
Atomic Force Microscopy and Single-Molecule Force
Microscopy Studies of Biopolymers
Nehal I. Abu-Lail
Terri A. Camesano
Worcester Polytechnic Institute, Worcester, Massachusetts, U.S.A.
INTRODUCTION
Biopolymers are macromolecules of biological origin,
which include nucleic acids (DNA and RNA), proteins,
peptides, and polysaccharides. Although these macro-
molecules influence biological processes in different
ways, most biological processes are associated to some
extent with the physical properties of biopolymers (chain
structure, flexibility, and excluded volume interactions).
For example, the conformation of bacterial surface bio-
polymers affects their adhesion to host tissue in the es-
tablishment of infection. In other biological processes
such as protein synthesis, the specific structural units of
the biopolymers (nucleic acids and proteins) control the
biological function.
[1]
Interest in analyzing the physical properties and
structural features of biopolymers stems from the wide
variety of functions they can perform in living systems of
humans,
[2]
animals,
[2,3]
plants,
[4]
bacteria,
[5]
and fungi
[6,7]
or the important roles they play in industrial operations.
The ultimate aim behind the characterization of bio-
polymer properties is to provide a better understanding
and control of their behavior in biological, medical, and/
or industrial processes. Examples of applications af-
fected by biopolymer properties are environmental biore-
mediation,
[8–10]
biomedical applications such as wound
healing,
[11–13]
gene therapy,
[14]
growth mechanisms of
macromolecular crystals,
[15]
food technology,
[16,17]
and
bacterial adhesion.
[18]
OVERVIEW
A wide range of techniques and instruments have been
used to characterize biopolymer properties. Examples
include the use of vibrational circular dichroism (VCD)
to investigate DNA condensation,
[19]
fluorescence corre-
lation spectroscopy (FCS) to study the diffusion proper-
ties, size, and conformation of native and denatured
schizophyllan in dilute solutions,
[20]
size exclusion
chromatography (SEC) to characterize Pseudomonas
putida KT2442 surface biopolymers,
[21]
X-ray diffraction
(XRD) to study the ordered conformation of gel-forming
polysaccharides,
[22]
transmission electron microscopy
(TEM) to image well-characterized algal cellulose micro-
fibrils,
[23]
scanning electron microscopy (SEM) to study
the extracellular matrix of nutrient-limited adherent
bacterial populations,
[24]
Fourier transform infrared
(FTIR) spectroscopy to study the effect of protein
immobilization on birnessite,
[25]
and laser light scattering
(LLS) to investigate the effect of pH on gelatin self-
association in dilute solutions.
[26]
Other techniques such
as optical tweezers
[27]
and the surface-force apparatus
[28]
have also been used to measure the interactions between
biopolymers and surfaces.
Because of the accelerating developments made in
atomic force microscopy (AFM) as a surface character-
ization technique, AFM is now a preferred instrument in
the study of biological macromolecules. Atomic force
microscopy is characterized by high lateral (6 A
˚ ) and
vertical (1 A
˚ ) resolutions
[29,30]
and a high signal-to-noise
ratio.
[31]
These features give rise to the AFM’s ability to
image detailed structures of individual or groups of
delicate biopolymers.
[31]
In contrast to conventional
biological imaging methods such as SEM and TEM,
AFM can be used to image biopolymers in their native
state without the need for staining,
[3]
labeling,
[31]
or
coating with a conducting gold layer.
[8]
In addition, AFM
can be used to probe biopolymers in ambient air or liquid
without the need to operate under vacuum.
[3]
In particular,
the ability to image biopolymers in liquid with AFM
allows for investigation of macromolecules under native
conditions for several hours or even days without
damage.
[31,32]
Although AFM can be used to image
biopolymers in air, precautionary measures have to be
taken to ensure that the sample is not damaged and that
artifacts are not created. When samples are allowed to air-
dry, there is the possibility of coagulation or rearrange-
ment of the molecules on the substrate during the drying
process.
[33]
Interaction forces between biopolymers and surfaces
should be measured in a liquid environment to minimize
the presence of large capillary forces that are present in
Dekker Encyclopedia of Nanoscience and Nanotechnology
119
DOI: 10.1081/E-ENN 120014171
Copyright
D 2004 by Marcel Dekker, Inc. All rights reserved.
A

air (
30 nN).
[34]
The high adhesive forces caused by
capillary forces are destructive to many biological sam-
ples and mask other interaction forces of lesser mag-
nitudes, such as van der Waals forces and electro-
static interactions.
Atomic force microscopy has evolved from an imaging
technique to a versatile tool that also allows for investi-
gation of molecular forces at interfaces with great detail.
Atomic force microscopy can also be used to probe the
chemical nature,
[35]
elasticity,
[36]
roughness,
[13]
and sur-
face charge
[37]
of biopolymers. We review the use of AFM
as a state-of-the-art tool to characterize biopolymers. Ex-
amples will be provided on the use of AFM to characterize
DNA, proteins, and polysaccharides.
PROBING DNA WITH ATOMIC
FORCE MICROSCOPY
Earlier Efforts to Establish High-Resolution
DNA Imaging
Since the invention of AFM, continuous improvements
have been made in the ability to image and characterize
deoxyribonucleic acid (DNA), which carries the genetic
code of all living organisms. Most early attempts to image
DNA were performed in air
[38]
because early attempts to
image DNA in liquid (especially water) showed that if the
molecules were not fixed properly, they could move
during the imaging process. Their movement limited the
ability to obtain reproducible results.
[39]
This challenge
was overcome by stable binding of double-stranded DNA
molecules to flat mica using chemical modification of the
mica with 3-aminopropyltriethoxysilane. DNA molecules
were bound to mica by this technique and molecules with
contour lengths of 20–80 nm were stably imaged under
repetitive scanning.
[40]
With techniques available at that
time, the highest resolution achievable in DNA imaging
was 2–3 nm. Because this was an order of magnitude less
than the resolution required for DNA sequencing,
[41]
better substrate and sample preparation methods were re-
quired. Continuous and accelerated efforts were focused
on reaching high-resolution AFM imaging of DNA. For
example, Bensimon et al.
[42]
invented a technique for
alignment of DNA on a silanated mica substrate, known as
‘‘molecular combing.’’ With this preparation technique,
DNA could be elongated and even minute quantities (10
3
molecules) could be imaged, which opened the way for
faster physical mapping of the genome and increased
detection abilities. Many variations of DNA immobiliza-
tion based on coating of a surface with an aminosilane
compound or a self-assembled monolayer can be found in
the literature.
[43–45]
Imaging of DNA Structure
Although there are numerous successful examples of
high-resolution DNA imaging, we will focus on selected
examples. Atomic force microscopy imaging has been
widely used to discern the structure of DNA. Single- and
double-stranded DNA were each imaged in propanol,
butanol, and air. Measured molecular lengths were
1
mm.
[46]
From imaging DNA on mica, single- and double-
stranded DNA could be differentiated.
[47]
The images of
the double-stranded DNA showed an open circular shape
without drastic contortions and a contour length within
7% of the calculated length. Single-stranded DNA was
present as compact open circles with nodes or lumps
almost uniformly distributed or as highly elongated circles
with a few nodes, the latter being more common.
[47]
Because of much effort, rapid characterization of the
structure of DNA by tapping mode AFM imaging in
Fig. 1
Top-view AFM images of different double-stranded (ds) DNA topologies on amino-terminated mica (vertical color scale = 3
nm) taken in AFM tapping mode under ambient conditions. (a) Linear l-DNA (with 48.5 kbp), (b) nontwisted circular DNA plasmids
(vector with 3.2 kbp) and (c) circular supercoiled DNA with twists and writhes due to internal supercoiling (supercoiled DNA ladder 2–
16 kbp). The measured width of dsDNA of all geometrical topologies was
3–7 nm and affected by the tip geometry. The molecular
height was
1 nm. (From Ref. [48] with permission from Wiley & Sons Inc.) (View this art in color at www.dekker.com.)
120
Atomic Force Microscopy and Single-Molecule Force Microscopy Studies of Biopolymers

ambient conditions has become a relatively routine
technique.
[48]
Examples on the use of tapping mode
AFM imaging to discern differences between linear
double-stranded l-DNA, circular double-stranded plasmid
DNA, and supercoiled double-stranded DNA plasmids
with twists and writhes are shown in Fig. 1. DNA
supercoiling has also been observed. The intramolecular
triplex H-DNA formed by mirror-repeated purine–pyrim-
idine repeats and stabilized by negative DNA supercoiling
was imaged. These images showed that the H-DNA is a
protrusion with a different thickness than the DNA
duplex (Fig. 2).
[49]
The conformation of DNA can be
also studied with AFM imaging. For example, pH was
shown to affect DNA conformation because l-DNA could
be denatured by HCl addition and renatured upon NaOH
addition.
