
344
Journal of Basic Microbiology 2007, 47, 344 – 350
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Research Paper
Yeasts colonizing the leaf surfaces
Elena Sláviková
1
, Renata Vadkertiová
1
and Dana Vránová
2
1
Culture Collection of Yeasts, Institute of Chemistry Slovak Academy of Sciences, Bratislava, Slovakia
2
Institute of Food Science and Technology, Faculty of Chemistry, BUT, Brno, Czech Republic
The yeasts were isolated from the leaf surfaces of ten species of trees. The study site was a
forest park (Železná Studnička) of the Small Carpathians mountain range. One hundred and
thirty seven yeast strains belonging to 13 genera were isolated from 320 samples of leaves and
needles. Seventeen yeast species were isolated, but only seven occurred regularly: Aureobasidium
pullulans, Cryptococcus laurentii, Pichia anomala, Metschnikowia pulcherrima, Saccharomyces sp.,
Lachancea thermotolerans, and Rhodotorula glutinis. The remaining species were isolated from the
leaves and needles of three or less tree species. A. pullulans, Cr. laurentii, and P. anomala were the
most frequently found species and they occurred on leaves and needles of all ten tree species.
Saccharomyces sp. occurred in leaf samples collected from eight kinds of trees. M. pulcherrima and
L. thermotolerans were found in samples collected from six species of trees. Both these species
occurred almost always on the leaves of deciduous trees. Rh. glutinis was the most frequently
isolated carotenoids producing species. We have found out that the ascomycetous and
basidiomycetous species were present in the leaf samples in approximately equal frequency,
contrary to the soil samples taken from this forest park, where the ascomycetous species were
found rarely.
Keywords: Yeasts/Leaf surfaces/Colonization/Isolation
Received: February 05, 2007; returned for modification: February 20, 2007; accepted February 27, 2007
DOI 10.1002/jobm.200710310
Introduction
*
The plant surfaces are colonized by a large number of
microorganisms. This environment is usually named the
phylloplane or phyllosphere. Most work on phyllos-
phere microbiology has focused on leaves, a more domi-
nant aerial plant structure (Lindow and Brandl 2003).
Yeasts are important members in many ecosystems
(Fleet 1998) and they form also a major component of
the population on leaves (Glushakova and Chernov
2004, Inácio et al. 2005, Nakase 2000). Little is known
about the ecological role of the phylloplane yeasts. The
leaf surface characteristics may affect, both qualitati-
vely and quantitatively, immigration of yeasts to the
phylloplane (Blakeman 1973). Leaves are covered, to the
various degrees, with surface waxes which function to
repel water due to their hydrophobic nature (Holloway
Correspondence: Dr. Elena Sláviková, Culture Collection of Yeasts,
Institute of Chemistry Slovak Academy of Sciences, Dúbravská cesta 9,
845 38 Bratislava, Slovakia
E-mail: Elena.Slavikova@savba.sk
1971). Possibly, the very waxy surface of leaves prevents
the nutrients from being available, since the presence
of free water on the leaves could contribute to the
enhanced leaching of nutrients, which is a positive
process and was shown to be associated with rain
(Tukey 1970). The abundance of nutrients can also vary
with the plant species, leaf age, and growing conditions
(Mercier and Lindow 2000). Yeasts are active as
competitors for nutrients, antagonists or symbiotic
associates or as victims of the behaviour of their
neighbours (Do Carmo-Sousa 1969). Leaf surfaces are
colonized by members of several genera of saprophitic
yeasts that provide a
natural buffer against plant
pathogen (Fokkema et al. 1979). The leaves are exposed
to rapidly fluctuating temperature and relative humi-
dity, which may have an impact on the yeast popu-
lation. Large fluxes of UV radiation are also one of the
most prominent features of the leaf surface environ-
ment to which micoorganisms have presumably had to
adapt (Lindow and Brandl 2003). Many plants contain a
number of compounds whose adaptive significance

Journal of Basic Microbiology 2007, 47, 344–350
Yeasts colonizing the leaf surfaces
345
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
may be a defense of the plant against invertebrates
and microorganisms (Robinson 1974). These com-
pounds also act, in some cases, as selective agents
which shape the yeast community composition (Lach-
ance 1990).
The external surface of the leaf as a habitat for yeasts
has been recognized more recently than the interior of
flowers or fruits of higher plants. In the past, several
years ago, the extensive isolation studies of yeasts from
plant materials were carried out (Middelhoven 1997,
Nakase 2000, Inácio et al. 2002). Some new yeast species
were isolated and described from these habitats.
