
Molecular oncology
ATM missense variant P1054R predisposes to prostate cancer
Andreas Meyer
a,
*
, Bettina Wilhelm
b
, Thilo Do
¨rk
b
, Michael Bremer
a
,
Rolf Baumann
a
, Johann Hinrich Karstens
a
, Stefan Machtens
c,d
a
Department of Radiation Oncology,
b
Department of Gynaecology and Obstetrics, and,
c
Department of Urology, Hannover Medical
School, Germany,
d
Department of Urology, Marien-Krankenhaus Bergisch Gladbach, Germany
Abstract
Background: Prostate cancer is associated with defective DNA strand break repair after DNA damage leading to genetic
instability and prostate cancer progression. The ATM (ataxia–telangiectasia mutated) gene product is known to play an
important role in cell cycle regulation and maintenance of genomic integrity. We investigated whether the prevalence of
the ATM missense substitution P1054R is increased in a hospital-based series of prostate cancer patients and whether
carriers are at increased risk for treatment-related side effects.
Materials and methods: A consecutive series of 261 patients treated for early-stage prostate cancer with I-125
brachytherapy (permanent seed implantation) between 10/2000 and 04/2006 at our institution and a comparison group
of 460 male control individuals were screened for the presence of the P1054R variant. Outcome of therapy regarding
morbidity was assessed prospectively and compared between carriers vs. non-carriers with the International Prostate
Symptom Score (IPSS), a Quality-of-Life-index (QoL) and the International Index of Erectile Function (IIEF-15) with its
subgroups (IIEF-5 and EF).
Results: The proportion of carriers of the P1054R variant was significantly higher among prostate cancer patients than
in the general population (25 out of 261 vs. 22 out of 460; OR 2.1; 95\% CI 1.2–3.8, p < 0.01). A subgroup of the carriers
additionally harboured the ATM missense variant F858L that was associated with a similar risk (OR = 2.2; 95% CI 1.1–4.6;
p = 0.03). After a mean follow-up of 18 months there were no statistically significant differences regarding IPSS
(p = 0.48), QoL (p = 0.61), IIEF-15 score (p = 0.78), IIEF-5 score (p = 0.83), and EF score (p = 0.80), respectively.
Conclusions: The ATM missense variant P1054R confers an about twofold increased risk for prostate cancer in our
series. The subgroup of patients with the second-site variant F858L is not at significantly higher risk. After 18 months,
there was no evidence for an increased adverse radiotherapy response in P1054R carriers.
c
2007 Elsevier Ireland Ltd. All rights reserved. Radiotherapy and Oncology 83 (2007) 283–288.
Keywords: Prostate cancer; Brachytherapy; ATM germline mutation; Late effects
Prostate cancer is the most common malignancy in the
male population in the European Union. The etiology of
prostate cancer is incompletely understood. Prostate can-
cer aggregates in families, indicating that genetic suscepti-
bility may be important, but the genes that may be involved
are largely unknown. The products of at least some suscep-
tibility genes might be involved in double-strand break (DSB)
repair as evidence has been presented that prostate cancer
is associated with genetic instability after DNA damage
DSB repair is initiated and monitored by ATM, the serine/
threonine kinase mutated in ataxia–telangiectasia (A–T)
and ATM activation is accompanied with earlier stages
of prostate tumorigenesis
Patients with A–T show hypersensitivity to ionising radi-
ation with devastating side effects
. In A–T hetero-
zygotes
there
is
evidence
for
intermediate
cellular
radiosensitivity and an increased risk of developing cancer
. One study has provided evidence that the
ATM missense substitution P1054R could be associated with
inherited prostate cancer risk
. Furthermore, it was re-
cently reported that ATM gene variants were predictive of
adverse radiotherapy reactions among patients treated for
prostate cancer with I-125 brachytherapy
.
In the present study, we aimed to replicate the associa-
tion of ATM variant P1054R with prostate cancer susceptibil-
ity and to investigate its potential association with defined
clinical variables in a hospital-based series of patients trea-
ted with I-125 brachytherapy for early stage prostate
cancer.
