
Biomaterials 23 (2002) 1353–1357
Bioactive titania gel layers formed by chemical treatment of Ti
substrate with a H
2
O
2
/HCl solution
Xiao-Xiang Wang
a,
*, Satoshi Hayakawa
b
, Kanji Tsuru
b
, Akiyoshi Osaka
b
a
Department of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China
b
Biomaterials Laboratory, Faculty of Engineering, Okayama University, Tsushima-Naka, Okayama-shi 700-8530, Japan
Received 30 January 2001; accepted 11 July 2001
Abstract
An amorphous titania gel layer was formed on the titanium surface after the titanium specimen was treated with a H
2
O
2
/0.1 m
HCl solution at 801C. The thickness of the gel layer increased almost linearly with the period of the treatment. A subsequent heat
treatment above 3001C transformed gradually the amorphous gel to the anatase crystal structure and the rutile started to appear
after heat treatment at 6001C. Meanwhile, the densification of the gel occurred significantly after heat treatment above 7001C.
Similar to the sol–gel derived titania gel coatings, titania gel layers obtained in the present study exhibited in vitro apatite deposition
ability after the gel layers exceeded a minimum thickness (0.2 mm) and was subsequently heated in a proper temperature range (400–
6001C). r 2002 Elsevier Science Ltd. All rights reserved.
Keywords:
Titanium; Surface modification; Apatite deposition; Bioactivity; Titania gel
1. Introduction
Titania and silica gels were able to induce the
formation of apatite when soaked in a simulated body
fluid (SBF) [1–4]. This property was considered as an
indication of bioactivity, the ability to bond with living
bone. Because the formation of a thin layer of apatite on
the implant surface was observed in in vivo experiments
and believed to be the first step of the bone bonding
process for the bioactive implants including silica and
titania gels [5,6]. Titania gel coating was therefore
considered to be one of the potential surface modifica-
tion techniques to improve the bioactivity of the
titanium implant and has been studied by several
researchers both in vitro and in vivo [1–3,6]. The
conventional sol–gel derived coatings are usually pro-
vided by dip-coating method that consists of several
coating–heating cycles to obtain necessary thickness for
apatite deposition and, thus, is quite time consuming
[1,2]. On the other hand, it was reported that titanium
could react with a H
2
O
2
solution and formed titania gel
[7,8]. Obviously, it could be a more convenient
technique for providing titania gel coating on titanium
substrate. The purpose of the present study was to
examine the microstructure and the in vitro bioactivity
of this gel layers.
2. Materials and methods
Specimens of 10 10 1 mm
3
in size were cut from a
sheet of commercially pure titanium and chemically
washed [9]. They were then chemically treated with a
solution containing 8.8 m H
2
O
2
and 0.1 m HCl (10 ml for
each specimen) at 801C for various time up to 1 h and
subsequently treated at various temperatures up to
8001C for 1 h in air atmosphere. Each of thus obtained
titanium specimens was immersed in 20 ml SBF of
Kokubo’s recipe [9–11] in a polystyrene vial. pHvalue
of the SBF was adjusted at 7.4 at 36.51C. After soaking
for various periods up to 7 days at 36.51C, the specimens
were gently washed with distilled water and dried in an
oven at 601C. The open surface of the specimen [11] was
examined by Fourier transform-infrared (FT-IR) reflec-
tion spectroscopy, scanning electron microscopy (SEM)
and low angle X-ray diffractometry (XRD). The FT-IR
reflection spectra were collected on a JASCO FT/IR-300
spectrometer with 100 scans at 4 cm
1
resolution using a
*Corresponding author. Fax: +86-571-795-1358.
E-mail address:
mse wangxx@dial.zju.edu.cn (X.-X. Wang).
0142-9612/02/$ - see front matter r 2002 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 1 ) 0 0 2 5 4 - X
Spectra Tech attachment Model 501. Low angle XRD
patterns were recorded with a Rigaku RAD-II diffract-
ometer using Cu K
a
radiation operating under 40 kV
and 25 mA acceleration at an angle of incidence of 11.
