Dipolar Cycloadditions in Alkaloid
Dipolar Cycloadditions in Alkaloid
Synthesis
Mariam Shamszad
Literature Group Meeting
February 25, 2009
H
Me
H
H
OH
H
OH
N
O
O
D
E
Selected Alkaloids
N
H
H
H
H
CH
2
OH
Kobusine
HN
O
N
N
O
O
Me
Me
H
N
MeO
OMe
H
Me
O
Aspidophytine
A
B
C
• Insecticidal properties
• Padwa and coworkers use
cyclization/dipolarcycloaddition
cascade to access pentacyclic
• Exhibits potent vasodilating
activity in vivo
• Partial synthesis achieved by
Gin and coworkers utilizing
dipolarcycloaddition to access
(-)-Spirotryprostatin B
HN
cascade to access pentacyclic
core
• Inhibits cell growth of various leukemia cell lines
• Williams and coworkers use dipolarcycloaddition to access
the spirooxindole core
dipolarcycloaddition to access
hetisine skeleton
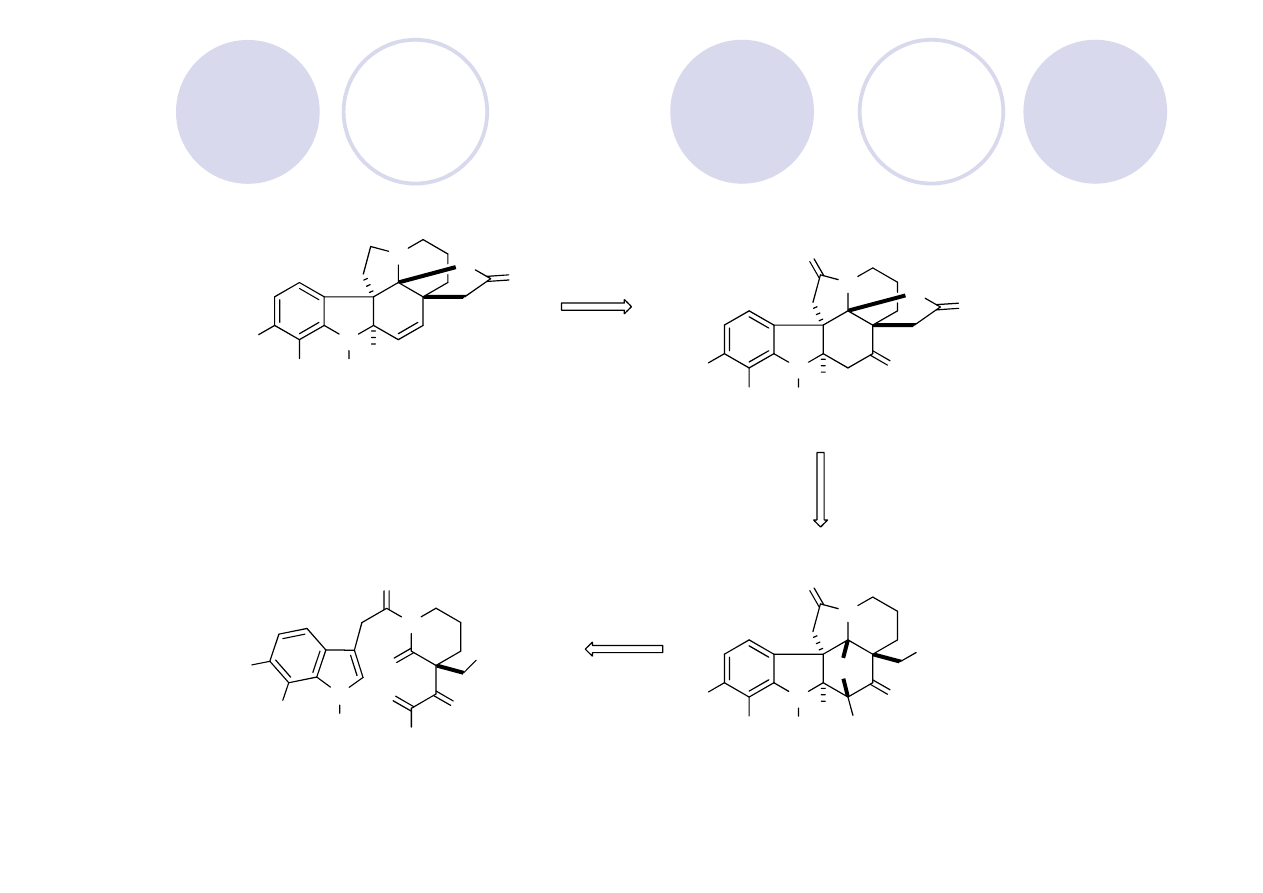