[50]
Characterization of DNA
Molecular Properties
Analyzing AFM images provides a wealth of information
on the size and conformation of DNA. For example, DNA
imaged under ambient conditions using near-contact mode
showed width values up to four times smaller than values
measured in noncontact mode, even with the same tip.
[51]
The discrepancy was attributed to the greater resolution
achievable through near contact compared with noncon-
tact mode imaging. Estimation of the length of double-
stranded DNA molecules as short as 100–200 base pairs
from AFM images in air was considered an advance in
DNA characterization. The measurements gave lengths
consistent with the known dimensions of A-DNA.
[52]
It
was not possible to image shorter DNA molecules (25–50
base pairs) because intermolecular cross-bridging and
base pairing in the molecules caused only globular forms
to be viewed.
[52]
In a revolutionary development, carbon nanotube AFM
tips are starting to provide a new dimension in AFM
imaging. These tips are ideal for AFM work because of
their small diameter, high aspect ratio, large Young’s
modulus, mechanical robustness, well-defined structure,
and unique chemical properties.
[53]
With the use of carbon
nanotube tips, high-resolution images of RecA-double
stranded DNA complexes were obtained (Fig. 3). The
images revealed the 10-nm pitch of RecA-double stranded
DNA complexes and RecA filaments as three-dimensional
surface topographical features, without reconstruction
Fig. 2
Atomic force microscopy images of DNA deposited
at pH = 5.0. These images represent high-resolution images of
H-DNA. The two images are schematics of the height and
width. (From Ref. [49]. Copyright (2001), with permission
from Elsevier.) (View this art in color at www.dekker.com.)
Fig. 3
Topographic AFM images of RecA–dsDNA filaments observed with A) a CNT tip and B) with a standard tip (TESP-type tip).
(From Ref. [54]. Copyright (2001), with permission from Elsevier.) (View this art in color at www.dekker.com.)
Atomic Force Microscopy and Single-Molecule Force Microscopy Studies of Biopolymers
121
A

analysis. The depth of the notch between two pitches was
< 1 nm.
[54]
New imaging modes also helped improve DNA
characterization. In a recent study, magnetic-mode AFM
was used to characterize the process of DNA adsorption
on a highly oriented pyrolytic graphite (HOPG) electrode
surface. The images of single- and double-stranded DNA
molecules showed that both types have the tendency to
self-assemble from solution onto the HOPG surface
(Figs. 4 and 5). The adsorbed film heights were dependent
on the DNA concentration and were held to the surface
with noncovalent interactions such as hydrogen bonding,
base stacking, and electrostatic, van der Waals, and
hydrophobic interactions.
[55]
Stretching DNA to Study
Biomechanical Properties
The biomechanical properties of DNA can be obtained
through stretching these molecules in a technique known
as single molecule force spectroscopy (SMFS). DNA
molecules are tethered to a surface at one end and
stretched through application of an external force, which
may be magnetic,
[56]
caused by hydrodynamic flow,
[56]
or
result from the AFM cantilever stiffness
[57]
or an electrical
field.
[58]
Typically, one DNA molecule is picked up from
an adsorbed layer of DNA molecules by the AFM tip
because of an applied contact force of several nanoNew-
tons. Upon retraction of the tip from the layer, the DNA
strand is stretched. The resulting force from this stretching
is measured as cantilever deflection, which can be
converted to force by accounting for the spring constant
of the cantilever. Several methods exist for determining
these spring constants, as reviewed in Ref. [59]. Stretching
experiments provide important information about the
mechanical properties of DNA. We will discuss three
examples: probing DNA elasticity, quantifying inter-
actions between complementary strands of DNA, and
DNA sequencing.
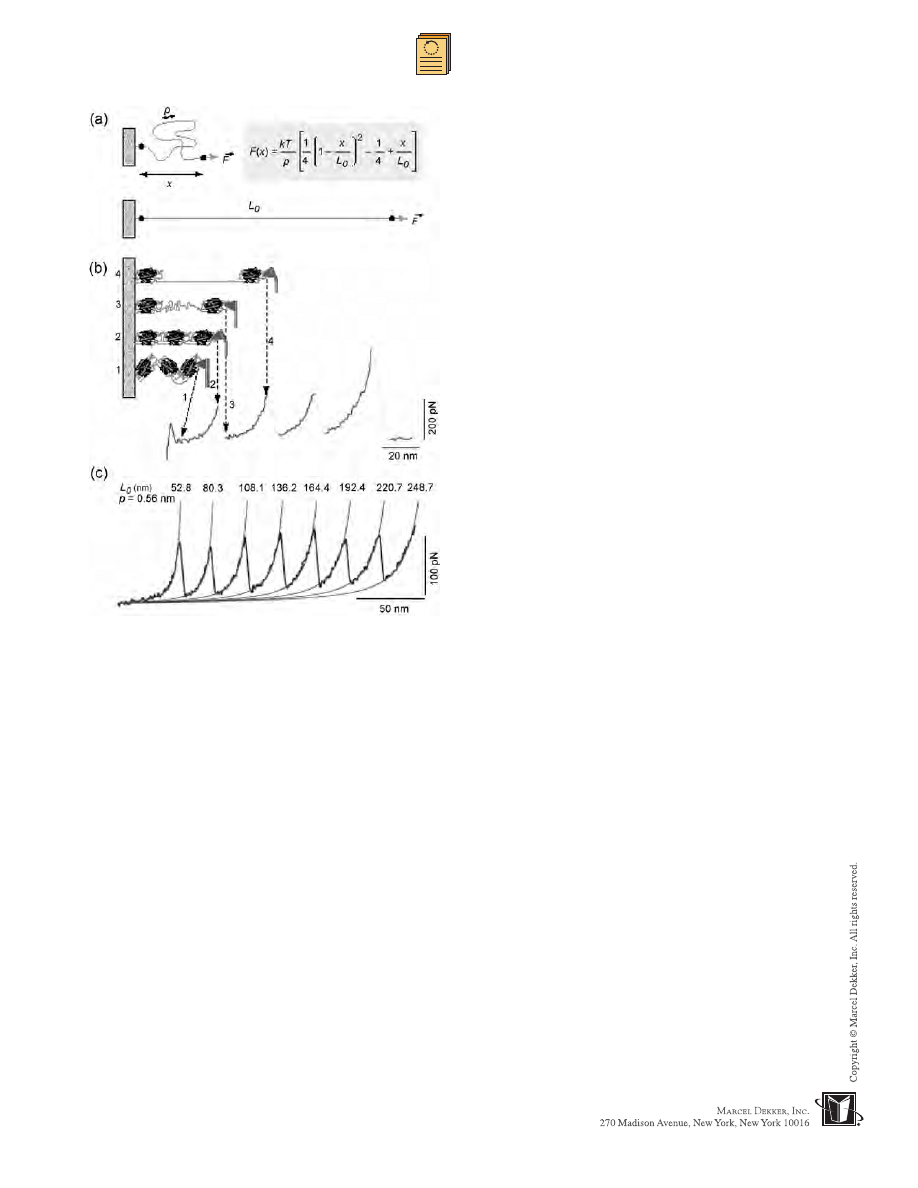
Elasticity of DNA
The elasticity of single molecules can be estimated
directly from force-extension measurements by applying
random-walk statistical mechanical-based models. The
most frequently used models are the freely jointed chain
(FJC), extensible freely jointed chain (FJC +), and
wormlike chain (WLC) models.
[36]
The elasticity of
DNA molecules was first estimated by applying the FJC
model to force-extension data between single DNA
molecules (chemically attached by one end to a glass
surface and by the other end to a magnetic bead) and an
AFM tip under three different salt concentrations.
[56]
The
FJC model failed to explain the force-extension data
because of the fact that it does not account for the
extensibility of the molecules. It appeared that the DNA
molecules could deform when exposed to stress. The
authors discounted the WLC model because they specu-
lated that the latter model would also fail due to the
inability to account for the extensibility of DNA.
However, in some cases, the WLC model was
appropriate for explaining DNA’s mechanical properties.
For example, the elasticity of l-phage DNA was ex-
plained well with the WLC model, although the FJC
model showed a large deviation from the experimental
data.
[58]
The WLC model provided a contour length of
32.8 ± 0.1 mm and a persistence length of 53.4 ± 2.3 nm.
[58]
An additional difference between the FJC and WLC
models is that the FJC model accounts for entropic
effects only, while the WLC model also accounts for
enthalpic interactions.
Fig. 4
Magnetic alternating contact (MAC) mode AFM topo-
graphical images in air of the DNA biosensor surface prepared
by 3 min of free adsorption onto HOPG from A) 10 mg/ml and
B) 5 mg/ml dsDNA in phosphate buffer (pH 7.0, 0.1 M). (From
Ref. [55]. Copyright (2001) American Chemical Society.) (View
this art in color at www.dekker.com.)
