REVIEW
Folia Microbiol. 53 (4), 275–287 (2008)
http://www.biomed.cas.cz/mbu/folia/
Transcriptional Regulators of Seven Yeast Species:
Comparative Genome Analysis
–
Review
E.
D
ROBNÁ
,
A.
B
IALKOVÁ
,
J.
Š
UBÍK
*
Department of Microbiology and Virology, Faculty of Natural Sciences, Comenius University, 842 15 Bratislava, Slovakia
fax +421 265 429 064
e-mail subik@fns.uniba.sk
Received 18 September 2007
Revised version 24 January 2008
ABSTRACT. The regulation of gene transcription allows yeast cells to respond properly to changing envi-
ronmental conditions. Several protein complexes take part in this process. They involve RNA polymerase
complexes, chromatin remodeling complexes, mediators, general transcription factors and specific transcrip-
tional regulators. Using Saccharomyces cerevisiae as reference, the genomes of six species (Ashbya gos-
sypii, Kluyveromyces lactis, K. waltii, Candida albicans, C. glabrata and Schizosaccharomyces pombe) that
are human pathogens or important for the food industry were analyzed for their complement of genes enco-
ding the homologous transcriptional regulators. The number of orthologs identified in a given species cor-
related with its phylogenetic distance from S. cerevisiae. Many duplicated genes encoding transcriptional
regulators in S. cerevisiae and C. glabrata were reduced to one copy in species diverged before the ancestral
whole genome duplication. Some transcriptional regulators appear to be specific for S. cerevisiae and pro-
bably reflect the physiological differences among species. Phylogenetic analysis and conserved gene order
relationships indicate that a similar set of gene families involved in the control of multidrug resistance and
oxidative stress response already existed in the common ancestor of the compared fungal species.
Abbreviations
CTD
C-terminal domain
PIC
pre-initiation complex
HAT(s)
histone acetyltransferase(s)
RNA pol II
RNA polymerase II
HDAC(s)
histone deacetylase(s)
TA(s)
transcriptional activator(s)
βHLH helix–loop–helix
(motif)
TAF(s) TBP-associated
factor(s)
HTH
helix–turn–helix (motif)
TBP
TATA-binding protein
MADS Mcm1p, Agamous, Deficiens, TF(s)
transcription
factor(s)
Serum response factor
TR(s)
transcriptional regulator(s)
MDR multidrug
resistance
CONTENTS
1 Introduction 275
2 Transcriptional regulation in the yeast 276
2.1
Chromatin remodeling, mediators and general transcription factors 276
2.2
Specific transcription factors 278
3 Comparative genomics of the yeast transcription factors 279
4 Inventory of transcriptional regulators involved in multidrug resistance 281
5 Conclusion 282
References 285
1 INTRODUCTION
In the past decades, along with experimental studies, the cell biology research also uses in silico
analysis of currently available genetic databases. Since the beginning of the nineties, when the first results of
microbial genome sequencing were published, large amounts of species with complete genome sequences
appeared. The availability of genetic information of a growing number of organisms, particularly microorga-
nisms, significantly contributed to the development of comparative genomics. The comparison of DNA se-
quences of both closely related and phylogenetically distant groups of microorganisms has brought new know-
ledge about the evolution of species and dynamics of genome changes during the evolution. Comparison of
*Corresponding author.
276 E. DROBNÁ et al.
Vol. 53
DNA sequences is frequently used to search for homologous genes to disclose the function of novel genes.
The results of in silico analysis represent a good starting point for subsequent experimental research. More-
over, they can also support the knowledge gained by the experimental work.
The regulation of transcription in the yeast represents a key step in the regulation of cellular pro-
cesses and the response of cells to changing environmental conditions. In this review we report on the regu-
lation of gene expression in Saccharomyces cerevisiae and we provide the inventory of TRs found in complete
genome sequences of seven yeast species (Ashbya gossypii, Kluyveromyces lactis, K. waltii, Candida albi-
cans, C. glabrata, Saccharomyces cerevisiae and Schizosaccharomyces pombe) widely spread over the evo-
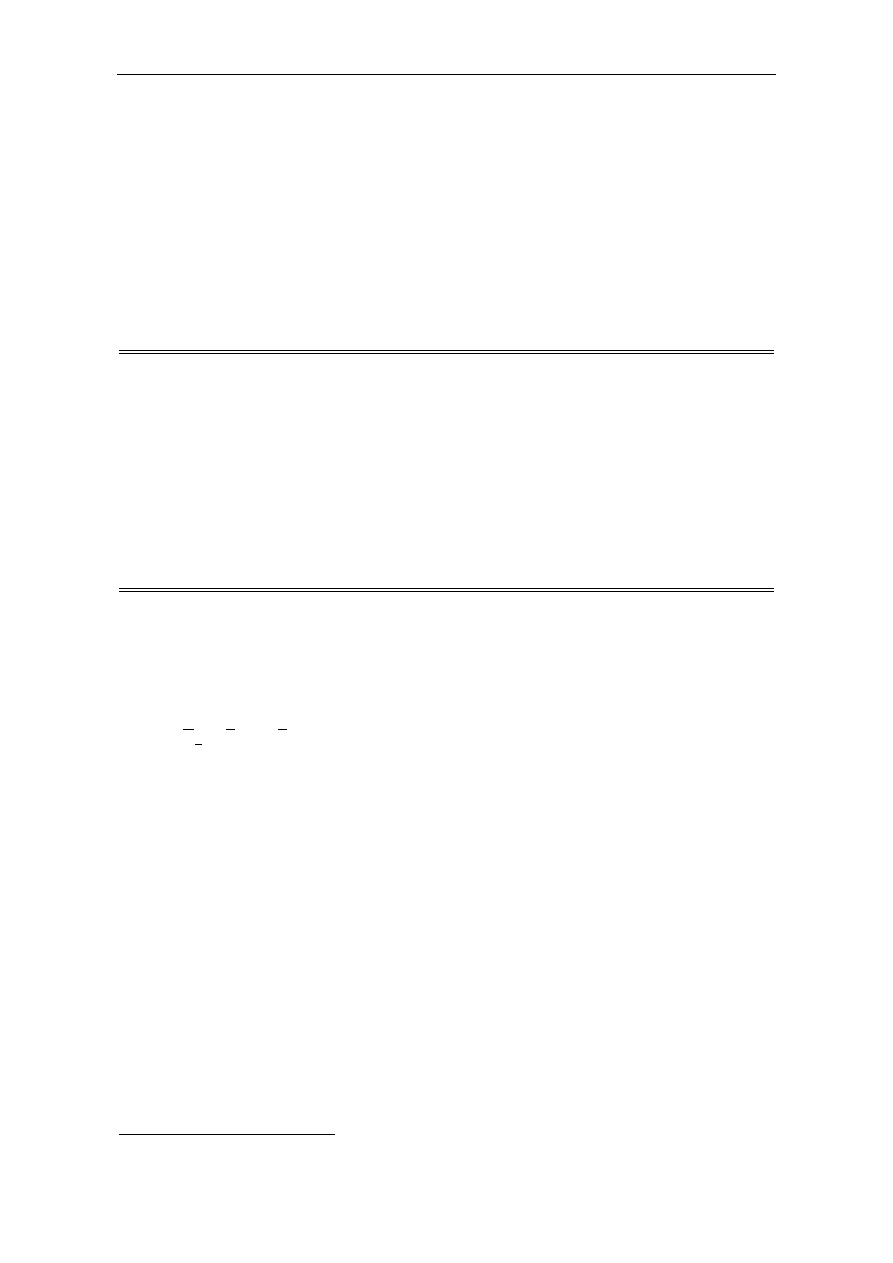
lutionary range of fungi (Fig. 1). These species represent biotechnologically and medically important eukaryo-
tic microorganisms frequently used in both fundamental and applied research.
Fig. 1. The phylogenetic tree of the species studied. The arrow indicates the
ancient whole genome duplication proposed by Wolfe and Shields (1997).
2 TRANSCRIPTIONAL
REGULATION IN THE YEAST
The regulation of gene transcription allows yeast cells to respond to changing environmental con-
ditions. Transcriptional activation or repression represents an important step in the regulation of gene expres-
sion. Several protein complexes participate in transcription and its regulation. Along with the enzyme complex
of RNA pol II synthesizing mRNA the activation of transcription requires general TFs, mediator complex
and complex of proteins responsible for the chromatin remodeling. Contrary to the basal transcription, the
induced one also requires specific TAs (Struhl 1995; Sellick and Reece 2005).
2.1
Chromatin remodeling, mediators and general transcription factors
In eukaryotic cells nuclear DNA associates with histones forming nucleosomes as the basic struc-
tural units of chromatin. The histone-bound DNA is inaccessible for the binding of other DNA-binding pro-
teins like TFs. Therefore, nucleosomes have an inhibitory effect on transcriptional activation (Workman et
al. 1998). The chains of nucleosomes can form a chromatin structure of a higher order resulting in silencing
of longer chromosome regions (Ramakrishnan 1997; Bell and Felsenfeld 1999).
The chromatin remodeling complexes are generally divided into two groups. The regulators of the
first group are able to modify the protruding N-terminal histone ends (Wu and Grunstein 2000) by acetyl-
ation and deacetylation of Lys residues, by ubiquitination, methylation of Arg residues and by Ser phosphoryl-
ation (Berger 2002). The second type of chromosome remodeling involves noncovalent histone modificat-
ions that are ATP-dependent and shift nucleosomes along DNA (Kingston and Narlikar 1999; Kornberg and
Lorch 1999; Vignali at al. 2000; Urnov and Wolffe 2001; Urnov 2002). The chromatin regulators do not re-
cognize specific DNA sequences and it is assumed that their binding to histones is mediated through inter-
actions with other proteins, such as TAs (Hampsey and Reinberg 2003).
The possible role of histone acetylation in the regulation of gene expression has already been sug-
gested by Alfrey et al. (1964). The breakthrough in the elucidation of connections between histone acetyl-
ation and transcription came after cloning of the gene encoding the HAT from Tetrahymena sp. which was
homologous to the GCN5 gene encoding the coactivator required for a full activity of certain TFs in S. cere-
visiae (Georgakopoulos and Thireos 1992). However, Gcn5p was found to be associated with other proteins
of the Ada and Spt groups forming the SAGA complex (Spt-Ada-Gcn5-acetyltransferase). Gcn5p possesses
the acetyltransferase activity and, together with Ada2p and Ada3p, they form a part of the SAGA complex
required for its binding to TAs. While the Spt3p and Spt8p subunits interact with TBP, Spt7p and Spt20p are

2008
TRANSCRIPTIONAL REGULATORS OF SOME YEASTS: COMPARATIVE GENOME ANALYSIS – review 277
required for the integrity of the entire complex (Grant et al. 1998). Along with Gcn5p other histone acetyl-
transferases were also described such as Hat1p (Kleff et al. 1995) and Esa1p in the Nua4 complex (Allard et
al. 1999).
HDACs are enzymes responsible for the removal of acetyl groups from histone and are associated
with the repression of transcription. Deacetylated chromatin regions are generally inactive, suggesting that the
histone deacetylation by HADC results in suppression of gene activity. In S. cerevisiae several different
HDACs were identified and divided into three groups (Ekwall 2005). The first group consists of Rpd3p,
Hos1p and Hos2p. The second group involves Hda1p and Hos3p. The third group, named “sirtuins”, con-
tains Sir2p, Hst1p, Hst2p, Hst3p and Hst4p. The sirtuins are enzymes dependent on NAD
+
(Bedalov et al.
