Appl Microbiol Biotechnol (2003) 61:309–313
DOI 10.1007/s00253-002-1213-3
O R I G I N A L P A P E R
I. Roy · A. Gupta · S. K. Khare · V. S. Bisaria ·
M. N. Gupta
Immobilization of xylan-degrading enzymes from
Melanocarpus
albomyces IIS 68 on the smart polymer Eudragit L-100
Received: 4 September 2002 / Revised: 29 November 2002 / Accepted: 29 November 2002 / Published online: 11 February 2003
Springer-Verlag 2003
Abstract
Xylanase of Melanocarpus albomyces IIS 68
was immobilized on Eudragit L-100. The latter is a
copolymer of methacrylic acid and methyl methacrylate
and is a pH-sensitive smart polymer. The immobilization
was carried out by gentle adsorption and an immobiliza-
tion efficiency of 0.82 was obtained. The enzyme did not
leach off the polymer even in the presence of 1 M NaCl
and 50% ethylene glycol. The K
m
of the enzyme changed
from 5.9 mg ml
–1
to 9.1 mg ml
–1
upon immobilization.
The V
max
of the immobilized enzyme showed an increase
from 90.9 mol ml
–1
min
–1
(for the free enzyme) to
111.1 mol ml
–1
min
–1
. The immobilized enzyme could
be reused up to ten times without impairment of the
xylanolytic activity. The immobilized enzyme was also
evaluated for its application in pre-bleaching of eucalyp-
tus kraft pulp.
Introduction
The enzymatic degradation of xylan has a variety of
biological functions. Industrial applications include the
use of xylanases in the paper and pulp industries, as food
additives to wheat flour, for the extraction of coffee and
plant oils, degumming of plant fibre sources and clarifi-
cation of juices and wines (Bajpai 1997; Beg et al. 2001).
One major application of xylanases is as a pre-bleaching
agent in treating Kraft pulps, used in most pulp and paper
mills, for paper manufacture. Such xylanase-pretreated
pulps result in lower generation of toxic, chlorinated
aromatic compounds and are therefore preferred as being
eco-friendly (Viikari et al. 1996; Bisaria et al. 2003).
Recently, purification, characterization and substrate
specificities of xylanase isoenzymes from Melanocarpus
albomyces IIS 68 have been described (Saraswat and
Bisaria 2000). In fact, multiplicity of xylanolytic enzymes
has been reported in several microorganisms. This has a
biochemical basis since degradation of xylan requires the
synergistic action of various xylanolytic enzymes (Beg et
al. 2001). Even in the case of M. albomyces, it was found
that the extent of xylan hydrolysis was higher when all the
isoenzymes were present (Saraswat and Bisaria 2000).
Recently, we reported the immobilization of Aspergil-
lus niger xylanase present in a commercial preparation on
Eudragit L-100, a copolymer of methacrylic acid and
methyl methacrylate (Sardar et al. 2000). Its stability in
aqueous solutions is pH-dependent and this has led to its
use in designing smart macroaffinity ligands (Roy and
Gupta 2002). As an immobilization matrix, its virtue lies
in the fact that it is soluble during catalysis, with its
insolubility below pH 4.0 enabling catalyst recovery by
lowering the pH. The soluble bioconjugate, of course, is
expected to have the considerably reduced mass transfer
constraints associated with enzymes immobilized on solid
supports (Roy et al. 2002).
Materials and methods
Xylan (from oat spelts) was purchased from Sigma, St. Louis, MO.
Eudragit L-100 was a product of Rohm Pharma, Weiterstadt,
Germany, and is a copolymer of methacrylic acid and methyl
methacrylate at a ratio of 1:1. All other chemicals used were of
analytical grade.
Production of xylanase
An ascomycetous fungus, M. albomyces, was isolated from
compost and soil. For xylanase production, the fungus was grown
in a medium containing (per liter): 10.0 g wheat straw, 0.5 g urea,
0.6 g KH
2
PO
4
, 0.4 g MgSO
4
·7H
2
O and 10 g yeast extract (Saraswat
and Bisaria 2000). The pH of the medium was adjusted to 6.0. The
growth temperature was 45C and agitation rate of the incubator
I. Roy and A. Gupta contributed equally to the work described here.
I. Roy · A. Gupta · S. K. Khare · M. N. Gupta (
)
)
Department of Chemistry, Indian Institute of Technology,
Delhi, Hauz Khas, 110016 New Delhi, India
e-mail: mn_gupta@hotmail.com
Tel.: +91-11-26591503
Fax: +91-11-26581073
V. S. Bisaria
Department of Biochemical Engineering and Biotechnology,
Indian Institute of Technology, Delhi, Hauz Khas,
110016 New Delhi, India
shaker was 230 rpm. The broth obtained after removal of mycelial
biomass and unspent wheat straw by centrifugation served as the
crude enzyme preparation (containing 68 U ml
–1
). The free enzyme
itself could be stored at 4C with no loss of activity for up to
16 weeks. Storage stability beyond this point was not checked.
