
28
Osmotic Dehydration of Fruits
and Vegetables
Piotr P. Lewicki and Andrzej Lenart
CONTENTS
28.1 INTRODUCTION
Water as a main constituent of most foods affects
food stability, microbial as well as chemical, and is
responsible for the consumer perception of many
organoleptic attributes, i.e.,
juiciness,
elasticity,
tenderness, and texture. It is generally accepted that
it is not the quantity of water in food but its thermo-
dynamic state that is responsible for its influence on
food stability and texture. The thermodynamic state
of water in food is expressed by its activity, which is 0
for absolutely dry material and 1 for pure water. The
lower the water activity the more stable is the food,
and the texture changes from juicy and elastic to
brittle and crunchy.
The lowering of water activity can be achieved in
two ways, either by addition of humectants or by
removal of solvent (i.e., water). The first way is mostly
unacceptable by consumers as it needs large amounts
of sodium chloride, sugars, or polyols to be added to
food. Moreover, this way is limited by nutritional
and toxicological restraints. The other way is energy
intensive, hence the final product is expensive.
The use of osmosis allows both ways of decreasing
water activity in food to be applied simultaneously.
The permeability of plant tissue is low to sugars and
high molecular weight compounds; hence, the mater-
ial is impregnated with the osmoactive substance in
the surface layers only. Water, on the other hand, is
removed by osmosis and the cell sap is concentrated
without a phase transition of the solvent. This makes
the process favorable from the energetic point of
view. The flux of water is much larger than the coun-
tercurrent flux of osmoactive substance. For this
reason the process is called osmotic dehydration or
osmotic dewatering.
ß
2006 by Taylor & Francis Group, LLC.
The food pro duced by this method has many
advantag eou s feat ures:
.
It is ready to eat and rehydrat ion is not need ed.
.
The amount of osmoact ive substa nce penetra t-
ing the tissue can be adjust ed to indivi dual re-
quirem ents.
.
The chemical co mposition of the food can be
regula ted acco rding to needs.
.
The mass of the raw mate rial is reduce d, usually
by half.
The os motic deh ydration does not reduce wat er ac-
tivity suffici ently to hinder the proliferation of micr o-
organis ms. The process extends , to some degree, the
shelf life of the mate rial, but it does not preser ve it.
Hence, the app lication of othe r preser vation methods ,
such as freez ing, pa steurizat ion, or drying is necessa ry.
Howev er, pro cessing of os motically dehydrat ed semi -
produc ts is much less ex pensive an d preserves most of
the charact eristics acqu ired dur ing the osmosi s.
28.2 THE NATURE OF OSMOTIC
DEWATERING
28.2.1 O
SMOTIC
P
RESSUR E
The therm odynami c stat e of wat er in solut ion is char-
acterized by wat er intera ctions with solut e. Becau se
each molec ule has its internal e nergy an d inter action s
also need energy e ach substa nce of the solut ion is in
the defi ned en ergetic stat e. This state referred to one
mole of the substa nce is called the chemi cal poten tial.
Chem ical potenti al is a function of concentra tion,
tempe rature, and pressur e. Un der isot hermal co ndi-
tions it is solely determ ined by conce ntration and
pressur e. The increa se of solut e concentra tion de -
crease s the ch emical poten tial of a solvent , which
can also be express ed by its a ctivity acco rding to the
followi ng relat ionshi p:
m
w
¼ m
ow
þ RT ln a
w
(28 : 1)
where m
w
—chemi cal potenti al of water
m
ow
—chemic al potenti al in a standar d stat e
R—gas constant
T—absol ute temperatur e
a
w
—wa ter acti vity coeffici ent
The inter actio n of two systems in different ene rgy
states is manif ested by the energy excha nge. Thi s
exchange proceed s until the equilibrium state is
achieved, which is the state in which chemical potentials
of tw o systems are the same.
The equilibrium state, under isothermal conditio n,
can be achieve d by the change of eithe r co ncentra tion
or pressure. Excess pressure needed to reach the state of
equilibrium between pure solvent and a solution is
called osmotic pressure and is expressed by the formula:
P
¼
RT
V
ln a
w
(28 : 2)
where V is molar volume of wat er.
As water is the solvent in food s, the above equ a-
tion can be sim plified to
P
¼ 4: 6063 10
5
T ln a
w
(28 : 3)
Osm otic pressur e is related to molar mass of the
solute; the smaller the mass , the high er will be the
pressur e at the same concen tration. Electr olytes show
higher osmot ic pressur e than nonelect rolytes because
each ion affects the chemi cal poten tial of a solvent.
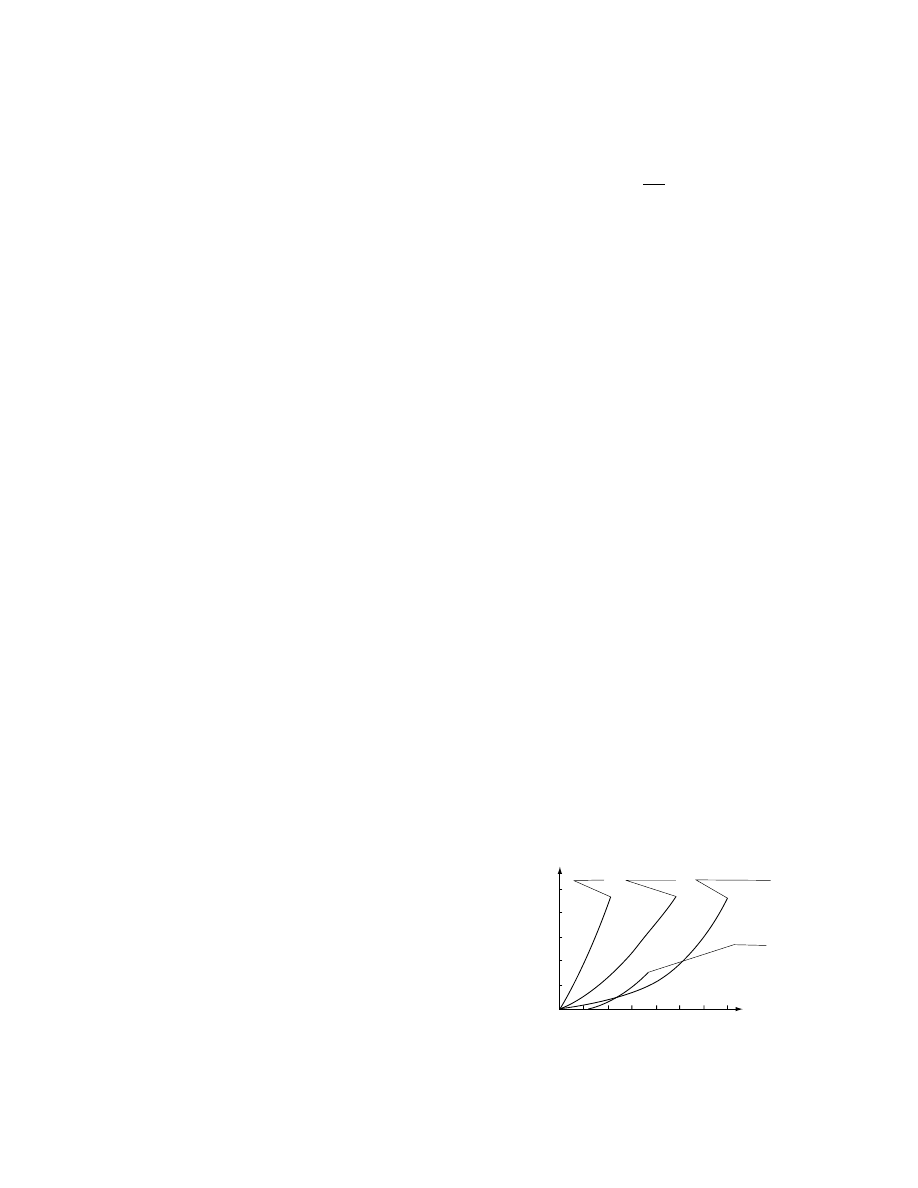
Rel ationsh ip betw een co ncentra tion and osmot ic
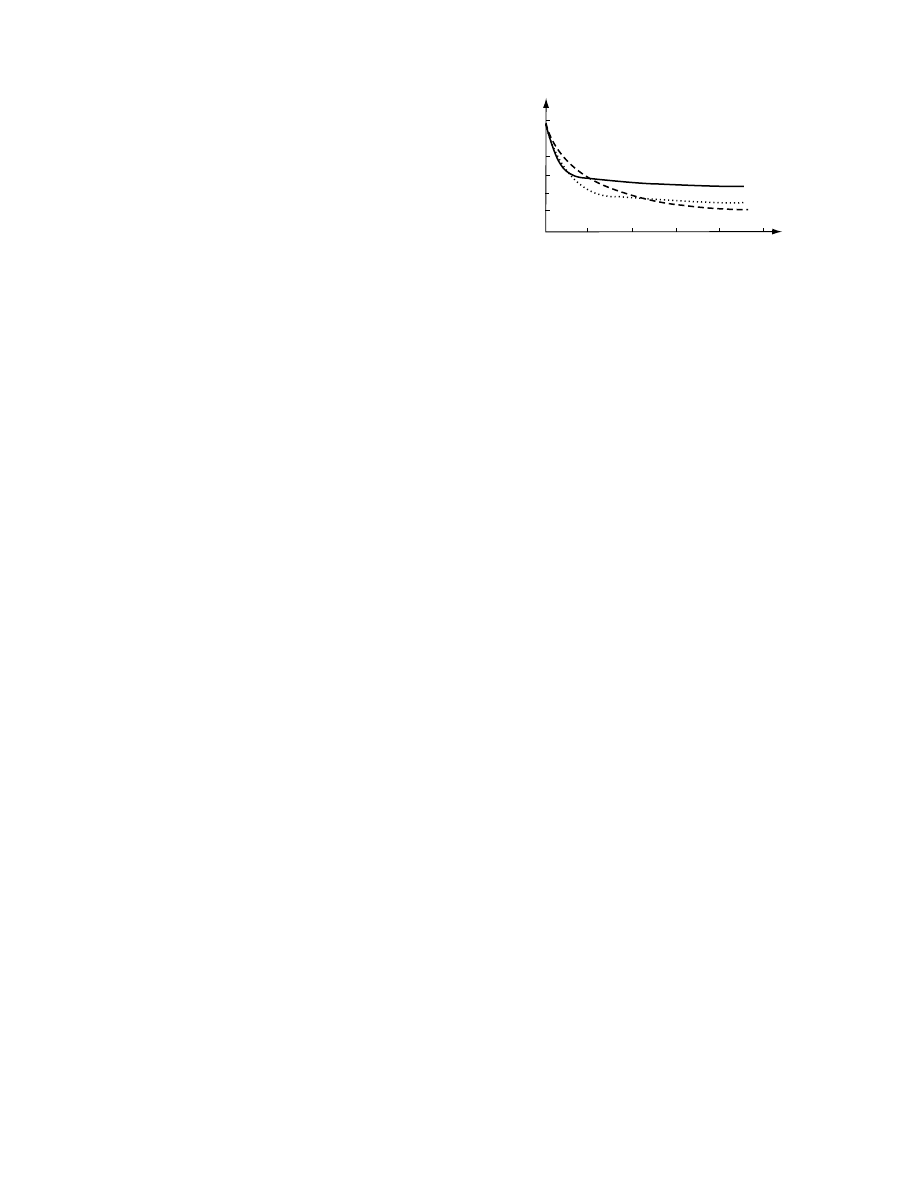
pressur e is shown in Figure 28.1. Osmoti c pr essure
has an inhibi tory effe ct on microo rganisms. Mo st
bacter ia, yeasts, an d mo ulds do not proli ferate a t
P >
12.7 MPa , P > 17.3 M Pa, and P > 30.1 M PA,
respectivel y. Hence, the shelf life of foods can be
regula ted by the osmot ic pressur e of the solut ion in
the material.
Difference in osmotic pressure of two systems is a
motive power for mass transfer, if the systems are
separated by a semipermeable membrane, i.e., the
membrane is permeable to solvent and impermeable
to solute molecules. This phenomenon is utilized in
osmotic dewatering of fruits and vegetables.
28.2.2 T
HE
S
TRUCTURE OF
P
LANT
T
ISSUE
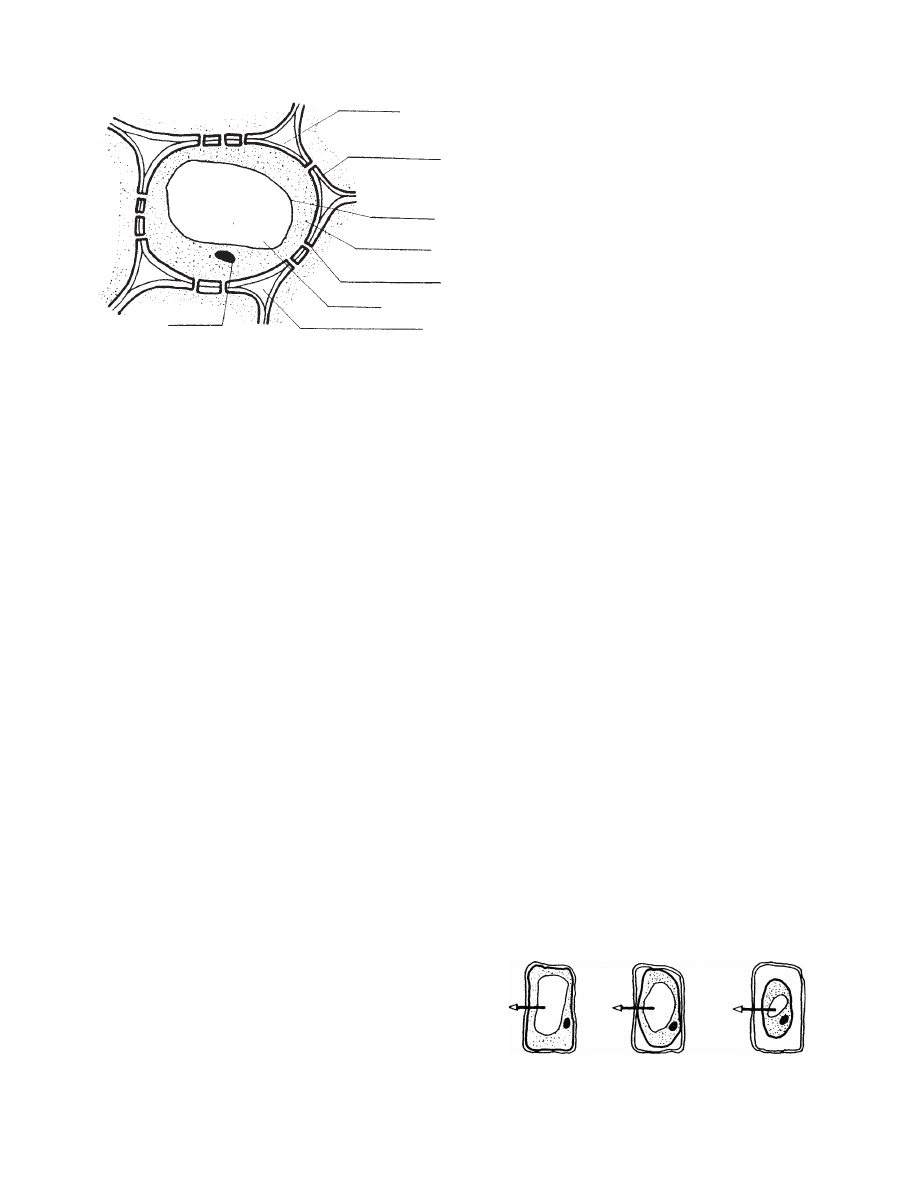
A plant cell can be simply pictured as a unit consisting
of two main components: the cell wall and the proto-
plast (
) . The cell wall is permea ble to wat er
and low molecular weight compounds and is not a
10
20 30
40 50 60 70
Concentration, %
5
10
15
20
25
NaCI
Glycerol
Saccharose
Milk
Osmotic pressure, MPa
FIGURE 28.1 Relationship between concentration and
osmotic pressure of solution.
ß
2006 by Taylor & Francis Group, LLC.
barrier in solute transport from and to the cell. The cell
wall is perforated and the channels are filled with thin
strands of protoplasm, assuring the contact between
protoplasts of neighboring cells. These strands of
protoplasm are called plasmodesmata. The diameter
of the strands is 20–70 nm and the average contact
area can be estimated as 0.2 m
2
/m
2
of the cell wall [1].
The protoplast is composed of protoplasm enclosed
in a membrane called plasmalemma, vacuoles, and
other structural elements such as the nucleus, plastids,
and so on. The plasmalemma is a protein–lipid layer
that regulates the contact between the protoplast
and the environment. It is 7.5–10 nm thick [2], per-
meable to water, and selectively permeable to other
substances. Protoplasm is a colloidal solution of pro-
teins and lipoproteins in water. The vacuole is sus-
pended in protoplasm and is enclosed in a membrane
called the tonoplast. It contains a solution of minerals,
sugars, and other organic compounds in water.
Most cells have dimensions between 10 and 100
m
m. Depending on their function they are loosely or
closely packed in a tissue. Cells are highly specialized.
