
INFECTION PREVENTION & CONTROL
CLEANING, DISINFECTION &
STERILISATION
Initiated by:
Infection Prevention & Control Team
Approved by:
Infection Prevention & Control Committee
Issue Date:
2010
Review Date:
2011
Version: 4
Doc Ref:
CDSv3 .09
Policy Title:
Cleaning, disinfection and Sterilisation
Executive Summary:
Details the differences between cleaning, disinfection and
sterilisation and how these are achieved and most appropriately
used within the Trust.
Supersedes:
V2 2007
Description of
Amendment(s):
Minor Wording
This policy will impact on:
clinical practices, employees and health & safety
Financial Implications:
None
Policy Area:
Infection Control Trust
Wide
Document
Reference:
CDSv3.09
Version Number:
3
Effective Date:
2009
Issued By:
Director of Infection
Prevention and Control
Review Date:
12.2011
Author:
Service Manager
Infection Prevention
and Control
Impact Assessment
Date:
06.09
APPROVAL RECORD
Committees / Group
Date
Consultation:
Infection Control Committee
06.09
Health Protection Unit
06.09
Approved by Director:
Director of Nursing & Patient
Care Standards
06.09
Received for information:

CONTENTS
Page
Policy Statement including Single Use Items
2
1. Introduction
2
2. Principles
3
3. Definitions
4
4. Risk Assessment for the Decontamination of Reusable Medical Devices
4
4.1 High Risk
4
4.2 Medium Risk
4
4.3 Low Risk
4
5. Methods of Disinfection and Sterilisation for Reusable Medical Devices
5
5.1 Heat Sterilisation
5
5.2 Chemical Sterilisation
5
5.3 Heat Disinfection Methods
5
5.4 Chemical Disinfection
6
6. Bench Top Steam Sterilisers
6
7. Endoscope Washer Disinfectors
7
7.1 Decontamination
7
8. Prion Disease
8
8.1 Prions
8
8.2 Creutzfeldt-Jacob Disease (CJD)
8
8.3 Identification and Tracing of Instruments
9
Legislation, Guidance and References
10
Appendices
I Risk Categories for the Decontamination of Reusable Medical Devices
II Quarantine of Surgical Instruments
III Disinfection Methods
Equality and Human Rights Policy Screening Tool

Clean,Disinfection,Sterilisationv3 2009
2 of 23
East Cheshire NHS Trust
POLICY STATEMENT FOR REPROCESSING MEDICAL DEVICES
The Trust has a responsibility to have a system in place to ensure that, as far as is
reasonably practicable, all re-usable medical devices are properly decontaminated prior to
use and that the risks associated with decontamination facilities and processes are
adequately managed.
In this policy the term
re-usable medical device applies to all such devices whether owned
by the organisation, rented, on loan or acquired by any other means.
SINGLE-USE DEVICES MUST NOT BE REPROCESSED
Single-use devices
must not be reprocessed and single patient use devices must not be
re-used outside the manufacturers guidance.
The processing and re-use of single-use devices has inherent risks such as safety,
performance and effectiveness, thereby exposing patients and staff to unnecessary risk and
represents a substantial litigation risk to the Trust.
All equipment purchased must comply with the European Union (EU) and United Kingdom
(UK) standards for reprocessing, packaging and ensuring the equipment is still suitable for
its intended use.
Guidance within this policy is provided for disinfection and sterilisation using heat and cold
chemical methods and advice for single-use items and guidance for CJD is also included.
Further detailed advice can be obtained from the Medical Devices Agency MDA DB2002
(05) July 2002 “Decontamination of endoscopes” and MDA DB2002 (06) October 2002
“Benchtop steam sterilisers – Guidance on Purchase, Operation and Maintenance”. These
documents should be available to all users of endoscope washer disinfectors and benchtop
sterilisers. Copies of the documents can be obtained from the Infection Prevention &
Control Team or
. Also refer to the Trust policy on single-use Medical
Devices: Implications and consequences of re-use.
1. INTRODUCTION
The choice of decontamination method depends on a number of factors, which include
the type of material to be treated, the organisms involved, the time available for
decontamination and the risks to staff and patients.
Disinfection, sterilisation and cleaning are necessary to prevent cross infection from
equipment, surfaces and skin of patients and health care staff (for skin decontamination
refer to the Good Practices policy). This policy relates to all areas.
Guidance is provided for disinfection and sterilisation using heat and cold chemical
methods and advice for single-use items and CJD is also included, refer also to the
Single-used Medical Devices: Implications and Consequences of Reuse policy.
Clean,Disinfection,Sterilisationv3 2009
3 of 23
East Cheshire NHS Trust
2. PRINCIPLES
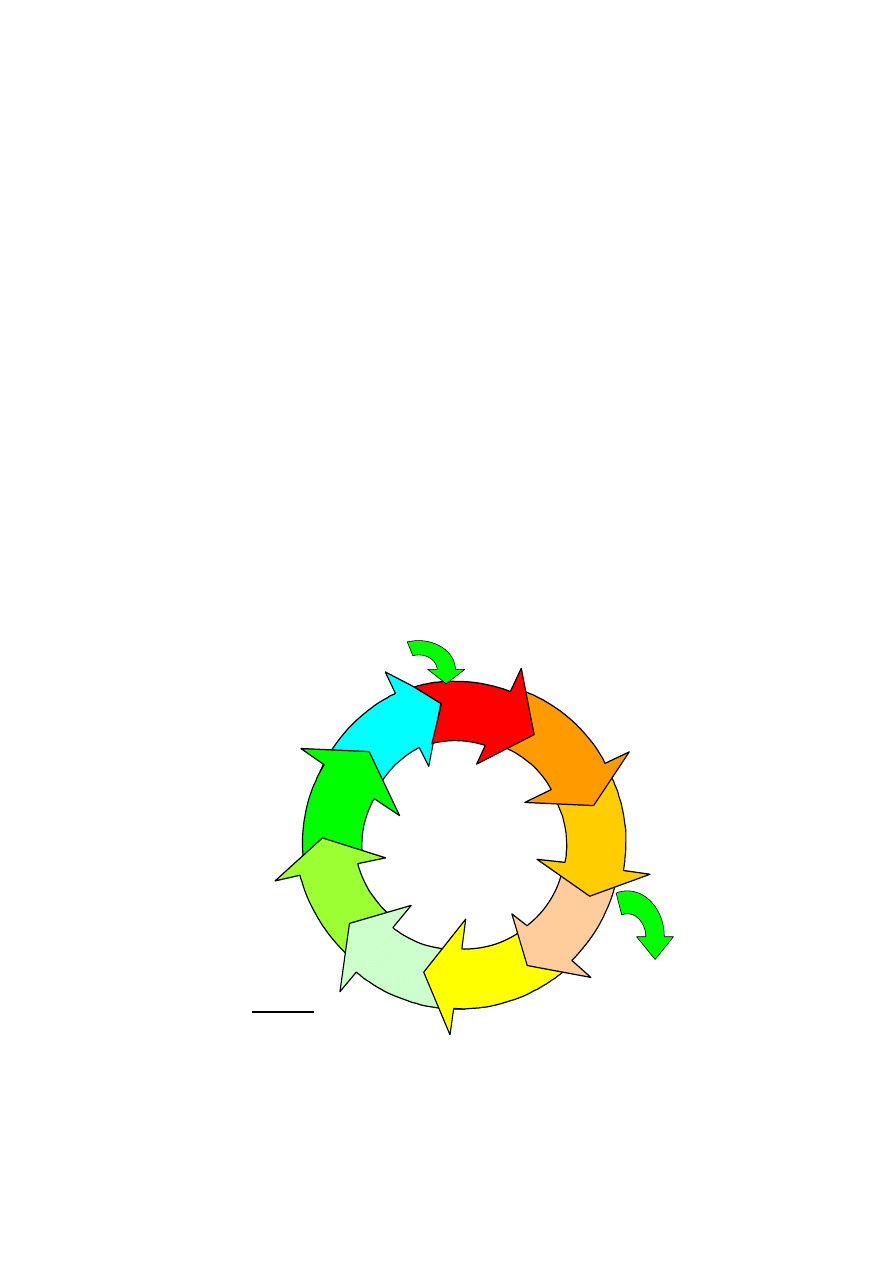
2.1 The re-usable medical device life cycle, shown in figure 1 comprises the following
processes – acquisition, cleaning, disinfection, inspection, packaging, sterilisation,
transportation, and storage before use. This cycle is used to render a re-usable
item safe for use. It is important that the Infection Prevention & Control Team is
involved at all stages including pre-purchasing.
