
FOOD
MICROBIOLOGY
www.elsevier.nl/locate/jnlabr/yfmic
Food Microbiology 20 (2003) 179–185
Exposure of Shigella flexneri to acid stress and heat shock enhances
acid tolerance
Gloria L. Tetteh, Larry R. Beuchat*
Center for Food Safety and Department of Food Science and Technology, University of Georgia, 1109 Experiment Street, Griffin, GA 30223-1797, USA
Received 1 April 2002; accepted 19 August 2002
Abstract
The effects of acid stress and heat shock on changes in acid tolerance of Shigella flexneri were determined. The pathogen was
grownat 371C for 18 h in tryptic soy broth (TSB) containing no glucose (TSBNG) (unadapted cells) and TSBNG supplemented with
1% glucose (TSBG) (acid-adapted cells). Cells growninTSBNG for 18 h thenheat shocked at 481C for 15 min(unadapted heat-
shocked cells) were also prepared. The three types of cells were inoculated into TSB (contains 0.25% glucose) acidified with acetic,
lactic, or propionic acids to pH 4.5, 4.0, and 3.5 and incubated at 371C. After incubating for 0.5, 1, 1.5, 2, 4, and 6 h, viable cells were
enumerated by plating acidified suspensions on tryptic soy agar (TSA). Populations of all three cell types inoculated into TSB
acidified to pH 3.5 with acetic, lactic, and propionic acids rapidly decreased, while a more gradual decline was observed at pH 4.0.
Populations of cells remained nearly constant at pH 4.5, regardless of acidulant used. Significantly (a ¼ 0:05) higher numbers of
acid-adapted cells and unadapted heat-shocked cells, compared to unadapted cells that were not heat shocked, were recovered from
TSB acidified (pH 3.5) with acetic or lactic acids. The populationof unadapted heat-shocked cells decreased approximately 3.5
log
10
cfu ml
1
, whereas unadapted cells that were not heat shocked decreased 5 log
10
cfu ml
1
after 30 mininTSB acidified to pH 3.5
with acetic acid. Chloramphenicol (100 mg ml
1
) prevented the development of acid tolerance in unadapted heat-shocked cells,
indicating a need for synthesis of heat-shock proteins for the development of acid resistance. Gel electrophoresis (sodium dodecyl
sulfate-polyacrylamide gel electrophoresis) revealed that acid-adapted cells contained more proteins than control, unadapted heat-
shocked, and unadapted, chloramphenicol-treated, heat-shocked cells. Results indicate that exposure of S. flexneri cells, unadapted
to an acidic environment, to a mild heat shock renders them more tolerant to acidic conditions and may enhance their survival and
ability to grow inhigh acid foods.
r
2002 Elsevier Science Ltd. All rights reserved.
Keywords: Shigella flexneri; Heat shock; Acid tolerance
1. Introduction
Cross-protectionof stressed foodborn
e pathogen
ic
bacteria against subsequent exposure to otherwise lethal
environmental stresses enhances the potential for
survival and growth (
;
). Shigella cansurvive for extended
periods under adverse conditions such as high acid or
high temperatures (
). Research has shownthat acid-adapted
Shigella flexneri cells have increased tolerance to acidic
conditions compared to cells that have not been adapted
to acid (
Tolerance of Shigella to low-pH environments may
enhance survival in acidic foods and also in the acidic
environment of the human stomach (
), thereby increasing the potential of causing
infection.
Treatment of foods with organic acids is effective in
reducing populations and controlling the growth of
many spoilage and pathogenic micro-organisms.
reported that treatment of alfalfa
seeds with acetic acid at 501C significantly reduced
the populationof Salmonella. Treatment of apples,
oranges, and tomatoes with a mixture of lactic acid and
hydrogenperoxide has beenshownto reduce popula-
tions of bacterial pathogens by >5 log
10
cfu fruit
1
*Corresponding author. Tel.: +1-770-412-4740; fax: +1-770-229-
3216.
E-mail address:
lbeuchat@cfs.griffin.peachnet.edu (L.R. Beuchat).
0740-0020/02/$ - see front matter r 2002 Elsevier Science Ltd. All rights reserved.
PII: S 0 7 4 0 - 0 0 2 0 ( 0 2 ) 0 0 1 1 9 - 3

reported that washing beef tissue with 1% acetic
and lactic acids reduced populations of Salmonella
typhimurium, Listeria monocytogenes, an d Escherichia
coli O157:H7. The level of acid tolerance in cells of these
and other pathogens that survive acid treatment of
foods has not been fully assessed.