No information on yeasts associated with the phyl-
losphere on the territory of Slovakia and neighbouring
countries is available. Only the yeasts associated with
fruits and grapes were studied in Slovakia. In our
previous investigations we studied the occurrence of
yeasts and yeast-like species in the soil taken from the
forest park Železná Studnička (Sláviková and Vadker-
tiová 2000), as well as in water of the fish-pond located
there, too (Sláviková and Vadkertiová 1995). The pur-
pose of this work was to investigate the yeast com-
munity colonizing the leaf surface of various tree
species during two consecutive years. The obtained
results enable to compare the yeast population colo-
nizing the leaves and needles of various tree kinds. The
monitored yeast community is actually fundamental to
the more comprehensive study of microbiological
functions in nature.
Materials and methods
The study site was a forest park (Železná Studnička)
frequently visited by inhabitants of Bratislava. It is a
typical forest of the Small Carpathians mountain range,
which represents the beginning of the Carpathians.
Plants in this territory have not been consistently
investigated as natural habitats for microorganisms in
general, and fungi in particular, and nothing is known
on the possible association between the members of the
phylloplane mycobiota and prevalent plant species.
The yeasts were isolated from the leaf surfaces of ten
species of trees.
The leaves were chosen at random from eight various
deciduous tree species: oak Quercus robur L. ex Simk.,
beech Fagus silvatica L., hornbeam Carpinus betulus L.,
maple Acer campestre L., acacia Robinia pseudacacia L., ash
Fraxinus excelsior L., linden Tilia cordata Mill., willow Salix
caprea L. and from two coniferous tree species: spruce
Picea abies Karst., pine Pinus silvestris L., which are
specific to this area (locality).
The collection of leaves and needles was made in the
springtime in the middle of June and in the autumn in
the late September during two consecutive years (June
2003 to September 2004). Eighty samples were collected
during each sampling. In total, this resulted in 320
samples from which yeasts and yeast-like organisms
were isolated. Leaves and needles were carefully ripped
out of the twigs and put into the sterile plastic bags,
transported to the laboratory, and processed within 2 h
after the collection. The amounts of 5 g of each samples
were cut up and placed in the 250-ml flasks containing
50 ml of sterile distilled water and shaken on a rotary
shaker for 2 h at 25
o
C. Leaf washings were serially
diluted and 0.1 ml of each dilution was spread on malt
agar (MEA; Oxoid) containing 80
µg · ml
–1
of strepto-
mycin. The plates were incubated at 25
o
C. After 3, 5
and 10 days the different colonies were picked and
were streaked pure on the malt agar plates. The
cultures were maintained on the malt agar slants.
The morphological and physiological characteristics
of isolates were examined by the methods described by
Yarrow (1998). Strains were identified according to
Kurtzman and Fell (1998) and Barnett et al. (2000).
The identification of the strains belonging to the
species Cryptococcus laurentii and Rhodotorula glutinis was
also confirmed by the PCR-RFLP analysis of the rRNA
gene internal transcribed spacer (ITS) regions according
to Esteve-Zarzoso et al. (1999) and Leaw et al. (2006).
DNA
preparation:
Cells were collected from a fresh
yeast colony. The DNA was extracted by using the Ultra
Clean Microbial DNA Isolation kit (MOBIO Laboratories,
USA) in accordance with the manufactures instruc-
tions. The extracted DNA was stored at –20 ºC.
PCR reaction and DNA digestion: To amplify the ITS
region two primers: ITS1 (5
′ TCCGTAGGTGAACCTGCGG
3
′) and ITS4 (5′ TCCTCCGCTTATTGATATGC 3′) were
used. The PCR was performed in a total reaction vol-
ume of 50
µl consisting of Taq polymerase buffer (Apli-
gene), 0.02 mM dNTPs, 0.02
µM of each primers, 1 unit
of Taq polymerase (Apligene), 3 – 10 ng DNA in 1 – 2
µl
of TE buffer. PCR amplification was carried out in PTC-
100 Programmable Thermal Controller (MJ Research,
Inc., USA). After an initial denaturation at 94 ºC for
4 min, 25 cycles of amplification were conducted as
follows: denaturation at 94 ºC for 1 min, annealing at
48 ºC for 30 sec, and
extension at 72 ºC for 1 min. The
final extension was at 72 ºC for 10 min. A negative con-
trol was performed with each run by replacing
the template DNA with sterile water in the PCR mix-
ture.
The amplification products were precipitated by
ethanol and diluted in an appropriate buffer. Ampli-
346 E.
Sláviková
et al.
Journal of Basic Microbiology 2007, 47, 340– 343
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Table 1. The occurrence of yeasts and yeast-like species isolated from tree leaves.
