
A review on recent approaches in the field of hot dip zinc galvanizing process
S.M.A. Shibli, B.N. Meena, R. Remya
PII:
S0257-8972(14)01198-0
DOI:
doi:
10.1016/j.surfcoat.2014.12.054
Reference:
SCT 19991
To appear in:
Surface & Coatings Technology
Received date:
7 November 2014
Accepted date:
22 December 2014
Please cite this article as: S.M.A. Shibli, B.N. Meena, R. Remya, A review on recent
approaches in the field of hot dip zinc galvanizing process, Surface & Coatings Technology
(2014), doi:
10.1016/j.surfcoat.2014.12.054
This is a PDF file of an unedited manuscript that has been accepted for publication.
As a service to our customers we are providing this early version of the manuscript.
The manuscript will undergo copyediting, typesetting, and review of the resulting proof
before it is published in its final form. Please note that during the production process
errors may be discovered which could affect the content, and all legal disclaimers that
apply to the journal pertain.

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
1
A review on recent approaches in the field of hot dip zinc
galvanizing process
S.M.A. Shibli*, B.N. Meena, R. Remya
Department of Chemistry, University of Kerala, Kariavattom Campus
Thiruvananthapuram, Kerala-695 581, India
Abstract
The recent developments in the field of hot dip zinc coating are reviewed with special
reference to different industrial applications. The improvements in physical and chemical
structural composition due to pre and post modification process are discussed. The present
review has the focus mainly on the readership of young researchers engaged in this field.
Very recent developments on the hot dip galvanization processes are highlighted. Their
industrial competencies with aluminium dipping are also briefly discussed. The scopes for
immediate future developments are also highlighted then and there.
Key words: Hot dip galvanization, Intermetallics, Metal/metal oxide incorporation,
Galvannealed coating, Aluminide coating.
----------------------------------------------------------------------------------------------------------
*Author for all correspondence: smashibli@yahoo.com
Phone: + 91 85470 67230 (mob), +91 471 2308682 (off), +91 471 2167 230 (Res)

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
2
1. Introduction
Steel of different forms, is an integral part of building and construction industry due
to its high strength and durability. Although many new and advanced materials have been
developed for engineering applications, steel is still considered to be the main construction
material for automobiles, appliances and industrial machinery [1]. Steel undergoes corrosion
when exposed to different environments. There are different methods to prevent corrosion
such as cathodic protection, anodic protection, addition of inhibitors, protective coatings and
metallic coatings. Zinc coatings are extensively used for the protection of steel. In such cases,
the more active zinc metal corrodes preferentially than the steel substrate by a cathodic
reaction that prevents steel from undergoing anodic corrosion reaction. Different types of
zinc coatings include: hot dip galvanizing (batch or continuous), electroplating, metalizing
(zinc spraying), mechanical plating and zinc rich paint. Among them, the hot dip
galvanization process, offers a unique combination of superior properties such as high
strength, formability, light weight, corrosion resistance, low cost and recyclability. In a
conventional hot dip galvanizing process, a steel article is cleaned, fluxed and then immersed
in a molten zinc bath at a temperature of about 450 °C [2]. Hot dip galvanized steels have
been extensively used in industrial fields such as automobiles, electrical home appliances or
construction due to their excellent corrosion resistance characteristics [3]. This technique has
been adopted as a well proven feasible process since 1800 soon after the exploration of iron
and zinc. The exploration of the process was attempted in 1742 when a French Chemist
Melouin presented a paper on hot dip galvanizing, and the process received commercial
momentum with patents mainly in the 1830‟s. The reason for the extensive use of hot dip
galvanization is the two-fold protective nature of the coating. As a barrier coating, it provides
a tough, metallurgically bonded zinc coating that completely covers the steel surface and
protects steel from corrosion. Additionally, the sacrificial action of zinc protects the steel
even when damage or a minor discontinuity occurs on its surface.
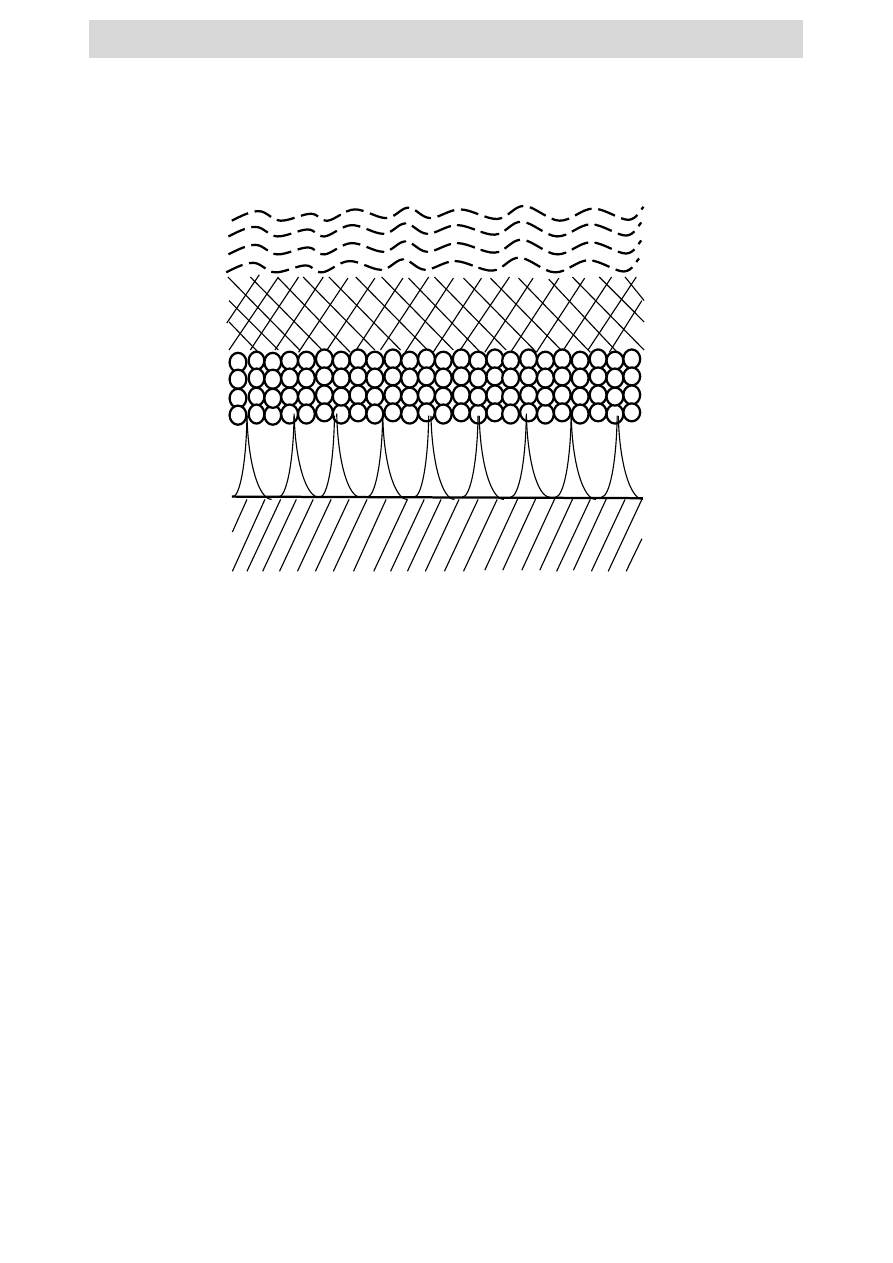
A hot dip galvanized coating consists of a heterogeneous assembly of different phases
which are formed due to metallurgical reactions between iron and zinc when a ferrite
substrate is immersed into molten zinc [4]. After solidification, the coating consists of an
outer layer of 100 % zinc (η-eta layer) and inner layers called alloy layers consisting of
intermetallic phases of iron and zinc such as zeta (ζ) layer (94 % Zn – 6 % Fe), delta (δ) layer
(90 % Zn – 10 % Fe) and gamma (Г) layer (75 % Zn – 25 % Fe) [5-7] (Fig. 1). These
intermetallic layers are relatively harder than the underlying steel and provide exceptional