Whena portionof the cells survive exposure to acid
stress, their tolerance to more extreme environmental
conditions may increase and virulence may be enhanced.
Studies with S. typhimurium (
and E. coli O157:H7 (
;
;
) have shownthat
suddenexposure of cells to reduced pH, described as
acid-adaptation or acid-shock, increases their resistance
to inactivation at lower pH. There is also evidence
indicating that microbial cells adapted to a particular
stress may exhibit increased resistance to unrelated
stresses (
;
;
). Bacterial cells
respond to various stresses by inducing the synthesis of
specific proteins characteristic to each stress (
). The induction of stress proteins upon
exposure to non-lethal assault has been shown to confer
protection against subsequent exposure to the otherwise
lethal effects of the same stress or other unrelated
stresses.
Mild heat treatment of foods containing Shigella may
result inheat-shocked cells that canmore easily survive
in high-acid foods or in the stomach and intestinal tract.
The potential of acid foods to serve as vehicles of
Shigella in outbreaks of infections prompted this study
to determine the fate of acid- and heat-stressed S.
flexneri upon exposure to an acidified organic environ-
ment. Another objective was to determine if synthesis of
proteins is required for the development of acid
tolerance.
2. Materials and methods
2.1. Preparation of inoculum
Shigella flexneri 2a (strainF340-MS1), obtained from
the Centers for Disease Control and Prevention (CDC)
in Atlanta, GA was confirmed for purity using
biochemical tests. Cultures maintained on tryptic soy
agar (TSA, pH 7.2; BBL/Difco, Sparks, Maryland,
USA) at 51C were revived by inoculating 10 ml of tryptic
soy broth (TSB, pH 7.3; BBL/Difco) and incubating for
24 h at 371C. Three consecutive 24-h transfers of culture
into 10 ml of TSB were made before cells were used as
inocula in experiments. Control (unadapted to acid),
acid-adapted, and unadapted heat-shocked cells were
prepared. Control cells were grown in TSB containing
no glucose (TSBNG) for 18 h at 371C and acid-adapted
cells were prepared by growing cells for 18 h at 371C in
TSBNG supplemented 1% glucose (TSBG). Cells grown
inTSBNG for 18 h at 371C were heat-shocked at 481C
for 15 minto produce unadapted heat-shocked cells.
2.2. Preparation of media
TSB containing 0.25% glucose and TSA (pH 7.2)
were prepared and sterilized according to the manufac-
turer’s instructions. Tryptic soy broth containing no
glucose (TSBNG) was supplemented to contain 1%
glucose (TSBG) by adding 10 g of glucose (Sigma
Chemical Co., St. Louis, Missouri, USA) per liter. To
prepare acidified broth, sterile 13 m lactic, 17.4 m acetic,
or 13 m propionic acids were added to sterile TSB to
reduce the pH to 4.5, 4.0, and 3.5; 9.9 ml were dispensed
into sterile 16 150-mm screw-capped test tubes.
Unacidified TSB (pH 7.3) served as a control broth.
2.3. Determination of acid tolerance
The ability of unadapted (control), acid-adapted, and
unadapted heat-shocked S. flexneri to survive or grow in
unacidified and acidified TSB was determined. Acidified
(pH 5.5, 4.5, and 3.5) and control (pH 7.3) TSB (9.9 ml)
inscrew-capped test tubes were inoculated with 0.1 ml of
cell suspensions (7.0 log
10
cfu ml
1
) of control, acid-
adapted, or unadapted heat-shocked cultures of S.
flexneri and incubated in a water bath at 371C for up
to 6 h. After incubation for 0.5, 1, 1.5, 2, 4, and 6 h,
undiluted suspensions were surface plated (0.25 ml in
quadruplicate and 0.1 ml in duplicate) on TSA. Samples
serially diluted insterile 0.1% peptone water were also
surface plated (0.1 ml induplicate) onTSA. Plates were
incubated at 371C for 48 h before colonies were counted.
To determine if synthesis of heat-shock proteins is
involved in the acid tolerance response of heat-stressed
S. flexneri, 18-h cultures of control cells were sedimented
by centrifuging at 2000 g for 15 min, then resuspended
in
TSBNG
supplemented
with
chloramphenicol
(100 mg ml
1
). Cells were incubated at 371C for 15 min
before subjecting to heat shock at 481C for 15 min. The
cells were then inoculated into TSB acidified (pH 4.5,
4.0, and 3.5) with lactic, acetic, and propionic acids and
incubated at 371C for up to 6 h ina water bath.
