Toxicity of Binary Chemical Mixtures: Effects on Reproduction
of Zebrafish (Brachydanio rerio)
U. Ensenbach,
1
R. Nagel
2
1
Centre of Environmental Research, Department of Chemical Ecotoxicology, P.O. Box 2, D-04301 Leipzig, Germany
2
Institute of Hydrobiology, Technical University Dresden, Germany
Received: 7 February 1996/Revised: 26 June 1996
Abstract. A complete life-cycle test with zebrafish was carried
out with different concentrations of the binary mixture 3,4-
dichloroaniline and lindane under flow-through conditions.
Length and weight of fish of the F
1
-generation were reduced,
even in the lowest test concentration of 2 µg/L 3,4-dichloroani-
line and 40 µg/L lindane. The same effects were found in the
early life stage test for the F
2
-generation. In the mixture of 100
µg/L 3,4-dichloroaniline and 40 µg/L lindane, fish which were
exposed for their whole life time stopped spawning, irrevers-
ibly; the fish population will become extinct. In an additional
experiment, fish were exposed to the same xenobiotic concentra-
tions after reaching maturity. In this case, egg production was
reduced. Cessation of egg production occurs in a concentration
of 200 µg/L 3,4-dichloroaniline and 40 µg/L lindane. Neverthe-
less, effects on spawning are influenced by duration of exposure
and the life stages of exposure.
To assess the chronic effects of xenobiotics on fish, established
test systems such as acute or prolonged toxicity tests are not
adequate because it is not possible to extrapolate from these
data to long-term effects. So it is necessary to develop new test
systems which are able to detect chronic effects. One of the
most important effects on fish populations is the impairment of
reproduction. The influence of xenobiotics on reproduction is
possible on different levels, e.g., development of maturing,
coupling, number of eggs produced, egg quality, hatching of
embryos and development of larvae (Donaldson and Scherer
1983). However, investigating many of these parameters, early
life stage tests have been shown to be inadequate, a full life
cycle test should be carried out.
Although in Germany, level two of the Chemical act requires
a long-term test that must include reproduction (Federal Envi-
ronmental Agency 1990), no satisfactory test guideline is
available up to now. European cold water fish such as Golden
ide (Leuciscus idus melanotus) used in acute toxicity tests need
three to four years to reach maturity. Holcombe et al. (1979)
estimated an experimental time of two years to conduct life
cycle tests with rainbow trout (Oncorhynchus mykiss). Because
development up to maturity takes only 3–4 months, zebrafish
(Brachydanio rerio) is a suitable model for this test system. We
have performed a zebrafish complete life cycle test with
different concentrations of the binary mixture of 3,4-dichloro-
aniline and lindane over a time period of six months. 3,4-
dichloroaniline (DCA) and lindane were chosen as model
compounds because of the results of two life cycle tests with
these chemicals (Nagel 1995) and we wanted to know if
synergistic effects occur when the chemicals were applied in
combination. The test was started by rearing of fertilized eggs,
and followed to mature animals, with subsequent examination
of egg production and fertilization rates. Subsequently, a second
breeding was conducted over 42 days. In an additional experi-
ment, adult zebrafish were exposed to different concentrations
of the binary mixture to determine how exposure time influ-
ences reproduction.
Material and Methods
Test Species
The experiments were performed with zebrafish (Brachydanio rerio,
Hamilton-Buchanan) obtained from the West Aquarium Co. in Bad
Lauterberg (FRG). The zebrafish is widely used in acute toxicity tests
(Cairns et al. 1965; Fogels and Sprague 1977; Zok et al. 1991; Gallo et
al. 1995). Fish measure 3–5 cm in the adult state. This species produces
gametes throughout the year and is not difficult to rear under laboratory
conditions (Cairns et al. 1965; Westerfield 1995).
Test Procedure
All life stages were exposed under flow-through conditions to the two
test substances, lindane and 3,4-dichloroaniline, at different concentra-
tions in two parallel groups (Figure 1). Two untreated groups were kept
under the same conditions as control. The flow-through test system
consisted of a water tank in which charcoal filtered tap water was
aerated and temperature was kept constant at 26°C (61°C). Tap water
was pumped (gamma/4-RS, prominent, Heidelberg, FRG) into the
mixing chambers. The toxicant solution was added by a diluter 401
(Gilson/Villiers le Bel, France) and a six-way valve (Anachem, Luton,
Correspondence to: U. Ensenbach
Arch. Environ. Contam. Toxicol. 32, 204–210 (1997)
A R C H I V E S O F
E
nvironmental
C
ontamination
a n d
T
oxicology
r
1997 Springer-Verlag New York Inc.
GB), controlled by a computer program, developed by abimed,
(Langenfeld, FRG). A photoperiod of 12 h was maintained.