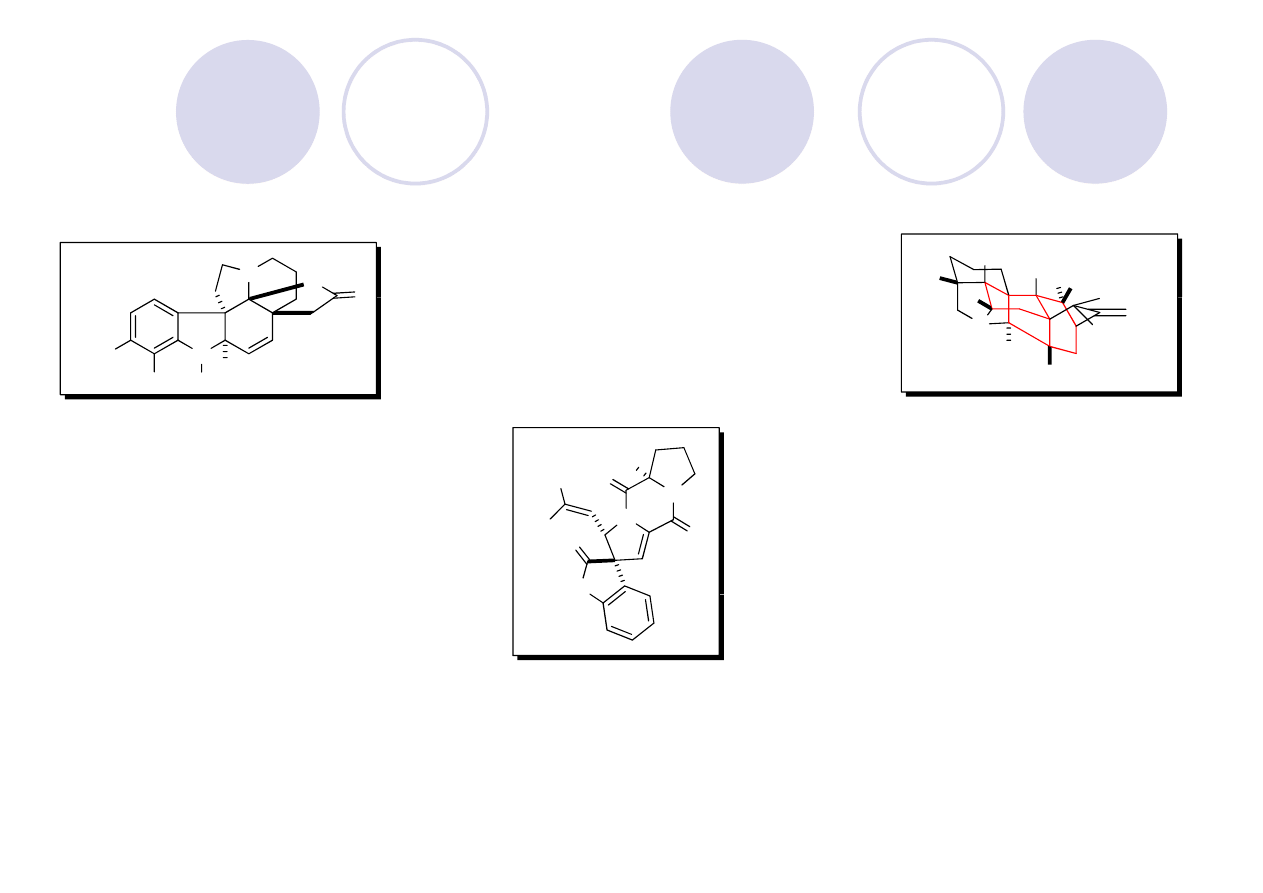
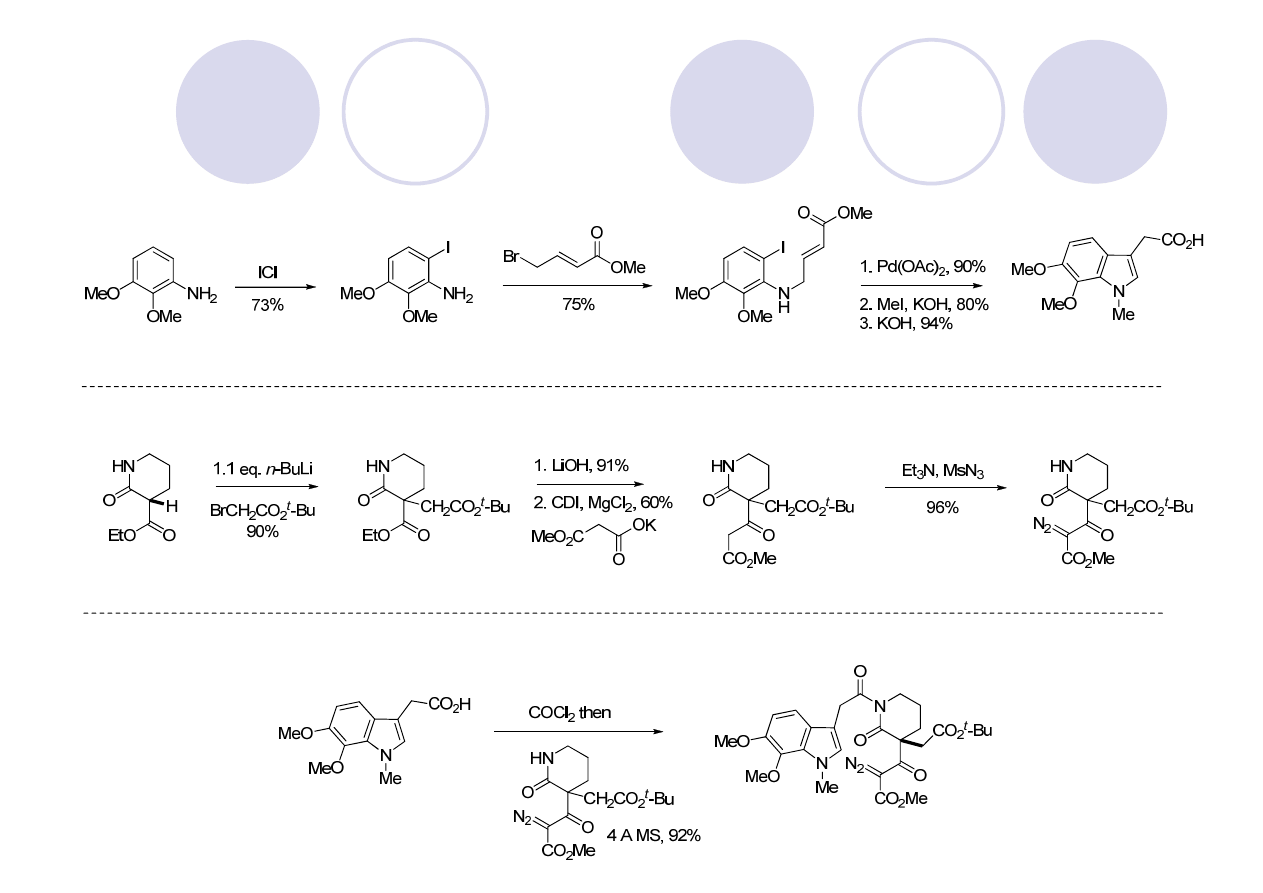
(
±
)-Aspidophytine: Retrosynthesis (Padwa)
N
O
O
N
O
O
Showcases their Rh (II) cyclization/dipolarcycloaddition cascase methodology
N
MeO
OMe
H
Me
Aspidophytine
N
MeO
OMe
H
Me
O
O
O
O
O
cyclization/cycloaddition
Lewis acid-mediated
rearrangement
N
MeO
OMe
H
N
Me
O
O
CO
2
t
-
Bu
N
Me
MeO
MeO
N
O
O
CO
2
Me
N
2