Materials and methods
A hospital-based series of 261 unselected patients, who
were treated for prostate cancer between 10/2000 and
04/2006 at our institution, was screened for the presence
Radiotherapy and Oncology 83 (2007) 283–288
www.thegreenjournal.com
0167-8140/$ - see front matter
c
2007 Elsevier Ireland Ltd. All rights reserved. doi:10.1016/j.radonc.2007.04.029

of the ATM missense variant P1054R (
). Indications
for permanent brachytherapy were biopsy-proven adenocar-
cinoma of the prostate, clinically localised low risk early
prostate cancer (T classification cT1a–cT2a) with a PSA
serum level <10 ng/ml and a Gleason sum <7. Brachytherapy
was administered via the transperineal approach. Intraoper-
ative dynamic planning and seed placement were performed
by using biplanar transrectal ultrasound imaging to direct
the placement of each radioactive source within the pros-
tate. The prescription dose for I-125 was 160 Gy. Post im-
plant dosimetry was carried out six weeks after the
implantation by using computed tomography. Outcome of
therapy regarding morbidity and PSA-response was com-
pared between carriers vs. non-carriers. Functional out-
come was measured prospectively with the International
Prostate Symptom Score (IPSS, ranging from 5 to 35 points
with a low score showing good function), the Quality-of-
Life-index (QoL, ranging from 0 to 6 points with a low score
showing good function), the International Index of Erectile
Function (IIEF-15, ranging from 5 to 75 points), a subgroup
of this test with 5 questions (IIEF-5, ranging from 1 to 25
points) and a subgroup consisting of 6 questions evaluating
the erectile function (EF, ranging from 1 to 30 points with
a high score showing good function), respectively. Biochem-
ical failure was defined using the ASTRO consensus defini-
tion
. Mean follow-up was 18 months for the total
cohort of prostate cancer patients. At the discretion of
the treating urologist, short-term (2–4 months pre-implan-
tation) neoadjuvant hormone therapy was given in 71 out of
the 261 patients. For comparison a series of 460 genomic
DNA samples was established at our hospital from random
healthy male blood donors.
We selectively chose the P1054R substitution (nucleotide
c.3161 C/G, rs1800057 in the SNP database at
www.ncbi.nlm.nih.gov/SNP/index.html
) as candidate poly-
morphism in the ATM gene based on the findings of Angele
et al.
. Allele frequencies were assessed using restriction
fragment length polymorphism (RFLP) analysis with AlwI
after polymerase chain reaction (PCR) amplification of a
genomic DNA fragment spanning the exons 23–24 with pre-
viously described primers
. PCR was carried out in a
total volume of 15 ll containing 50 ng DNA, 120 lM of each
dNTP, 1.5 mM MgCl
2
, 0.5 lM primer and 0.1 U Taq DNA poly-
merase. The cycling conditions were 94
°C 5 min, followed
by 37 cycles of 94
°C 60 s, 59 °C 60 s, 72 °C 60 s, with a final
extension at 72
°C for 5 min. PCR products were digested
with AlwI (New England BioLabs) according to the manufac-
turer’s instructions and the fragments were analysed by
electrophoresis on a 3% NuSieve agarose gel (FMC Biozym).
Next, we investigated a second-site missense variant,
F858L (rs1800056), that is known to be located in cis on a
subset of P1054R alleles and may be associated with a
Table 1
Patient’s characteristics of carriers vs. non-carriers of P1054R variant
Carrier
Non-carrier
p-value
Mean age at diagnosis (years)
63.8
65.5
0.14
T classification
0.20
cT1c
0
5
cT2a
20
174
cT2b
3
45
cT2c
2
4
Unknown
0
8
Mean Gleason sum
5.88
5.67
0.17
3
0
4
4
3
14
5
2
47
6
15
164
7
5
6
8
0
1
Mean PSA level at diagnosis (ng/ml)
6.6
7.0
0.43
</= 4
3
25
>4–10
20
185
>10–20
2
26
Neoadjuvant hormone therapy
7
67
0.52
Mean preimplant ultrasound prostate volume (ccm)
37.3
38.9
0.59
Total activity (mCi)
28.4
30.3
0.40
Mean number of needles
22.4
22.5
0.91
Mean number of seeds
63.5
65.5
0.41
Mean dose to 90% of the prostate (Gy)
173.1
170.1
0.73
Mean dose to 90% of the apex of the prostate (Gy)
171.7
161.1
0.35
Mean dose to 90% of the base of the prostate (Gy)
146.7
147.6
0.93
Mean dose to 90% of the penile bulb (Gy)
77.5
83.7
0.66
Mean volume of rectum receiving 100% of prescription dose (ccm)
1.0
1.1
0.72
Mean volume of the apex of the prostate receiving 100% of prescription dose (ccm)
3.2
3.0
0.65
Mean volume of the base of the prostate receiving 100% of prescription dose (ccm)
4.1
3.7
0.5
Mean volume of the penile bulb receiving 100% of prescription dose (ccm)
0.5
0.5
0.81
284
ATM variant and prostate cancer
similar or even higher increase in risk for certain malignan-
cies
. The F858L variant was assessed by RFLP
analysis following a previously published protocol
. In-
formed consent was obtained from all patients before blood
sampling, and the research project has been approved by
the local Ethical Committee.
