
Journal of Archaeological Science (1999) 26, 1127–1133
Article No. jasc.1999.0408, available online at http://www.idealibrary.com on
Determining the Technological Origins of Iron and Steel
David Starley
Ancient Monuments Laboratory, English Heritage, 23 Savile Row, London, WIX 1AB, U.K.
The potential for identifying the technology of production of iron artefacts is investigated through the examination of
two series of well-dated iron samples. Compositional variations were investigated in both the metal matrices, using
scanning electron microscope (SEM)-based wavelength dispersive analysis, and in the slag inclusions by an energy
dispersive detector. This combination of data allowed the partitioning of elements between the two phases to be
calculated, providing a measure of furnace conditions. A first study looked at high quality iron and steel from the Late
Mediaeval and Renaissance, through the analysis of 60 samples from 44 plate armours. A change to the use of superior
steels by south German and English armourers from
1500 is suggested to derive from the high bloomery smelting
process, on the basis of the partitioning of manganese. More recent work examined architectural ironwork with the aim
of investigating 18th and 19th century innovative coke-fired reverberatory processes for the conversion of cast iron to
wrought iron. The results have now been tested against previously suggested models for the composition of these alloys.
1999 Academic Press
Keywords: IRON, SLAG, INCLUSION, ARMOUR, ARCHITECTURAL, FINING, PUDDLING.
Introduction
F
rom the Late Mediaeval through the industrial
period, iron and steel production and trade were
of major importance to the economies of both
the increasingly specialized iron-producing regions of
Europe and the many nations heavily dependent on
imports. This continent-wide trade pattern shifted,
often as a result of technological innovations. Two
particularly significant changes were the move from
direct (bloomery) iron and steel to indirect (blast
furnace/finery) alloys and the change from charcoal to
coke as fuel for furnaces and hearths of the industry.
Iron smelting technology in Europe followed three
broad traditions. The bloomery or ‘‘direct’’ process.
had developed from, but retained essentially the same
principles as, the earliest furnaces. This was a batch
operation in which each smelt was terminated to
remove the solid bloom. The iron, although containing
considerable slag as inclusions, was su
fficiently low in
carbon (ferritic iron or steel) that it could be worked
without further treatment, apart from the expulsion of
slag during forging. From the Late Mediaeval period
the blast furnace became increasingly important with
the sole product being cast iron; this typically had a
much higher carbon content, above 2·5% and was not
malleable. The third technique, often considered as
intermediate between the other two, was the high
bloomery (stu¨ckofen) which allowed the flexibility to
produce either cast iron or malleable alloys, the latter
being noted (
) for their steely nature.
Whilst the casting of objects direct from the furnace
was often an attractive option, the greater need
continued to be for malleable iron and a series of
conversion processes were developed to decarburize
cast iron. Until the 18th century this was achieved by
remelting in a finery, a highly oxidizing charcoal-fired
hearth. However, through that century other tech-
niques were developed based on the use of reverber-
atory furnaces which, by separating the fuel and iron,
allowed coal or coke to be used as fuel without
excessive transfer of sulphur to the metal (
). A patent by John Wood in 1761
involved fining of cast iron with coal followed by
granulation and heating with flux in crucibles in a
reverberatory furnace. The term stamping and potting
was applied to this and similar processes operating
around this date. In 1783 Cort patented what became
known as the dry puddling process. This dispensed
with use of a separate finery hearth and decarburized
the cast iron on a bed of sand in a reverberatory
furnace. The originality of his method was the subject
of a lawsuit and there is reason to believe aspects of his
process were not without precedent (ibid.). A further
development, beyond 1816, was wet puddling, in which
the furnace bottom was lined with iron plates protected
by furnace cinder. This allowed a slag bath to build up
which very e
ffectively oxidized elements from the cast
iron. These developments, together with the use of
coke in blast furnaces, were crucial for the continued
expansion of the British iron industry, previously
hindered by high charcoal costs. From 1720, when
more bar iron was imported than produced, the
country had become a net exporter by the end of the
century (
Despite the diversity of production methods, the
range of ‘‘wrought iron’’ products have proved
remarkably di
fficult to differentiate. Reasons include
1127
0305–4403/99/081127+07 $30.00/0
1999 Academic Press

the heterogeneity within samples, the very low levels of
trace elements which might be co-smelted with the
iron, and the great variation in the composition of
ores, fluxes and inadvertently added compounds, such
as those deriving from furnace linings. Previous
approaches to the subject have compared slag inclu-
sions within the iron to bulk slags recovered from
production centres (
) and
against archival iron samples (
). How-
ever, for many of the less well-known processes, reli-
ably identified residues are not yet available. Without
such material of known technological provenance, the
work described below adopted a methodology of
examining large groups of artefacts, reliably dated
across a broad period of time. Compositional changes
observed in the data were matched against historically
known details of production methods.