CO
2
t
-Bu
CO
2
Me
cyclization/cycloaddition
cascade
Mejia-Oneto, J. M.; Padwa, A. Org. Lett. 2006, 8, 3275.
(
±
)-Aspidophytine - Padwa
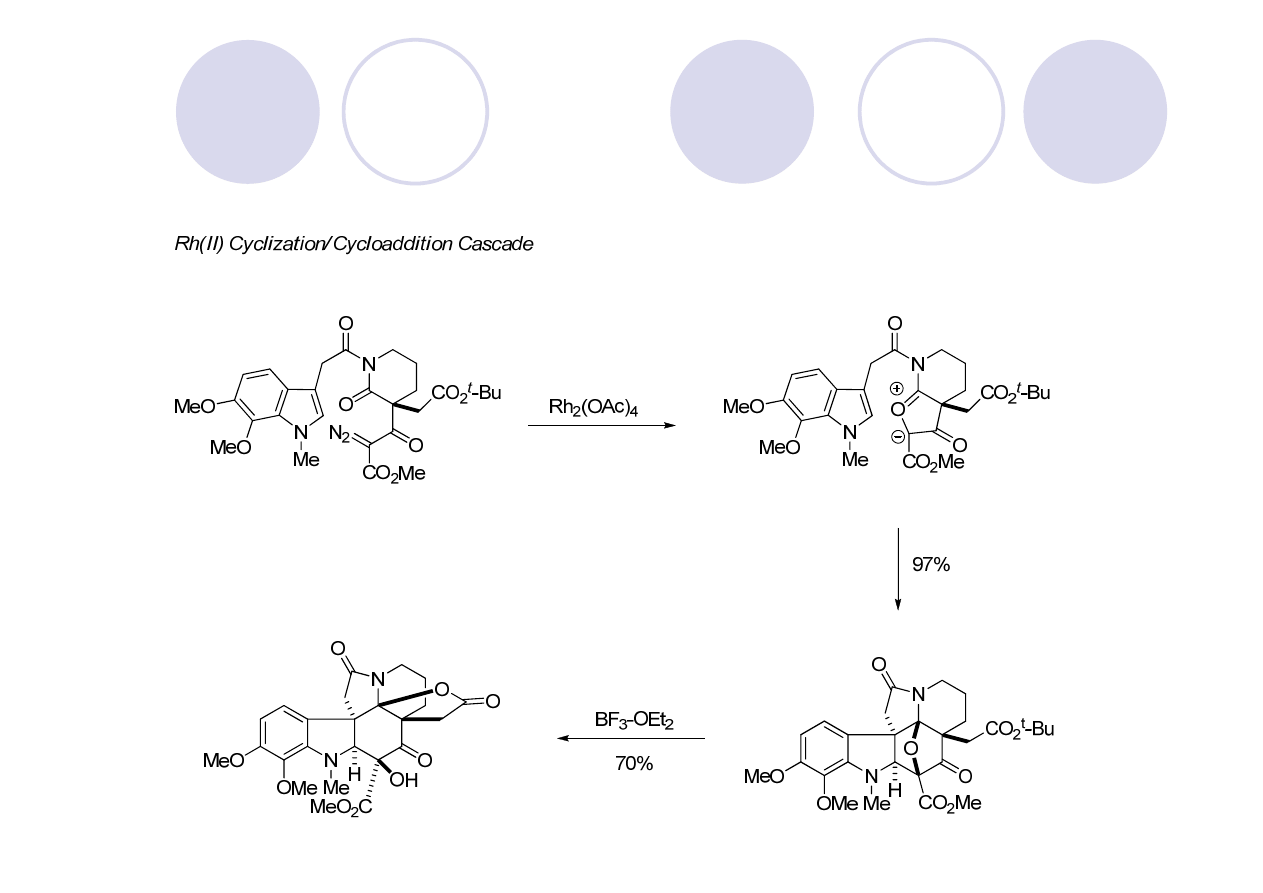
(
±
)-Aspidophytine - Padwa
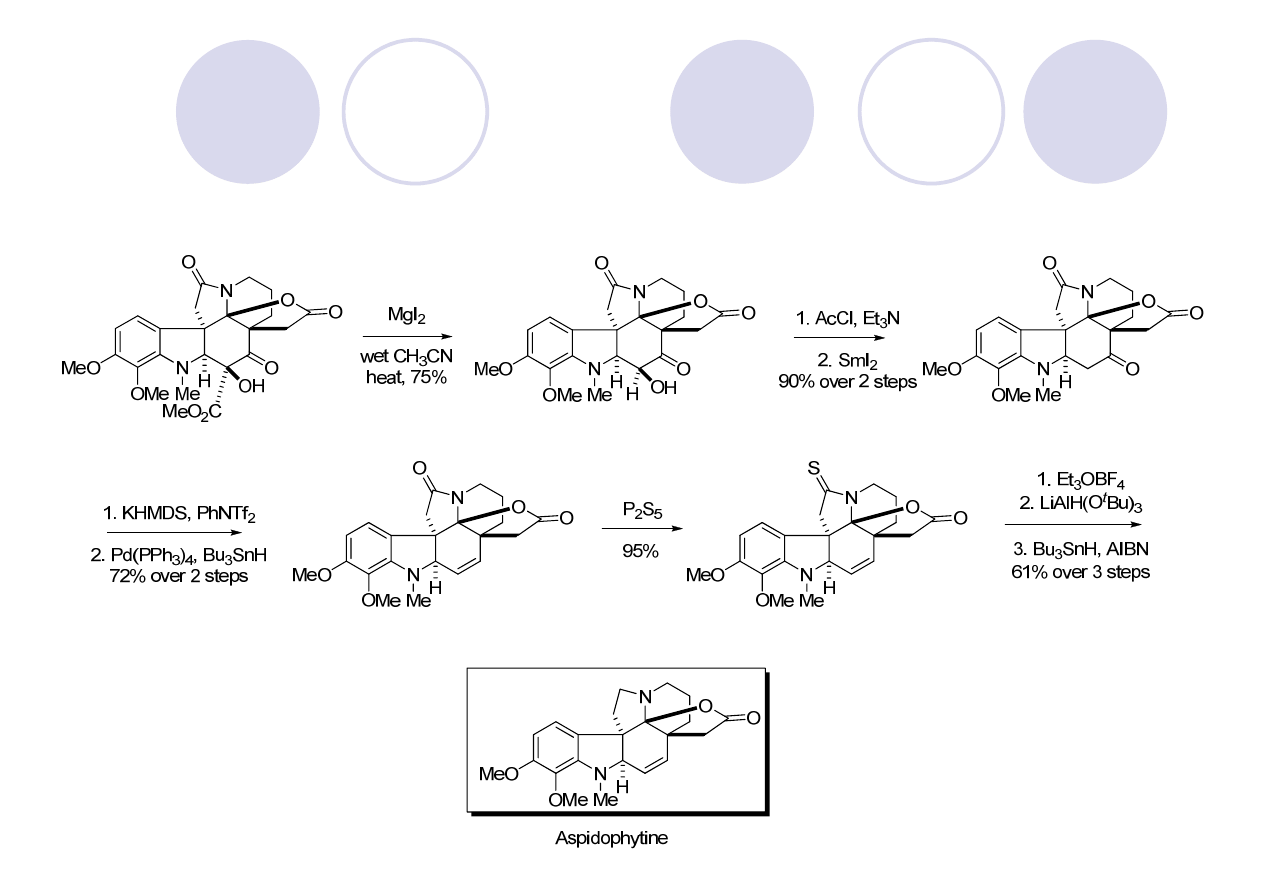
(
±
)-Aspidophytine - Padwa
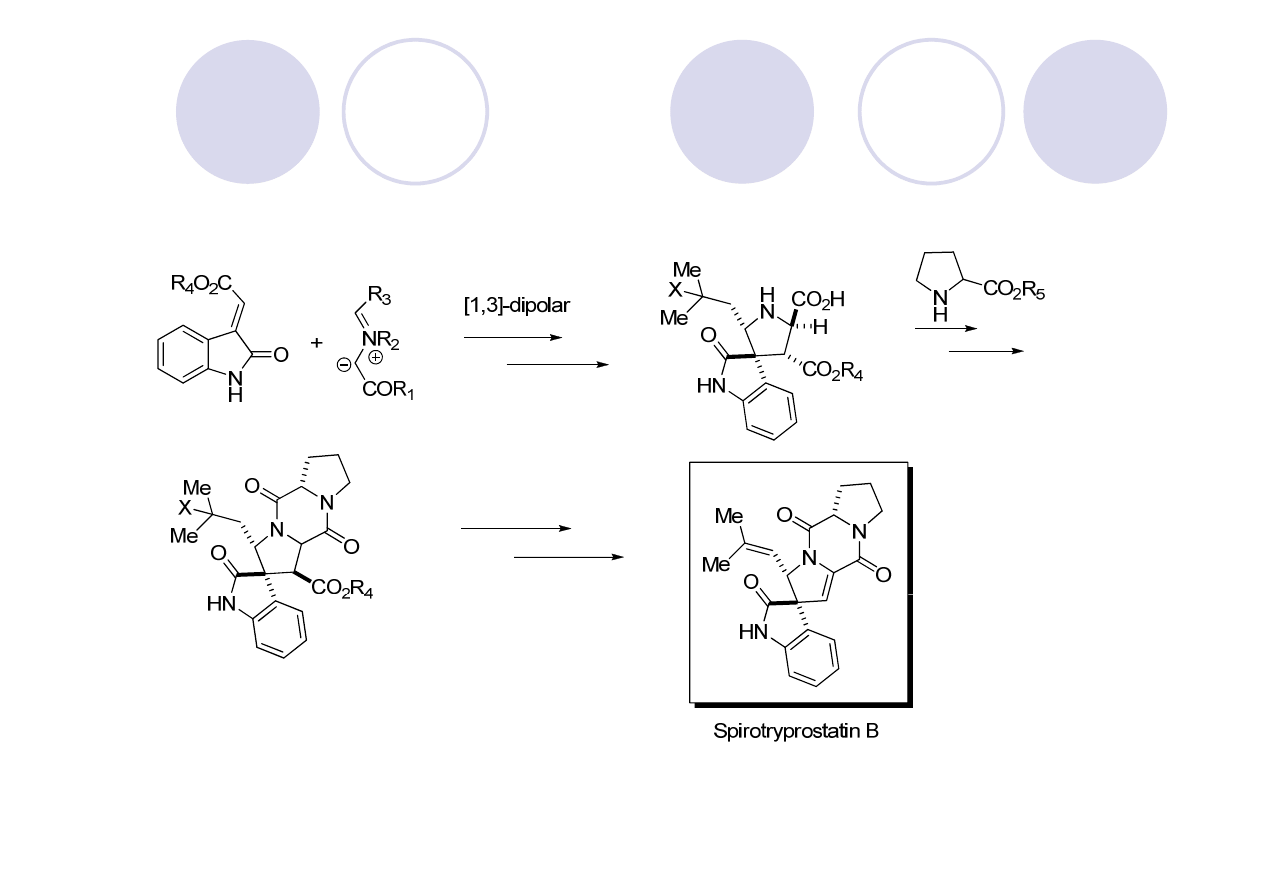
(-)-Spirotryprostatin B - Williams
Sebahar, P. R.; Williams, R. M. J. Am. Chem. Soc. 2000, 122, 5666.
(-)-Spirotryprostatin B - Williams
OHC
M
Me
OMe
HN
O
O
Ph
Ph
N
O
O
Ph
Ph
Me
M O
CO Et
toluene
82%
O
N
O
Me
Me
O
Ph
Ph
H
MeO
N
H
O
EtO
2
C
Me
MeO
Me
HN
O
CO
2
Et
3A mol. sieves
HN
CO
2
Et
(-)-Spirotryprostatin B - Williams
O
Ph
Ph
OHC
Me
Me
OMe
O
N
O
Me
Me
O
Ph
Ph
MeO
H
2
, PdCl
2
THF EtOH
O
H
N
Me
Me
MeO
CO
2
H
H
1. D-pro-OBn, BOP
Et
3
N, MeCN, 74%
2 H Pd/C EtOH
HN
O
mol. sieves, tol.