Statistical considerations regarding differences in allele
and genotype frequencies between cases and controls
were made using Pearson’s v
2
and Log Odds Ratio tests
(Statistix 7.0). No adjustments for multiple testing were
required as the P1054R variant was the first genetic variant
tested in this case-control series. Analyses were performed
using the Statistical Package for Social Sciences (SPSS
V14.0) software. Differences in proportions were derived
using the Fisher’s exact t-test. A two-sided p value of
<0.05 was considered to be significant. The outcomes
regarding changes of the different functional scores during
time were statistically compared using the mixed model
analysis of variance.
Results
Twenty-five out of 261 prostate cancer patients (9.6%)
were identified as heterozygous carriers of the ATM se-
quence variant P1054R. In the control series, 22 out of
460 males (4.8%) were found to be carriers, including 1
homozygote. The proportion of P1054R carriers was signifi-
cantly higher among the prostate cancer patients than in
the controls (OR 2.1; 95% CI 1.2–3.8; p = 0.01). Among
these, 17 out of the 25 prostate cancer patients and 14
out of the 22 male controls (incl. 1 homozygote) were iden-
tified to be carriers of the second-site variant F858L in exon
19 of the ATM gene (OR = 2.2; 95% CI 1.1–4.6; p = 0.03). The
P1054R allele in the absence of the F858L substitution still
appeared to confer a non-significant increase in prostate
cancer risk (OR 1.8; 95% CI 0.7–4.8).
Patients who were heterozygous for the P1054R substitu-
tion tended to have an earlier age at diagnosis than non-car-
riers
but this
observation
did
not
reach
statistical
significance (p = 0.14, 1-sided median test). We then inves-
tigated whether P1054R carriers showed a different clinical
outcome of brachytherapy compared with non-carriers.
There were no statistically significant differences between
the two groups regarding the dose volume histograms
(DVH) obtained from the postimplant CT. Before implanta-
tion the mean IPSS for carriers vs. non-carriers was 6.0 vs.
6.7 (p = 0.45), QoL 1.17 vs. 1.35 (p = 0.46), IIEF-15 score
44.58 vs. 43.11 (p = 0.78), IIEF-5 score 14.88 vs. 14.13
(p = 0.73), and EF score 18.29 vs. 17.62 (p = 0.79), respec-
tively. Six weeks after implantation the scores for carriers
vs. non-carriers showed their maximum respective minimum
peak with a mean IPSS of 17.1 vs. 16.9 (p = 0.94), QoL of 3.2
vs. 3.1 (p = 0.57), IIEF-15 score of 30.5 vs. 28.8 (p = 0.73),
IIEF-5 score of 9.4 vs. 8.6 (p = 0.72), and EF score of 11.6
vs. 10.8 (p = 0.77), respectively. After mean follow-up of
18 months mean IPSS for carriers vs. non-carriers was 9.9
vs. 11.7 (p = 0.45), QoL 1.7 vs. 2.1 (p = 0.32), IIEF-15 score
37.7 vs. 37.0 (p = 0.92), IIEF-5 score 11.5 vs. 11.5
(p = 0.99), and EF score 14.2 vs. 14.4 (p = 0.95), respectively
). If the scores are statistically compared using
the mixed model analysis of variance there were no statisti-
cally significant differences regarding IPSS (p = 0.48), QoL
(p = 0.61), IIEF-15 score (p = 0.78), IIEF-5 score (p = 0.83),
and EF score (p = 0.80), respectively. One carrier developed
a proctitis of grade 2 regarding the Common Terminology
Criteria for Adverse Events v3.0 (CTCAE v3.0) vs. 7 non-car-
riers (p = 0.78). One patient not carrying the P1054R variant
showed three consecutive rises of the PSA level 24 months
after seed implantation and was treated with antihormone
therapy.