A group of cells that is designated to play a special
role in a plant is called tissue.
In general, three types of tissues are recognized.
Epidermal tissue forms the outermost layer of cells
that are thick walled and covered, in many cases, with
cuticle containing a waxy substance known as cutin.
Parenchymatous tissue forms the essential part of
organs and serves to produce and store nutritional
substances. The cells are predominantly large, thin
walled, and highly vacuolated. Usually, parenchyma
cells are loosely arranged in the tissue and intercellu-
lar spaces are formed. The volume of intercellular
spaces depends on the kind of plant and its part.
Leaves have large intercellular spaces whereas roots
are little porous. For example, intercellular spaces in
potato tuber occupy about 1% of total volume,
whereas in apple fruit this volume is as large as 20%.
Intercellular spaces form a continuous system of
channels that is filled with air.
A particular type of tissue is the vascular one. It
contains xylem and phloem, which form bundles.
Xylem is present in elongated cells with perforated
end walls that no longer contain viable protoplasm.
Xylem, in other words, forms open dead vessels that
provide a way of transportation for minerals and
water from roots to other parts of a plant. Phloem is
present in elongated viable cells that have sieve end
plates. Phloem translocates a solution of sugars,
amino acids, and other nutritious substances.
A solution in a vacuole has an osmotic pressure
that pushes protoplasm and plasmalemma toward the
cell wall. The protoplast is tightly pressed to the cell
wall and the cell is in a turgor state. The difference
between the osmotic pressure in the cell and in its
surroundings is called the turgor pressure.
28.2.3 M
ASS
T
RANSFER IN
O
SMOTIC
P
ROCESS
If the cell and the surroundings have the same os-
motic pressure then turgor pressure is zero and the
system is in thermodynamic equilibrium. Osmotic
pressure of the surroundings lower than that of the
cell causes transfer of water into the cell. The cell
swells, but the rigid cell wall limits the extent of
swelling. A cell placed in a hypertonic solution
(osmotic pressure higher than that of the cell) will
lose water. The dehydration of a protoplast causes
decrease of its volume and, in consequence, detach-
ment of plasmalemma from the cell wall. This process
is called plasmolysis (Figure 28.3). As the cell wall is
permeable the volume between the cell wall and
plasmalemma fills with the hypertonic solution.
Osmotic dehydration occurs on a piece of material
and not on a single cell. Hence, it should be assumed
that the piece exists in all kinds of plant tissue. As a
rule, a skin is removed from the raw material; there-
fore, epidermal cells and cuticle are absent in most
cases. A piece of fruit or vegetable thus will contain
parenchymatous and vascular tissue and intercellular
spaces, as well.
Cell wall
Plasmalemma
Tonoplast
Protoplasm
Plasmodesmata
Vacuole
Intercellular space
Nucleus
FIGURE 28.2 Plant cell (simplified).
Flux
of water
FIGURE 28.3 Plasmolysis.
ß
2006 by Taylor & Francis Group, LLC.
Fr om the process point of view, a plant material
can be co nsider ed as a cap illary-p orous body that is
divide d inter nally in num erous repeat ing units . Some
capillari es an d por es are filled wi th a solution ,
wherea s others are empty (i.e., contai n air). M ost
capillari es an d pores are ope n. Repeat ing units can
exchange water betwe en ea ch oth er.
The inter nal structure of a body is not a hom oge-
neous one as far as trans port of water is co nsider ed.
Cell walls are built from microfi brils, and intermi-
crofibri llar spaces are some 10 nm in cross section
[3]. These spaces are large enough to allow water,
ions, an d small molec ules to pass through them. As
cell wal ls are interconn ected in the tissue, a c ontinu-
ous matr ix c apable of transp orting wat er and smal l
molec ules is formed. Thi s continuum is call ed the
apoplast . In a major ity of cell s, protopl asm of neigh-
boring cell s is intercon nected through plasm odesmata
and another continuou s ne twork is form ed. The sys-
tem of protopl asts and connec ting plasmo desmata
is wi dely known as sympl ast. Becau se plasmod es-
mata permi t the passage of solut es [1], they un-
doubted ly permi t the pa ssage of water also. The
apoplast an d sympl ast as networks capable of trans -
portin g water are separat ed from each other by plas-
malemma. Eac h vacu ole is enclosed in tonoplas t and
they are not inter connected. Hen ce, vacuoles form a
discont inuity in the syst em.
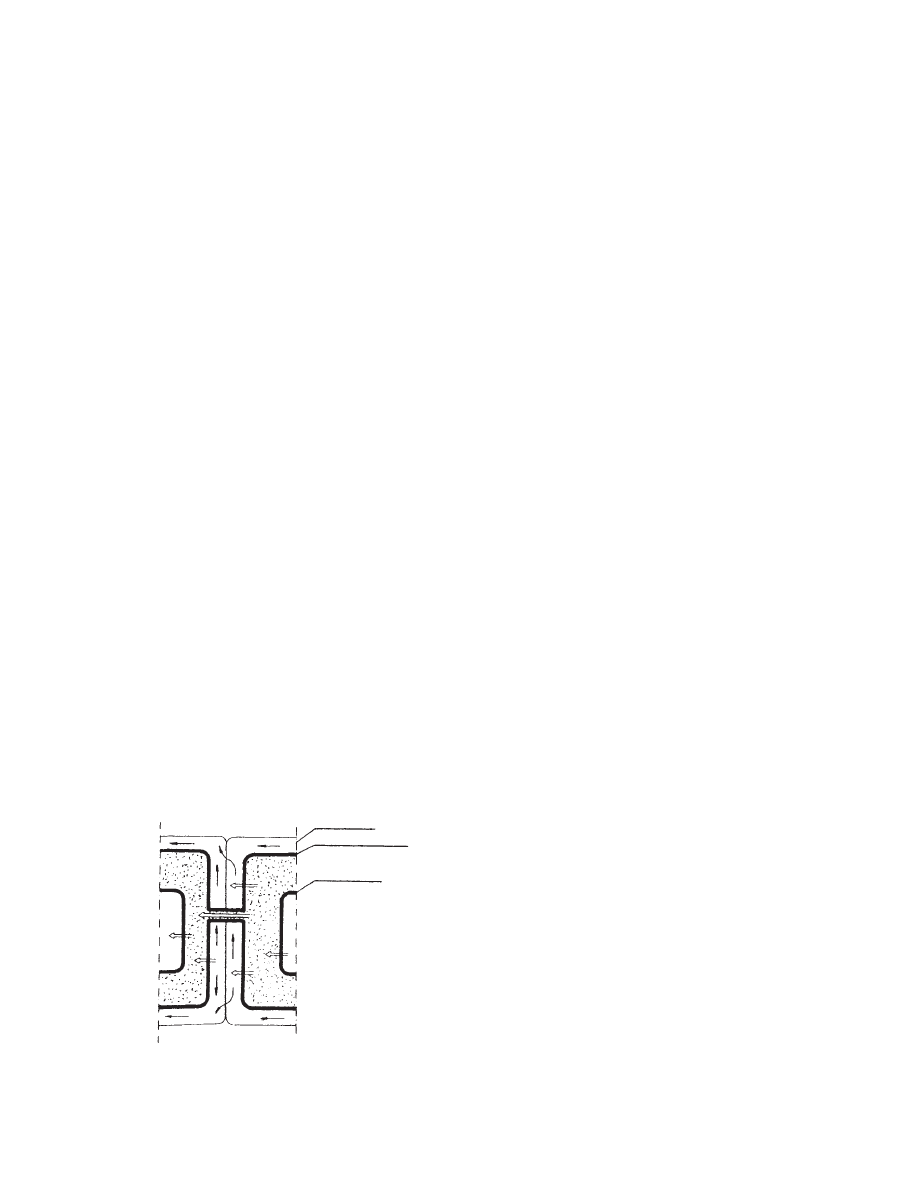
Two ways of wat er trans port in a plant have been
recogni zed: apoplasm ic and symplasmi c (F igure
28.4). It is general ly agreed that the cell walls prov ide
the major pa thway of water movem ent in plant ma-
terial. The rati o of volume flows in the apoplasm ic
and sympl asmic (vacuo le-to-va cuole) pa thways is of
the order of 50:1 in leaf tissue [1]. For the root cortex ,
the rati o is lower.
The capillary and porous system of the body exists
in vascular tissue and intercellular spaces. Xylem forms
an open conduit of relatively low hydraulic resistance
that is filled with diluted mineral solution. Phloem
exists in cells with a width ranging from 10 to 70 mm
and a length from 100 to 500 mm in dicotyledons [4].
Their turgor is around 2 MPa (beetroot is 1.83 MPa)
with a pressure gradient of 0.02–0.03 MPa/m [5,6]. As
phloem transports substances of very different molecu-
lar weight, shape, charge, and surface activity along
with water, it is presumed that the mechanism is an
osmotically driven solution flow [6].
The intercellular system of channels has the vol-
ume dependent on the kind of tissue. In potatoes, it
occupies 1–3%, whereas in beetroot 25% of volume is
attributed to cell walls and intercellular spaces [7].
There is no doubt that all these structures of the
transport system in the plant tissue will participate in
the process of osmotic dehydration.
Contacting plant tissue with the hypertonic solu-
tion, a sequence of mass transfer processes can be
envisa ged (
) as follows :
.
In intercellular spaces a capillary suction will
occur. The channels will fill in with the hyper-
tonic solution and the gas phase will be com-
pressed or pushed out until the equilibrium state
will be achieved.
.
Xylem and phloem containing solutions of
lower osmotic pressure than the hypertonic so-
lution will be penetrated by the osmoactive sub-
stance by diffusion. Osmotic pressure flow can
also take place.
.
Cell walls in contact with hypertonic solution
will lose water due to diffusion and osmotic
flow. Osmoactive substance will penetrate cell
walls by diffusion.
.
Change of osmotic pressure in xylem and
phloem and the dewatering of the cell walls
will initiate the symplasmic movement of water
in the material. The dehydration of the cells will
take place and plasmolysis will be induced.
The sequence presented above also suggests the
kinetics of the osmotic dehydration process. As long
as the mass transfer processes are not strongly depen-
dent on the symplasmic pathway, the water flux will
predominate over the osmoactive substance flux. This
is due to osmotic pressure flow, which will reduce the
countercurrent diffusion of osmoactive substance but
it will not strongly affect the diffusive flux of water as
the self-diffusion of water in a solution is of the same
order of magnitude as that for solute. When plas-
molysis occurs and the hypertonic solution fills in
the volume between cell walls and plasmalemma, the
π
1
π
1
>
π
2
π
2
Cell wall
Plasmalemma
Tonoplast
FIGURE 28.4 Apoplasmic and symplasmic transport
of water.
ß
2006 by Taylor & Francis Group, LLC.
process of dewatering will be substituted by the
impregnation of the tissue. The flux of osmoactive
substance will be equal to or will surpass the flux
of water.
The above picture of the mechanism of osmotic
dehydration suggests that the plasmalemma resist-
ance to mass transfer affects the process to only a
small extent. The process will be rather dependent
on the internal resistance to osmotic flow and apo-
plast dewatering and, to some extent, on external
resistance to mass transfer.
28.2.4 M
ODELING THE
O
SMOTIC
D
EHYDRATION
P
ROCESS
From the previous description of the structure of the
plant material and processes that can be involved in
the mass transfer between plant tissue and the os-
motic solution, it is evident that the modeling of
osmotic dehydration is not simple.
Models have been developed that describe the
behavior of the plant tissue under normal growing
conditions, i.e., when the osmotic pressure in the
tissue is higher than that in the surroundings and
the plant cell is in the turgor state [8–14]. They shed
some light on the possible ways the water and solute
molecules move in a plant tissue during osmotic
dewatering, and that was presented in previous chap-
ter. Moreover, the models were used, in some cases,
to quantify the osmotic dewatering process.
In the last five years a lot of work has been done
on modeling the osmotic dehydration process.
Mostly, the theory of molecular diffusion in the
solid has been used to predict the water loss during
the process. An unsteady unidirectional diffusion de-
scribed by the second Fick’s equation was used to
quantify the process by the effective diffusivity. The
resulting diffusivity is generally correlated with the
concentration and the temperature of the hypertonic
solution [15–28].
The models based on the second Fick’s equation
do not necessarily simulate the osmotic dehydration
process. In this process countercurrent fluxes of water
and the osmoactive substance occur. Moreover, a flux
of soluble solids from the tissue accompanies a flux of
water (Figure 28.6). Hence, there is a simultaneous
mass transfer and probable interactions between
flows cannot be taken into account. The estimated
effective diffusivities are affected by the countercur-
rent flows and they cannot be used to predict the
contribution of each flux to the process. Moreover,
in these models the resistance at the surface of the
Vacuole
Tonoplast
Intercellular space
Air
Plasmalemma
Cell wall
Mass transfer
surface
Hypertonic solution
Cell sap
Vascular
tissue
FIGURE 28.5 Possible ways of transport of water in a plant tissue during osmotic dehydration.
Hypertonic
solution
Water
Material
Osmoactive
substance
Natural
solubles
FIGURE 28.6 Mass transfer in osmotic dehydration.
ß
2006 by Taylor & Francis Group, LLC.

solid is assumed to be negligible, thus the whole re-
sistance to mass transfer is in the solid. Last, the
models do not take into account the possible effect
of the living cell on the mass transfer process.
A pseudo-diffusion approach [29] to model mass
transfer in osmotic dewatering showed that effective
diffusivity was not a unique function of Fourier num-
ber, as it would be expected in pure diffusion. The
developed model had no predictive ability. On the other
hand, experiments done on the frozen apple tissue
showed that mass transfer was only diffusive [30].
Besides all the limits and contradictory results, the
models based on the second Fick’s equation proved to
be quite successful. Hawkes and Flink plotted the
normalized solids content of apple versus the square
root of time and obtained a straight line, the slope of
which was called the mass transfer coefficient [15]. This
approach was used in numerous works [16,31–38].
A model was developed based on the irreversible
process thermodynamics in which the cell membrane
characteristics, the cell volume changes, tissue shrink-
age, internal volumetric rearrangements, and diffu-
sion of nonpermeating and permeating species are
taken into account [39,40]. The set of equations
solved numerically showed the model as satisfactorily
representing the behavior of parenchymatous storage
tissue undergoing osmotic dehydration. Moreover,
the simulations have shown that the cell membrane
represents the major resistance to mass transfer in
such systems. The model needed simultaneous adjust-
ment of four constants to obtain a good fit, hence its
practical usefulness is rather questionable.
Marcotte et al. improved Toupin’s model by giv-
ing a closer thermodynamic description of forces in-
volved in the osmotic dehydration process [41]. The
transmembrane transport is modeled on the basis of
irreversible thermodynamics whereas transport in the
intercellular space is modeled by relations derived
from the second Fick’s equation.
Further development of this model incorporating
diffusion, bulk flow, transmembrane flux, and matrix
shrinkage [42–44] showed that the cell membrane is
the main barrier to mass transfer only for single cells
or thin slices of tissue. When the thickness of the
sample increases, the extracellular space may become
the limiting factor [45].
The models based on the irreversible process
thermodynamics show that the cell membrane (plas-
malemma) represents the major resistance to mass
transfer. This is contradicted by findings of Raoult-
Wack et al. [46–48], who showed that membranes are
not necessary for osmotic dehydration and merely
diffusive properties of the material are responsible
for high water flux with only marginal sugar penetra-
tion. These authors suggest the following mechanism.
At the beginning of the process the removal of
water concentrates the superficial layer of solute
in the surface of the material. This layer is detri-
mental to further solute incorporation but is favor-
able to water removal as it creates a pronounced
concentration gradient [48–50]. The compartmental
model was developed that provided good fit for
the different situations tested. The solution of the
set of differential equations was done by numerical
methods.
The role of intercellular space and capillary flow
in osmotic dehydration was well documented by Fito
et al. [51–53]. On this basis the nondiffusional mass
transfer model was developed incorporating hydro-
dynamic mechanism (HDM). Studies done by the
same group [19,54] showed that long time of osmotic
process is needed to obtain a fully developed water
and sugar concentration profiles. A model based on
the advancing disturbance front (ADF) was proposed
that allows prediction of sample concentration during
osmotic dehydration.
The above models of osmotic dewatering were
developed taking the processes into account that
take place in plant tissue when the tissue is contacted
with hypertonic solution. Empirical models are also
proposed in the literature.
Mass transport during osmotic dehydration was
described by the first order kinetics in which the
rate constant is a function of main process parameters
[55] or by an empirical equation with two para-
meters, which correspond to the initial rate of mass
transfer and to equilibrium conditions, respectively
[56]. A kinetic model based on the theory of the
decreasing nucleus was also proposed to describe the
osmotic process [57].
Phenomenological or empirical models describing
osmotic dehydration are of limited use. The phenom-
enological models try to explain processes taking
place in plant tissue during osmotic dewatering. Be-
cause of that they are general and do not account for
individual response of the tissue to osmotic stress.
And a response of cells to osmotic stress depends on
the origin and morphology of plant tissue [58]. On the
other hand, empirical models are developed for the
investigated product and treatment conditions, and
they cannot be used to model the process in general.