Species Spruce
Pine
Willow
Oak
Beech
Maple
Hornbean
Linden
Acacia
Ash
Aureobasidium pullulans
57* 41
75 75
63 63 63
63 33 50
Candida catenulata
4
8
Candida krusei
4
Cryptococcus albidus
9
8
Cr. laurentii
26 41
13 20
38 21 16
36 25 42
Geotrichum candidum
13
Hanseniaspora vineae
4
Lachancea thermotolerans
13
5
25
8
11
8
Metschnikowia pulcherrima
4
5
13
17
5
9
Pichia anomala
26
27
13
5
25
21
11
18
17
17
Pseudozyma flocculosa
4
4
9
Rhodotorula glutinis
4
4
25
5
8
8
Rh. mucilaginosa
4
Rh. muscorum
4
4
13
Saccharomyces sp.
13
18
20
13
47
18
8
25
Sporobolomyces roseus
4
4
5
9
Guehomyces pullulans
13
* The number gives % of positive samples
cons were digested with HaeIII, TaqI, TruI, HinfI, HhaI and
Eco88I restriction endonucleases (Promega). The digests
were analyzed by 2% agarose gel electrophoresis in TBE
buffer. Gels were stained with ethidium bromide and
visualised under UV light (Ultra. Lum, Inc.). The amplifi-
cation products of the unknown strains were compared
with the amplification products of standard strains.
Results and discussion
One hundred and thirty seven yeast strains belonging
to 13 genera and 17 species were isolated from 320
samples of leaves and needles. Table 1 provides a list of
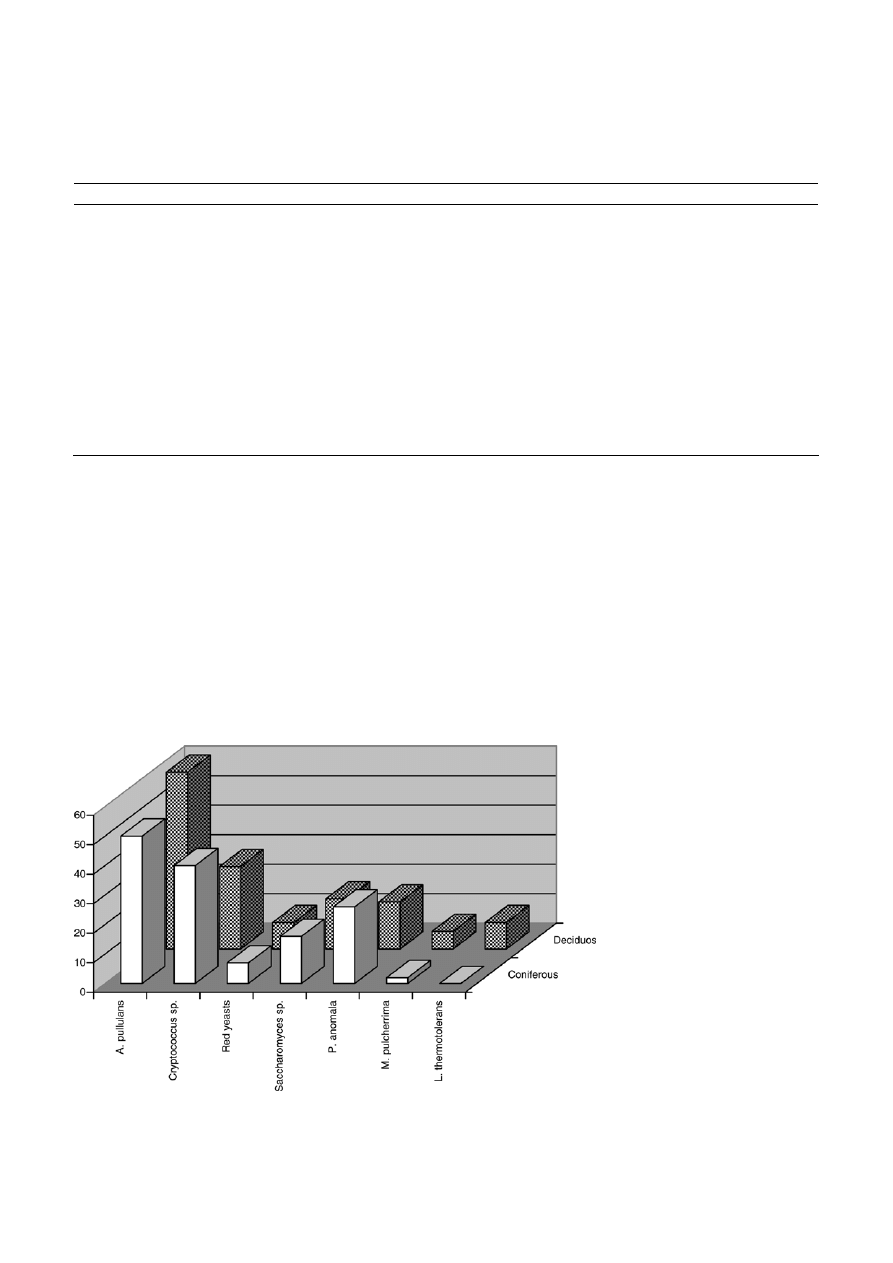
isolated species. Aureobasidium pullulans, Cryptococcus lau-
rentii, and Pichia anomala were the most frequently
isolated species and they occurred on leaves and
needles of all ten trees species; A. pullulans was present
in 33 – 75% of the samples, Cr. laurentii in 16 – 42% of
samples, and P. anomala in 5–27% of the samples (Table 1,
Fig.
