
Chlorine: State of the Art
Richard B. Evans, MD, MPH
Cayuga Medical Center, Ithaca, NY, 14850, USA
Abstract. Chlorine is a widely used industrial chemical. Individuals can be
exposed to chlorine through transportation accidents, industrial exposures or
misuse of domestic cleaners. While most exposed individuals recover normal
pulmonary function, chlorine can cause a variety of lung injuries including
pulmonary edema, restrictive lung disease, and obstructive disease, including
Reactive Airways Dysfunction Syndrome. Residual effects of chlorine exposure
are a function of intensity of exposure, minute ventilation during exposure, and
host characteristics such as cigarette smoking and atopy. This monograph will
summarize uses of chlorine, the potential for accidents, the mechanism of
chlorine toxicity in the lung, and review acute and chronic effects of chlorine
exposure on the lung, as well as systemic effects of massive chlorine exposure.
Key words: Chlorine—Chemical warfare agent—Asthma, chemically induced;
Pulmonary disease—Chronic obstructive—Transportation methods.
Introduction
Chlorine, a greenish-yellow gas, is a commonly used and highly toxic industrial
commodity. In the US, approximately 15million tons of chlorine are produced
annually, the majority of which is transported in rail cars to end users. In Europe,
annual chlorine production is approximately 10 million tons, and 90
% is pro-
duced and consumed on site. Given the widespread use of chlorine with the
potential for transportation accidents and the toxicity of chlorine to the lung,
pulmonary physicians may be presented with cases of chlorine poisoning. Given
the shipment of large amounts of chlorine through populated areas, physicians
Correspondence to:
R.B. Evans, Cayuga Medical Center, Ithaca, NY, 14850; email:
revans@cayugamed.org
Lung (2004) 183:151–167
DOI: 10.1007/s00408-004-2530-3

practicing in areas far from chlorine production facilities or chlorine end users
may have to deal with chlorine poisoning.
Discovery and Early Uses of Chlorine
Chlorine and its bleaching quality was discovered in 1772, by Carl Wilheln
Scheele, a Swedish pharmacist but it was Berthollet, working in Javelle, France,
who recognized that chlorine could be used to remove color from cloth [63]. While
Berthollet used a potash aqueous solution of chlorine gas, Tennant in 1799
substituted limestone (CaCO3) and made a bleaching powder (calcium hypo-
chlorite), which was safer to transport, and remained in widespread industrial use
through the 1920Õs [63].
Demand for chlorine increased markedly in the early 1900Õs, for bleaching of
wood pulp newsprint and as chlorination of water supplies became increasingly
widespread following 1912.
Chlorine and Disinfection
Following a typhoid outbreak in Maidstone, UK in 1897, a water main was
disinfected with chlorine bleach (sodium hypochlorite). In the US, bleaching
powder was used in Chicago, and sodium hypochlorite was used in Jersey City for
disinfection of water starting in 1908. In 1911, following a typhoid outbreak, the
first large scale water disinfection using chlorine gas was performed in Niagara
Falls, New York; the site of one of the first chlorine gas production facilities.
The effect of chlorination of the water supply in the US was dramatic. Prior to
1908, the death rate from typhoid fever in the US approached 30/100,000 pop-
ulation. By 1920 the death rate had decreased to 8/100,000 and by 1950 was 0/
100,000.
Military Use of Chlorine as an Asphyxiant,WWI
At the battle of Ypres (4/22/1915), 5,730 cylinders capable of releasing 180,000 kg
of chlorine gas were dug into a six-kilometer-long front. The gas was then released
in 5minutes. It has been reported that the gas attack caused 15
,000 Allied
casualties, 5,000 of them fatal. More reasonable estimates are that out of 15,000
exposed French troops, 800 were killed and 2500 to 3000 were incapacitated from
chlorine gas [32].
By the Fall of 1915, phosgene was being used in combination with chlorine,
and thus the number of troops exposed to chlorine alone is [relatively] small
compared to the number of troops exposed to combinations of chlorine and
phosgene, or the number of troops exposed to mustard gas, or combinations of
mustard and phosgene. Of soldiers who survived the chlorine attacks, the
152
R. B. Evans

majority were able to return to duty, however, some were left with residual
respiratory abnormalities [7, 11, 24, 25, 47, 58].
Modern Uses of Chlorine
Chlorine is one the top ten chemicals produced (by gross weight) and it is used for
the following purposes:
28% for plastics production, with the majority going to PVC (Polyvinyl
chloride) production.
14% for pulp & paper production, both as a bleaching agent, and as a biocide.
