The Treatment of the Behavioral Sequelae of Autism with
Dextromethorphan: A Case Report
Cooper Woodard,
1,2
June Groden,
1
Matthew Goodwin,
1
Cori Shanower,
1
and Joanne Bianco
1
Dextromethorphan is the d-isomer of levorphenol, and an ingredient in antitussive
preparations. A 10 year-old male diagnosed with Autistic Disorder, Pervasive Developmental
Disorder, and Generalized Anxiety Disorder was administered this medication initially to treat
a medical condition. This became a quasi-experimental ABAB design (A = baseline,
B = treatment), with improvements during treatment phases shown in tracked behavioral
data and anecdotal reports. Several candidate mechanisms to explain the improvements are
offered, including glutamate receptor antagonism. While dextromethorphan is not commonly
administered for the treatment of behavioral challenges in this or any population, the results
suggest the need for larger-scale, adequately controlled, and methodologically rigorous studies
of the potential clinical effects of dextromethorphan.
KEY WORDS: Autism; dextromethorphan; glutamate.
Dextromethorphan (d-3-methoxy-N-methylmor-
phinian) is an antitussive preparation that is the d-
isomer of the codeine analog levorphenol. Unlike the
l
isomer, it has no analgesic or addictive properties
and does not act through opioid receptors. Although
not indicated as a psychotropic agent, reports have
emerged of its psychotherapeutic properties in per-
sons with neurodevelopmental disorders.
Welch and Sovner (1992) documented behav-
ioral improvements of an individual with severe
mental retardation and organic mental disorder when
he was administered dextromethorphan. This person
displayed severe aggression and self-injurious behav-
ior (SIB), irritability, and a chaotic sleep pattern
characterized by frequent awakenings and minimal
sleep duration (less than 4 hours per night). He had
not responded to trials of multiple pharmacological
interventions, including haloperidol, thioridazine,
chlorpromazine, imipramine, hydroxyzine, carba-
mazepine, and clonazepam. He was administered
60 mgs. BID of dextromethorphan for the treatment
of an upper respiratory infection, and showed a
marked decrease in aggression, SIB, and overactivity.
His sleep pattern also normalized. At week 26,
dextromethorphan was increased to 90 mgs. BID,
and low rates of target behaviors were maintained.
Philips, Hamid, and McGrew (1999) reported
that dextromethorphan significantly improved behav-
ioral problems for two children diagnosed with
autism, who also had elevated levels of glutamine
or glycine or both on repeated studies. Both children
were administered dextromethorphan at 5 mg/kg/day
divided BID. Staff who worked with these children
reported significant improvement in language skills,
attention, socialization with peers, and motor plan-
ning. Upon discontinuation of the medication,
regression was noted in the same areas by staff
members who were blind to the treatment conditions.
1
The Groden Center, Providence, Rhode Island.
2
Correspondence should be addressed to: Cooper Woodard, The
Groden Center, 86 Mount Hope Ave, Providence, Rhode Island
02906; Tel.: (401) 274-6310, ext. 1036; Fax: (401) 421-3280; e-mail:
cwoodard@grodencenter.org
515
0162-3257/05/0800-0515/0
Ó 2005 Springer ScienceþBusiness Media, Inc.
Journal of Autism and Developmental Disorders, Vol. 35, No. 4, August 2005 (
Ó 2005)
DOI: 10.1007/s10803-005-5041-z

Medication administration was resumed, and the
children again responded in a positive manner.
Here we report on the effects of dextromethor-
phan in a child with autism, evaluated retrospectively
in a single-subject reversal design.
METHOD
Participant
The participant was a 10-year-old male, diag-
nosed with Autistic Disorder, Pervasive Develop-
mental Disorder, and Generalized Anxiety Disorder.
The child had trials of sertraline for 13 months and
citalopram for 2 months, with no clinical benefit for
the symptoms of anxiety, perseveration, and tantrum
behavior. At the time of the present dextromethor-
phan trial, the child had been receiving .5 mg. BID of
guanfacine for a 3 month period, and this dosage
continued through the dextromethorphan trial.
Measurement
Evaluation of treatment effects involved two
sources of data. First, the child’s teachers provided
frequency counts of defined target behaviors (leaving
the classroom and aggressive tantrum) during each
day of the trial. Second, anecdotal reports of the
child’s behavior were provided by his mother and
teachers.
Design
A quasi-experimental ABAB (A = baseline,
B = treatment) single-subject design began with an
initial
baseline
assessment
period
that
lasted
3 months. The first treatment phase was initiated by
the parents to treat a medical condition, and involved
administration of 30 mgs. BID of dextromethorphan
for 1 week, the active ingredient in an over-the-
counter cough medication. This was followed by a
1 week return to baseline due to the child’s improved
medical status. The second treatment evaluation
phase lasted 3 weeks, and was initiated by the parents
in response to an increase in behavioral challenges.