2003). Contrary to HATs able to bind to upstream activation sites in transcriptionaly active genes along the
entire genome, HDACs bind rather to certain genome regions. Rpd3p is known as a negative regulator of
genes of earlier meiosis as well as those of genes involved in the cell cycle. Hst1p interacts with Sum1p
repressor and is involved in the repression of genes of the middle phase of sporulation (Robert et al. 2004).
Hos1p and Hos2p associate with hyperacetylated intergenic regions of rDNA loci and Hos2p affects the
acetylation of promoter regions in genes encoding ribosomal proteins. Hda1p binds to subtelomeric chromo-
somal regions containing genes that are active during nutrition stress or carbon source changes and are re-
pressed by the Tup1p–Ssn6p complex (Ekwall 2005).
Besides covalent modifications, the ATP-dependent complexes, such as the SWI–SNF, ISWI, RSC
and Ino80p, are also involved in chromatin remodeling. The best studied SWI–SNF complex consists of 11
subunits (Cairns et al. 1994). Its Swp29p is identical with the Tfg3p subunit of a general TF IIF and with the
TAF
II
30 subunit of a general TF IID (Cairns et al. 1996). ATPase activity is executed by the Swi2p/Snf2p
subunit which contains a bromodomain required for the recognition of acetylated Lys residues in histones
(Yang 2004). The RSC complex consists of 15 subunits, some of them homologous with the subunits of the
SWI–SNF complex (Cairns et al. 1996). In cells this complex is present at 10 times higher amount than the
SWI–SNF complex. Several genes encoding the subunits of the RSC complex are essential. Their deletions
result in the arrest of cells in the transition from G
2
to M phase of the cell cycle (Hampsey 1998).
The mediator is another protein complex required for the initiation of transcription. The results of
in vitro experiments revealed that for the transcription dependent on specific TFs a simple presence of general
TFs together with specific TAs is not sufficient and the cell extract was required for activation of RNA pol II
transcription. The unknown components of the cell extract required for transcriptional activation were
named mediator (Flanagan et al. 1991). The combination of biochemical and genetic approaches allowed
identification of all 20 subunits of the mediator complex. In the yeast this complex consists of three functio-
nally and physically different modules (Biddick et al. 2005). The first module plays a major role in trans-
cription and is responsible for the mediator binding to the CTD of RNA pol II (Lee and Kim 1998; Lee and
Young 2000). The second module, called a middle domain, interacts both with CTD RNA pol II and TFIIE.
The last module apparently participates in the recognition and binding to the activator (Bhoite et al. 2001).
Electron microscopic images have revealed that the mediator can occur in two conformation states: in com-
pact structure in the absence of RNA pol II, and in elongated structure in its presence (Austrias et al. 1999).
At the beginning the mediator was thought to make a bridge between the TA and RNA pol II binding them
into PIC. Latter other functions also were revealed. Its Med5p subunit was found to possess histone acetyl-
transferase activity and the mediator can directly interact with nucleosomes (Lorch et al. 2000). The mediator
participates in phosphorylation of CTD RNA pol II through the Cdk8p subunit leading to its release from
transcription PIC at the beginning of elongation (Kim et al. 1994). The mediator can function not only as
a coactivator but also as a repressor (Jiang and Stilman 1992; Papamichos-Chronakis et al. 2000; Nishizawa
et al. 2001; Halberg et al. 2004). Since the mediator may also be regulated one can assume that it may func-
tion as a control point in transcriptional initiation. It may prevent the RNA pol II binding to DNA template
under unfavorable conditions or may serve as a backbone helping to recruit RNA pol II and initiate trans-
cription (Biddick et al. 2005).
The yeast RNA pol II, responsible for mRNA synthesis, represents a multiprotein complex consist-
ing of 12 subunits (Cramer 2004). The basal transcription requires also other protein complexes named gene-
ral TFs such as TFIIB, TFIID, TFIIE, TFIIF and TFIIH. The TFIID complex contains TBP that binds to TATA
box in the promoter and bends DNA together with 14 TAFs. They bind to surrounding DNA sequences and
mediate interaction of TFIID with other TFs. TFIIB binds directly to RNA pol II and is responsible for its
proper localization on the promoter. It is recruited to transcription PIC after TBP binding to TATA box. It
also interacts with subunits of TFIID and TFIIF; TFIIF displays some similarity with the bacterial
σ factor.
It is firmly bound to RNA pol II, prevents nonspecific binding of RNA pol II to DNA and stabilizes PIC.
One of the TFIIF subunits named Tfg3p is identical with the Swp29p subunit of the SWI–SNF chromatin re-
modeling protein complex (Cairns et al. 1996) and with the TAF
II
30 subunit of TFIID (Poon et al. 1995).
278 E. DROBNÁ et al.
Vol. 53
So, Tfg3p establishes a connection between the basal and the regulatory components of the transcriptional
machinery (Cairns et al. 1996; Henry et al. 1994). TFIIE recruits to PIC after RNA pol II and before TFIIH
(Buratowski et al. 1989). It interacts with nonphosphorylated RNA pol II and with TFIIF and TFIIH. TFIIE
stimulates helicase and kinase activities of TFIIH (Lee and Young 2000; Ohkuma 1997). TFIIH also regu-
lates the transition from transcription initiation to elongation presumably mediated by CTD phosphorylation
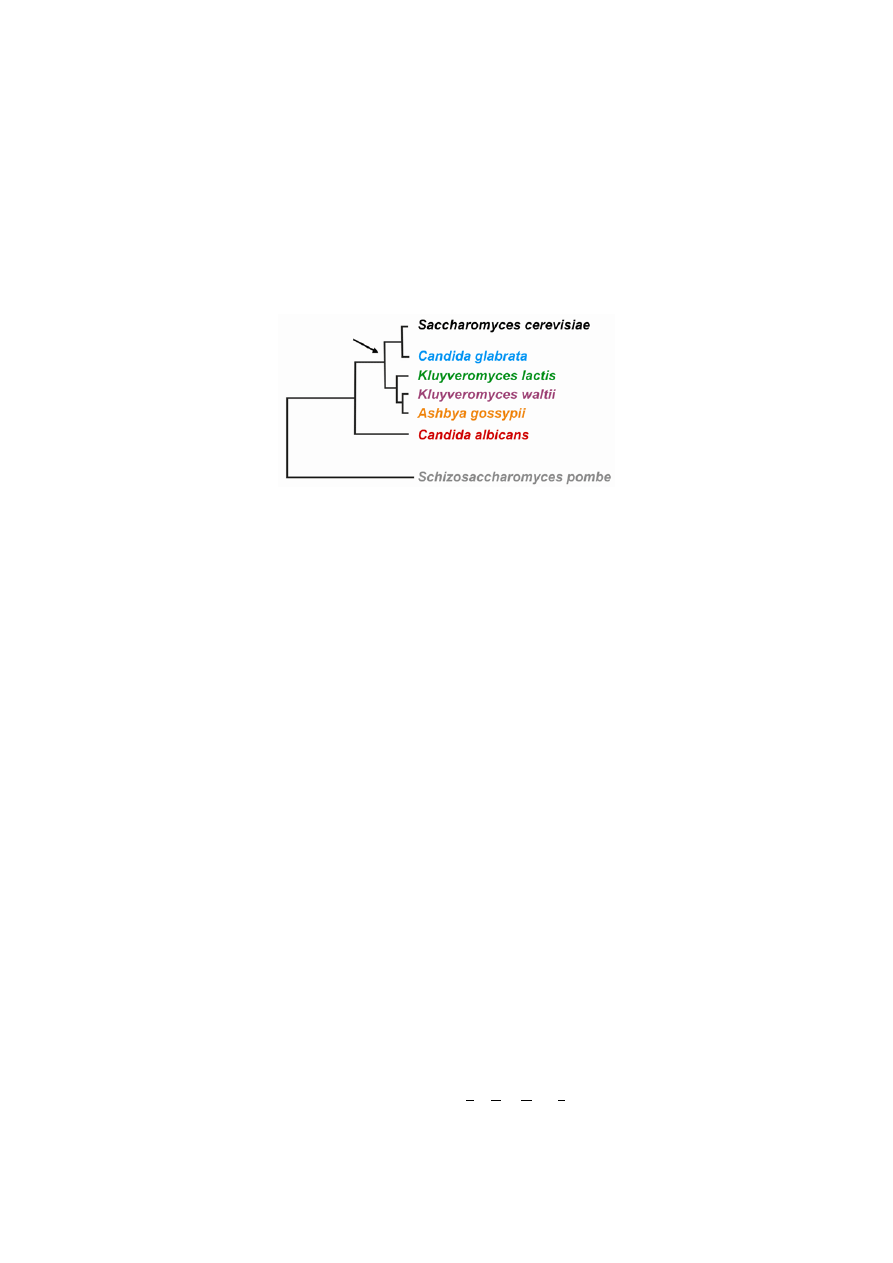
(Hampsey 1999). Schematic depiction of transcription PIC is shown in Fig. 2.
Fig. 2. Schematic depiction of the transcription pre-iniciation complex. Its assembly is nu-
cleated by TBP binding to the TATA box, inducing a bend in the DNA template, followed
by association of general TFs and RNA pol II. TBP – TATA-binding protein, RNA pol II
– RNA polymerase II, TFIIB, TFIIE, TFIIF, TFIIH – yeast general transcription factors.
2.2
Specific transcription factors
Besides general TFs that are highly conserved during evolution in eukaryotes, transcriptional acti-
vation also requires the sequence-specific TFs; these proteins usually possess a modular structure. In the case
of TAs they contain the DNA-binding domain and the activation domain. The DNA-binding domain recog-
nizes the specific promoter sequence and is responsible for sequence specificity of TR. The activation domain
interacts with coactivators, such as the subunits of chromatin remodeling complexes and mediator, but also
with general TFs. Due to such protein–protein interactions individual components of transcription machinery
are recruited and properly bound to the promoter of the activated gene resulting in transcription PIC form-
ation and transcription initiation. Taking into account the amino acid composition most activation domains
belong to those containing acidic, Pro-rich or Gln-rich domains (Ptashne and Gann 1997).
Specific TFs are classified according to the structural motifs that constitute their DNA-binding do-
mains. Major classes of the yeast TFs include those with the HTH, the
βHLH, the Leu zipper motif (βZip)
and proteins binding metal, such as those with the Zn finger motif. The first DNA-binding protein motif to
be recognized was the HTH (Harrison and Aggarwal 1990). It is constructed from two
α-helices connected
by a short chain of amino acids which constitutes the
β-turn (Brown 2002). The βHLH motif is characterized
by two
α-helices connected by a loop (Massari and Murre 2000). It should not be confused with the HTH
motif. In fact, these two structures are quite different but the
βHLH looks like a Leu zipper. The TFs with
βHLH forms both homo- and heterodimers, such as Ino2p and Ino4p (Hoshizaki et al. 1990; Nikoloff et al.
1992). Beside the
βHLH motif some TFs (βHLH–Zip) also contain the Leu zipper motif, such as TFs Rgt1p
(Liao and Butow 1993) and Cbf1p (Cai and Davis 1990).
The DNA-binding domain in TFs containing the Leu zipper motif consists of 60–80 amino acid re-
sidues forming a region of the Leu zipper and the basic region contacting DNA. The Leu zipper is formed by
two
α-helices in which every seventh amino acid is Leu. The helices are held together by hydrophobic inter-
actions between Leu residues. The Leu zipper proteins bind to DNA as dimers (Pabo and Sauer 1992).