Populations were determined by surface plating suspen-
sions on TSA as described above.
2.4. Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) analysis of S. flexneri
The SDS-PAGE method described by
was used with some modifications. Fifty milli-
liters each of control, acid-adapted, unadapted heat-
shocked,
and
chloramphenicol-treated
unadapted
heat-shocked cultures were centrifuged in a bench
G.L. Tetteh, L.R. Beuchat / Food Microbiology 20 (2003) 179–185
180

top Centra-CL2 centrifuge (International Equipment
Company, Needham Heights, Massachusetts, USA) at
2000 g for 10 min. The cells were washed with 45 ml of
20 mm Tris–hydrochloride (Tris–HCl, pH 7.1) and
centrifuged again at 2000 g for 10 min. The pellet
was resuspended in 6 ml of a solution containing 20 mm
Tris–HCl and 10 mm ethylenediaminetetraacetic acid
(EDTA), followed by sonication with a Branson model
250/450 sonifier (Branson Ultrasonics Corporation,
Danbury, Connecticut, USA) for 30 s and cooling in a
slurry of ice and ethanol for 90 s. The procedure was
repeated until about 95% of the cells were disrupted.
This suspension was centrifuged at 2000 g for 5 minto
remove some of the solid debris and the supernatant was
transferred into a microcentrifuge tube. The membrane
material was separated from the proteinsuspensionby
centrifuging at 12,000 g for 30 minina Compac
microcentrifuge, (Micro 16, Labnet International, Inc.,
Woodbridge, New Jersey, USA). The pellet was washed
with 20 mm Tris–HCl (pH 7.1) and centrifuged again at
12,000 g for 30 min; 500 ml of 20 mm Tris–HCl (pH
7.1) was added to the pellet and the protein content in
these samples was determined based on the dye-binding
method described by
. The concentra-
tionof proteininextracts of the four different cell types
was adjusted to be approximately equal by diluting in
Laemmli sample buffer, (3.8 ml of distilled water, 1 ml of
0.5 m Tris–HCl [pH 6.8], 0.80 ml of glycerol, 1.6 ml of
10% [w/v] SDS, 0.4 ml of 2-mercaptoethanol, and 0.4 ml
of 0.05% [w/v] bromophenol blue); solution was mixed
and heated at 951C for 5 min. The proteins in the
samples were separated by SDS-PAGE using a PRO-
TEAN II xi cell (Bio-Rad Laboratories, Hercules,
California, USA) with the separating and stacking gel
concentrations at 12% and 4%, respectively. Silver stain
SDS-PAGE molecular weight standards ranging from
14,400 to 97,400 Da (Bio-Rad Laboratories) were used
as the molecular mass markers. Separationof proteins
was done at 25 mA when proteins were moving through
the stacking gel and then at 35 mA for the rest of the
run. The gel was stained with 0.1% coomassie blue in
10% acetic acid and 40% methanol, and destained with
10% acetic acid and 40% methanol solution.
2.5. Statistical analysis
Three replicate trials were done for each experiment.
Data were analysed using the general linear model and
analysis of variance of the Statistical Analysis Systems.
Significant differences (a
p0:05) inpopulation
s as
affected by test parameters, i.e., various organic acids,
cell type (control, acid-adapted, and unadapted heat-
shocked), and pH were determined. Least significant
difference and the Duncan’s multiple range tests were
used to determine sources of differences (
3. Results and discussion
The pH values of 18-h cultures of S. flexneri grownin
TSBNG and TSBNG supplemented with 1% glucose
(TSBG) were 6.82
70.05 and 4.8570.03, respectively.