SEM photographs were taken using a JEOL JSM-6300
microscope with the specimens being sputter coated with
a 20 nm gold layer.
3. Results
3.1. The formation of titania gel layers
The reaction between the Ti specimen and the H
2
O
2
solution resulted in the formation of a layer of
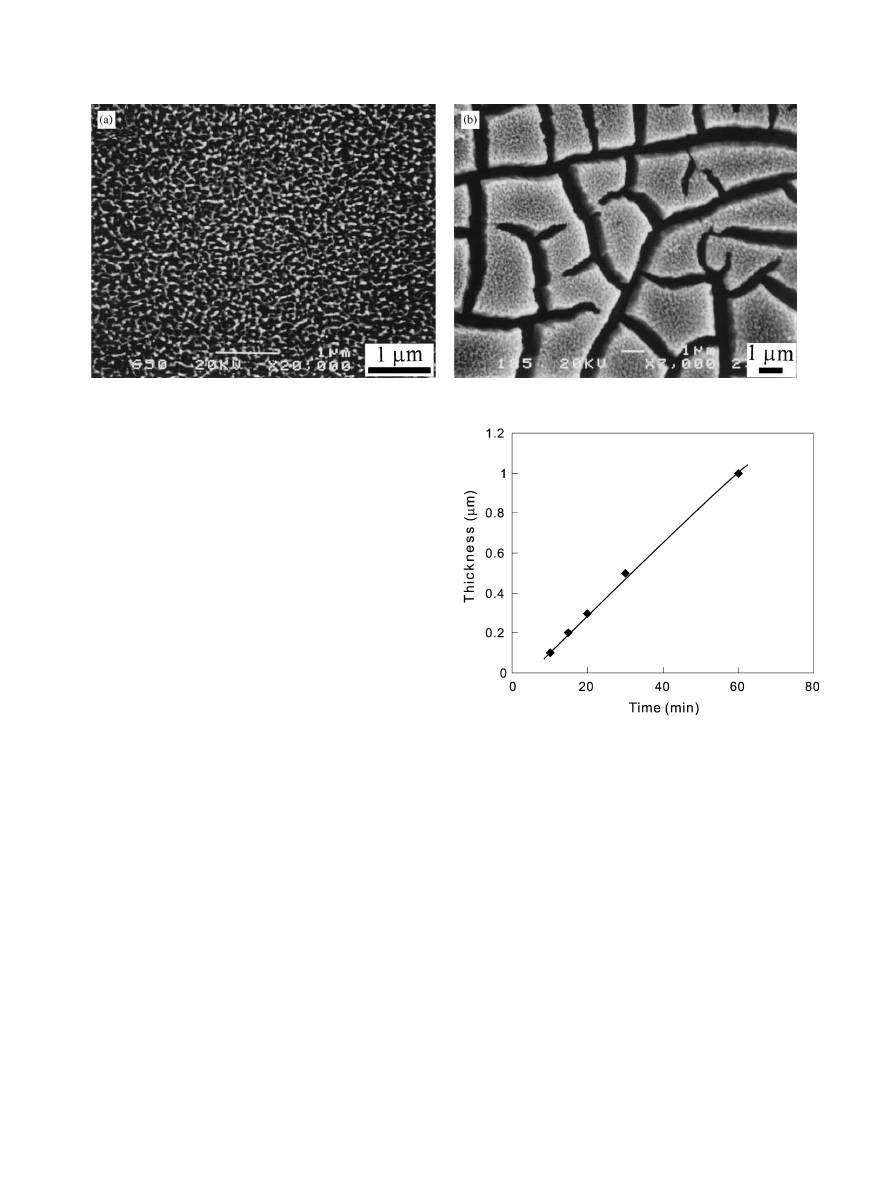
amorphous titania gel on the Ti surface. Shorter
chemical treatment time yielded a thinner gel layer with
premature porosity. After 20 min chemical treatment, a
titania gel layer of about 0.3 mm in thickness and with
mature porosity was obtained (Fig. 1a). The pores were
in the size of submicrometer. Further extending the
treatment time to 1 h resulted in a cracked thicker gel
layer with thickness of about 1 mm (Fig. 1b). The
thickness of the titania gel layers depended almost
linearly on the period of time of the chemical treatment