Partitioning of Elements
The approach of this study is to consider the behaviour
of di
fferent elements in the alternative processes,
particularly those elements which tend to partition
between the metal and slag phases. This di
fference is
unlikely to a
ffect those elements which always pass into
the slag: potassium, sodium, calcium and magnesium,
or those that are invariably reduced into the iron:
copper, nickel and cobalt. However, for certain ele-
ments (phosphorus, manganese, sulphur and silicon)
the degree to which they are reduced into the metal will
depend on furnace temperature. Sulphur’s detrimental
e
ffect on iron is such that high-sulphur raw materials
were avoided by smelters. Silicon is only likely to be
reduced into the cast iron at high temperatures, cer-
tainly when coke is used as fuel, but is removed rapidly
during the early stages of fining. A large proportion of
phosphorus will be reduced into the iron but the
tendency for the element to di
ffuse between metal and
inclusions during hot working makes it a less suitable
identifier of smelting technology.
Manganese is potentially a reliable indicator of
smelting conditions. The e
ffect of temperature on
the reduction of manganese has been discussed by
. Their calculations, which
assume the activity of carbon to be unity and the
partial pressure of carbon monoxide to be one atmos-
phere, suggest that a rise from 1327 to 1527
C would
increase the recovery of manganese 11-fold. Hence,
given similar ores, cast iron from the higher tempera-
ture blast furnace might be expected to contain levels
of manganese an order of magnitude greater than
would be expected from a bloomery.
The subsequent e
ffect of the fining processes on the
dissolved elements is dramatic. In the conversion pro-
cesses silicon in the pig iron oxidizes most rapidly;
followed in succession by manganese, phosphorus then
carbon. Thus, a high proportion of any manganese and
phosphorus present will oxidize into the finery/
puddling slag. Their final content there will be depen-
dent not only on the amount of the two elements in the
cast iron but also on the bulk of slag present and
the extent to which other materials were added (par-
ticularly hammerscale and haematite ore during wet
puddling). However, it would appear likely that slag
from the decarburizing processes would contain
very significantly elevated levels of manganese and
phosphorus.
Examination and Analysis
After mounting and preparation, metallographic
examination was carried out to allow carbon content,
grain size, inclusion content and any heat treatment of
samples to be assessed.
shows a typical
microstructure of wrought iron, with a high slag inclu-
sion content and little if any carbon in the metal.
Microanalysis of the samples used scanning electron
microscope (SEM) based techniques. The metal
matrices were analysed using a wavelength dispersive
detector, which with detection limits such as 0·004 wt%
for phosphorus and 0·009 wt% for manganese was
su
fficiently sensitive to detect trace levels of impurities
present. Slag inclusion analysis utilized a faster but
less sensitive energy dispersive detector to provide
quantitative analysis of all elements present with
atomic number greater than oxygen. Inclusion size was
recorded during analysis to enable a check on one
possible source of error; elements such as phosphorus
are known to di
ffuse between inclusions and the metal
during hot working and it might be expected that the
composition of small inclusions, with high surface area
to volume ratio, would be more significantly changed
as a result. However, no significant link between
inclusion size and composition was identified.
Study 1. Technological Origins of Iron and
Steel in European Plate Armour
This research (
investigated 44 plate
armours of 14th to 17th century date from the
Figure 1. Micrograph of sample from staircase upright, Coventry
Hall, Streatham 1800. Etched in nital
100.
1128 D. Starley
collections of the Royal Armouries in HM Tower of
London. The project aimed to use this well-preserved,
remarkably closely dated, material to identify a change
from the use of bloomery iron to blast furnace/finery
products. The research was carried out against the
widely held view of art historians that the 15th century
comprised the ‘‘great period’’ of plate armour and that
little significant later development occurred. Metallo-
graphic examination revealed a di
fferent picture. The
major armour-producing centre of south Germany
(together with the English Greenwich Armoury
which employed German craftsmen) began, from the
early 16th century, to utilize remarkably homogenous
steel, subsequently heat treated to optimize its
e
ffectiveness.