The life-cycle test was started with 100 fertilized eggs per glass petri
dish (8-cm height, 15-cm in diameter, 500-ml test solution). Eggs were
obtained according to the method of Nagel (1986). From day six,
young larvae were fed twice a day with AZ 25 (Tetrawerke, Melle,
FRG). From day 8 newly hatched brine shrimp (Artemia spec.) and
pulverized dry food (Tetramin) were added. After two weeks, larvae
were transferred into glass vessels (20 3 15 3 30 cm, H 3 W 3 L,
8-L test solution). Feeding with Tetramin AZ 25 was gradually reduced.
Excrement and surplus food were removed daily. Hatching rates,
morphological abnormalities and mortality during the test were re-
corded. After 42 days of exposure, length, weight and survival rates of
the juvenile fish were determined. Surviving fish were kept in larger
glass aquaria (30 3 40 3 25 cm, H 3 W 3 L, 28-L test solution) until
sexual maturity was reached. Water exchange was once a day (Sprague
1973; Bresch et al. 1990). Depending on fish density in the aquaria,
sexual maturity took place after 12–14 weeks. From day 109, groups of
8 male and 4 female fish were taken from every concentration and from
the controls. Length and weight of the remaining fish were determined.
Then egg production was examined for a period of three weeks. To
study the quality of offspring of fish, which were long-term exposed to
xenobiotics, an additional early life stage test was carried out with the
F
2
-generation.
In the experiment where 4 month old adult fish were exposed, the
same equipment was used as during the full life-cycle test. The effects
of four different xenobiotic concentrations were investigated with two
parallels each. In every basin 8 male and 4 female fish were exposed.
To identify differences of the treated groups to the control groups the
procedure of Williams (1971) modified by Gelber et al. (1985) was
used for the following parameters: hatching rate, morphological
abnormality and survival rate. Comparison of body length and weight
was made by the rank test of Mann and Whitney (1947).
Egg Production
Eggs were collected in glass dishes covered with stainless steel wire
mesh. The dishes were placed at the bottom of the glass aquaria. Green
glass trees served as spawning substrate. Immediately after switching
on the light in the morning, coupling began and was completed within
20 min. Two hours after lighting, glass dishes were removed from the
aquaria and eggs were counted. The eggs were transferred to petri
dishes and 24 h later fertilization rates were determined. Unfertilized
eggs denature after 6–7 h; thus, it is simple to distinguish unfertilized
from fertilized eggs.
Chemicals and Chemical Analysis
Lindane (CAS no.: 58-89-9, chemical purity 99.8%) was provided by
CelaMerck (Ingelheim, FRG), 3,4-dichloroaniline (CAS no.: 95-76-1,
chemical purity 99.5%) was obtained from Aldrich (Steinheim, FRG).
Lindane and 3,4-dichloroaniline were extracted with toluene from the
test water. Analysis was performed weekly by gas chromatography,
using an electron capture detector (5890 workstation, Hewlett-Packard;
capillary column: crosslinked 5% phenyl silicone, 25 m 3 0.32
mm 3 0.52 µm). Toxicant concentrations were quantified by linear
regression of external standards. To analyze the concentration of
3,4-dichloroaniline, it was necessary to derivatize the substance
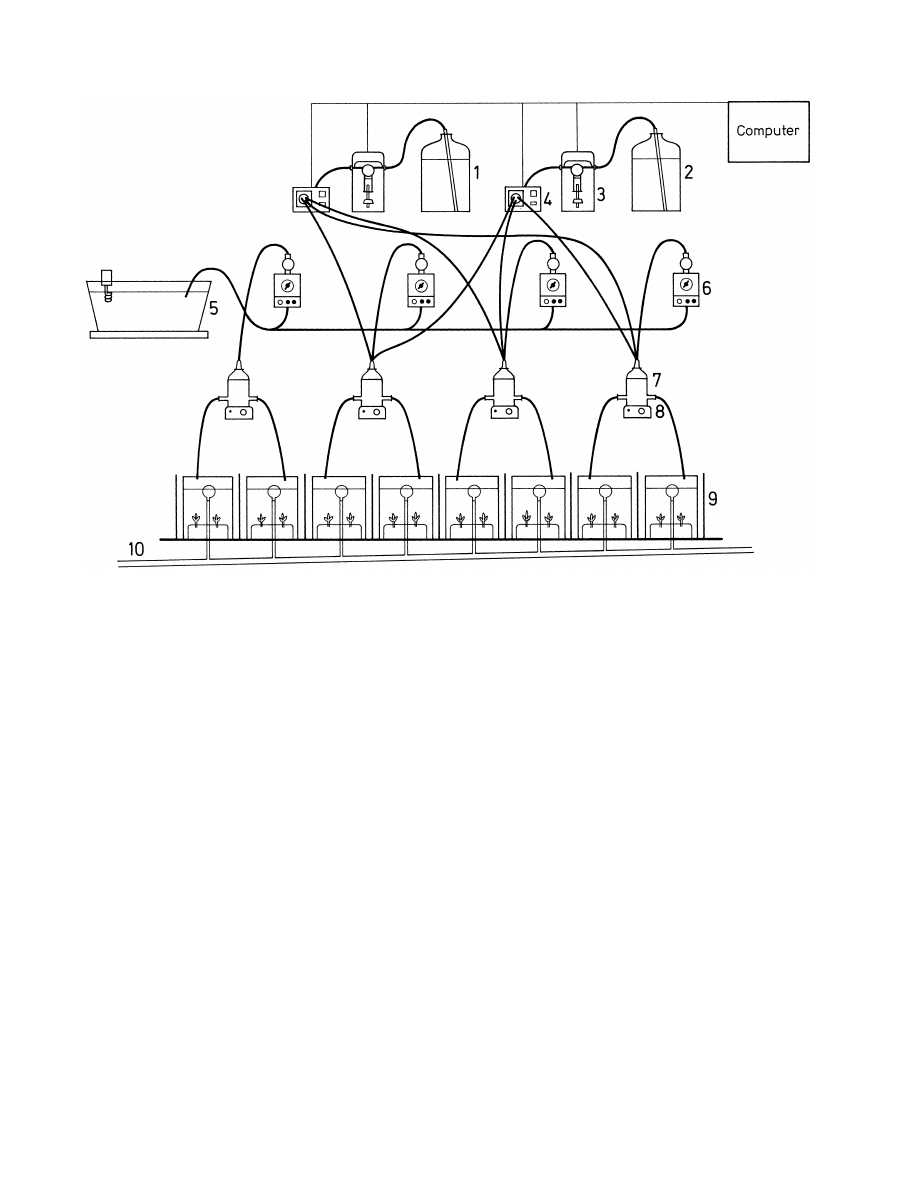