2.2 Prior to purchasing equipment Trust staff must ensure that the item can be
decontaminated effectively and that the suppliers offers clear instructions on the
cleaning, disinfection and sterilisation methods suitable for individual pieces of
equipment, the Infection Prevention & Control Team can also provide advice.
2.3 Routine decontamination of equipment and the environment can be undertaken by
cleaning with soap/detergent. This same process must also be used for the
preparation of equipment prior to the appropriate disinfection/sterilisation method if
required.
Heat disinfection/sterilisation methods can be used on all heat-stable equipment.
Heat-sensitive equipment such as fireoptic scopes must be sterilised/disinfected
using cold chemical processes or gas sterilisation with ethylene oxide.
Re-Usable Medical Device Life Cycle
At all stages:
Location
Facilities
Equipment
Management
Policies/Procedures
CLEANING
DISINFECTION
INSPECTION
PACKAGING
STERILIZATION
TRANSPORT
STORAGE
USE
TRANSPORT
DISPOSAL
1. Scrap
2. Return to lender
ACQUISITION
1. Purchase
2. Loan
Figure 1

Clean,Disinfection,Sterilisationv3 2009
4 of 23
East Cheshire NHS Trust
3. DEFINITIONS
Decontamination: is the combination of processes, including cleaning, disinfection
and/or sterilisation, used to render a reusable item safe for further use.
Cleaning: is the process which physically removes large numbers of micro-organisms
and the organic material on which they survive.
Disinfection: is described as a process used to reduce the number of viable micro-
organisms but which may not necessarily inactivate some microbial agents, such as
viruses and bacterial spores.
Sterilisation: the removal of viable micro-organisms and spore on an object.
4. RISK ASSESSMENT FOR THE DECONTAMINATION OF RE-USABLE MEDICAL
DEVICES
The choice of appropriate decontamination methods is undertaken on a risk assessment
basis, with three categories, high, medium and low risk. A description of these
categories is given below and also summarised in Appendix I.
4.1 High Risk
These devices penetrate skin or mucous membranes, enter the vascular system or
sterile spaces, for example surgical instruments, and require sterilisation prior to
their use. Other sterile devices such as cardiac or urinary catheters, implants,
needles, etc, are single use (and sterilised by the manufacturer) and
must not be
reprocessed.
4.2 Medium
Risk
These devices come into contact with intact mucous membranes or may be
contaminated with particularly virulent (pathogenic) or readily transmissible
organisms. They require high-level disinfection to remove vegetative bacteria.
These items have the potential to be in contact with mucous membranes, damaged
skin, infected lesions, blood/body fluids. This category also applies to items used
on immuno-compromised patients. These items are made safe by a disinfection
process.
4.3 Low
Risk
These devices either come into contact with intact skin or do not come into contact
with the patient. Whilst this policy focuses mainly on the high and medium risk
devices, it is equally applicable to the lower risk devices, which are frequently
decontaminated locally in a clinical unit.

Clean,Disinfection,Sterilisationv3 2009
5 of 23
East Cheshire NHS Trust
5. METHODS OF DISINFECTION AND STERILISATION AVAILABLE FOR RE-USABLE
DEVICES
The method of decontamination will depend largely on the nature of potential pathogens
present and the infection risk associated with the device (Appendix I). To prevent
damage to a device, other factors such as the heat, pressure and chemical tolerance as
well as the manufacturers advice, must be taken into account before reprocessing
devices, there must be compliance with each of the stages identified in figure 1.
5.1 Heat
Sterilisation
Heat sterilisation – using autoclaves with steam under pressure either in the
Hospital Sterilisation and Disinfection Unit (HSDU) or bench top steam sterilisers,
which are covered in detail in section 6.
The following are the temperatures and cycle times for autoclaves in HSDU or
bench top steam sterilisers in clinical areas:
Porous load (wrapped)
134 - 138
o
for 3 minutes
Unwrapped instruments
121 - 124
o
for 15 minutes or 115
o
c for 15 minutes
5.2 Chemical
Sterilisation
Cold sterilisation – using chemicals such as chlorine dioxide for heat labile
equipment. For sterilisation, immersion in chlorine dioxide is required for 5
minutes.
Gas Sterilisation – can be used for the sterilisation of heat sensitive equipment
(ethylene oxide is used, although not available in HSDU – for further advice contact
the HSDU manager ext. 1920).
5.3 Heat Disinfection Methods
Disinfection by low temperature steam –this is moist head disinfection/
pasteurisation process (73
o
c for 10 minutes).
Washer disinfection – the use of washer/disinfectors uses two principles of
disinfection (thermal disinfection and chemical disinfection), although the main
emphasis is on the mechanical/physical removal of tissue and debris prior to
disinfection (see also HTM 2030 washer-disinfector).