Behavior data was collected on each day of each of
the 4 phases of the trial, and the child’s mother and
his teachers provided anecdotal reports during each
phase.
RESULTS
Target Behavior Data
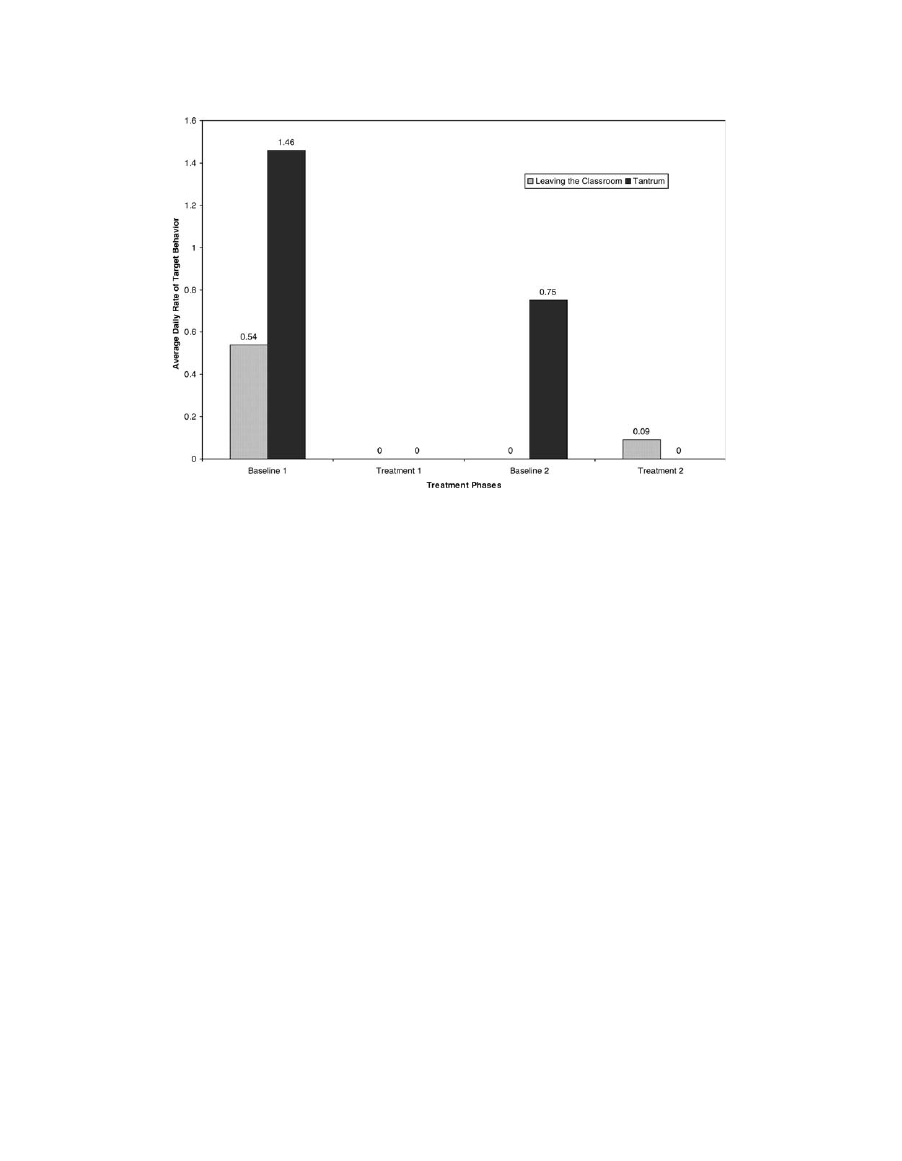
The results of the assessment of target behaviors
are presented in Fig. 1. During the first baseline
phase, the average daily rate of leaving the classroom
was .54 incidents/day, and the average daily rate of
tantrum was 1.46 incidents/day. During the first
treatment phase, the average daily rates were 0/day
for both behavioral categories. The average daily rate
of leaving the classroom was 0/day during the second
baseline phase, and the rate of tantrum was .75/day.
During the second treatment phase, average daily
rates were .09/day for leaving the classroom and
0/day for tantrums.
Anecdotal Information
During the first baseline phase, the child’s
mother reported aggressive tantrum behavior and
generalized fears. In school, teachers reported signif-
icant anxiety, which interfered with learning. During
the first dextromethorphan phase, both parents and
teachers reported a distinct behavioral improvement.
He communicated fewer anxiety-related concerns,
coped more easily with behavioral consequences,
became more cooperative, and began to show empa-
thy. When the medication was discontinued for the
second baseline phase, anecdotal reports indicated
that behavior immediately deteriorated in the home
and at school. Anxiety symptoms and tantrum
behavior were reported to decrease during the second
treatment phase.