18% for the production of chlorinated solvents which are used in metalworking,
dry cleaning & electronics
5% for water purification, including municipal water systems, swimming pools
and water parks
35% for other chemical production, including pharmaceuticals.
Transportation
Chlorine production is 15million tons per annum in the US and 10 million tons
per annum in Europe. In Europe, 90
% of produced chlorine is used on site, while
10
% is transported. In the US, chlorine is produced at 44 plants in 21 states, and is
then transported to every city in the US. This transportation is predominantly by
rail car. Rail car accidents are rare, but potentially catastrophic, as the rupture of
a 90-ton rail car could release a potentially lethal, 20-mile-wide cloud of chlorine
[39].
Accidental Exposure to Chlorine
Given the widespread production of chlorine, shipment from these plants to end
users in 90,000 gallon railroad tank cars, and end use in a variety of industries,
there are multiple opportunities for chlorine leaks to occur. Over a five-year
period in the 1990s, according to an Environmental Protection Agency Toxic
Release Inventory, chlorine was released in 518 serious accidents in the United
States, second only to ammonia. Kales [34] reported that 36
% of chlorine acci-
dents led to ER visits. In the primary production industry, and to a lesser extent in
end users, workers are educated in safety maneuvers regarding chlorine accidents,
and morbidity and mortality from industrial chlorine accidents are low [40].
Frequent ÔgassingsÕ are common in paper and pulp mills [42], and are also seen in
aluminum billet production (where chlorine gas is bubbled through liquid alu-
minum to remove air pockets). Plants subject to frequent gassings often have
chlorine detectors installed, and in plants without monitoring equipment, workers
can detect chlorine by odor alone (odor noticed at 0.3 ppm, odor identifiable as
Chlorine: State of Art
153

chlorine at 0.7 ppm) [2]. After working with chlorine for 2-5years, however, some
workers lose the ability to detect chlorine at levels as high as 2.8 ppm [38]. In
addition to heavy industry exposure and transportation accidents, chlorine
exposure can occur in small volume users, such as swimming pools, in school
chemistry lab accidents, and in accidental mixtures of acid cleaners (phosphoric
acid) with bleach (sodium hypochlorite) [1, 18, 54].
Mechanism of Chlorine Toxicity
The toxicity of an irritant gas is a function of water solubility of the gas, con-
centration of the gas, duration of exposure, minute ventilation of the exposed
individual and individual host characteristics, such as cigarette smoking. Water
soluble compounds such as ammonia react rapidly with tissues of the upper
airway and lead to ocular, nasal and upper airway damage [41]. Compounds with
limited water solubility, such as phosgene, cause limited upper airway problems,
but are associated with alveolar damage and non-cardiogenic pulmonary edema
[10].
Chlorine has intermediate water solubility, and as such, can lead to upper or
lower airway damage. Chlorine on contact with water vapor (as in the airway
mucosa) produces both hydrochloric and hypochlorous acids:
Equation 1 : Cl
2
þH
2
O Ð OCl
þ 2H