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
3
protection against coating damage. The characteristics of the intermetallic phases of hot dip
zinc coatings are compared in Table1 [8-11].
2. The process
2.1. Selection of substrate
The composition of a steel substrate has great influence on the hot dipping process
and the performance of the resultant hot dip zinc coating. The change in the composition of
the substrate not only influences the rate of attack of steel by molten zinc but also changes the
mode of attack at a given galvanizing temperature [12]. The compositional variation of the
steel used for hot dip zinc coating is an important factor that determines the zinc drainage,
coating morphology and protection capacity of the coating. The chemical composition of the
steel substrate also influences the metallurgical properties of the hot dip zinc coatings. The
presence of Si, P, C and Mn in the steel substrate can influence the Fe-Zn solidification
mechanism depending on their concentration. The presence of critical amount of silicon and
phosphorus in the steel substrate is necessary to control the coating weight and the presence
of carbon & phosphorus accelerates the growth of the alloy layer, thereby improving the
adherence of the coating [13,14]. There are mainly two groups of steels that are used for hot
dip galvanization namely low carbon, non-killed steel with low silicon and reactive steel with
high silicon content.
2.2. Preparation of the base surface
Surface preparation is an important step in hot dip galvanization because zinc does
not metallurgically react with steel surface when it is not completely clean. The effective
cleaning of steel substrate can be achieved by a variety of processes. Surface preparation
typically consists of degreasing, pickling and fluxing. Oils, greases and other saponificable
compounds present in the steel substrates are removed through degreasing. Alkaline solutions
are normally used for this purpose since they are less expensive than vapour degreases using
costly organic solvents. Yuttanant et al. [15] and R. Sa-nguanmo et al. [2] used to degrease
the low carbon cold rolled steel substrate using 10 % NaOH solution at 60 °C for 10 minute
prior to hot dip galvanization. The steel substrate could also be degreased using 5 % NaOH
solution at 50 °C to ensure that the substrate is free from foreign materials [16].

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
4
Normally steel substrate contains oxides of iron, even after the removal of greasy
substances and that are to be removed by pickling. The warm/hot acid solutions such as HCl
and H
2
SO
4
are commonly used for the pickling process as both provide same pickling effect.
But most of the researchers prefer HCl as pickling agent due to its use at low temperature,
with less volume. Moreover, it is easy to inhibit and the steel substrate doesn‟t require any
caustic treatment [17]. Shibli et al. have reported about the usage of 8 % HCl as a pickling
agent for steel substrate [16]. It should be noted that steel coupons could also be pretreated by
pickling in 14 % HCl at room temperature for 20 min [15, 2]. Stieglitz et al. have reported the
use of a pickling solution of HCl and H
3
PO
4
to ensure a stable coating [18].
Fluxing is required to dissolve any oxide films formed on the steel surface after
pickling. It should be ensured that a clear metal surface contacts the molten zinc during the
galvanization process. The fluxing treatment provides good adherence of liquid zinc on the
steel substrate and facilitates adequate metallurgical interaction between zinc and steel. It also
suppresses in-situ oxidation of the steel surface by atmospheric oxidation. Fluxing solutions
consist of alkali and alkaline earth metal chlorides or fluorides with zinc chloride. The
conventional fluxing solution consists of a mixture of zinc chloride (ZnCl
2
) and ammonium
chloride (NH
4
Cl) in 1:3 mole ratio [19]. The presence of ammonium chloride in the flux
promotes drying of the flux on the steel substrate before its entry into the molten zinc bath
and zinc chloride protects the steel from corrosion or surface oxidation prior to its entry into
the molten zinc bath. The flux coated steel substrate is dried at a temperature of about 120 °C
since the flux decomposes at about 150 °C. A salt mixture containing zinc, ammonium,
sodium, potassium, cobalt and lead chloride has been formulated based on the decomposition
temperature of individual chloride salts for Zn – 5 wt. % Al alloy coating on wire surface
[20]. Yuttanant et al. have reported that a fluxing solution that contains NiCl
2
could affect the
growth morphology of zeta phase and reduce its growth rate, resulting in the reduction of the
overall thickness of the coating [15]. The use of NiCl
2
in a fluxing solution serves as a simple
and effective method for preventing excessive growth of galvanized coatings. Balloy et al.
have reported the use of vegetable oil like linseed oil as fluxing agent rather than
conventional industrial chloride flux [21]. The use of a mineral oil with an acid function such
as HCl has also been reported as a fluxing agent during hot dip galvanization. However the
usage of vegetable or mineral oils is yet to be proved commercially. There is no scope for
commercialization of such processes as conventional fluxing does not involve with much
cost. Similar is the case regarding the proposal by Balloy et al. who has proposed a process
called “single bath” in order to merge the steps of pickling & fluxing. In this case the same