Temperature ranges and time periods
65
o
c for 10 minutes
or
71
o
c for 3 minutes
or
80
o
c for 1 minute
Clean,Disinfection,Sterilisationv3 2009
6 of 23
East Cheshire NHS Trust
5.4 Chemical
Disinfection
Chemical disinfection – Chemical disinfectants are used when alternatives are
required for heat-sensitive items. The microbicidal activity of the chemical and the
contact time are important considerations when choosing chemical disinfectants,
for example, hypochlorite or chlorine dioxide.
IMPORTANT DOS AND DON’TS OF CHEMICAL DISINFECTION
DO
DON’T
Take care to measure your disinfectant
correctly
Use a disinfectant for sterilisation
Add the disinfectant to the right amount
of water, to make a solution for use
Add detergent to a disinfectant; this
may inactivate both
Use a clean, dry container for the
solution
Store instruments or cleaning tools in a
disinfectant
Wash away dirt, where you can, before
using disinfectant
Top up solution: make up a fresh one
when expiry time reached, e.g. Virkon 7
days shelf life
Remember that if disinfectants are used
carelessly, they may grow microbes
Use two disinfectants together
Check expiry dates
Bring in your own disinfectant to the
hospital
Give adequate time for disinfectant to
work
Disinfect if cleaning is sufficient
6. BENCH TOP STEAM STERILISERS
Wherever possible, all instruments and other equipment should be sterilised in
HSDU. Benchtop steam sterilisers are intended for the sterilisation of unwrapped
instruments for use in the immediate environment and have the following features:
Fully automated pre-determined sterilisation cycle
Steam generated internally
Single manually operated door
Electrically heated
Before purchase advice should be sought from an Authorised Person for sterilisers
(Contact Estates Department, at MDGH ext 1616) regarding the use, ranges of
sterilisers available and installation and commissioning. HTM 2010 contains detailed
advice on specification, purchase, installation, maintenance and operation. Validation
is also required prior to the use of the steriliser and all records of the validation
process retained by the owner/user.
Periodic testing of benchtop steam sterilisers and maintenance tasks must be
undertaken and retrospective testing is not recommended. Routine monitoring of the
process and periodic testing on a daily basis, weekly, quarterly and annually must be
undertaken and recorded (HTM 2010 part 4).

Clean,Disinfection,Sterilisationv3 2009
7 of 23
East Cheshire NHS Trust
Owners of benchtop steam sterilisers should ensure that the steriliser is subject to a
planned and documented schedule of preventative maintenance. Guidance is
provided in HTM 2010.
The user should follow the recommendations for the use and quality of the water to
fill the reservoir, the frequency of changing the water and routine maintenance of the
reservoir (HTM 2010, 2030, 2031). The quality of steam is also an important factor in
the sterilisation process and should be assessed regularly in line with HTM 2031.
In order to comply with HTM standards benchtop steam sterilisers must only be used
in association with an automatic washer disinfector and benchtop steam sterilisers
must have an automatic water cycle (filling and emptying).
All forms of wrapping material, including pouches, are considered as being
inappropriate for use with benchtop steam sterilisers. Benchtop steam sterilisers
are also
not suitable for processing porous loads such as swabs, towel dressings,
gowns and drapes.
Therefore, only those benchtop steam sterilisers equipped with a vacuum stage
(porous load) are only suitable for processing wrapped instrument and instruments
with lumens, and then only they have a pre-sterilising vacuum cycle.
7. ENDOSCOPE WASHER DISINFECTORS
Endoscopes can be categorised by their design into rigid or flexible, and all flexible and
most rigid scopes are meant to be re-used. When purchasing endoscopes or
accessories, consideration must be given to the ease of decontamination of reusable
equipment and alternative single use items. Flexible scopes contain a wide range of
materials which render them heat sensitive, and chemical disinfection is the choice of
reprocessing method.
The diverse range of materials incorporated into scopes and their accessories, including
automated endoscopes reprocessors (AERs) make it essential to obtain advice from
manufacturers on chemical compatibility and decontamination methods (detailed
information can be found in MDA DB2002 05).
7.1 Decontamination
The effective decontamination of equipment requires input from the following areas
of expertise:
Manufacturers of scopes and accessories
Manufacturers of AERs
Infection Prevention & Control Team
Endoscopy Department
Authorised persons for decontamination
Clinicians
Manufacturers of chemical disinfectants

Clean,Disinfection,Sterilisationv3 2009
8 of 23
East Cheshire NHS Trust
All procedures for the purchase, cleaning, disinfection/sterilisation, maintenance
and storage of scopes and AERs should be undertaken following expert guidance
from the above list of people and appropriate MDA Bulletins and HTM’s listed in the
reference section. Advice on the validation, maintenance and the periodic testing
is provided in HTM 2030.
Traceability of devices during their life cycle is also important, therefore, systems
have to be put in place to ensure all equipment used including AERs can be
identified to individual patients and recorded. (see also section 8.3 and Appendix II)
The use of mains water of appropriate quality can be a contributory factor to the
contamination of the scopes and AERs. Some machines have a pre-programmed
self-disinfection cycle. Which ever disinfection regime is chosen, it is essential that
all parts of the machine that come into contact with the water is accessed during
the self-disinfection cycle of the AER. It is also essential that water tanks and fluid
pathways of the machine are drained and left dry when not in use. Detailed advice
can be sought from the HTM 2031 on the required water quality for endoscopes
and AERs.
For information on disinfection methods see Appendix III.
8. PRION DISEASES
The abnormal proteins associated with prion disease are very resistant to all
conventional methods of decontamination.
8.1 Prions
Prions are infectious agents smaller than viruses and unlike any other pathogens.
Their only known component is an abnormal conformed protein. These abnormal
proteins then accumulate in the central nervous system. Prion diseases are fatal,
infectious, neuro-degenerative disorders with no known immunisation or treatment.
Currently there are four known human prion diseases:
Kuru
Gerstmann-straussler-scheinker-syndrome (GSS)
Fatal Familial Insomnia
Creutzfeldt-Jakob disease (CJD) and variant Creutzfeldt-Jakob disease (vCJD)
Most chemical and physical means of cleaning, disinfection and sterilisation are
only partially effective in inactivating prion disease.
8.2 Creutzfeldt-Jacob Disease (CJD)
For all patients irrespective of known or suspected prion disease: single use
devices must be used for lumber punctures and surgery relating to the eye, tonsils,
brain or spinal cord.

Clean,Disinfection,Sterilisationv3 2009
9 of 23
East Cheshire NHS Trust
For known and suspected cases of CJD for all types of surgery: it is important that
the
Infection Prevention & Control Team and Hospital Sterilising and
Disinfection Unit Manager are informed by clinicians of ALL suspected or
known patients with CJD, so that disposable instruments can be used for all
surgery on those patients (wherever possible). All non-disposable instruments on
such patients must be quarantined (suspected cases) or destroyed (known cases).