at the present treatment temperature (801C), as in-
dicated in Fig. 2. Subsequent heat treatment above
3001C transformed gradually the gel from amorphous to
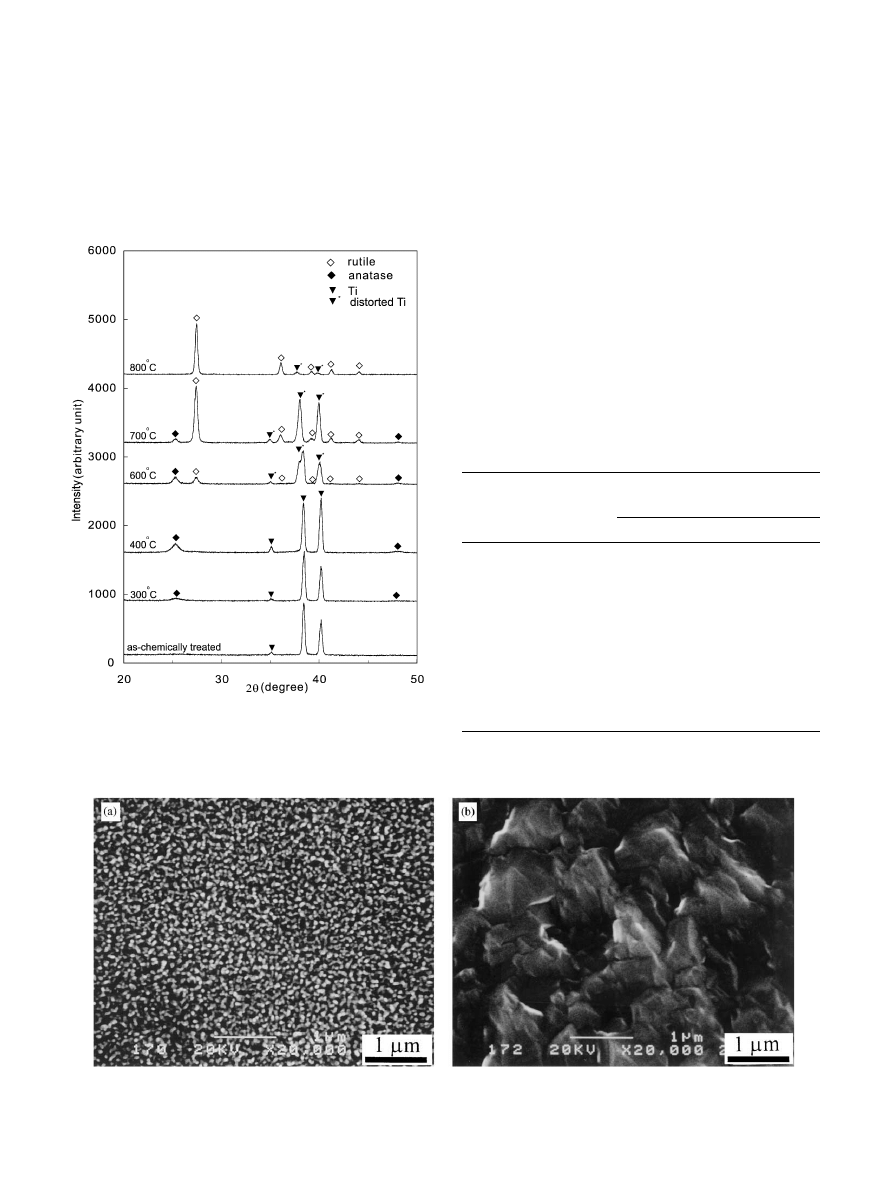
crystalline state. The XRD patterns in Fig. 3 exhibit the
transformation: the as-chemically treated amorphous
gel layer transformed mainly to anatase as the heat
treatment temperature was below 6001C while the rutile
became dominant above 7001C. The SEM observation
revealed that the subsequent heat treatment at tempera-
tures lower than 6001C hardly changed the morphology
of the pore of the gel layer. Large spherical particles of
titania started to appear after heat treatment at 7001C
(Fig. 4a), apparently due to the coalescence of small
particles. Further increasing the heating temperature to
8001C resulted in a fully densified titania layer (Fig. 4b).