Fig. 5
Magnetic alternating contact mode AFM topographical
images in air of the DNA biosensor surface prepared by 3 min of
free adsorption onto HOPG from a 5 mg/ml single-stranded DNA
in phosphate buffer (pH 7.0, 0.1 M). (From Ref. [55], Copyright
(2001) American Chemical Society.) (View this art in color at
www.dekker.com.)
122
Atomic Force Microscopy and Single-Molecule Force Microscopy Studies of Biopolymers
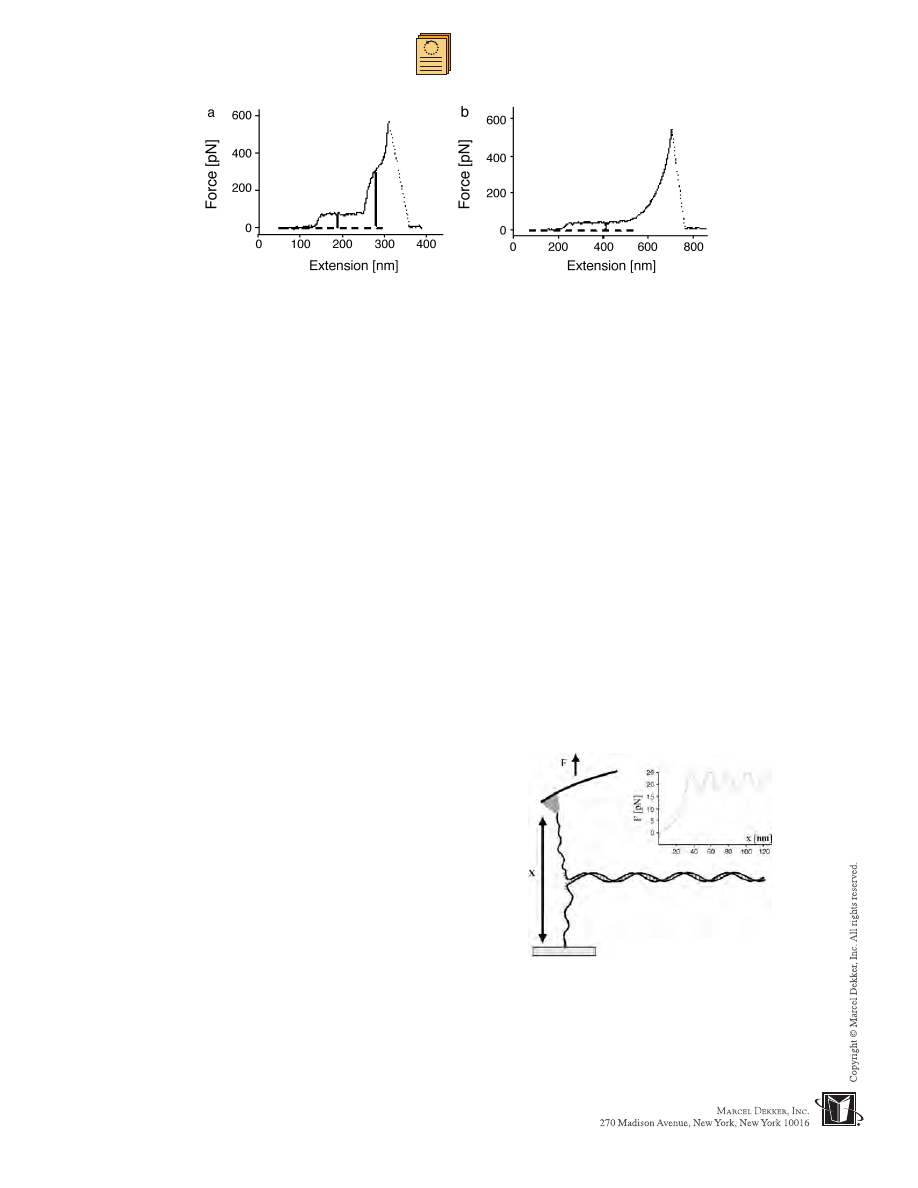
In another example, the elasticity of a single super-
coiled DNA molecule was probed via SMFS. Sharp
transitions were observed in the elasticity of the molecules
at
0.45 and 3 pN for underwound and overwound
molecules, respectively. These transitions were attributed
to the possibility of the formation of alternative DNA
superstructures or because of DNA transcription and
replication.
[60]
Interaction Forces Between Complementary
Strands of DNA
Understanding the intermolecular forces within the DNA
double helix is important to control the behavior of DNA
in various applications, such as DNA sequencing. In the
first effort to measure the forces between single DNA
strands, DNA oligonucleotides were covalently attached
to a spherical probe and to a silica surface.
[57]
Force
measurements between these strands showed three distinct
force regimes, centered at 1.52, 1.11, and 0.83 nN. The
forces were directly associated with the rupture of the
interaction between a single pair of molecules involving
20, 16, and 12 base pairs, respectively. This study demon-
strated the importance of AFM in detecting the presence
of, and relative positions of, specific base sequences with
angstrom resolution.
[57]
In a study on double-stranded l-phage DNA, the split
of the molecule into single strands was observed via force
microscopy.
[61]
Stretching experiments revealed a transi-
tion in the force-extension measurement at 65 pN attrib-
uted to the conversion of B-DNA to a new overstretched
conformation called S-DNA. This transition was followed
by a nonequilibrium melting transition at 150 pN (Fig. 6).
The melting transition is the part of the curve at which
the double-stranded DNA split into single strands that
fully recombined upon relaxation.
[61]
DNA Sequencing
Because of the continuous increase in the resolution of
AFM to the angstrom level, sequencing of DNA became
possible. The principle behind this application is that the
force-extension curve that arises when DNA is stretched
is sequence-dependent. In one of the first studies to ad-
dress DNA sequencing, a comparison was made between
the force measurements on poly(dG–dC) and poly(dA–
dT), where ‘‘d’’ represents the deoxynucleotide.
[61]
With
knowledge of the melting transition for l-phage DNA,
[62]
single strands of poly(dG–dC) and poly(dA–dT) were
prepared. Upon relaxation, these strands reannealed into
hairpin structures as a result of their self-complementary
sequences.
[61]
Studying the unzipping of these hairpins
with AFM directly revealed the base pair unbinding forces
Fig. 6
The mechanical compliance of DNA strongly depends on the specific base paring in the double helix. a) For double-stranded
poly(dG–dC) DNA, there was a transition from B-DNA to a new overstretched form (S-DNA) that occurred at 65 pN (see arrows),
similar to the transition observed in l-DNA. The melting transition occurred at 300 pN for this DNA, compared to 150 pN in l-DNA. b)
In duplex poly(dA–dT) DNA, the force of the B to S transition is reduced to 35 pN and the strands melt during this transition, so that no
separate melting transition can be observed. (From Ref. [61]. Copyright (1999) Nature Publishing Group ( http://www.nature.com/ ).)
Fig. 7
Complementary DNA oligonucleotides are chemically
attached to an AFM tip and a glass surface. As the tip is brought
in contact with the surface, a double strand forms, which can
subsequently be unfolded upon retraction of the tip. (From
Ref. [63]. Copyright (2003) American Chemical Society.) (View
this art in color at www.dekker.com.)
Atomic Force Microscopy and Single-Molecule Force Microscopy Studies of Biopolymers
123
A

for G–C and for A–T, which were 20 ± 3 and 9 ± 3 pN,
respectively. In another study of DNA unzipping, the
force needed to open the double strands upon tip retraction
was measured as a function of extension of the DNA
molecule (Fig. 7). The required force was between 10 and
20 pN on a length scale of 10 bases. The force profiles
were characteristic for each specific DNA sequence.
[63]
PROBING PROTEINS WITH ATOMIC
FORCE MICROSCOPY
High-Resolution Imaging of Proteins
High-resolution protein imaging with AFM is a well-
established technique. Proteins are often bound to mica for
imaging because their tight binding facilitates imaging.
[64]
Alternately, proteins are covalently bound to chemically
modified surfaces.
[65]
Examples of AFM imaging studies
on proteins are too numerous and diverse to be contained
in this review. We focus on two illustrative examples: 1)
imaging of ligand–receptor interactions between cholera
toxin B-oligomers bound to bilayers of biologically
relevant lipids
[66]
and 2) imaging microtubules, protein
structures of eukaryotic cells.
[67]
In the first example, the interactions between cholera
toxin B-oligomers (CTX-B) and a dipalmitoylphosphati-
dylcholine (DPPC) bilayer containing 10 mol% GM1 (the
membrane receptor for CTX-B) were characterized.
Images taken before and after the CTX-B oligomers were
added to the lipid bilayer confirmed the high binding
affinity of CTX-B to the receptor GM1 (Fig. 8). The high
quality of the images revealed the ability of AFM to image
membrane proteins without the need for cross-linking.
As a second example, a comparison was made between
AFM, scanning tunneling microscopy (STM), and trans-
mission electron microscopy (TEM) for their abilities to
image microtubules isolated from pig brains. Atomic
force microscopy was the easiest to use and most repro-
ducible imaging technique.