Discussion
Ionising radiation produces its biological effects mainly
through the generation of short-lived but highly reactive
radicals that result in DNA breaks. These trigger cellular
processes required for DNA damage recognition and DNA re-
pair by means of recombinational repair or nonhomologous
end-joining of DSBs, or base excision repair of other types
of single or simple clustered lesions, all of which are modu-
lated by genetic variation. Despite the advances in radio-
therapy
delivery
and
modalities
to
increase
the
0
2
4
6
8
10
12
14
16
18
before impl. 1,5 months
post impl.
6 months
post impl.
12 months
post impl.
18 months
post impl.
24 months
post impl.
Carrier
Non-Carrier
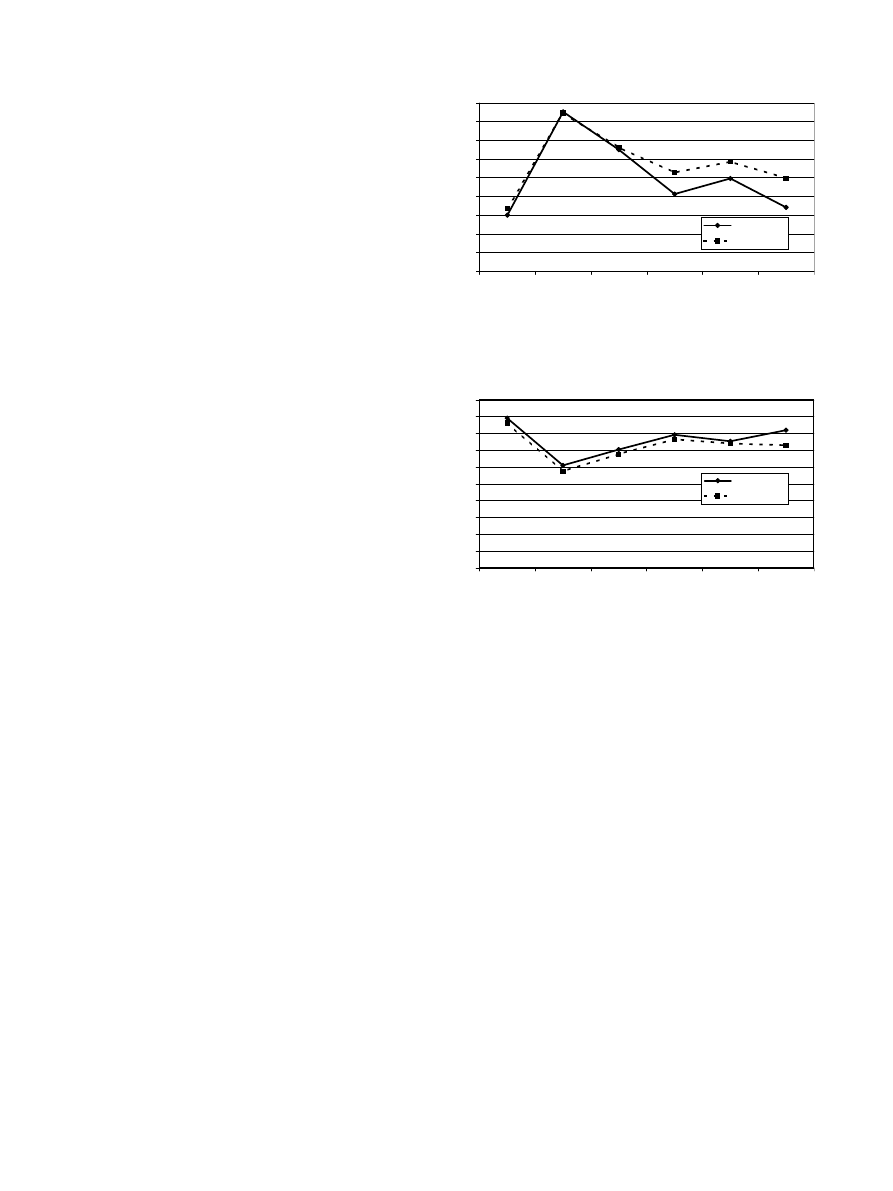
Fig. 1. Illustration of the International Prostate Symptom Score
(IPSS) of carrier vs. non-carrier. The IPSS ranges from 5 to 35 points
with a low score showing a good function.
0
5
10
15
20
25
30
35
40
45
50
before impl. 1,5 months
post impl.
6 months
post impl.
12 months
post impl.