3.2. The apatite deposition on the titania gel layers
The correlation of apatite deposition ability with the
period of the chemical treatment and the temperature of
heat treatment is summarized in Table 1. Apatite
deposition was observed only with the specimens
chemically treated for longer than 15 min and heat
treated subsequently between 4001C and 6001C. Fig. 5
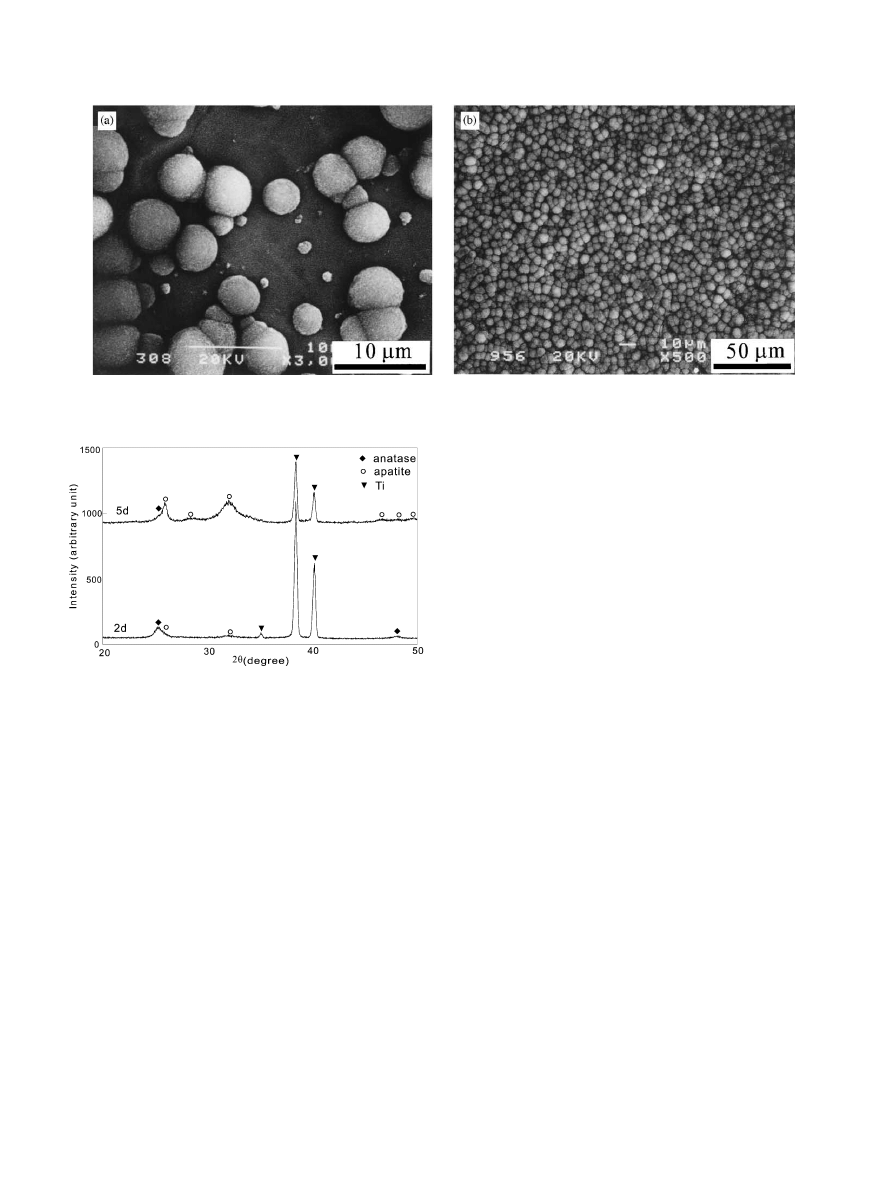
shows the SEM images of the apatite deposited on the
specimen subjected to the chemical treatment for 30 min
and subsequent heating at 4001C for 1 h. As we can see,
apatite particles scatter on the gel layer after 2 days of
soaking (Fig. 5a) and cover the whole surface after 5
days of soaking (Fig. 5b). These apatite particles have
the SEM morphology same as those deposited on
Fig. 1. SEM images of the titania gel layers formed on the Ti surfaces after treatment with the H
2
O
2
/HCl solution at 801C for (a) 20 min; (b) 60 min.