[67]
The AFM images revealed
the linear structure of the microtubules (Fig. 9) and the
possibility of crossing of various tubules.
The capabilities of AFM imaging of proteins were
extended to many other interesting applications such as
capturing the conformational changes of proteins under
physiological conditions
[68]
and visualization of DNA–
protein complexes.
[69]
Folding and Unfolding of Proteins
Proteins are molecules that are composed of a sequence of
amino acids. This sequence of amino acids determines the
complex helical shape that the protein will assume. The
protein helix can be denatured under special conditions
(chemical or thermal) and refold to its native state upon
the removal of the denaturing source.
[70]
Each protein has
a unique structure and a specific folding pattern for its
polypeptide chain that is required for proper biological
function.
[71,72]
Understanding the process of folding and
unfolding of proteins can help in controlling protein
function for many applications.
The force-extension curves measured with AFM can
characterize protein folding and unfolding.
[73,74]
For
example, the mechanical properties of titin immuno-
globulin (a giant sarcomeric protein of striated muscle)
Fig. 8
A) A typical AFM image of a DPPC bilayer with 10
mol% GM1 before CTX-B was added. B) The CTX-B bound to
the ganglioside in the bilayer is clearly seen. The coverage is
complete indicating that the distribution of the ganglioside is
uniform. (From Ref. [66]. Copyright (1995), with permission
from Elsevier.) (View this art in color at www.dekker.com.)
Fig. 9
Atomic force microscopy top-view image of chemically
immobilized microtubules (MTs) on silicon imaged under buffer
solution (MES buffer with 0.7 M glycerol). Protein concentra-
tion was 2.0 mg/ml at the start of polymerization. Microtubules
fixed with 5% glutaraldehyde before immobilization and imaged
without damage under liquid, demonstrating an improved
resistance to tip pressure. (From Ref. [67]. Copyright (1995),
with permission from Elsevier.)
124
Atomic Force Microscopy and Single-Molecule Force Microscopy Studies of Biopolymers
were studied by repetitive stretching of an individual titin
molecule with AFM.
[71]
The stretching force was recorded
as a function of molecule elongation. At large extensions,
the force extensions exhibited a sawtooth-like pattern,
with a periodicity that varied between 25 and 28 nm. These
peaks were attributed to unfolding of individual domains
of the protein. The forces required to unfold these domains
were 150–300 pN and were dependent on the pulling
speed. Refolding of protein was observed upon relaxation.
In a similar study, the folding and unfolding of
fibronectin (a modular extracellular matrix protein) was
investigated via AFM.
[75]
Statistical analysis of the force-
extension curves clearly revealed the unfolding of three
different types of fibronectin. The unfolding of fibronectin
was irreversible within the timescale of one extension-
relaxation cycle. The folding and unfolding of other
proteins such as human tenascin-C
[72]
(Fig. 10), titin
Ig,
[76]
and barnase
[77]
were also studied with AFM.
Probing Ligand–Receptor Interactions
Ligand–receptor interactions can occur during the forma-
tion of double-stranded DNA, in enzymatic reactions, and
in antigen–antibody recognition.
[78]
To measure these
interactions, the AFM tip is functionalized with either the
ligand or the receptor and the surface is functionalized with
the other component.
[79]
In the first AFM study to measure
the interactions between ligands and receptors, the inter-
actions between a model receptor, streptavidin, and its
ligand, biotin, were probed under physiological condi-
tions.
[80]
The adhesion forces between the two function-
alized surfaces were 3–8 times higher than the nonspecific
interactions measured between blocked streptavidin and
biotinylated surfaces. Statistical analysis of the adhesive
forces revealed the maximum number of streptavidin–
biotin interactions and the force required to rupture the
ligand–receptor bond. In another study of the interactions
between avidin and biotin, the unbinding forces of discrete
complexes were proportional to the enthalpy change of the
complex formation, but independent of the free energy.
[81]
As another example, the interactions between P-selectin
and P-selectin glycoprotein ligand-1 were probed with
SMFS.
[82]
By modeling the resulting intermolecular
forces, knowledge of the rupture forces, elasticity, and
kinetics of the interactions of the P-selectin/P-selectin
glycoprotein ligand-1 interactions were obtained.
PROBING POLYSACCHARIDES WITH
ATOMIC FORCE MICROSCOPY
Polysaccharides are a large group of molecules that exist
as components of plant, animal, algal, bacterial, and yeast
cells. They provide structural support and act as an energy
reservoirs in plants and animals.
[23]
Polysaccharides also
Fig. 10
The entropic elasticity of proteins and domain
unfolding. a) The entropic elasticity of proteins can be described
by the WLC model, which expresses the relationship between
force (F) and extension (x) of proteins using its persistence
length (p) and its contour length (L
c
); k is Boltzmann constant
and T is temperature. b) The sawtooth pattern of peaks observed
when force was applied to extend the protein corresponds to
sequential unraveling of individual domains of modular proteins.
As the distance between the substrate and the cantilever
increases (from state 1 to state 2), the protein elongates,
generating a restoring force that bends the cantilever. When a
domain unfolds (state 3), the free length of the protein increases,
returning the force of the cantilever to near zero. Further
extension results in force on the cantilever (state 4). The last
peak represents the final extension of the unfolded protein prior
to detachment from the AFM tip. c) Consecutive unfolding
peaks of recombinant human tenascin-C were fit using WLC
model. The contour length (L
c
) for each fit is shown; the
persistence length (p) was fixed at 0.56 nm. (From Ref. [72].
Copyright (1999), with permission from Elsevier.) (View this art
in color at www.dekker.com.)
Atomic Force Microscopy and Single-Molecule Force Microscopy Studies of Biopolymers
125
A
play an important role in microbial activity, including
evasion of host defense systems and attachment to host
tissue in infections. Polysaccharides are important in
biofilm formation, which affects such diverse microbial
processes as the uptake of trace metals in soil,
[83]
failure
of medical implants such as artificial heart valves,
[84]
the success of bioremediation,
[85]
and the virulence of
pathogenic infections.
[86]
Polysaccharides are also linked
with cancer pathology.
[87]
In many of these fields, the
mechanisms by which polysaccharides control the biolog-
ical processes are not well known. A better understanding
of the properties of polysaccharides and their subsequent
biological functions can be obtained by high-resolution
studies using AFM imaging and force measurements.
Quantitative Characterization of
Polysaccharide Morphology by Atomic
Force Microscopy Imaging
Atomic force microscopy imaging of polysaccharides
can be used to obtain quantitative information on the
molecule’s height,
[88]
thickness,
[88]
width,
[89]
contour
length,
[33]
persistence length,
[34]
end-to-end distance,
[88]
and the polydispersity and distribution of polysaccharides
on the surfaces of living microbial cells.
[90]
Conformational transitions of polysaccharides can be
investigated with AFM imaging. For example, the
denaturation/renaturation process for the xanthan triple
helix was observed with tapping mode AFM imaging
under ambient conditions. The triple helix denatured upon
heating and renatured when cooled only if sufficient salt
was present in solution.
[88]
The effect of different solvent
chemistries (pH and ionic strength) on polysaccharide
conformation was also investigated with tapping mode
AFM. For example, the conformation of succinoglycan
deposited on mica was observed in the presence and
absence of salt. When there was no salt in the aqueous
solution, a combination of rigid and flexible chains was
observed, while only flexible single chains were observed
in the presence of 0.01 M KCl.
[89]
Another exciting application of AFM with respect
to polysaccharide research is the imaging of dynamic
biological processes. Gunning et al.
[91]
imaged molecular
motion of a water-soluble wheat pentosan polysaccharide
extracted from wheat flour. Parts of the molecules
desorbed and readsorbed onto the mica surface during
tapping mode imaging in 10-mM HEPES buffer. Loops,
trains, and tails were directly observed, confirming that
polymer chains were desorbing and readsorbing. The use
of AFM to study other dynamic biological processes,
including enzymatic breakdown of polysaccharides, is
therefore not far from being realizable.
Force Microscopy for Mechanical
Characterization of Polysaccharides
Studying the force spectra recorded on polysaccharides
(pure polysaccharides or polysaccharides on a microbial
surface) provides useful information on the elastic, me-
chanical, and sometimes chemical nature of the macro-
molecules. Examples of the types of information that can
be deduced from SMFS are the following: quantitative
information about polysaccharide elasticity, estimated
by applying polymer statistical models;
[92]
identifying
the components of a mixture of pure polysaccharides;
[93]
probing the elasticity of polysaccharides on microbial
cells;
[7,36,94]
and qualitative prediction of the conforma-
tion of microbial biopolymers.
[95]
We will discuss some of
these examples in detail.