18 months
post impl.
24 months
post impl.
Carrier
Non-Carrier
Fig. 2. Illustration of the International Index of Erectile Function
with 15 questions (IIEF-15) of carrier vs. non-carrier. The IIEF-15
ranges from 5 to 75 points with a high score showing good function.
A. Meyer et al. / Radiotherapy and Oncology 83 (2007) 283–288
285

therapeutic ratio, the inherent biological complexity among
individuals due to variations in their genome has been a lim-
iting factor in predicting normal tissue radiosensitivity. The
ATM protein assumes a central role orchestrating the cellu-
lar response to DNA double-strand breaks
. While biall-
elic ATM gene mutations result in ataxia–telangiectasia
(A–T), a rare radiation sensitivity syndrome, it is estimated
that approximately 1% of the population is heterozygous for
a single ATM mutation and there is evidence for at least
some effect of monoallelic ATM gene mutations manifesting
as intermediate cellular radiosensitivity and an increased
risk of developing cancer in A–T heterozygotes
In the study presented here, we have addressed the prev-
alence and clinical impact of a particular ATM missense var-
iant, P1054R. Bioinformatic analysis using two different
software tools predicts this variant as damaging (SIFT,
<
http://blocks.fhcrc.org/sift/SIFT.html
>) or probably dam-
aging (PolyPhen, <
http://genetics.bwh.harvard.edu/pph/
>).
A putative role for the P1054R variant in cellular radiosensi-
tivity and prostate cancer etiology was previously suggested
by two lines of evidence. First, P1054R carrying cell lines
have been found to exhibit an increased cellular radiosensi-
tivity in vitro
. Because genetic instability after DNA
damage is a feature of prostate cancer cells
, P1054R
could be one of the factors involved. Secondly, a large
case-control study of 624 prostate cancer patients and 417
controls has been reported with a significantly higher prev-
alence of the P1054R variant in cases
, suggesting that
P1054R could be a prostate cancer susceptibility allele.
We have confirmed a higher prevalence of the ATM se-
quence variant P1054R in our hospital-based series of pros-
tate cancer patients in comparison to population controls
from the same geographic region, supporting the findings
of Angele et al. that this variant is associated with an about
twofold increased prostate cancer risk. We furthermore
tested whether P1054R or a second-site variant F858L
underlies this increase in risk. The F858L variant is known
to occur on a subset of P1054R alleles
and has been
reported to confer a higher risk for leukemia than P1054R
. Our data indicate that the F858L variant, found on
the majority of P1054R alleles, is also associated with pros-
tate cancer susceptibility, but as its relative contribution
was similar in P1054R heterozygous cases and controls, it
does not appear to strongly increase the risk that is con-
ferred by the P1054R allele. The available data thus indicate
that the ATM variant P1054R represents a low-penetrance
prostate cancer susceptibility allele and, combining our
study with the study by Angele et al.
, is associated with
an about twofold increase in risk (Mantel–Haenszel Odds
Ratio 2.12; 95% CI 1.39–3.24; p < 0.001). Apart from pros-
tate cancer, previous association studies did not find strong
support for a role of the P1054R variant in breast cancer sus-
ceptibility although these data may still be compatible with
low to moderate risks
. Our results are in line, how-
ever, with recent observations that P1054R may represent a
susceptibility allele for leukemia and for colorectal cancer
. Further research will be needed to fully eluci-
date the spectrum of malignancies that are influenced by
the ATM variant P1054R.
The question whether inherited variations in repair genes
modulate the effectiveness and clinical toxicity of radiation
therapy is important as permanent interstitial brachyther-
apy is an increasingly popular approach for the treatment
of early and localised prostate cancer. Long-term data show
results of biochemical and local tumour control to be as
effective as after radical prostatectomy or external beam
radiotherapy
. Dose escalation has been suggested
to improve prostate cancer control by the use of three-
dimensional conformal radiotherapy, intensity-modulated
radiotherapy and brachytherapy. In prostate seed implanta-
tion correlation between implanted dose and freedom from
PSA failure could be demonstrated by Stock et al., who
found that a D90 value of more than 140 Gy was associated
with an improved biochemical control rate
. Most pa-
tients with permanent seed implantation have normaliza-
tion of their urinary complaints by one year postimplant,
as could be shown by Bottomley et al.