Fig. 2. The plot of the thickness of titania gel layers against the
periods of chemical treatment with H
2
O
2
/HCl solution at 801C.
X.-X. Wang et al. / Biomaterials 23 (2002) 1353–1357
1354
bioactive glass and conventional titania gel layers
[3,4,12]. XRD experimental results in Fig. 6 and FTIR
spectra (see Ref. [9]) indicate they are carbonate-
incorporated hydroxyapatite with low crystillinity, that
is bonelike apatite.
4. Discussion
The titania gel coatings, which are traditionally
derived by the sol–gel technique by hydrolysis of
titanium alkoxides, have been widely studied with
respect to bioactivity in recent years. Those sol–gel
derived coatings can induce apatite deposition in SBF if
the coating exceeds a certain thickness (about 200 nm
[2,13]) and experiences a subsequent heat treatment at a
proper temperature range (400–5501C [2]). In the
present study, we showed that the titania gel coatings
could be produced by the chemical treatment of
titanium with the H
2
O
2
/HCl solution. This titania gel
layers exhibited a similar behavior with respect to the
apatite deposition: a certain thickness and a subsequent
heat treatment are necessary for apatite to deposit. The
present technique is much simpler than the sol–gel
Fig. 4. SEM images of the titania gel layers on the Ti surfaces treated with the H
2
O
2
/HCl solution at 801C for 30 min and subsequently heat treated
at (a) 7001C and (b) 8001C for 1 h, indicating the change in morphology of the gel layers.
Fig. 3. XRD patterns of the specimens treated with the H
2
O
2
/HCl
solution at 801C for 30 min and subsequently heat treated at various
temperatures for 1 h, indicating the transformation of crystal structure
of the gels.
Table 1
Summary of apatite deposition, assessed with FTIR technique, on the
titanium surfaces after soaking in SBF for various time
Periods of
chemical
treatment (min)
a
Heat
treatment
(1C at 1 h)
Periods of soaking in SBF (days)
1
2
3
5
7
5
400
n
n
n
n
n
10
400
n
n
n
n
n
15
400
n
n
n
y
y
20
400
n
y
y
y
y
60
400
n
y
y
y
y
30
No
n
n
n
n
n
30
300
n
n
n
n
n
30
400
n
y
y
y
y
30
500
n
y
y
y
y
30
600
n
n
y
y
y
30
700
n
n
n
n
n
30
800
n
n
n
n
n
a
The chemical treatment was conducted with the H
2
O
2
/0.1 m HCl
solution at 801C.
X.-X. Wang et al. / Biomaterials 23 (2002) 1353–1357
1355
processing in which the reactions are difficult to control
due to the fast kinetics of the alkoxides of transition
metals and it would take several cycles of coating and
heating to attain a required thickness. In addition,
cracking and chipping prevailed in a sol–gel derived
titania gel coating of about 380 nm in thickness [2,13]
whereas the gel layers of less than 0.5 mm in thickness
formed after the chemical treatment of shorter than
30 min in the present study did not show any cracking
and chipping, suggesting that the gel layer derived by the
chemical treatment in the present study may have
stronger adhesion to the titanium substrate than the
sol–gel-derived coating. Therefore, it may be concluded
that the H
2
O
2
treatment is a superior technique over the
traditional sol–gel coating technique in producing
bioactive titania gel coatings on titanium surfaces.