Metal binding TFs involve mainly proteins containing a domain that utilizes Zn as an important
component of the DNA-binding region. Amino acid residues such as Cys and His tetrahedrally coordinate
the Zn atom. Together they form a finger-like projection that is in close contact with DNA. Three major clas-
ses of Zn finger proteins have been established in eukaryotes, based on their unique and highly conserved
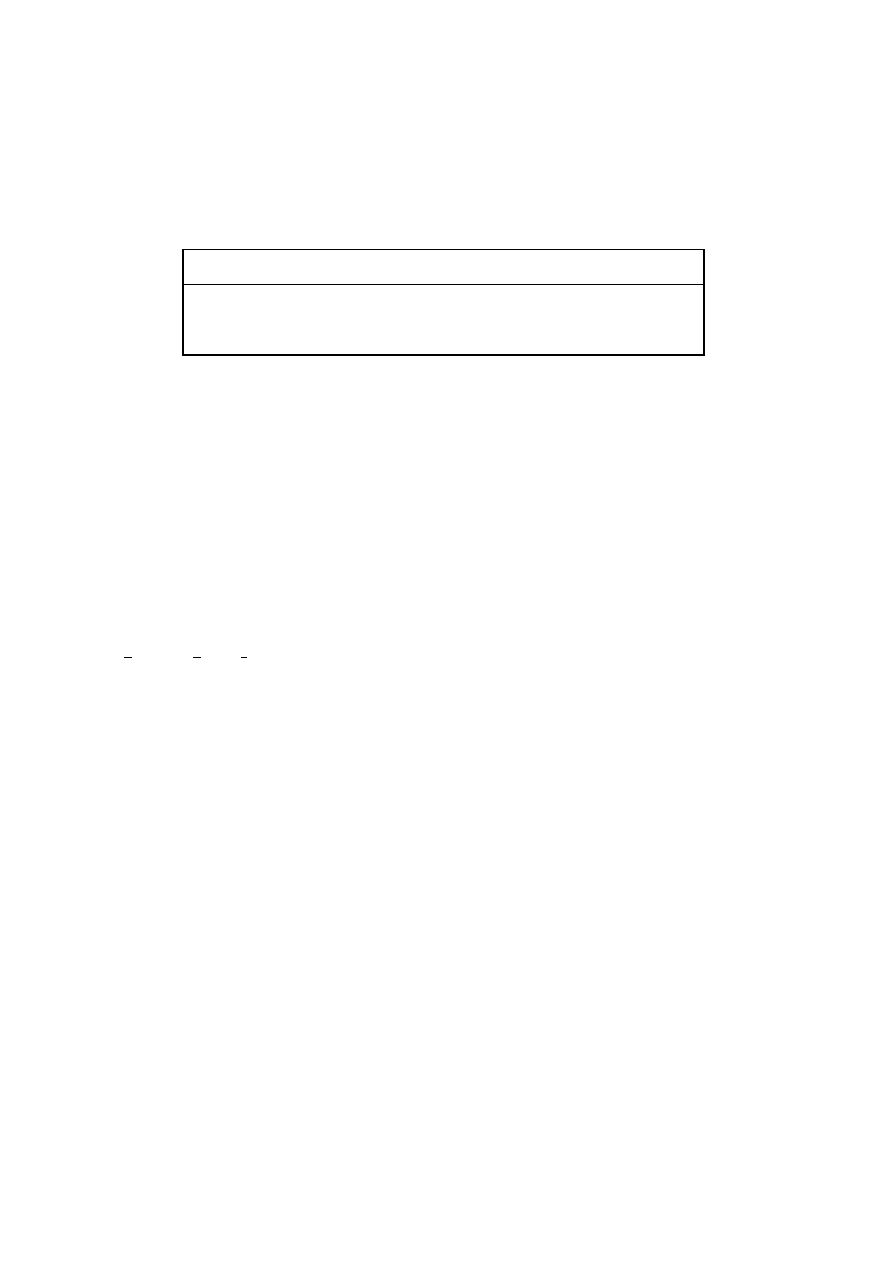
consensus amino acid sequences (McPherson et al. 2006); they are summarized in Table I.
Class I encompasses the Cys
2
His
2
(C
2
H
2
) proteins and is often referred to as the classical Zn finger
(Böhm et al. 1997). They can be subdivided according to the number and positions of Zn fingers to proteins
containing two Zn fingers in tandem arrangement (C
2
H
2
–Zf tandem) or more Zn fingers scattered through
the entire protein sequence (C
2
H
2
–Zf multiple) (Busserau et al. 2003).
2008
TRANSCRIPTIONAL REGULATORS OF SOME YEASTS: COMPARATIVE GENOME ANALYSIS – review 279
Class II represents the Cys
4
(C
4
) Zn fingers. The first four Cys residues bind to the Zn
II
ion and the
last four bind to another one. Unlike the first class, these proteins usually contain one Zn finger unit binding
to DNA as homo- or heterodimers consisting of two C
4
proteins. Usually, homodimers recognize inverted
repeats within the target nucleic acid sequence, whereas heterodimers bind to direct repeats (Laity et al.
2001).
Table I. Zinc finger transcriptional regulators
Zinc finger class
Consensus amino acid sequence
a
I (Cys
2
His
2
) Cys–X
2–4
–Cys–X
12
–His–X
3–5
–His
II (Cys
4
) Cys–X
2
–Cys–X
n
–Cys–X
2
–Cys–X
n
–Cys–X
2
–Cys–X
n
–Cys–X
2
–Cys
III (Cys
6
) Cys–X
2
–Cys–X
6
–Cys–X
5–12
–Cys–X
2
–Cys–X
6–8
–Cys
a
X – any amino acid.
Class III (C
6
) Zn finger proteins contain a DNA-binding domain that consists of six Cys residues
bound to two Zn atoms (Schjerling and Holmberg 1996). This Zn binuclear cluster or Zn
II
2
Cys
6
proteins has
been identified exclusively in fungi and Gal4p was one of the first eukaryotic TFs to be characterized (John-
son and Hopper 1982; Laughon and Gesteland 1982). This class of TFs is unique in that these proteins con-
tain only one Zn finger unit that binds two Zn atoms (Todd and Andrianopulos 1997; Vallee et al. 1991). They
can interact with DNA as monomers, homo- or heterodimers (Mamnum et al. 2002; Akache et al. 2004).
Some yeast TFs belong to proteins containing the MADS-box or the forkhead motif. MADS-box
proteins are able to bind DNA as homodimers in vitro but in vivo they usually interact with cofactors helping
them to recognize a specific binding site on DNA. The MADS box is a phylogenetically conserved region
consisting of 80 amino acid residues representing DNA-binding and dimerization domains sufficient for inter-
action with required cofactors (Abraham and Vershon 2005).
The forkhead motif in the DNA-binding domain has been named according to the shape formed by
helices and loops. It is also named winged helix. This structure has been described for the first time in HNF3
(hepatocyte nuclear factor) in rats, mice and Drosophila melanogaster (Lai et al. 1993). Later, proteins with
this structure, such as Fhk1p and Fhk2p, have also been found in S. cerevisiae (Hollenhorst et al. 2000).
3
COMPARATIVE GENOMICS OF THE YEAST TRANSCRIPTION FACTORS
The advances in DNA sequencing have opened the way for determination of complete genome se-
quences of several organisms. The first eukaryotic entirely sequenced genome in S. cerevisiae significantly
contributed to the development of functional genomics (Goffeau et al. 1996). However, the completed geno-
me sequences of other species in the last six years have put baker’s yeast into a leading position in the area
of comparative genomics (Piskur and Langkjaer 2004). Today, complete or almost complete genome sequen-
ces of dozen yeast species represent a set of genomic information that has no analogy in other eukaryotic
groups (Dujon 2005). The results of comparative genomics help to elucidate the molecular mechanisms that
were used in the evolution (Souciet et al. 2000). They also help to interpret the complex genomes of higher
eukaryotes (Dujon 2005).
The first results from the comparison of genomes in related organisms led to the improvement of
the annotation of individual DNA sequences. Improved annotation in S. cerevisiae is helpful for the annotat-
ion of genes in other yeast species and other eukaryotes. Despite the fact that some yeast species are consi-
dered to be related, the phylogenetic distances among them are higher than those among chordates (Dujon
2005). Although each yeast species contains certain specific genes most of the genes are common to all spe-
cies or they are universally conserved (Kellis et al. 2003; Dujon et al. 2004).
In S. cerevisiae, some functionally related genes occur as a pair of homologous genes, twin genes,
named paralogs. The analysis of chromosomal localization of them revealed the existence of original dupli-
cated chromosomal blocks. The whole genome duplication is considered as one of the advantageous ways
during evolution because duplicated genes create the background for the development of new functions due
to mutations and selection. During evolution one of the two paralogs is usually preserved its original funct-
ion while the second one gained a new function. The occurrence of homologous chromosomal regions in
S. cerevisiae is supposed to be the result of an ancient duplication of the whole genome followed by a mas-

280 E. DROBNÁ et al.
Vol. 53
sive loss of genes along with duplicated number of chromosomes (Wolfe and Shields 1997). This hypothesis
was later supported by the complete genome sequences of other species such as Ashbya gossypii, Kluyvero-
myces waltii and K. lactis. Their genomes have not undergone duplication and their genes display the syn-
teny (conservation of gene order) at least with one of the homologous genes in S. cerevisiae (Dietrich et al.
2004; Kellis et al. 2004; Dujon et al. 2004). The whole genome duplication also occurred in pathogenic
C. glabrata but most of the duplicated genes were lost (Dujon et al. 2004).
The ability to respond to the changing environmental conditions belongs to the basic properties of
living cells. They can find a good opportunity how to do it by the regulation of the gene expression at the
level of transcription initiation. Specific TFs regulating the expression of target genes play a key role here.
However, genes encoding specific TFs do not belong to the group of highly conserved genes. Rather, their
coding sequences were subjected to relatively fast development during the evolution resulting in the increased
functional variability and ability to accommodate to different environmental conditions typical of the life of
individual species (Byrne and Wolfe 2005, 2007; Tirosh and Barkai 2007).
The available genomic sequences of seven yeast species (A.
gossypii, C.
albicans, C.
glabrata, K.
lac-
tis, K. waltii, S. cerevisiae, Schizosaccharomyces pombe) allow us to analyze the regulatory capabilities en-
coded by their genomes. In this review we have also attempted to identify the full set of TRs in these spe-
cies. S. cerevisiae was used as reference due to its highest number of annotated genes and comprehensive
results of biochemical and genetic analyses. A total of 163 TFs (Teixeira et al. 2006) were selected from the
list of 169 TRs described in the Yeastract database (
http://www.yeastract.com
). Annotated genes
were gained from several genome databases and their encoded protein sequences were compared with the
BLAST software. The list of TRs homologous to S. cerevisiae TFs and their basic characteristics for six spe-
cies are summarized in the Online Supplementary Table II –
http://www.cssm.info/priloha/fm2008_drobna_tab2.pdf
TRs are divided into several groups according to their DNA-binding motifs. As can be inspected,
the lowest number of orthologs was found for S. pombe reflecting its high evolutionary distance from S. cere-
visiae. Certainly, the total number of TRs in S. pombe is higher but they were not revealed by their amino
acid composition. In some proteins a certain homology has been recognized but often only in the regions
encoding the DNA-binding motifs.