Fig. 1. Flow-through test system for full life-cycle tests: (1,2) stock solution for 3,4-dichloroaniline and lindane, (3) dilutor, (4) six-way valve, (5)
tap-water container, (6) water pump, (7) mixing chamber, (8) magnetic mixer, (9) glass test vessel, (10) waste pipe.
Toxicity of Binary Chemical Mixtures on Zebrafish
205
because of its low detection limit. First the pH of 100-ml solution was
adjusted to 8.6 with KOH. After extraction with 5 ml toluene, 50 µl
perfluorobutyric acid were added. The solution was heated 35 min at
58°C. Excess perfluorobutyric acid was eliminated by washing three
times with 25 ml distilled water. The results of the chemical analyses
are presented in Table 1. During the experiments water temperature
was 26 6 1°C. Dissolved oxygen exceeded 80% of the saturation
value, pH ranged from 7.8 to 8.4. Total hardness was 20–24°dH and
carbonate hardness 13–18°dH, respectively.
Results and Discussion
Development to Sexual Maturity
The results from the early life stage test of the F
1
-generation up
to day 42 were described by Ensenbach and Nagel (1995). From
day 43 to day 80 all fish developed normally. Subsequently fish
in the highest concentration lost weight. In one of these
parallels 13 fish died between day 84 and 108. More than 50%
of fish developed surface leasons. From day 95 of treatment, a
white film appeared on the walls of the aquaria. A bacteria or
fungus infection was suspected. No infection was found in all
other test groups. Fish in the infected group were sacrificed
after day 108 and their length and weight measured. All
equipment was disinfected.
Selected fish from the different treatments were sent to Dr. F.
Krupp (Natural Museum Senkenberg (Frankfurt/Main)) for
analysis. In one aquarium of the highest test concentration, an
infection of Ichthyosporidium at an advanced stage was diag-
nosed. In skin, muscle and intestine mainly mycelium was
found and in liver and heart muscle capsulated mycelium stages
predominated. Hansen et al. (1971) reported similar effects on
the skin of fish after exposure to PCB.
Weight and Length of Zebrafish (Age 18 Weeks) Exposed
During the Whole Lifetime
In comparison to the controls, a significant difference in length
and weigth of fish was found after treatment (Table 2). Weight
was reduced in the lowest concentration about 22% and in the
medium and highest concentration about 29 and 36%, respec-
tively.
Fish length was reduced about 8–12%, but there was no
dose-dependent reduction of length. Nagel (1995) observed no
effect on growth in a full life cycle test with zebrafish exposed
to lindane up to 150 µg/L. Similarly, Nagel (1989) observed no
effect on growth when fish were exposed to a dose of 20 µg/L
3,4-dichloroaniline. It can be concluded that the effect on fish
length is produced by the combination of both chemicals.
Egg Production and Fertilization Rates of Zebrafish
Exposed During the Whole Lifetime
From day 100, glass dishes were installed in the aquaria to
assess whether spawning had already begun. From day 109 of
treatment eggs were found in all dishes. The number of animals
was reduced to 8 male and 4 female fish in each aquarium. Egg
production was subsequently investigated from day 112 to day
133.
The number of eggs in controls were 66 per female per day.
Fertilization rates were between 52 and 60%. The quantity of
produced eggs laid within the range known from the literature.