Estimation of protein
Protein was estimated by the dye-binding method (Bradford 1976),
using bovine serum albumin as the standard protein.
Measurement of enzyme activity
Xylanase activity was determined as described by Bailey et al.
(1992) using oat-spelt xylan as the substrate. One unit of xylanase
activity is described as the amount of enzyme required to produce
1 mol reducing sugar (measured as xylose) per minute under assay
conditions (Saraswat and Bisaria 2000). As the polymer-linked
enzyme was soluble at the pH used in the assay (pH 6.0), the
activity of the immobilized preparation could be determined and
expressed in the same manner as the free enzyme.
Preparation of Eudragit L-100 solution
Eudragit L-100 (1 g) was dissolved with constant stirring in 40 ml
distilled water by adding 3 M NaOH dropwise until the pH
increased to 11.0. After the polymer had dissolved completely, the
pH of the solution was decreased to 7.0 by adding 3 M acetic acid
and the total volume made up to 50 ml with distilled water. The
solution was stored at 4C until further use.
Immobilization of xylanase to Eudragit L-100
Varying volumes (0.6–2.7 ml) of the clear culture broth of M.
albomyces IIS 68 were added to 0.75 ml 2% Eudragit L-100 and the
final volume was made up to 5.0 ml with 0.05 M acetate buffer,
pH 6.0. After incubation for 1 h at 25C, the polymer was
precipitated by lowering the pH to 4.0 with 130 l 0.1 N acetic acid.
After 20 min, the suspension was centrifuged at 12,000 g for 20 min.
The precipitate was washed with 4.0 ml acetate buffer (0.01 M,
pH 4.0) until no enzyme activity could be detected in the washings.
Enzyme activity and protein concentration were measured in the
supernatant and washings.
Determination of pH optimum and thermal stability
Effect of pH on free and immobilized xylanase was studied by
assaying both preparations at different pH values. Thermal stability
was studied at 60C by incubating the two enzymes at that
temperature. Appropriate aliquots of free and immobilized xy-
lanases were withdrawn at different time intervals and the activities
were determined.
Reusability of the immobilized preparation
The reusability of the immobilized preparation was assessed at
60C by carrying out the hydrolysis of 36 mg xylan in 4.0 ml 0.05 M
phosphate buffer, pH 6.0 containing 2.6 U immobilized enzyme
activity, and monitoring the amount of reducing sugar liberated
after each cycle. After each cycle of hydrolysis, the undegraded
xylan was removed by centrifugation at 2,000 g for 10 min. The pH
of the supernatant was lowered to 4.0 by the addition of 130 l 0.1 N
acetic acid when the immobilized enzyme precipitated. This was
collected by centrifugation (12,000 g, 20 min), the pH of
supernatant adjusted back to 6.0 and the amount of reducing sugar
estimated using the dinitrosalicylic acid (DNSA) method (Miller
1959). For running the second cycle, the immobilized enzyme was
redissolved in 5.0 ml fresh buffer and added to undegraded xylan
and processed the same way as before.
Hydrolysis of xylan from oat spelt and kraft pulp
by xylanase preparations
Free and immobilized xylanases (2.6 U) were incubated with oat
spelt xylan (1%, in 0.05 M phosphate buffer, pH 6.0) at 60C. The
amount of reducing sugars produced was estimated by the DNSA
method (Miller 1959) by withdrawing appropriate aliquots at
regular time intervals for 6 h.
In the case of kraft pulp, the enzyme was incubated with 5%
pulp (in 0.05 M phosphate buffer, pH 6.0) at 60C and the amount
of reducing sugar formed was estimated as before after 2 h and 5 h
of incubation.
Determination of Km and Vmax
K
m
and V
max
values of free and immobilized xylanases were
determined by measurement of initial rate of xylan hydrolysis at
various concentrations of xylan. The Michaelis constant was
calculated using the Leonora software program (Cornish-Bowden
1995). This software uses the Lineweaver-Burk equation to
calculate the K
m
value. Each experiment was run in duplicate and
the error margin was less than 5% in each case.