Among the hemiascomycetous species a pathogenic C. albicans is phylogenetically most distant from
other yeast species analyzed although its phylogenetic distance from S. cerevisiae is lower than that from
S. pombe. Compared to S. cerevisiae, the number of orthologs found in the C. albicans genome (Jones et al.
2004) was lower and usually only one ortholog of the pairs of S. cerevisiae paralogs was identified. Some of
the putative homologous genes may not be detected because the sequences have diverged too much and were
not recognizable by standard sequence comparison programs, such as BLAST. In the identified homologs
the lowest similarity in amino acid composition of encoded proteins was found for C. albicans and the highest
one for TRs in C. glabrata (Table II).
In the second pathogenic species, C. glabrata, which is phylogenetically highly related to S. cere-
visiae, the reduction of paralogs has been observed only in a few cases. In the group of TRs containing the
Zn
II
2
Cys
6
DNA-binding motif instead of the gene pair PDR1–PDR3 only a single ortholog CAGL0A00451g
was found. Along with paralogs, C. glabrata apparently also lost some genes occurring as a single ortholog
even in S. cerevisiae. They are represented by homologs of ACA1 (YER045c) and ARR1 (YPR199c) of
βZip
TFs and SRD1 (YCR018c) of Cys
4
-Zf TRs. Remarkable is the loss of several homologs of Zn
II
2
Cys
6
TRs,
some of which are involved in galactose (GAL4) and maltose (MAL13, MAL23) metabolism.
Many duplicated (or triplicated) genes encoding TAs in S. cerevisiae are also reduced to one (or
two) copy in species diverged before the occurrence of the ancestral whole genome duplication, such as K. lac-
tis, K. waltii and A. gossypii (Kellis et al. 2003, 2004; Dujon et al. 2004; Bussereau et al. 2006). This con-
cerns the gene pair PHD1–SOK2 containing the
βHLH–Zip DNA-binding motif and gene groups, such as
YAP1–CAD1 = YAP2, YAP5–YAP7, CIN5 = YAP4–YAP6 and ACA1–CST6, encoding TRs bearing the
βZip
motif, NRG1–NRG2, GIS1–RPH1, MET31–MET32, MIG1–MIG2–MIG3, MSM2–MSM4, USV1–RGM1 and
SWI5–ACE2 encoding TRs bearing the C
2
H
2
–Zf motif, the GZF3–DAL80 gene pair containing Cys
4
–Zf
motif, FKH1–FKH2 encoding TRs bearing the forkhead motif as well as gene pairs HAL9–TBS1, UPC2–
ECM22, SUT1–SUT2, PDR1–PDR3–YER184c and YRM1–YRR1–PDR8 coding for proteins bearing the
Zn
II
2
Cys
6
DNA-binding motif. According to the phylogenetic analysis and synteny some of them could be
assigned correctly to the corresponding S. cerevisiae orthologs. Some of the
βZip and Zn cluster TRs appear
to be specific for S. cerevisiae only and they have probably evolved in response of its cells to specific envi-
ronmental conditions.
2008
TRANSCRIPTIONAL REGULATORS OF SOME YEASTS: COMPARATIVE GENOME ANALYSIS – review 281
4
INVENTORY OF TRANSCRIPTIONAL REGULATORS INVOLVED
IN MULTIDRUG RESISTANCE
MDR is a ubiquitous biological phenomenon hampering efficient antimicrobial therapy and tumor
treatment. In fungi, this phenomenon has been described for the first time in S. cerevisiae and later also in
pathogenic species, such as C. albicans, C. dubliniensis and C. glabrata (Balzi and Goffeau 1994; Jungwirth
and Kuchler 2006; Prasad et al. 2002; Sanglard 2007; Bialková and Šubík 2006). The most important me-
chanism leading to MDR is the drug efflux mediated by overexpressed membrane associated transporters. The
expression of the transporter encoding genes involved in MDR is under the control of two regulatory net-
works PDR and YAP. They include TRs belonging to proteins harboring the binuclear Zn
II
2
Cys
6
Zn finger
DNA-binding motif and proteins possessing the basic Leu zipper motif. Some drug efflux transporters are
also induced in the Msn2p-dependent manner (Gbelská et al. 2006). Msn2p is the Cys
2
His
2
Zn finger pro-
tein acting as a regulator in the general stress response pathway (Martinez-Pastor et al. 1996).
Putative orthologs of MDR TRs characterized in S. cerevisiae were identified in most of the ana-
lyzed species but the twin genes often occurring in S. cerevisiae and C. glabrata genomes are reduced to one
copy in the genomes of K. lactis, K. waltii and A. gossypii (Figs 3 and 5). The phylogenetic trees for multi-
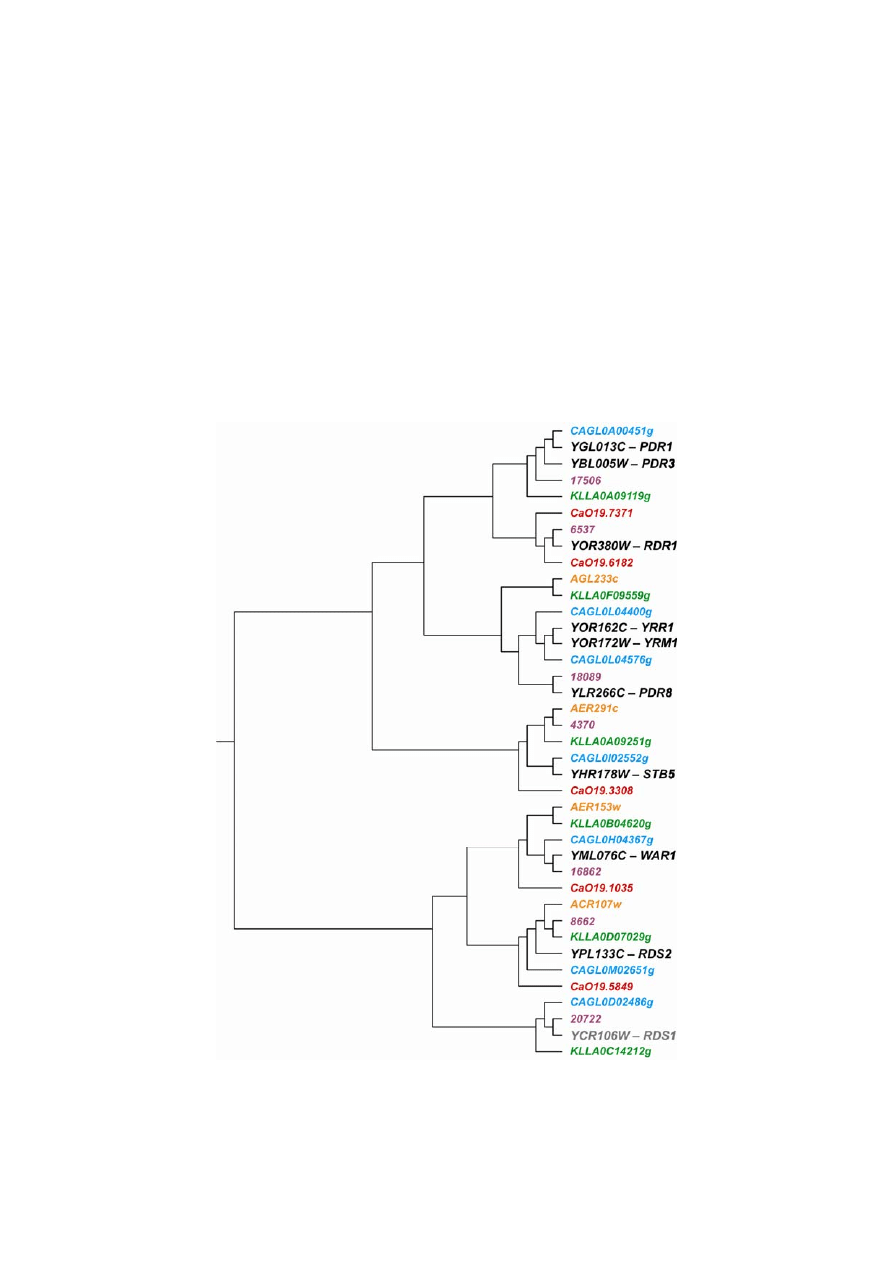
Fig. 3. Phylogenetic relationship of MDR TFs harboring the Zn
II
2
Cys
6
DNA-binding motif. The protein sequences
obtained were aligned with the multiple sequence alignment program ClustalW at GenomeNet (http://www.geno
me.jp
) using default parameters. The program was used for phylogenetic tree construction and for the identification
of gene clusters; the beginning of the cluster marked with A – A. gossypii, CAGL – C. glabrata, Ca – C. albicans,
KLLA – K. lactis, Y – S. cerevisiae, numbers without the letters – K. waltii genes; colors as in Fig. 1.
282 E. DROBNÁ et al.
Vol. 53
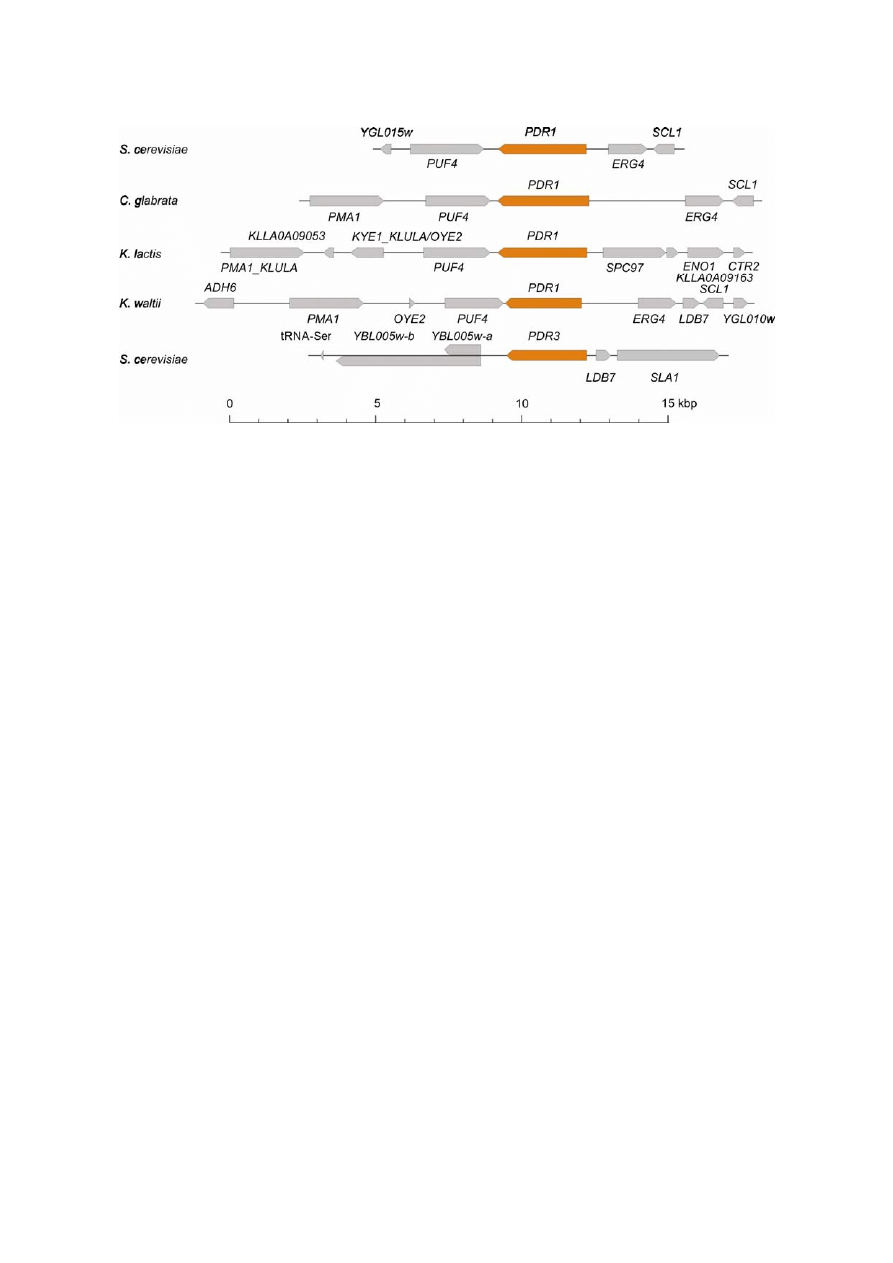
Fig. 4. Synteny of PDR1–PDR3 homologs in different species. The order and orientation of genes annotated according to the Gen-
bank entry were recorded on each DNA fragment. The gene order browser (Byrne and Wolfe 2005) was consulted for comparison.