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
5
bath has HCl at the bottom to accelerate the pickling of steel while the mineral oil at the top
is used as flux [21]. Such complicated processes have limited scope of reaching industry as
many more recent developments have been emerged for this purpose.
2.3. Hot dip galvanization
During hot dip galvanization, the steel substrate is completely immersed in a bath
consisting of a minimum of 98 % pure molten zinc. The bath chemistry is specified by the
ASTM in specification B6. The bath temperature is maintained at about 450 °C. The zinc
metal then reacts with the iron on the steel surface to form a zinc/iron intermetallic alloy. The
articles are withdrawn slowly from the galvanizing bath and the excess zinc is removed by
draining, vibrating or centrifuging. The articles are cooled in air immediately after
withdrawal from the bath. The characteristics of the resultant hot dip zinc coatings depend on
the following parameters.
2.3.1. Bath temperature
Conventionally the hot dip bath is maintained at around 440-460 °C. However, most
of the significant chemical reactions between iron-zinc alloy coatings and the molten zinc
occur at 480 °C. Below 480 °C, a compact Zn-Fe alloy is formed at the surface of the steel
with the alloying action eventually ceasing up and above this critical temperature,
fragmentation of the alloy layer is happened that stimulates zinc to penetrate into the metal
resulting in the formation of very large amount of dross. The bath temperature can be
controlled to tune the nature and thickness of the intermittent layers to suit the purpose of the
specific application.
2.3.2. Dipping time
The alloying reactions proceed after about 20 seconds of immersion of the substrate
into the molten zinc bath. Conventionally 4-5 minutes duration is maintained for optimum
alloying reactions. In certain cases where heavy articles are to be galvanized, they are kept
immersed for longer durations to enhance zinc penetration as well as the overall coating
thickness. The dipping duration can be controlled to tune the nature and thickness of the
intermittent layers and also the extent of coverage.

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
6
2.3.3. Withdrawal speed
The steel substrate is taken out of the bath when the reaction between iron and zinc
completed. The thickness of hot dip galvanized coatings depends on withdrawal speed.
Conventionally an optimum withdrawal rate of 1.5m per minute is maintained for the
formation of a bright shiny zinc coating. If the withdrawal speed is too slow, a uniform
unalloyed zinc layer is formed while in the case of faster withdrawal speed, an uneven
coating is formed. Hence, the withdrawal speed should be controlled to tune the nature,
thickness and the surface finishing of the coating.
2.3.4. Bath composition
The extent of effective life of hot dip zinc coating not only depends on the coating
composition but also on the metallurgical characteristics of the coating. It is necessary to
increase the quality and thus the service life of the coating by making improvements in the
galvanizing techniques. Several additives are incorporated into molten zinc bath in order to
improve the performance of hot dip zinc coatings. The presence of elements such as
aluminium, magnesium, and nickel plays an important role in improving the galvanic
performance of hot dip zinc coatings. Aluminium is one of the commonly used additives in
molten zinc bath. Aluminium has the ability to reduce the rate of oxidation of molten zinc
and to reduce the spangle size thereby improves the uniformity of the coating. The effect of
aluminium on the bath has also been extensively studied for improving the corrosion
resistance [22,23]. During hot dip galvanization, the presence of aluminium in the molten
zinc bath suppresses the growth of Fe-Zn intermetallic layer and reduces iron loss into the
bath through the formation of Fe
2
Al
5
and FeAl
3
intermetallic layers. It has been reported that
the presence of Al (0.15 – 0.2 wt. %) in molten zinc bath can prevent the growth of Fe-Zn
intermetallic layer at the steel coating interface [24]. Liberski et al. has reported that hot dip
zinc coating containing 5 wt. % Al could possess excellent corrosion resistance than
conventional zinc coatings [25]. The most commonly used industrial hot dip zinc coatings
with aluminium as additive are the galvanized iron (Zn – 0.2 wt. % Al), galvalume (55 wt. %
Al, 43.5 wt. % Zn, 1.5 wt. % Si) and galfan (Zn – 5 wt. % Al) [26]. The addition of lead and
antimony in small concentrations (0.004 – 0.200 %) could significantly improve the
uniformity and adherence of the coating [27]. Pistofidis et al. have found that the addition of
bismuth into the galvanizing bath could yield excellent adhesion and corrosion resistance