þ
þ Cl
$ HOCl þ HCl
Martin [45], studying chlorine toxicity in mice, noted that the hypothetical
mechanisms of chlorine-induced airway injury are several. He noted that early
investigators had attributed the effects of chlorine on the airways to acid injury
from hydrochloric and hypochlorous acids. However, in animal studies, chlorine
gas is 35times more toxic than hydrochloric acid fumes [4]. Martin noted that the
enhanced toxicity of chlorine compared to acid aerosol HCl indicated that other
mechanisms other than acid injury must be involved. At physiologic pH and
water vapor saturation, the dissociation of chlorine to HCl and HOCl is driven to
the right, so that in the airway, HOCl concentration is 120,000 times Cl
2
gas
concentration [50]. Eiserich [20] demonstrated that, in vitro, hypochlorous acid
[HOCl] interacts with nitrite (NO
2
)
) [the auto-oxidation product of nitric oxide]
to form reactive intermediates capable of nitrating, chlorinating, and dimerizing
aromatic amino acids. NO
2
)
is felt to react with HOCl to form an intermediate
species, either nitryl chloride (Cl-NO
2
) or chlorine nitrite (Cl-ONO) which are
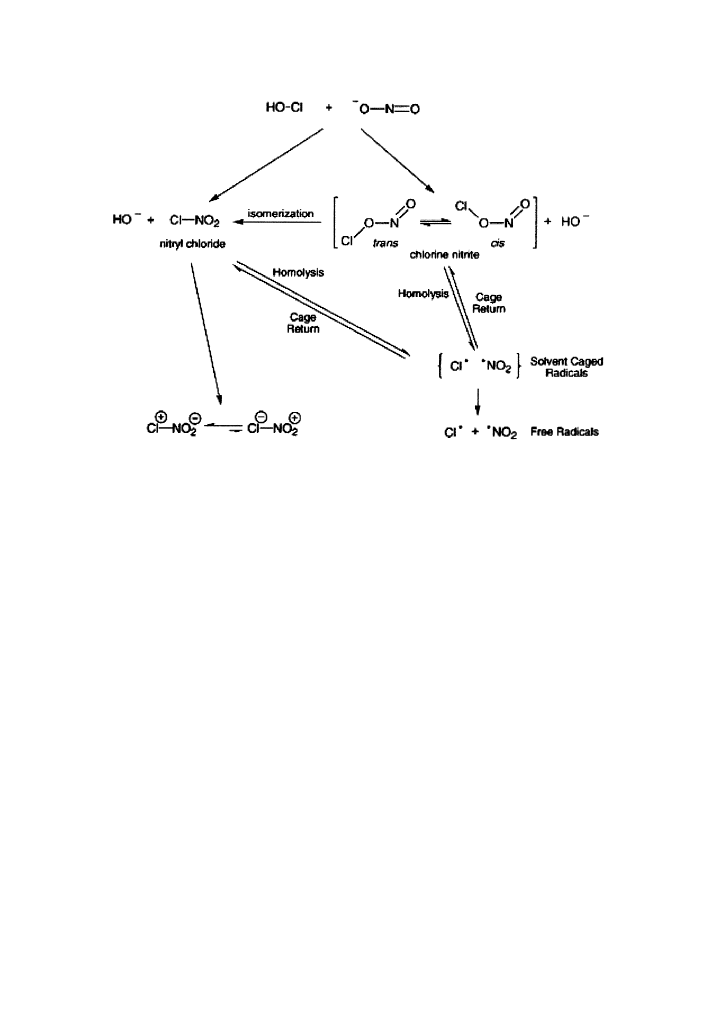
capable of nitrating, chlorinating or dimerizing aromatic amino acids (Fig. 1).
Airway levels of nitric oxide are normally quite low, but rise rapidly in
pathologic conditions [52]. If EiserichÕs findings apply in vivo as well as in vitro,
initial inflammation in the airway from HCl and HOCl would recruit neutrophils
and macrophages, leading to increased local nitric oxide concentrations. The
reaction of nitric oxide (nitrite) with HOCl then leads to the severe tissue damage
154
R. B. Evans
seen in chlorine exposure. Increased production of nitric oxide following gas
exposure has been demonstrated in pulp mill workers after ozone gassings [52]
and in mice following chlorine exposure [52]. Hypochlorous acid may also interact
with hydrogen peroxide released from neutrophils at the site of injury to produce
hydroxyl radical [52].
Distribution of Chlorine in the Airway
Chlorine exposure can lead to a wide variety of respiratory injury varying from
nasal irritation to pulmonary edema. Long-term, low-level exposure studies in
rats and mice (which are obligate nasal breathers) have shown both predomi-
nant nasal injury and anterior-posterior attenuation of the injury [66]. However,
rhesus monkeys, which can breathe orally as well as nasally have shown both
nasal and tracheal injury, although again with anterior to posterior attenuation
[50]. Based on these animal studies, Nodelman and Ultman [50] hypothesized
that as a highly soluble gas, the principal site of absorption of chlorine is most
likely the upper airways; and that there is a progressive loss of Cl
2
from the
inhaled gas stream as it passes over more and more airway surface. To examine
this hypothesis, Nodelman and Ultman examined the distribution of chlorine
gas in the airway of normal, non-smoking [human] volunteers. Airway distri-
Fig. 1 Proposed mechanism for the reaction of NO
2
)
with HOCl, leading to the formation of reactive
intermediates capable of nitrating, chlorinating, and dimerizing aromatic amino acids. (Reprinted with
permission from Eiserach et al. [20]).