Eaton and Farley (1974) found 23 up to 60 eggs per female per
day. Nagel (1988) ascertained variations from 40 up to 160 eggs
and Bresch et al. (1990) published a mean value of 100 eggs per
female per day.
On the concentrations of 2/40 and 20/40 µg/L 3,4-dichloro-
aniline/lindane, no negative effect on egg production could be
detected (Table 3). Despite of the smaller size of fish in these
treatments, 3 of 4 groups produced more eggs than the controls.
A significant effect on egg production was, however, found in
the highest xenobiotic concentration. The number of eggs
decreased 85% and the fertilization rate 55%. From day 125 of
Table 1. Xenobiotic concentrations (µg/L) during the life cycle test (I) and the exposition of adult zebrafish (II)
DCA/Lindane
DCA/Lindane
DCA/Lindane
DCA/Lindane
I
Nominal values
2.00/40
20.0/40
100.0/40
Real values
x
1.98/34
19.8/40.1
85.4/45.3
s
0.57/9.8
10.0/13.2
23.1/14.7
II
Nominal values
0/40
100.0/40
200.0/40
200.0/0
Real values
x
—/41.9
92.9/33.5
205.0/42.3
196.5/—
s
—/10.7
24.3/2.1
26.7/10.2
66.0/—
x 5 mean value; s 5 standard deviation; DCA 5 3,4-dichloroaniline
Table 2. Mean values of weight and length of F
1
-zebrafish (age 18
weeks) exposed during the whole lifetime to the binary mixture of
3,4-dichloroaniline (DCA) and lindane
DCA/Lindane
(µg/L)
Survival
rate
a
Length
(mm)
s
Weight
(mg)
s
0/0
40
34.9
1.8
457
81
0/0
59
33.6
2.1
420
87
2/40
49
31.1*
1.8
314*
71
2/40
64
31.1*
1.6
317*
57
20/40
54
30.9*
1.3
327*
60
20/40
34
32.3*
1.7
363*
65
100/40
30
30.1*
1.4
281*
52
100/40
b
29
26.5
2.5
179
41
(s) 5 standard deviation; * significant difference to control (p , 0.05);
a
start with 100 fertilized eggs each;
b
fish were measured after 15
weeks and were not taken for statistical evaluation because of an
infection of Ichthyosporidium
U. Ensenbach and R. Nagel
206

the experiment egg production had totally stopped at this
concentration. This effect was linked to the bad condition of fish
and behavioural changes. No courtship of the males occurred.
Normally, healthy fish react very quickly if food is placed into
the aquaria, but in this case fish were very lethargic. They did
not swim to the surface and ate only, when food reached the
bottom of the basin. After 140 days of treatment, 30% of fish
showed wounded flanks. The abdomens were red spotted. This
disorder was more marked in females than in males. Male fish
grew thinner. At the end of this experiment, the remaining fish
were sent to Prof. Ko¨rting (Tiera¨ztliche Hochschule Hannover)
for diagnosis of fish disease. No infectious diseases were found.
Only a few species of Aeromonas could be isolated and
cultivated. But these species have no pathogenic relevance for
fish.
Exactly whether the effect on egg production was caused by
the xenobiotic mixture or by one of the individual chemicals
3,4-dichloraniline or lindane was in question by that time. A
concentration of 40 µg/L lindane does not effect reproduction of
zebrafish (Nagel 1988). Also, Macek et al. (1976) found no
effects for 3 different fish species up to the highest investigated
concentration of 23.5 µg/L lindane. For 3,4-dichloroaniline no
reproduction data for a concentration of 100 µg/L were
available. The full life-cycle test with this substance (Nagel
1995) was carried out with concentrations of 2, 20, and 200
µg/L. The fish treated with 200 µg/L died before reaching
maturity and no effect occurred at 20 µg/L.
Early Life Stage Test of F
2
-Generation in the Binary
Mixture over 42 Days
Because egg production decreased in the highest concentration
of 100 µg/L 3,4-dichloroaniline and 40 µg/L lindane, it was not
possible to start the early life stage test with a hundred fertilized
eggs. So, only one group with 36 eggs could be tested at this
concentration. The results are summarized in Table 4.
Hatching and Developmental Abnormalities
Contrary to the F
1
-generation, F
2
-eggs were directly spawned
into xenobiotic treated water. But that did not influence
hatching rates, and there was no increase in developmental
abnormalities. In all but one group all rates were less than 3%.
Seven larvae exposed to 2/40 µg/L 3,4-dichloroaniline/lindane
showed edema and died before day 14. It could be assumed that
the observed deformation rate is natural, for these results agree
with data from Bresch et al. (1990), who found less than 5%
deformations during an experimental time over two fish genera-
tions.
Survival Rates
After 6 weeks of treatment, about 50% of juvenile fish survived
in all concentrations. No effect on survival rates could be
detected (Table 4). Most nonsurviving fish died within the
second week. This effect is connected with a nutritional change.