8.3 Identification and Tracing of Instruments
All instruments must be traceable and the system must identify the patient and the
date of each individual occasion when the instrument has been used.
As stated above, instruments used on known or suspected cases of CJD, have to
be clearly identifies and destroyed or quarantined. This would include the whole
pool of instruments of a given type if individual items can not be identified.
Therefore, all instruments must have a unique identifier in order to accurately trace
them.

Clean,Disinfection,Sterilisationv3 2009
10 of 23
East Cheshire NHS Trust
LEGISLATION, GUIDANCE AND REFERENCES
ACDP (1998)
Transmittable Spongiform Encephalopathy Agents: Safe Working and
the Prevention of Infections
Advisory Committee on Dangerous
Pathogens Protection Against Bloodborne Infections
in the Workplace – HIV & Hepatitis
Ayliffe G.A.J., Coates D. and Hoffman P.N. (1986)
Chemical Disinfection in Hospitals
PHLS LONDON
Ayliffe G.A.J., Lowbury E.J.L., Geddes A.M. and Williams J.D. (1992)
Control of Hospital
Infection – A Practical Handbook (3
rd
Edition) Chapman and Hall Medical, LONDON
British Medical Association (1989)
A Code of Practice for the Sterilisation of Instruments and Control of Cross Infection
BMA LONDON
Control of Substances Hazardous to Health Regulations 1999 (COSHH)
Department of Health and Public Health Laboratory Service (1995)
Hospital Infection
Control – Guidance on the Control of Infection in Hospital Department of Health,
LONDON
Department of Health
The Health & Social Care Act 2008 – Code of Practice for the
Prevention and Control of Health Care Associated Infection
Horton R., Parker L. (1997)
Informed Infection Control Practice Churchill Livingstone
DOH (2000)
Control Assurance Standards – Decontamination of re-usable devices
DOH (1999)
Decontamination Guidance (CD Rom Version 1.0) NHS Estates
MDA DB 9605 (1996)
The Purchase Operation and Maintenance of Benchtop Steam
Sterilisers
MDA DB 2000 (04)
Single-use Medical Devices: Implications and Consequences of
Re-Use
MDA DB 2002 (05)
Decontamination of Endoscopes
MDA DB 2002 (6)
Benchtop Steam Sterilisers – Guidance to the Purchase, Operation
and Maintenance
WHC (99) 157
Decontamination of Medical Devices
WHC (99) 158
Variant Creutzfeldt-Jakob Disease (vCJD)
Clean,Disinfection,Sterilisationv3 2009
11 of 23
East Cheshire NHS Trust
RISK CATEGORIES FOR THE DECONTAMINATION
OF REUSABLE MEDICAL DEVICES
Appendix I
Items in close contact with a break in skin or
mucous membrane or introduced into a normally
sterile body area
Definition
Examples
Surgical instruments
Syringes and needles
Intrauterine devices
Dressings
High Risk
Suitable methods
Sterilisation required
Items in contact with mucous membranes or other
items contaminated with particularly virulent or
readily transmissible organisms; or items to be used
on highly susceptible people
Definition
Examples
Respiratory equipment
Gastroscopes
Intermediate
Risk
Suitable methods
Disinfection required, by heat where possible
Items in contact with normal and intact skin
Definition
Examples
Washing bowls
Floors
Low Risk
Suitable method
Cleaning and drying usually adequate

APPENDIX II
Clean,Disinfection,Sterilisationv3 2009
2 of 23
East Cheshire NHS Trust
QUARANTINING OF SURGICAL INSTRUMENTS
Paragraph 4.28 of the Advisory Committee on Dangerous Pathogens (ACDP) Spongiform
Encephalopathy Advisory Committee (SEAC) guidance on “Transmissible spongiform
encephalopathy agents: Safe working and the prevention of infection” allows for instruments
that have been used on a patient suspected of having CJD of any type to be quarantined
pending a confirmation of diagnosis. Although it is not expected that this facility will need to
be used widely, the following supplementary advice has been prepared for reference in
those instances where such quarantining may be appropriate.
At the completion of a surgical procedure undertaken on a patient suspected of suffering
with CJD of any type, single-use instruments should be separated and disposed of by
incineration, re-usable instruments should be washed to remove gross soil. Care should be
taken to avoid splashing and generating aerosols by holding instruments below the surface
of the water in a sink into which water is running and draining out continuously.
Instruments should not be held directly under a flowing tap, as this is likely to generate
splashes. Operatives should wear protective gloves and either a visor or goggles and care
must be taken to avoid penetrating injuries.
Instruments should be placed in a disposable instrument tray and allowed to air dry. They
should then be placed in an impervious rigid plastic container with a close fitting lid. The lid
should be sealed with heavy-duty tape (e.g. autoclavable tape) and labelled with the
patient’s identification (i.e. hospital number, name and date of birth, the surgical procedure in
which the instruments were used and the name of the responsible person (e.g. the theatre
manager).
The sealed box should be stored indefinitely in a suitable designated place (within main
theatre) until the outcome of any further investigation is known. The instrument tray should
be disposed of by incineration.
If the patient is confirmed as suffering from CJD of any type, the box and its contents should
be incinerated without further examination. If an alternative, definitive diagnosis is
confirmed, the instruments may be removed from the box by the responsible person
(Theatre Manager or a deputy) and sent to the HSDU for processing in the usual way.
Records must be kept of all decisions, and the HSDU must be told of the decision before the
instruments are sent for routine processing.
Prolonged autoclaving or supplemental disinfection is not necessary for instruments
removed from quarantine, which had been used on a patient
not suffering from CJD of any
type.
Clean,Disinfection,Sterilisationv3 2009
1 of 23
East Cheshire NHS Trust
DISINFECTION METHODS APPENDIX III
Disinfection is the removal of most pathogenic organisms from an object. (Disinfectants
cannot sterilise. Where sterility is required heat treatment is
always preferable.) Simple cleaning with detergent will remove the majority of micro-organisms and is an essential first step in disinfection. There
are several types of disinfectant.
DISINFECTANTS
TYPE
EXAMPLE
(Brand may be changed from time to time)
NOTES
Alcohol
Industrial methylated spirits (70%).
70% Alcohol impregnated swabs and wipes
e.g. Mediswab, Azowipes.
Alcohol used for soaking items must be
disposed of at the end of each day.
Chlorine Dioxide
Tristel
Must only be used in specified areas.
Hypochlorite
Haztabs, Haztab Granules and Titan 500 mg
Hypochlorite sanitising powder e.g. Endbac
Neutralised by organic material.
Corrosive to metal.
Effective against viruses.