Chlorine: State of Art
155
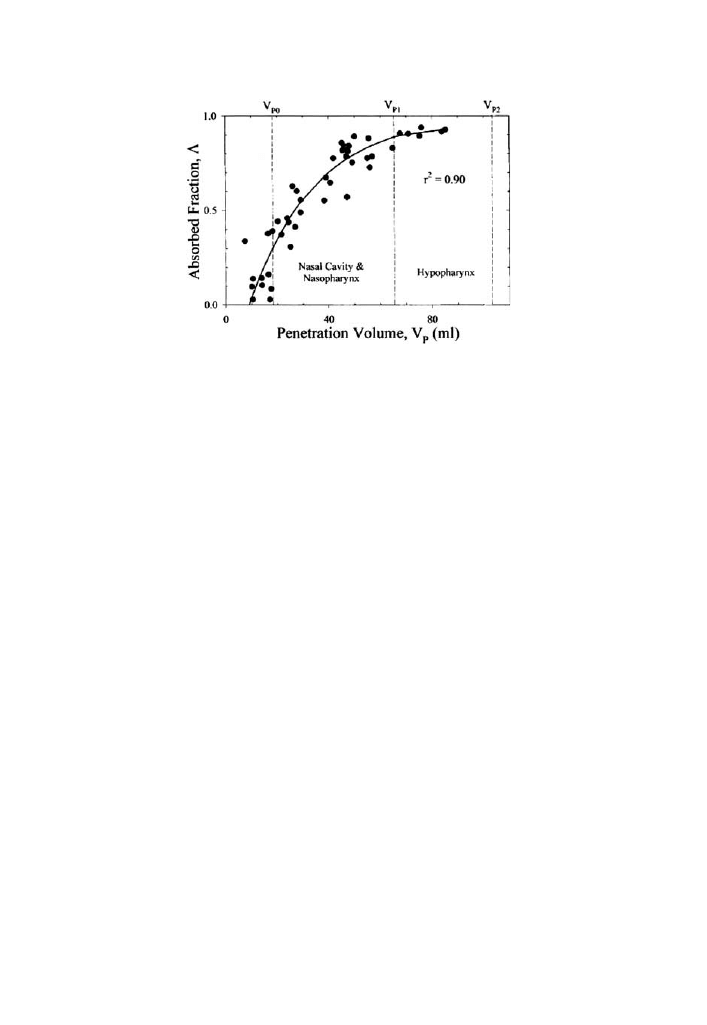
bution of single breath, low level concentrations (<2.5ppm) of chlorine were
measured and the authors demonstrated that as opposed to ozone, single
breaths of low level chlorine are effectively scrubbed by the nasal mucosa [51]
(Fig. 2).
Nodelman and Ultman noted that in physiologic concentrations of 0.16 mol/l
of [Cl
)
] and a pH of 6.6 in airway mucus, the concentration of Cl
2
in hydrolyzed
form [HOCl] is 120,000 times the gaseous phase [Cl
2
]. As HOCl will interact with
NO and tissue proteins, the ÔscrubbingÕ of chlorine in the upper airway is ex-
plained. The authors concluded that because this study revealed that almost all
inhaled Cl
2
was absorbed in the nose during nasal breathing and the mouth during
oral breathing, it is reasonable to conclude that the upper airways are the most likely
site of long-term Cl
2
-induced tissue damage in humans [50].
Dose-response Relationship of Chlorine Exposure
In keeping with the findings of Nodelman and Ultman, chlorine is a potent
irritant to the eyes of humans, the upper respiratory tract, and the lungs. The
Environmental Protection Agency has reported tickling of the nose at 0.014 to
0.054 parts per million (ppm); tickling of the throat at 0.04 to 0.097 ppm; itching
of the nose and cough, stinging, or dryness of the nose and throat at 0.06 to 0.3
ppm; burning of the conjunctiva and pain after 15minutes at 0.35to 0.72 ppm;
and discomfort ranging from ocular and respiratory irritation to coughing,
shortness of breath, and headaches above 1.0 ppm.
However, while Nodelman and Ultman concluded that the upper airways are
the most likely site of long-term Cl
2
-induced tissue damage in humans,
concentra-
tions of >50 ppm are associated with pulmonary edema; and long-term respi-
Fig. 2 Regression of diffusion model to Cl
2
distribution data obtained during nasal breathing in 1
subject. Each point represents absorption fraction (A) obtained from a bolus test breath; smooth curve,
splined regression of these data (Reprinted with permission from Nodelman et al. [50].
156
R. B. Evans

ratory effects following unknown [but presumably high concentration] accidental
exposures are associated in some individuals with obstructive disease, restrictive
disease and RADS. There are 2 potential reasons for the difference between the
upper airway deposition found by Nodelman and Ultman and the clinical reports
of infrequent, but serious ongoing lower respiratory disease, often for years after
chlorine exposure has ceased.
1) Nodelman and Ultman studied young (age 18–40), healthy, non-smoking
volunteers. They specifically excluded anyone who had smoked within 3 years,
and excluded those with a history of hay fever, asthma, allergic rhinitis, chronic
respiratory disease, or cardiovascular disease. In reports of individuals following
chlorine exposure, ongoing problems are usually reported in smokers or those
with pre-existent asthma. Thus, Nodelman and Ultman studied a non-susceptible
population while chlorine accidents expose the general population (both suscep-
tible and non-susceptible individuals).