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
7
[28]. However the compositional modification using elements such as lead and antimony is
seemed to have no scope for receiving any commercial attention.
3. Evaluation of coating composition
The important properties that concern the use of zinc coatings are primarily corrosion
and formability and other properties involved are weldability & paintability. The progress of
corrosion in different types of galvanized coatings can be investigated by using
Electrochemical Impedance Spectroscopic (EIS) technique. V. Barranco et al. have compared
the corrosion rate of hot dip galvanized coatings especially pure zinc, Zn – 5 wt. % Al and Zn
– 10 wt. % Fe in 3 % NaCl, based on EIS measurements. The EIS results revealed that
corrosion of the pure zinc & Zn – 5 wt. % Al coatings progresses in an almost uniform way
while the corrosion rate of the Zn – 10 wt. % Fe coating experiences a decrease after 10 days
of exposure [29]. J.H. Hong et al. have reported that the Mossbauer properties can be used for
the phase formation and transformation studies as a function of the process parameters. The
Mossbauer spectra of different Fe-Zn intermetallic phases are different from each other.
According to them, the gamma and gamma-1 phases are composed of four sub spectra, while
the delta and zeta phases reveal three and one sub spectrum respectively [30]. H. Liu et al.
have studied the influence of H
2
and water vapour content on selective oxidation during
continuous hot dip galvanization by thermo-calc and Wagner model. As per the model, the
nature of oxidation process (internal/external) can be changed by altering H
2
content in the
annealing atmosphere. A study based on thermodynamic characteristics of the process has
revealed that simple oxides such as MnO
2
, Cr
2
O
3
etc produced by selective oxidation may be
reduced by effective Al in Zn liquid [31]. Thus, in-situ and post evaluation of the coating
characteristics could yield significant information to alter the composition of the bath and the
coating.
4. The phase reaction and formation of intermetallics
Dutta et al. have reported about the formation of MgZn
2
on the top surface and
described about the morphology and properties of Zn-Mg & Zn-Mg-Al coatings [32]. They
also discussed about the Mg gradient in the coating. Liu et al. have studied the relationship
between the annealing atmosphere and microstructure of Zn-Al coated dual phase steel. They
discussed about the coexistence of Fe-Al intermetallic compounds and needle like Fe-Zn

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
8
compound as the result of reduction in aluminothermic reaction during hot-dip zinc process
[33]. Song et al. have found that zinc coating on dual phase steel consists of a zinc layer and
columnar
–FeZn
13
particles on the top of a thin inhibition layer next to steel substrate. These
inhibition layers consist of lenticular –Fe
2
Al
5-x
Zn
x
particles [34]. Yang et al. have studied the
influence of strip entry temperature on the formation of interfacial layer during hot dip
galvanization of hardened steel. He has illustrated that the size of
-phase was increased with
strip entry temperature and was formed at the base steel surface when temperature was 480
°C. According to Yang et al. the Fe-Al intermetallic layer was fully formed at strip entry
temperature ranging from 440-480 °C [35].
Cheng et al. reported the formation of a new quaternary phase in the interdentritic
area of a laser treated Zn-Al-Mg-Si coating [36]. Yang et al. studied the growth of
2
intermetallic compounds on hot dipped Zn-Ti coating. The
2
particles generated in the η
layer prevents the generation of compact
layer in Zn – 0.05 % Ti coating. According to
them, higher Ti content increases the number of precipitated particles [37]. Peng et al.
reported that a typical shiny, feathery and dull spangle can be obtained by batch hot dip
galvanizing in Zn – 0.05 Al – 0.20 Sb bath. A large number of precipitated
-Sb
3
Zn
4
particles
get distributed randomly on the shiny spangle surface. Both
-Sb
3
Zn
4
particles and the
dentritic segregation of Sb result in spacing of feathery spangles on the surface [38]. The bath
parameters including the elemental and other additives such composites should be controlled
to tune the nature and thickness of the intermittent layers to suit the purpose of the specific
application of the coating.
5. Metal and metal oxide addition in molten zinc bath
Mg and Mg-Al additions in the galvanizing bath are a proven technique to regulate
the intermittent reactions improving the coating morphology and properties of low carbon
steel. The hardness of Zn-Mg & Zn-Mg-Al coatings is normally higher than that of pure zinc
coating, a favorable character for scratch resistance. The Zn - 0.5 wt. % Mg – 0.25 wt. % Al
coating has more corrosion resistance than pure zinc & Zn-Mg coatings [32]. Duchoslav et al.
have studied the initial progress of atmospheric corrosion of tenancy alloyed Zn-Mg-Al
coatings in aqueous NaCl environment. They have also reported that two fundamental
processes are involved in the initial corrosion attack. This results in dissolution of the surface
layer consisting of MgO and preferential anodic dissolution of both binary and ternary
eutectic phases constituting the coating [39]. Kondratiuk et al. have reported about the

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
9
formation of a ZnO layer on Zn-Ni coating during heat treatment. The electroplated uniphase
intermetallic Zn-Ni coatings are highly suitable for hot sheet metal forming applications
owing to their good thermal stability compared to hot dip zinc coatings [40]. The presence of
Mg in zinc coatings causes a remarkable improvement in the corrosion resistance of painted
systems. Hausbrand et al. have studied the corrosion of painted MgZn
2
with a defect, under
constant conditions of high humidity and an electrolyte covered defect [41]. They discussed
about the galvanic coupling among the defects and also about the galvanic coupling between
defect and intact interface. According to them, no cathodic delamination is possible due to an
unfavorable potential gradient between the defect and the intact interface in the case of
MgZn
2
. An anodic delamination is possible through the migration of ions at the metal oxide
interface [41]. LeBozec et al. have compared the corrosion performance of Zn-Mg-Al
coatings with conventional zinc coatings such as hot dip galvanized, electrogalvanized,
Galvannealed and galfan coatings. The performance of ZnMgAl coatings is depending on the
testing conditions and the configuration of the samples and these coatings provide a major
improvement during neutral salt spray analysis [42].
High performance hot dip zinc coatings can be developed by the incorporation of
individual and mixed metal oxides such as nano TiO
2
, CeO
2
-TiO
2
and Al
2
O
3
-ZrO
2
into the
hot dip zinc coating [43-45]. The incorporation of Al
2
O
3
-ZrO
2
mixed oxide composite into
galvanizing bath yields aluminium rich zinc coatings with high sliding and wear resistance.
The interior layers of the coatings are also known to possess high stability against corrosion.
The incorporation of nano TiO
2
in the hot dip zinc coating has been known to facilitate
phosphating and also good paintability. The hot dip galvanized coatings with high corrosion
resistance, effective barrier protection, good antifouling characteristics and improved surface
quality can be achieved by CeO
2
-TiO
2
incorporation. These composites not only yield
individual characteristics to the coating but also give a new range of reaction modification
during the process.
6. Annealing of hot dip zinc coatings
The annealing of hot dip galvanized coatings (galvannealed) leads to the formation of
Fe-Zn intermetallic phases. Galvannealed coatings differ from pure zinc coatings that they
consist of a layered structure of different Fe-Zn intermetallic phases and have superior