N
H
O
EtO
2
C
HN
O
O
H
CO
2
Et
82%
THF, EtOH
60 psi
99%
HN
CO
2
Et
2. H
2
, Pd/C, EtOH
3. BOP, Et
3
N, MeCN
94% over 2 steps
HN
O
N
Me
Me
MeO
CO
2
Et
N
O
O
H
H
TsOH ( 1eq)
toluene, heat
82-89%
HN
O
N
Me
Me
CO
2
Et
N
O
O
H
H
1. LiI, py., heat
70-74%
2. DCC, DMAP
N
HO
HN
O
N
N
O
O
Me
Me
12
HN
HN
BrCCl
3
, heat, 34-43%
3. NaOMe, MeOH
S
(-)-spirotryprostatin B
2:1 (-)-spirotryprostatin B: 12-epi-spirotryprostatinB
(+)-spirotryprostatin B accessible from
HN
O
O
Ph
Ph
N
Bn
OEt
O
D-pro-OBn
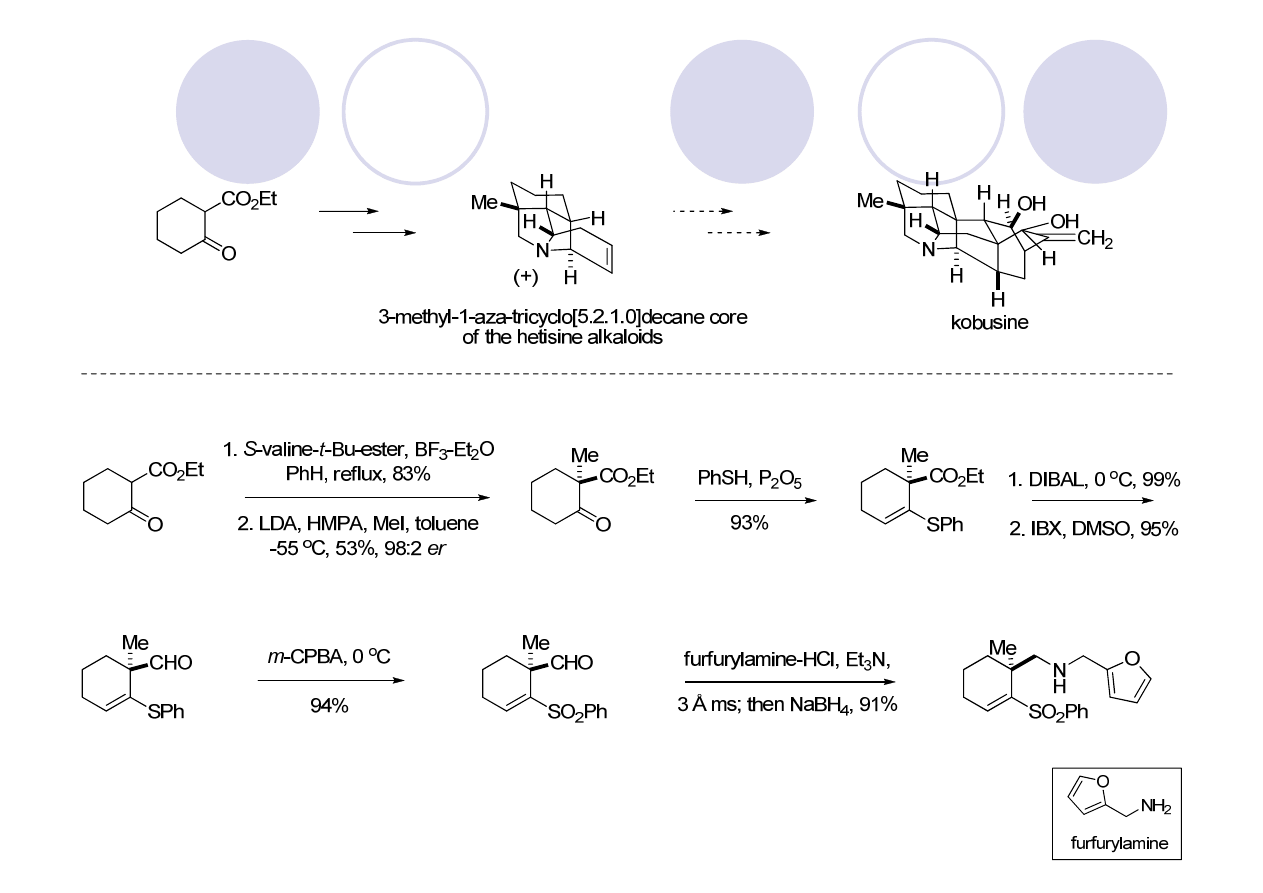
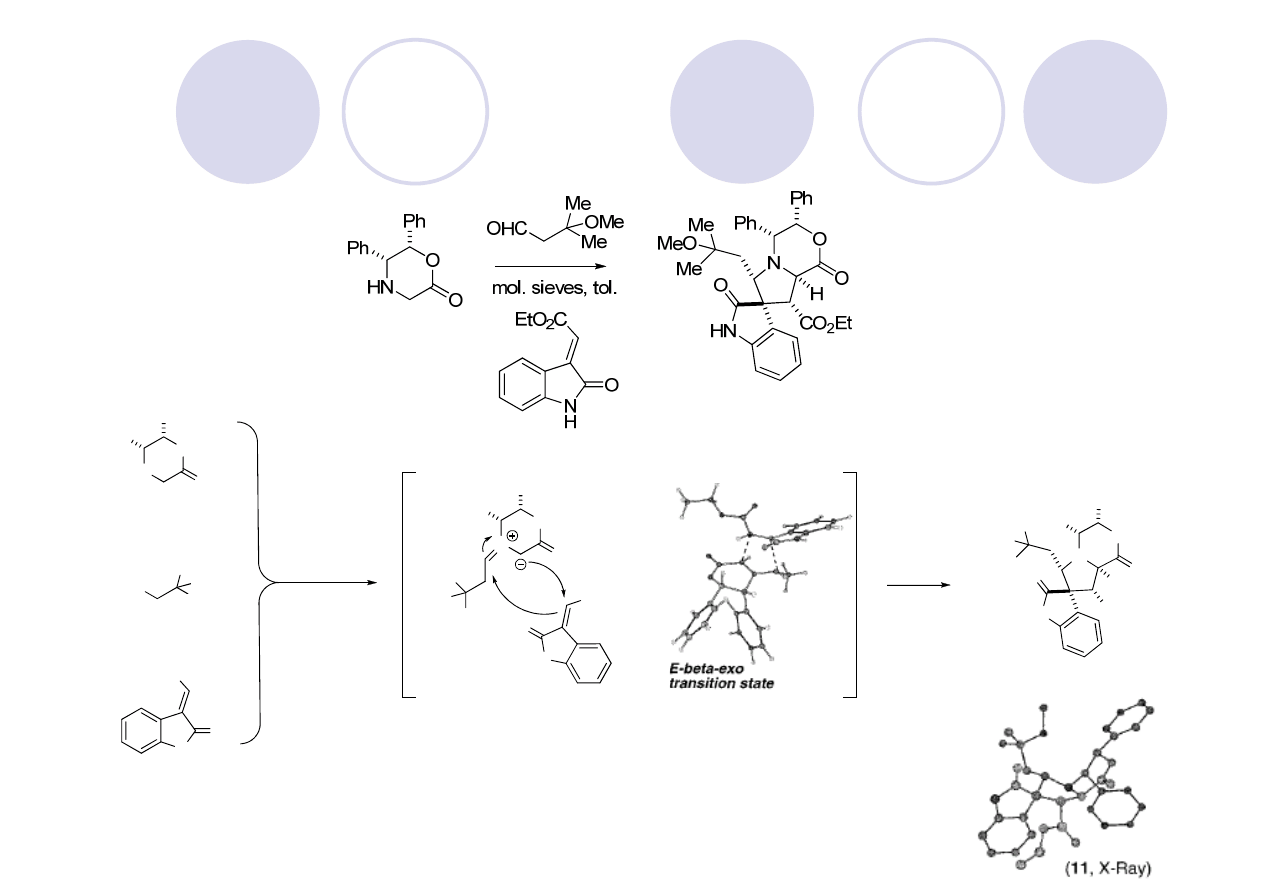
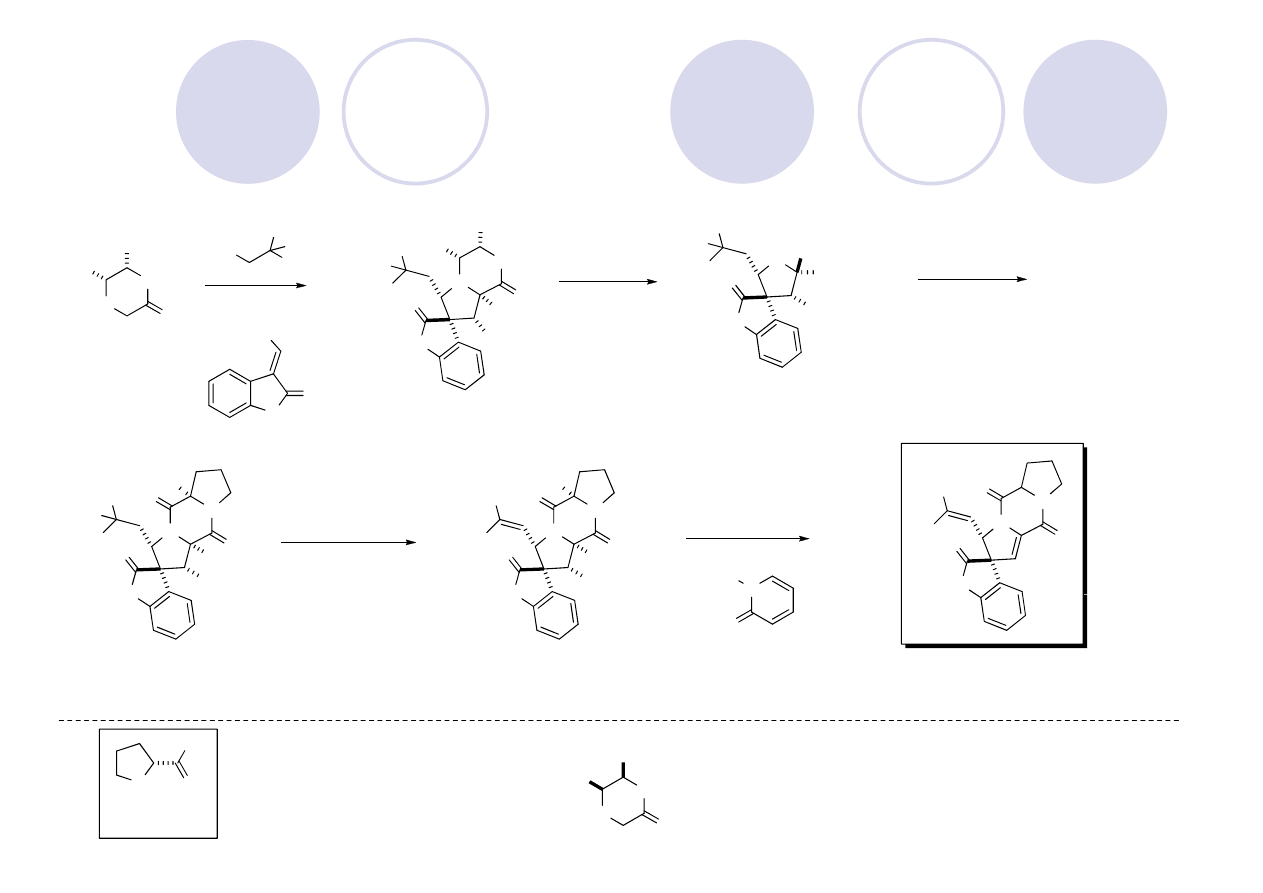
Hetisine Skeleton: Kobusine (Gin)
Peese, K. M.; Gin, D. Y. Org. Lett. 2005, 7, 3323.