Acid adaptationof cells growninTSBG simulated a
process that could occur during fermentation or spoilage
of foods by acid-producing micro-organisms. The
method used to achieve acid adaptationof S. flexneri
was similar to that originally described for E. coli
O157:H7 (
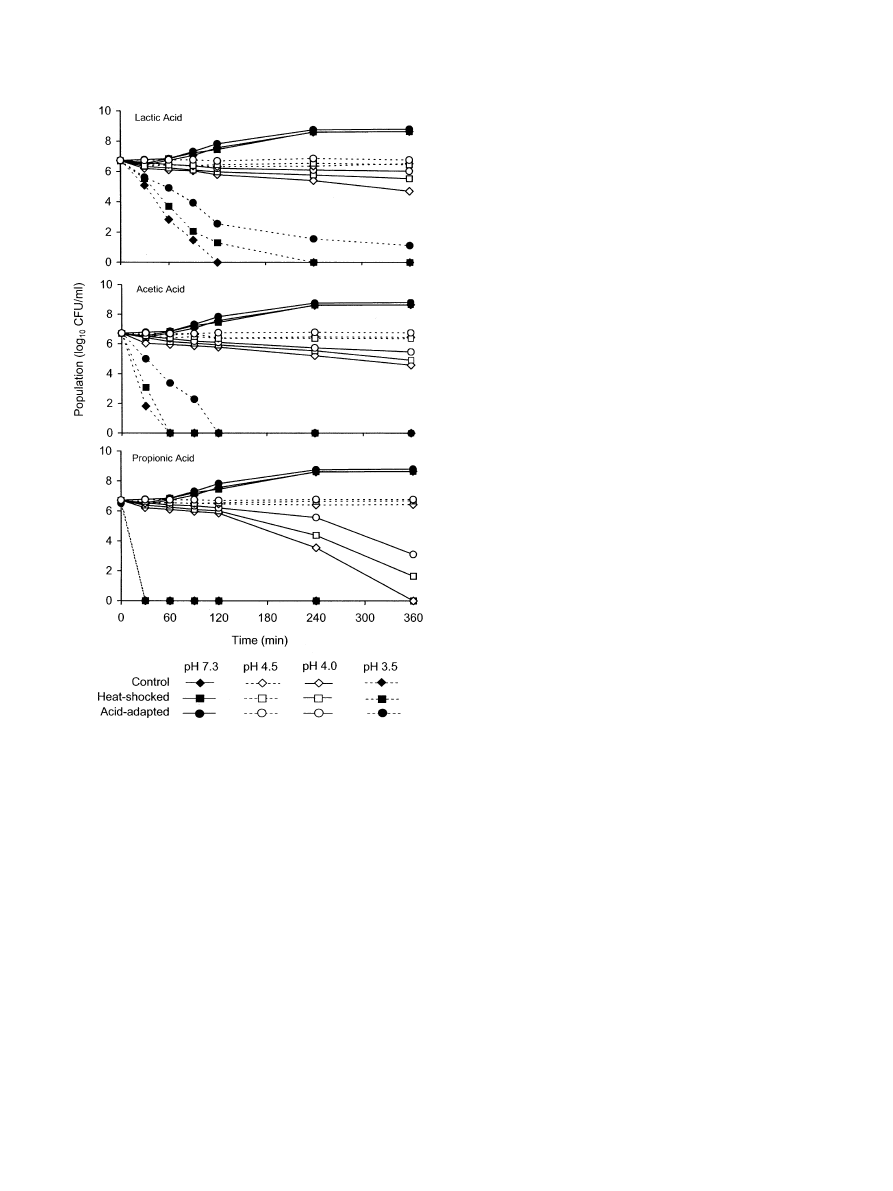
Growth and inactivation curves of unadapted S.
flexneri cells that were not heat-shocked (control cells),
acid-adapted cells, and unadapted heat-shocked cells in
TSB (pH 7.3) and TSB acidified (pH 3.5, 4.0, and 5.0)
with lactic, acetic, and propionic acids are shown in
. All three cell types grew inTSB at pH 7.3.
Populations of cells inoculated into TSB acidified to pH
3.5 with all three acids rapidly decreased, while a more
gradual decrease was observed at pH 4.0. Populations of
cells remained nearly constant at pH 4.5, regardless of
acidulant used. Compared to unadapted cells that were
not heat shocked (control cells), unadapted heat-
shocked cells and acid-adapted cells exhibited higher
resistance to acid stress. Heat-shocked S. flexneri cells at
aninitial populationof 6.7 log
10
cfu ml
1
had increased
ability to survive for at least 2 h inTSB acidified to pH
3.5 with lactic acid and to survive at pH 4.0 or 4.5 for
6 h, compared to control cells. The number of un-
adapted heat-shocked cells decreased approximately 3.5
log
10
cfu/ml inTSB acidified to pH 3.5 with acetic acid,
whereas unadapted cells that were not heat shocked
decreased 5 log
10
cfu ml
1
after 30 min.