2) NodelmanÕs subjects inhaled a tidal volume of 500 ml, with an inspi-
ratory flow of 250 ml/sec. At a Ôpredetermined timeÕ during inhalation, the
data-acquisition system automatically injected a 10-ml Cl
2
bolus into the in-
spired airflow. Chlorine concentration was 3.0 ppm for oral breathing and 0.5
ppm for nasal breathing. In chlorine accidents, exposures can be as high as 400
ppm, and tidal volumes can easily exceed 500 ml in one running from the scene
of an accident, and the ÔbolusÕ of inhaled chlorine at the site of an accident
exceeds 10 ml.
Also, while Nodelman reported predominant upper airway exposure from
chlorine inhalation, exposures to concentrations greater than 15ppm have tra-
ditionally been associated with lower respiratory effects [65]. However, as will be
discussed, the effects of chlorine inhalation are dependent not only on concen-
tration but on duration of exposure, minute ventilation, tidal volume and host
susceptibility (Table 1).
Systemic Absorption of Chlorine
As chlorine is highly reactive at local sites, it is not felt to be absorbed systemi-
cally. There are however, 2 reports of brain and liver damage with overwhelming
exposures. Baader [3] reported results of autopsies on 3 workers who had died
from chlorine inhalation.
Small hemorrhages were found in the white matter of the brain. Leube and
Kreiter [43] reported on 90 persons exposed when chlorine blew across a factory
and found that
15% had abnormal SGOT, 40% had abnormal SGPT. LDH values were
normal.
2 patients with exceptionally high SGPT values had liver biopsies; one showed
swollen liver epithelia. There are no studies on the metabolism of chlorine after
inhalation or dermal exposure.
Chlorine: State of Art
157

Respiratory Effects of Acute Chlorine Exposure
Acute Effects of Very Low Level Exposures in Healthy Volunteers
Rotman et al. [57] studied the effects of acute low level exposures (0.5 or 1.0 ppm)
to chlorine on normal volunteers. While Nodelman and UltmanÕs work would
suggest that such low level exposures would have no effect on respiratory health,
Rotman found that while the 0.5ppm exposure had no effect, exposure to 1.0
ppm effected pulmonary function. After 4 hours of exposure, there were signifi-
cant changes in FEV1, peak flow, airways resistance, functional residual capacity
and total lung capacity. By 48 hours post-exposure, all pulmonary function tests
returned to normal, although the decrease in FEV1 and increase in FRC persisted
for 24 hours. As compared to Nodelman and UltmanÕs subjects, RotmanÕs were
exposed in a chamber and exercised on a stationary cycle for 15minutes of every
hour. This threshold effect (no effect on respiratory function, no effect on markers
of nasal inflammatory mediators with exposures less than or equal to 0.5ppm)
was confirmed by Schins et al. [59].
Reactive Airways Dysfunction Syndrome from Acute Exposure
In 1985, Brooks et al. [12] described a new syndrome: irritant-induced asthma
developing in previously healthy individuals after a single exposure to an irri-
tating gas or fume, which they labeled Reactive Airways Dysfunction Syndrome
or RADS. Initially, it was felt that chlorine did not lead to RADS, as most case
studies from 1940-1990 had shown no residual effects from chlorine exposure [28,
37, 64). However, starting in 1990, case reports of individuals developing RADS
after exposure to chlorine began to appear. The case reports suggest that indi-
vidual host characteristics are crucial for the development of RADS following
chlorine exposure. Of the 9 reported cases of RADS, all were smokers, ex-
smokers or individuals with prior atopic disease. Schwartz et al. [61] examined 13
workers 12 years after they had been trapped on a building rooftop adjacent to a
leaking rail car. They found that 3/13 (23
%) had Ôhyper-reactive airwaysÕ by
methacholine testing; all 3 were current or ex-smokers at the time of the accident.
Donnelly and FilzGeraid [19] reported on a non-smoking worker with a child-
Table 1. Dose response relationship of chlorine (Reprinted with permission from Winder [65]).