Results
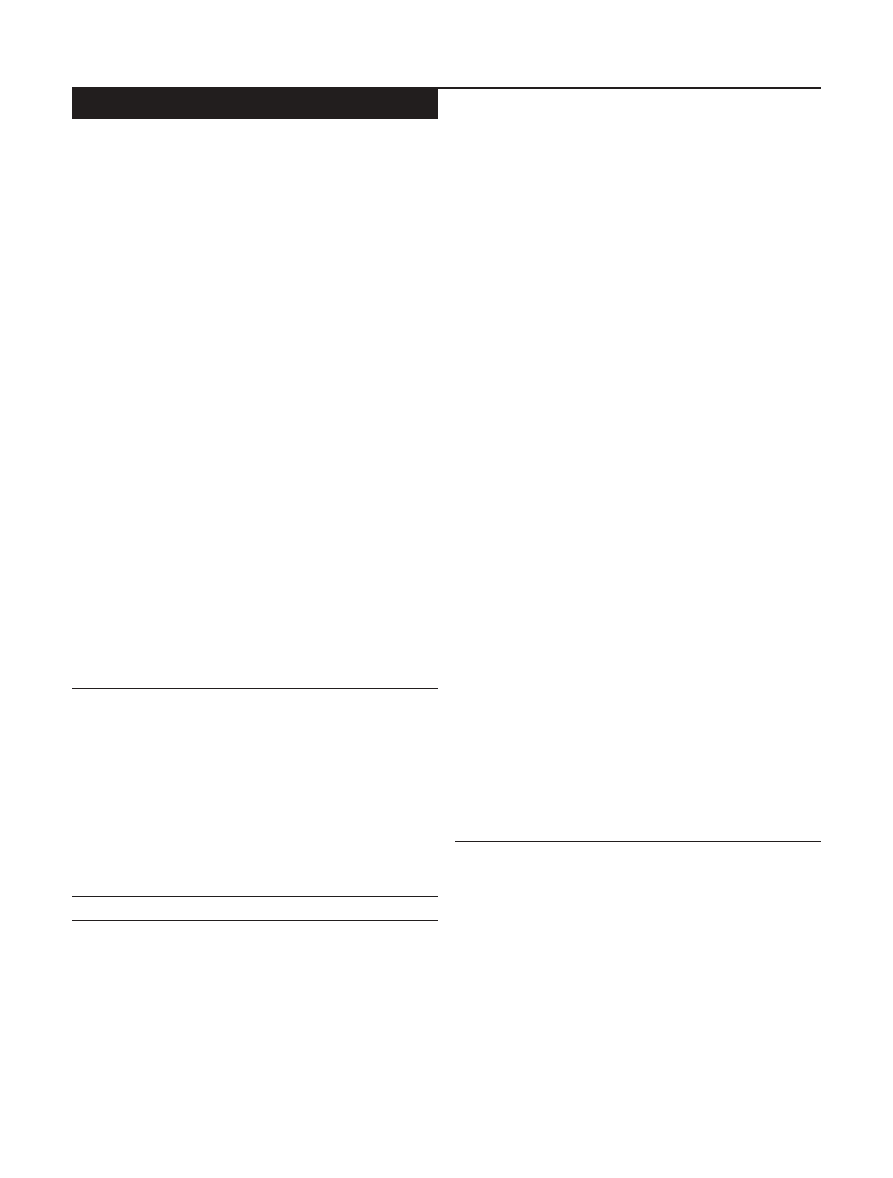
Figure 1 shows (1) that the xylan-degrading activity can
be immobilized on Eudragit L-100 and (2) the variation of
“immobilization efficiency” with the amount of enzyme
adsorbed per unit mass of the polymer. The latter follows
the trend generally observed during immobilization. The
decrease with larger enzyme load is generally attributed
to “overcrowding” of the enzyme on the surface (Sardar
et al. 2000). With a reasonable enzyme load (120 U), an
Fig. 1
Immobilization of xylanase on Eudragit L-100. Various
aliquots of the free enzyme were coupled to the matrix as described
in Materials and methods. A represents the amount of enzyme
theoretically bound to the matrix. This is calculated by subtracting
the unbound activity in the supernatant from the initially added
enzyme. B represents the expressed activity of the particular
immobilized preparation, measured after incubating the immobi-
lized enzyme (after dissolving) with the substrate
310
immobilization efficiency of 0.82 could be achieved. No
detectable activity was found to leach when the immo-
bilized enzyme was washed with 1 M NaCl or 50%
ethylene glycol. This is the preparation that was charac-
terized further.