The yolk sac of larvae is consumed and larvae have to search
food in the aquaria. When larvae are not able to seek their food,
they die by starvation at the end of the second week. Subse-
quently, mortality rate decreased slightly and was higher in
treated basins than in controls.
Growth
Growth of juvenile fish was reduced in the concentrations of
2/40 and 20/40 µg/L 3,4-dichloroaniline/lindane. This effect did
not occur in the highest concentration. Because of the lower fish
density in this concentration a possible effect could be over-
looked. Only 17 fish survived in this concentration whereas in
the other treatments 40 to 50 fish survived. Comparing the
effects in early life stage tests of F
1
- (Ensenbach and Nagel
1995) and F
2
-generation, no additional effects appeared. So,
raising of parent fish in the xenobiotic mixture did not influence
the quality of their young.
Egg Production and Fertilization Rates of Zebrafish
after Exposure of Adult Animals to 3,4-Dichloroaniline
and Lindane
After an exposure time of 6 weeks egg production was
investigated for 4 weeks. The higher the xenobiotic concentra-
tion of 3,4-dichloroaniline, the lower were the number of eggs
produced (Table 5). The number of eggs were significantly
reduced compared with the groups which were not treated with
Table 3. Egg production and fertilization rates of F
1
-zebrafish exposed
during the whole life-time to the binary mixture of 3,4-dichloroaniline
(DCA) and lindane (mean values of three weeks)
DCA/Lindane
(µg/L)
Eggs/Group/Day
(s)
Fertilization
Rate (s)
0/0
259
(67)
53.4 (11.6)
0/0
275
(85)
53.9 (10.3)
2/40
308
(76)
55.4
(8)
2/40
454 (132)
60
(11.6)
20/40
362 (158)
52.2 (11.4)
20/40
233
(59)
58.4 (10.6)
100/40
41* (55)
24.5*
(18)
(s) 5 standard deviation; * significant difference to control (p , 0.05);
egg production was investigated from week 16 to 18
Table 4. Effects of the binary mixture of 3,4-dichloroaniline (DCA)
and lindane on the development of F
2
-zebrafish (duration 42 days, start
with 100 fertilized eggs)
DCA/Lindane
µg/L
Survival
Rates %
Length
a
(mm)
s
Weight
a
(mg)
s
0/0
46
16.4
1.8
86.7
26.3
0/0
54
15.7
1.6
76.9
22.9
2/40
59
13.9*
2.1
49.5*
23.2
2/20
46
14.9*
1.8
58.7*
21.3
20/40
40
15.1*
2.3
62.4*
25.0
20/40
41
14.9*
2.6
61.5*
26.6
100/40
b
47
16.7
2.3
100.2
34.2
a
Mean values, (s) 5 standard deviation;
b
start with 36 fertilized eggs;
*significant difference to control (p , 0.05)
Toxicity of Binary Chemical Mixtures on Zebrafish
207

3,4-dichloroaniline. Egg production of fish exposed to 200 µg/L
3,4-dichloroaniline was slightly higher than that of those fish
additionaly exposed to lindane, but the difference between these
groups was not significant. So the extra treatment with lindane
did not effect egg production when adult fish were exposed.
Fertilization rate was reduced only in one group exposed to 200
µg/L 3,4-dichloroaniline and 40 µg/L lindane. Cessation of egg
production occurred after 8 weeks of exposition in the highest
concentration of 3,4-dichloroaniline and lindane and after 9
weeks when fish were exposed only to 3,4-dichloroaniline.
Male fish lost weight during exposure. Females had swollen
abdomen and the flanks showed slightly redness. Because male
fish did not couple anymore, they were replaced by untreated
adult fish. But this procedure did not effect egg production.
The remaining fish were kept for two additional months in
untreated water. At this time, no eggs were found in all groups
which were exposed to the xenobiotic mixture. Fish formerly
exposed only to 200 µg/L 3,4-dichloroaniline again produced
eggs after keeping fish in untreated conditions. For two more
weeks eggs were counted. The number of eggs/group/day was
249 (s 5 51.3) and 266 (s 5 75), respectively. Fertilization
rates amount to 48.4 and 49.1%. These fish were exposed a
second time to 200 µg/L 3,4-dichloroaniline for 14 days and
after 10 days egg production was stopped. Afterwards fish were
kept in untreated water for two more months, but no more eggs
were produced. So the conclusion that changing fish from
treated to untreated water functions as an on/off switch for egg
production is incorrect.
Treatment with the single substance 3,4-dichloroaniline leads
to similar irreversible effects as exposition to the binary mixture
of 3,4-dichloroaniline and lindane. But the exposition to the
binary mixture accelerates the occurrence of the effect.
Very important for the occurrence of xenobiotic effects is the
time period of treatment. To detect an effect on egg production
an exposure time of ten weeks was necessary. No uniform test
duration could be defined, because the occurrence of an effect
depends on xenobiotic concentration, duration of exposition
and the toxicodynamics of the chemical.