Phenolic –
only for use prior to
macerator repair – available from
Pharmacy on request
Hycolin (dilute to 2% solution in water)
Toxic and corrosive to skin.
Kills bacteria and fungi.
For specific infections the Infection Prevention & Control Team may offer individual advice on the choice and use of disinfectant.
Don't Forget -
C.O.S.H.H. regulations apply to all Disinfectants.
-
Always follow manufacturers instructions regarding dilutions.
-
Ensure adequate ventilation (especially for Hypochlorites and Aldehydes).
-
Always wear gloves and apron.
-
Know what action to take if you accidentally splash any on to your skin or into your eyes and mouth. See Good Practices
Policy for Infection Prevention & Control.
The following list is a guide for adequate disinfection. Additional information may be required by community staff working within the patient’s home environment. If
you have any problems or doubts about procedures, please contact either:
Infection Prevention & Control Nurses, Ext.
1597, 1417 or 1769, or Bleep 3034, or Consultant Microbiologist, Et 1810 or Bleep 3102
ITEM
ROUTINE
ADDITIONAL
RECOMMENDATIONS
Airways Disposable.
Ambubags
Detergent and hot water. Dry thoroughly.
Ambulift
Detergent and hot water. Dry thoroughly.
Hypochlorite if fouled, or after potentially infected patients.
Ampoules and Vials
Wipe neck with alcohol impregnated swab.
Auroscopes
Disposable single patient use
Baths
Clean at least daily, and between patients, with
detergent in hot water, rinse and dry.
After use by known or potentially infected patient, clean with
Hypochlorite sanitiser, rinse and allow to dry.
Bath Mats
Disposable single patient use
Bed Frames
Detergent in hot water between patients.
After patient with a specific infection, consult Infection Control Nursing
Plan.
Bed Pans
Non disposable.
Disposable
Bed/slipper pans, place directly into washer/disinfector.
Place directly into macerator.
Bed Pan Disposal Units
(Macerators)
Wash outside with detergent and hot water.
Report any leakage’s around the lid to the Nurse in Charge. Add 100
ml of Hycolin to machine prior to servicing and repairs.
Bed Pan Washer/Disinfectors
Wash outside with detergent and hot water.
Report any leakage’s to Nurse in Charge.
Bed Pan Holders
Wash in detergent and hot water, then rinse well and
store dry after each use.
ITEM
ROUTINE
ADDITIONAL
RECOMMENDATIONS
Bidets
Wash with detergent and hot water, rinse and dry
between patients.
Clean with hypochlorite sanitiser after use by known or potentially
infected patients and when any blood spillage’s are visible, rinse
and dry
Blood Glucose Meters
Wipe any blood contamination with alcohol impregnated
wipes.
Carpets
Vacuum clean daily.
Faeces & Vomit – Remove as much organic matter as possible
using paper towels, discard directly into yellow bag. Clean area
using a Hypochlorite solution and rinse with water.
Blood - Haztab granules and Hypochlorite, rinse well. (See
instructions in blood spillage pack)
Cleaning Cloths
Disposable.
Use different cloths in different areas, i.e.
Green
- Kitchens
Red
-
Bathrooms & Toilets
Blue
- General
Areas
Commodes (and Sanichairs)
Frame:
Approved cleaner e.g. Steri7 Wipes.
Bed pan: Disposable or bed pan washer.
Commodes must be labelled as clean
After patient with enteric infection wipe frame with Hypochlorite
sanitiser, rinse and dry.
Commodes must b e labelled as clean
Dressing Trolley
Clean with detergent and hot water before commencing
dressings. Disinfect top with alcohol impregnated wipes
between patients.
Emergency Pocket Masks
Wash with detergent and hot water, rinse and dry.
Replace disposable valve following each patient use.
Endoscope
See local guidelines.
Feeding Bottles
Send to HSDU.
Floors
Detergent only.
For blood contaminated spillage use hypochlorite 10,000 ppm, or
granules (see blood spillage pack). For other known or potentially
infected spillage use hypochlorite solution 10,000 ppm.
Flower Vases
Detergent in hot water, then rinse and dry.
Do not wash in the kitchen.
ITEM
ROUTINE
ADDITIONAL
RECOMMENDATIONS
Food Preparation Areas
Hypochlorite sanitiser solution and rinse with water.
Important to wipe dry, especially metal surfaces.
Food Trolleys
Detergent and hot water.
Disconnect heated trolley first!
Furniture
Detergent in hot water.
After Specific Infection Control Nursing, special arrangements will
be made.
Handbasins
Clean daily with hypochlorite sanitiser. Wash with detergent
in hot water and rinse and dry between patients.
After use by known or potentially infected patients, disinfect with
Hypochlorite sanitiser, rinse and allow to dry.
Hands
Soap and water, rinse and dry well or if appropriate use
alcohol hand sanitiser.
Antiseptic cleansing agent after contact with infected patients or
materials and prior to aseptic techniques ONLY. See hand
cleansing guidelines in Good Practices Policy.
Humidifiers
Wash daily with soap and water, then rinse and dry before
refilling with sterile water.
In between patient use send to HSDU.
Ice Making Machine
Defrost, wash inside with detergent and hot water, rinse and
dry, weekly.
Infant Incubator
Detergent and hot water, then Hypochlorite solution, 125
ppm available chlorine (one Haztabs tablet in 2.5 litres
water). Hypochlorite 125 ppm.
Important to wipe dry, especially metal parts.
Injection Trays/Kidney
Dishes
Disposable or detergent and hot water daily. Alcohol
impregnated wipes between patients.
Instruments
Autoclave or disposable.
Laryngoscope Blades
Disposable or wash in hot soapy water, rinse and dry. Wipe
blade with alcohol impregnated wipe.
Lavatory Brush
Rinse in last flush and store dry.
Linen
Launder.
Blood contaminated linen and linen from patients with specific
infections (isolation nursing) -
Red linen bag with soluble liner
Faecal contaminated linen -
White bag with red plastic liner
Soiled/dirty linen (including urine stained) -
White bag

ITEM
ROUTINE
ADDITIONAL
RECOMMENDATIONS
Mattress
Detergent in hot water, rinse and dry.
Hypochlorite sanitiser solution after contamination.
Measuring Jug (for use in
dirty utility room only)
Disposable
Medicine Pots
Disposable or detergent in hot water, rinse and dry.
Manual Handling Aids:
Machinery
Fabric Slings belts &
Slides
Detergent in hot water and dry. Launder unless single
patient use.
Must be identified per patient use with ID bracelet
Mops – Domestic
Wash mop head in detergent, rinse and squeeze dry. Store
dry – separate from other mop heads or launder detachable
mop heads daily if service available.