The slower rate of inactivation of the unadapted heat-
shocked cells at pH 3.5 suggests that high-acid foods
(pH 3.5–4.5) that rely onlow pH to control growth of S.
flexneri could serve as vehicles for the pathogen, the risk
being influenced by previous exposure to mild heat. At a
given pH, propionic acid was the most inhibitory of the
three acids evaluated. These results are similar to those
observed inanearlier study showing that S. flexneri was
able to survive longer in TSB when lactic acid was used
as an acidulant compared to propionic acid, which
inactivated cells within 30 min at pH 3.5 (
). The order of sensitivity of S. flexneri to
acids at pH 4.0 and 3.5 was lactic acid
oacetic
acid
opropionic acid.
re-
ported that E. coli O157:H7 was also more sensitive to
acetic acid thanto lactic acid.
observed that the order of tolerance of acid-adapted E.
coli O157:H7 cells at a givenpH was acetic>citric>
malic acid. Differences in sensitivity of acid-shocked S.
typhimurium cells to various organic acids have also
beenreported (
). Clearly, the anion
concentration and molarity of acids, as well as pH,
influences the sensitivity of enteric pathogens. Tolerance
G.L. Tetteh, L.R. Beuchat / Food Microbiology 20 (2003) 179–185
181
to acid stress is enhanced by pre-exposure of cells to
acidic environments.
Acid-adapted cells were more resistant to acid stress
than were unadapted heat-shocked cells or control cells,
regardless of the acidulant. These observations suggest
that some types of fermented, acidified, or high-acid
foods could provide environments that would enhance
survival and promote the development of acid-adapted
cells.
shows the number of control, unadapted heat-
shocked, and unadapted heat-shocked, chlorampheni-
col-treated cells of S. flexneri recovered from TSB
acidified with lactic, acetic, or propionic acids over a 6-h
incubation period. Chloramphenicol, which inhibits
protein synthesis, prevented the development of acid
tolerance in heat-shocked cells, suggesting that synthesis
of heat-shock proteins is a prerequisite for acid
resistance. Bacterial cells are known to respond to
physical and chemical stresses by synthesizing proteins
that confer enhanced stress resistance. Our study shows
that sub-lethal heat treatment may have triggered the
productionof heat-shock proteins that cross protect S.
flexneri to acid challenge. Heat shock response has also
been observed in other pathogens. According to
, when E. coli O157:H7 cells are acid
stressed at pH 4.0, they exhibit greater resistance to heat
at 561C compared to cells that have not been exposed to
acid. Salmonella enteritidis phage type 4 also shows a
marked increase in acid and heat tolerance when cells
are heat shocked at 461C (
). In
non-pathogenic E. coli, a rapid induction of heat shock
proteins has been observed following a temperature shift
from 301C to 421C (
SDS-PAGE was used to detect heat-shock proteins
produced by heat-shocked and acid-adapted S. flexneri
cells and estimate their molecular weights. Gels revealed
that acid-adapted cells contained more proteins than the
other three types of cells (
). This suggests that
synthesis of proteins in S. flexneri is induced when cells
are exposed to sub-lethal acidic environments, which
may result in enhanced resistance to subsequent
exposure to acidic environments. Gels revealed no
differences in the number or amount of proteins in
control cells, unadapted heat-shocked cells, and un-
adapted heat-shocked, chloramphenicol-treated cells
(
). However, differences were observed in toler-
ance of these cell types to acidified TSB (
suggesting that heat-shocked proteins are produced.
It may be that these proteins did not separate or
were present at concentrations too low to detect by
one-dimensional
SDS-PAGE
gel
electrophoresis.
Two-dimensional gel electrophoresis may separate these
proteins.
A better understanding of factors influencing adapta-
tionof S. flexneri to acid stress will be valuable when
predicting its survival and growth in acidic foods.
However, the behavior of acid-adapted and heat-
shocked unadapted cells as affected by pH and
composition of foods needs to be determined before
an accurate assessment can be made. It is known that
enteric pathogens are protected against death in extreme
acidic conditions (pH 2.5) when inoculated into some
types of foods (
). Acid-
adapted E. coli O157:H7 survives better thanunadapted
cells during sausage fermentation, and shows enhanced
survival indry salami an
d apple cider (
). Acid adaptationen
han
ces survival of S. typhi-
murium during milk fermentation and in cheeses stored
at 51C (
). Strategies of Shigella
for survival at low pH have beenreviewed (
A basic strategy is to neutralize incoming H
+
by
coupling H
+
transport with amino acid transport.