For practical reasons, the experimental time should not be too
long. Conduction of a life cycle test with zebrafish could be the
way to get toxicological data of parameters relevant for the
population. Fish are exposed for four months to the xenobiotic
and all developmental stages are recordable. In comparison to
experiments with adult fish, much more toxicological data can
be collected, which leads to a better assessment of the toxic
potential of chemicals.
This is shown by the results of egg production by comparing
data for adult and whole lifetime exposed fish to 100/40 µg/L
3,4-dichloroaniline/lindane. Fish which were exposed in the
adult stage reduced the number of produced eggs, whereas fish
exposed for their whole lifetime stopped their egg production
irreversibly. These results have different consequences. In case
of whole lifetime exposure, it must be concluded, that the
experimental population of zebrafish will die out.
Nevertheless, some reduction of egg production did not
influence the zebrafish population. Scha¨fers et al. (1989)
investigated the population dynamics of zebrafish in a micro-
cosm system. Based on this data, a computer simulation model
was developed for zebrafish (Oertel et al. 1991). The model
showed that the number of eggs produced is not the most
relevant parameter for the preservation of the fish population.
Apparently, a reduction of egg production of about 50% does
not influence the survival of the population. The basis of this
calculation is a small population in a laboratory system and it is
not known if the results are transferable to field conditions.
Wannemacher et al. (1992) investigated the effects of single
doses of 2,3,7,8,-tetrachlorodibenzo-p-dioxin (TCDD) applied
with the food, on oogenesis and reproduction of zebrafish.
Doses of 5 ng TCDD (corresponding to 1.7–2.0 µg/kg body
weight) led to loss of weight and completely suppressed
spawning. The suppression of spawning coincided with a
significant reduction of mature oocytes and an increased
number of atretic follicles. It is not known if these effects on
reproduction are irreversible.
An explanation of the mechanistic level for the obstruction of
egg production is not yet available. Braunbeck (1989) studied
isolated hepatocytes, derived from zebrafish, which were used
in life cycle tests exposed to 3,4-dichloroaniline and lindane
(Nagel 1988, 1989, 1995). In hepatocytes of fish exposed to 20
µg/L 3,4-dichloroaniline he found fenestration of rough endo-
plasmatic reticulum, enlarged mitochondria and an increased
number of lysosomes. Fish exposed to lindane (40 µg/L)
showed liver adipose. These hepatocytic changes caused no
effect on egg production, but it is possible, that at higher
xenobiotic concentrations, a greater damage of liver cells leads
to the effects found in our investigation.
Several authors found influences of xenobiotics on the
endocrine system (Mattison and Thomford 1989; Donaldson
and Scherer 1983). Thomas (1989, 1990) investigated the
effects of heavy metals, benzo(a)pyrene, and a mixture of
polychlorinated biphenyls (Arochlor 1254) on endocrine func-
tions of fish (Micropogonias undulatus), which influenced
reproduction. These organic compounds decreased the growth
of the ovaries, estrogen and gonadotropin production were
degraded. Thomas assumed that the primary effect of xenobiot-
ics was the reduction of gonadotropin secretion, which is
responsible for stimulation of sexual hormone formation in the
reproductive glands. Sivarayah et al. (1978) found a connection
between PCB effected stimulation of liver enzyme activities
and decreasing concentrations of sex hormones in the blood of
fish. The induction of mixed function oxidases by xenobiotics
was shown by several authors (Stegeman et al. 1981, 1982;
Oikari et al. 1988; Levine et al. 1994; Holm et al. 1994; Gagnon
Table 5. Egg production and fertilization rates of zebrafish after
exposition of adult animals to 3,4-dichloroaniline (DCA) and lindane
(mean values of four weeks)
DCA/Lindane
(µg/L)
Eggs/Group/Day
Fertilization
Rate (%)
0/40
426 (174)
48.9 (10.6)
0/40
450 (155)
51.5
(7.1)
100/40
270* (148)
50.3
(8.8)
100/40
305* (107)
44.2
(9.9)
200/40
174* (144)
37.1* (8.6)
200/40
174* (137)
49.7
(7.5)
200/0
223* (101)
56.5
(9.5)
200/0
211* (92)
50.9
(8.5)
* Significant difference to control (p , 0.05); data in parenthesis 5
standard deviation; age of fish was 4 months when starting the
experiment; total exposition time was 10 weeks; egg production was
investigated from week 7 to 10
U. Ensenbach and R. Nagel
208

et al. 1994). Increasing activity of liver enzymes by PCBs leads
to breakdown of steroid hormones (Richter 1974). The insecti-
cide fenitrothion leads to inhibition of steroid synthesis in the
gonads (Kapur et al. 1978). Johnson et al. (1993) studied the
influence of different contaminated sites on ovarian develop-
ment in different species of sole. The results showed that female
English sole (Pleuronectes vetulus) from heavily contaminated
sites had lower plasma-estradiol levels than fish from the
relatively uncontaminated sites and were less likely to enter
vitellogenesis and undergo normal ovarian development. In
laboratory experiments the field observations were supported
when sole were injected with extracts of contaminated sedi-
ment. Similar effects were found by Gagnon et al. (1994). They
compared reproductive parameters of white sucker (Castosto-
mus commersoni) exposed to bleached kraft mill effluents with
unexposed populations. In females, testosterone and 17b-
estradiol levels were significantly reduced at the exposed
stations relative to the reference stations.