There was no significant change in the xylan hydrol-
ysis activity versus pH curve upon immobilization (data
not shown). The temperature optimum for xylanolytic
activity also did not change (data not shown).
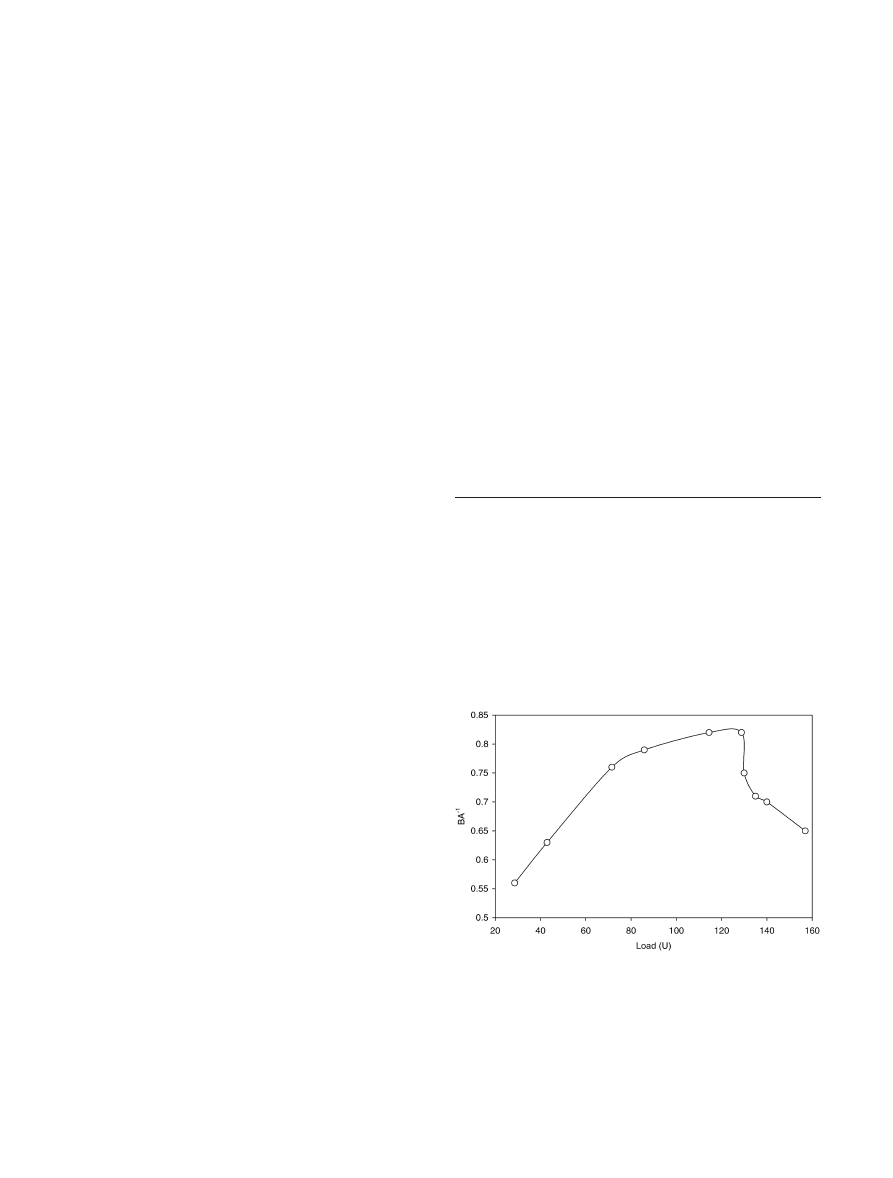
Figure 2 shows that there was no significant change in
the xylanolytic activity of the immobilized preparation up
to ten cycles. It is noteworthy that changing the pH of the
free enzyme to 4.0 destroyed all its activity in a single
exposure. The K
m
changed from 5.9 mg ml
–1
for the free
enzyme to 9.1 mg ml
–1
upon immobilization on the
soluble polymer. V
max
of the immobilized enzyme,
however, showed a marginal increase to 111.1 mol ml
–1
min
–1
(from 90.9 mol ml
–1
min
–1
for the free enzyme).