1). The strains of Saccharomyces sp. was also
regularly isolated. It was present in 8 – 47% of the
samples and did not occur only on the leaves of beeches
and willows. Another ascomycetous yeast species
Metschnikowia pulcherrima and Lachancea thermotolerans
were found in the samples collected from six species of
Figure 1. Yeast species most frequently isolated from the leaves of coniferous and decidous trees (the number gives % of positive samples).

Journal of Basic Microbiology 2007, 47, 344–350
Yeasts colonizing the leaf surfaces
347
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
trees and were present at most in 17 and 25% of
samples, respectively. Both these species occurred
almost always on the leaves of deciduous trees.
Rhodotorula
glutinis was the most frequently isolated
carotenoids producing species. It colonized the leaves
and needles of six tree species and was found in 4 – 25%
of the samples. Rh. glutinis has a world-wide distribution
and some studies have suggested that this species
might encompass more than one species (Gadanho and
Sampaio 2002). Therefore, the sequence analysis of the
rRNA gene internal transcribed spacer (ITS) regions
ensued the phenotypic identification of tree leaves
isolates. As it can be seen, the representative strains no.
3, 40a, 16f, 38
1
, and 22 classified as Rh. glutinis exhibited
similar profiles as the type strain of this species (Fig. 2).
Another red-pigmented Rhodotorula sp. and Sporo-
bolomyces roseus occurred less frequently. The remain-
ing seven species (Cryptococcus albidus, Guehomyces pul-
lulans, Geotrichum candidum, Pseudozyma flocculosa, Han-
seniaspora vineae, Candida catenulata, and Candida krusei)
were isolated from the leaves and needles of three or
less species.
Table 1 shows that the ascomycetous and basidio-
mycetous species were present in the leaf samples in
approximately equal frequency, contrary to the soil
samples taken from this forest park (Sláviková and
Vadkertiová 2000), where the ascomycetous species
were found rarely. On the other hand, Cr. laurentii was
the dominant species in both surroundings, in the soil
and on the leaf surfaces. This species is reported to be
heterogenous based on DNA G + C content, whole-cell
protein electrophoretic patterns, and the sequences of
1
2
3
4
5
6
7
8
9
10 11 12
13
M
bp
1 000
500
100
Figure 2. PCR-RFLP analysis of the ITS region Cryptococcus
laurentii and Rhodotorula glutinis strains.
Lanes 1 – 5: isolates from tree leaves no. 6b, 14
1
, 16, 27
1,
and 10
phenotypically identified as Cryptococcus laurentii, lane 6 – Cr.
laurentii CCY 17-3-2 (Type); Lanes 7 – 11: isolates from tree leaves
no. 3, 40a, 16f, 38
1
, and 22 phenotypically identified as Rhodotorula
glutinis, lane 12 – Rh. glutinis CCY 20-2-34 (Type); 13 – DNA size
marker; M – molecular size marker; DNA cleaved with HhaI.
the D
1
/D
2
region of 26S rDNA and ITS regions (Sugita
et al. 2000, Takashima et al. 2003). The representative
strains no. 6b, 14
1
,
16, 27
1
, and 10 classified as Cr. lau-
rentii also show some heterogenity among the strains of
this species (Fig. 2).
The habitat of A. pullulans is quite broad; it has been
isolated from different substrates and samples (bark,
roots, marine sediments, waters). This “black yeast” is
from an ecological point of view the ubiquitous species,
found mainly on the phylloplane (Inácio et al. 2002,
Pereira et al. 2002, Woody et al. 2003). A. pullulans was
the dominant species of water samples taken in
autumn from the fish-pond (situated also in the forest
park Železná Studnička) (Sláviková and Vadkertiová
1995) and artificial lake waters (located in the Lowland
of Záhorie) (Sláviková and Vadkertiová 1992) when the
water contained many fallen leaves. On the other hand,
A. pullulans was seldom presented in the soil samples
taken from the forest park Železná Studnička (Slávi-
ková and Vadkertiová 2000). A. pullulans contributes to
a fast decomposition of the organic material through
the production of cellulosolytic, pectinolytic, and lig-
ninolytic enzymes (Domsh et al. 1980).