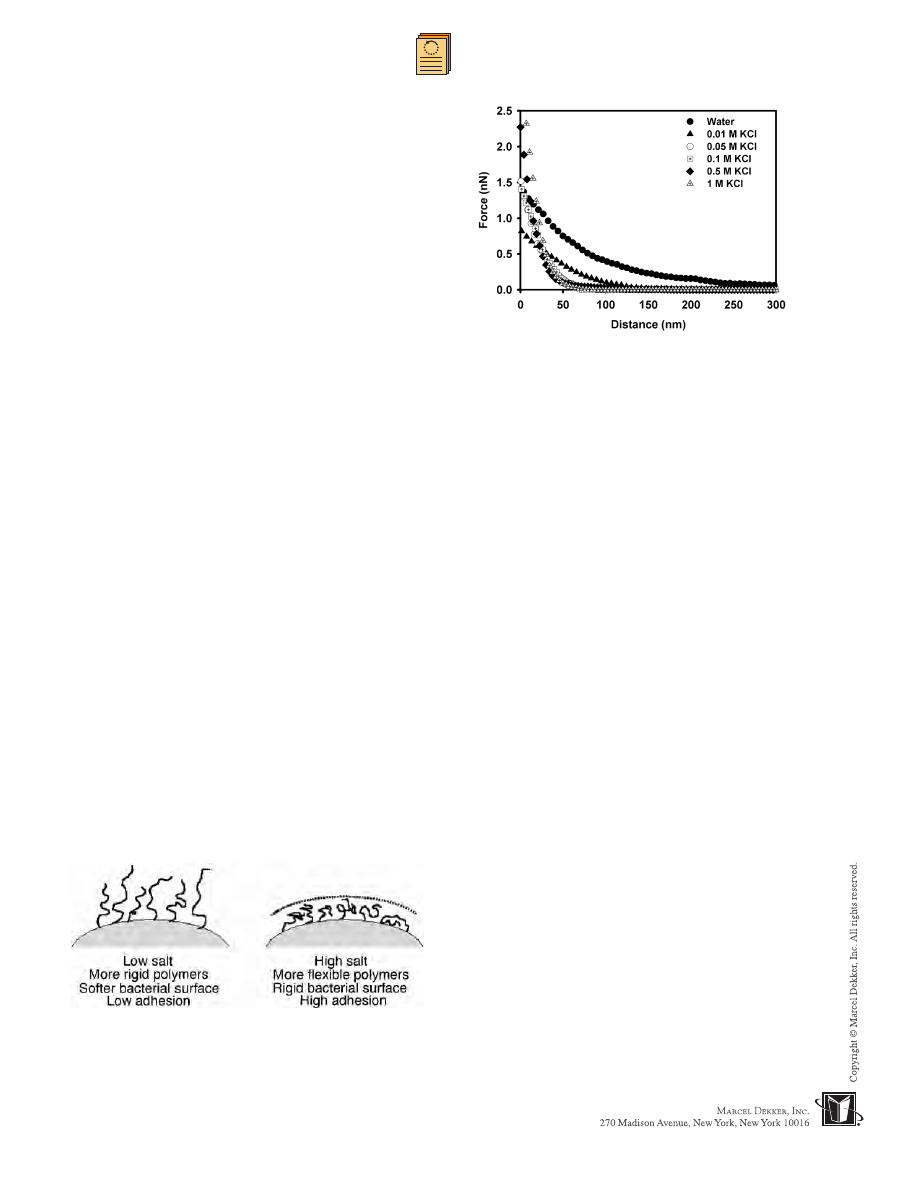
In the first study to quantify the elasticity of macro-
molecules on a microbial surface, surface macromolecules
of dormant spores of Aspergillus oryzae were probed via
Fig. 11
Conceptual representation of the conformation of bac-
terial surface biopolymers at low and high salt concentrations.
(View this art in color at www.dekker.com.)
Fig. 12
A comparison between average approach curves
(each curve is an average of 25 individual force measurements)
for P. putida KT2442 in various solutions. Slopes of the
compliance region of these curves are
0.014,
0.010,
0.054,
0.035,
0.109, and
0.114 nN/nm, from water to
1 M KCl, respectively.
126
Atomic Force Microscopy and Single-Molecule Force Microscopy Studies of Biopolymers

SMFS.
[94]
The elongation forces were well described by
the FJC + model, with estimated values of the Kuhn length
and the segment elasticity in agreement with reported
values for the structural properties of the polysaccharides
dextran and amylose.
Single molecule force spectroscopy was also used to
qualitatively predict the conformation of biopolymers,
predominately polysaccharides, on the surface of the
bacterium P. putida KT2442. Forces were measured on
individual bacterial cells in solutions with varying added
salt concentrations (water–1 M KCl). The biopolymers on
the microbial surface adopted a more flexible conforma-
tion with increasing solution salt concentration.
[95]
The
flexibility of the molecules was quantified using the FJC
model for polymer elasticity. Because of the increased
flexibility of the biopolymers in high salt solutions, the
biopolymers collapsed onto the surface of the bacterium,
leading to a more rigid surface when in high salt (Fig. 11).
The transition in polysaccharide conformation was related
to the slope of the compliance region of the approach
curves measured between the bacterial cells and the AFM
silicon nitride tip (Fig. 12). Changes in biopolymer
conformation and the biomechanical properties of the
bacterium were related to bioadhesion.
[95]
Atomic force microscopy can also be used as a
spectroscopic technique for chemical fingerprinting of
polysaccharides. Transitions in the flexibility of xanthan,
amylose, and dextran upon the cleavage of the pyranose
ring were observed by AFM and linked with the chemical
structure of the molecules.
[96]
Specifically, the pyranose
ring was identified as the structural unit controlling the
molecules’ elasticities. Cleavage of the pyranose ring with
5 mM sodium metaperiodate eliminated the extra enthal-
pic component of the elasticity and made the force
transition disappear. The transitions were attributed to
force-induced elongations of the ring structure and, for
some molecules, to transitions in the pyranose ring from a
chair to boat structure. These transitions produced
fingerprints in the extension-force spectrum that were
characteristic of the ground-energy conformations of the
pyranose ring and the type of glycosidic linkage present in
each polysaccharide
[93,97]
(Fig. 13).
CONCLUSION
The use of AFM and SMFS to characterize the physico-
chemical properties of biopolymers was reviewed. Atomic
force microscopy is preferred over other surface charac-
terization techniques because of several unique advan-
tages, including 1) high lateral and vertical resolutions, 2)
high signal-to-noise ratio, 3) ability to probe biopolymers
in their native environment with minimal sample prepa-
ration, 4) ability to measure interaction forces at inter-
faces, and 5) ability to obtain quantitative information
about the chemical structure of macromolecules.
High-resolution imaging of single molecules of DNA,
proteins, and polysaccharides, including polysaccharides
on microbial surfaces, can now be routinely performed
with AFM. Images can provide quantitative information
on molecular properties and can be used to elucidate
biopolymer conformation. Among the more interesting
examples of probing biopolymers with SMFS are DNA
sequencing, quantifying ligand–receptor interactions be-
tween proteins and lipids, protein folding and unfolding,
identifying polysaccharide components from mixtures,
and probing the distribution of macromolecules on living
cells.
Fig. 13
Force-extension curves for single-pectin molecules
reveal a two-step transition. A) The shape of the curves for
molecules with varying lengths reveals two enthalpic extensions
at
300 and 800–900 pN. B) High-resolution normalized plot
of the force-extension relationship for a single-pectin molecule.
The thin lines are fits of the FJC model modified to include the
extensibility of the monomers. (From Ref. [97] (PNAS, vol. 96)
# (1999) by the National Academy of Sciences, Courtesy of the
National Academics Press, Washington, D.C.)
Atomic Force Microscopy and Single-Molecule Force Microscopy Studies of Biopolymers
127
A

New advancements continue to be made in the
formulation of more sensitive AFM probes, improved
instrumentation, less-invasive sample preparation techni-
ques, and facilitated data analysis. Undoubtedly, AFM
will continue to be a key tool for probing the properties of
delicate biopolymers at the nanoscale. Knowledge of the
relationships between molecular structure and function of
biopolymers will benefit such diverse fields as biotech-
nology, food safety, environmental science, pharmaceu-
tics, and medicine.
ACKNOWLEDGMENTS
This publication was made possible in part by a CAREER
Award to TAC from the National Science Foundation
(Grant Number BES-0238627).
REFERENCES
1.
Grosberg, A.Y.; Khokhlov, A.R. Statistical Physics
of Macromolecules. Polymers and Complex Materi-
als; Larson, R., Pincus, P.A., Eds.; American
Institute of Physics: New York, 1994.
2.
Gunning, A.P.; Morris, V.J.; Al-Assaf, S.; Phillips,
G.O. AFM studies of hylan and hyaluronan.
Carbohydr. Polym. 1996, 30, 1 – 8.
3.
Hanssen, E.; Franc, S.; Garrone, R. AFM and
modeling of natural elastic fibrillin polymers. Biol.