A lot of work has been done to understand osmotic
dewatering of food products. The research done on
micro- as well as macroscopic scale presents fairly
well processes occurring in plant tissue during os-
motic dewatering. However, the great variability of
plant tissue structure and its response to osmotic
stress makes it difficult to control the main variables
of the process. Hence, current technologies are still
somewhat empirical.
ß
2006 by Taylor & Francis Group, LLC.

28.3 DESIGN OF OSMOTIC DEHYDRATION
Osmotic dehydration is a versatile process, which
makes it possible to produce a variety of products
based on the same raw material. By a proper choice
of process parameters, a wide range of products can
be obtained starting with highly dehydrated and low
solute infiltrated products and ending with highly
impregnated and little dehydrated products. If hyper-
tonic solution contains other solutes the chemical
composition of the final product can be formulated,
according to the request [59]. Hence, the design of
the osmotic process depends on the expected quality
attributes of the final product.
28.3.1 P
REDEHYDRATION
T
REATMENT
Fruits and vegetables undergoing processing come
from different parts of a plant. They are roots (car-
rots, parsley, beetroots), stems (kohlrabi, potatoes),
shoots (asparagus, onions), leaves (cabbages, spin-
ach), flowers (cauliflower, broccoli), fruits (tomatoes,
cucumbers, pumpkins, apples, pears, plums, green
bean), and seeds (green peas, beans) and must be
appropriately prepared for the osmotic process.
The epidermal tissue has very low permeability for
water and solutes, hence the skin must be removed
before osmotic treatment. In the case of small fruits
such as berries and grapes the skin permeability must
be increased. It can be done by treatment in NaOH
solution containing ethyl oleate. It proved to be
effective for tomato [60] and strawberries [61].
Most of fruits and vegetables are cut into pieces
before they are contacted with hypertonic solution.
Shape and size of the material pronouncedly affect
the rate of the process. Osmosed fruits and vegetables
have different forms that come from the technology
and consumer requirements [62,63]. Plums were de-
hydrated in whole or in halves [64]; apples were
cutinto 12 segments [65,66] or sliced into 3-mm slices
[67] or 3–4 mm thick [15]. Peaches were cut into 6 or 8
segments and pears into 8 segments [65]. Carrots were
cut into cubes of 5 or 10 mm [68,69]. Potatoes were
sliced 5 and 10 mm thick [70], or diced [20]. Papaya
was cut into cubes.
Lenart and Lewicki have shown that the thickness
of the material should not exceed 10 mm [71,72].
Taking into account further processing following os-
motic dehydration and use of the product, they con-
sidered a cube with a side dimension close to 10 mm
as an optimal size and shape for most materials.
Lewicki et al. [73–77] and Lerici et al. [78] dehydrated
apples, carrots, and potatoes as cubes of 8–10 mm on
a side. Flink, as well as Simal et al. [20], likewise used
this shape in most of his studies [79].
Blanching of fruits and vegetables before osmotic
treatment strongly affects the course of the process.
Blanching of carrots and potatoes reduces water loss
and increases solids gain [73,77,80]. Hence, its effect is
detrimental to osmotic dehydration of these materials.
Steam or microwave blanching of strawberries affected
volatile profile of the product inhibiting formation of
esters of furanones [81]. Steam blanching gave better
results than microwave treatment [82,83]. Blanching of
apple pieces either by high temperature short time
(HTST) or low temperature long time (LTLT) process
resulted in softening of tissue [84] and adversely affected
quality of the dried apricots [85].
It has been reported that immersion of some ma-
terials in CaCl
2
solution prior to osmotic dehydration
affects the properties of the product. Texture of apple
was improved [84]. Brining of cashew apple in NaCl
solution before osmotic dehydration resulted in fir-
mer texture of the candied product [86].
Immersion of cut material in ascorbic acid [87] or
citric acid [88] is used to prevent browning of the tissue.
The effect of high pressure on osmotic dewatering
was studied by Rastogi et al. [89,90]. It was shown
that the treatment has a damaging effect on tissue and
results in higher permeability to water and solutes.
The process of osmotic dehydration is facilitated by
high-pressure pretreatment.
High-intensity electric field pulses accelerated os-
motic dehydration of carrot [18]. A Fickian diffusion
coefficient for water and solute increased exponen-
tially with electric field strength. This effect was at-
tributed to increased cell wall permeability, which was
also manifested by the softening of product.
Most of the above-described treatments are aimed at
increasing permeability of plant tissue and facilitating
the osmotic dehydration. Damage to the tissue structure
results in increased permeability to water as well as to
solute. Hence, the pretreatment has no selective effect
and faster water removal is accompanied by greater
infiltration of solute into osmosed material. In many
cases, it is desirable to reduce infiltration of solute into
tissue as much as possible to obtain dehydrated product
with little or no change in its chemical composition.
The influx of osmoactive substance into the tissue
can be hindered by special pretreatment or by artifi-
cial semipermeable membranes. It was shown that
convective drying of apple cubes for time as short as
10 min forms a type of skin on the surface which does
not affect the flux of water but reduces the flux of
sugar during further osmotic dehydration [91]. Artifi-
cial semipermeable membranes are formed from pec-
tins or starch [91–95], alginate or low-methoxyl pectin
[96]. Coatings significantly reduce solute incorpor-
ation into tissue and result in increased weight loss
during osmotic treatment.
ß
2006 by Taylor & Francis Group, LLC.
28.3.2 O
SMOACTIVE
S
UBSTANCE
Osmoactive substances used in food must comply
with special requirements. They have to be edible
with accepted taste and flavor, nontoxic, inert to
food components, and if possible, highly osmoactive.
Quality of the final product was the main aim of
most experiments testing the suitability of different
osmoactive substances. Their technological applicabil-
ity is estimated on water loss rate and final water con-
tent in the material. Usually, saturated solutions, or the
solutions at the same concentrations, are compared.
Flink [97] used two criteria to rate different osmoactive
substances: water loss and the amount of the substance
penetrating the material that is osmosed. Lowering of
water activity in the material was also used as an
indicator of suitability of an osmoactive substance [98].
Solutions of sugars are mostly used to dehydrate
fruits; and glycerol, starch syrup, and sodium chlo-
ride are used for vegetables [62,73,91,99]. Sucrose is
the most frequently used substance [17,65,100–104].
The control of pH of sucrose solution is recom-
mended for banana slices osmotic dehydration [105].
It was also shown that control of pH of sucrose
solution affects the course of osmotic dehydration of
apple and carrot [106]. Addition of ascorbic acid to
sugar solution is practiced to minimize browning of
fruit pieces during osmotic process [72]. Sucrose can
be substituted in part by lactose [15].
Glucose and fructose give a similar dehydration
effect [107,108]. In other publications it is reported
that fructose increases the dry matter content by 50%
as compared with sucrose. Water activity of the final
product was also lower with fructose as a hypertonic
solution [109]. In apple, banana, and kiwifruit, glu-
cose caused higher water loss and solids gain than
sucrose [102]. Starch syrup makes it possible to have
similar final water content in dehydrated material as
that obtained with sucrose but at a much lower influx
of osmoactive substance into tissue [87,101,110]. The
dextrose equivalent of the syrup strongly affected the
ratio between water loss and solids gain. Corn syrup
solids [111], cane sugar syrup [112], palm sugar syrup
[113], and hydrolyzed lactose syrup [114] were also
used in osmotic dehydration of fruits and vegetables.
The effect of the kind of osmoactive substance on the
water content of osmosed material is presented in
Figure 28.7 [115].
Mixtures of osmoactive substances are also used.
Maltini et al. [116] used sucrose and starch syrup in
a ratio of 1:1. Lerici et al. [117] dehydrated apples in a
solution containing 42% fructose, 52% sucrose, 3%
maltose, 3% polysaccharides, and 0.5% sodium chlor-
ide in dry matter. Mastrocola et al. [118] used solut-
ions containing sucrose and fructose in varying
proportions. Water loss was similar for all solutions
tested but the penetration of the osmoactive substance
was different. Peaches dehydrated in solutions of glu-
cose and fructose were especially suitable to pasteur-
ization [119]. Solution of sucrose and glucose yielded
high drying rate of apple slices [17]. Mixture of crys-
talline sucrose and glucose lowered water activity of
guava to 0.77 whereas sucrose alone yielded a water
activity of 0.80 [120]. Mixture of sucrose and sodium
chloride is used to osmose fruits and vegetables [121–
123]. The presence of NaCl facilitates the process.
Mixture of sucrose and citrate was used in osmotic
dehydration of peas [124–126] and papaya [127].
Sodium chloride was used to dehydrate vegetables.
Speck et al. [68] used 10% solutions to dehydrate car-
rots. Lewicki et al. [80] used 15% NaCl to dehydrate
carrots and potatoes. Adambounou et al. [128] dehy-
drated paprika, tomatoes, and eggplant in saturated
salt solutions, getting water activity as low as 0.8.
Vijayanand et al. [129] used 5–25% NaCl solution to
dehydrate cauliflower. Use of sodium and potassium
chlorides made it possible to regulate sodium and
potassium content in dehydrated corn and green peas.
It has been found that the addition of low molecu-
lar weight substances such as sodium chloride, malic
acid, lactic acid, and hydrochloric acid in concentra-
tions of 1–5% to sugars or starch syrups improves the
process of osmotic dehydration. In general, they pro-
mote removal of water from the material. Calcium
chloride and malic acid were added to sucrose to
improve the texture of osmosed apples [130].
28.3.3 P
ROCESSING
P
ROCEDURES AND
P
ARAMETERS
Osmotic dehydration can be done basically in two
ways: by static or dynamic process. In a static process,
5
5
4
3
2
1
10
Water content, g/gd·m
Time, h
15
20
25
6
FIGURE 28.7 The effect of osmoactive substance on
the
course
of
osmotic
dehydration
of
apples
at
308C
(. . . . . .,
glucose; ------, saccharose; ........., starch
syrup). (From Lenart, A. and Lewicki, P.P., of in IDS ’89
Mujumdar, A.S. and Roques, M., eds., Hemisphere Publ.
Co., New York, 1990, p. 501.)
ß
2006 by Taylor & Francis Group, LLC.
the material is mixed with an osmoactive substance,
which can be used as crystals or solution, and the
mixture is left motionless until the desired water loss
is achieved. It has been shown that the mass transfer
resistance in this method is higher than that observed
in a dynamic process [131,132].
In a dynamic process, the mixture is mixed; dif-
ferent methods of mixing can be used. Movement of
food particles in a stationary solution, mixing of the
whole suspension, and the flow of the osmoactive
substance through the stationary layer of food pieces
are the commonly used designs of the dynamic pro-
cess. If crystals of the osmoactive substance are
used, the fluidized bed is the solution for the dynamic
process. It has been shown that the rate of motion
has little effect on the rate of osmotic dehydra-
tion [71,133]. It is just sufficient to induce motion of
particles or solution in the system to have increased
mass transfer rates. Moreover, it was shown that the
motion of osmotic solution in a turbulent region
affected water flux but no difference in solids gain
occurred in comparison with laminar flow [134].
Azuara et al. [121] applied centrifugal force to
suspension of potato and apple slices in hypertonic
solution. The force affected solids gain much more
than the water loss. In comparison with static
method, the application of centrifugal force resulted
in larger water flux and smaller solids gain.
Reduction of pressure during osmotic dehydra-
tion increases the rate of the process [115,135]. It
has also been observed that low pressure facilitates
penetration of the osmoactive substance into the tis-
sue [136,137]. Osmotic dehydration under reduced
pressure is done in two ways: reduced pressure is
kept continuously or reduction of pressure is done
in pulses [83,127,137–139]. In general, pulsed vac-
uum osmotic dehydration gives better results than
vacuum osmotic dewatering.
Apple cubes subjected to osmotic process and
treated by ultrasound, dewatered faster than the
nontreated ones [20]. Water and solute transport
rates were significantly higher in sonicated samples in
comparison with those not sonicated during osmosis.
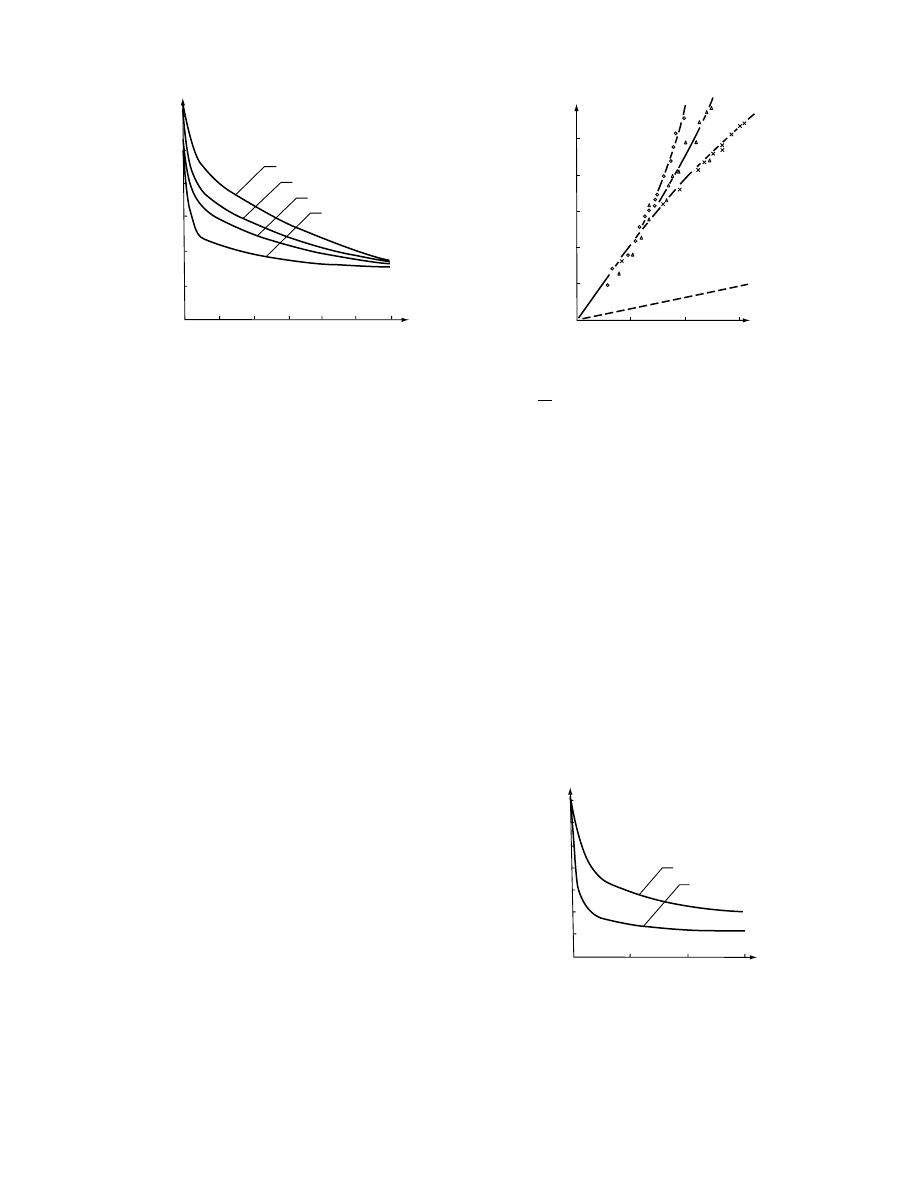
The rate of osmosis increases with increase of
concentration of the osmoactive substance (Figure
28.8) [23,66]. The weight loss of mango and papaya
is linearly dependent on sucrose concentration up to
60% [140]. Similar results were obtained for apple,
carrot, and pumpkin. At higher concentrations, a
lower rate is observed and the impregnation of fruits
with saccharose is high. Ponting [65] used 65–70%
sucrose solutions to dehydrate apples. Pinnavaia
et al. [101] recommend 70% starch syrup for osmode-
hydration of apples. Lenart and Cerkowniak used
glucose, saccharose, and starch syrup solutions to
dehydrate apples [141]. Rastogi et al. [23] recommend
40–708Bx sugar solution for osmotic dehydration
of bananas. A high rate of dehydration of carrot
and potato was obtained with 15% sodium chloride
solution [142,143].
Crystalline osmoactive substance is used at a
weight ratio of 1:1 to fruits [45,105]. For solutions,
investigations were done at weight ratios of 1:1 to 1:6
[87,144]. Osmotic dehydration of fruits and veget-
ables is recommended to be done at a weight ratio
of 1:4 to 1:5 of food to osmoactive solution [77,145].
Temperature has a substantial effect on the course
of osmotic dehydration. It not only affects the rate of
the process but also influences the chemical compos-
ition and properties of the product. Increased tempera-
ture increases the rate of chemical reactions and mass
transfer processes as well. Viscosity of hypertonic solu-
tion is lowered and the diffusion coefficient of water
increases with the increase of temperature [21,77].
Andreotti et al. [31] recognize a temperature of
438C as the optimal for osmotic dehydration of cher-
ries and pears in glucose or glucose–fructose syrup.