The present experimental results showed that the
titania gel heated between 4001C and 5001C and thus
with anatase crystal structure exhibited excellent bioac-
tivity. Increasing heating temperature resulted in in-
creasing amount of rutile in the gel and the deterioration
of the bioactivity. This phenomenon, however, does not
necessarily mean the titania gel with rutile crystal
structure would lack bioactivity because the densifica-
tion of the gel occurred concurrently when the heat
treatment temperature was raised above 6001C. It is well
known that the porosity is critical for a gel to show
bioactivity. Li [3] has shown that dense single crystal
anatase lacked bioactivity although the porous one
could induce apatite deposition. It follows that the
densification of the gel after the heat treatment above
6001C should contribute to the deterioration of the
bioactivity in the present case. Under this condition, it is
unclear how and to what extent the formation of rutile
in the gel affected the bioactivity. In this regard, further
investigation is required into the effect of crystal
structure of the titania gel on the bioactivity.
Although the mechanism of the titania gel formation
through the reaction between titanium metal and H
2
O
2
solution has not been studied yet in present study and
thus is not well understood, we found the value of pHof
the H
2
O
2
solution had an effect on the gel formation. An
acidic H
2
O
2
solution by addition of HCl could speed up
the reaction and produce a clear gel morphology with
the pore size uniform all over the specimen surface (see
Figs. 1 and 7b). Another important factor involved in
this treatment is temperature. Lower temperature
tended to cause intergranular corrosion of the titanium
substrate before a certain thickness of titania gel layer
was formed, as shown in Fig. 7a in which the specimen
was treated at 251C for 1 day in the same solution. In
contrast, higher temperature can ensure the reaction to
take place uniformly all over the titanium surface, as
shown in Fig. 7b. Because intergranular corrosion on
the surface will damage severely the fatigue property of
the titanium implants, a higher temperature, as the
temperature of 801C chosen in the present study, was
Fig. 5. SEM images of apatite particles deposited on the Ti surfaces after soaked in SBF for (a) 2 days and (b) 5 days. The Ti surfaces have been
treated with the H
2
O
2
/HCl solution at 801C for 30 min and subsequently heat treated at 4001C for 1 h.
Fig. 6. XRD patterns of the specimens after soaking in SBF for 2 and
5 days. The Ti specimens have been chemically treated with the H
2
O
2
/
HCl solution at 801C for 30 min and subsequently heat treated at
4001C for 1 h.
X.-X. Wang et al. / Biomaterials 23 (2002) 1353–1357
1356

critical for the treatment of titanium implant in the
practical application of this treatment.
5. Conclusions
An amorphous titania gel layer was formed by the
treatment of Ti with the H
2
O
2
/0.1 m HCl solution. The
thickness of the gel layer was controllable through the
periods of the treatment. The subsequent heat treatment
above 3001C transformed the gel to anatase. Rutile was
dominant as the heating temperature was raised to
between 7001C and 8001C, meanwhile the densification
of the gel occurred significantly. The minimum thickness
of the titania gel layer and the optimal temperature of
heat treatment are about 0.2 mm and 400–5001C,
respectively, with respect to bioactivity.
Acknowledgements
One of the authors, X.X. Wang, gratefully acknowl-
edges the financial support of the Venture Business
Laboratories (VBL) in the Graduate School of Natural
Sciences of Okayama University. This work was
performed when XXW was on leave from Department
of MSE of Zhejiang University, China. A part of this
work was supported by Mikiya Science Foundation and
RSP-activity of Okayama Prefecture, sponsored by the
Science and Technology Agency of Japan.
References
[1] Li P, Kangasniemi I, DeGroot K, Kokubo T. Bone hydro-
xyapatite induction by a gel-derived titania on a titanium
substrate. J Am Ceram Soc 1994;77:1307–12.
[2] Peltola T, Patsi M, Rahiala H, Kangasiemi I, Yli-Urpo A.