Microanalyses of the iron show this later German
material to be uniformly high in manganese (averaging
0·04 wt%) and low in phosphorus compared with ear-
lier German and Italian material of both periods
). The date for this change coincides with the
expansion of the blast furnace, particularly in the Low
Countries (
). However the data from analy-
sis of more than 700 inclusions show that manganese
levels in inclusions from these late German armours
are not significantly higher than those for other
samples, giving rise to relatively low partition coef-
ficients (wt% in slag/wt% in metal). These results
suggest that the final process in which the slag inter-
acted with the metal was, relative to the bloomery,
more reducing. It was therefore concluded that this
material most probably derived from the high
bloomery rather than the highly oxidizing finery. For
this specialized product at least, the demand for high
quality metal took precedence over any economic
incentive to use mass produced iron from the indirect
process.
Study 2. Converting Processes for
Architectural Wrought Iron
Architectural ironwork shares two advantages with
armour as a source of samples. Firstly, samples may be
dated with precision unattainable in archaeologically
recovered artefacts and secondly, corrosion is rarely a
problem. Furthermore, it is more representative of
bulk iron production, importation and use than
specific artefacts and it potentially provides a very wide
date range. In this study samples of ‘‘wrought iron’’,
mainly from the Architectural Study Collection of
English Heritage, were investigated for compositional
changes, particularly those which could be associated
with the innovative 18th and 19th century conver-
sion processes which had such a dramatic e
ffect on
the expansion of the British iron industry and the
industrial revolution in general.
discuss the characteris-
tics of bulk slags from the wide range of iron conver-
sion processes operating through this period.
examined dry puddling slags and con-
cluded that it was possible to distinguish these from
finery and bloomery slags on the basis of microstruc-
tural constituents. Neither of these studies extended to
the consideration of the evidence for stamping and
potting, although the economic importance of these
processes was recognized in stating that they were
responsible for half the production of bar iron in
Britain by 1780 (ibid.).
A major di
fficulty with any study of historical iron-
working debris is the scarcity of reference material
for which the production process is known with
certainty. Like the study of armour this research used
samples, for which the technology of production was
unknown, but which were closely dated; in this case
from their architectural contexts. It is strictly only
possible to say whether a sample predates a specific
technique. The recycling of old iron and the continued
supply of iron from more traditional techniques will
inevitably lead to a blurred transition rather than an
immediate displacement of old by new. However,
unlike the study of the specialized production of steel
armour the choice of architectural ironwork should
more closely reflect the availability of bulk iron at
any particular time. Considering the major economic
importance of the processes of interest, very extensive
sampling should not be necessary to ensure that
material from the full range of techniques will be
represented.
A large proportion of the samples reported on here
are from the later 18th century but some undoubted
bloomery samples date from as early as 1200 and some
Mn
Mean matrix analysis (wt%)
±
1
σ
North Italian
P
S
Ni
South German
and Greenwic
h
0
0.1
0.2
Before
1500
After
1500
Mn
P
S
Ni
Mn
P
S
Ni
Before
1500
After
1500
Mn
P
S
Ni
Figure 2. Mean (
one standard deviation) levels of manganese,
phosphorus, sulphur and nickel in the metal of the armour samples.
Determining the Technological Origins of Iron and Steel 1129
ornamental ironwork is from the last decade of the
19th century. Material was generally obtained from
buildings whose history was well documented and a
series of short wall ties (
) which incorporated
the year of construction were a very welcome addition.
The 26 samples studied can only be considered as a
pilot project. However, the results do show patterns in
composition which are interesting to compare with
suggestions made by other workers for determining the
technology used to produce artefacts.