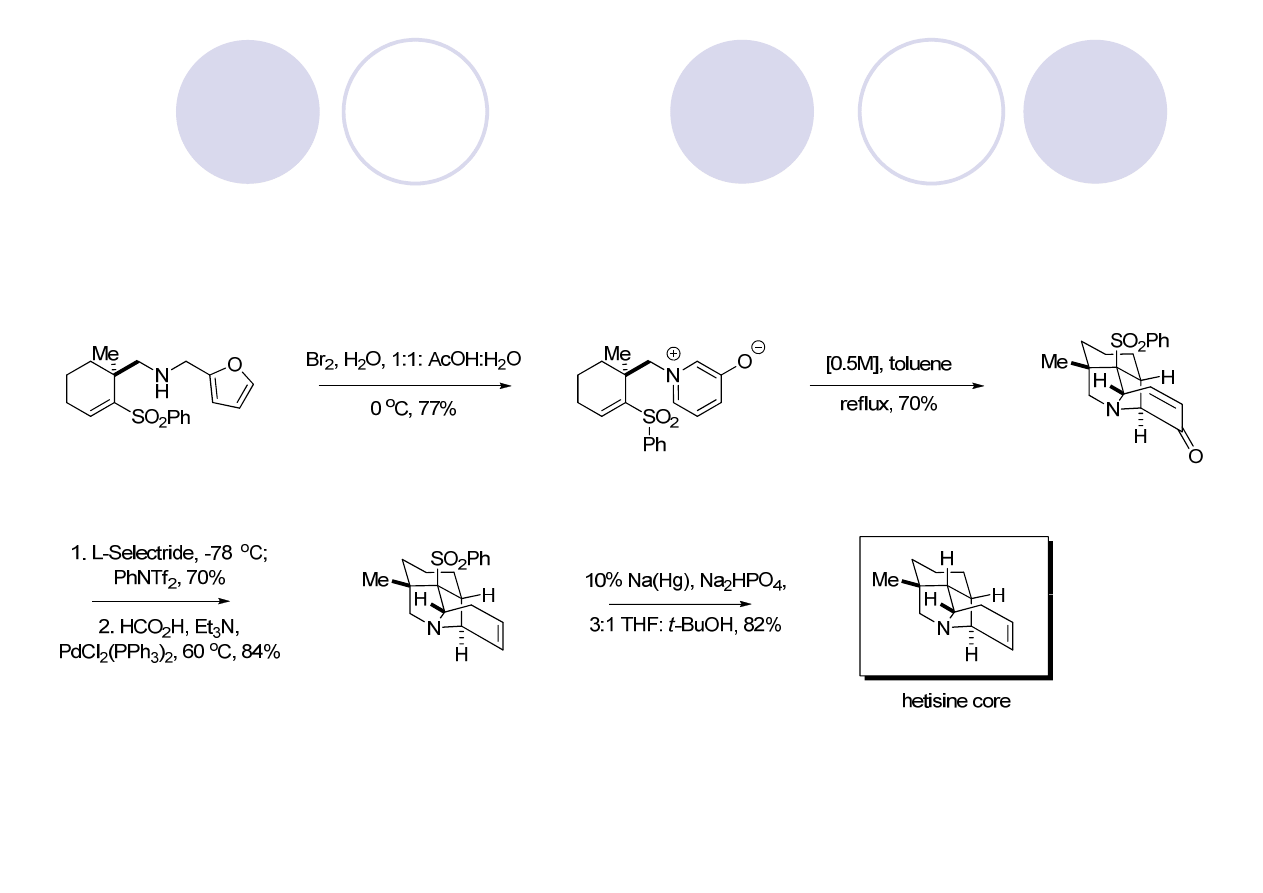
Hetisine Skeleton: Kobusine (Gin)
Wyszukiwarka
Podobne podstrony:
więcej podobnych podstron