Cell 1998, 90 (3), 223 – 228.
4.
Morris, V.J.; Gunning, A.P.; Kirby, A.R.; Round, A.;
Waldron, K.; Ng, A. AFM of plant cell walls, plant
cell wall polysaccharides and gels. Int. J. Biol.
Macromol. 1997, 21, 61 – 66.
5.
Kirby, A.R.; Gunning, A.P.; Morris, V.J.; Ridout,
M.J. Observation of the helical structure of bacterial
polysaccharide acetan by AFM. Biophys. J. 1995,
68
, 360 – 363.
6.
Dufreˆne, Y.F.; Boonaert, C.J.P.; Gerin, P.A.; Asther,
M.; Rouxhet, P.G. Direct probing of the surface
ultrastructure and molecular interactions of dormant
and germinating spores of Phanerochaete chrysos-
porium. J. Bacteriol. 1999, 181, 5350 – 5354.
7.
Van der Aa, B.C.; Asther, M.; Duˆfrene, Y.F. Surface
properties of Aspergillus oryzae spores investigated
by atomic force microscopy. Colloids Surf., B
Biointerfaces 2002, 24, 277 – 284.
8.
Wilkinson, K.J.; Balnois, E.; Leppard, G.G.; Buffle,
J. Characteristic features of the major components of
freshwater colloidal organic matter revealed by
transmission electron and atomic force microscopy.
Colloids Surf., A Physicochem. Eng. Asp. 1999,
155
, 287 – 310.
9.
Stoll, S.; Starchev, K.; Wilkinson, K.J.; Choda-
nowski, P.; Balnois, E.; Leng, X.; Buffle, J. The
study of environmental biopolymers by mathemati-
cal modeling and single molecule detection techni-
ques. Chimia 2001, 55 (3), 190 – 195.
10.
Santschi, P.H.; Balnois, E.; Wilkinson, K.J.; Zhang,
J.; Buffle, J. Fibrillar polysaccharides in marine
macromolecules organic matter as imaged by AFM
and TEM. Limnol. Oceanogr. 1998, 43 (5), 896 –
908.
11.
Jandt, K.D.; Finke, M.; Cacciafesta, P. Aspects of
the physical chemistry of polymers, biomaterials,
and mineralised tissues investigated with AFM.
Colloids Surf., B Biointerfaces 2000, 19, 301 – 314.
12.
Andrew, R.; Argyrios, M. Production and mass
transfer characteristics of non-Newtonian biopoly-
mers for biomedical applications. Crit. Rev. Bio-
technol. 2002, 22 (4), 355 – 374.
13.
Kayirhan, N.; Denizli, A.; Hasirci, N. Adsorption of
blood proteins on glow-discharge-modified polyure-
thane membranes. J. Appl. Polym. Sci. 2001, 81 (6),
1322 – 1332.
14.
Yokota, H.; Sunwoo, J.; Sarikaya, M.; Engh, G.;
Aebersold, R. Spin-stretching of DNA and protein
molecules for detection by fluorescence and AFM.
Anal. Chem. 1999, 71, 4418 – 4422.
15.
Kuznetsov, Y.G.; Malkin, A.J.; McPherson, A.
AFM studies of the nucleation and growth mechan-
isms of macromolecular crystals. J. Cryst. Growth
1999
, 196, 489 – 502.
16.
Round, A.N.; MacDougall, A.J.; Ring, S.G.; Morris,
V.J. Unexpected branching in pectin observed by
AFM. Carbohydr. Res. 1997, 303, 251 – 253.
17.
Morris, V.J.; Mackie, A.R.; Wilde, P.J.; Kirby, A.R.;
Mills, E.C.N.; Gunning, A.P. AFM as a tool for
interpreting the rheology of food biopolymers at the
molecular level. Lebensm.-Wiss. Technol. 2001, 34,
3 – 10.
18.
Jucker, B.A.; Zehnder, A.J.B.; Harms, H. Quantifi-
cation of polymer interactions in bacterial adhesion.
Environ. Sci. Technol. 1998, 32, 2909 – 2915.
19.
Andrushchenko, V.; Leonenko, Z.; Cramb, D.; van
de Sande, H.; Wieser, H. Vibrational CD (VCD) and
atomic force microscopy (AFM) study of DNA
interaction with Cr
+ 3
ions: VCD and AFM evidence
of DNA condensation. Biopolymers 2002, 61, 243 –
260.
20.
Leng, X.; Starchev, K.; Buffle, J. Applications of
fluorescence correlation spectroscopy: Measure-
ments of size–mass relationship of native and
denatured schizophyllan. Biopolymers 2001, 59,
290 – 299.
21.
Camesano, T.A.; Abu-Lail, N.I. Heterogeneity in
bacterial surface polysaccharides, probed on a
128
Atomic Force Microscopy and Single-Molecule Force Microscopy Studies of Biopolymers

single-molecule basis. Biomacromolecules 2002, 3
(661–667).
22.
Arnott, S. Ordered conformations of gel forming
polysaccharides obtained by X-ray diffraction anal-
ysis of oriented fibers. Dev. Food Carbohydr. Ser.
1977
, 1, 43 – 60.
23.
Hanely, S.J.; Giasson, J.; Revol, J.-F.; Gray, D.G.
Atomic force microscopy of cellulose microfibrils:
Comparison with TEM. Polymer 1992, 33 (21),
4639 – 4642.
24.
Gilbert, P.; Allison, D.G.; Evans, D.J.; Handley,
P.S.; Brown, M.R. Growth rate control of adherent
bacterial populations. Appl. Environ. Microbiol.
1989
, 55 (5), 1308 – 1311.
25.
Naidja, A.; Liu, C.; Huang, P.M. Formation of
protein–birnessite complex: XRD, FTIR, and AFM
analysis. J. Colloid Interface Sci. 2002, 251, 46 –
56.
26.
Lin, W.; Yan, L.; Mu, C.; Li, W.; Zhang, M.; Zhu,
Q. Effect of pH on gelatin self-association investi-
gated by laser light scattering and AFM. Polym. Int.
2002
, 51, 233 – 238.
27.
Sun, Y.; Luo, Z.; An, K. Stretching short biopoly-
mers using optical tweezers. Biochem. Biophys.
Res. Commun. 2001, 286 (4), 826 – 830.
28.
Hartley, P.G.; Bailey, A.I.; Luckham, P.F.; Batts, G.
Non-specific interactions between heparin and
poly(
L
-lysine) surfaces. Colloids Surf., A Physico-
chem. Eng. Asp. 1993, 77 (3), 191 – 198.
29.
Scheuring, S.; Fotiadis, D.; Moller, C.; Muller, S.A.;
Engel, A.; Muller, D.J. Single proteins observed by
AFM. Single Mol. 2001, 2, 59 – 67.
30.
Binning, G.; Quate, C.F.; Gerber, C.H. Atomic
force microscope. Phys. Rev. Lett. 1986, 56 (9),
930 – 933.
31.
Muller, D.J.; Anderson, K. Biomacromolecular
imaging using atomic force microscopy. Trends
Biotechnol. 2002, 20 (8), S45 – S49.
32.
Brant, D.A. Conformation Behavior of Polysacchar-
ides in Solution. In The Biochemistry of Plants;
Academic Press, Inc., 1980; 425 – 469.
33.
Balnois, E.; Wilkinson, K.J. Sample preparation
techniques for the observation of environmental
biopolymers by AFM. Colloid Surf., A Physico-
chem. Eng. Asp. 2002, 207, 229 – 242.
34.
Vuppu, A.K.; Garcia, A.A.; Vernia, C. Tapping
mode AFM of scleroglucan networks. Biopolymers
1997
, 42, 89 – 100.
35.
McMaster, T.J.; Miles, M.J.; Wannerberger, L.;
Eliasson, A.-C.; Shewry, P.R.; Tatham, A.S. Iden-
tification of microphases in mixed alpha and omega
gliadin protein films investigated by AFM. J. Agric.
Food Chem. 1999, 47, 5093 – 5099.
36.
Abu-Lail, N.I.; Camesano, T.A. Elasticity of
Pseudomonas putida KT2442 biopolymers probed
with single-molecule force microscopy. Langmuir
2002
, 18, 4071 – 4081.
37.
Muller, D.J.; Engel, A. The height of biomolecules
measured with the atomic force microscope depends
on electrostatic interactions. Biophys. J. 1997, 73
(3), 1633 – 1644.
38.
Hansma, H.G.; Sinsheimer, R.L.; Groope, J.; Bruice,
T.C.; Elings, V.; Gurley, G.; Bezanilla, M.; Mas-
trangelo, I.A.; Hough, P.V.C.; Hansma, P.K. Recent
advances in AFM of DNA. Scanning 1993, 15 (5),
296 – 299.
39.