Chlorine exposure concentration
Effect on human health
1-3 ppm
Mild irritation of mucous membranes
> 5ppm
Eye irritation
> 15ppm
Throat irritation
15–30 ppm
Cough, choking, burning
>50 ppm
Chemical pneumonitis
430 ppm
Death after 30 minutes exposure
> 1000 ppm
Death within minutes
158
R. B. Evans

hood history of asthma who was symptom free and off medication until a faulty
gas cylinder exploded spraying chlorine into his face. Six years following the
exposure, DonnellyÕs worker continued with mild reversible obstructive airways
disease. Moore and Sheriman [48] reported on a sewer worker who was both a
smoker and had a history of childhood asthma. The worker was trapped for 2
hours in a room with chlorine gas, developed RADS and continued with
reversible obstructive airways disease for 4 years following the exposure. Des-
champs et al. [18] reported on an individual with prior atopy but no asthma who
mixed household bleach and acid-based cleaners, leading to a 5-minute exposure
to an unknown chlorine concentration; after 44 days asthma developed. The
patient continued to have asthma 2 years after the exposure, and PC20 was 0.8
mg/dl. Schonhofer et al. [60] reported on 3 police officers who spent 2 hours
within 50 meters of a leaking chlorine tank. Two were ex-smokers, the other non-
smoker had had mild seasonal allergic rhinitis since childhood. All 3 developed
persistent hyperactive airways.
Chlorine Exposure in Individuals with Prior Bronchial Hyperreactivity
Shortly after case reports of RADS following chlorine exposure began to appear,
DÕAllesandro [17] from BlancÕs group at the University of California at San
Francisco noted that individuals with increased baseline airway reactivity were
known to have heightened responses to sulfur dioxide, but that the response to
chlorine in this group had not been addressed. DÕAllesandroÕs group was inter-
ested in whether pre-existing airway hyper-responsiveness would predispose to a
more severe outcome following chlorine exposure. They exposed Ôhyper-respon-
siveÕ individuals to 0.4 ppm and 1.0 ppm of chlorine, and also exposed normal
individuals to the higher level of 1.0 ppm. The Ôhyper-responsiveÕ group included 7
individuals: all had methacholine PC20 < 8 mg/dl; 4/7 had a clinical history of
asthma, although only 1 was treated regularly with inhaled or systemic steroids.
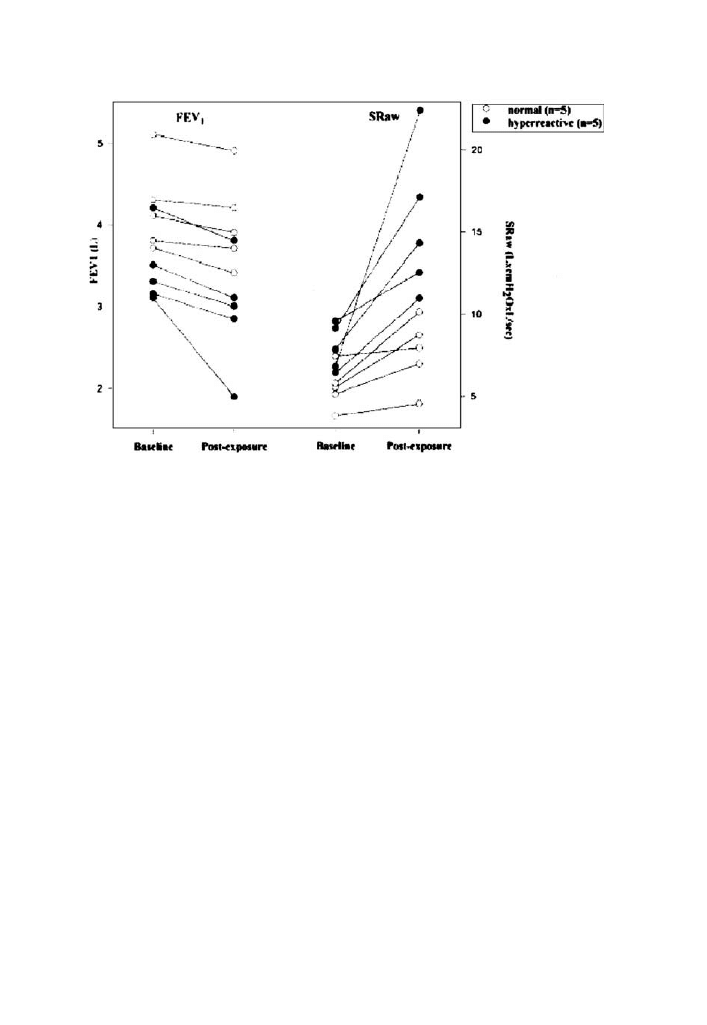
DÕAllesandro found that while both normal and hyper-reactive individuals had
decreases in air-flow (FEV1) and increases in airways resistance (Sraw) following
chlorine exposure that the hyper-reactive group had significantly greater changes
in both measures (figure 3 below):
Residual Pulmonary Effect of Chlorine Exposure in General Populations
Nodelman et al. studied healthy, non-smoking, non-atopic volunteers with low
concentration exposures. DÕAllesandro studied asthmatics, but only with low level
exposures. In chlorine accidents, intensity of exposure is high, and both suscep-
tible and non-susceptible individuals are exposed. Also, minute ventilation, which
was low in both the Nodelman and DÕAllesandro et al. [17] studies, can be quite
high in an individual running away from a chlorine spill. In addition to the studies
published during and after WWI, there are 21 published case reports of accidental
chlorine exposure [1, 3, 5, 14–16, 26, 28, 30, 31, 33, 35, 37, 40, 43, 49, 54–56, 62,
Chlorine: State of Art
159
64]. These 21 case reports summarize the effects of acute accidental-exposure to
doses of chlorine. Approximately 3,069 individuals were exposed, most to massive
doses of gas. Chlorine may cause severe irritation, and in some cases leads to