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
10
resistance to corrosion, improved paintability and better weldability than pure zinc coatings
[46]. Chakraborty et al. have studied the root cause of hitherto unknown and uncommon
defect that has been encountered in the coating of an industrial galvannealed HIF steel. The
root cause of the defect lies in the presence of some pickling resistant sticky iron oxide on the
surface of the hot rolled sheet, which exists even after cold rolling. According to them, the
subsequent galvannealing operation has no effect on Fe-Zn reaction at the spots covered by
the oxide layer and it causes defects in the final product [47]. H. Y. Ha et al. examined the
dissolution process of a galvannealed coating layer on a dual phase steel and the variation in
the corrosion rate during the stripping process. According to them, the corrosion rate gets
accelerated when the outermost
phase is completely dissolved and the galvanic couple of
the δ-Г substrate is exposed to the 1M NaCl + 0.01M H
2
SO
4
. However uniform and thick
layer of
phase is required for enhanced corrosion prevention [48]. Kim et al. have evaluated
the mechanical properties of galvannealed steel sheets used for automotive exposed panel and
the failure phenomenon of its coating layer using finite element method. They have reported
that the failure of the coating layer occurs when tensile deformation mode is activated at the
coating layer and equivalent local plastic strain becomes more than 0.28 [49].
Okamoto et al. have studied the compression deformability of
and
Fe-Zn
intermetallics to mitigate detachment of brittle intermetallic coating of galvannealed steels.
The
phase is the most deformable followed by the
phase, while the
1
,
1k
and
1P
phases
are all highly brittle based on compression tests of micrometer sized single phase specimens
[50]. Manna & Dutta have reported that the charge transfer resistivity of galvannealed
coatings with different intermediate layers is better than similar galvanized coatings and also
reported that the galvanized and galvannealed coatings with prior metal flash coatings show
better mechanical performance compared to the coatings obtained without prior metal flash
coating [51]. Choi et al. have studied the effect of pre-electroplating with prior to reduction of
annealing on the surface quality and resultant corrosion characteristics of galvannealed high
strength DP steel. The degree of Fe-Zn alloying can be significantly improved due to the
formation of a homogeneous interfacial Fe
2
Al
5
layer at the coating/substrate interface during
the galvanizing process, resulting in an increase in the thickness of the coating layer. These
changes arise due to the suppression of the segregation and selective oxidation of small
amount of alloying elements during reduction in the annealing process prior to the
galvanization [52]. Thus, apart from the bath reactions which are mainly controlled by the
composition and the dipping conditions, some superficial reactions can be tuned by

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
11
secondary parameters such as energy and strain during the annealing process. Such controlled
and post treated coatings only find extensive industrial applications.
7. Recent developments in hot dip galvanization of high strength steel
The performance of automotive designs requires high strength materials with good
formability, fatigue resistance and toughness [53]. The use of advanced high strength steels in
automotive industry has saved weight and enables economy of fuel. The protection of high
strength steel against corrosion by hot dip galvanization became an important issue in recent
years because it is an integral part of automotive industry. The developments in hot dip
galvanized high strength steels have been reported extensively. Some of the recent catches
are discussed in this review. The high strength steels undergo recrystallization annealing prior
to hot dip galvanization. The annealing in the reducing atmosphere causes segregation and
selective oxidation of alloying elements, making the steel surface unsuitable for
galvanization. The wetting nature of zinc is decreased by the presence of external oxides of
elements such as Al, Si and Mn on the surface of steel [54]. Frenznick et al. have reported
about the influence of Si, Mn oxides and their surface coverage on wetting kinetics of zinc
coatings. According to them, the Cassie equation along with Avrami growth law was a good
approximation for the wetting kinetics and the growth mechanism of reaction layer. They
found the reactive wetting dynamics depends not only on the overall oxide coverage but also
on the size of the oxide islands [55]. H. Liu et al. (2012) have reported about the surface
selective oxidation of alloying elements and mechanical property degradation of the hot dip
galvanized high strength dual phase steel by comparing three dual phase steels such as Mn-Si
steel, Cr steel and Cr-Mo steel. According to them, the surface segregation and the selective
oxidation are greatly influenced by the dew point of annealing atmosphere and the steel
composition [56]. Blumenau et al. have studied the effect of pre oxidation on improving the
reactive wetting of high manganese alloyed steel during hot dip galvanization. It becomes
clear that the pre oxidation offers several advantages regarding technical feasibility [57].
8. Competency with aluminium dipping
8.1. The advantages of aluminium hot dipping
Aluminium and aluminium alloy coatings are used in automobile and construction
industries as an alternative for zinc coating in order to reduce weight, provide better