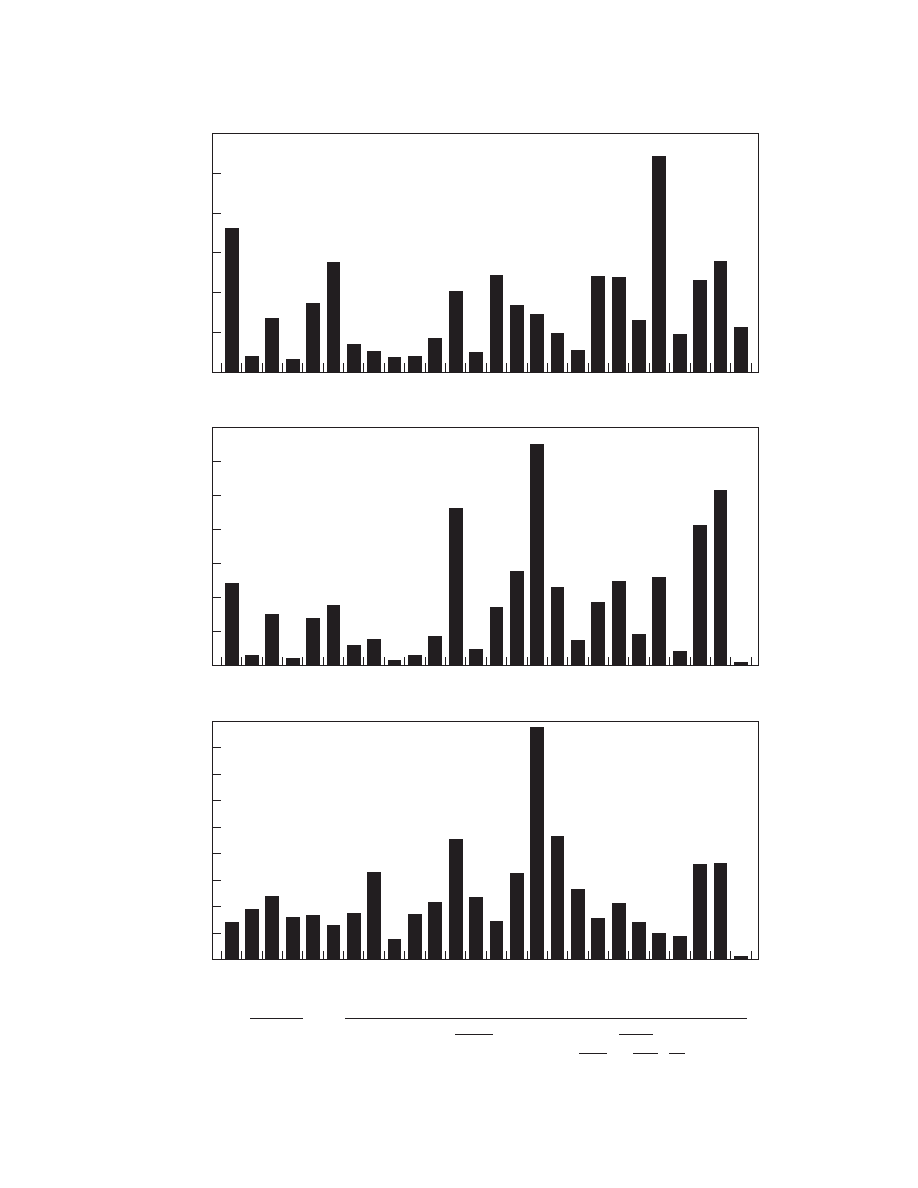
Low sulphur levels have generally been regarded as
indicative of bloomery iron and charcoal fined iron.
The inclusion data in
show the changing
levels of sulphur in inclusions with time. For clarity
only the latest possible dates for the samples were
plotted on the x-axis. Generally, values show an
upward trend, with values above 0·4% all dating to
1799 or later and exceptionally high levels from a
‘‘wrought iron’’ gate of 1891. Despite this trend, ab-
solute di
fferences over the entire period are narrow and
it would appear that little confidence can be placed
in sulphur as a discriminator for the fuel used in
conversion processes.
The alkalis, potash and soda, originate from either
charcoal or coke ash.
note
that very low levels of Na
2
O and K
2
O occur in dry
puddling slags, where the reverberatory furnace kept
the fuel separate from the iron and slag. This contrasts
to bloomery and finery slags which form in contact
with fuel ash.
looked at
inclusions and extended this argument to suggest that
low alkali content of inclusions could be used as a
discriminator of process. In
it can be seen
that slag inclusions in late 18th and 19th century
samples are generally low in Na
2
O and K
2
O and so
conform to this model (a small staple of 1825 may well
be one example of recycled iron).
‘‘probable upper limit’’ of 1% alkali certainly fits
most of the ironwork used in the period when
puddling was at its height. However, low alkali con-
tents also appear to characterize iron from the decade
before Cort’s patent for the puddling process of 1784.
These are unlikely to derive from the stamping and
potting process which, although it also used reverber-
atory furnaces, also used highly alkaline fluxes to
remove sulphur picked up in an earlier refining stage.