Pichia
anomala and Saccharomyces sp. belonged to the
most frequently found ascosporogenous yeasts isolated
from the surfaces of leaves and needles. The pellicle-
forming P. anomala usually occurs in association with
trees (Spencer et al. 1974). It was often isolated, similar
to A. pullulans, from the fish-pond and lake waters.
S. cerevisiae predominated in the phyllosphere of wild
plants (Kvasnikov et al. 1975) and it has been often
closely associated with water pollution (Hagler and
Mendonca-Hagler 1981, Grabiňska-Loniewska et al.
1993). This species represented approximately one
quarter of the yeast community of the river Danube
water (Sláviková and Vadkertiová 1997). Both these
species are able to ferment saccharides very well.
L.
thermotolerans has the obvious natural affinities
with some Saccharomyces and Zygosaccharomyces species
and usually has a fruit origin (Kurtzmann and Fell
1998). It was isolated only from the leaves of deciduous
tree species collected in the autumn.
The ascomycetous yeast species of the genus
Metschnikowia frequently dominates the mycobiota of
flowers and fruits (Lachance et al. 2001, Phaff and
Starmer 1987), and it is interesting that the species
M. pulcherrima was also found as the dominant leaf
colonist.
The red yeast species of the genera Rhodotorula and
Sporobolomyces belong to the most frequently yeasts
occurred on the leaf surfaces (Inácio et al. 2002, Nakase
2000, Phaff and Starmer 1987). Rh. glutinis was the
348 E.
Sláviková
et al.
Journal of Basic Microbiology 2007, 47, 344 – 350
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
common red species found in our samples of leaves and
needles. A surprising result was the low incidence of
ballistoconidia-forming yeasts. Inácio et al. (2002)
obtained the same results during their study of
phylloplane mycobiota on Mediterranean plants and
they are of the opinion that this could be attributed
either to their lower relative concentration or to the
stronger attachment of these yeasts to the leaf surfaces
preventing their recovery by the isolation procedure
employed in the study.
Although the ballistoconidia-forming yeast species
Sp. roseus occurred on leaves and needles only of four
tree species and was present in 4 – 9% of the samples, it
was the dominant species of the fish-pond water
samples taken in autumn (Sláviková and Vadkertiová
1995). We support the opinion that this could be
attributed to the stronger attachment of these yeasts to
the leaf surface preventing their recovery. It is possible,
that some yeasts are better released from the decom-
posed leaves in the water. We observed the ability of
the yeast Sp. roseus isolated from the leafy material to
modify the lignin derived from beechwood pulping
(Košíková and Sláviková 2004).
Middelhoven (1997) found out that the phyllosphere
yeasts have the wide biodegradative activities. From his
study it is clear that phyllosphere yeasts are able to
attack and to assimilate many high-molecular and low-
molecular plant constituents and that they may benefit
by many compounds leaking out of the plant. By
successful competing for nutrients yeasts may protect
the plant against phytopathogenic fungi.
The representation of the species in the yeast com-
munity colonizing the leaves seems to be very similar
in the various territories, but the frequency of indi-
vidual species is distinct. We found out that the
ascomycetous and basidiomycetous species were pre-
sent in the leaf samples in approximately equal fre-
quency, but Middelhoven (1997) reported only about
one third of the strains frequently found on plants
growing in an arid climate as basidiomycetous species.
On the other hand, the vast majority of isolates
obtained from leaves of trees and shrubs growing in a
Portuguese Mediterranean ecosystem were of basidio-
mycetous affinity (Inácio et al. 2002).
The aim of this work was to investigate the yeast
community colonizing the leaf surface of various tree
species during two consecutive years. We intended to
find out whether the leaves of different tree species
are colonized by the same yeast community or not.