They recommended a temperature of 208C for os-
motic dehydration of apricots. Bananas were osmot-
ically dehydrated at 608C [146]; however, it was
shown that optimal temperature was dependent on
the concentration and pH of the osmotic solution
[105]. Pineapple was dehydrated at 42–478C [96] but
application of vacuum and temperature higher than
408C resulted in loss of volatiles [112]. Osmotic dehy-
dration of plums is done at 508C [147,148], kiwifruit
at 378C, and peas at 50–708C [124].
Kowalska et al. [149] recognize a temperature of
508C as the optimal for osmotic dehydration of straw-
berries and cherries in glucose, sucrose, and starch
syrup solution. They recommended osmosing fruits
at 30–508C. Nsonzi and Ramaswamy [21] osmotically
dehydrated blueberries at temperature 37–608C and
2
60
Relative weight, %
70
80
90
100
4
Time, h
6
8
FIGURE 28.8 The effect of sugar syrup concentration on
the course of osmotic dehydration of apples at 508C (708Bx;
------, 608Bx; ........., 508Bx). (Adapted from Farkas, D.F.
and Lazar, M.E., Food Technol., 23, 688, 1969.)
ß
2006 by Taylor & Francis Group, LLC.
sucrose solution conc entration 47–70 8 Bx. Apples
were dehydrat ed at tempe ratur e 30–90 8 C [115] (Fig-
ure 28.9) . It has be en sho wn that the increa se of
tempe rature in the range of 30–80 8 C su bstantial ly
shorte ns the tim e of deh ydration [133]. How ever, in-
crease d tempe rature promot es penetra tion of
osmoact ive substa nce into the tissue [77] .
A HTS T proce ss was propo sed for osmotic de hy-
dration by Ma strocola et al. [118], Lerici et al. [78] ,
Levi et al. [150], and Da˛ browsk a an d Lena rt [151] .
The process is con ducted at 65–90 8 C at a tim e of
1–20 min. The de gree of dehydrat ion is equival ent to
that at 20 8C last ing for 2 h. The HTS T pro cess also
gives the effect of blanch ing, whi ch inact ivates en -
zymes and remove s part of the air from the inter cel-
lular sp ace. To obtain a high ratio between water loss
and soli ds gain (F igure 28.10) , a tempe ratur e between
20 8 C and 40 8 C is recomm ended [117, 152,153]. The
degree of dehyd ration is regula ted by the time of
osmosi s.
The cou rse of mass loss versus tim e is cu rvilinear .
The highest rates occur at the be ginning of the pr o-
cess [154]. Porosit y greatly increa ses during the first
period of osmot ic deh ydration . This impl ies that a
part of the air vo lume in the struc ture is replac ed by
the exter nal solution [155]. The most significan t
changes of water content , water loss, and soli ds ga in
take place during the first 30 min of dewat ering at
30 8 C. Rate of wat er loss is 5–10 times higher than the
rate of solids gain and depend s on the advan cement
of the dewateri ng pr ocess [156]. Accor ding to Lena rt
[157], opt imal tim e of apple de hydration is 5–6 h at
20 8 C, 3–4 h at 30 8 C, and 1–1.5 h at 40 8 C. A redu ction
of mass by 50% can be achieve d after 2.5–3 h of
osmosis at 5 08C.
It is well recognized tha t the charact eristics of the
material undergoing dehydration by osmosis
strongly affect the c ourse of the proc e ss. U nder
identical c onditions some materials l ose w ater faster
than others (Figure 28.11). The penetration of the
osm oactive sub s t ance dif fers m arkedly (
); he nce, properties of the product and its c on-
sumer acceptance are strongly affected by the i nitial
properties of the material, supposedly by its tissue
structure.
2
2
3
3
Time, h
4
4
5
6
1
1
1
Water content, g/gi·d·m
2
3
4
5
6
FIGURE 28.9 The effect of temperature on the course of
osmotic dehydration of apples in saccharose solution (1,
308C; 2, 508C; 3, 708C; 4, 908C). (From Lenart, A. and
Lewicki, P.P., in IDS ’89, Mujumdar, A.S. and Roques,
M., eds., Hemisphere Publ. Co., New York, 1990, p. 501.)
0.3
Water loss, g/gi·d·m
1
2
3
4
5
Solids gain, g/gi·d·m
0.6
0.9
FIGURE 28.10 Relationship between water loss and solids
gain in apples during osmotic dehydration in saccharose
solution (
, 208C;
; 308C; ++, 408C; . . . . . . ; diag-
onal). (From Lenart, A. and Lewicki, P.P., in Drying ’87,
Vol. 2 Mujumdar, A.S. ed., Hemisphere Publ. Co., New
York, 1987, p. 239.)
5
Time, h
Water content, g/gi·d·m
10
15
1
1
2
2
4
3
5
6
7
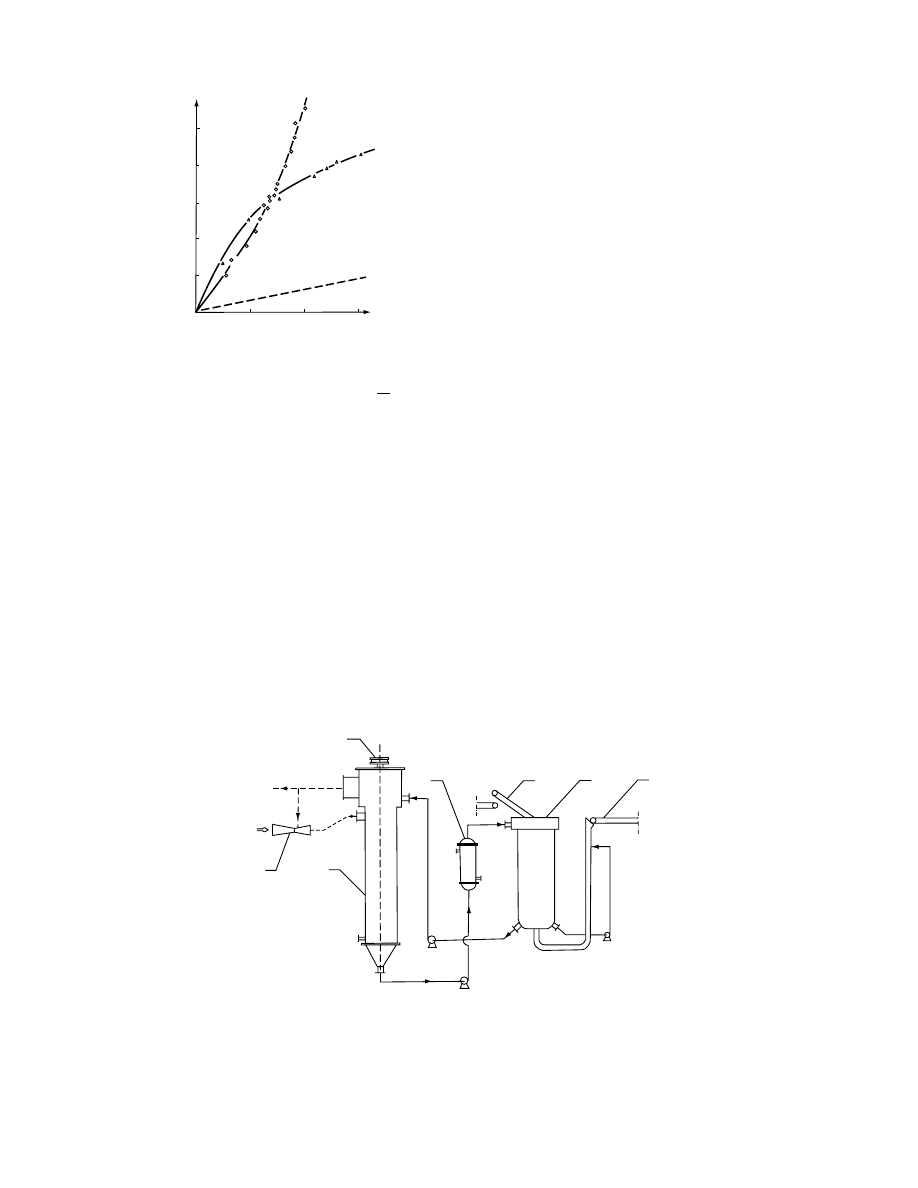
FIGURE 28.11 The effect of the kind of material on the
course of osmotic dehydration in saccharose solution at
208C. 1-apple, 2-carrot.
ß
2006 by Taylor & Francis Group, LLC.
Concluding the effect of procedures and process-
ing parameters on the rate and efficiency of osmotic
dehydration it can be stated that all of them are
equally important. The kind and concentration of
the osmoactive substance, the weight ratio of the
solution to food, the kind of osmosed material, its
size and shape, temperature and pressure, and the
pretreatment of the material prior to osmosis affect
strongly not only the course of the process but also,
first of all, influence the quality and organoleptic
attributes of the final product. To reach reques-
ted quality of the product the process of osmotic
dehydration must be individually designed.
28.3.4 O
SMOTIC
S
OLUTION
M
ANAGEMENT
Water withdrawn from the material dilutes the hyper-
tonic solution. Hence, it is important to keep its
concentration constant, either by a continuous evapo-
ration of excess water [133,144] (Figure 28.13) or by
dissolution of osmoactive substance [144]. Both
methods make it possible to use the same hypertonic
solution several times.
Dilution of the hypertonic solution depends on
the ratio between solids and the solution. At high
ratio, i.e., 1:10, dilution is low and amounts to few
percent, whereas at high ratio such as 1:2 the increase
of volume of osmotic solution can be as large as
several percent [158].
Evaporation of excess water, membrane separation,
or dissolution of osmoactive substance can bring the
concentration of hypertonic solution to the required
value but the solution is not the same as that used in
the beginning of the process. Leaching of solubles from
fruits or vegetables [159,160] to the hypertonic solution
changes its physical, chemical, and sensory properties.
Moreover, debries of fruits and seeds are present in
the solution after its use. And finally, may be this is
the most important issue, the solution becomes a
good medium for microbial growth.
Concentration of substances leaching from the
processed material stabilizes after few uses depending
on the kind of osmosed material, and reaches the level
similar to that of the osmotically processed fruits or
vegetables.
Designing the process of the reuse of osmotic solution
the coarse filtration, pasteurization, and decolorization
0.3
Water loss, g/gi·d·m
1
2
3
4
5
Solids gain, g/gi·d·m
0.6
0.9
FIGURE 28.12 The effect of the kind of material on the
relationship between water loss and solids gain in osmotic
dehydration in saccharose solution at 208C (
, apple;
4—4, carrot; ------, diagonal).
2
1
3
4
5
7
6
FIGURE 28.13 Osmotic dehydration with reconcentration of hypertonic solution (1) feeding conveyor; (2) osmotic dehy-
drator; (3) redler conveyor; (4) heat exchanger; (5) scaraped surface evaporator; (6) thermocompressor; (7) driven wheel; flow
of hypertonic solution; ------, vapor; ), high pressure steam; ------, heating steam).
ß
2006 by Taylor & Francis Group, LLC.

must be taken into account. The sanitation of the solu-
tion is a priority in the recycling process.
Microbial contamination of the solution comes
from different sources but its water activity 0.90–
0.95 limits the growth of nonosmotolerant bacteria
and yeasts. Processing of fruits and vegetables results
in contamination of osmotic solution with moulds,
yeasts, and lactic bacteria. Reported microbial loads
of osmotic solution range from 10
2
to 10
5
cfu/ml after
long-time use [158]. Mild heat pasteurization is suffi-
cient to lower the microbial load of the solution to
value as low as 10
2
cfu/ml [161].
Heat treatment of sugar solutions containing
acids and proteins results in nonenzymatic browning.
The presence of 5-(hydroxymethyl)-2-furfuraldehyde
(HMF) was shown to be a good indicator of Maillard
reactions [158]. Decolorization of the solution can be
done with activated carbon, charcoal as filtration
coadjuvant and polyvinylpyrrolidone [132,160,161].
Filtered, pasteurized, and decolorized syrup can
be used few to several times depending on the pro-
cessed material and organization of the process. In
continuous processing more recycling can be done
in comparison to the process in which runs are not
done consequently. The possibility to reuse the os-
motic solution more than 20 times was reported
[158]. However, number of cycles is dependent on:
.
Kind of processed material
.
Type of reconcentration technology
.
Pasteurization parameters
.
Organization of the process
.
Individual adaptation to the given process
Osmotic solution, even after several uses must
be disposed. In the case of fruit processing, some
ways of further use of osmotic solution have been
proposed [158]:
.
Syrup for fruit canning
.
Pproduction of jams
.
Mixing with fruit juices
.
Production of fruity soft drinks
.
Production of natural flavorings
.
Bee feeding
Processing of vegetables, especially with sodium
chloride yields solution which further management
is not solved until today.
Spent solutions if not used in other processes must
be discharged as wastewater. High carbohydrate con-
tent and the presence of other organic materials cause
very high demand for oxygen. The biological oxygen
demand (BOD
5
) of the osmotic solution is high and
efficient wastewater treatment is needed. The presence
of salt in osmotic solution creates additional prob-
lems and probably the use of reverse osmosis is the
rational way of this spent-liquor treatment.
28.3.5 E
NERGY
A
SPECTS OF
O
SMOTIC
D
EHYDRATION
Osmotic dehydration is distinctive in that water is
removed from the product without undergoing the
phase change. It offers a considerable potential for
energy saving in comparison with convection drying.
Most publications consider energy consumption
in a drying process that is preceded by osmotic dehy-
dration [162] or analyze the process under laboratory
conditions [142,163,164].
Energy consumption in the osmotic dehydration
process arises from the following [165]:
.
Heating of the material and osmoactive solution
to the required temperature and making up a
heat loss
.
Solution mixing or pumping and recirculation,
depending on the variant applied
.
Dissolution of hypertonic substance in a diluted
solution
.
Evaporation of water in an appropriate eva-
porator
It is estimated that dissolution of osmoactive sub-
stance in a hypertonic solution needs some 1 kJ/kg of
water removed from the material. Hence, this process
affects energy consumption in osmotic dehydration
negligibly.
The amount of water removed during osmotic
dehydration is not large. Processing of 1 ton of fruits
or vegetables per h will give, at the most, 450 kg of
surplus solution (i.e., some 5.5 tons of water evapor-
ated per day). Hence, a single-effect evaporator with
vapor recompression will meet the needs.
Energy use in the evaporator consists of electric
energy for syrup circulation and heat for water evap-
oration. Electric energy use is estimated to be equal to
10 kJ/kg of evaporated water, and heat consumption
is some 1.8 MJ/kg of evaporated water.
The increase of temperature shortens the osmotic
process. The use of energy for syrup mixing or circu-
lation is estimated as 17.2, 10.0, and 4.3 kJ/kg of
water removed at temperatures 208C, 308C, and
408C, respectively. To keep the process running at a
desired temperature, a supply of heat is necessary.
Depending on the amount of water removed from
the material, the heat supply amounts to 180–240
kJ/kg at 308C and 380–500 kJ/kg of water removed
at 408C [166].
Energy consumption in osmotic dehydration of
fruits and vegetables under industrial conditions is
ß
2006 by Taylor & Francis Group, LLC.
estimat ed to be between 100 an d 2400 kJ/kg of wat er
remove d (Figur e 28.14), depending on the tempe ra-
ture of the process an d the way the surplus solution is
managed. It is wort hwhile to notice that con vection
drying needs some 5 MJ/kg of evap orated water,
which is at least twice as much as is needed in osmot ic
dehydrat ion.
28.4 EQUIPMENT FOR OSMOTIC
DEWATERING
Depending on the aim of osmotic process and desired
produc t charact eris tics the process can be de signed as
far as process ing parame ters are concerned. To im-
pleme nt the de signed proce ss a specia l equ ipment is
needed which must assure control of pro cessing
parame ters and effici ency and econo mics as wel l.
Choi ce of the equipment is based on the following
criteri a:
.
Type of process ing; periodi c or continuous
.
Resist ance of food to mechani cal damage
.
Shap e of food; whole or cut into pieces
.
Suscepta bility of food to oxidat ion in contact
with air
.
Relati ve mo vement of phases, soli d and liquid
.
Possibi lity to control pro cessing pa rameters
.
Invest ment and run ning co st
Accor ding to M arouze´ et al. [167] process es of
osmot ic deh ydration can be categor ized as foll ows:
.
Thos e in whi ch foo d is imm ersed in the osmot ic
solut ion
.
Thos e in whi ch solut ion is intr oduced onto the
food
.
Those in whi ch osmot ic substa nce in solid state
is con tacted with food
.
Those in whi ch reduced pr essure is used to fa-
cilitate mass trans fer
28.4.1 F
OOD
I
MMERSED IN
S
OLUTION
The sim plest way to contact foo d with osmo tic solu-
tion is to immerse a basket with food into solut ion.
The movem ent of solut ion is sli ght due to natural
convecti on. M ass trans fer is slow and most of pro -
cessing parame ters are not controlled. The method
can be used to soft frui ts.