drug resistance TRs clearly support the proposition by Wolfe and Shields (1997) that K. lactis, K. waltii and
A. gossypii are placed before the ancestral whole genome duplication. The S. cerevisiae and C. glabrata ortho-
logs are always more clearly related than the orthologs in the other fungal species compared. The duplicated
genes of S. cerevisiae Zn
II
2
Cys
6
regulators PDR1–PDR3 are single in both Kluyveromyces spp. and C. glab-
rata genomes. The phylogenetic analysis and synteny (Figs 3 and 4) indicate that PDR3 homologs are lack-
ing in all the analyzed fungal genomes. In A. gossypii even the PDR1 ortholog is absent. In C. albicans two
very distantly related PDR1–PDR3 homologs were experimentally identified, such as FCR1 (CaO19.6817)
and TAC1 (CaO19.3188). However, the search for TAC1 homologs did not identify significant equivalents
in S. cerevisiae and C. glabrata. While in S. cerevisiae the FCR1 gene product behaved as a TA, in C. albi-
cans it acts as a negative regulator (Talibi and Raymond 1999). Only Tac1p has been experimentally proved
to function as a TA of C. albicans drug efflux transporter genes, such as CDR1 and CDR2 (Coste et al.
2004).
In the phylogenetic tree the C. albicans PDR1 and RDS1 homologs appeared in the cluster of the
RDR1 gene which encodes a repressor of drug resistance in S. cerevisiae. While the RDS1 homolog is absent
in A. gossypii, the STB5, RDS2 and WAR1 genes are present as a single copy in the six genomes compa-
red. The chromosomal environments of these orthologs are also conserved and the yeast gene browser
(
http://wolfe.gen.tcd.ie/ygob/
) can provide an easy access to such data (Byrne and Wolfe 2005).
The YRM1–YRR1–PDR8 gene family is also reduced in K. lactis, K. waltii and A. gossypii. The MSN2–MSN4
genes occurring as twin genes in S. cerevisiae and C. glabrata are single in the genomes of K. lactis, K. wal-
tii and A. gossypii. Two genes (MSN4–CaO19.4752 and MNL1–CaO19.6121) encoding Msn2/4-like pro-
teins are present in the C. albicans genome but they play no obvious roles in the stress responses of this
pathogen (Nicholls et al. 2004).
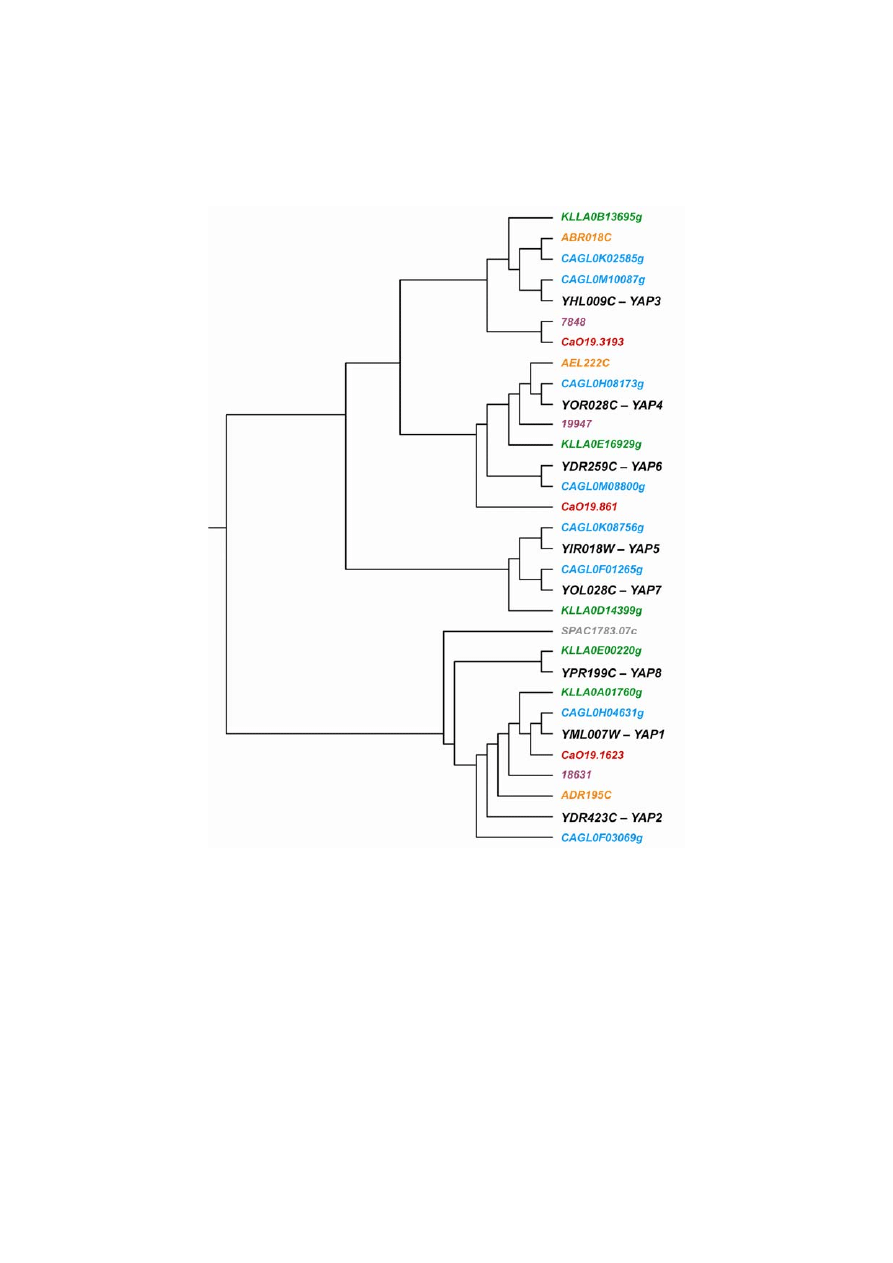
The
βZip family of TRs with 8 members involved in the oxidative stress response and drug resis-
tance regulation in S. cerevisiae and C. glabrata genomes is reduced to 5 orthologs in K. lactis, 3 orthologs
in K. waltii, A. gossypii and C. albicans, and 1 ortholog in S. pombe (Table II, Fig. 5). The chromosomal
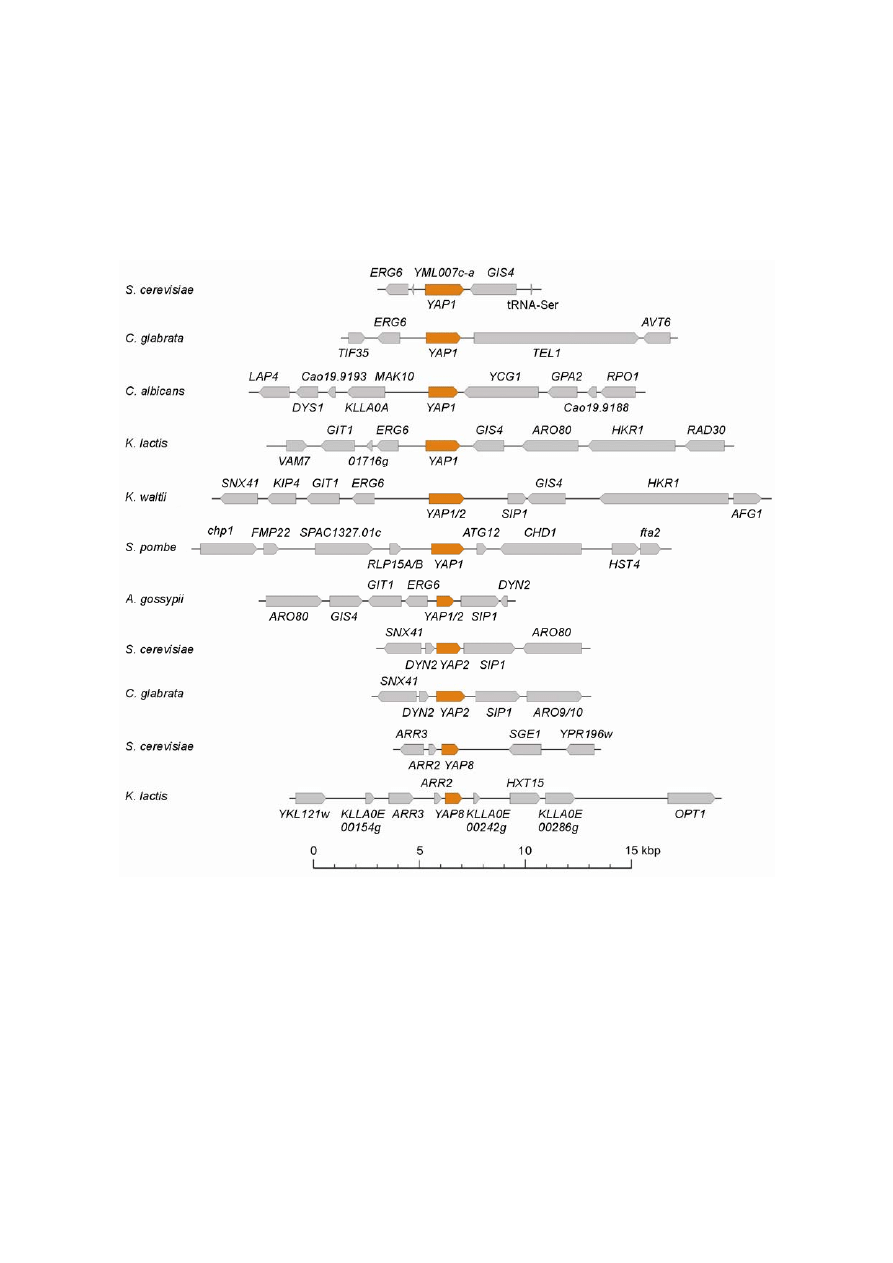
environments of YAP homologs in S. cerevisiae and selected species are highly conserved during evolution
(Fig. 6). The results of the phylogenetic analysis and synteny data demonstrate that a similar set of gene fami-
lies involved in the control of oxidative stress and MDR already existed in the common ancestor of the
compared fungal species.
5 CONCLUSION
The basic components of the transcriptional machinery and principles of the transcriptional regulat-
ion in eukaryotic cells are highly conserved during evolution. The life of various yeast species in different
environmental conditions has led to the development of specific TFs that regulate a different subset of genes
2008
TRANSCRIPTIONAL REGULATORS OF SOME YEASTS: COMPARATIVE GENOME ANALYSIS – review 283
in response to extra- and intracellular signals. Generally, the genes encoding the specific TFs belong to the
weakly conserved and are amenable to faster development during evolution (Todd and Andrianopulos 1997;
Dujon et al. 2004). This apparently helps to reach physiological differences among species (Hittinger et al.