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
12
mechanical properties and yield high temperature oxidation resistance compared to hot dip
zinc coating. The corrosion resistant characteristics of aluminized steel are due to the
formation of a stable thin film of aluminium oxide. Hot dip aluminizing is a diffusion coating
formation process used to deposit high temperature oxidation and corrosion resistant coatings
on stainless steels and low alloy steels [58-60]. The dipping of steel in molten aluminium
causes interdiffusion of the aluminium and iron leads to the formation of different
intermetallics [61]. The coating on hot dipped aluminide steel is composed of an aluminium
top coat and a thick (10-100
m) brittle Fe-Al intermetallic layer. The Fe-Al intermetallic
layer comprises an outer FeAl
3
layer (often called Fe
4
Al
13
[61]) and an inner Fe
2
Al
5
layer
[62]. The Fe
2
Al
5
layer determines the development of coating phases and the final coating
properties [63]. Hot dipped aluminide mild steels have been widely used in boilers and
exhaust pipes in high temperature environments, due to the formation of fine, dense Al
2
O
3
with good oxidation resistance on the surface of steel [64]. In order to improve the lifetime of
hot dipped aluminide steel in high temperature conditions, it is essential to create a diffusion
barrier between the steel substrate and the aluminide coating.
8.2. Influence of alloying elements in the formation of intermetallic layers vs aluminium
Cheng & Wang have studied the effect of silicon addition in the Al bath on the
oxidation behaviour of hot dipped aluminide mild steel [65]. Aluminide steel with high Si
having poor isothermal oxidation resistance is attributed to its thin aluminide layer and
formation of a large number of phase transformation induced voids. Aluminide steel with a
thick Fe
2
Al
5
layer in the low Si content aluminide layer has low cyclic oxidation resistance
because the low fracture toughness of Fe
2
Al
5
phase enhances crack formation in the
aluminide layer [65]. Windmann et al. have reported about the transformation of brittle Al-Si
coatings into more ductile phase of type Al-Fe during austenitization and have studied the
phase formation as a function of coating thickness and Si content [66]. The presence of
silicon influences the diffusivity of Al & Fe in the Al-Fe rich intermetallics and promotes the
formation of Si rich intermetallics of type Al
8
Fe
2
Si, Al
13
Fe
4
and Al
5
Fe
2
. The transformation
of Al rich intermetallic phases of type Al
5
Fe
2
& Al
13
Fe
4
into iron rich phase of type AlFe can
be enhanced by higher Si content (10 mass%) and by decreasing the coating thickness [66].
Cheng et al. have reported about the factors affecting the thickness and morphology of
the Al-Si coated CLAM steel. Fingerlike intermetallic layers are formed under optimum
conditions of 1073 K, 3 minute and Al – 5.8 at. % Si. The existence of certain amount of Si in

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
13
the molten Al bath suppresses the growth of Fe
2
Al
5
, builds up the thickness of FeAl
3
slightly
that causes reduction in the thickness of intermetallic layer [67]. Later Danzo et al. have
reported the microstructural and crystallographic features of steel during hot dipping with Al-
Si bath followed by diffusion annealing treatment (900-1200 °C for 1 hour). Columnar grain
shape can be achieved during diffusion annealing and the intermetallic layers formed during
hot dipping can be vanished, as they serve as an Al source for columnar grain growth [68].
Takata et al. have also studied the morphology and growth of Fe-Al intermetallic
layers formed on pure Fe sheets dipped in Al – 8.2 Mg – 4.8 Si alloy melt at 750 °C. The
intermetallic layer exhibits a dual layer structure which consists of a continuous
-FeAl
3
and
a large η-Fe
2
Al
5
phase layers. The presence of Si and Mg prevents the diffusion of Fe into Al
melt and thereby the growth of η phase layer and promotes the growth of
phase layer. The
phase acts as the diffusion barrier in Al-Si-Mg aluminide coating [69]. Cheng et al. have
found that a thin intermetallic layer composed of (Fe-Ni)
2
Al
9
, NiAl
3
and Ni
2
Al
3
and a thick
intermetallic layer composed of FeAl
3
and Fe
2
Al
5
are formed in the aluminide/nickel duplex
coating on mild steel. In such case, nickel pre-plating has no effect on the growth rates of
FeAl
3
& Fe
2
Al
5
as it only slows down the initial growth rate for FeAl
3
and Fe
2
Al
5
which can
be attributed to the presence of Ni
2
Al
3
, NiAl
3
and (Fe, Ni)
2
Al
9
in the intermetallic layers
before the formation of FeAl
3
& Fe
2
Al
5
. As the immersion time is increased, nickel pre-
plating layer is fully consumed due to its dissolution into the Al bath resulting in intermetallic
layers contained only outer minor FeAl
3
and inner major Fe
2
Al
5
. Among all the phases
formed, Fe
2
Al
5
had the fastest growth rate [62].
8.3. Corrosion resistant and oxidation resistant characteristics of aluminide coating vs
aluminium
Frutos et al. have reported the oxidation behaviour of hot dipped AISI 316 LVM
stainless steel in molten Al - 31at. % Si bath. At 900 °C, the rapid transformation of less
protective aluminas into the protective
–Al
2
O
3
enhances the oxidation resistance of the
aluminized material compared with uncoated one. Below 900 °C, the chromia layer is more
protective than the scale consisting of less protective aluminas. As a result the coating had no
beneficial effect on the oxidation resistance of stainless steel [70]. Lemmens et al. have
compared the electrochemical behavior of hot dipped DC06 European grade steel in Al & Al-
Si bath. According to them the crates do not corrode while the outer Al layer can dissolve
sacrificially to protect all underlying layers and the steel substrate in chloride environments