death. However, of the approximately 3,069 individuals exposed, there were 18
deaths, a case fatality rate of 0.58
%. Autopsy following fatalities that resulted
from acute exposure to chlorine revealed inflamed bronchi, pulmonary edema,
and small foci of bronchopneumonia in the lungs [3, 56]. The vast majority of
patients, even those exposed to tons of chlorine, suffered non-fatal injuries
ranging from cough to pulmonary edema. While chlorine is known to be a skin
irritant, this has not been reported in cases of accidental exposures. Somewhat
surprisingly, given the sometimes severe nature of the initial injury, almost all
exposed individuals regained full pulmonary function; a few developed permanent
lung disease and obstructive, restrictive, mixed obstructive-restrictive and RADS
have been reported.
Chassis et al. [15] studied 418 patients exposed to a chlorine tank leak into the
NYC subway system; 33 individuals were felt to be severely affected and 23 had
pulmonary edema. However, at 16 months post-exposure, none were found to
have residual pulmonary effects.
Weill [64] studied 12 individuals 7 years after being exposed to a 36 ton rail
car spill. Exposure estimates were 400 ppm 75yards from the spill and 10 ppm on
the fringe of the chlorine cloud. Sixty-five persons were treated in hospital; 4 were
unconscious on admission and 10 had pulmonary edema. Weill studied 12 of the
Fig. 3 Increased responsiveness to chlorine Its asthmatics (solid circles) (Reprinted with permission
from DÕAllessandro et al. [17]).
160
R. B. Evans

most severely affected 7 years after the accident. He found no residual pulmonary
disability.
While Weill and Chassis et al. found no residual pulmonary injury, even in
those with pulmonary edema at the time of acute exposure, Kowitz et al. [37]
found residual pulmonary effects in smokers at 19–25months following exposure.
However, at 2-3 years post exposure, Kowitz et al. noted improvement in these
workers and concluded that lung repair after high level acute exposure would take
at least 6 months to 2 years to occur. Sessa et al. [62] reported severe restrictive
disease in 6/12 workers exposed, but did not report the interval between chlorine
exposure and pulmonary testing, nor the estimated concentration or duration of
chlorine exposure. Barret and Faure [5] found that of 129 exposed workers, that
43
%, acutely, had abnormal pulmonary function tests, with 27 showing an
obstructive pattern, 11 restrictive and 3 mixed, as well as 27 workers with hyp-
oxemia. However, within one month after exposure, pulmonary function tests had
returned to normal. Similarly, Moulick et al. [49] reported obstructive, restrictive
and mixed pattern in 84
% of 82 patients hospitalized following chlorine exposure;
however, by 1 month, all had returned to normal.
Low-Grade Chronic Exposures to Chlorine Gas
The studies cited above describe either transportation or storage accidents, with
exposures level as high as 400 ppm. Such exposures can be fatal, but as noted, in
most cases individuals recover function. While most individuals, even with severe
chlorine intoxication, recover normal pulmonary function, Kaufman and Berkons
[35] found ongoing airways obstruction in 4/5 workers after a high level accidental
exposure. These workers had frequent low level chlorine exposures for years prior
to the single high level exposure. As individuals with isolated high level exposure
tend to recover function, but KaufmanÕs chronically exposed workers developed
obstructive disease, it may be that chronic low level exposure predisposes workers
to permanent injury following acute exposure.