Osm otic dewat ering can be facilitated by de -
creasing mass trans fer resistance . This can be
done eithe r by circul ation of solut ion or by slow
movem ent of food. Ci rculation of solut ion is done
by install ation of circul ation pump to a vessel in
which basket with food is immersed. Movement of
food in the solut ion is done by vibrat ion (Figur e
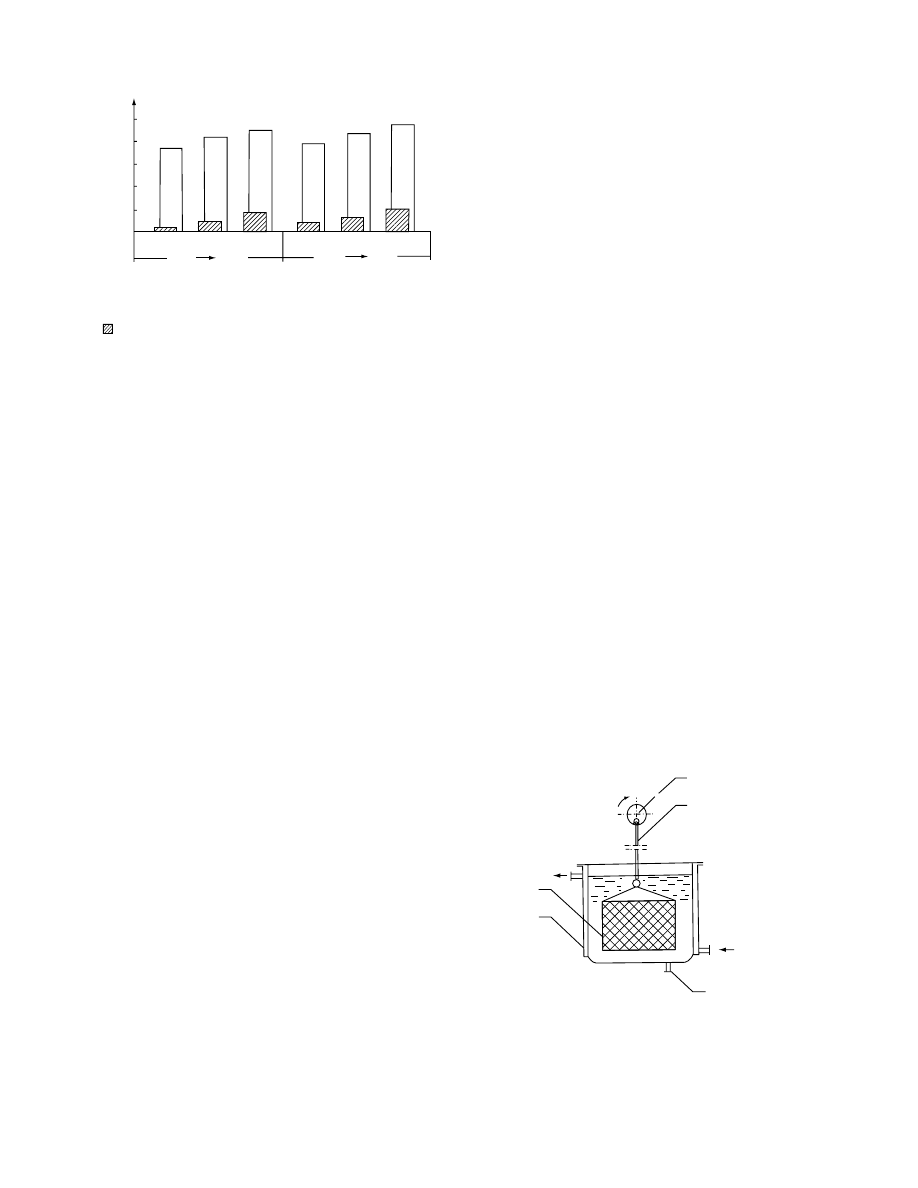
28.15) or by a conveyo r (
). The latter
solution is used in Poland in the process ing of app le
slices [168,169] .
Com binati on of solution circulatio n and mov e-
ment
of
food
particles
is
combined
in
such
equipment as vibrating plate mixer (
and percolated bed with slow displacement of food
(
) . In vibrated plate mixe r [133] , osmot ic
solution is circulating in two loops, one is a feed loop
by which food is fed into a mixer, and the second
loop maintains constant temperature of the solution.
Food moves from bottom of the mixer to its top
7.6
Energy consumption, MJ
1
20
⬚C
30
⬚C
40
⬚C
20
⬚C
30
⬚C
40
⬚C
4.0
2.0
7.6
2
FIGURE 28.14 Energy use in osmotic dehydration ex-
pressed per kilogram of removed water. Temperature of
the process and the degree of dewatering are the parameters
( , diluted hypertonic solution completed with osmoactive
substance; &, diluted hypertonic solution concentrated in
evaporator).
1
5
2
3
4
FIGURE 28.15 Osmotic dehydration with a vibrating bas-
ket (1, jacketed vessel; 2, basket; 3, shaft; 4, eccentric; 5,
spout). (From Lenart, A. and Lewicki, P.P., in IDS ’89,
Mujumdar, A.S. and Roques, M., eds., Hemisphere Publ.
Co., New York, 1990, p. 501. With permission.)
ß
2006 by Taylor & Francis Group, LLC.
through a series of perfor ated vibrat ing horizont al
plates mou nted on a v ertical axis. In a pe rcolated
bed, food is delivered at the bottom of the tank by a
hydrauli c feed an d forms a poro us be d. The bed
moves slowly to the top of the tank an d is extracted
by a redler or buck et co nveyor. Solutio n is fed a t
the top of the tank an d is circul ated through the
feed leg. In this equipment, a countercurrent movement
of food and solution occurs. A cocurrent movem en t of
food and solut ion as a percola ted bed was also
designe d for osmot ic process [167] .
M ovement of solut ion an d pa rticles can be
done by mechanical mixing. Mixing device can be
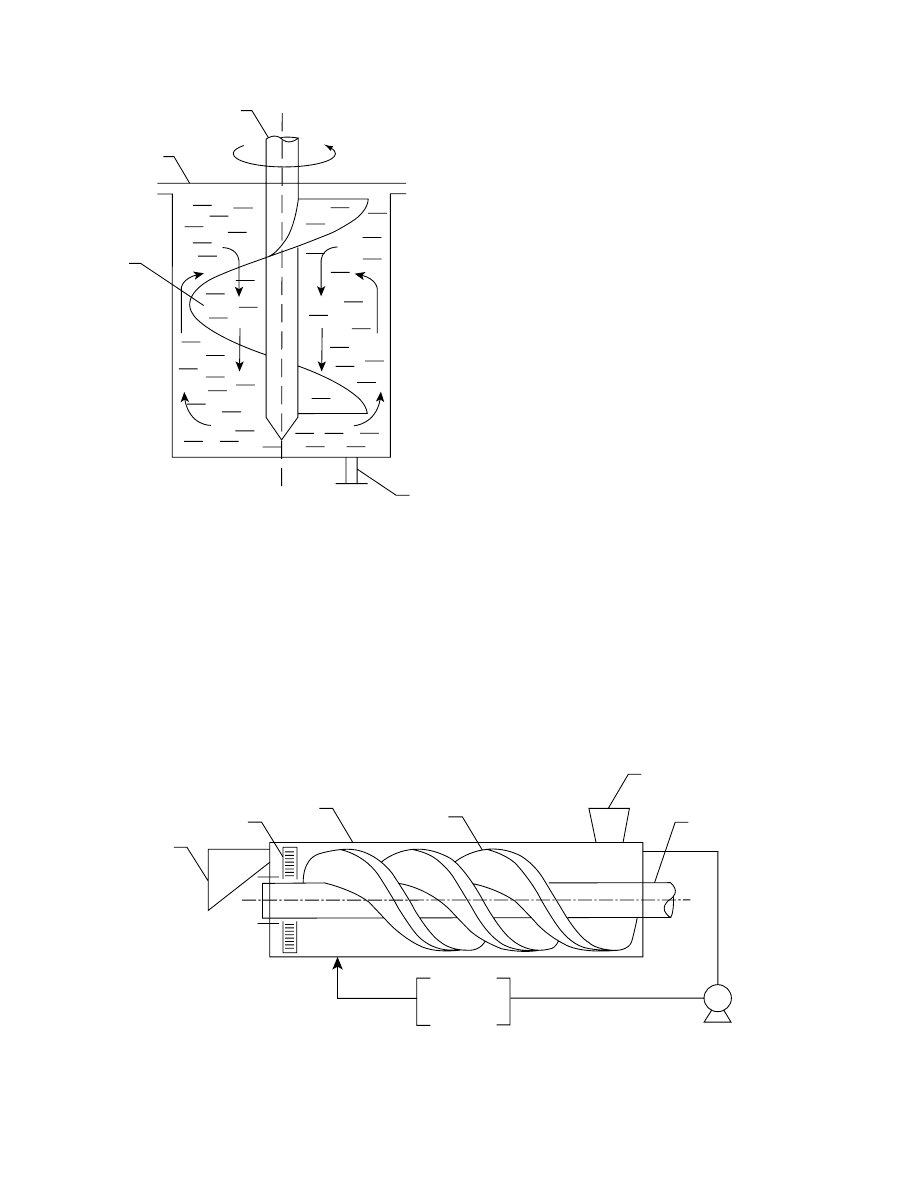
install ed vertical ly (
) or horizont ally
(
) . In the first design, a worm screw is
placed coaxially inside a vertical cylindrical tank. The
screw moves particles of food from top to the bottom
of the tank. Then the pieces rise toward the surface
under the buoyancy force. In the second technical
solution or design, the screw is mounted horizontally.
The food pieces together with the solution are moved
along the cylinder axle. Pieces of food are carried in
rotation toward the end of the cylinder where a de-
flector catches the pieces and directs them to the
outlet.
Designs of equipment with mechanical motion of
food pieces exert some force on processed material.
Hence some disintegration and deformation of food
can take place and increased pulp content in the
solution can be observed.
1
2
3
4
Reconstitution
system
FIGURE 28.16 Conveyor osmotic dehydration (1, perforated conveyor with osmosed material; 2, vessel with hypertonic
solution; 3, conveyor preventing apple slices from floating; 4, pump).
1
7
2
6
3
4
5
7
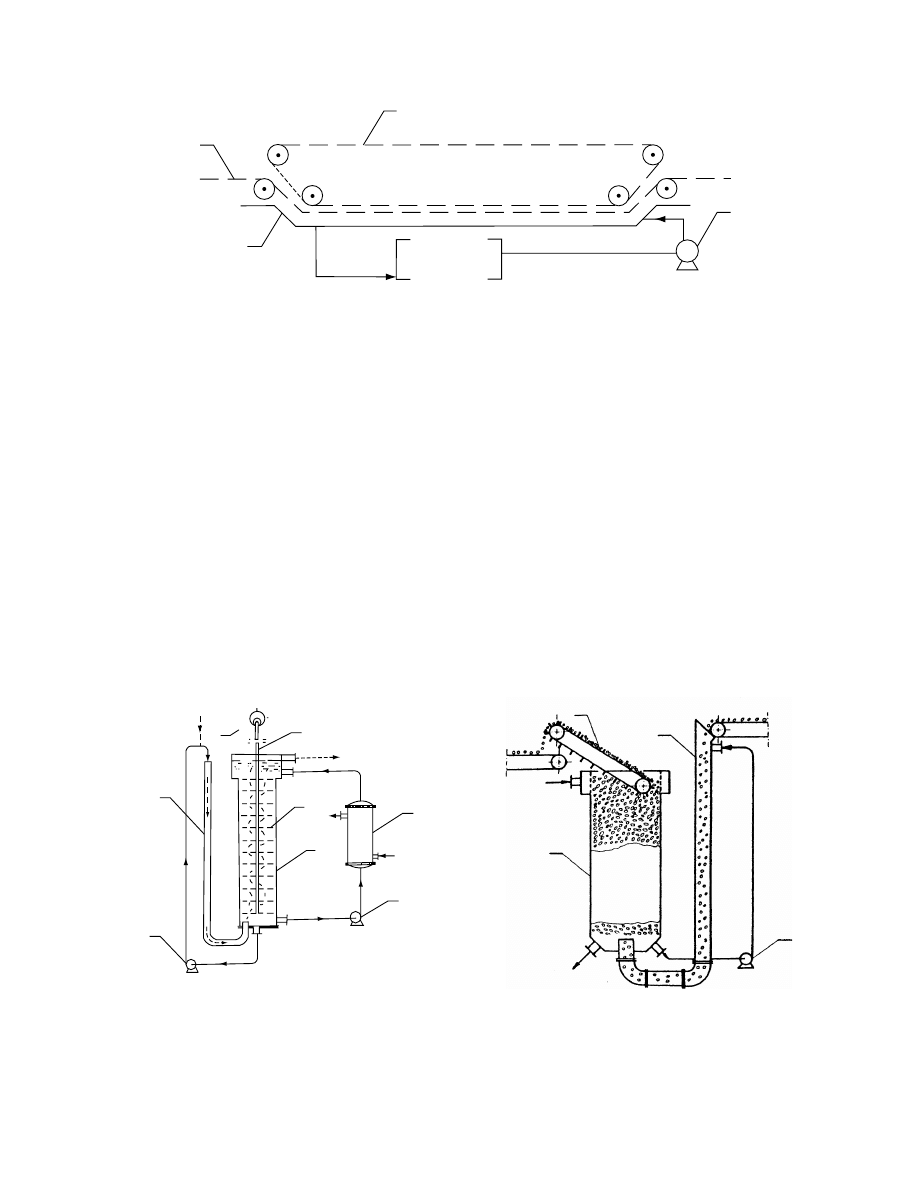
FIGURE 28.17 Osmotic dehydration with a vibrating plate
mixer (1, feed leg; 2, vessel; 3, vibrating mixer; 4, shaft; 5,
eccentric; 6, heat exchanger; 7, pump). (Adapted from
Pavasovec, V., Stefanovic, M., and Stefanovic. P., Drying
’86 Vol 2, Mujumdar, A.S., ed., Hemisphere Pub Co.,
New York, 1986, p. 761)
2
3
1
4
FIGURE 28.18 Osmotic dehydrator—a packed bed unit
(1, vessel; 2, redler conveyor; 3, feed leg; 4, pump). (Adapted
from Pavasovec, V., Stefanovic, M., and Stefanovic.
P., Drying ’86 Vol 2, Mujumdar, A.S., ed., Hemisphere
Pub Co., New York, 1986, p. 761)
ß
2006 by Taylor & Francis Group, LLC.
28.4.2 S
OLUTION
S
PRAYED ONTO THE
F
OOD
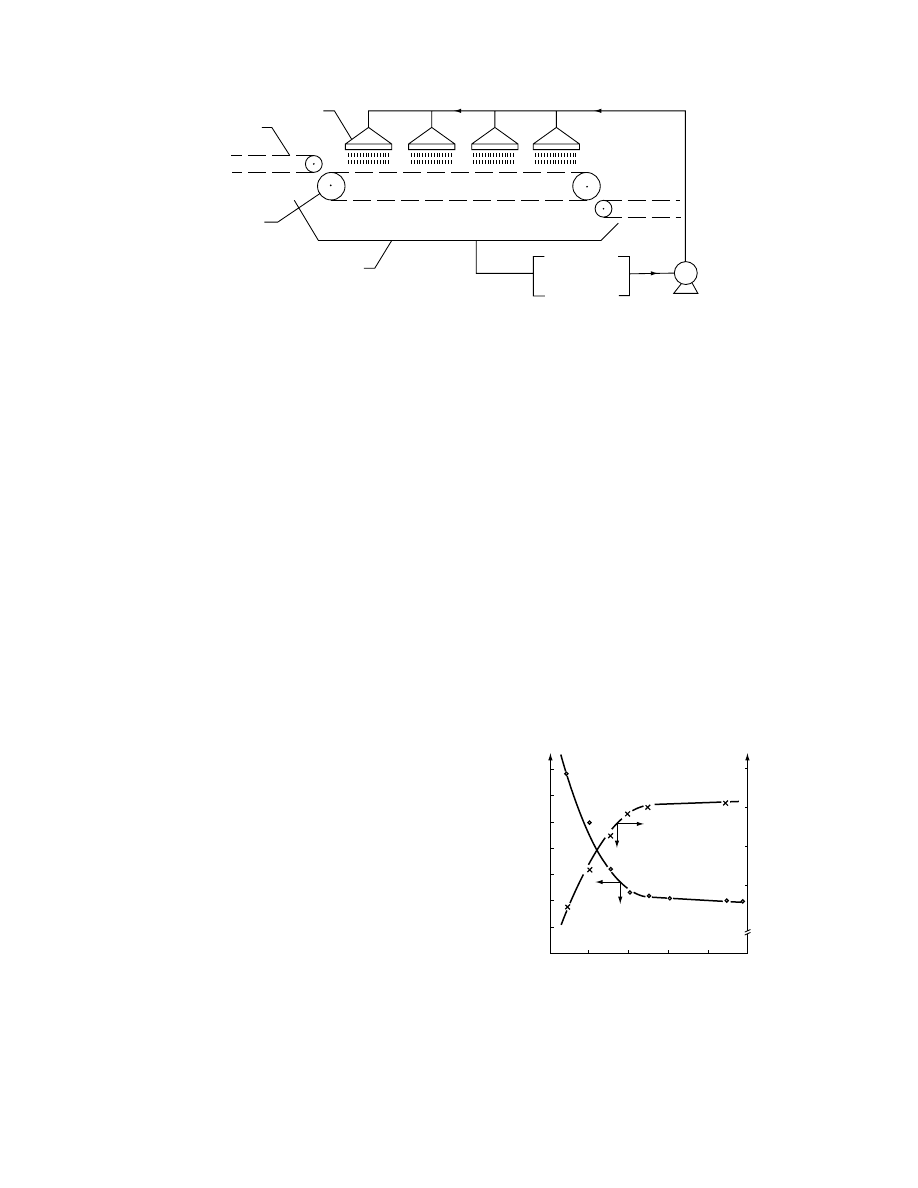
Reduct ion of solution and food ratio can be done by
applic ation of thin layer of hyp ertonic so lution to
food pieces . It is done by placi ng food pieces on
perfor ated conv eyor and sprayi ng con centrated solu-
tion on process ed mate rial (
). The de sign
is wel l suit ed to continuou s process ing but requir es
food pieces to be spread on the conveyo r in a single
layer. Hence, a large area of the conveyo r is needed to
process an y given quantity of foo d. This techni cal
solution of osmot ic de wateri ng was proposed by Le
Maguer [170] and Dall a Rosa et al. [161] .