DISCUSSION
In this case study, a significant improvement was
documented both with formal behavioral data on
target symptoms and anecdotal reports during the
periods when the child was administered dextrometh-
orphan. Although it is difficult to rule out other
factors that may have contributed to behavioral
changes in a single case, there were no obvious
changes in this child’s situation that might account
for the improvements noted and the reversal design
helps to establish the apparent role the medication
played
Given existing models of the pathophysiology of
autistic symptoms, several candidate mechanisms
seem plausible. Perhaps the most simple model of
516
Woodard, Groden, Goodwin, Shanower, and Bianco
the effects of dextromethorphan would involve it’s
potential to relieve discomfort and irritability sec-
ondary to the treatment of an illness or infection.
However, in the present case study and the cases
reported by Philips et al. (1999), dextromethorphan’s
effects were observed independent of periods of
obvious illness. A second potential mechanism would
be the clinical benefit of sedation secondary to the use
of dextromethorphan. Mild drowsiness is a possible
side effect of this medication, and may have contrib-
uted to the behavioral improvements noted in the
present case study. Finally, there is preliminary data
available on the putative mechanism of action for
dextromethorphan, which is glutamate receptor
antagonism. Based on aberrations in brain areas
containing high densities of glutamate neurons and
symptom similarities between persons with autism
and healthy participants given NMDA antagonists,
Carlsson (1998) suggests that autism may be a
hypoglutamatergic disorder. He discusses the poten-
tial benefits and risks of treatment with glutamate
agonists
, as well as other substances designed to
modulate the NMDA receptor complex. McDougle
(2002) reviews additional literature addressing gluta-
matergic dysfunction and autistic disorder. In con-
trast to the hypoglutamatergic model proposed by
Carlsson but consistent with the results reported by
Philips et al., drug trials that attenuated cortical
release of glutamate (lamotrigine) or were NMDA
receptor antagonists (amantadine) had promising
results. While the available research was limited, the
results warranted additional research on glutamater-
gic mechanisms.
The results of this case report add to those of the
previous case studies of dextromethorphan effects
(Welch & Sovner, 1992; Philips et al., 1999), and
support the hypothesis that dextromethorphan may
have psychotherapeutic properties in at least a subset
of children with autism. However, all available
reports of dextromethorphan effects come from
uncontrolled case studies and thus interpretation of
the available reports in this area must be tempered by
the lack of methodological rigor in these case studies.
Together, these case studies simply point to the need
for larger-scale, adequately controlled studies of the
potential clinical effects of dextromethorphan.
REFERENCES
Carlsson, M. L. (1998). Hypothesis: Is infantile autism a
hypoglutamatergic disorder? Relevance of glutamate—
serotonin interactions for pharacotherapy. Journal of
Neural Transmission
, 105, 525–535.
Fig. 1. Average rates of leaving the classroom and aggressive tantrum behavior per day during the baseline
and dextromethorphan treatment phases.
Dextromethorphan Treatment
517

McDougle, C. J. (2002). Current and Emerging Therapeutics of
Autistic Disorder and Related Pervasive Developmental Dis-
orders. In K. L. Davis, D. Charney, J. T. Coyle, & C Nemeroff
(Eds.), .(pp. 566–577). Philadelphia: Lippincott Williams and
Wilkins.
Phillips, J. A., Hamid, R., & McGrew, S. (1999, October). Effects
of dextromethorphan in autistic children with abnormal amino
acid profiles
. Program 2861 presented at the annual meeting of
the American Society of Human Genetics (ASHG), San
Francisco, CA.
Welch, L., & Sovner, R. (1992). The treatment of a chronic organic
mental disorder with dextromethorphan in a man with severe
mental retardation. British Journal of Psychiatry, 161, 118–
120.
518
Woodard, Groden, Goodwin, Shanower, and Bianco

Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
Wyszukiwarka
Podobne podstrony:
APA practice guideline for the treatment of patients with Borderline Personality Disorder
Flashback to the 1960s LSD in the treatment of autism
Periacetabular osteotomy for the treatment of dysplastic hip with Perthes like deformities
(IV)The effect of McKenzie therapy as compared with that of intensive strengthening training for the
Kinesio taping compared to physical therapy modalities for the treatment of shoulder impingement syn
The term therapeutic relates to the treatment of disease or physical disorder
(autyzm) The History of Autism 2004
Geoffrey Hinton, Ruslan Salakhutdinov Reducing the dimensionality of data with neural networks
Jóźwiak, Małgorzata; Warczakowska, Agnieszka Effect of base–acid properties of the mixtures of wate
The Neurobiology of Autism New Pieces of the Puzzle Autyzm
From Small Beginnings; The Euthanasia of Children with Disabilities in Nazi Germany
Shel Leanne, Shelly Leanne Say It Like Obama and WIN!, The Power of Speaking with Purpose and Visio
Latour Pursuing the Discussion of Interobjectivity With a Few Friends
Understanding the Nature of Autism And A Edward R Ritvo
(autyzm) The age of autism by dan olmstead and mark blaxill
The Keys of Basilus With Commentary
więcej podobnych podstron