Fig. 1. Populations of unadapted S. flexneri cells that were not heat
shocked (control cells), unadapted heat-shocked cells, and acid-
adapted cells recovered from TSB (pH 7.3) and TSB acidified (pH
3.5, 4.0, 4.5) with lactic, acetic and propionic acids.
G.L. Tetteh, L.R. Beuchat / Food Microbiology 20 (2003) 179–185
182

Ta
ble
1
Pop
ulations
(log
10
cfu
ml
1
)
o
f
contro
l
(unada
pted),
unad
apted
heat
-shocked
,
a
nd
u
nad
apted,
chlora
mphen
icol-
treated,
he
at-shocke
d
S
.
fle
xneri
cells
recovere
d
fr
o
m
acidifi
ed
TS
B
a
pH
3.5
pH
4.0
pH
4.5
pH
7.3
Acidulant
Incubation
time
(min)
Control
Heat
shocked
Chloramphenicol
treated,
heat
shocked
Control
Heat
shocked
Chloramphenicol
treated,
heat
shocked
Control
Heat
shocked
Chloramphenicol
treated,
heat
shocked
Control
Heat
shocked
Chloramphenicol
treated,
heat
shocked
Lactic
acid
30
5.09
B
5.53
A
4.76
B
6.21
B
6.34
A
6.11
B
6.50
A
6.36
B
6.39
B
6.45
A
6.54
A
6.43
A
60
2.83
B
3.70
A
2.56
B
6.11
B
6.24
A
5.82
B
6.47
A
6.44
A
6.33
A
6.74
A
6.85
A
6.66
A
90
1.48
B
2.06
A
1.19
C
6.05
A
6.11
A
5.70
B
6.37
A
6.40
A
6.32
A
7.07
B
7.24
A
7.02
B
120
0
B
1.31
A
0
B
5.80
A
5.98
A
5.63
B
6.34
A
6.46
A
6.33
A
7.58
A
7.46
A
7.42
A
240
0
0
0
5.41
B
5.78
A
5.25
B
6.37
A
6.56
B
6.32
A
8.61
A
8.63
A
8.68
A
360
0
0
0
4.71
B
5.54
A
4.64
B
6.54
A
6.46
A
6.28
B
8.66
A
8.65
A
8.71
A
Acetic
acid
30
1.82
B
3.07
A
1.73
B
6.04
B
6.42
A
5.99
B
6.63
A
6.55
A
6.43
A
6.45
A
6.54
A
6.43
A
60
0
0
0
5.96
B
6.16
A
5.67
C
6.61
A
6.40
A
6.42
A
6.74
A
6.85
A
6.66
A
90
0
0
0
5.88
B
6.06
A
5.58
C
6.69
A
6.50
B
6.43
B
7.07
B
7.24
A
7.02
B
120
0
0
0
5.79
B
5.93
A
5.52
C
6.40
A
6.38
A
6.37
A
7.58
A
7.46
A
7.42
A
240
0
0
0
5.21
B
5.55
A
5.17
B
6.48
A
6.38
A
6.31
A
8.61
A
8.63
A
8.68
A
360
0
0
0
4.58
B
4.91
A
4.41
C
6.45
A
6.37
A
6.42
A
8.66
A
8.65
A
8.71
A
Propionic
acid
30
0
0
0
6.22
B
6.39
A
6.17
B
6.53
A
6.64
A
6.31
B
6.45
A
6.54
A
6.43
A
60
0
0
0
6.11
B
6.27
A
6.05
B
6.56
A
6.56
A
6.26
B
6.74
A
6.85
A
6.66
A
90
0
0
0
5.98
A
6.12
A
6.00
A
6.52
A
6.52
A
6.27
B
7.07
B
7.24
A
7.02
B
120
0
0
0
5.87
B
6.02
A
5.84
B
6.49
A
6.56
A
6.31
B
7.58
A
7.46
A
7.42
A
240
0
0
0
3.55
B
4.39
A
3.15
C
6.42
AB
6.66
A
6.29
B
8.61
A
8.63
A
8.68
A
360
0
0
0
0
B
1.65
A
0
B
6.44
AB
6.68
A
6.28
B
8.66
A
8.65
A
8.71
A
a
Valu
es
in
the
sam
e
row
w
ithin
the
sam
e
p
H
(3.5,
4.0,
4.5,
and
7.3)
that
are
not
follo
wed
by
the
same
letter
are
signi
ficantly
differ
ent
(a
¼
0
:05);
zero
(0)
represents
o
1
cfu
ml
1
.