Use different coloured mops and buckets in different areas i.e.:
Green - Kitchen
Red
- Bathrooms
and
Toilets
Blue
- General
Areas
Mops – Isolation
For Domestic and Spillage use.
Yellow/Gold -
Isolation Nursing
Mops – Spillage
After use with disinfectants, rinse well and store dry.
White
Nailbrushes
Use not recommended.
Single use only.
Nappies Disposable.
Nebulisers & Volumatics
Wash daily with detergent in hot water, rinse and dry. For
single patient use only.
Must be stored dry when not in use.
Potties
Empty into sluice. If not available, empty down a toilet,
avoiding splashing. Clean in a designated sink using
disposable paper, detergent and hot water, rinse and store
dry. Ensure handles are also cleaned.
Use for individual patients only. Send to HSDU for disinfection once
no longer required by patient.
Razor – Electric
Should be patients own razor.
Razor – Wet
Disposable type only, unless patients own then wash with
detergent in hot water. Store dry.
Dispose of used blades and disposable razors in sharps disposal
box immediately after use.
Resuscitation Mask
Detergent in hot water. Store dry.
After infected patient dispose of appropriately.
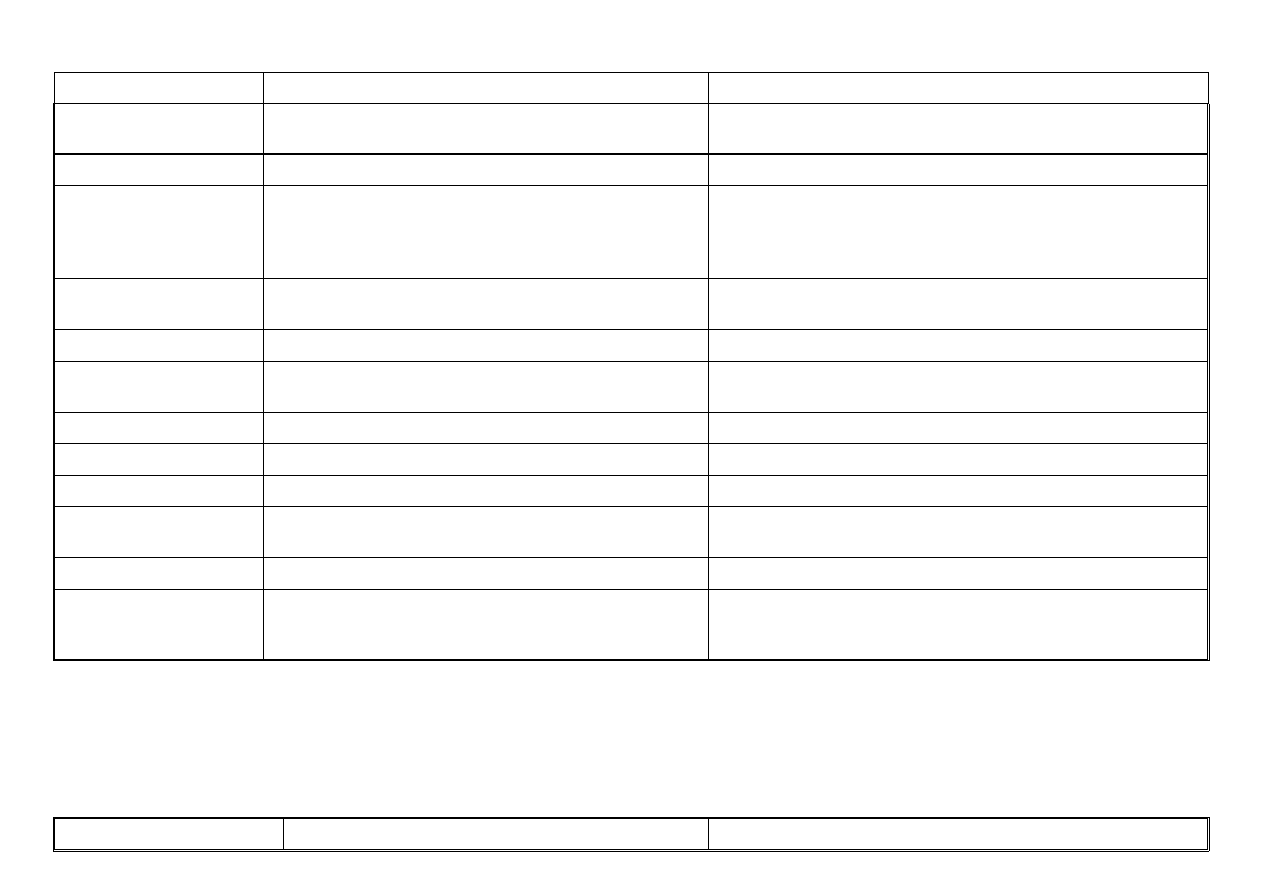
ITEM
ROUTINE
ADDITIONAL
RECOMMENDATIONS
Razor – Wet
Disposable type only, unless patients own then wash with
detergent in hot water. Store dry.
Dispose of used blades and disposable razors in sharps disposal
box immediately after use.
Resuscitation Mask
Detergent in hot water. Store dry.
After infected patient dispose of appropriately.
Rooms
(cleaning after a patient
with a specific infection)
Scrubbing Machines
Detergent/ Hypochlorite sanitiser.
Detergent and hot water (including scouring pads). Store
dry.
After patient with specific infections special arrangements will be
made with the Domestic staff.
Discard pads after potentially or known infected area cleaned.
Sharps Boxes
Wipe any blood contamination with alcohol impregnated
wipe.
Shaving Brushes
Use not recommended. Use aerosol foam.
Shower Cubicle/Chairs
Wash with detergent in hot water, between patients.
After potentially or known infected patient use hypochlorite sanitiser,
rinse and allow to dry.
Stethoscope Heads
Wipe with alcohol impregnated swab.
Suction Jars
Detergent and hot water. Store dry.
Autoclave after use on infected patients.
Thermometer - Electronic
Disposable cover must be disposed of after use.
Tonometer Prisms
Soak in 70% alcohol for 15 minutes between patients and
store dry.
Toothglass
Disposable or dishwater (80
o
c)
Toys
Vinyl/plastic toys wash in hot soapy water, rinse and dry,
weekly or when visibly soiled. Soft toys are not
recommended for general use.
Hospital toys must be washed with detergent and hot water, rinsed
and dried or cleaned immediately on removal from the room of a
child who has been isolated.
ITEM
ROUTINE
ADDITIONAL
RECOMMENDATIONS

Urinals Non
disposable.
Disposable
Place in Bed Pan Washer/Disinfector.
Place directly into macerator.
Ventilators
Send to HSDU.
Ventstream
Wash daily with detergent in hot water, rinse and dry.