How far these modifications in hormone concentrations lead
to cessation of egg production is not known. Most of these
hormonal effects are reversible when exposure to the chemical
is stopped. This is in contrary to the irreversible effect on egg
production of zebrafish by the binary mixture of 3,4-
dichloroaniline and lindane.
References
Braunbeck T (1989) Cytopathologische Vera¨nderungen in der Fischle-
ber durch Umweltchemiekalien. dissertation thesis, University of
Heidelberg
Bresch H, Beck H, Ehlermann D, Schlaszus H, Urbanek M (1990) A
long-term toxicity test comprising reproduction and growth of
zebrafish with 4-chloroaniline. Arch Environ Contam Toxicol
19(3):419–427
Cairns J, Scheier A, Loss JJ (1965) A comparison of the sensitivity to
certain chemicals of adult zebra danios Brachydanio rerio (Hamil-
ton-Buchanon) and zebra danio eggs with that of adult bluegill
sunfish Lepomis macrochirus Raf. Notulae Naturale 381:1–9
Donaldson EM, Scherer E (1983) Methods to test and assess effects of
chemicals on reproduction in fish. In: Vouk VB, Sheehan PJ (eds)
Methods for assessing the effects of chemicals on reproductive
functions. Wiley and Sons, Chichester, pp 365–405
Eaton RC, Farley R (1974) Spawning cycle and egg production of
zebrafish in the laboratory. Copeia 1:195–204
Ensenbach U, Nagel R (1995) Toxicity of complex chemical mixtures:
Acute and long-term effects on different life stages of zebrafish
(Brachydanio rerio). Ecotoxicol Environ Saf 30(2):151–157
Federal Environmental Agency (1990) Chemicals Act—Principles for
the assessment of new chemicals under the chemicals act. Texte
28/90 E
Fogels A, Sprague JB (1977) Comparative short-term tolerance of
zebrafish, flagfish and rainbow trout to five poisons including
potential reference toxicants. Water Res 11:811–817
Gagnon MM, Dodson JJ, Hodson PV, Van der Kraak G, Carey JH
(1994) Seasonal effects of bleached kraft mill effluent on reproduc-
tive parameters of white sucker (Catostomus commersoni) popula-
tions on the St. Maurice river, Quebec, Canada. Can J Fish Aquat
Sci 51:337–347
Gallo D, Merendino A, Keizer J, Vittozzi L (1995) Acute toxicity of
two carbamates to the guppy (Poecilia reticulata) and the zebrafish
(Brachydanio rerio). Sci Total Environ 171:131–136
Gelber RD, Lavin PT, Metha CR, Schoenfeld DA (1985) Statistical
analysis. In: Rand GM Petrocelli SR (eds) Fundamentals of
Aquatic Toxicology. Hemisphere Publishing Corp, NY, pp 110–
123
Hansen DJ, Parrish PR, Lowe JI, Wilson AJ, Wilson PD (1971)
Chronic toxicity, uptake and retention of aroclor 1254 in two
estuarine fishes. Bull Environ Cont Toxicol 6(2):113–119
Holcombe GW, Benoit DA, Leonard EN (1979) Longterm effects of
zinc exposure on brook trout (Salvelinus fontinalis). Trans Am
Fish Soc 108:76–87
Holm G, Lundstro¨m J, Andersson T, Norrgren L (1994) Influences of
halogenated organic substances on ovarian development and
hepatic EROD in the three-spined stickleback, Gasterosteus
aculeatus, and rainbow trout, Oncorhynchus mykiss. Aquat Toxi-
col 29:241–256
Johnson L, Casillas E, Sol S, Collier T, Stein J, Varanasi U (1993)
Contaminant effects on reproductive success in selected benthic
fish. Mar Environ Res 35:165–170
Kapur K, Kamaldeep K, Toor HS (1978) The effect of fenitrothion on
reproduction of a teleost fish, Cyprinus carpio communis Linn.: a
biochemical study. Bull Environ Contam Toxicol 20:438–442
Levine ST, Oris JT, Wissing TE (1994) Comparison of P-4501A1
monooxygenase induction in gizzard shad (Dorosoma cepedia-
num) following intraperitoneal injektion or continuous waterborne-
exposure with benzo(a)pyrene: Temporal and dose-dependent
studies. Aquat Toxicol 30:61–75
Macek KJ, Buxton KS, Derr SK, Dean JW, Sauter S (1976) Chronic
toxicity of lindane to selected aquatic invertebrates and fishes.
