000). Reporting analyses of covariance structures.
Structural Equation Modeling, 7, 461–483.
Bowirrat, A., & Oscar-Berman, M. (2005). Relationship between
dopaminergic neurotransmission, alcoholism, and reward deficiency
syndrome. American Journal of Medical Genetics Part B-Neuropsy-
chiatric Genetics, 132B, 29–37.
Byrne, B. M. (2001). Structural equation modelling with AMOS. Mahwah,
NJ: Erlbaum.
Carver, C. S., & White, T. L. (1994). Behavioral inhibition, behavioral
activation, and affective responese to impending reward and punish-
ment: The BIS/BAS Scales. Journal of Personality and Social
Psychology, 67, 319–333.
Caseras, X., Avila, C., & Torrubia, R. (2003). The measurement of
individual differences in behavioural inhibition and behavioural
activation systems: A comparison of personality scales. Personality
and Individual Differences, 34, 999–1013.
Cochrane, C., Malcolm, R., & Brewerton, T. (1998). The role of weight
control as a motivator for cocaine abuse. Addictive Behaviors, 23,
201–207.
Cohen, M. X., Young, J., Baek, J.-M., Kessler, C., & Ranganath, C.
(2005). Individual differences in extraversion and DA genetics predict
neural reward responses. Cognitive Brain Research, 25, 851–861.
Corwin, R. L. (2006). Bingeing rats: A model of intermittent excessive
behavior? Appetite, 46, 11–15.
Corwin, R. L., & Hajnal, A. (2005). Too much of a good thing:
Neurobiology of non-homeostatic eating and drug abuse. Physiology &
Behavior, 86, 5–8.
Davis, C., Strachan, S., & Berkson, M. (2004). Sensitivity to reward:
Implications for overeating and overweight. Appetite, 42, 131–138.
Delahanty, L., Meigs, J. B., Hayden, D., Williamson, D. A., & Nathan, D.
M. (2002). Psychological and behavioral correlates of baseline BMI in
the Diabetes Prevention Program (DPP). Diabetes Care, 25,
1992–1998.
Depue, R. A., & Collins, P. F. (1999). Neurobiology of the structure of
personality: DA, facilitation of incentive motivation, and extraversion.
Behavioral Brain Science, 22, 491–517 (discussion 518–569).
Di Chiara, G., Bassareo, V., Fenu, S., De Luca, M. A., Spina, L., Cadoni,
C., et al. (2004). DA and drug addiction: The nucleus accumbens shell
connection. Neuropsychopharmacology, 47(Suppl. S), 227–241.
Drewnowski, A., Krahn, D. D., Demitrack, M. A., Nairn, K., & Gosnell,
B. A. (1992). Taste responses and preferences for sweet high-fat
foods—evidence for opioid involvement. Physiology & Behavior, 51,
371–379.
Epstein, L. H., & Leddy, J. J. (2006). Food reinforcement. Appetite, 46,
22–25.
Evans, A. H., Lawrence, A. D., Potts, J., MacGregor, L., Katzenschlager,
R., Shaw, K., et al. (2006). Relationship between impulsive sensation
seeking traits, smoking, alcohol and caffeine intake, and Parkinson’s
disease. Journal of Neurology Neurosurgery and Psychiatry, 77,
317–321.
Franken, I. H. A., & Muris, P. (2005). Individual differences in reward
sensitivity are related to food craving and relative body weight in
healthy women. Appetite, 45, 198–201.
Freedman, D. S., Khan, L. K., Serdula, M. K., Galuska, D. A., & Dietz,
W. H. (2002). Trends and correlates of class 3 obesity in the United
States from 1990 through 2000. Journal of the American Medical
Association, 288, 1758–1761.
Geiselman, P. J., Anderson, A. M., Dowdy, M. L., West, D. B., Redmann,
S. M., & Smith, S. R. (1998). Reliability and validity of the
macronutrient self-selection paradigm and a Food Preference Ques-
tionnaire. Physiology & Behavior, 63, 919–928.
Halmi, K. A., Falk, J. R., & Schwartz, E. (1981). Binge-eating and
vomiting—A survey of a college population. Psychological Medicine,
11, 697–706.
James, A. G., Gold, M. S., & Liu, Y. (2004). Interaction of satiety and
reward responses to food stimulation. Journal of Addictive Diseases,
23, 23–37.
Kaplan, D. (1990). Evaluating and modifying covariance structure
models: A review and recommendation (with discussion). Multivariate
Behavioral Research, 25, 137–155.
Kelley, A. E., Schiltz, & Landry, C. F. (2005). Neural systems recruited by
drug- and food-related cues: Studies of gene activation in corticolimbic
regions. Physiology & Behavior, 86 11–14.