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
14
[61]. According to Cheng et al., Ni-Al alloys possess better corrosion resistance than Fe-Al
alloy in chloride containing atmospheres. Therefore, Ni-aluminide coating has been widely
used to improve the hot corrosion resistance of the substrate material [62]. Shi et al. have
studied the effect of dipping temperature and heat treatment on the aluminide coatings
characteristics. The thickness of the intermetallic layer increases with increasing dipping
temperature. The transformation of multiphase structures of aluminizing layer into a single
phase (Fe
3
Al) through heat treatment favors improvement on chemical stability, toughness,
corrosion and wear resistance of T91 steel [71]. Ni et al. have studied the corrosion resistant
characteristics of aluminium coating with and without annealing against molten carbonate
using electrochemical impedance spectroscopy. These annealed coatings generally exhibit
better corrosion resistance than Al-Fe intermetallic coating formed in-situ during the
corrosion process. The main cause for the degradation of aluminide coatings is not only due
to the corrosion of the coating in contact with molten carbonate but also due to the aluminium
depletion through the interdiffusion of aluminium and the substrate [72].
9. Conclusions
The role of composition of the substrate, the bath as well as the process parameters
including annealing conditions significantly tune the structural characteristics and
applicability of the coatings. Since the hot dipping process is an alloying reaction, the process
conditions significantly extent/suppress/delaying the targeted reaction. Thus the process
parameters of the whole processes including pretreatment, dipping and curing/annealing have
a greater role than the role of the composition of the bath or other solutions involved in the
process. The significance of presence of optimum amount of silicon and phosphorus in the
steel substrate has been found to be crucial during the hot dipping process. Apart from the
bath composition, the pretreatment and the dipping conditions alter the resultant coating to a
great extent. Tuning of intermetallics and multiphase interdentrices can fulfill the formation
of targeted characteristics of the hot dip coatings. Control of other in-situ physicochemical
reactions such as control of segregation, selective oxidation of doping elements and
suppression of selective intermetallic reactions are considered to be crucial during the hot
dipping process.

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
15
Acknowledgement
The authors thank the Head of the Department of Chemistry, University of Kerala for
extending support to carry out the research work.
References
[1] A. Amadeh, B. Pahlevani, S. Heshmati-Manesh, Corros. Sci. 44 (2002) 2321.
[2] R. Sa-nguanmoo, E. Nisaratanaporn, Y. Boonyongmaneerat, Corros. Sci. 53 (2011)
122.
[3] S. Maeda, Prog. Org. Coat. 28 (1996) 227.
[4] G. Reumont, J.B. Vogt, A. Iost, J. Foet, Surf. Coat. Technol. 139 (2000) 265.
[5] P.R. Sere, J.D. Culcai, C.I. Elsner, A.R.D. Sarli, Rev. Metal. Madrid. 33 (1997) 376.
[6] C.S. Lin, M. Meshi, Metall. Mater. Trans. B. 25B (1994) 721.
[7] S. Felin Jr, V. Barranco, Acta Mater. 51 (2003) 5413.
[8] P. Rajak, U. Tewary, S. Das, B. Bhattacharya, N. Chakraborti, Comput. Mater. Sci. 50
(2011) 2502.
[9] C.H.E. Belin, R.C.H. Belin, J. Solid State Chem. 151 (2000) 85.
[10] W.H. Zhu, H.M. Jin, P. Wu, H.L. Liu, Phys. Rev. B 70 (2004) 165419.
[11] A.S. Koster, J.C. Schoone, Acta Crystallogr. B 37 (1981) 1905.
[12] D. Horstmann, „The influence of impurities in iron on attack by molten zinc‟, 4
th
Int.
Galv. Conf. Milan (1956).
[13] C.E. Jordan, A.R. Marder, J. Mater. Sci. 32 (1997) 5603.
[14] M.S. Kozdras, P. Niessen, Mater. Sci. Technol. 6 (1990) 681.
[15] Y. Boonyongmaneerat, K. Saengkiettiyut, P. Rattanawaleedirojn, C. Angkaprasert, J.
Wanichsampan, S. Saenapitak, J. Iron Steel Res. Int. 17(8) (2011) 74.
[16] S.M.A. Shibli, R. Manu, Surf. Coat. Technol. 197 (2005) 103.
[17] T.H. Cook, Met. Finish. 89 (6) (1991) 107.
[18] U. Stieglitz, M.W.D. Schulz, H. Kulker, V. Brucken, Metalloberflaeche 56 (2002) 11.
[19] R.P. Krepski, Met. Finish. 87 (10) (1989) 37.
[20] M. Manna, Surf. Coat. Technol. 205 (2011) 3716.
[21] D. Balloy, J.Y. Dauphin, J.C. Tissier, Surf. Coat. Technol. 202 (2007) 479.
[22] L. Allegra, J.C. Zoccola, Mater. Performance 22 (1983) 5.
[23] S.M.A. Shibli, A.C. Jayalekshmi, R. Remya, Surf. Coat. Technol. 201 (2007) 7560.
[24] S. Maeda, Prog. Org. Coat. 28 (1996) 227.