. The incontinence
rates vary between 0% and 19%, a grade 3 urinary morbidity
has been found to occur in 1–3% of the patients, and rectal
complications such as proctitis range from 1% to 21%. The
occurrence of erectile dysfunction is most significantly
influenced by the pre-treatment erectile function
The best strategy to identify patients who are potentially
at risk for the development of radiation-induced late effects
on the one hand and for patients who may benefit most from
brachytherapy on the other hand is currently unknown. In
these instances it could be very helpful to predict radiosen-
sitive patients in order to avoid enhanced late toxicities.
Up to now only few studies have examined the relation-
ship of polymorphism in the ATM gene and clinical outcome
of prostate cancer in terms of acute and late side effects
after exposure to ionising radiation. Weissberg et al. evalu-
ated the medical records of obligate A–T heterozygotes
treated with radiation therapy for breast (n = 11) or pros-
tate cancer (n = 2). They found no instances of soft tissue
necrosis or other apparent serious injuries to normal tissues
. Hall et al. examined 17 prostate cancer patients with
severe late sequela, specifically proctitis or cystitis, after
high-dose external-beam conformal radiation therapy. They
reported that three of them (17.6%) carried mutations in
the ATM gene vs. no patient in the control group
. Dam-
araju et al. explored the possible relationship between 49
single nucleotide polymorphisms (SNPs) in certain candidate
genes with clinical radiation toxicity in a retrospective co-
hort of 83 patients previously treated with three-dimen-
sional
conformal
radiotherapy
for
prostate
cancer.
Significant associations with toxicity were found for SNPs
in LIG4, ERCC2 and CYP2D6 genes. The authors suggested
SNPs in the above-mentioned genes as putative markers to
predict individuals at risk for complications arising from
radiation therapy in prostate cancer. However, regarding
SNPs in the ATM gene, only the D1853N and not the
P1054R polymorphism was screened
. Cesaretti et al.
examined 37 patients treated with I-125 prostate brachy-
therapy with a follow-up of more than 12 months. They
screened for DNA sequence variations in all 62 coding exons
of the ATM gene. Ten out of 16 patients (63%) harbouring
one of 21 ATM sequence alterations exhibited at least one
form of adverse response versus 3 out of 21 patients (14%)
who did not harbour an ATM sequence variation. The
authors suggested that ATM sequence variants, particularly
those encoding amino acid substitutions, were predictive
286
ATM variant and prostate cancer

for the development of adverse radiotherapy responses.
However, only one patient carried a P1054R mutation, and
this patient did not exhibit adverse effects after a follow-
up of 27 months
.
Given the previously reported association of ATM se-
quence alterations with radiation related side-effects, we
might have expected a higher incidence of adverse radio-
therapy responses among P1054R carriers. However, we
could not demonstrate that heterozygosity for this ATM se-
quence variant is predictive for the development of an ad-
verse
radiotherapy
response
regarding
IPSS,
erectile
function and proctitis. One reason could be that our fol-
low-up may be too short to detect any statistically signifi-
cant differences. The increased cellular radiosensitivity
may render tumour cells more susceptible to the cell killing
effect of ionising radiation potentially leading to an en-
hanced therapeutic ratio. However, all patients included
in this study had low-risk prostate cancer, and were treated
with optimum implants based upon evaluation of their post-
brachytherapy dosimetric studies. Additionally, the follow-
up is short yet to detect a PSA failure, and therefore, it
was not possible to examine whether carrier showed in-
creased tumour radiosensitivity. Finally, it is likely that
ATM is not the only genetic variant that may predispose pa-
tients to adverse radiotherapy responses. Thus, the patients
in this series who exhibited pronounced radiation-related
morbidity but proved negative for the ATM sequence variant
P1054R may possess other ATM sequence variants or altera-
tions in genes other than ATM associated with adverse nor-
mal tissue radiation response. More comprehensive genetic
screening of radiotherapy patients for DNA sequence varia-
tions in candidate genes associated with adverse radiation
response could contribute to the definition of predictive risk
models in individual patients with prostate cancer to im-
prove the therapeutic ratio. The knowledge of the interac-
tion of various SNPs in candidate genes may ultimately
result in a prediction of radiotherapy late effects leading
to a more individualised therapy.
In summary, we have confirmed the proposed associa-
tion of the ATM
*
P1054R missense substitution with an in-
creased prostate cancer risk. However, we could not
detect the increased side effects between carriers and
non-carriers previously described by others. Further stud-
ies of candidate gene variants for radiosensitivity will be
needed and might have important clinical implications
for prostate cancer.