2004; Malpertuy et al. 2000; Scannell et al. 2007).
Fig. 5. Phylogenetic relationship of MDR TFs harboring the
βZIP DNA-binding motif; A – A. gossypii, CAGL –
C. glabrata, Ca – C. albicans, KLLA – K. lactis, S – S. pombe, Y – S. cerevisiae, numbers without the letters
– K. waltii genes (see also Fig. 3) ; colors as in Fig. 1.
The life of S. cerevisiae is associated with the function of ≈170 TRs harboring several different DNA-
binding motifs. In yeast, the Zn cluster TFs represent the most abundant group of TRs. C. glabrata which is
phylogenetically very close to S. cerevisiae, possesses narrower repertoire of TRs and is able to utilize only
a limited number of substrates due to its regressive evolution connected with the loss of 29 genes involved in
the metabolism of galactose and other sugars, nitrogen, sulfur and phosphate (Dujon et al. 2004). The geno-
mes of other species (K. lactis, K. waltii, A. gossypii) that during evolution have not undergone the whole
genome duplication event contain even a lower number of TRs. Apparently, genome duplication and muta-
tions allowed yeast cells to better tune their metabolism and accommodate to changing environmental con-
ditions. Compared to gene evolution in K. waltii a faster evolution has been observed between paralogs in
C. glabrata (2 % of twin genes in genome) and S. cerevisiae (8 %) (Kellis et al. 2004; Dujon et al. 2004).
One of the twin genes evolved slowly and preserved its original function while the second gained a new
284 E. DROBNÁ et al.
Vol. 53
function due to an accelerated evolution (Benard et al. 1999; Kellis et al. 2004; Byrne and Wolfe 2005;
Tirosh and Barkai 2007). However, the structural changes during evolution were not limited to TRs only but
also involved the intergenic regions (Gasch et al. 2004). A recent analysis of TF responsive elements in pro-
moters of drug efflux transporter genes in five related species revealed that only approximately a half of them
match the known sequence motifs for MDR regulators described in S. cerevisiae (Gbelská et al. 2006). Pro-
bably, this may be a reason why homologous TRs often fail to activate transcription of specific genes in the
heterologous yeast background.
Fig. 6. Synteny of YAP homologs in different species. The order and orientation of genes annotated according to the GenBank entry
were recorded on each DNA fragment. The gene order browser (Byrne and Wolfe 2005) was consulted for comparison.
Apparently, more information about the regulatory capability of different yeast species will be
obtained by functional analysis of their annotated TRs using more sophisticated experimental approaches,
such as the electrophoretic mobility shift assay (Garner and Revzin 1981; Yang 1998), serial analysis of gene
expression (Velculescu et al. 1995, 2000), DNA microarray (DeRisi et al. 1997), chromatin immunopreci-
pitation (Ren et al. 2000; Taverner et al. 2004) and the Dam methyltransferase identification (DamID)
(Van Steensel and Henikoff 2000; Van Steensel et al. 2001). So far, most of such studies have been car-
ried out only with S. cerevisiae and C. albicans.
This work was supported by grants from the Slovak Research and Developmental Agency (APVV-20-00604, LPP-0022-06)
and the Slovak Grant Agency of Science (VEGA 1/3250/06).

2008
TRANSCRIPTIONAL REGULATORS OF SOME YEASTS: COMPARATIVE GENOME ANALYSIS – review 285
REFERENCES
A
BRAHAM
D.S.,
V
ERSHON
A.K.: N-Terminal arm of Mcm1 is required for transcription of a subset of genes involved in maintenance of
the cell wall. Eukar.Cell 4, 1808–1819 (2005).
A
KACHE
B.,
M
CPHERSON
S.,
S
YLVAIN
M.A.,
T
URCOTTE
B.: Complex interplay among regulators of drug resistance genes in Sac-
charomyces cerevisiae. J.Biol.Chem. 279, 27855–27860 (2004).
A
LFREY
V.G.,
F
AULKNER
R.,
M
IRSKY
A.E.: Acetylation and methylation of histones and their possible role in the regulation in RNA
synthesis. Proc.Nat.Acad.Sci.USA 51, 786–794 (1964).
A
LLARD
S.,
U
TLEY
R.T.,
S
AVARD
J.,
C
LARK
A.,
G
RANT
P.,
B
RANDL
C.J.,
P
ILLUS
L.,
W
ORKMAN
J.L.,
C
ROTE
J.: Nua4, an essential trans-
cription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related factor Tra1p. EMBO J. 18,
5108–5119 (1999).
A
LTSCHUL
S.F.,
M
ADDEN
T.L.,
S
CHAFFER
A.A.,
Z
HANG
J.,
Z
HANG
Z.,
M
ILLER
W.,
L
IPMAN
D.J.: Gapped BLAST and PSI-BLAST: a new
generation of protein database search programs. Nucl.Acids Res. 25, 3389–3402 (1997).
A
USTRIAS
F.J.,
J
IANG
Y.W.,
M
YERS
L.C.,
G
USTAFSSON
C.M.,
K
ORNBERG
R.D.: Conserved structure of mediator and RNA polymerase II
holoenzyme. Science 283, 985–987 (1999).
B
ALZI
E.,
G
OFFEAU
A.: Genetics and biochemistry of yeast multidrug resistance. Biochim.Biophys.Acta 1187, 152–162 (1994).
B
EDALOV
A.,
H
IRAO
M.,
P
OSAKONY
J.,
N
ELSON
M.,
S
IMON
J.A.: NAD
+
-dependent deacetylase Hst1p controls biosynthesis and cellular
NAD
+
levels in Saccharomyces cerevisiae. Mol.Cell Biol. 19, 7044–7054 (2003).
B
ELL
A.C.,
F
ELSFENFELD
G.: Stopped at the border, boundaries and insulators. Curr.Opin.Genet.Dev. 9, 191–198 (1999).
B
ENARD
L.,
C
ARROLL
K.,
V
ALLE
R.C.,
M
ASISON
D.C.,
W
ICKNER
R.B.: Ski7 antiviral protein is an EF1-
α homolog that blocks expres-
sion of non-poly(A)mRNA in Saccharomyces cerevisiae. J.Virol. 73, 2893–2900 (1999).
B
ERGER
S.L.: Histone modifications in transcriptional regulation. Curr.Opin.Genet.Dev. 12, 142–148 (2002).
B
HOITE
L.T.,
Y
U
Y.,
S
TILMAN
D.J.: The Swi5 activator recruits the mediator complex to the HO promoter without RNA polymerase II.
Genes Dev. 15, 2457–2469 (2001).
B
IALKOVÁ
A., Š
UBÍK
J.: Biology of the pathogenic yeast Candida glabrata. Folia Microbiol. 51, 3–20 (2006).
B
IDDICK
R., Y
OUNG
E.T.: Yeast mediator and its role in transcriptional regulation. C.R.Biol. 328, 773–782 (2005).
B
ÖHM
S.,
F
RISHMAN
D.,
M
EWES
H.W.: Variation of C
2
H
2
zinc finger motif in the yeast genome and classification of zinc finger pro-
tein. Nucl.Acid Res. 25, 2464–2469 (1997).
B
ROWN
T.A.: Genomes, 2nd ed. BIOs Scientific Publishing, Oxford (UK) 2002.
B
URATOWSKI
S.,
H
AHU
S.,
G
UARENTE
L.,
S
HARP
P.A.: Five intermediate complexes in transcription initiation by RNA polymerase II.
Cell 56, 539–561 (1989).
B
USSEREAU
F.,
L
AFAY
L.F.,
B
OLOTIN
-F
UKUHARA
M.: Zinc finger transcriptional activators of yeast. FEMS Yeast Res. 4, 445–458
(2004).
B
USSEREAU
F.,
C
ASAREGOLA
S.,
L
AFAY
L.F.,
B
OLOTIN
-F
UKUHARA
M.: The Kluyveromyces lactis repertoire of transcriptional regula-
tors. FEMS Yeast Res. 6, 325–335 (2006).
B
YRNE
K.P., W
OLFE
K.H.: The yeast gene order browser: contex reveals gene fate in polyploid species. Genome Res. 15, 1456–1461
(2005).
B
YRNE
K.P., W
OLFE
K.H.: Consistent pattern of rate asymmetry and gene loss indicate widespread neofunctionalization of yeast genes
after whole genome duplication. Genetics 175, 1341–1350 (2007).
C
AI
M., D
AVIS
R.W.: Yeast centromere binding protein CBF1, of the helix–loop–helix protein family, is required for chromosome sta-
bility and methionine prototrophy. Cell 61, 437–446 (1990).
C
AIRNS
B.R.,
K
IM
Y.J.,
S
AYRE
M.H.,
L
AURENT
B.C.,
K
ORNBERG
R.D.: A multisubunit complex containing the SWI1/ADR6,
SWI2/SNF2, SWI3, SNF5 and SNF6 gene products isolated from yeast. Proc.Nat.Acad.Sci.USA 91, 1950–1954 (1994).
C
AIRNS
B.R.,
H
ENRY
N.L.,
K
ORNBERG
R.D.: TFG3/TAF30/ANC1, a component of the yeast SWI/SNF complex that is similar to the
leucomogenic proteins ENL and AF-9. Mol.Cell.Biol. 16, 3308–3316 (1996a).
C
AIRNS
B.R.,
L
ORCH
Y.,
L
I
Y.,
Z
HANG
M.C.,
L
ACOMIS
L.,
E
RDJUMENTBROMAGE
H.,
T
EMPST
P.,
D
U
J.,
L
AURENT
B.,
K
ORNBERG
R.D.:
RSC an essential, abundant chromatin-remodeling complex. Cell 87, 1249–1260 (1996b).
C
OSTE
A.T.,
K
ARABABA
M.,
I
SCHER
F.,
B
ILLE
J.,
S
ANGLARD
D.: TAC1, transcriptional activator of CDR genes, is a new transcription
factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot.Cell 3, 1639–1652
(2004).
C
RAMER
P.: RNA polymerase II structure to functional complexes. Curr.Opin.Gen.Dev. 14, 218–226 (2004).
C
UI
Z.,
S
HIRAKI
T.,
H
IRATA
D.,
M
IYAKAWA
T.: Yeast gene YRR1, which is required for resistance to 4-nitroquinoline N-oxide,
mediates transcriptional activation of the multidrug resistance transporter gene SNQ2. Mol.Microbiol. 29, 1307–1315 (1998).
D
ERISI
J.,
V
AN
D
EN
H
AZEL
B.,
M
ARC
P.,
B
ALZI
E.,
B
ROWN
P.,
J
ACQ
C.,
G
OFFEAU
A.: Genome microarray analysis of transcriptional
activation in multidrug resistant yeast mutants. FEBS Lett. 470, 156–160 (2000).
D
IETRICH
F.S.,
V
OEGELI
S.,
B
RACHAT
S.,
L
ERCH
A.,
G
ATES
K.,
S
TEINER
S.,
M
OHR
C.,
P
ÖHLMANN
R.,
L
UEDI
P.,
C
HOI
S.,
W
ING
R.A.,
F
LAVIER
A.,
G
AFFNEY
T.D.,
P
HILIPPSEN
P.: The Ashbya gossypii genome as a tool for mapping the ancient Saccharomyces
cerevisiae genome. Science 304, 304–307 (2004).
D
UJON
B.: Hemiascomycetous yeasts at the forefront of comparative genomics. Curr.Opin.Genet.Dev. 15, 614–620 (2005).