We found out that even if the leaves as habitats for
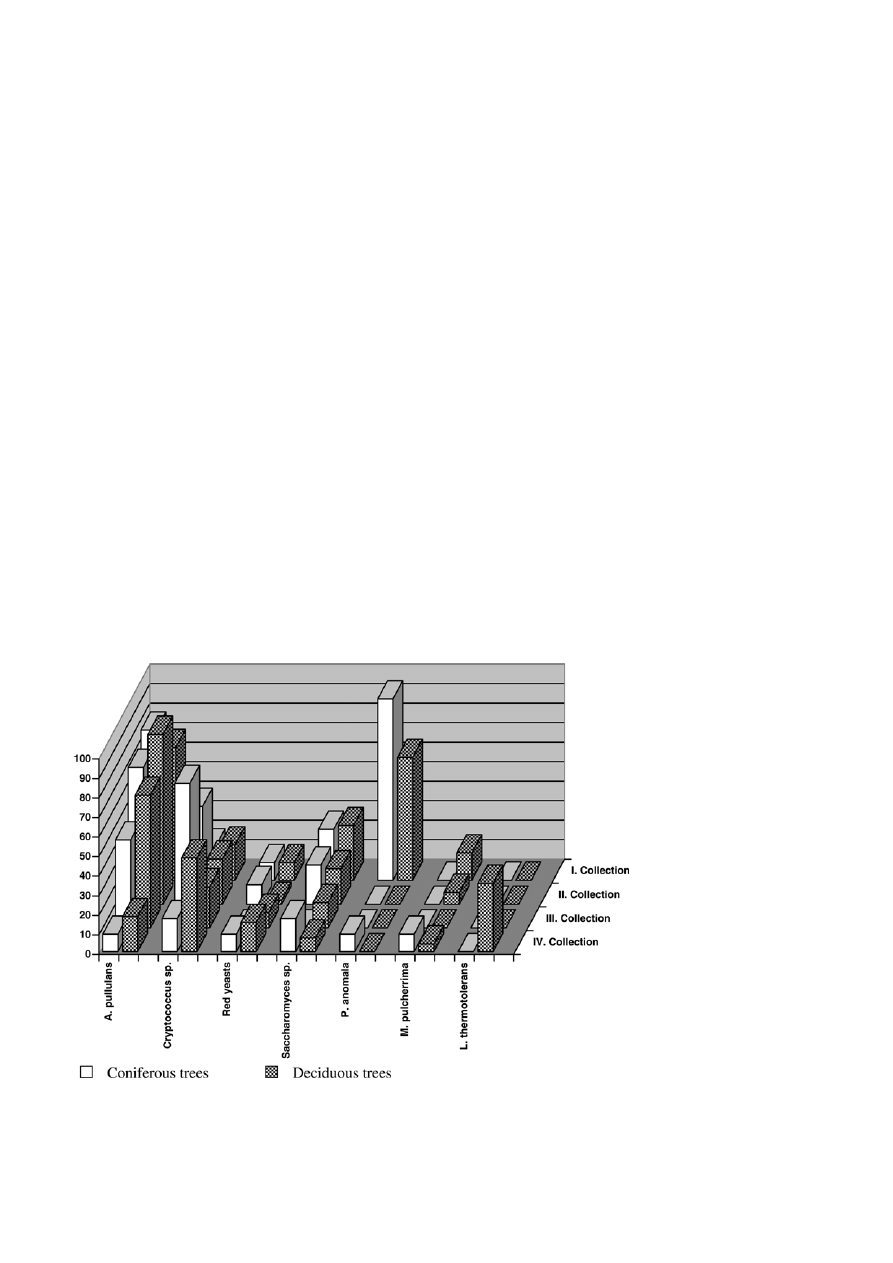
Figure 3. Yeast species most frequently isolated from the leaves of coniferous and deciduous trees during four collections (the number
gives % of positive samples).

Journal of Basic Microbiology 2007, 47, 344–350
Yeasts colonizing the leaf surfaces
349
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
microbial colonization came from various tree species,
only a little differences in the yeast community were
observed (Table 1). The dominant species occurred
regularly on the majority of the leaves. There does not
appear to be a significant difference in the yeast
community found on the leaves of deciduous trees and
on the needles of coniferous trees (Fig. 1).
On the other hand, the colonization of the leaves and
needles by yeasts varied throughout the year, but no
periodicity was found (Fig. 3). Sampling I and III were
made in the springtime and sampling II and IV were
made in the autumn during two consecutive years. The
differences in the quantitative representation of indi-
vidual yeast species ascertained during four sampling
are evident. The most marked differences are in the
case of species P. anomala and L. thermotolerans. The
quantity of red pigmented yeasts and the yeasts of
Saccharomyces sp. changed the least of all.
The phyllosphere has many features that make it an
excellent habitat for studying microbial ecology. Thus,
phyllosphere microbiology has much to offer to the
field of microbial ecology and promises to contribute to
the more effective and less environmentally damaging
means of plant protection (Lindow and Brandl 2003).
The ability of some yeasts to attach to hyphae or coni-
dia of phytopathogenic fungi has been speculated to
contribute to the biocontrol activity on plant surfaces
(Allen et al. 2004). Some isolates of the species A. pullu-
lans, P. anomala, and Cr. laurentii showed antagonistic
activity against a number of pathogenic fungi (Schena
et al. 2002, Fredlund et al. 2002, Allen et al. 2004).
It is possible that some of the isolated strains may
have the biological control potential against foliar and
post harvest diseases, but it remains the aim of further
studies to investigate this capability. Our preliminary
results indicate that also some of the isolated strains
have various degradative abilities.
Acknowledgements
This work was supported by a grant from the VEGA for
biological and ecological sciences No. 2/7031/27 and
grant from the Ministry of Education (FRVŠ) No.
2774/F4.