G.L. Tetteh, L.R. Beuchat / Food Microbiology 20 (2003) 179–185
183

Synthesis of proteins is also involved. Temperature and
sodium chloride have a marked influence the ability of
Shigella to survive at reduced pH (
Clearly, the ability of Shigella to survive inacid-stress
environments such as acidic foods may be influenced by
prior exposure to acidic pH and, perhaps, also heat
shock. Experiments to test this hypothesis are being
conducted in our laboratory.
Acknowledgements
This study was supported, inpart, by the US Agency
for International Development Bean/Cowpea Colla-
borative Research Support Program.
References
Abdul-Raouf, U.M., Beuchat, L.R., Ammar, M.S., 1993. Survival and
growth of E. coli O157:H7 inground, roasted beef as affected by
pH, acidulants, and temperature. Appl. Environ. Microbiol. 59,
2364–2368.
Ars
"ene, F., Tomoyasu, T., Bukau, B., 2000. The heat shock response
of Escherichia coli. Int. J. Food Microbiol. 55, 3–9.
Baik, H.S., Bearson, S., Dunbar, S., Foster, J.W., 1996. The acid
tolerance response of Salmonella typhimurium provides protection
against organic acids. Microbiology 142, 3195–3200.
Bradford, M.M., 1976. A rapid and sensitive method for the
quantitation of microgram quantities of protein utilizing the
principle of protein–dye binding. Anal. Biochem. 72, 248–254.
Buchanan, R., Edelson, S.G., 1996. Culturing enterohemorrhagic
Escherichia coli in the presence and absence of glucose as a simple
means of evaluating the acid tolerance of stationary-phase cells.
Appl. Environ. Microbiol. 62, 4009–4013.
Deng, Y., Ryu, J.H., Beuchat, L.R., 1999. Tolerance of acid adapted
and non-adapted Escherichia coli O157:H7 cells to reduced pH as
affected by type of acidulant. J. Appl. Microbiol. 86, 203–210.
Dickson, J.S., Siragusa, G.R., 1994. Survival of Salmonella typhimur-
ium, Escherichia coli O157:H7 and Listeria monocytogenes during
storage on beef sanitized with organic acids. J. Food Safety 14,
313–327.
Farber, J.M., Pagotto, F., 1992. The effect of acid shock onthe heat
resistance of Listeria monocytogenes. Lett. Appl. Microbiol. 15,
197–201.
Foster, J.W., Hall, H.K., 1990. Adaptive acidificationtoleran
ce
response of Salmonella typhimurium. J. Bacteriol. 172, 771–778.
Gill, C., O’Driscoll, B., Booth, I., 1995. Acid adaptationand food
poisoning microorganisms. Int. J. Food Microbiol. 28, 245–254.
Gorden, J., Small, P.L.C., 1993. Acid resistance in enteric bacteria.
Infect. Immunol. 61, 364–367.
Humphrey, T.J., Richardson, N.P., Statton, K.M., Rowbury, R.J.,
1993. Effects of temperature shift onacid and heat tolerance in
Salmonella enteritidis phage type 4. Appl. Environ. Microbiol. 59,
3120–3122.
Jenkins, D.E., Schultz, J.E., Matin, A., 1988. Starvation-induced cross
protectionagain
st heat or H
2
O
2
challenge in Escherichia coli.
J. Bacteriol. 170, 3910–3914.
Jordan, K.N., Oxford, L., O’Bryne, C.P., 1999. Survival of low-pH
stress by Escherichia coli O157:H7: correlationbetweenalterations
in the cell envelope and increased acid tolerance. Appl. Environ.
Microbiol. 65, 3048–3055.
Leyer, G.J., Johnson, E.A., 1992. Acid adaptation promotes survival
of Salmonella spp. in cheese. Appl. Environ. Microbiol. 58,
2075–2080.
Leyer, G.J., Johnson, E.A., 1993. Acid adaptation induces cross-
protection against environmental stresses in Salmonella typhimur-
ium. Appl. Environ. Microbiol. 59, 1842–1847.
Leyer, G.J., Wang, L.L., Johnson, E.A., 1995. Acid adaptation of
Escherichia coli O157: H7 increases survival in acidic foods. Appl.
Environ. Microbiol. 61, 3752–3755.
Lin, J., Lee, I., Frey, J., Slonczewski, J.L., Foster, J.W., 1995.
Comparative analysis of extreme acid survival in Salmonella
typhimurium, Shigella flexneri, an d Escherichia coli. J. Bacteriol.
177, 4097–4104.