28.4.3 S
OLID
O
SMOTIC
S
UBSTANCE
C
ONTACTED
WITH
F
OOD
The low est solut ion and food rati o is obtaine d when
solid osmo tic substa nce is co ntacted wi th food. Cry s-
tals of sugar or mixt ure of sugar and salt are mixed
with food pieces in appropri ate proporti on an d tum-
bled in slowly rotating cylind rical tank . The amoun t
of osmo tic substa nce used should be such that wate r
remove d from foo d pieces form s no solut ion in the
tank. W et but solid osmot ic sub stance is sep arated
from food on v ibrating screen. How ever, some cryst als
stick to the foo d surfa ce an d can creat e prob lems in
packaging or furt her process ing of osmo sed material.
28.4.4 E
QUIPMENT
W
ORKING UNDER
R
EDUCED
P
RESSURE
Static or pulsed- vacuum proc essing of immersed frui t
or vegeta ble pro ducts facilita tes os motic de waterin g.
Equipmen t used in this process c an be of any type
previously presented but requires hermetic design.
28.5 PRODUCT CHARACTERISTICS
Osmotic dehydration is a complex process of counter-
current mass transfer between the plant tissue and
hypertonic solution. This leads to dehydration of
the material and changes in its chemical composition
1
2
4
3
FIGURE 28.19 Osmotic dehydrator—a vertical mixer (1,
worm screw; 2, vessel; 3, shaft; 4, spout). (Adapted from
Marouze, A., Groux, F., Collignan, A., and River, M.,
J. Food Eng., 49,207,2001. With permission)
2
3
Regenerating
system
1
6
4
5
FIGURE 28.20 Osmotic dehydrator—a horizontal mixer (1, feed hopper; 2, vessel; 3, worm screw; 4, deflector; 5, discharge
hopper; 6, shaft). (From Lenart, A. and Lewicki, P.P., Zesz. Nauk. SGGW-AR, Technol. Roln.-Spo_zz., 14, 33, 1981. With
permission.)
ß
2006 by Taylor & Francis Group, LLC.
as well. Hence, it must be expected that the proper-
ties of the material dehydrated by osmosis will dif-
fer substantially from those dried by convection
[5,10,76,171].
The flux of osmoactive substance penetrating the
osmosed tissue changes its chemical composition. It
has been shown that the content of sucrose increases
in cell sap during osmotic dehydration [15,67,172],
and the sucrose flux is increased by the presence of
sodium chloride [70]. On the other hand, use of starch
syrup gives only a small influx of sugars to the mater-
ial [144]. Glucose seems more effective than sucrose in
the water loss and in the solids gain by fruits [102].
Sodium chloride penetrates tissue very effectively,
hence contacting of the material with this substance
leads to salting rather than to dewatering of the tis-
sue [73,80]. There is also a flux of native substances
leaving the tissue. Concentration of organic acids is
lowered and native sugars are replaced by sucrose
[67,117,173].
Penetration of an osmoactive substance, except
sodium chloride, is a surface process. Sugars pene-
trate to the depth of 2–3 mm whereas changes in
water content are observed up to the depth of 5 mm
(Figure 28.22) [109,174,175]. When sodium chloride
is used, it penetrates carrot tissue to a depth exceeding
12 mm.
Concentration of the cell sap and influx of
osmoactive substance lower the water activity in the
tissue to a value dependent on processing parameters.
The water-binding capacity of the tissue is also
affected by the osmotic process, although changes
are observed only in surface layers [176]. Osmotic
dehydration done for 0.5 h led to a sixfold decrease
of water-binding forces at the surface of apple in
comparison to the raw material [177]. Water removal
from the tissue by osmosis shows a much stronger
effect on water-binding forces than by the convection
drying done to the same final water content [178].
As it has been stated previously, osmotic dehydra-
tion cannot be treated as a food preservation process
per se. It is a pretreatment that removes a certain
amount of water from the material; to achieve shelf
stability, a further processing of the product is
needed. Hence, the interaction of osmotic dehydra-
tion with further processing is important for quality
assurance.
Use of osmotic dehydration practically eliminates
the need to use preservatives such as sulfur dioxide in
fruits. The process removes a substantial amount of
air from the tissue, thus blanching prior to osmotic
dehydration also can be omitted [166].
It has been shown that apples dried by osmosis
and then frozen compared favorably with the conven-
tional frozen fruits [144,179]. Osmotic dehydration
preceding freeze drying shortens the time of the pro-
cess and yields fruits superior to those not treated by
osmosis [15,79,97]. Osmotic dehydration followed by
Reconstitution
system
4
3
2
1
FIGURE 28.21 Spray osmotic dehydrator (1, spray nozzles; 2, feed conveyor; 3, perforated conveyor with material
undergoing osmotic dehydration; 4, collector for used hypertonic solution).
1
10
20
30
2
3
4
Distance, mm
Total sugars, %
0.6
0.7
0.8
0.9
1.0
Water content, g/g cell sap
FIGURE 28.22 The depth of osmotic substance penetration
and cell sap concentration in apple osmosed in 68.5%
saccharose solution at 408C for 4 h. (From Lenart, A. and
Lewicki,
P.P.,
Zesz.
Nauk.
SGGW-AR,
Technol.
Roln.-Spo_zz., 14, 33, 1981. With permission.)
ß
2006 by Taylor & Francis Group, LLC.

vacuum drying gives products that are very stable
upon storage [65].
Most research has been directed toward combin-
ing osmotic dehydration with convection drying
[75,88,180–183]. The approach is of special interest
due to the growing consumer demand for commod-
ities in the freshlike state. The IMF comply well with
consumer expectations [184–186].
Osmotic pretreatment before microwave-assisted
air drying increase the final overall quality of the
product [187]. Fruits and vegetables treated by osmo-
sis can be further dehydrated in a convection dryer to
lower the water activity to the level of 0.65–0.90. At
those water activities, water content in the material is
still high and the product presents such organoleptic
attributes as chewiness, softness, elasticity, and plas-
ticity [76,188–190]. The product has a natural color,
well-preserved flavor, and high retention of vitamins
[132]. Its shrinkage is much smaller when compared
with that observed in convection-dried products at
the same water activity.
REFERENCES
1. R.M. Spanswick, Symplasmic transport in tissues. In
Encyclopedia of Plant Physiology. Vol. 2. Transport in
Plants II, Part B. Tissues and Organs (U. Luttge and
M.G. Pitman, eds.), Springer Verlag, Berlin, 1976,
p. 35.
2. A. Nason and R.L. Dehaan, The Biological World,
John Wiley & Sons, New York, 1973.
3. A. Lauchli, Apoplasmic transport in tissues. In En-
cyclopedia of Plant Physiology. Vol. 2. Transport in
Plants II, Part B. Tissues and Organs (U. Luttge
and M.G. Pitman, eds.), Springer Verlag, Berlin,
1976, p. 3.
4. N.V. Parthasarathy, Sieve-element structure. In En-
cyclopedia of Plant Physiology, Vol. 2. Transport in
Plants I. Phloem Transport (M.H. Zimmermann and
J.A. Milburn, eds.), Springer Verlag, Berlin, 1975, p. 3.
5. H. Ziegler, Nature of transported substances. In En-
cyclopedia of Plant Physiology, Vol. 2. Transport in
Plants I. Phloem Transport (M.H. Zimmermann and
J.A. Milburn, eds.), Springer Verlag, Berlin, 1975,
p. 59.
6. J.A. Milburn, Pressure flow. In Encyclopedia of Plant
Physiology, Vol. 2. Transport in Plants I. Phloem
Transport (M.H. Zimmermann and J.A. Milburn,
eds.), Springer Verlag, Berlin, 1975, p. 328.
7. R.J. Poole, Transport in cells of storage tissue. In
Encyclopedia of Plant Physiology, Vol. 2. Transport in
Plants II. Part B. Tissues and Organs (U. Luttge and
M.G. Pitman, eds.), Springer Verlag, Berlin, 1976,
p. 227.
8. J.R. Philip, The osmotic cell, solute diffusibility and
the plant water economy, Plant Physiol., 33:264
(1958).
9. J.R. Philip, Propagation of turgor and other proper-
ties through cell aggregations, Plant Physiol., 33:271
(1958).
10. J.R. Philip, Osmosis and diffusion in tissue: half-times
and internal gradients, Plant Physiol., 33:275 (1958).
11. F.J. Molz and G.M. Hornberger, Water transport
through plant tissues in the presence of a diffusible
solute, Soil Sci. Soc. Am. Proc., 37:883 (1973).
12. F.J. Molz and E. Ikenberry, Water transport through
plant cell and cell walls: theoretical development, Soil
Sci. Soc. Am. Proc., 38:699 (1974).
13. F.J. Molz, Water transport through plant tissue: the
apoplasm and symplasm pathways, J. Theor. Biol.,
59:277 (1976).
14. F.J. Molz, D.V. Kerns, C.M. Peterson, and J.H. Dane,
A circuit analog model for studying qualitative water
relations of plant tissue, Plant Physiol., 64:712 (1979).
15. J. Hawkes and J.M. Flink, Osmotic concentration of
fruit slices prior to freeze dehydration, J. Food Proc.
Preserv., 2:265 (1978).
16. J. Conway, F. Castaigne, and X.V. Pickard, Mass
transfer considerations in the osmotic dehydration of
apples, Can. Inst. Food Sci. Technol., 16:1 (1983).
17. F. Kaymak-Ertekin and M. Sultanoglu, Modelling of
mass transfer during osmotic dehydration of apples.
J. Food Eng., 45:243 (2000).
18. N.K. Rastogi, M.N. Eshtiaghi, and D. Knorr, Accel-
erated mass transfer during osmotic dehydration of
high intensity electrical field pulse pretreated carrots,
J. Food Sci., 64:1020 (1999).
19. D. Salvatori, A. Andres, A. Chiralt, and P. Fito, Os-
motic dehydration progression in apple tissue. I. Spa-
tial distribution of solutes and moisture content,
J. Food Eng., 42:124 (1999).
20. S. Simal, J. Benedito, E.S. Sanchez, and C. Rosello, Use
of ultrasound to increase mass transport rates during
osmotic dehydration, J. Food Eng., 36:323 (1998).
21. F. Nsonzi and H.S. Ramaswamy, Osmotic dehydra-
tion kinetics of blueberries, Drying Technol., 16(3/5):
725 (1998).
22. K.N. Waliszewski, N.I. Texon, M.A. Salgado, and
M.A. Garcia, Mass transfer in banana chips during
osmotic dehydration, Drying Technol., 15(10): 2597
(1997).
23. N.K. Rastogi, K.S.M.S. Raghavarao, and K. Niran-
jan, Mass transfer during osmotic dehydration of ba-
nana: Fickian diffusion in cylindrical configuration,
J. Food Eng., 31:423 (1997).
24. Kwang Sup Youn and Yong Hee Choi, Mass transfer
characteristics during the osmotic dehydration process
of apples, J. Korean Soc. Food Sci. Nutr., 25:824
(1996) (FSTA 1997-11-J0048).
25. S. Simal, F. Bauza de Mirabo, E. Deya, and C. Rosello,
A simple model to predict mass transfers in de-
hydration by osmosis, Z. Lebensmit. Untersuch und
Forsch., 204(3): 210 (1997).
26. Kwang Sup Youn and Yong Hee Choi, Mass transfer
characteristics in the osmotic dehydration process of
carrot, Korean J. Food Sci. Technol., 27(3):387 (1995)
(FSTA 1996-12-J0076).
ß
2006 by Taylor & Francis Group, LLC.

27. N.K. Rastogi and K.S.M.S. Raghavarao, Kinetics of
osmotic dehydration of coconut, J. Food Process Eng.,
18(2):187 (1995).
28. E. Azuara, R. Cortes, H.S. Garcia, and C.I. Beristain,
Kinetic model for osmotic dehydration and its rela-
tionship with Fick’s second law, Int. J. Food Sci.
Technol., 27(4):409 (1992).
29. Z. Yao and M. Le Maguer, Possibility of using
pseudo-diffusion approach to model mass transfer in
osmotic dehydration, Trans. ASAE, 41:409 (1998).
30. R. Saurel, A.L. Raoult-Wack, R. Rios, and S. Guil-
bert, Mass transfer phenomena during osmotic dehy-
dration of apple. II. Frozen plant tissue, Int. J. Food
Sci. Technol., 29:543 (1994).
31. R. Andreotti, M. Tomasicchino, and L. Machiavell,
La disidratazione parziale della frutta per osmosi, Ind.
Conserve., 58:88 (1983).
32. M. Tomasicchino, R. Andreotti, and A. De Giorgi,
Desidratazione partiale della frutta per osmosi. II.
Ananas, fragole e susine, Ind. Conserve., 61:108 (1986).
33. D. Torreggiani, R. Giangiacomo, G. Bertolo, and E.
Abbo, Ricerche sulla disidratazione osmotica della
frutta. I. Idoneita varietale delle ciliege, Ind. Conserve.,
61:101 (1986).
34. M. Dalla Rosa, G. Pinnavaia, and C.R. Lerici, La
disidratazione della frutta mediante osmosi diretta.
II. Esperienze di laboratorio su alcumi generi di frutta,
Ind. Conserve., 57:3 (1982).
35. R. Giangiacomo, D. Torreggiani, and E. Abbo, Os-
motic dehydration of fruit. Part I. Sugars exchange
between fruit and extracting syrups, J. Food Process.
Preserv., 11:183 (1987).
36. D. Torreggiani, R. Giangiacomo, G. Bartolo, and E.
Abbo, Ricerche sulla disidratazione osmotica della
frutta. II. Idoneita varietale della albicocche, Ind. Con-
serve., 61:226 (1986).
37. T.R.A. Magee, A.A. Hassaballah, and W.R. Murphy,
Internal mass transfer during osmotic dehydration of
apple slices in sugar solutions, Ir. J. Food Sci. Tech-
nol., 7:147 (1983).
38. I. Beristain, E. Azuara, R. Cortes, and H.S. Garcia,
Mass transfer during osmotic dehydration of pine-
apple rings, Int. J. Food Sci. Technol., 25:576 (1990).
39. J. Toupin, M. Marcotte, and M. Le Maguer, Osmot-
ically-induced mass transfer in plant storage tissue.
A mathematical model. Part I, J. Food Eng., 10:13
(1989).
40. J. Toupin and M. Le Maguer, Osmotically-induced
mass transfer in plant storage tissue. A mathematical
model. Part II, J. Food Eng., 10:97 (1989).
41. M. Marcotte, C.J. Toupin, and M. Le Maguer, Mass
transfer in cellular tissues. Part I. The mathematical
model, J. Food Eng., 13:199 (1991).
42. Z. Yao and M. LeMaguer, Mathematical modelling
and simulation of mass transfer in osmotic dehydra-
tion processes. I. Conceptual and mathematical
models, J. Food Eng., 29:349 (1996).
43. Z. Yao and M. LeMaguer, Osmotic dehydration: an
analysis of fluxes and shrinkage in cellular structure,
Trans. ASAE, 39:2211 (1996).
44. Z. Yao and M. LeMaguer, Finite element modelling of
osmotic dehydration processes, Food Res. Int.,
27(2):211 (1994).
45. Z. Yao and M. LeMaguer, Mathematical modelling
and simulation of mass transfer in osmotic dehydra-
tion processes. III. Parametric study, J. Food Eng.,
32:33 (1997).
46. A.-L. Raoult-Wack, F. Lafont, G. Rios, and S. Guil-
bert, Osmotic dehydration: study of mass transfer in
terms of engineering properties. In IDS ’89 (A.S.
Mujumdar and M.A. Roques, eds.), Hemisphere
Publ. Co., Washington, 1990, p. 487.
47. A.-L. Raoult-Wack, G. Rios, R. Saurel, F. Giroux,
and S. Guilbert, Modelling of dewatering and impreg-
nation soaking process (osmotic dehydration), Food
Res. Int., 27:207 (1994).
48. A.-L. Raoult-Wack, S. Guilbert, M. Le Maguer, and
G. Rios, Simultaneous water and solute transport in
shrinking media Part 1. Application to dewatering and
impregnation soaking process analysis (osmotic dehy-
dration), Drying Technol., 9:589 (1991).