Several much earlier samples also contain only low
alkali concentrations. Thus, whilst earlier researchers
are correct in stating that puddled iron normally
contains low alkali inclusions, the presence of low
alkali inclusions cannot be cited as proof of this
process.
The value of manganese partitioning in the architec-
tural samples is limited by the very low concentrations
of the element in the metal and inclusions and so this is
not discussed further here. By contrast most of the
samples from early to late contained significant levels
of phosphorus (
. This provided an oppor-
tunity to test the findings of
who
suggested, on the basis of inclusion and bulk metal
analyses, that distinctly higher proportions of phos-
phorus pass into the slag of fined and puddled iron
than bloomery iron. Whilst some di
ffusion of phos-
phorus will occur during hot working, this will be
relatively minor in comparison to the changes taking
place during the high temperature, highly oxidizing
conditions of the decarburizing processes.
shows very considerable variation between the mean
phosphorus content of inclusions, demonstrating the
quantities of this element which could be stored up in
the non-metallic phases. However, it is di
fficult to
discern patterns in the data until the partitioning data
is displayed (
. Values on the y-axis are
calculated as percentage phosphorus pentoxide in the
slag divided by mean percentage phosphorus in the
iron. Early samples, at least before the late 17th
century, are relatively consistent and set a baseline for
what might be expected from the bloomery process. In
fact many later samples show very similar levels.
However, a scattering of samples dated as widely as
1699 to 1876 show a technique or techniques which
were able to extract greater proportions of phosphorus
from the metal into the slag. Wet puddling, from 1816
or slightly later (
), had a reputation for
the superior removal of phosphorus (and silicon) from
cast iron. Five samples date beyond this introduction
but none show greater partitioning of phosphorus than
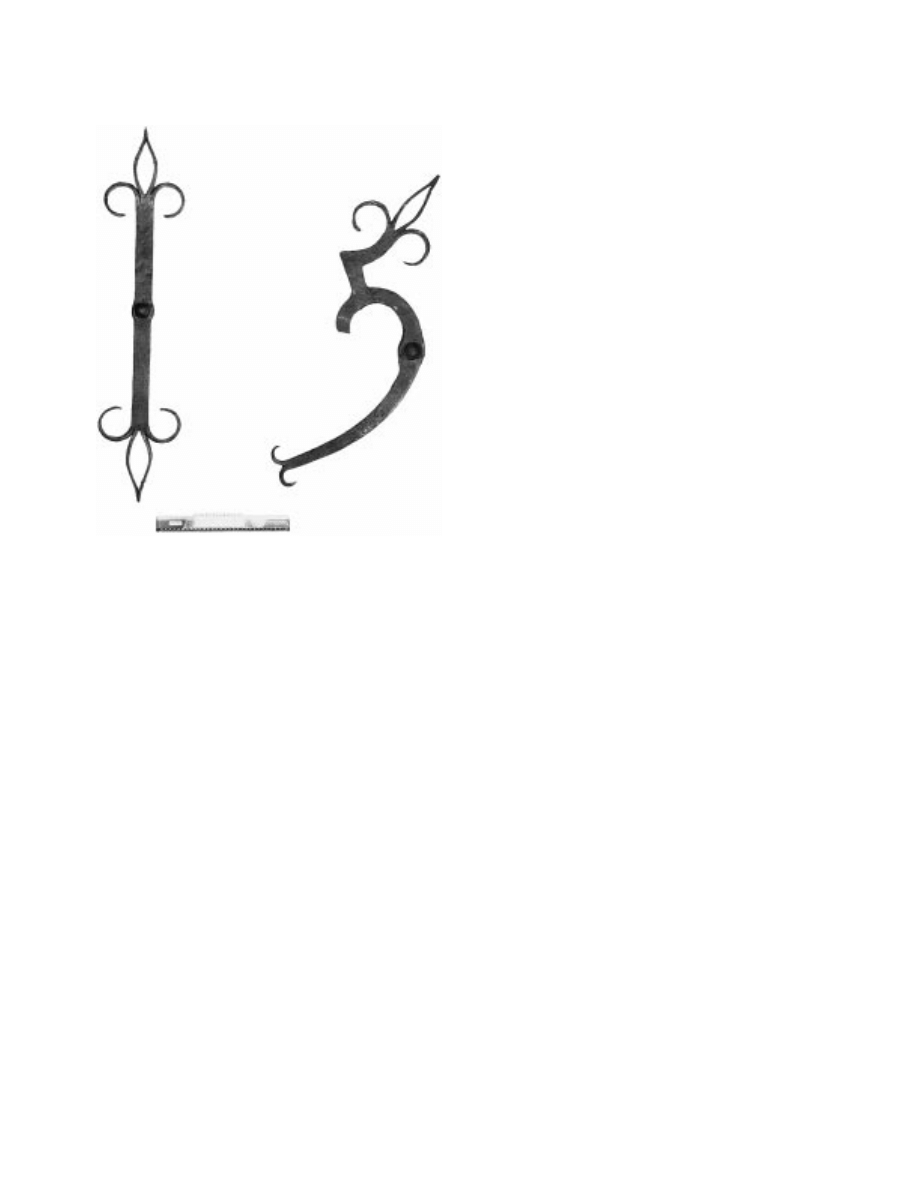
Figure 3. Two ‘‘short wall ties’’, Great Yarmouth, 156 . . . The final
digit was missing from this oldest and largest known set of wall
anchors from the town. Copyright English Heritage Photographic
Library.