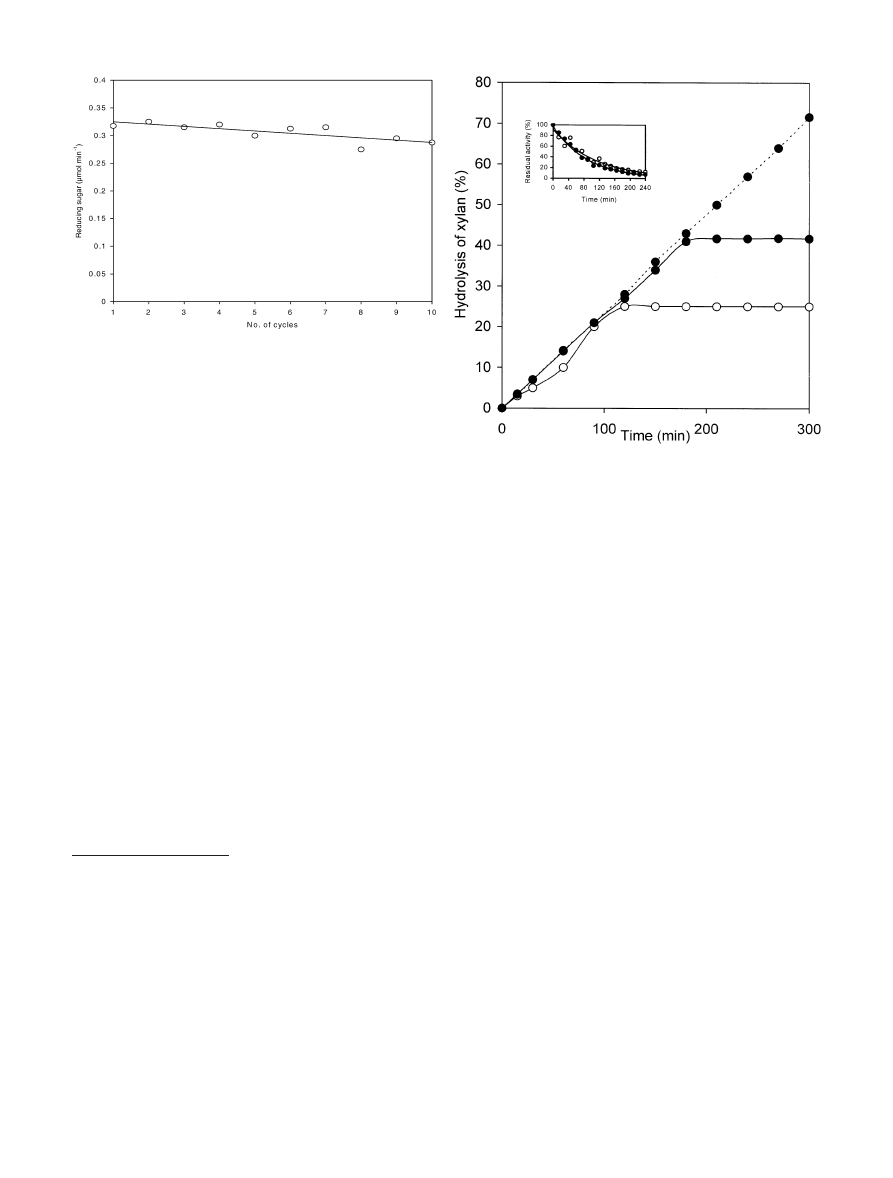
Figure 3 shows a time course hydrolysis by free and
immobilized enzyme. Hydrolysis % was calculated as
follows:
g xylose produced
0:9
g initial xylan
100
ð1Þ
In the case of the free enzyme, there was no further
hydrolysis after 120 min whereas hydrolysis continued up
to 180 min with immobilized enzyme. The final xylan
hydrolysis was 25% for the free enzyme and 42% for the
immobilized enzyme. The reason for the difference in the
performance of the free and immobilized enzymes did not
originate from a difference in thermal stability. Both free
and immobilized enzymes show similar thermal stabilities
at 60C (the temperature used for hydrolysis (inset in
Fig.3). [It should be added that, although the enzyme
alone is not very stable at 60C, its assay in this and
earlier work (Saraswat and Bisaria 2000) has been carried
out at 60C since the presence of xylan, the substrate,
during the assay protects the enzyme from thermoinac-
tivation. This kind of stabilization by the substrate
towards denaturing conditions has been observed in many
cases (Gray 1993)]. Thus, a likely reason for hydrolysis
stopping at 120 min (in the case of free enzyme) is
product inhibition (Hoshino et al. 1989). The immobilized
enzyme presumably has a different K
i
and allows
hydrolysis to proceed further. The broken line in Fig. 3
shows the effect of removing product inhibition factor,
which resulted in 70% xylan hydrolysis compared to 42%
in the case of the product-inhibited reaction. This is
possible only in the case of immobilized enzyme that can
be removed from the hydrolysis system and reintroduced
at any time.