Rowe, M.T., Kirk, R., 1999. An investigation into the phenomenon of
cross-protectioninEscherichia coli O157:H7. Food Microbiol. 19,
157–164.
Ryu, J.-H., Beuchat, L.R., 1998. Influence of acid tolerance responses
onsurvival, growth, and thermal cross-protectionof Escherichia
coli O157:H7 in acidified media and fruit juices. Int. J. Food
Microbiol. 45, 185–193.
Ryu, J.-H., Beuchat, L.R., 1999. Changes in heat tolerance of
Escherichia coli O157:H7 after exposure to acidic environments.
Food Microbiol. 16, 317–324.
SAS Institute, 1987. Statistical Analysis System. SAS Institute, Cary,
NC.
Fig. 2. SDS-PAGE profiles of acid- and heat-stressed S. flexneri cells.
Lane 1, marker; lane 2, control (unadapted); lane 3, acid adapted; lane
4, unadapted heat-shocked; lane 5, unadapted chloramphenicol-
treated, heat-shocked cells.
G.L. Tetteh, L.R. Beuchat / Food Microbiology 20 (2003) 179–185
184

Small, P.L.C., 1998. Shigella and Escherichia coli strategies for survival
at low pH. Jpn. J. Med. Sci. Biol. 51 (Suppl.), S81–S89.
Small, P., Blankenhorn, D., Welty, D., Zinser, E., Slonczewski, J.L.,
1994. Acid and base resistance in Escherichia coli and Shigella
flexneri: role of rpoS and growth pH. J. Bacteriol. 176, 1729–1737.
Smith, J.L., 1987. Shigella as a foodborne pathogen. J. Food Prot. 50,
788–801.
Tetteh, G.L., Beuchat, L.R., 2001. Sensitivity of acid-adapted and
acid-shocked Shigella flexneri to reduced pH achieved with acetic,
lactic, and propionic acids. J. Food Prot. 64, 975–981.
Venkitanarayanan, K.S., Lin, C.-M., Bailey, H., Doyle, M.P., 2002.
Inactivation of Escherichia coli O157:H7, Salmonella enteritidis,
and Listeria monocytogenes on apples, oranges, and tomatoes by
lactic acid with hydrogenperoxide. J. Food Prot. 65, 100–105.
Waterman, S.R., Small, P.L.C., 1998. Acid-sensitive enteric pathogens
are protected from killing under extremely acidic conditions of pH
2.5 when they are inoculated onto certain solid food sources. Appl.
Environ. Microbiol. 64, 3882–3886.
Weissinger, W.R., McWatters, K.H., Beuchat, L.R., 2001. Evaluation
of volatile chemical treatments for lethality to Salmonella onalfalfa
seeds and sprouts. J. Food Prot. 64, 442–450.
Zaika, L.L., Engel, L.S., Kim, A.H., Palumbo, S.A., 1989. Effect of
sodium chloride pH and temperature on growth of Shigella
flexneri. J. Food Prot. 52, 356–359.
G.L. Tetteh, L.R. Beuchat / Food Microbiology 20 (2003) 179–185
185
Document Outline
Wyszukiwarka
Podobne podstrony:
The pathogenesis of Sh flexneri infection lessons from in vitro and in vivo studies
business management McGraw Hill Leaning Into Six Sigma A Parable of the Journey to Six Sigma and
A proteogenomic analysis of Sh flexneri
In Defense of the Representational Theory of Qualia (Replies to Neander, Rey, and Tye)
Applications of Genetic Algorithms to Malware Detection and Creation
Drying kinetics and drying shrinkage of garlic subjected to vacuum microwave dehydration (Figiel)
Attribution of Hand Bones to Sex and Population Groups
Guide to the properties and uses of detergents in biology and biochemistry
Law of Attraction How to Attract Money, Love, and Happiness
Epidemiology and Prevention of Viral Hepatitis A to E
[13]Role of oxidative stress and protein oxidation in the aging process
An Introduction to USA 2 Geographical and Cultural Regions of the USA
Drying kinetics and drying shrinkage of garlic subjected to vacuum microwave dehydration (Figiel)
Attribution of Hand Bones to Sex and Population Groups
Guide to the properties and uses of detergents in biology and biochemistry
Berklee Shares Essential Guide to Lyric Form and Structure Number of Phrases, Getting Your Balance
A1 3 CARVALHO, João M S (2013) The Crucial Role of Internal Communication Audit to Improve Internal
więcej podobnych podstron