1130 D. Starley

several late 18th century samples. Whilst initial indi-
cations, based on so few samples should be treated
with some caution, given the apparent rapidity with
which wet puddling became the standard technique for
the production of wrought iron in Britain it would be
unlikely that none of these five samples derived from
the process.
Conclusions
Metallography and microanalysis are powerful tech-
niques for characterizing ferrous alloys and determin-
ing the later working and heat treatment histories of
artefacts. This study extended these techniques to the
investigation of iron production technology, particu-
larly with the aim of di
fferentiating the products of
direct process from those of indirect production.
Microanalysis of both inclusions and metal matrices
allowed quantification of elemental partitioning be-
tween the two phases which reflects the conditions in
the furnace or hearth. The methodology was tested on
two groups of material. Firstly, samples from the
specialized manufacture of defensive armour showed
a shift in the use of materials, identifiable by metal-
lography and microanalysis. The partitioning behav-
iour of the element manganese suggested a specific, if
unexpected, technological source: the high bloomery.
The second group of material comprised samples of
architectural ironwork covering a much wider date
range. Of particular interest were the origins of mid- to
late 18th century material. Hopes of identifying
less well documented conversion processes such as
stamping and potting were not fulfilled. Whether this is
due to methodological weakness or poor choice of
Dry
/
Wet puddling
4.5
0.0
1200
Sample date
P
ercentage of K
2
O + Na
2
O in inc
lusions
2.0
4.0
3.5
3.0
2.5
1.5
1.0
0.5
1400
1569
1601
1637
1651
1691
1699
1729
1729
1743
1770
1775
1779
1779
1782
1800
1822
1825
1872
1876
1891
(b)
1779
1799
1799
1799
5.2
0.0
1200
P
ercentage of sulphur in inc
lusions
0.2
5.0
0.8
0.6
0.4
1400
1569
1601
1637
1651
1691
1699
1729
1729
1743
1770
1775
1779
1779
1782
1800
1822
1825
1872
1876
1891
(a)
1779
1799
1799
1799
Fining
?
Potting and stamping
?
Figure 4. Concentrations of (a) sulphur and (b) oxides of potassium and sodium in architectural iron inclusions.
Determining the Technological Origins of Iron and Steel 1131
180
0
1200
Sample date
P
ercentage of phosphorus pentoxide (slag)/
percentage of P (iron)
80
160
140
120
100
60
40
20
1400
1569
1601
1637
1651
1691
1699
1729
1729
1743
1770
1775
1779
1779
1782
1800
1822
1825
1872
1876
1891
(c)
1779
1799
1799
1799
14.0
0.0
1200
P
ercentage of phosphorus pentoxide in inc
lusions
4.0
12.0
10.0
8.0
6.0
2.0
1400
1569
1601
1637
1651
1691
1699
1729
1729
1743
1770
1775
1779
1779
1782
1800
1822
1825
1872
1876
1891
(b)
1779
1799
1799
1799
0.300
0.000
1200
P
ercentage of phosphorus in iron
0.050
0.250
0.200
0.150
0.100
1400
1569
1601
1637
1651
1691
1699
1729
1729
1743
1770
1775
1779
1779
1782
1800
1822
1825
1872
1876
1891
(a)
1779
1799
1799
1799
Fining
?