Must be returned to HSDU between patients.
Vials
Wipe rubber stopper with alcohol impregnated swab.
Wash Bowls
Detergent in hot water.
Dry thoroughly inside and outside and store dry. Clean
daily with Hypochlorite sanitiser.
Each patient should have an individual wash bowl.
W.C.
At least daily with hypochlorite sanitiser and rinse well
After potentially infected patient use hypochlorite sanitiser and rinse
well at least 4 times daily or after each use.
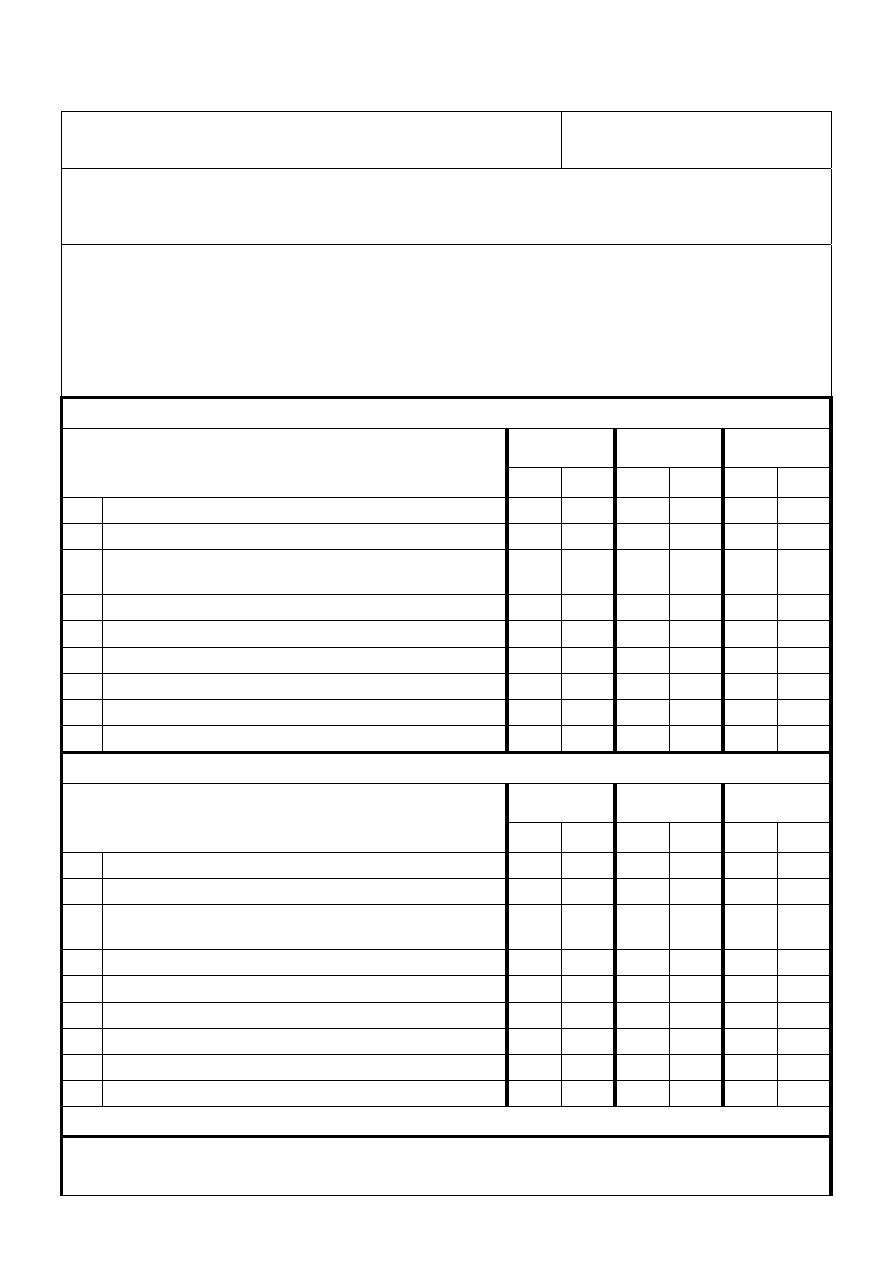
Equality and Human Rights Policy Screening Tool
Policy Title: Cleaning, Disinfection & Sterilisation
Directorate: Nursing & Patient
Care Standards
Name of person/s auditing / authoring policy:
Service Manager Infection Prevention & Control & Infection Prevention & Control Committee
Policy Content:
For each of the following check whether the policy under consideration is sensitive to people of a
different age, ethnicity, gender, disability, religion or belief, and sexual orientation?
The checklist below will help you to identify any strengths and weaknesses of the policy and to
check whether it is compliant with equality legislation.
1. Check for DIRECT discrimination against any minority group of PATIENTS:
Response
Action
required
Resource
implication
Question: Does the policy contain any statements which
may disadvantage people from the following groups?
Yes
No
Yes No Yes No
1.0 Age?
√
√
√
1.1 Gender (Male, Female and Transsexual)?
√
√
√
1.2 Learning Difficulties / Disability or Cognitive
Impairment?
√
√
√
1.3 Mental Health Need?
√
√
√
1.4 Sensory Impairment?
√
√
√
1.5 Physical Disability?
√
√
√
1.6 Race or Ethnicity?
√
√
√
1.7 Religious Belief?
√
√
√
1.8 Sexual Orientation?
√
√
√
2. Check for DIRECT discrimination against any minority group relating to EMPLOYEES:
Response
Action
required
Resource
implication
Question: Does the policy contain any statements which
may disadvantage employees or potential employees from
any of the following groups?
Yes
No
Yes No Yes No
2.0 Age?
√
√
√
2.1 Gender (Male, Female and Transsexual)?
√
√
√
2.2 Learning Difficulties / Disability or Cognitive
Impairment?