D
UJON
B.,
S
HERMAN
D.,
F
ISCHER
G.,
D
URRENS
P.,
C
ASAREGOLA
S.,
L
AFONTAINE
I.,
D
E
M
ONTIGNY
J.,
M
ARCK
C.,
N
EUVEGLISE
C.,
T
ALLA
E.,
G
OFFARD
N.,
F
RANGEUL
L.,
A
IGLE
M.,
A
NTHOUARD
V.,
B
ABOUR
A.,
B
ARBE
V.,
B
ARNAY
S.,
B
LANCHIN
S.,
B
EC
-
KERICH
J.M.,
B
EYNE
E.,
B
LEYKASTEN
C.,
B
OISRAME
A.,
B
OYER
J.,
C
ATTOLICO
L.,
C
ONFANIOLERI
F.,
D
E
D
ARUVAR
A.,
D
ES
-
PONS
L.,
F
ABRE
E.,
F
AIRHEAD
C.,
F
ERRY
-D
UMAZET
H.,
G
ROPPI
A.,
H
ANTRAYE
F.,
H
ENNEQUIN
C.,
J
AUNIAUX
N.,
J
OYET
P.,
K
ACHOURI
R.,
K
ERREST
A.,
K
OSZUL
R.,
L
EMAIRE
M.,
L
ESUR
I.,
M
A
L.,
M
ULLER
H.,
N
ICAUD
J.M.,
N
IKOLSKI
M.,
O
ZTAS
S.,
O
ZIER
-K
ALOGEROPOULOS
O.,
P
ELLENZ
S.,
P
OTIER
S.,
R
ICHARD
G.F.,
S
TRAUB
M.L.,
S
ULEAU
A.,
S
WENNEN
D.,
T
EKAIA
F.,
W
ESOLOWSKI
-L
OUVEL
M.,
W
ESTHOF
E.,
W
IRTH
B.,
Z
ENIOU
-M
EYER
M.,
Z
IVANOVIC
I.,
B
OLOTIN
-F
UKUHARA
M.,
T
HIER
-
RY
A.,
B
OUCHIER
C.,
C
AUDRON
B.,
S
CARPELLI
C.,
G
AILLARDIN
C.,
W
EISSENBACH
J.,
W
INCKER
P.,
S
OUCIET
J.L.: Genome
evolution in yeasts. Nature 430, 35–44 (2004).
E
KWALL
K.: Genome-wide analysis of HDAC function. Trends Genet. 21, 608–615 (2005).

286 E. DROBNÁ et al.
Vol. 53
F
LANAGAN
P.M.,
K
ELLEHER
I.R.J.,
S
AYRE
M.H.,
T
SCHOCHNER
H.,
K
ORNBERG
R.D.: A mediator required for activation of RNA poly-
merase II transcription in vitro. Nature 350, 436–438 (1991).
G
ARNER
M.M., R
EVZIN
A.: A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions, application
to components of Escherichia coli lactose operon regulatory system. Nucl.Acids Res. 9, 3047–3060 (1981).
G
ASCH
A.P.,
M
OSES
A.M.,
C
HIANG
D.Y.,
F
RASER
H.B.,
B
ERARDINI
M.,
B
ERMAN
J.,
B
ARKAI
N.: Conservation and evolution of cis-
regulatory systems in ascomycete fungi. PLOS Biol. 2, E398 (2004).
G
BELSKÁ
Y.,
K
RIJGER
J.J.,
B
REUNIG
K.D.: Evolution of gene families: the multidrug resistance transporter genes in five related yeast
species. FEMS Yeast Res. 6, 345–355 (2006).
G
EORGAKOPOULOS
T.,
T
HIREOS
G.: Two distinct yeast transcriptional activators require the function of the GCN5 protein to promote
normal level of transcription. EMBO J. 11, 4145–4152 (1992).
G
OFFEAU
A.,
B
ARREL
B.G.,
B
USSEY
H.,
D
AVIS
R.W.,
D
UJON
B.,
F
ELDMANN
H.,
G
ALIBERT
F.,
H
OHEISEL
J.D.,
J
ACQ
C.,
J
OHNSTON
M.,
L
OUIS
E.J.,
M
EWES
M.W.,
M
URAKAMI
Y.,
P
HILIPPSEN
P.,
T
ETHELIN
H.,
O
LIVER
S.G.: Life with 6000 genes. Science 274,
546–563 (1996).
G
RANT
P.A.,
S
TERNER
D.E.,
D
UGGAN
L.J.,
W
ORKMAN
J.L.,
B
ERGER
S.L.: The SAGA unfolds, convergence of transcription regulators
in chromatin-modifying complexes. Trends Cell Biol. 8, 193–197 (1998).
H
ALBERG
M.,
P
OLOZKOV
G.V.,
H
U
G.Z.,
B
EVE
J.,
G
USTAFSSON
C.M.,
R
ONNE
H.,
B
JORKLUND
S.: Site-specific Srb10-dependent phos-
phorylation of the yeast mediator subunit Med2 regulates gene expression from the 2-micron plasmid. Proc.Nat.Acad.Sci.
USA 101, 3370–3375 (2004).
H
AMPSEY
M.: Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol.Mol.Biol.Rev. 62, 465–503
(1998).
H
AMPSEY
M.,
R
EINBERG
D.: Tails of intrigue, phosphorylation of RNA polymerase II mediates histone methylation. Cell 113, 429–432
(2003).
H
ARRISON
S.C., A
GGARWAL
A.K.: DNA recognition by proteins with the helix–turn–helix motif. Ann.Rev.Biochem. 59, 933–999 (1990).
H
ENRY
N.L.,
C
AMBELL
A.M.,
F
EAVER
W.J.,
P
OON
D.,
W
EIL
P.A.,
K
ORNBERG
R.D.: TFIIF-TAF–RNA polymerase II connection. Genes
Dev. 8, 2868–2878 (1994).
H
ITTINGER
C.T.,
R
OKAS
A.,
C
ARROLL
S.B.: Parallel inactivation of multiple GAL pathway genes and ecological diversification
in yeasts. Proc.Nat.Acad.Sci.USA 101, 14144–14149 (2004).
H
OLLENHORST
P.C.,
B
OSE
M.E.,
M
IELKE
M.R.,
M
ÜLLER
U.,
F
OX
C.A.: Forkhead genes in transcriptional silencing, cell morphology
and the cell cycle, overlapping and distinct functions for FKH1 and FKH2 in Saccharomyces cerevisiae. Genetics 154,
1533–1548 (2000).
H
OSHIZAKI
D.K.,
H
ILL
J.E.,
H
ENRY
S.A.: The Saccharomyces cerevisiae INO4 gene encodes a small, highly basic protein required for
derepression of phospholipid biosynthetic enzymes. J.Biol.Chem. 265, 4736–4745 (1990).
J
IANG
Y.W., S
TILMAN
D.J.: Involvement of the SIN4 global transcriptional regulator in the chromatin structure of Saccharomyces cere-
visiae. Mol.Cell.Biol. 12, 4503–4514 (1992).
J
OHNSTON
S.A., Hopper J.E.: Isolation of the yeast regulatory gene GAL4 and analysis of its dosage effects on the galactose/melibiose
regulon. Proc.Nat.Acad.Sci.USA 79, 6971–6975 (1982).
J
ONES
T.,
F
EDERSPIEL
N.A.,
C
HIBANA
H.,
D
UNGAN
J.,
K
ALMAN
S.,
M
AGGE
B.B.,
N
EWPORT
G.,
T
HORSTENSON
Y.R.,
A
GABIAN
N.,
M
A
-
GEE
P.T.,
D
AWIS
P.W.,
S
CHERER
S.: The diploid genome sequence of Candida albicans. Proc.Nat.Acad.Sci.USA 101, 7329–
7334 (2004).
J
UNGWIRTH
H., K
UCHLER
K.: Yeast ABC transporters – a tale of sex, stress, drugs and aging. FEBS Lett. 580, 1131–1138 (2006).
K
ELLIS
M.,
P
ETTERSON
N.,
E
NDRIZZI
M.,
B
IRREN
B.,
L
ANDER
E.S.: Sequencing and comparison of yeast species to identify genes and
regulatory elements. Nature 423, 241–254 (2003).
K
ELLIS
M.,
B
IRREN
B.W.,
L
ANDER
E.S.: Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces
cerevisiae. Nature 428, 617–624 (2004).
K
IM
Y.J.,
B
JORKLUND
S.,
L
I
Y.,
S
AYRE
M.H.,
K
ORNBERG
R.D.: A multiprotein mediator of transcriptional activation and its interaction
with the C-terminal repeat domain of RNA polymerase II. Cell 77, 599–608 (1994).
K
INGSTON
R.E., N
ARLIKAR
G.J.: ATP-dependent remodeling and acetylation as regulator of chromatin fluidity. Genes Dev. 13, 2339–
2352 (1999).
K
LEFF
S.,
A
NDRULISH
E.D.,
A
NDERSON
C.W.,
S
TERNGLANZ
R.: Identification of a gene encoding a yeast histone H4 acetyltransferase.
J.Biol.Chem. 270, 24674–24677 (1995).
K
ORNBERG
R.D.,
L
ORCH
Y.: Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98, 285–
294 (1999).
L
AI
E.,
C
LARK
K.L.,
B
URLEY
S.K.,
D
ARNELL
J.E.: Hepatocyte nuclear factor 3/forkhead or “winged helix” proteins, a family of trans-
cription factors of diverse biologic functions. Proc.Nat.Acad.Sci.USA 90, 10421–10423 (1993).
L
AITY
J.H.,
L
EE
B.M.,
W
RIGHT
P.E.: Zinc finger proteins, new insights into structural and functional diversity. Curr.Opin.Struct.Biol.
11, 39–46 (2001).
L
AUGHON
A.,
G
ESTELAND
R.F.: Isolation and preliminary characterization of the GAL4 gene, a positive regulator of transcription
in yeast. Proc.Nat.Acad.Sci.USA 79, 6827–6831 (1982).
L
EE
Y.C.,
K
IM
Y.J.: Requirement for a functional interaction between mediator components Med6 and Srb4 in RNA polymerase II
transcription. Mol.Cell.Biol. 18, 5364–5370 (1998).
L
EE
T.I.,
Y
OUNG
T.A.: Transcription of eukaryotic protein-coding genes. Ann.Rev.Genet. 34, 77–137 (2000).
L
EE
S.W.,
T
OMASETTO
C.,
S
AGER
R.: Positive selection of candidate tumor-supressor genes by subtractive hybridization. Proc.Nat.
Acad.Sci.USA 88, 2825–2829 (1991).
L
ORCH
Y.,
B
EVE
J.,
G
USTAFSSON
C.M.,
M
YERS
L.C.,
K
ORNBERG
R.D.: Mediator–nucleosome interaction. Mol.Cell 6, 197–201 (2000).
M
ACPHERSON
S.,
L
AROCHELLE
M.,
T
URCOTTE
B.: A fungal family of transcriptional regulators, the zinc cluster proteins. Microb.Mol.
Biol.Rev. 70, 583–604 (2006).