The free and immobilized enzyme preparations were
also evaluated for treatment of kraft pulp. The kraft pulp
(derived from eucalyptus) was treated with either immo-
bilized or free enzyme for 2 h or 5 h. The effectiveness of
the two enzymes was compared in terms of liberation of
sugars from the xylan component of the pulp. As shown
Fig. 2
Reusability of the immobilized preparation. After one cycle
of incubation of the immobilized enzyme and the substrate at 60C,
xylan was removed by centrifugation followed by removal of the
polymer-bound enzyme, as described in Materials and methods.
The immobilized enzyme was redissolved and incubated again with
fresh substrate to initiate the second cycle of hydrolysis. Each
hydrolysis cycle lasted 30 min
Fig. 3
Hydrolysis of xylan by immobilized xylanase. The free (m)
and immobilized (l) xylanases were incubated with xylan under
assay conditions. Aliquots were withdrawn from the suspension
after regular intervals, the pH lowered to 4.0, followed by
centrifugation to remove undegraded xylan and immobilized
enzyme. The amount of reducing sugar was estimated in the
supernatant after increasing the pH to 6.0. In the case of free
enzyme, the degree of hydrolysis was measured without lowering
the pH. The effect of product inhibition was evaluated by carrying
out the hydrolysis as described, except that the pH of the reaction
mixture was lowered and centrifugation carried out to precipitate
xylan and immobilized enzyme (broken line). These were then
redissolved in fresh buffer and the hydrolysis allowed to proceed.
Inset Thermal stability of the free and immobilized enzymes at
60C
311
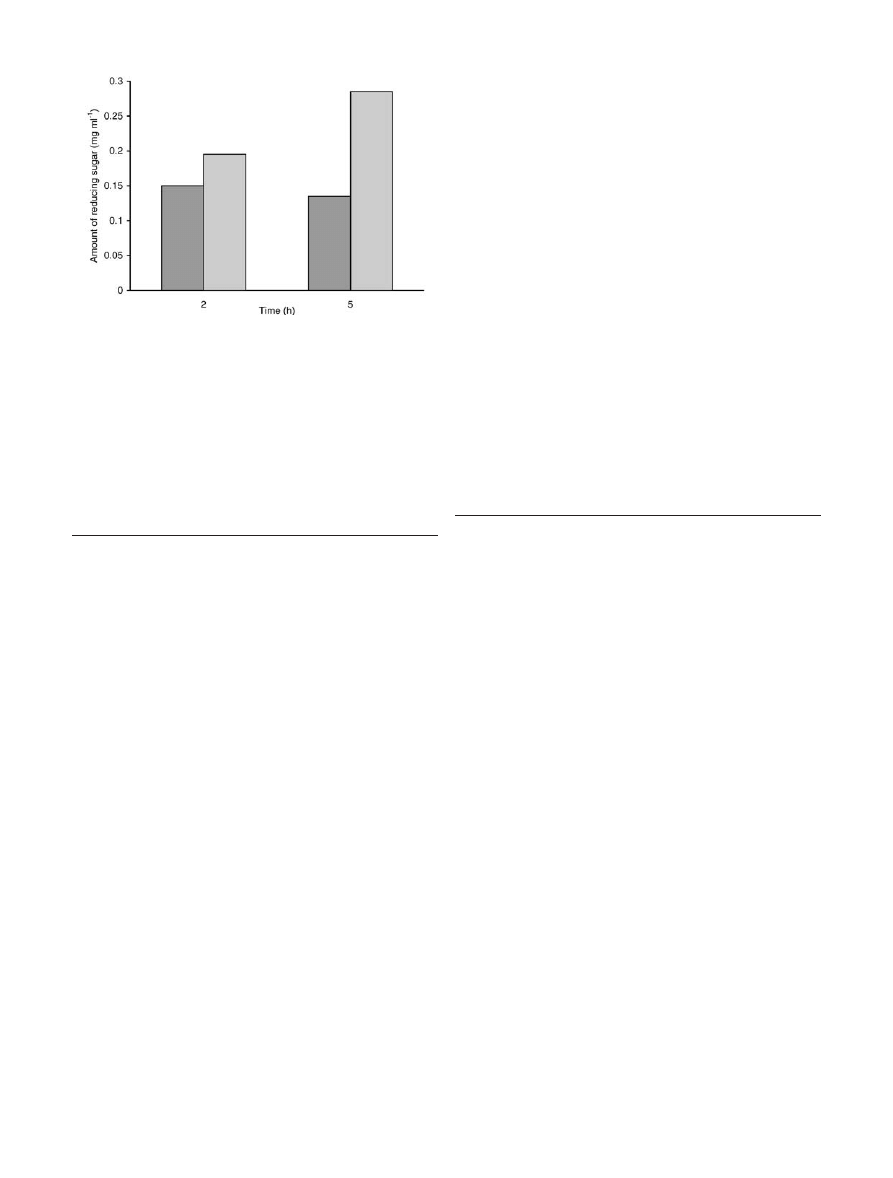
in Fig. 4, the immobilized xylanase was more effective
for the pre-treatment of kraft pulp, presumably due to its
higher stability under pre-treatment conditions. Thus, the
use of immobilized xylanase may be a better option to
pre-bleach kraft pulps.