√
√
√
2.3 Mental Health Need?
√
√
√
2.4 Sensory Impairment?
√
√
√
2.5 Physical Disability?
√
√
√
2.6 Race or Ethnicity?
√
√
√
2.7 Religious Belief?
√
√
√
2.8 Sexual Orientation?
√
√
√
TOTAL NUMBER OF ITEMS ANSWERED ‘YES’ INDICATING DIRECT DISCRIMINATION = 0
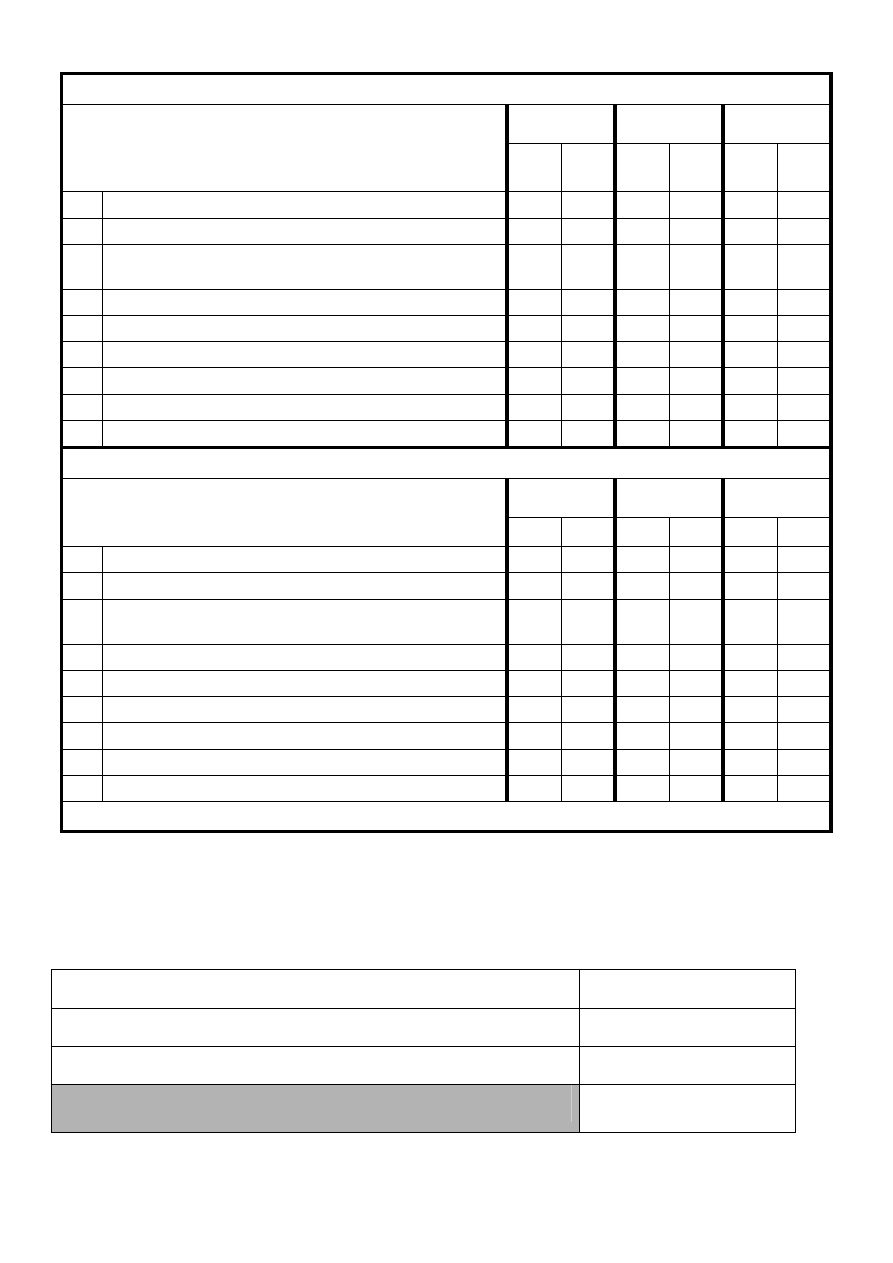
3. Check for INDIRECT discrimination against any minority group of PATIENTS:
Response
Action
required
Resource
implication
Question: Does the policy contain any conditions or
requirements which are applied equally to everyone, but
disadvantage particular people because they cannot
comply due to:
Yes
No
Yes No Yes No
3.0 Age?
√
√
√
3.1 Gender (Male, Female and Transsexual)?
√
√
√
3.2 Learning Difficulties / Disability or Cognitive
Impairment?
√
√
√
3.3 Mental Health Need?
√
√
√
3.4 Sensory Impairment?
√
√
√
3.5 Physical Disability?
√
√
√
3.6 Race or Ethnicity?
√
√
√
3.7 Religious, Spiritual belief (including other belief)?
√
√
√
3.8 Sexual Orientation?
√
√
√
4. Check for INDIRECT discrimination against any minority group relating to EMPLOYEES:
Response
Action
required
Resource
implication
Question: Does the policy contain any statements which
may disadvantage employees or potential employees from
any of the following groups?
Yes
No
Yes No Yes No
4.0 Age?
√
√
√
4.1 Gender (Male, Female and Transsexual)?
√
√
√
4.2 Learning Difficulties / Disability or Cognitive
Impairment?
√
√
√
4.3 Mental Health Need?
√
√
√
4.4 Sensory Impairment?
√
√
√
4.5 Physical Disability?
√
√
√
4.6 Race or Ethnicity?
√
√
√
4.7 Religious, Spiritual belief (including other belief)?
√
√
√
4.8 Sexual Orientation?
√
√
√
TOTAL NUMBER OF ITEMS ANSWERED ‘YES’ INDICATING INDIRECT DISCRIMINATION = 0
Signatures of authors / auditors:
Chris McGinley Service Manager Infection Prevention & Control
Dr Alan Wills DIPC, on behalf of the IPCC Date: 06.2009
Equality and Human Rights Compliance / Percentage Calculation
Number of ‘Yes’ answers for DIRECT discrimination.
0
Number of ‘Yes’ for INDIRECT discrimination.
0
Total answers for POLICY CONTENTS discrimination.
0
Percentage content non compliant
= 0%
Document Outline
- CLEANING, DISINFECTION & STERILISATION
- CONTENTS
- Legislation, Guidance and References 10
- Appendices
- II Quarantine of Surgical Instruments
- III Disinfection Methods
- DISINFECTION METHODS APPENDIX III
- Equality and Human Rights Compliance / Percentage Calculation
Wyszukiwarka
Podobne podstrony:
Disinfection and Sterilization in Health Care Facilities
8Sterilization, High Level Disinfection, and Environmental Cleaning
Best Practice Guidelines Cleaning Disinfection Sterilization Medical Devices
Monetary and Fiscal Policy Quick Overview of the U S ?on
Persson Tabellini Constitutions and Economic Policy
Bubbles and Monetary Policy Roubini
Kwiek, Marek The Changing Attractiveness of European Higher Education Current Developments, Future
Contamination, Disinfection, and Cross Colonization o infekcjach
Common Security and Defense Policy (CSDP) Is the EU ready to take action
Canadian current health care guidelines Hand Washing, Cleaning,Disinfection
Mancuso Roman Military and Diplomatic Policy in the East
dyncorp iraq civpol media relations and confidentiality policy 2007
IQ and Immigration Policy Jason Richwine (2009)
5year cleaning and disinfection eng OK
Cleaning and Disinfection in Endoscopy
Cleaning and Disinfection Protocol for Incubators
5year cleaning and disinfection eng OK
więcej podobnych podstron