49. A.-L. Raoult-Wack, F. Petitdemange, F. Giroux, S.
Guilbert, G. Rios, and A. Lebert, Simultaneous water
and solute transport in shrinking media. Part 2.
A compartmental model for the control of dewatering
and impregnation soaking process, Drying Technol.,
9:631 (1991).
50. A.-L. Raoult-Wack, O. Botz, S. Guilbert, and G. Rios,
Simultaneous water and solute transport in shrinking
media. Part 3. A tentative analysis of the spatial distri-
bution of the impregnating solute in the model gel,
Drying Technol., 9:631 (1991).
51. P. Fito, Modelling of vacuum osmotic dehydration of
food, J. Food Eng., 22:313 (1994).
52. P. Fito and R. Pastor, Non-diffusional mechanisms
occurring during vacuum osmotic dehydration, J. Food
Eng., 21:513 (1994).
53. X.Q. Shi and P. Fito, Mass transfer in vacuum os-
motic dehydration of fruits: a mathematical model
approach, LWT, 27:67 (1994).
54. D. Salvatori, A. Andres, A. Chiralt, and P. Fito, Os-
motic dehydration progression in apple tissue. II. Gen-
eralized
equations
for
concentration
prediction,
J. Food Eng., 42:133 (1999).
55. N. Panagiotou, V.T. Karathanos, and Z.B. Maroulis,
Mass transfer modelling of the osmotic dehydration of
some fruits, Int. J. Food Sci. Technol., 33:267 (1998).
56. G. Sacchetti, A. Gianotti, and M. Dalla Rosa,
Sucrose-salt combined effects on mass transfer kinet-
ics and product acceptability. Study on apple osmotic
treatments, J. Food Eng., 49:163 (2001).
57. E. Azuara, C.I. Beristain, and G.F. Gutierrez, Kinetic
model to explain the osmotic dehydration stages, Pro-
ceedings of IFT Annual Meeting (1996), p. 87.
58. M. Ferrando and W.E.L. Spiess, Cellular response of
plant tissue during the osmotic treatment with sucrose,
maltose and trehalose solutions, J. Food Eng., 49:115
(2001).
59. P. Fito, A. Chiralt, N. Betoret, M. Gras, M. Cha´fer,
J. Martinez-Monzo´, A. Andre´s, and D. Vidal, Vacuum
ß
2006 by Taylor & Francis Group, LLC.

impregnation and osmotic dehydration in matrix en-
gineering. Application in functional fresh food devel-
opment, J. Food Eng., 40:175 (2001).
60. J.X. Shi, M. Le Maguer, S.L. Wang, and A. Liptay.
Application of osmotic treatment in tomato process-
ing—effect of skin treatment on mass transfer in os-
motic dehydration of tomatoes, Food Res. Int., 30:669
(1997).
61. K. Venkatachalapathy and G.S.V. Raghavan, Com-
bined osmotic and microwave drying of strawberries,
Drying Technol., 17:837 (1999).
62. A. Lenart, Aspekty in_zzynieryjne osmotycznego odwad-
niania jabłek, Przem. Spo_zz. 30:216 (1976).
63. A. Lenart and R. Da˛browska, Mass transfer during
osmotic dehydration of apples with pectin coatings, In
Proceedings of the 11th International Drying Sympo-
sium Drying’98. (C.B. Akritidis, D. Marinos-Kouris,
G.D. Saravacos, and A.S. Mujumdar, eds.), ZITI Edi-
tions, Thessaloniki, 1998, p. 903.
64. W.M. Camirand, R.R. Forrey, K. Popper, F.P. Boyle,
and W.C. Stanley, Dehydration of membrane coated
foods by osmosis, J. Food Agric. Chem., 19:472 (1968).
65. J.D. Ponting, Osmotic dehydration of fruits—Recent
modifications and applications, Process. Biochem.,
8:18 (1973).
66. D.F. Farkas and M.E. Lazar, Osmotic dehydration of
apple pieces: effect of temperature and syrup concen-
tration on rates, Food Technol., 23:688 (1969).
67. M. Dixon, J.J. Jen, and V.A. Paynter, Tasty apple
slices result from combined osmotic-dehydration and
vacuum-drying
process,
Food
Process.
Develop.,
10:634 (1976).
68. P. Speck, F. Escher, and J. Solms, Effect of salt pre-
treatment on quality and storage stability of air-dried
carrots, LWT, 70:308 (1977).
69. G. Mazza, Dehydration of carrots. Effect of pre-dry-
ing treatments on moisture transport and product
quality, J. Food Technol., 18:113 (1983).
70. M.N. Islam and J.M. Flink, Dehydration of potato.
II. Osmotic concentration and its effect on air drying
behaviour, J. Food Technol., 17:387 (1982).
71. A. Lenart and P.P. Lewicki, Osmotyczne odwadnianie
jabłek w cyrkuluja˛cym syropie cukrowym, Zesz.
Probl. Post. Nauk Roln., 243:223 (1980).
72. A. Lenart and P.P. Lewicki, Owoce i warzywa utrwa-
lone
sposobem
osmotyczno-owiewowym,
Przem.
Spo_zz., 50:70 (1996).
73. P.P. Lewicki, A. Lenart, and D. Turska, Diffusive
mass transfer in potato tissue during osmotic dehydra-
tion, Ann. Warsaw Agric. Univ., Food Technol. Nutr.,
16:25 (1984).
74. P.P. Lewicki, A. Lenart, and G. Młynarczyk, Suszenie
osmotyczno-owiewowe jabłek. I. Analiza kinetyki
odwadniania osmotycznego jabłek, Przem. Ferm. i
Rol., 21(10):21 (1977).
75. Sitkiewicz, A. Lenart, and P.P. Lewicki, Mechanical
properties of osmotic-convection dried apples, Pol.
J. Food Nutr. Sci., 5/46:1 (1996).
76. D. Witrowa-Rajchert, P.P. Lewicki, and A. Lenart,
The influence of osmotic pretreatment on dry carrot
reconstitution properties. In Proceedings of the 10th
International Drying Symposium. Drying’96 (Cz. Stru-
miłło, Z. Pakowski, and A.S. Mujumdar, eds.), Ło´dz
Technical University, Ło´dz, vB: 1996, p. 847.
77. P.P. Lewicki, H. Kowalska, and A. Lenart, Effect of
temperature on mass transfer during osmotic dehydra-
tion of plant tissue, In Industrial Application of Os-
motic Dehydration/Treatments of Food (M. Dalla Rosa
and W.E.L. Spiess, eds.), Forum, Udine, 2000, p.31.
78. R. Lerici, D. Mastrocola, and G. Pinnavaia, Esper-
ienze di osmosi diretta ad alta temperatura per tempi
brevi, Ind. Conserve, 61:223 (1986).
79. M. Flink, Methods for improving freeze drying econ-
omy and their influence on product quality, Report to
Danish Agricultural Veterinary Research Council,
Copenhagen, 1981, p. 1.
80. P.P. Lewicki, A. Lenart, and M. Małkowska, Diffu-
sive movement of substance in the carrot dehydrated
osmotically in the sodium chloride solution, Ann.
Warsaw Agric. Univ., Food Technol. Nutr., 17:45
(1987).
81. I. Escriche, A. Chiralt, J. Moreno, and J.A. Serra,
Influence of blanching-osmotic dehydration treat-
ments on volatile fraction of strawberries, J. Food
Sci., 65:1107 (2000).
82. J. Moreno, A. Chiralt, I. Esriche, and J.A. Serra,
Effect of blanching/osmotic dehydration combined
method on quality and stability of minimally pro-
cessed strawberries, Food Res. Int., 33:609 (2000).
83. J. Moreno, A. Chiralt, I. Esriche, and J.A. Serra,
Stabilizing effect of combined enzyme inactivation/os-
motic dehydration methods on minimally processed
strawberries, Alimentaria, 295:49 (1998).
84. J.M. Del-Valle, V. Aranguiz, and H. Leon, Effect of
blanching and calcium infiltration on PPO activity,
texture, microstructure and kinetics of osmotic dehy-
dration of apple tissue, Food Res. Int., 31:557 (1998).
85. B.C. Prain, C.J.A. Olaeta, and M.P. Undurraga,
Evaluation of the behaviour of apricot (Prunus arme-
niaca L) cultivars Katy, Tilton and Imperial, in two
maturite stages, being dehydrated by four different
methods, Alimentos, 19(3):19 (1994).
86. G. Bidaisee and N. Badrie, Osmotic dehydration of
cashew apples (Anacardium occidentale L.): quality
evaluation of candied cashew apples, Int. J. Food
Sci., Technol., 36:71 (2001).
87. A. Lenart and H. Kowalska, Wpływ odwadniania
osmotycznego na przebieg suszenia konwekcyjnego
ziemniako´w, Przem. Spo_zz., 51:35 (1997).
88. P.P. Lewicki and A. Łukaszuk, Effect of osmotic
dewatering on rheological properties of apple sub-
jected to convective drying, J. Food Eng., 45:119
(2000).
89. N.K. Rastogi, A. Angersbach, and D. Knorr, Syner-
gistic effect of high hydrostatic pressure pretreatment
and osmotic stress on mass transfer during osmotic
dehydration, J. Food Eng., 45:25 (2000).
90. N.K. Rastogi and K. Niranjan, Enchanced mass
transfer during osmotic dehydration of high pressure
treated pineapple, J. Food Sci., 63:508 (1998).
ß
2006 by Taylor & Francis Group, LLC.

91. P.P. Lewicki, A. Lenart, and W. Pakuła, Influence of
artificial semi-permeable membranes on the process of
osmotic dehydration of apples, Ann. Warsaw Agric.
Univ., Food Technol. Nutr., 16:17 (1984).
92. R.D. Scott, Dehydration and packaging of foodstuffs
by dialysis, U.S. Patent 3,758,313 (1983).
93. A. Lenart and R. Da˛browska, Kinetics of osmotic
dehydration of apples with pectin coatings, Drying
Technol., 17:1359 (1999).
94. A.Lenart and R. Da˛browska, Osmotic dehydration of
apples with polysaccharide coatings, Pol. J. Food
Nutr. Sci., 6/47(4):105 (1997).
95. R. Da˛browska and A. Lenart, Influence of edible
coatings on osmotic treatment of apples. In Osmotic
Dehydration and Vacuum Impregnation. Applications in
Food Industry. (P. Fito, A. Chiralt, J.M. Barat, W.E.L.
Spiess, and D. Behsnilian, eds.), Technomic Publ. Co.,
Lancaster, 2001, p. 43.
96. H.M.C. Azeredo and J.G. Jardine, Optimization of
osmotic dehydration of pineapple applied to com-
bined methods technology, Ciencia e Tecnologia de
Alimentos, 20(1):74 (2000).
97. J.M. Flink, Dehydrated carrot slices: influence of os-
motic concentration on drying behaviour and product
quality, Food Proc. Eng., 1:412 (1980).
98. R. Lerici and D. Mastrocola, Nuove tecnologie nella
transformazione della frutta. 1. La disidratazione
osmotica, Notizario Tecnico, 18:2 (1985).
99. A. Lenart, Osmotyczne odwadnianie produkto´w
spo_zzywczych, Przem. Spo_zz., 30:86 (1976).
100. T.L. Adambounou and F. Castaigne, Deshydration
partielle par osmose des bananes et determination de
courbes de sorption isotherme, LWT, 16:230 (1983).
101. G. Pinnavaia, M. Dalla Rosa, and C.R. Lerici, La
disidratazione mediante osmosi diretta per la valoriz-
zazione di produtti vegetali. In Atti 28 Convegno
Nazionale Nutrizione Ambiente Lavoro (M. Coachi
and T.G. Modena, eds.), 1983, p. 313.
102. N.M. Panagiotou, V.T. Karathanos, and Z.B. Mar-
oulis, Effect of osmotic agent on osmotic dehydration
of fruits, Drying Technol., 17:175 (1999).
103. J.M. del Valle, T.R.M. Cuadros, and J.M. Aguilera,
Glass transition and shrinkage during drying and stor-
age of osmosed apple pieces, Food Res. Int., 31:191
(1998).
104. A. Quintero-Ramos, C. de la Vega, E. Hernandez, and
A. Anzaldua-Morales, Effect of the conditions of os-
motic treatment on the quality of dried apple dices,
AIChE Symp. Series, 89(297):108 (1993).
105. K.N. Waliszewski, H.D. Cortes, V.T. Pardio, and
M.A. Garcia, Color parameter changes in banana
slices during osmotic dehydration, Drying Technol.,
17:955 (1999).
106. P. Lewicki and E. Mazur, The effect of pH on the
course of osmotic dehydration. In Osmotic Dehydra-
tion of Fruits and Vegetables (A. Lenart and P.P.
Lewicki, eds.) Warsaw Agricultural University Press,
Warsaw, 1995, p. 20.
107. E. Maltini and D. Torreggiani, La disidratazione
osmotica. II. Possibilita d’impiego della concentra-
zione osmotica per la preparazione di conserve di
frutta. In Monografia No. 4. Progressi della Tecniche
di Disidratazione di Frutta e Ortaggi, CNR-IPRA,
Rome, p. 1985, 149.
108. H. Sarosi and A. Polak, Possibilities of application of
osmotic drying in the food industry, Tudomanyos
Kozlemenyck. Elelmiszeripari Foiskola, 6:63 (1976).
109. H.R. Bolin, C.C. Huxsoll, and R. Jackson, Effect of
osmotic agents and concentration on fruit quality,
J. Food Sci., 48:202 (1983).
110. A. Lenart and E. Grodecka, Wpływ rodzaju substancji
osmotycznej na kinetyke˛ suszenia owiewowego jabłek
i marchwi, Ann. Warsaw Agric. Univ., Food Technol.
Nutr., 18:21 (1989).
111. A. Argaiz, A. Lopez-Malo, E. Palou, and J. Welti,
Osmotic dehydration of papaya with corn syrup sol-
ids. Drying Technol., 12:1709 (1994).
112. J.A. Pino, D. Castro, P. Fito, J. Barat, and F. Lopez,
Multivariate statistical analysis of volatile compounds
as a criterion for selecting technological parameters in
the osmotic dehydration of pineapple, J. Food Qual.,
22:653 (1999).
113. A. Parjoko, S.M. Rahman, K.A. Buckle, and C.O.
Perera, Osmotic dehydration kinetics of pineapple
wedges using palm sugar, LWT, 29:452 (1996).
114. D. Torregiani, E. Forni, M.L. Erba, and F. Longoni,
Functional properties of pepper osmodehydrated in
hydrolized cheese whey permeate with or without
sorbitol, Food Res. Int., 28:161 (1995).
115. A. Lenart and P.P. Lewicki, Osmotic dehydration of
apples at high temperature. In IDS ’89 (A.S. Mujum-
dar and M. Roques, eds.), Hemisphere Publ. Co., New
York, 1990, p. 501.
116. E. Maltini, D. Torreggiani, G. Bertolo, and M.
Stecchini, Recent developments in the production of
shelf-stable fruit by osmosis. Proceedings of 6th Inter-
national Congress Food Science and Technology, 1983,
p. 177.
117. R. Lerici, N. Pepe, and G. Pinnavaia, La disidrata-
zione della frutta mediante osmosi diretta. I. Resultati
di esperienze effettuate in laboratorio, Ind. Conserve.,
52:1 (1977).
118. D. Mastrocola, C. Severini, and C.R. Lerici, Prove di
disidratazione per via osmotica della carota, Ind. Con-
serve., 62:33 (1987).
119. R. Andreotti, Conservazione di pesche parzialmente
disidrate per osmosi diretta, Ind. Conserve., 60:96
(1985).
120. M. Ayub, R. Khan, S. Wahab, A. Zeb, and
J. Muhammad, Effect of crystalline sweeteners on the
water activity and shelf stability of osmotically dehy-
drated guava. Sarhad J. Agric., 11:755 (1995) (FSTA
1996-05-J0081).
121. W. Azuara, H.S. Garcia, and C.I. Beristain, Effect of
the centrifugal force on osmotic dehydration of pota-
toes and apples, Food Res. Int., 29:195 (1996).
122. G. Donsi, G. Ferrari, R. Nigro, and P. di Matteo,
A combined technology for the production of dried
vegetables: osmotic dehydration/freeze drying, Ital.
Food Beverage Technol., 15:9, 17 (1999).
ß
2006 by Taylor & Francis Group, LLC.

123. A.M. Sereno, R. Moreira, and E. Martinez, Mass
transfer coefficients during osmotic dehydration of
apple in single and combined aqueous solutions of
sugar and salt, J. Food Eng., 47:43 (2001).
124. S. Bhuvaneswari, V.V. Sreenaryanan, R. Kailappan,
and K. Parvathy, Osmotic dehydration of peas, Indian
Food Packer, 53(2):10 (1999).
125. F.K. Ertekin and T. Cakaloz, Osmotic dehydration of
peas. I. Influence of process variables on mass trans-
fer, J. Food Process. Preserv., 20:87 (1996).
126. F.K. Ertekin and T. Cakaloz, Osmotic dehydration of
peas. II. Influence of process variables on mass trans-
fer, J. Food Process. Preserv., 20:105 (1996).