Dry
/
Wet puddling
Stamping and potting
?
Figure 5. The distribution of phosphorus in architectural iron samples (mean values). (a) Elemental phosphorus in iron matrix. (b) Phosphorus
pentoxide in slag inclusions. (c) Partitioning of phosphorus between slag inclusions and iron.
1132 D. Starley

samples is unclear and will need to be tested by
more specific sampling as the project moves beyond
the pilot stage. Meanwhile it would appear that the
identification of production processes of ‘‘wrought
iron’’ alloys by quantification of the sulphur or alkali
content of their inclusions should be viewed with some
caution.
Acknowledgements
Many thanks to those who provided samples, in par-
ticular the sta
ff of the Royal Armouries and those
associated with English Heritage’s Architectural
Collections and Study Centre. The work on armour
was undertaken as a Ph.D. at Bradford University,
funded by the Science and Engineering Research
Council. Wavelength dispersive analysis was under-
taken by Chris Salter at The Research Laboratory
for Archaeology and the History of Art, Oxford
University.
References
Awty, B. G. (1981). The continental origins of Wealden ironworkers,
1451–1544. Economic History Review 34(4), 524–539.
Bodsworth, C. & Bell, H. B. (1972). Physical Chemistry of Iron and
Steel Manufacture. 2nd edn. London: Longman.
Gordon, R. B. (1983). English iron for American arms: laboratory
evidence on the iron used at the Springfield Armoury in 1860.
Historical Metallurgy 17(2), 91–98.
Gordon, R. B. (1984) The quality of wrought iron evaluated by
microprobe analysis. In A. D. Romig Jr. & J. I. Goldstein, Eds)
Microbeam analysis 1984. Proceedings of the 19th annual confer-
ence of the Microbeam Analysis Society, Bethlehem, Pennsylvania
1984. San Franscisco: San Francisco Press, pp. 231–234.
Killick, D. J. & Gordon, R. B. (1987). Microstructures of puddling
slags from Fontley, England and Roxbury, Connecticut, U.S.A.
Historical Metallurgy 21 (1), 28–36.
Morton, G. R. & Mutton, N. (1967). The transition to Cort’s
puddling process. Journal of the Iron and Steel Institute 205,
722–728.
Morton, G. R. & Wingrove, J. (1969). Slag, cinder, and bear. Bulletin
of the Historical Metallurgy Group 3 (1), 55–61.
Mott, R. A. (1977–78). Dry and wet puddling. Transactions
Newcomen Society 49, 153–158.
Percy, J. (1864). Metallurgy: Iron and Steel. London: John Murray.
Rostoker, W. & Dvorak, J. R. (1990). Wrought irons: distinguishing
between processes. Archeomaterials 4 (2), 153–166.
Starley, D. (1992). Medieval iron and steel production. Unpubl. Ph.D.
Thesis. Bradford University.
Tylecote, R. F. (1976). A History of Metallurgy. London: Metals
Society.
Determining the Technological Origins of Iron and Steel 1133
Document Outline
Wyszukiwarka
Podobne podstrony:
Serneels Archeometallurgy of Iron and Steel
[2007] Hoffmann, T Aquinas And Intellectual Determinism; The Test Case Of Angelic Sin
Kissinger and Rockefeller the Origins of AIDS and Ebola
We have the widest range of equipment and products worldwide
Best Available Techniques for the Surface Treatment of metals and plastics
The Intellectual Origins Of Modern Catholic Social Teaching On Economics
The Pernicious Blend of Rumination and Fearlessness in NSSI
The Cambodian Campaign during the Vietnam War The History of the Controversial Invasion of Cambodia
Chavda The hidden power of prayer and fasting
The Inner Nature of Music and the Experience of Tone
The Specious Origins of Liberalism Anthony Ludovici (1967)
Hoppe Hans H The Political Economy of Democracy and Monarchy and the Idea of a Natural Order 1995
Yasunari Kawabata The Dancing Girl of Izu and Other Stories (rtf)
Волощук Medieval Slovakia and Croatia as the second homeland of nobility and peoples from the Rus’ i
the illict preparation of morphine and heroin from pharmaceutical products containing codeine homeba
British Patent 6,481 Improvements relating to the Electrical Transmission of Power and to Apparatus
Technologie w Hearts of Iron II
Virato, Swami Interview With Sogyal Rinpoche On The Tibetan Book Of Living And Dying (New Frontier
więcej podobnych podstron