References
Allen, T.W., Burpee, L.L. and Buck, J.W., 2004. In vitro attach-
ment of phylloplane yeasts to Botrytis cinerea, Rhizoctonia so-
lani, and Sclerotinia homoeocarpa. Can. J. Microbiol.,
50,
1041 – 1048.
Barnett, J.A., Payne, R.W. and Yarrow, D., 2000. Yeasts: Cha-
racteristics and Identification (Third Edition). Cambridge
University Press.
Blakeman, J.P., 1973. The chemical environment of leaf surfa-
ces with special reference to spore germination of pathoge-
nic fungi. Pestic. Sci.,
4, 575 – 588.
Do Carmo-Sousa, L., 1969. Distribution of yeasts in nature.
In: The Yeasts (A.H. Rose and J.S. Harrison, eds.), Vol. I,
pp. 79 – 105. Academic Press London.
Domsh, K.H., Gaams, W. and Anderson, T.H., 1980. Compen-
dium of Soil Fungi, Vol.
1, Academic Press London.
Esteve-Zarzoso, B., Belloch, C., Uruburu, F. and Qerol A., 1999.
Identification of yeasts by RFLP analysis of the 5.8S rRNA
gene and the two ribosomal internal transcribed spacers.
International J. Systematic Bacteriol.,
49, 329 – 337.
Fleet, G.H., 1998. Yeasts in natural habitats. Food Technol.
Biotechnol,
36, 285 – 289.
Fokkema, N.J., den Houter, J.G., Kosterman, Y.J.C. and Nelis,
A.L., 1979. Manipulation of yeasts on field-grown wheat
leaves and their antagonistic effect on Cochliobolus sativus
and Septoria nodorum. Trans. Br. Mycol. Soc.,
72, 19 – 29.
Fredlund, E., Druvefors, U., Boysen, M.E., Lingsten, K.-J. and
Schnürer, J., 2002. Physiological characteristics of the bio-
control yeast Pichia anomala J121. FEMS Yeast Research,
2,
395 – 402.
Gadanho, M. and Sampaio, J.P. 2002. Polyphasic taxonomy of
the basidiomycetous yeast genus Rhodotorula: Rh. glutinis
sensu stricto and Rh. dairensis comb. nov. FEMS Yeast Re-
search,
2, 47 – 58.
Glushakova, A.M. and Chernov, I.Yu., 2004. Seasonal dynam-
ics in a yeast population on leaves of the common wood
sorrel Oxalis acetosella L. Microbiology (Moscow),
73, 184 –
188, Translated from Mikrobiologiya,
73, 226 – 232.
Grabiňska-Loniewska, A., Sláviková, E., Furmanska, M. and
Slomczynski, T., 1993. Fungi in activated sludge biocenosis.
Acta Microbiologica Polonica,
42, 303 – 313.
Hagler, A.N. and Mendonca-Hagler, L.C., 1981. Yeast from
marine and estuarine waters with defferent levels of pollu-
tion in the state of Rio de Janeiro, Brazil. Appl. Environ.
Microbiol.,
41, 173 – 178.
Holloway, P.J., 1971. The chemical and physical characteristics
of leaf surfaces. In: Ecology of Leaf Surface Microorganisms
(T.F. Preece and C.H. Dickinson, eds.), pp. 39 – 54. Academic
Press New York.
Inácio, J., Pereira, P., de Carvalho, M., Fonseca, Á., Amaral-
Collaço, M.T. and Spencer-Martins, I., 2002. Estimation and
diversity of phylloplane mycobiota on selected plants in a
Mediterranean-type ecosystem in Portugal. Microb. Ecol.,
44, 344 – 353.
Inácio, J., Portugal, L., Spencer-Martins, I. and Fonseca, Á.,
2005. Phylloplane yeasts from Portugal: Seven novel ana-
morphic species in the Tremellales lineage of the Hyme-
nomyces (Basidiomycota) producing orangr-coloured colo-
nies. FEMS Yeast Res.,
5, 1167 – 1183.
Košíková, B. and Sláviková, E., 2004. Biotransformation of
lignin polymers derived from beech wood pulping by Sporo-
bolomyces roseus isolated from leafy material. Biotechnology
Letters,
26, 517 – 519.

350 E.
Sláviková
et al.
Journal of Basic Microbiology 2007, 47, 344 – 350
© 2007 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
www.jbm-journal.com
Kurtzmann, C.P. and Fell J.W., 1998. The Yeasts, a Taxonomic
Study (Fourth Revised and Enlarged Edition). Elsevier Ams-
terdam.
Kvasnikov, E.I., Nagornaia, S.S. and Shchelokova, I.F., 1975.
Yeast flora of plant rhizosphere and phyllosphere. Mikrobi-
ologiia,
44, 339 – 346.
Lachance, M.A., 1990. Yeast selection in nature. In: Yeast
Strain Selection (Ch. J. Panchal, ed.),
8, 2 – 41. M. Dekker
New York.