Discussion
Simple adsorption is a gentle method for noncovalent
immobilization of enzymes (Gupta and Mattiasson 1992).
The high immobilization efficiency observed in the
present case agrees with this general experience.
As Tischer and Kasche (1999) pointed out recently, the
real advantage of immobilization lies more often in
reusability rather than in stabilization. This stabilization
of the enzyme towards exposure to low pH is obviously
crucial to the reusability of the immobilized preparation.
The strategy of immobilization used in this case will not
be applicable to those enzymes that are not stable at such
low pH or are not stabilized upon immobilization. We
have previously observed similar stabilization of A. niger
xylanase upon immobilization on Eudragit L-100 (Sardar
et al. 2000). Reusable enzyme derivatives immobilized on
Eudragit have also been described with amylase (Cong et
al. 1995) and pectinlyase (Dinnella et al. 1995).
The change in kinetic parameters upon immobilization
is also critical for evaluating the success of an immobi-
lization protocol. Unfortunately, kinetic parameters for
enzymes immobilized on smart polymers have quite often
not been reported (Fujimura et al. 1987; Hoshino et al.
1989). There is some limited data available for compar-
ison. Cong et al. (1995) report marginal changes in K
m
and V
max
upon immobilization of amylase on Eudragit L-
100. Dinnella et al. (1995) report no change in K
m
but a
decrease in V
max
. While more extensive experience with
such systems is required before a clear analysis can
emerge, the increase in K
m
in this case, in spite of the
matrix being a soluble polymer, points towards factors
other than steric ones operating. An increase in V
max
upon
immobilization is generally not observed. However, such
a result is not unprecedented (Suh et al. 1987; Dumitriu et
al. 1989). This marginal increase in V
max
may arise from a
polar product (xylan fragments) being pushed away from
the microenvironment due to the somewhat hydrophobic
nature of Eudragit L-100. Incidentally, this will also
explain the increase in K
m
of the enzyme as the substrate
xylan is also polar in nature.
The soluble and reusable immobilized enzyme de-
scribed here can be used for numerous biotechnological
applications associated with xylanase. As the matrix
Eudragit L-100 is a food grade polymer and the immo-
bilization involves simple adsorption (thus avoiding the
need for toxic and harsh chemicals generally associated
with covalent coupling methods; Williams and Blanch
1994; Tyagi et al. 1998), applications in the area of food
processing can be safely considered.
Acknowledgements
The partial supports provided by the Council
for Scientific and Industrial Research (CSIR) (Extramural Division
and Technology Mission on Oilseeds, Pulses and Maize) and the
Department of Science and Technology, both Government of India
organizations, are gratefully acknowledged. A.G. is grateful to IIT
Delhi for financial support.
References
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of
methods for assay of xylanase activity. J Biotechnol 23:257–
270
Bajpai P (1997) Microbial xylanolytic enzyme system: properties
and applications. In: Neidleman S, Laskin A (eds) Advances in
applied microbiology. Academic Press, New York, pp 141–194
Beg QK, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial
xylanases and their industrial applications: a review. Appl
Microb Technol 56:326–338
Bisaria VS, Mishra S, Sahai V, Jain JK, Mathur RM (2003) Pre-
bleaching of kraft pulp with xylanase enzyme for paper
manufacture. In: Proceedings of the International Symposium
on "New Horizons in Biotechnology", Trivandrum, India, April
2001 (in press)
Bradford MM (1976) A rapid and sensitive method for the
quantitation of microgram quantities of protein utilizing the
principle of protein-dye binding. Anal Biochem 72:248–254
Cong L, Kaul R, Dissing U, Mattiasson B (1995) A model study on
Eudragit and polyethyleneimine as soluble carriers for a-
amylase for repeated hydrolysis of starch. J Biotechnol 42:75–
84
Cornish-Bowden A (1995) Analysis of enzyme kinetic data. Oxford
University Press, Oxford, pp 27–36
Dinnella C, Lanzarini G, Ercolessi P (1995) Preparation and
properties of an immobilized soluble-insoluble pectinylase.