Conflict of Interest Statement
All authors disclose any financial and personal relation-
ships with other people or organisations that could inappro-
priately influence their work.
Acknowledgements
We thank Jo
¨rn Hageman and Ju
¨rgen Serth for their support in
the recruitment of patients. This work was supported by an intra-
mural research grant at Hannover Medical School and by funds from
the Lower Saxonian Cancer Society.
* Corresponding author. Andreas Meyer, Department of Radia-
tion Oncology, Medical School Hannover, Carl-Neuberg-Str. 1, 30625
Hannover, Germany. E-mail address:
Received 22 March 2007; received in revised form 30 April 2007;
accepted 30 April 2007; Available online 14 May 2007
References
[1] Angele S, Falconer A, Edwards SM, Do
¨rk T, et al. ATM
polymorphisms as risk factors for prostate cancer develop-
ment. Br J Cancer 2004;91:783–7.
[2] ASTRO Consensus Panel. Consensus statement:Guidelines for
PSA following radiation therapy. Int J Radiat Oncol Biol Phys
1997;37:1035–41.
[3] Athma P, Rappaport R, Swift M. Molecular genotyping shows
that ataxia–telangiectasia heterozygotes are predisposed to
breast cancer. Cancer Genet Cytogenet 1996;92:130–4.
[4] Bottomley D, Ash D, Al-Qaisieh B, et al. Side effects of
permanent I125 prostate seed implants in 667 patients treated
in Leeds. Radiother Oncol 2007;82:46–9.
[5] Cesaretti JA, Stock RG, Lehrer S, et al. ATM sequence variants
are predictive of adverse radiotherapy response among
patients treated for prostate cancer. Int J Radiat Oncol Biol
Phys 2005;61:196–202.
[6] Damaraju S, Murray D, Dufour J, et al. Association of DNA
repair and steroid metabolism gene polymorphisms with
clinical late toxicity in patients treated with conformal
radiotherapy
for
prostate
cancer.
Clin
Cancer
Res
2006;12:2545–54.
[7] Do
¨rk T, Bendix R, Bremer M, et al. Spectrum of ATM gene
mutations in a hospital-based series of unselected breast
cancer patients. Cancer Res 2001;61:7608–15.
[8] Fan C, Quan R, Feng X, et al. ATM activation is accompanied
with earlier stages of prostate tumorigenesis. Biochim Biophys
Acta 2006;1763:1090–7.
[9] Fan R, Kumaravel TS, Jalali F, Marrano P, Squire JA, Bristow
RG. Defective DNA strand break repair after DNA damage in
prostate cancer cells: implications for genetic instability and
prostate cancer progression. Cancer Res 2004;64:8526–33.
[10] Guedea F, Aguilo F, Polo A, et al. Early biochemical outcomes
following permanent interstitial brachytherapy as mono-
therapy in 1050 patients with clinical T1–T2 prostate cancer.
Radiother Oncol 2006;80:57–61.
[11] Gotoff SP, Amirmokri E, Liebner EJ. Ataxia telangiectasia.
Neoplasia, untoward response to X-irradiation, and tuberous
sclerosis. Am J Dis Child 1967;114:617–25.
[12] Gutierrez-Enriquez S, Fernet M, Do
¨rk T, et al. Functional
consequences of ATM sequence variants for chromosomal
radiosensitivity. Genes Chromosomes Cancer 2004;40:109–19.
[13] Hall EJ, Schiff PB, Hanks GE, et al. A preliminary report:
frequency of A–T heterozygotes among prostate cancer
patients with severe late responses to radiation therapy.
Cancer J Sci Am 1998;4:385–9.
[14] Lo
¨brich M, Jeggo PA. The two edges of the ATM sword: co-
operation between repair and checkpoint functions. Radiother
Oncol 2005;76:112–8.
[15] Machtens S, Baumann R, Hagemann J, et al. Long-term results
of interstitial brachytherapy (LDR-Brachytherapy) in the treat-
ment of patients with prostate cancer. World J Urol
2006;24:289–95.
[16] Meier M, den Boer ML, Hall AG, et al. Relation between
genetic variants of the ataxia telangiectasia-mutated (ATM)
gene, drug resistance, clinical outcome and predisposition to
childhood T-lineage acute lymphoblastic leukaemia. Leukemia
2005;19:1887–95.