Calcium phosphate induction by sol–gel-derived titania coatings
on titanium substrates in vitro. J Biomed Mater Res 1998;41:
504–10.
[3] Li P, Ohtsuki C, Kokubo T, Nakanishi K, Soga N, DeGroot K.
The role of hydrated silica, titania, and alumina in inducing
apatite on implants. J Biomed Mater Res 1994;28:7–14.
[4] Peltola T, Jokinen M, Rahiala H, Levanen E, Rosenholm JB,
Kangasiemi I, Yli-Urpo A. Calcium phosphate formation on
porous sol–gel-derived SiO
2
and CaO–P
2
O
5
–SiO
2
substrates
in vitro. J Biomed Mater Res 1999;44:12–21.
[5] Li P, Ye X, Kangasniemi I, DeBlieck-Hogervorst JMA,
Klein CPAT, DeGroot K. In vivo calcium phosphate formation
induced by sol–gel-prepared silica. J Biomed Mater Res
1995;29:325–8.
[6] Li P, DeGroot K. Calcium phosphate formation within sol–gel
prepared titania in vitro and in vivo. J Biomed Mater Res 1993;
27:1495–500.
[7] Tengvall P, Lundstrom I, Sjoqvist L, Elwing H. Titanium–
hydrogen peroxide interaction: model studies of the influence of
the inflammatory response on titanium implants. Biomaterials
1989;10:166–75.
[8] Tengvell P, Elwing H, Lundstrom I. Titanium gel made from
metallic titanium and hydrogen peroxide. J Colloid Interface Sci
1989;130:405–13.
[9] Wang XX, Hayakawa S, Tsuru K, Osaka A. Improvement of
bioactivity of H
2
O
2
/TaCl
5
-treated titanium after subsequent heat
treatments. J Biomed Mater Res 2000;52:171–6.
[10] Hench LL. Characterization of bioceramics. In: Hench LL,
Wilson J, editors. An introduction to bioceramics. Singapore:
World Science, 1993. p. 319–34.
[11] Wang XX, Hayakawa S, Tsuru K, Osaka A. A comparative study
of in vitro apatite deposition on heated-, H
2
O
2
-, and NaOH-
treated titanium substrates. J Biomed Mater Res 2001;54:
172–8.
[12] Vallet-Regi M, Romero AM, Ragel CV, LeGeros RZ. XRD,
SEM-EDS, and FTIR studies of in vitro growth of an apatite-like
layer on sol–gel glasses. J Biomed Mater Res 1999;44:416–21.
[13] Jokinen M, Patsi M, Rahiala H, Peltola T, Ritala M, Rosenholm
JB. Influence of sol and surface properties on in vitro bioactivity
of sol–gel-derived TiO
2
and TiO
2
–SiO
2
films deposited by dip-
coating method. J Biomed Mater Res 1998;42:295–302.
Fig. 7. SEM images of the Ti surfaces after treatment with the H
2
O
2
/HCl solution at (a) 251C for 1 day and (b) 801C for 30 min, showing the severe
intergranular corrosion for the former and uniform titania gel layer without intergranular corrosion for the latter.
X.-X. Wang et al. / Biomaterials 23 (2002) 1353–1357
1357
Wyszukiwarka
Podobne podstrony:
Osteoinduction of porous bioactive titanium metal
Preparation of bioactive titanium metal via anodic oxidation
[38]QUERCETIN AND ITS DERIVATIVES CHEMICAL STRUCTURE AND BIOACTIVITY – A REVIEW
titanic wordsearch
Titanic Secondclass crossword
Titanic?cts and Figures
titanik plyvet
Titanic
Informacje o titanicu i jego katastrofie
Titanic Firstclass Passengers
MECHANICAL PROPERTIES TITANIUM
Nowy Titanic
Titanic Lifeboats crossword
Titanic Blame crossword
titanic quiz
was titanic unsinkable
titanic crossword
Ladley, Titania You’ve Got Irish Male!
Titanic bogów atlantyda, Atlantyda
więcej podobnych podstron