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
16
[25] P. Liberski, K. Henryk, P. Podolski, A. Gierek, Ochrona przed Korozja, 47 (2004) 264.
[26] A. R. Marder, Prog. Mater. Sci. 45 (2000) 191.
[27] P.R. Sere, J.D. Culcasi, C.I. Elsner, A.R. Di Sarli, Surf. Coat. Technol. 122 (1999) 143.
[28] N. Pistofidis, G. Yourlias, S. Konidaris, E. Pavilidou, A. Stergiou, G. Stergioudis,
Mater. Lett. 61 (2007) 994.
[29] V. Barranco, S. Feliu Jr., S. Feliu, Corros. Sci. 46 (2004) 2203.
[30] J.H. Hong, S.J. Oh, S.J. Kwon, Intermetallics 11 (2003) 207.
[31] H. Liu, Y. He, L. Li, Appl. Surf. Sci. 256 (2009) 1399.
[32] M. Dutta, A.K. Halder, S.B. Singh, Surf. Coat. Technol. 205 (2010) 2578.
[33] H. Liu, F. Li, W. Shi, R. Liu, L. Li, Surf. Coat. Technol. 205 (2011) 3535.
[34] G.M. Song, T. Vystavel, N. van der Pers, J.Th.M. De Hosson, W.G. Sloof, Acta
Mater. 60 (2012) 2973.
[35] H. Yang, S. Zhang, J. Li, X. Liu, H. Wang, Surf. Coat. Technol. 240 (2014) 269.
[36] Z. Chen, C.T. Peng, Q. Liu, R. Smith, D. Nolan, J. Alloys Compd. 589 (2014) 226.
[37] Y. Gui, Q. Xu, Y. Guo, J. Iron Steel Res. Int. 21(3) (2014) 396.
[38] S. Peng, J. Lu, C. Che, G. Kong, Q. Xu, Appl. Surf. Sci. 256 (2010) 5015.
[39] J. Duchoslav, M. Arndt, R. Steinberger, T. Keppert, G. Luckeneder, K.H. Stellnberger,
J. Hagler, C.K. Riener, G. Angeli, D. Stifter, Corros. Sci. 83 (2014) 327.
[40] J. Kondratiuk, P. Kuhn, E. Labrenz, C. Bischoff, Surf. Coat. Technol. 205 (2011) 4141.
[41] R. Hausbrand, M. Stratmann, M. Rohwerder, Corros. Sci. 51 (2009) 2107.
[42] N. LeBozec, D. Thierry, A. Peltola, L. Luxem, G. Luckeneder, G. Marchiaro, M.
Rohwerder, Mater. Corros. 64 (2013) 969.
[43] S.M.A. Shibli, F. Chacko, C. Divya, Corros. Sci. 52 (2010) 518.
[44] S.M.A. Shibli, F. Chacko, Appl. Surf. Sci. 257 (2011) 3111.
[45] S.M.A. Shibli, F. Chacko, Surf. Coat. Technol. 205 (2011) 2931.
[46] C. Xhoffer, H. Dillen, B.C. Cooman, J. Appl. Electrochem. 29 (1999) 209.
[47] A. Chakraborty , M. Dutta , R. Pais , R.K. Ray, Surf. Coat. Technol. 204 (2010) 3481.
[48] H.Y. Ha, S.J. Park, J.Y. Kang, H.D. Kim, M.B. Moon, Corros. Sci. 53 (2011) 2430.
[49] S.I. Kim, J.U. Her, Y.C. Jang, Y. Lee, Trans. Nonferrous Met. Soc. China 21 (2011)
s111.
[50] N.L. Okamoto, D. Kashioka, M. Inomoto, H. Inui, H. Takebayashi, S. Yamaguchid
Scripta Mater. 69 (2013) 307.
[51] M. Manna, M. Dutta, Surf. Coat. Technol. 251 (2014) 29.
[52] Y.I. Choi, E.S. Shin, K. Kuroda, M. Okido, C.J. Park, Corros. Sci. 58 (2012) 152.

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
17
[53] J. Sun, H. Yu, Mat. Sci. Eng. A 586 (2013) 100.
[54] H.J. Grabke, V. Leroy, H. Viefhaus, ISIJ Int. 35 (1995) 95.
[55] S. Frenznick, S. Swaminathan, M. Stratmann, M. Rohwerder, J. Mater. Sci. 45 (2010)
2106.
[56] H. Liu, F. Li, W. Shi, S. Swaminathan, Y. He, M. Rohwerder, L. Li, Surf. Coat.Technol.
206 (2012) 3428.
[57] M. Blumenau, M. Norden, F. Friedel, K. Peters, Surf. Coat. Technol. 206 (2011) 559.
[58] C.J. Wang, S.M. Chen, Surf. Coat. Technol. 201 (2006) 3862.
[59] C.C. Tsaur, J.C. Rock, Y.Y. Chang, Mater. Chem. Phys. 91 (2005) 330.
[60] C.J. Wang, C.C. Li, Surf. Coat. Technol. 177–178 (2003) 37.
[61] B. Lemmens, Y.G. Garcia, B. Corlud, J.D. Strycker, I.D. Graeve, K. Verbeken,
Surf. Coat. Technol. (2014) Article in Press, DOI: 10.1016/j.surfcoat.2014.06.064.
[62] W.J. Cheng, C.J. Wang, Mater. Charact. 69 (2012) 63.
[63] M. Dutta, S.B. Singh, Scripta Mater. 60 (2009) 643.
[64] W.J. Cheng, C.J. Wang, Surf. Coat. Technol. 204 (2009) 824.
[65] W.J. Cheng, C.J. Wang, Appl. Surf. Sci. 274 (2013) 258.
[66] M. Windmann, A. Röttger, W. Theisen, Surf. Coat. Technol. 246 (2014) 17.
[67] X. Chen
, Q. Huang, Z. Yan, Y. Song, S. Liu, Z. Jiang, J. Nucl. Mater. 442 (2013) S597.
[68] I.I. Danzo, Y. Houbaert, K. Verbeken, Surf. Coat. Technol. 251 (2014) 15.
[69] N. Takata, M. Nishimoto, S. Kobayashi, M. Takeyama, Intermetallics 54 (2014) 136.
[70] E. Frutos, P. Adeva, J.L. González-Carrasco, P. Pérez, Surf. Coat. Technol. 236 (2013)
188.
[71] Z. Shi, J. Cao, F. Han, J. Nucl. Mater. 447 (2014) 77.
[72] C.S. Ni, L.Y. Lu, C.L. Zeng, Y. Niu, J. Power Sources 261 (2014) 162.

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
18
Table 1: Characteristics of Fe-Zn intermetallic phases of hot dip zinc coatings [8-11]
η phase
ζ phase
δ phase
Г1 phase
Г phase
Stoichiometry
Zn
FeZn13
FeZn10
Fe5Zn21
Fe3Zn10
Wt % of iron
0
5-6
7-11.5
17-19.5
23.5-28
Crystal structure
HCP
Monoclinic Hexagonal
FCC
BCC
Atoms/unit cell
6
28
555
408
52
Source: Comput. Mater. Sci., Vol. 50, 2011, pp. 2502 (Elsevier)
ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
19
eta
zeta
delta
gamma
steel
Fig. 1. The intermetallic layers present in a typical / conventional hot dip galvanized
coating.

ACCEPTED MANUSCRIPT
ACCEPTED MANUSCRIPT
20
Highlights
highlighted.
iscussed.
Wyszukiwarka
Podobne podstrony:
więcej podobnych podstron