M
ALPERTUY
A.,
T
EKAIA
F.,
C
ASARÉGOLA
S.,
A
IGLE
M.,
A
RTIGUENAVE
F.,
B
LANDIN
G.,
B
OLOTIN
-F
UKUHARA
M.,
B
ON
E.,
B
ROT
-
TIER
P.,
DE
M
ONTIGNY
J.,
D
URRENS
P.,
G
AILLARDIN
C.,
L
ÉPINGLE
A.,
L
LORENTE
B.,
N
EUVÉGLISE
C.,
O
ZIER
-K
ALOGERO
-
POULOS
O.,
P
OTIER
S.,
S
AURIN
W.,
T
OFFANO
-N
IOCHE
C.,
W
EŚOLOWSKI
-L
OUVEL
M.,
W
INCKER
P.,
W
EISSENBACH
J.,
S
OU
-

2008
TRANSCRIPTIONAL REGULATORS OF SOME YEASTS: COMPARATIVE GENOME ANALYSIS – review 287
CIET
J.,
D
UJON
B.: Genomic exploration of the hemiascomycetous yeasts. 19. Ascomycetes-specific genes. FEBS Lett. 487,
113–121 (2000).
M
AMNUN
Y.R.,
P
ANDJAITAN
R.,
M
AHE
Y.,
D
ELAHODDE
A.,
K
UCHLER
K.: The zinc finger regulators Pdr1p and Pdr3p control pleio-
tropic drug resistance (PDR) as homo- and heterodimers in vivo. Mol.Microbiol. 46, 1429–1440 (2002).
M
ARTINEZ
-P
ASTOR
M.T.,
M
ARCHLER
G.,
S
CHILLER
C.,
M
ARCHLER
-B
ONER
A.,
R
UIS
H.: The Saccharomyces cerevisiae zinc finger pro-
teins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). EMBO J. 15,
2227–2235 (1996).
M
ASSARI
M.E.,
M
URRE
C.: Helix–loop–helix proteins, regulators of transcription in eukaryotic organisms. Mol.Cell.Biol. 20, 249–440
(2000).
N
ICHOLS
J.,
S
TRAFFON
M.,
E
NJALBERT
B.,
N
ANTEL
A.,
M
ACASKILL
S.,
W
HITEWAY
M.,
B
ROWN
A.J.P.: Msn2/4-like transcription factors
play no obvious roles in the stress responses of the fungal pathogen, Candida albicans. Eukaryot.Cell 3, 1111–1123 (2004).
N
IKOLOFF
D.M.,
M
CGRAW
P.,
H
ENRY
S.A.: The INO2 gene of Saccharomyces cerevisiae encodes a helix–loop–helix protein that is re-
quired for activation of phospholipid synthesis. Nucl.Acids Res. 20, 3253–3258 (1992).
N
ISHIZAWA
M.: Negative regulation of transcription by the yeast global transcription factors, Gal11 and Sin4. Yeast 18, 1099–1110 (2001).
O
HKUMA
Y.: Multiple functions of general transcription factors TFIIE and TFIIH in transcription, possible points of regulation by
trans-acting factors. J.Biochem. 122, 481–489 (1997).
P
ABO
C.O.,
S
AUER
R.T.: Transcription factors, structural families and principles of DNA recognition. Ann.Rev.Biochem. 61, 1053–1095
(1992).
P
APAMICHOS
-C
HRONAKIS
M.,
C
ONLAN
R.S.,
G
OUNALAKI
N.,
C
OPF
T.,
T
ZAMARIAS
D.: Hrs1/Med3 is a Cyc8-Tup1 corepressor target
in the RNA polymerase II holoenzyme. J.Biol.Chem. 275, 8397–8403 (2000).
P
ISKUR
J., L
ANKJAER
R.B.: Yeast genome sequencing: the power of comparative genomics. Mol.Microbiol. 53, 381–389 (2004).
P
OON
D.,
B
AI
Y.,
C
AMBELL
A.M.,
B
JORKLUND
S.,
K
IM
Y.J.,
Z
HOU
S.,
K
ORNBERG
R.D.,
W
EIL
P.A.: Identification and characterization
of a TFIID-like multiprotein complex from Saccharomyces cerevisiae. Proc.Nat.Acad.Sci.USA 92, 8224–8228 (1995).
P
RASAD
R.,
P
ANWAR
S.L.,
S
MRITI
A.: Drug resistance in yeasts – an emerging scenario. Adv.Microb.Physiol. 46, 155–201 (2002).
P
TASHNE
M., G
ANN
A.: Transcriptional activation by recruitment. Nature 386,
569–577
(1997).
R
AMAKRISHNAN
V.: Histone H1 and chromatin higher-order structure. Crit.Rev.Eukaryot.Gene Expr. 7, 215–230 (1997).
R
EN
B.,
R
OBERT
F.,
W
YRICK
J.J.,
A
PARICIO
O.,
J
ENNINGS
E.G.,
S
IMON
I.,
Z
EITLINGER
J.,
S
CHREIBER
J.,
H
ANNETT
N.,
K
ANIN
E.,
V
OL
-
KERT
T.L.,
W
ILSON
C.J.,
B
ELL
S.P.,
Y
OUNG
R.A.: Genome-wide location and function of DNA binding proteins. Science
290, 2306–2309 (2000).
R
OBERT
F.,
P
OKHOLOK
D.K.,
H
ANNET
N.M.,
R
INALDI
N.J.,
C
HANDY
M.,
R
OLFE
A.,
W
ORKMAN
J.L.,
G
IFFORD
D.K.,
Y
OUNG
R.A.: Glo-
bal position and recruitment of HATs and HDACs in the yeast genome. Mol.Cell 16, 199–209 (2004).
S
ANGLARD
D.: Genomic view on antifungal resistance mechanisms among yeast and fungal pathogens, pp. 359–383 in Candida, Com-
parative and Functional Genomics (C. d’Enfert, B. Hube, Eds). Caister Academic Press, Norfolk (UK) 2007.
S
CANNELL
D.,
B
UTLER
G.,
W
OLFE
K.H.: Yeast genome evolution – the origin of the species. Yeast 24, 929–942 (2007).
S
CHJERLING
P.,
H
OLMBERG
S.: Comparative amino acid sequence analysis of the C
6
zinc cluster family of transcriptional regulators.
Nucl.Acids Res. 24, 4599–4607 (1996).
S
ELLICK
C.A.,
R
EECE
R.J.: Eukaryotic transcription factors as direct nutrient sensors. Trends Biochem.Sci. 30, 405–412 (2005).
S
OUCIET
J.L.,
A
IGLE
M.,
A
RTIGUENAVE
F.,
B
LANDIN
G.,
B
OLOTIN
-F
UKUHARA
M.,
B
ON
E.,
B
ROTTIER
P.,
C
ASAREGOLA
S.,
D
E
M
ON
-
TIGNY
J.,
D
UJON
B.: Genomic exploration of the hemiascomycetous yeast. FEBS Lett. 487, 3–147 (2000).
S
TRUHL
K.: Yeast transcriptional regulatory mechanisms. Ann.Rev.Genet. 29, 651–674 (1995).
T
ALIBI
D.,
R
AYMOND
M.: Isolation of putative Candida albicans transcriptional regulator involved in pleiotropic drug resistance by
functional complementation of pdr1pdr3 mutations in Saccharomyces cerevisiae. J.Bacteriol. 181, 231–240 (1999).
T
AVERNER
N.V.,
S
MITH
J.C.,
W
ARDLE
F.C.: Identifying transcriptional targets. Genome Biol. 5, 210–216 (2004).
T
EIXEIRA
M.C.,
M
ONTEIRO
P.,
J
AIN
P.,
T
ENREIRO
S.,
F
ERNANDES
A.R.,
M
IRA
N.P.,
A
LENGUER
M.,
F
REITAS
A.T.,
O
LIVEIRA
A.L.,
S
A
-
C
ORREIA
I.: The Yeastract database: a tool for the analysis of transcription regulatory associations in Saccharomyces cere-
visiae. Nucl.Acids Res. 34, D446–D451 (2006).
T
IROSH
I., B
ARKAI
N.: Comparative analysis indicates regulatory neofunctionalization of yeast duplicates. Genome Biol. 8, R50 (2007).
T
ODD
R.B., A
NDRIANOPULOS
A.: Evolution of a fungal regulatory gene family, the Zn
II
2
Cys
6
binuclear cluster DNA binding motif.
Fungal Genet.Biol. 21, 388–405 (1997).
U
RNOV
F.D.: A feel for the template, zinc finger protein transcription factors and chromatin. Biochem.Cell.Biol. 80, 321–333 (2002).
U
RNOV
F.D., W
OLFFE
A.P.: Chromatin remodeling and transcriptional activation, the cast (order in appearance). Oncogene 20, 2991–
3006 (2001).
V
ALLEE
B.L.,
C
OLEMAN
J.E.,
A
ULD
D.S.: Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc.Nat.Acad.
Sci.USA 88, 999–1003 (1991).
V
AN
S
TEENSEL
B.,
H
ENIKOFF
S.: Identification in vivo DNA targets of chromatin proteins using tethered Dam methyltransferase. Nat.
Biotechnol. 18, 379–380 (2000).
V
AN
S
TEENSEL
B.,
D
ELROW
J.,
H
ENIKOFF
S.: Chromatin profiling using targeted DNA adenine methyltransferase. Nat.Genet. 27, 304–
308 (2001).
V
ELCULESCU
V.E.,
Z
HANG
L.,
V
OGELSTEIN
B.,
K
INZLER
K.W.: Serial analysis of gene expression. Science 270, 484–487 (1995).
V
ELCULESCU
V.E.,
V
OGELSTEIN
B.,
K
INZLER
K.W.: Analysing uncharted transcriptomes with SAGE. Trends Genet. 16, 423–425 (2000).
V
IGNALI
M.,
H
ASSAN
A.H.,
N
EELY
K.E.,
W
ORKMAN
J.L.: ATP-dependent chromatin-remodeling complexes. Mol.Cell.Biol. 20, 1899–
1910 (2000).
W
OLFE
K.H.,
S
HIELDS
D.C.: Molecular evidence for ancient duplication of the entire yeast genome. Nature 387, 708–713 (1997).
W
ORKMAN
J.L.,
K
INGSTON
R.E.: Alteration of nucleosome structure as a mechanism of transcriptional regulation. Ann.Rev.Biochem.
65, 545–579 (1998).
W
U
J.,
G
RUNSTEIN
M.: 25 years after the nucleosome mode, chromatin modifications. Trends Biochem.Sci. 25, 619–623 (2000).
Y
ANG
V.W.: Eukaryotic transcription factors: identification, characterization and function. J.Nutr. 128, 2045–2051 (1998).
Z
HANG
L.,
G
UARENTE
L.: The yeast activator HAP1 – a GAL4 family member – binds DNA in a directly repeated orientation. Genes
Dev. 8, 2110–2119 (1994).
Wyszukiwarka
Podobne podstrony:
Regulation of pleiotropic drug resistance in yeast
Development of a highthroughput yeast based assay for detection of metabolically activated genotoxin
Bcl 2 family regulator of apoptosis
hawthornes symbols in the house of seven gables
The?C v Pacifica Foundation Regulation of Radio Broadca
The Transcendence ( Uluw) of Allaah , Refuting Doubts and Misconceptions
BOOK OF SEVEN DELIGHTS By Betina Krahn
Bcl 2 family regulator of apoptosis
Regulation of multidrug resistance in pathogenic fungi
General Explanation of Seven Point Attitude Training
Evaluation of biomass quality of selected woody species depending on the method of soil enrichment a
MicroRNA Regulation of Cancer Stem Cells and Therapeutic Implications
Explanation of Seven Point Attitude Training
Inci H , Kappeler T , Topalov P On the regularity of the composition of diffeomorphisms (MEMO1062, A
Dos Santos Ferreira D , Staubach W Global and local regularity of Fourier integral operators on weig
~$aluation of biomass quality of selected woody species depending on the method of soil enrichment a
więcej podobnych podstron