Hansma, H.G.; Weisenhorn, A.L.; Gould, S.A.C.;
Sinsheimer, R.L.; Gaub, H.E.; Stucky, G.D.; Zar-
emba, C.M.; Hansma, P.K. Progress in sequencing
deoxyribonucleic acid with AFM. J. Vac. Sci.
Technol., B Microelectron. Nanometer Struct.
1991
, 9 (2. Pt.2), 1282 – 1284.
40.
Yuri, L.L.; Alexander, A.G.; Lyuda, S.S.; Rodney,
E.H.; Bertram, L.J.; Patrick, I.O.; Stuart, M.L. AFM
imaging of double stranded DNA and RNA. J.
Biomol. Struct. Dyn. 1992, 10 (3), 589 – 606.
41.
Hansma, H.G.; Hansma, P.K. Potential applica-
tions of AFM of DNA to the human genome pro-
ject. Proc. SPIE Int. Soc. Opt. Eng. 1993, 1891, 66 –
70.
42.
Bensimon, A.; Simon, A.; Chiffaudel, A.; Croquette,
V.; Heslot, F.; Bensimon, D. Alignment and
sensitive detection of DNA by a moving interface.
Science 1994, 265, 2096 – 2098.
43.
Rivetti, C.; Guthold, M.; Bustamante, C. Scanning
force microscopy of DNA deposited onto mica:
Equilibration versus kinetic trapping studied by
statistical polymer chain analysis. J. Mol. Biol.
1996
, 264 (5), 919 – 932.
44.
Lyubchenko, Y.; Shlyakhtenko, L.; Harrington, R.;
Oden, P.; Lindsay, S. Atomic force microscopy of
long DNA—Imaging in Air and under water. Proc.
Natl. Acad. Sci. U. S. A. 1993, 90 (6), 2137 –
2140.
45.
Hansma, H.G.; Golan, R.; Hsieh, W.; Lollo, C.P.;
Mullen-Ley, P.; Kwoh, D. DNA condensation for
gene therapy as monitored by atomic force
microscopy. Nucleic Acids Res. 1998, 26, 2481 –
2487.
46.
Hansma, H.G.; Sinsheimer, R.L.; Li, M.Q.; Hansma,
P.K. AFM of single and double stranded DNA.
Nucleic Acids Res. 1992, 20 (14), 3585 – 3590.
47.
Thundat, T.; Allison, D.P.; Warmack, R.J.; Doktycz,
M.J.; Jacobson, K.B.; Brown, G.M. AFM of single
and double stranded deoxyribonucleic acid. J. Vac.
Sci. Technol., A, Vac. Surf. Films 1993, 11 (4,
Pt.1), 824 – 828.
48.
Dario, A.; Jurgen, F.; Benjamin, S.; Xavier, F.-B.
Atomic Force Microscopy and Single-Molecule Force Microscopy Studies of Biopolymers
129
A

Single molecule DNA biophysics with AFM. Single
Mol. 2000, 1 (1), 53 – 58.
49.
Tiner, W.J.; Potaman, V.N.; Sinden, R.R.; Lyub-
chenko, Y.L. The structure of intramolecular triplex
DNA: AFM study. J. Mol. Biol. 2001, 314 (3), 353 –
357.
50.
Wei, L.J.; Fang, T.; Chen, W.; Li, B.C.; Hua, C.E.
Possible multistranded DNA induced by acid
denaturation–renaturation. J. Vac. Sci. Technol., B,
Microelectron. Nanometer Struct. 1997, 15 (5),
1637 – 1640.
51.
Frank, N.; Bernd, G.; Norbert, H. Improvement of
DNA-visualization in dynamic mode AFM in air.
Scanning 2001, 23 (3), 175 – 181.
52.
Hansma, H.G.; Irene, R.; Kerry, K.; Daniel, L.E.
AFM of long and short double stranded and triple
stranded nucleic acids. Nucleic Acids Res. 1996, 24
(4), 713 – 720.
53.
Hafner, J.H.; Cheung, C.-L.; Woolley, A.T.; Lieber,
C.M. Structural and functional imaging with carbon
nanotube AFM probes. Prog. Biophys. Mol. Biol.
2001
, 77 (1), 73 – 110.
54.
Kazuo, U.; Jun, K.; Takayuki, U.; Nami, C.;
Shukuko, I.; Taro, N.; Takehiko, S.; Yoshikazu,
N.; Shinji, K.; Akira, M.; Hiroshi, T.; Mitsuru, I.;
Reiko, K. AFM of RecA–DNA complexes using a
carbon nanotube tip. Biochem. Biophys. Res.
Commun. 2001, 281 (2), 390 – 395.
55.
Oliveira, B.A.M.; Ana-Maria, C. AFM of DNA
immobilized onto a highly oriented pyrolytic
graphite electrode surface. Langmuir 2003, 19 (9),
3830 – 3839.
56.
Smith, S.B.; Finzi, L.; Bustamante, C. Direct
mechanical measurements of the elasticity of single
DNA molecules by using magnetic beads. Science
1992
, 258, 1122 – 1126.
57.
Lee, G.U.; Chrisey, L.A.; Colton, R.J. Direct
measurement of the forces between complemen-
tary strands of DNA. Science 1994, 266, 771 –
773.
58.
Marko, J.F.; Siggia, E.D.; Smith, S. Entropic
elasticity of lambda-phage DNA. Science 1994,
265
, 1599 – 1600.
59.
Burnham, N.A.; Chen, X.; Hodges, C.S.; Matei,
G.A.; Thoreson, E.J.; Roberts, C.J.; Davies, M.C.;
Tendler, S.J.B. Comparison of calibration methods
for atomic-force microscopy cantilevers. Nanotech-
nology 2003, 14, 1 – 6.
60.
Strick, T.R.; Allemand, J.-F.; Bensimon, D.; Bensi-
mon, A.; Croquette, V. The elasticity of a single
supercoiled DNA molecule. Science 1996, 271,
1835 – 1837.
61.
Rief, M.; Clausen-Schaumann, H.; Gaub, H.E.
Sequence-dependent mechanics of single DNA
molecules. Nat. Struct. Biol. 1999, 6 (4), 346 –
349.
62.
Smith, S.B.; Cui, Y.; Bustamante, C. Overstretching
B-DNA: The elastic response of individual double-
stranded DNA molecules. Science 1996, 271, 795 –
799.
63.
Rupert, K.; Rief, M.; Gaub, H.E. Unzipping DNA
oligomers. Nano Lett 2003, 3 (4), 493 – 496.
64.
Czajkowski, D.M.; Shao, Z. Submolecular resolu-
tion of single macromolecules with AFM. FEBS
Lett. 1998, 430, 51 – 54.
65.
Wadu-Mesthridge, K.; Amro, N.A.; Liu, G.Y.
Immobilization of proteins on self-assembled mono-
layers. Scanning 2000, 22 (6), 380 – 388.
66.
Mou, J.; Yang, J.; Shao, Z. AFM of cholera toxin B-
oligomers bound to bilayers of biologically relevant
lipids. J. Mol. Biol. 1995, 248, 507 – 512.
67.
Vinckier, A.; Heyvaert, I.; D’Hoore, A.; McKittrick,
T.; Haesendonck, C.V.; Engelborghs, Y.; Helle-
mans, L. Immobilizing and imaging microtubules by
AFM. Ultramicroscopy 1995, 57, 337 – 343.
68.
Muller, D.J.; Sass, H.J.; Muller, S.; Buldt, G.;
Engel, A.J. Surface structures of native bacterior-
hodopsin depend on the molecular packing arrange-
ment in the membrane. J. Mol. Biol. 1999, 285 (5),
1903 – 1909.
69.
Ohad, M.; Joseph, E.; Reinhard, G.; Joseph, S. AFM
imaging in solution of protein–DNA complexes
formed on DNA anchored to a gold surface.
Ultramicroscopy 2002, 90 (2/3), 103 – 112.
70.
Stanier, R.; Ingraham, J.L.; Wheelis, M.L.; Painter,
P.R. The Microbial World, 5th Ed.; Prentice-Hall:
New Jersey, 1986.
71.
Rief, M.; Gautel, M.; Oesterhelt, F.; Fernandez,
J.M.; Gaub, H.E. Reversible unfolding of individual
titin immunoglobulin domains by AFM. Science
1997
, 276, 1109 – 1111.
72.
Fisher, T.E.; Oberhauser, A.F.; Carrion-Vazquez,
M.; Marszalek, P.E.; Fernandez, J.M. The study of
protein mechanics with AFM. Trends Biochem. Sci.
1999
, 24, 379 – 384.
73.
Fernandez, J.M.; Rief, G.M.; Gaub, H.E. Single
molecule force spectroscopy with individual pro-
teins. NATO Sci. Ser. Ser. C, Math. Phys. Sci. 1999,
519
, 319 – 336.
74.
Carrion-Vazquez, M.; Oberhauser, A.F.; Fisher,
T.E.; Marszalek, P.E.; Li, H.; Fernandez, J.M.