Lachance, M.-A., Starmer, W.T., Rosa, C.A., Bowles, J.M., Bar-
ker, J.S.F. and Janzen, D.H., 2001. Biogeography of the
yeasts of ephemeral flowers and their insects. FEMS Yeast
Research,
1, 1 – 8.
Leaw S.N., Chang, H.C., Sun, H.F., Barton, R., Bouchara, J.P.
and Chang, T.C., 2006. Identification of medically impor-
tant yeast species by sequence analysis of the internal
transcribed spacer regions. J. Clin. Microbiol.,
44, 693 – 699.
Lindow, S.E. and Brandl, M.T., 2003. Microbiology of the phyl-
losphere. Appl. Environ. Microbiol.,
69, 1875 – 1883.
Mercier, J. and Lindow, S.E., 2000. Role of leaf surface sugars
in colonization of plants by bacterial epiphytes. Appl. Envi-
ron. Microbiol.,
66, 369 – 374.
Middelhoven, W.J., 1997. Identity and biodegradative abilities
of yeasts isolated from plants growing in an arid climate.
Ant. van Leeuwenhoek,
72, 81 – 89.
Nakase, T., 2000. Expanding world of ballistosporous yeasts:
distribution in the phyllosphere, systematics and phyloge-
ny. J. Gen. Appl. Microbiol.,
46, 189 – 216.
Pereira, P.T., de Carvalho, M.M., Girio, F.M., Roseiro, J.C. and
Amaral-Collaco, M.T., 2002. Diversity of microfungi in the
phylloplane of plants growing in a Mediterranean ecosys-
tem. J. Basic Microbiol.,
42, 396 – 407.
Phaff, H.J. and Starmer, W.T., 1987. Yeasts associated with
plants, insects and soil. In: The Yeasts. (2
nd
ed., A.H. Rose
and J.S. Harrison, eds.),
1, 123-180. Academic Press Lon-
don.
Robinson, T., 1974. Metabolism and function of alcaloids in
plants. Science,
184, 430 – 435.
Schena, L., Finetti, S.M. and Gallitelli, D., 2002. Molecular
detection of strain L 47 of Aureobasidium pullulans, a bio-
control agent of postharvest diseases. Plant Dis.,
86, 54 – 60.
Spencer, J.F.T., Gorin, P.A.J. and Gardner, N.R., 1974. Yeasts
occurring in the effluent disposal basins of a pulp mill in
Saskatchewan. Can. J. Microbiol.,
20, 993 – 998.
Sláviková, E. and Vadkertiová, R., 1992. Yeasts isolated from
artificial lake waters. Can. J. Microbiol.,
38, 1206 – 1209.
Sláviková, E. and Vadkertiová, R., 1995. Yeasts and yeast-like
organisms isolated from fish-pond waters. Acta Microbiol.
Polonica,
44, 181 – 189.
Sláviková, E. and Vadkertiová, R., 1997. Seasonal occurrence
of yeasts and yeast-like organisms in the river Danube. Ant.
van Leeuwenhoek,
72, 77 – 80.
Sláviková, E. and Vadkertiová, R., 2000. The occurrence of
yeasts in the forest soils. J. Basic Microbiol.,
40, 207 – 212.
Sugita, T., Takashima, M., Ikeda, R., Nakase, T. and Shinoda,
T., 2000. Intraspecies diversity of Cryptococcus laurentii as re-
vealed by sequences of internal transcribed spacer regions
and 28S rRNA gene and taxonomic position of C. laurentii
clinical isolates. J. Clin. Microbiol.,
38, 1468 – 1471.
Takashima, M., Sugita, T., Shinoda, T. and Nakase, T., 2003.
Three new combinations from the Cryptococcus laurentii
complex: Cryptococcus aureus, Cryptococcus carnescens and Cryp-
tococcus peneaus. Int. J. Syst. Evol. Microbiol.,
53, 1187 –
1194.
Tukey, H.B., Jr., 1970. The leaching of substances from plants.
Annu. Rev. Plant Physiol.,
21, 305 – 324.
Woody, S.T., Spear, R.N., Nordheim, E.V., Ives, A.R. and And-
rews, J.H., 2003. Single-leaf resolution of the temporal po-
pulation dynamics of Aureobasidium pullulans on apple lea-
ves. Appl. Environ. Microbiol.,
69, 4892 – 4900.
Yarrow, D., 1998. Methods for isolation, maintenance and
identification of yeasts. In: The Yeasts, a Taxonomic Study
(4
rd
ed., C.P. Kurtzman and J.W. Fell, eds.), pp. 77 – 100. Else-
vier Science Amsterdam.
Wyszukiwarka
Podobne podstrony:
więcej podobnych podstron