USEPA, Duluth, MN, EPA 600/3-76-046, 49 pp
Mann HB, Whitney DR (1947) On a test of whether one or two random
variables is stochastically larger than the other. Ann Math Statist
18:50
Mattison DR, Thomford PJ (1989) The mechanisms of action of
reproductive toxicants. Toxicol Pathol 17(2):364–376
Nagel R (1986) Untersuchungen zur Eiproduktion beim Zebraba¨rbling
(Brachydanio rerio, Ham.-Buch.). J Appl Ichthyol 4:173–181
——— (1988) Umweltchemikalien und Fische—Beitra¨ge zu einer
Bewertung. Habilitationsschrift, Universita¨t Mainz
——— (1989) Erprobung und Absicherung des Reproduktionstests am
Fisch (Stufe 2 ChemG). Umweltbundesamt, Forschungsbericht
10603043/01
——— (1995) Complete life cycle tests with zebrafish—a critical
assessment of the results. In: Mu¨ller R, Lloyd R (eds) Sublethal
and chronic effects of pollutants on freshwater fish. FAO, Fishing
New Books, pp 188–195
Oertel D, Poethke H-J, Seitz A, Scha¨fers C, Nagel R (1991) Monte-
Carlo-simulation of the population dynamics of zebrafish in a
complex experimental system. Verh Ges O
¨ kologie 20(2):865–869
Oikari A, Lindstro¨m-Seppa¨ P, Kukkonen J (1988) Subchronic meta-
bolic effects and toxicity of a simulated pulp mill effluent on
juvenile lake trout, Salmo trutta m. lacustris. Ecotoxicol Environ
Saf 16:202–218
Richter E (1974) Untersuchungen u¨ber die Ausscheidung polychlori-
erter Biphenyle in das Hu¨hnerei und ihre Verteilung zwischen
Eiweiß und Dotter. Dissertation thesis, University of Mu¨nchen
Scha¨fers C, Nagel R, Seitz A (1989) Verhalten, Reproduktion und
Populationsdynamik des Zebraba¨rblings (Brachydanio rerio) in
einem naturnahen Laborsystem. Fischo¨kologie 1(2):45–59
Sivarayah K, Franklin CS, Williams WP (1978) The effects of
polychlorinated biphenyls on plasma steroid levels and hepatic
microsomal enzymes in fish. J Fish Biol 13:401–409
Sprague JB (1973) The ABC’s of pollutant assay using fish. In: Biol.
methods for the assessment of water quality. ASTM special
technical publication No. 528, pp. 6–30
Stegemann JJ, Klotz AV, Woodin BR, Pajor AN (1981) Induction of
hepatic cytochrome P-450 in fish and the indication of environmen-
tal induction in scup (Stenotomus chrysops). Aquat Toxicol
1:197–212
Stegemann JJ, Pajor AM, Thomas P (1982) Influence of estradiol and
testosterone on cytochrome P-450 and monooxygenase activity in
Toxicity of Binary Chemical Mixtures on Zebrafish
209

immature brook trout (Salvelinus fontinalis). Biochem Pharmacol
31:3979–3989
Thomas P (1989) Effects of aroclor 1254 and cadmium on reproductive
endocrine function and ovarian growth in atlantic croaker. Mar
Biol Res 28:499–503
Thomas P (1990) Teleost model for studying the effects of chemicals on
female reproductive endocrine function. J Exp Zoo Suppl 4:126–
128
Wannemacher R, Rebstock A, Kulzer E, Schrenk D, Bock KW (1992)
Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproduction and
oogenesis in zebrafish (Brachydanio rerio). Chemosphere 24(9):
1361–1368
Westerfield M (1995) The zebrafish book. Institute of Neuroscience,
University of Oregon
Williams DA (1971) A test for differences between treatment means
when several dose levels are compared with zero dose control.
Biometrics 27:103–117
Zok S, Go¨rge K, Kalsch W, Nagel R (1991) Bioconcentration,
metabolism and toxicity of substituted anilines in the zebrafish
(Brachydanio rerio). Sci Total Environ 109–110:411–421
U. Ensenbach and R. Nagel
210
Wyszukiwarka
Podobne podstrony:
The?fects of Industrialization on Society
Effect of?renaline on survival in out of hospital?rdiac arrest
effect of varying doses of caffeine on life span D melanogaster
3 The influence of intelligence on students' success
6 The importance of motivation on students' success
Comparative eco toxicity of nanoscale TiO2, SiO2, and ZnO
Monetary and Fiscal Policy Quick Overview of the U S ?on
effect of AVR on survival
Effect of caffeine on fecundity egg laying capacity development time and longevity in Drosophila
Effect of caffeine on short hole borer beetle
Curseu, Schruijer The Effects of Framing on Inter group Negotiation
PBO-PD01-F08 Protocol of training on Qsms, Akademia Morska, Chipolbrok
The?fects of Race on Sentencing in?pital Punishment?ses
effects of divorce on children
The influence of Aristotle on Alfarabi
influence of parents on thier childrens sexual orientation
więcej podobnych podstron