Kleiner, K. D., Gold, M. S., Frost-Pineda, K., Lenz-Brunsman, B., Perri,
M. G., & Jacobs, W. S. (2004). Body mass index and alcohol use.
Journal of Addictive Diseases, 23, 105–118.
Lluch, A., Herbeth, B., Mejean, L., & Siest, G. (2000). Dietary intakes,
eating style and overweight in the Stanislas family study. International
Journal of Obesity, 24, 1493–1499.
Loxton, N. J., & Dawe, S. (2001). Alcohol abuse and dysfunctional eating
in adolescent girls: The influence of individual differences in sensitivity
to reward and punishment. International Journal of Eating Disorders,
29, 455–462.
Noble, E. P., Noble, R. E., Ritchie, T., Syndulko, K., Bohlman, M. C.,
Noble, L. A., et al. (1994). D
2
DA receptor gene and obesity.
International Journal of Eating Disorders, 15, 205–217.
Risinger, F. O., Freeman, P. A., Rubinstein, M., Low, M. J., & Grandy,
D. K. (2000). Lack of operant ethanol self-administration in DA D-2
receptor knockout mice. Psychopharmacology, 152, 343–350.
Torrubia, R., Avila, C., Molto, J., & Caseras, X. (2001). The Sensitivity to
Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a
measure of Gray’s anxiety and impulsivity dimensions. Personality and
Individual Differences, 31, 837–862.
Van Strien, T., Frijters, J. E., Bergers, G. P., & Defares, P. B. (1986). The
Dutch Eating Behavior Questionnaire (DEBQ) for assessment of
restrained, emotional, and external eating behavior. International
Journal of Eating Disorders, 5, 295–315.
Volkow, N. D., Wang, G.-J., Fowler, J. S., Logan, J., Jayne, M.,
Francesschi, D., et al. (2002). ‘‘Nonhedonic’’ food motivation in
humans involves DA in the dorsal striatum and methyphenidate
amplifies this effect. Synapse, 44, 175–180.
World Health Organization [WHO]. (1998). Obesity: Preventing and
managing the global epidemic. Report of a WHO consultation on
obesity. Geneva: World Health Organization.
Wang, G. J., Volkow, N. D., Felder, C., Fowler, J. S., Levy, A. V.,
Pappas, N. R., et al. (2002). Enhanced resting activity of the oral
somatosensory cortex in obese subjects. Neuroreport, 13, 1151–1155.
Wang, G.-J., Volkow, N. D., Logan, J., Pappas, N. R., Wong, C. T., Zhu,
W., et al. (2001). Brain DA and obesity. The Lancet, 357, 354–357.
ARTICLE IN PRESS
C. Davis et al. / Appetite 48 (2007) 12–19
18

Wang, G. J., Volkow, N. D., Thanos, P. K., & Fowler, J. S. (2004).
Similarity between obesity and drug addiction as assessed by
neurofunctional imaging: A concept review. Journal of Affective
Disorders, 23, 39–53.
Wansink, B., Cheney, M. M., & Chan, N. (2003). Exploring comfort food
preferences across gender and age. Physiology and Behavior, 79,
739–747.
Wansink, B., Painter, J. E., & North, J. (2005). Bottomless bowls: Why
visual cues of portion size may influence intake. Obesity Research, 13,
93–100.
Warren, M., Frost-Pineda, K., & Gold, M. (2005). Body mass index and
marijuana use. Journal of Addictive Diseases, 24, 95–99.
West, S. G., Finch, J. F., & Curran, P. J. (1995). Structural equation
models with non-normal variables. Thousand Oaks, CA: Sage.
ARTICLE IN PRESS
C. Davis et al. / Appetite 48 (2007) 12–19
19
Document Outline
Wyszukiwarka
Podobne podstrony:
Progressing from imitative to creative exercises
opow from rags to riches
Least squares estimation from Gauss to Kalman H W Sorenson
Complex Numbers from Geometry to Astronomy 98 Wieting p34
From empire to community
FROM COMMODITY TO
Idea of God from Prehistory to the Middle Ages
Manovich, Lev The Engineering of Vision from Constructivism to Computers
Adaptive Filters in MATLAB From Novice to Expert
From dictatorship to democracy a conceptual framework for liberation
Notto R Thelle Buddhism and Christianity in Japan From Conflict to Dialogue, 1854 1899, 1987
Zizek From politics to Biopolitics and back
From Sunrise to Sundown
From Village to City
Create Oracle Linked Server to Query?ta from Oracle to SQL Server
lecture 16 from SPC to APC
Introducing Children's Literature From Romanticism to Postmodernism
Russian and Chinese Methods of going from Communism to?moc
from new to new age archaeology
więcej podobnych podstron