Mechanical design of proteins studied by single-
molecule force spectroscopy and protein engineer-
ing. Biophys. Mol. Biol. 2000, 74 (1–2), 63 – 91.
75.
Oberdorfer, Y.; Fuchs, H.; Janshoff, A. Conforma-
tional analysis of native fibronectin by means of
force spectroscopy. Langmuir 2000, 16 (26), 9955 –
9958.
130
Atomic Force Microscopy and Single-Molecule Force Microscopy Studies of Biopolymers

76.
Fowler, S.B.; Best, R.B.; Jose, L.T.H.; Trevor, J.R.;
Annette, S.; Emanuele, P.; Martin, K.; Jane, C.
Mechanical unfolding of a titin Ig domain:structure
of unfolding intermediate revealed by combining
AFM, molecular dynamic simulations, NMR and
protein engineering. J. Mol. Biol. 2002, 322 (4),
841 – 849.
77.
Best, R.B.; Li, B.; Steward, A.; Daggett, V.; Clarke,
J. Can non-mechanical proteins withstand force?
Stretching barnase by AFM and molecular dynamic
simulations. Biophys. J. 2001, 81 (4), 2344 – 2356.
78.
Janshoff, A.; Neitzert, M.; Oberdorfer, Y.; Fuchs, H.
Force spectroscopy of molecular systems—Single
molecule spectroscopy of polymers and biomole-
cules. Angew. Chem., Int. Ed. 2000, 39, 3212 –
3237.
79.
Lisa, W.M.; Stephanie, A.; Martyn, C.D.; Saul,
J.B.T.; Philip, W.M.; Clive, R.J. Bifunctional AFM
probes for molecular screening applications. Anal.
Chim. Acta 2003, 479 (1), 77 – 85.
80.
Lee, G.U.; Kidwell, D.A.; Colton, R.J. Sensing
discrete streptavidin–biotin interactions with atomic
force microscopy. Langmuir 1994, 10, 354 – 357.
81.
Moy, V.T.; Florin, E.-L.; Gaub, H.E. Intermolecular
forces and energies between ligands and receptors.
Science 1994, 266, 257 – 259.
82.
Fritz, J.A.D.; Katopodis, A.G.; Kolbinger, F.;
Anselmetti, D. Force-mediated kinetics of single
P-selectin/ligand complexes observed by AFM.
Proc. Natl. Acad. Sci. U. S. A. 1998, 95 (21),
12283 – 12288.
83.
Brantley, S.L.; Liermann, L.; Bau, M.; Wu, S.
Uptake of trace metals and rare earth elements from
hornblende by a soil bacterium. Geomicrobiol. J.
2001
, 18, 37 – 61.
84.
Verheyen, C.C.P.M.; Dhert, W.J.A.; de Blieck-
Hogervorst, J.M.A.; van der Reijen, T.J.K.; Petit,
P.L.C.; de Groot, K. Adherence to a metal, polymer
and composite by Staphylococcus aureus and
Staphylococcus epidermidis. Biomaterials 1993,
14
, 383 – 391.
85.
Caccavo, F.J.; Das, A. Adhesion of dissimilatory
Fe(III)-reducing bacteria to Fe(III) minerals. Geo-
microbiol. J. 2002, 19, 161 – 177.
86.
Mecsas, J.; Strauss, E.J. Molecular mechanisms of
bacterial virulence: Type III secretion and pathoge-
nicity islands. Emerg. Infect. Dis. 1996, 2, 271 – 288.
87.
Kwakman, J.M.; Freihofer, H.P.; van Waas, M.A.
Needs for implant therapy in cancer patients, a
retrospective study. Ned. Tijdschr. Tandheelkd.
2000
, 107 (8), 318 – 321.
88.
Camesano, T.A.; Wilkinson, K.J. Single molecule
study of xanthan conformation using atomic force
microscopy. Biomacromolecules 2001, 2, 1184 –
1191.
89.
Balnois, E.; Stoll, S.; Wilkinson, K.J.; Buffle, J.;
Rinaudo, M.; Milas, M. Conformations of succino-
glycan as observed by atomic force microscopy.
Macromolecules 2000, 33, 7440 – 7447.
90.
Gad, M.; Itoh, A.; Ikai, A. Mapping cell wall
polysaccharides of living microbial cells using
atomic force microscopy. Cell Biol. Int. 1997, 21,
697 – 706.
91.
Gunning, P.A.; Mackie, A.R.; Kirby, A.R.; Kroon,
P.; Williamson, G.; Morris, V.J. Motion of a cell
wall polysaccharide observed by AFM. Macromo-
lecules 2000, 33 (15), 5680 – 5685.
92.
Li, H.; Rief, M.; Oesterhelt, F.; Gaub, H.; Zhang, X.;
Shen, J. Single-molecule force spectroscopy on
polysaccharides by AFM nanomechanical finger-
print of alpha- (1–4) linked polysaccharides. Chem.
Phys. Lett. 1999, 305, 197 – 201.
93.
Marszalek, P.E.; Li, H.; Fernandez, J.M. Finger-
printing polysaccharides with single-molecule
force spectroscopy. Nat. Biotechnol. 2001, 19,
258 – 262.
94.
Van der Aa, B.C.; Michel, R.M.; Asther, M.;
Zamora, M.T.; Rouxhet, P.G.; Duˆfrene, Y.F.
Stretching cell surface macromolecules by atomic
force microscopy. Langmuir 2001, 17, 3116 –
3119.
95.
Abu-Lail, N.I.; Camesano, T.A. Role of ionic
strength on the relationship of biopolymer confor-
mation, DLVO contributions, and steric interactions
to bioadhesion of Pseudomonas putida KT2442.
Biomacromolecules 2003, 4, 1000 – 1012.
96.
Marszalek, P.E.; Oberhauser, A.F.; Pang, Y.-P.;
Fernandez, J.M. Polysaccharide elasticity governed
by chair-boat transitions of the glucopyranose ring.
Nature 1998, 396, 661 – 666.
97.
Marszalek, P.E.; Pang, Y.-P.; Li, H.; El Yazal, J.;
Oberhauser, A.F.; Fernandez, J.M. Atomic levers
control pyranose ring conformation. Proc. Natl.
Acad. Sci. U. S. A. 1999, 96, 7894 – 7898.
Atomic Force Microscopy and Single-Molecule Force Microscopy Studies of Biopolymers
131
A

Request Permission/Order Reprints
Reprints of this article can also be ordered at
http://www.dekker.com/servlet/product/DOI/101081EENN120014171
Request Permission or Order Reprints Instantly!
Interested in copying and sharing this article? In most cases, U.S. Copyright
Law requires that you get permission from the article’s rightsholder before
using copyrighted content.
All information and materials found in this article, including but not limited
to text, trademarks, patents, logos, graphics and images (the "Materials"), are
the copyrighted works and other forms of intellectual property of Marcel
Dekker, Inc., or its licensors. All rights not expressly granted are reserved.
Get permission to lawfully reproduce and distribute the Materials or order
reprints quickly and painlessly. Simply click on the "Request Permission/
Order Reprints" link below and follow the instructions. Visit the
for information on Fair Use limitations of U.S.
copyright law. Please refer to The Association of American Publishers’
(AAP) website for guidelines on
.
The Materials are for your personal use only and cannot be reformatted,
reposted, resold or distributed by electronic means or otherwise without
permission from Marcel Dekker, Inc. Marcel Dekker, Inc. grants you the
limited right to display the Materials only on your personal computer or
personal wireless device, and to copy and download single copies of such
Materials provided that any copyright, trademark or other notice appearing
on such Materials is also retained by, displayed, copied or downloaded as
part of the Materials and is not removed or obscured, and provided you do
not edit, modify, alter or enhance the Materials. Please refer to our
Wyszukiwarka
Podobne podstrony:
Dekker Encyclopedia Of Nanoscience And Nanotechnology Nanostructured Catalytic Materials Design A
Encyclopedia of Explosives and Related Items Volume 02
Cho Chikun's encyclopedia of life and death part three a
Cho Chikun's encyclopedia of life and death part one ele
encyclopedia of herbs and mind enhancing substances 2000 group
Encyclopedia of Explosives and Related Items Volume 03
Encyclopedia of Explosives and Related Items Volume 07
Encyclopedia of Explosives and Related Items Volume 10
Encyclopedia of Explosives and Related Items Volume 03
encyclopedia of herbs and mind enhancing substances 2000 group
Encyclopedia of Explosives and Related Items Volume 10
Encyclopedia of Explosives and Related Items Volume 07
Cho Chikun s encyclopedia of life and death part three a
Atomic Force Microscopy
Comparative eco toxicity of nanoscale TiO2, SiO2, and ZnO
Encyclopedia of Mind Enhancing Foods, Drugs and Nutritional Substances ~ [TSG]
An Examination of the Evolution of Army and Air Force
więcej podobnych podstron