A. Meyer et al. / Radiotherapy and Oncology 83 (2007) 283–288
287

[17] Morgan JL, Holcomb TM, Morrissey RW. Radiation reaction in
ataxia telangiectasia. Am J Dis Child 1968;116:557–8.
[18] Neubauer S, Arutyunyan R, Stumm M, et al. Radiosensitivity of
ataxia telangiectasia and Nijmegen breakage syndrome homo-
zygotes and heterozygotes as determined by three-color FISH
chromosome painting. Radiat Res 2002;157:312–21.
[19] Renwick A, Thompson D, Seal S, et al. ATM mutations that
cause ataxia–telangiectasia are breast cancer susceptibility
alleles. Nat Genet 2006;38:873–5.
[20] Rudd MF, Sellick GS, Webb EL, Catovsky D, Houlston RS.
Variants in the ATM-BRCA2-CHEK2 axis predispose to chronic
lymphocytic leukemia. Blood 2006;108:638–44.
[21] Sandoval N, Platzer M, Rosenthal A, et al. Characterization of
ATM gene mutations in 66 ataxia telangiectasia families. Hum
Mol Genet 1999;8:69–79.
[22] Shiloh Y. The ATM-mediated DNA-damage response: taking
shape. Trends Biochem Sci 2006;31:402–10.
[23] Spurdle AB, Hopper JL, Chen X, et al. No evidence for
association of ataxia–telangiectasia mutated gene T2119C
and C3161G amino acid substitution variants with risk of breast
cancer. Breast Cancer Res 2002;4:R15.
[24] Stock RG, Stone NN, Tabert A, Iannuzzi C, DeWyngaert JK. A
dose–response study for I-125 prostate implants. Int J Radiat
Oncol Biol Phys 1998;41:101–8.
[25] Thompson D, Duedal S, Kirner J, et al. Cancer risks and
mortality in heterozygous ATM mutation carriers. J Natl
Cancer Inst 2005;97:813–22.
[26] Vorechovsky I, Luo L, Ortmann E, Steinmann D, Do
¨rk T.
Missense mutations at ATM gene and cancer risk. Lancet
1999;353:1276.
[27] Webb EL, Rudd MF, Sellick GS, et al. Search for low
penetrance alleles for colorectal cancer through a scan of
1,467 non-synonymous SNPs in 2575 cases and 2707 controls
with validation by kin-cohort analysis of 14,704 first-degree
relatives. Hum Mol Genet 2006;15:3263–71.
[28] Weissberg JB, Huang DD, Swift M. Radiosensitivity of normal
tissues in ataxia–telangiectasia heterozygotes. Int J Radiat
Oncol Biol Phys 1998;42:1133–6.
[29] West CM, Elyan SA, Berry P, Cowan R, Scott D. A comparison of
the radiosensitivity of lymphocytes from normal donors,
cancer patients, individuals with ataxia–telangiectasia (A–T)
and A–T heterozygotes. Int J Radiat Biol 1995;68:197–203.
288
ATM variant and prostate cancer
Document Outline
Wyszukiwarka
Podobne podstrony:
ATM polymorphisms as risk factors for prostate cancer development
Evaluation of the role of Finnish ataxia telangiectasia mutations in hereditary predisposition to br
Missense Variants in ATM in 26,101 Breast Cancer Cases an 29,842 Controls
ATM SEQUENCE VARIANTS ARE PREDICTIVE OF ADVERSE RADIOTHERAPY RESPONSE AMONG PATIENTS TREATED FOR PRO
Population Based Estimates of Breast Cancer Risks Associated With ATM Gene Variants c 7271T4G and c
Functional and Computational Assessment of Missense Variants in the Ataxia Telangiectasia Mutated (A
ATM POLYMORPHISM IVS6260GA IS NOT ASSOCIATED WITH DISEASE AGGRESSIVENESS IN PROSTATE CANCER
Risk of Cancer by ATM Missense Mutations in the General Population
Perceived risk and adherence to breast cancer screening guidelines
Natural Strategies to Kill Cancer
Alternative approaches to cervical cancer screening — kopia
Five Reasons To Get Cancer John Tarrant (Zen, Buddhism, Koan)
Protein Degradation in the Large Intestine Relevance to Colorectal Cancer
Prostate Cancer 2010 June 17th Nieznany
Progress in clinical genetics of prostate cancer
Morbidity and mortality due to cervical cancer in Poland
więcej podobnych podstron