127. M.S. Tapia, R. Consuegra, P. Corte, A. Lopez-Malo,
and J. Welti, Minimally processed papaya by osmotic
vacuum impregnation techniques, Ciencia y Tecnolo-
gia de Alimentos Internacional, 5(1):41 (1999).
128. T.L. Adambounou, F. Castaigne, and I.C. Dillon,
Abaissement de l’activite de 1’eau de legumes tropi-
caux par deshydration osmotique partielle, Sci. Ali-
ment., 3:551 (1983).
129. P.V. Vijayanand, N. Chaud, and W.E. Eipeson, Opti-
mization of osmotic dehydration of cauliflower,
J. Food Process. Preserv., 19:229 (1995).
130. W. Hoover and N.C. Miller, Factors influencing im-
pregnation of apple slices and development of a con-
tinuous process, J. Food Sci., 40:698 (1975).
131. G.W. Hope and D.G. Vitale, Osmotic Dehydration,
International Development Research Center Mono-
graph, IDRC-004e, Ottawa, Canada, 1972, p. 1.
132. D.R. Bongirwar and A. Sreenivasan, Studies on os-
motic dehydration of banana, J. Food Sci. Technol.,
74:104 (1977).
133. V. Pavasovi
cc, M. Stefanovi
cc, and R. Stefanovi
cc, Os-
motic dehydration of fruit. In Drying ’86 Vol. 2 (A.S.
Mujumdar, ed.), Hemisphere Publ. Co., Italic NOT
ALLOWEDNew York, 1986, p. 761.
134. N.E. Mavroudis, V. Gekas, and I. Sjo¨holm, Osmotic
dehydration of apples—effect of agitation and raw
material characteristics, J. Food Eng., 35:191 (1998).
135. X.Q. Shi, P. Fito, and A. Chiralt, Influence of vacuum
treatment on mass transfer during osmotic dehydra-
tion of fruits, Food Res. Int., 28:445 (1995).
136. G.W. Dalla Rosa, G. Pinnavaia, and C.R. Lerici, La
disidratazione della frutta mediante osmosi diretta. II.
Esperienze di laboratorio su alcumi generi di frutta,
Ind. Conserve., 57:3 (1982).
137. I. Escriche, R. Garcia-Pinchi, A. Andres, and P. Fito,
Osmotic dehydration of kiwifruit (Actinidia Chinen-
sis): fluxes and mass transfer kinetics, J. Food Process
Eng., 23:191 (2000).
138. D. Castro, O. Treto, P. Fito, G. Panades, M. Nunez,
C. Fernandez, and J.M. Barat, Osmotic dehydration
of pineapple under pulsed vacuum. Study of process
variables, Alimentaria, 282:27 (1997).
139. N.K. Rastogi and K.S.M.S. Raghavarao, Kinetics of
osmotic dehydration under vacuum, LWT, 29:669 (1996).
140. H. Moy, N.B. Lau, and A.M. Dollar, Effects of
sucrose and acids on osmovac-dehydration of tropical
fruits, J. Food Process. Preserv., 2:131 (1978).
141. A. Lenart and M. Cerkowniak, Osmo-convective dry-
ing of apples. In Proceedings of the Third Main Meet-
ing: Process Optimisation and Minimal Processing
of Foods, Vol. 3 (J.C. Oliveira and F.A.R. Oliveira
eds.), Escola Superior de Biotecnologia Press. Porto,
1998, p. 19.
142. P. Lewicki, A. Lenart, and G. Młynarczyk, Poro´wna-
nie energochłonnos´ci usuwania wody z jabłek metoda˛
suszenia konwekcyjnego i odwadniania osmotycz-
nego, Przem. Ferm. i Owoc.-Warz., 24(8/9):24 (1980).
143. A. Lenart and J.M. Flink, An improved proximity
equilibration cell method for measuring water activity
of food, LWT, 16:84 (1983).
144. E. Contreras and T.G. Smyrl, An evaluation of os-
motic concentration of apple rings using corn syrup
solids solutions, Can. Inst. Food Sci. Technol. J.,
14:310 (1981).
145. A. Lenart and J.M. Flink, Osmotic concentration of
potato, I. Criteria for the end-point of the osmosis
process, J. Food Technol., 19:45 (1984).
146. R. Garcia, J.F. Menchu, and C. Rolz, Tropical fruit
drying, A comparative study. In Proceedings of 4th
International Congress Food Science and Technology,
1974, p. 13.
147. K.D. Sharma and B.B. Lalkanshal, Mass transfer dur-
ing osmotic dehydration and its influence on quality of
canned plum, J. Sci. Ind. Res., 58:711 (1999).
148. K.D. Sharma and B.B. Lalkanshal, Effect of partial
osmotic dehydration prior to canning on drained
weight and quality of three varieties of plum, J. Food
Sci. Technol., India, 36:136 (1999).
149. H. Kowalska, A. Lenart, and M. Janowicz, Wymiana
masy w czasie odwadniania osmotycznego truskawek i
wis´ni,
Zeszyty
Naukowe
Politechniki
Opolskiej,
Mechanika, 254:135 (2000).
150. A. Levi, S. Gagel, and B. Juven, Intermediate moisture
tropical fruits products for developing countries. I.
Technological data on papaya, J. Food Technol.,
18:667 (1983).
151. R. Da˛browska and A. Lenart, Wpływ błon pektyno-
wych na odwadnianie osmotyczne jabłek, Materiały
VIII Konferencji Naukowo-Technicznej ‘‘Budowa i Eks-
ploatacja Maszyn Przemysłu Spo_zzywczego.’’ Dział
Wydawnictw i Poligrafii Politechniki Białostockiej.
Białystok, 59 (1998).
152. A. Lenart and P.P. Lewicki, Kinetics of osmotic de-
hydration of the plant tissue. In Drying ’87, Vol. 2
(A.S. Mujumdar, ed.), Hemisphere Publ. Co., New
York, 1987, p. 239.
153. A. Lenart and P.P. Lewicki, Mechanizm odwadniania
osmotycznego tkanki ros´linnej, Materiały XVII Sesji
Naukowej Komit. Techn. i Chem. _
Z
Zywn. PAN, Ło´dz,
1986, p. 558.
154. E. Azuara, C.I. Beristain, and G.F. Gutierrez,
A method for continuous kinetic evaluation of os-
motic dehydration, LWT, 31:317 (1999).
155. J.M. Barat, A. Albors, A. Chiralt, and P. Fito,
Equilibrium of apple tissue in osmotic dehydra-
tion: microstructural
changes, Drying Technol.,
17:1375 (1999).
ß
2006 by Taylor & Francis Group, LLC.

156. H. Kowalska and A. Lenart, Mass exchange during
osmotic pretreatment of vegetables, J. Food Eng.,
49:137 (2001).
157. A. Lenart, Sacharoza Jako Czynnik Modyfikuja˛cy
Osmotyczno-Owiewowe Utrwalanie Jabłek, Wydaw-
nictwo SGGW, Warszawa (1988).
158. M. Dalla Rosa and F. Giroux, Osmotic treatments
(OT) and problems related to the solution manage-
ment, J. Food Eng., 49:223 (2001).
159. A. Valdez-Fragoso, J. Welti-Chanes, and F. Giroux,
Properties of a sucrose solution reused in osmotic de-
hydration of apples. Drying Technol., 16:1429 (1998).
160. J.A. Szymczak, W.J. Płocharski, and D. Konopacka,
The influence of repeated use of sucrose syrup on the
quality of osmo-convectively dried sour cherries, In
Proceedings of the 11th International Drying Sympo-
sium, IDS’98 Vol. A (C.B. Akritidis, D. Marinos-
Kouris, G.D. Saravacos and A.S. Mujumdar, eds.),
Ziti Editions, Thessaloniki, 1998, p. 895.
161. M. Dalla Rosa, F. Bressa, D. Mastrocola, and P.
Pittia, Use of osmotic treatments to improve the qual-
ity of high moisture-minimally processed fruits. In
Osmotic Dehydration of Fruits and Vegetables (A.
Lenart and P.P. Lewicki, eds.), Warsaw Agricultural
University Press, Warsaw, 1995, p. 69.
162. F. Girod, A. Collignan, A. Themelin, and A.-L.
Raoult-Wack, Energy study of food processing by
osmotic dehydration and air drying, Precedes de
De´shydration-Impre´gnation
par
Immersion
(D11)
dans des Solutions Concentrees, Montpellier (1990).
163. A. Lenart and P.P. Lewicki, Energy consumption dur-
ing osmotic and convective drying of plant tissue, Acta
Aliment. Pol., 14:65 (1988).
164. D. Mastrocola, M. Dalla Rosa, and C.R. Lerici, Pro-
cessi di scambio e caratteristiche chimico-fisiche dei
prodotti ricostituiti, Technologie Alimentari, 1:70
(1999).
165. P. Lewicki and A. Lenart, Energy consumption during
osmo-convection drying of fruits and vegetables. In
Drying of Solids (A.S. Mujumdar, ed.), Inter. Sci.
Publ., New York, and Oxford & IBH Publ. Co.,
New Delhi, 1992, p. 354.
166. A. Lenart, Osmo-convection drying of fruits and
vegetables: technology and application, Drying Tech-
nol., 14:391 (1996).
167. A. Marouze´, F. Giroux, A. Collignan, and M. Rivier,
Equipment design for osmotic treatments, J. Food
Eng., 49:207 (2001).
168. S. Smarkusz, P.P. Lewicki, A. Lenart, and C. Stygar,
Urza˛dzenie do krojenia warzyw i owoco´w, głowica
no_zzowa do tego urza˛dzenia oraz no´_zz kra˛_zzkowy do tej
głowicy. Patent P-329 263 (1998).
169. S. Smarkusz, J. Kiełkiewicz, W. Raczko, P.P. Lewicki,
A. Lenart, and W. Płocharski, Linia produkcyjna do
wytwarzania czipso´w, Patent P-329 264 (1998).
170. M. Le Maguer, Osmotic dehydration: review and fu-
ture directions. In Prog. Food Preservation Processes,
Vol. 1, CERIA, Brussels, 1988, p. 283.
171. Z. Pałacha and A. Danak, Effect of temperature on
water sorption properties of osmo-convection dried
pumpkin and carrot, In Properties of Water in Foods
(P.P. Lewicki, ed.), Warsaw Agricultural University
Press, Warsaw, 1997, p. 130.
172. H.N. Lazarides, P. Fito, A. Chiralt, V. Gekas, and
A. Lenart, Advances in osmotic dehydration, In
Processing Foods. Quality Optimization and Process
Assessment (F.A.R. Oliveira and J.C. Oliveira, eds.),
CRC Press, Washington, 1999, p. 175.
173. M. Janowicz, H. Kowalska, W. Pomaran´ska-Łazuka,
and A. Lenart, Mass exchange during osmotic dehy-
dration of fruits, In Properties of Water in Foods (P.P.
Lewicki, ed.), Warsaw Agricultural University Press,
Warsaw, 2000, p. 144.
174. A. Lenart and P.P. Lewicki, Dyfuzja wody w tkance
jabłka podczas osmotycznego odwadniania, Zesz.
Nauk. SGGW-AR, Technol. Roln.-Spo_zz., 14:33 (1981).
175. A. Lenart, Wpływ włas´ciwos´ci tkanki ros´linnej na
dyfuzje˛ substancji osmoaktywnych, Zesz. Probl. Post.
Nauk Roln., 297:351 (1986).
176. A. Lenart, P.P. Lewicki, and Z. Pałacha, Water bind-
ing in the apple tissue during its diffusive processing.
In Drying ’86 (A.S. Mujumdar, ed.), Hemisphere Publ.
Co., New York, 1986, p. 516.
177. Z. Pałacha, A. Lenart, and P.P. Lewicki, Desorpcja
wody z jabłka po obro´bce dyfuzyjnej w zakresie
aktywnos´ci wody 0.9–1.0, Zesz. Nauk. Politech.
Ło´dz. In_zz. Chem., 14:56 (1987).
178. J. Wiczkowska and D. Witrowa-Rajchert, Wpływ sus-
zenia
osmotyczno-konwekcyjnego
na
włas´ciwos´ci
rekonstytucyjne suszu, Zeszyty Naukowe Politechniki
Ło´dzkiej, In_zzynieria Chemiczna i Procesowa, 25:147
(1999).
179. Z. Pałacha and R. Babski, Wpływ wste˛pnej obro´bki
osmotycznej
na
przebieg
procesu
zamra_zzania
marchwi, Zeszyty Naukowe Politechniki Opolskiej,
Mechanika, 254: 229 (2000).
180. D. Witrowa-Rajchert, P.P. Lewicki, and A. Lenart,
Reconstitution properties of osmo-convective dried
plant tissue, In Engineering and Food, Part II
(R. Jowitt, ed.), Sheffield Academic Press, London,
1997, G 45.
181. D. Nowak, A. Danak, P.P. Lewicki, and A. Lenart,
Zmiany włas´ciwos´ci rekonstytucyjnych jabłek suszo-
nych sposobem osmotyczno-konwekcyjnym w czasie
przechowywania, Zeszyty Problemowe Poste˛po´w Nauk
Rolniczych, 454:501 (1998).
182. D. Piotrowski and A. Lenart, Recent advances in the
drying of apples under variable process conditions, In
Processing Foods. Quality Optimization and Process
Assessment (F.A.R. Oliveira and J.C. Oliveira, eds.),
CRC Press, Washington, 1999,229.
183. C.K. Sankat, F. Castaigne, and R. Maharaj, The air
drying behaviour of fresh and osmotically dehydrated
banana slices, Int.J. Food Sci. Technol., 31:123 (1996).
184. D. Nowak, P.P. Lewicki, and A. Lenart, Stability of
apples during storage, Proceedings of the Final Work-
shop ‘‘Shelf Life Prediction’’ Copernicus Project CIPA-
CT94-0120, ATO-DLO Press, Wageningen, 1997, p. 81.
185. A. Lenart, D. Piotrowski, and J. Doman˜ski, The
influence of edible coatings on the temperature
ß
2006 by Taylor & Francis Group, LLC.

changes of dried apples, Drying’98. Proceedings of the
11th International Drying Symposium (C.B. Akritidis, D.
Marinos-Kouris, G.D. Saravacos, and A.S. Mujumdar,
eds.), ZITI Editions, Thessaloniki, 1998, p. 1074.
186. Z. Pałacha and P.P. Lewicki, Włas´ciwos´ci sorpcyjne
marchwi suszonej sposobem osmotyczno-konwekcyjnym,
Zeszyty
Naukowe
Politechniki
Ło´dzkiej,
In_zzynieria
ChemicznaiProcesowa, 25:105 (1999).
187. F. Prothon, L.M. Ahrne, T. Funebo, S. Kidman,
M. Langton, and I. Sjo¨holm, Effect of combined os-
motic and microwave dehydration of apple on texture,
microstructure and rehydration characteristics, LWT,
4:95 (2001).
188. I. Sitkiewicz, A. Lenart, and P.P. Lewicki, Rheological
properties of osmo-dehydrated plant tissue, In Engin-
eering and Food, Part II (R. Jowitt, ed.), Sheffield
Academic Press, London, 1997, G 41.
189. P.P. Lewicki and I. Sitkiewicz, Wpływ obro´bki wste˛p-
nej przed suszeniem konwekcyjnym na włas´ciwos´ci
reologiczne suszonej cebuli, Zeszyty Problemowe Post-
e˛po´w Nauk Rolniczych, 454:447 (1998).
190. P.P. Lewicki and A. Lenart, Effect of osmotic pre-
treatment on convection drying of selected fruits, Pro-
ceedings of the First Seminar on Osmotic Treatment,
Osmotic Pretreatments for the Food Industry, FEUP
Edic¸oes, Porto, Portugal, 1999, p. 29.
ß
2006 by Taylor & Francis Group, LLC.

Document Outline
- Table of Contents
- Chapter 028: Osmotic Dehydration of Fruits and Vegetables
Wyszukiwarka
Podobne podstrony:
025 Drying of Fruits and Vegetables
Combined osmotic and microwave vacuum dehydration of Apple and strawberries
fruits and vegetables
12 FRUITS AND VEGETABLES
Drying Fruits and Vegetables 2nd Edition
Modelling vacuum and convective dehydration of Vegetables (Patricia Gerla, Jorge Martínez Garreiro)
Drying kinetics and quality of vacuum microwave dehydrated garlic cloves and slices
Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum microwav
Approaches to improving the quality of dried fruit and vegetables
The Big Book of Juicing 150 of the Best Recipes for Fruit and Vegetable Juices, Green Smoothies, and
Dehydration of Carrots by a Combination of Freeze Drying, Microwave Heating and Air or Vacuum Drying
effect of high fiber vegetable fruit diet on the activity of liver damage and serum iron level in po
Historia gry Heroes of Might and Magic
Overview of Exploration and Production
Blanchard European Unemployment The Evolution of Facts and Ideas
Magnetic Treatment of Water and its application to agriculture
ABC Of Arterial and Venous Disease
68 979 990 Increasing of Lifetime of Aluminium and Magnesium Pressure Die Casting Moulds by Arc Ion
ABC Of Occupational and Environmental Medicine
więcej podobnych podstron