Process Biochem 30:151–157
Dumitriu S, Popa M, Artenie V, Dan F (1989) Bioactive polymers.
56:
Urease
immobilization
on
carboxymethylcellulose.
Biotechnol Bioeng 34:283–290
Fujimura M, Mori T, Tosa T (1987) Preparation and properties of
soluble-insoluble immobilized proteases. Biotechnol Bioeng
29:747–752
Gray CG (1993) Stabilization of enzymes with soluble additives.
In: Gupta MN (ed) Thermostability of enzymes. Springer,
Berlin Heidelberg New York, pp 124–143
Gupta MN, Mattiasson B (1992) Unique applications of immobi-
lized proteins in bioanalytical systems. In: Suelter CH, Kricka,
Fig. 4
Continuous hydrolysis of xylan from kraft pulp by immo-
bilized xylanase. The hydrolysis of kraft pulp was carried out by
free (vertically hatched) and immobilized (cross-hatched) xylanase
preparations as described in Materials and methods
312

L (eds) Bioanalytical applications of enzymes, vol 36. Wiley,
New York, pp 1–34
Hoshino K, Taniguchi M, Netsu Y, Fujii M (1989) Repeated
hydrolysis of raw starch using amylase immobilized on a
reversibly soluble-insoluble carrier. J Chem Eng Jpn 22:54–59
Miller GL (1959) Use of dinitrosalicylic acid reagent for determi-
nation of reducing sugar. Anal Chem 31:426–428
Roy I, Gupta M N (2002) Macroaffinity ligands in bioseparation.
In: Gupta MN (ed) Methods for affinity-based separation of
proteins/enzymes, Birkhauser, Basel, pp 130–147
Roy I, Sharma S, Gupta MN (2002) Smart biocatalysts: design and
applications. Adv Biochem Eng Biotechnol (in press)
Saraswat V, Bisaria VS (2000) Purification, characterization and
substrate specificities of xylanase isoenzyme from Melanocar-
pus albomyces IIS 58. Biosci Biotechnol Biochem 64:1167–
1180
Sardar M, Roy I, Gupta MN (2000) Simultaneous purification and
immobilization of Aspergillus niger xylanase on the reversibly
soluble polymer Eudragit
TM
L-100. Enzyme Microb Technol
27:672–679
Suh WC, Lim BS, Chun M, Sernetz M (1987) Immobilization of
nuclease P1 from Penicillium citrinum and production of 5-
nucleotides by bioreactor. Korean Biochem J 20:17–23
Tischer W, Kasche V (1999) Immobilized enzymes: crystals or
carriers? Trends Biotechnol 17:326–335
Tyagi R, Roy I, Agarwal R, Gupta MN (1998) Carbodiimide
coupling of enzymes to the reversibly soluble-insoluble poly-
mer Eudragit S-100. Biotechnol Appl Biochem 28:201–206
Viikari L, Suurnakki A, Buchert J (1996) Enzyme-aided bleaching
of kraft pulps: fundamental mechanisms and practical applica-
tions. In: Jeffries TW, Viikari L (eds) Enzymes for pulp and
paper processing. ACS Symposium series 665, Oxford Univer-
sity Press, Oxford, pp 15–24
Williams RA, Blanch HW (1994) Covalent immobilization of
protein monolayers for biosensor applications. Biosens Bio-
electron 9:159–167
313
Wyszukiwarka
Podobne podstrony:
Xylan Degrading enzymes from Aspergillus niger
Xylan degrading enzymes from the Yeast
Production of xylan degrading enzymes from Amazon forest fungal
Screening for distinct xylan degrading enzymes in complex shake flask
A xylan degrading strain of Sulfolobus solfataricus
O'Reilly How To Build A FreeBSD STABLE Firewall With IPFILTER From The O'Reilly Anthology
Degradable Polymers and Plastics in Landfill Sites
Estimation of Dietary Pb and Cd Intake from Pb and Cd in blood and urine
pages from xm 754sx 3
Inny świat – hołd złożony człowiekowi i dokument degradacji człowieka, język polski
Does the number of rescuers affect the survival rate from out-of-hospital cardiac arrests, MEDYCYNA,
Test 3 notes from 'Techniques for Clasroom Interaction' by Donn Byrne Longman
How to draw Donkey from Shrek
Mapowanie genów na przykładzie Drosophila melanogaster(1)
big profits from a very dirty business
więcej podobnych podstron