The problem of chronic low level chlorine has been studied in 3 populations:
pulp and paper mill workers, chlorine production (electrolytic cell) workers and
aluminum flux workers. In the production of paper, trees are debarked, ground
up, and digested in a sulfur dioxide bath. The chips are then bleached with
chlorine and chlorine dioxide. Kennedy et al. [36] noted that accidental releases of
chlorine dioxide and chlorine is a frequent occurrence in pulp and paper mills as a
result of leaks, discharges, unstable operating conditions, and equipment mal-
functions. They also noted that Ôsuch short-term emissions are difficult to detect
and quantifyÕ but given the nature of the work, it is Ôoften, the same workers who
are exposed . . .on repeated occasions.Õ In the recycling of aluminum cans, liquid
chlorine is injected into molten aluminum. This fluxing removes impurities (Na,
Ca, Mg, Li) but results in the emission of chlorine gas and HCl. Thus, while
chlorine accidents involving transportation or storage are rare, workers in
industries involved in the end use of chlorine (paper mills for bleaching of paper,
Chlorine: State of Art
161

chlorine fluxing of recycled molten aluminum) are exposed on a more frequent
basis to repetitive low level exposures to chlorine.
Concern regarding workers with chronic low grade exposure date back to
McCord [46] in 1926. He reported on a worker who was employed in 1920 to
shovel paper bleached with chlorine out of a cellar room, load it onto hand trucks,
and transport it to another room. The worker said the odor of chlorine was
always present, but that he knew of no gross exposure. No information was
available on chlorine levels, nor is there information on smoking history. After 4
years of exposure, the worker first noticed the development of an intermittent
slight cough, associated with sneezing and burning in the eyes. The coughing
became increasingly severe. Symptoms worsened during the 5
th
year of exposure,
and the patient became disabled. McCord noted that the patient might have had a
decrease in pulmonary function because of chlorine exposure.
Bates and Christie [6] reported a chlorine exposure in a 59-year-old worker
who had been exposed to chlorine in an aluminum fluxing operation. The worker
had had at least 5known exposures to chlorine between 1942 and 1960. With each
exposure he reported having temporary cough and shortness of breath; after the
fourth exposure, he developed severe, persistent dyspnea which was brought on
by even mild exertion or talking. The results of pulmonary function tests showed a
reduction in VC and an increase in RV. Smoking status and exposure levels to
chlorine were not stated. Ferris et al. [22], in 1967, were the first to investigate
chronic respiratory diseases in pulp and paper mill workers. Ferris found a non-
significant increase in chronic non-specific respiratory disease among pulp mill
workers (exposed to sulfur dioxide, chlorine and chlorine dioxide) as compared to
paper mill workers (exposed to paper dust, but not to sulfur dioxide, chlorine or
chlorine dioxide). No significant difference in FVC, FEV1 or PEFR was found
between the pulp and paper mill workers. Within the pulp mill, Ferns differen-
tiated between sulfite plant workers (where the wood chips are digested by sulfur
dioxide) and bleachery workers exposed to chlorine and chlorine dioxide. No
difference was noted in pulmonary function tests of 118 pulp-mill workers ex-
posed to sulfur dioxide and the 73 exposed to chlorine or chlorine dioxide. While
pulmonary function did not differ between sulfur dioxide and chlorine workers,
the chlorine workers were more likely to complain of being gassed at work, having
phlegm in the past 3 years, and of Ôgrade 3 or moreÕ shortness of breath.
Capadoglio [13] et al. examined 52 workers employed for an average of 10
years in a chlorine production plant. All complained of intermittent irritation
from exposure to chlorine, although none had been incapacitated; 18 determi-
nations of chlorine concentration in the plant were made, and the average con-
centration was 0.298 ppm (sd 0.181). Capadoglio et al. compared their 52 exposed
workers to 27 non-exposed healthy workers from the same plant, obtained
smoking histories and performed pulmonary function tests on both groups. They
then divided the workers into 4 groups—exposed smokers, non-exposed smokers,
exposed non-smokers and non-exposed non-smokers and concluded that con-
trolling for smoking, prior accidental exposure to chlorine was associated with a
decreasing diffusion capacity. However, exposure to chlorine had no effect on
FEV1, FVC or FEV1/FVC ratio.
162
R. B. Evans

In 1970, Patil et al. [53] studied 600 workers from 25 chlorine-producing
plants in North America. Chlorine exposure data were available on 268 workers.
On average, the workers had been exposed for 10.9 years. The control group
consisted of 382 non-exposed workers from the same plants. Time-weighted
average exposures to chlorine ranged from 0.006 to 1.42 ppm, with a mean of 0.15
ppm (below the current OSHA PEL of 0.5ppm). All but 6 of the 332 workers had
time-weighted average (TWA) exposures of less than 1 ppm and only 21 had
TWAÕs above 0.52 ppm. Sixty percent of both exposed workers and controls were
smokers. No significant dose-response correlation was found when chlorine
exposure was related to VC, MVV, FEV, and forced expiratory volume at 3
second (FEV 3) values. Patil concluded that there was no evidence of permanent
lung damage attributable to chlorine at the levels reported.
Barret and Faure [5] in their report of 186 patients, occupationally exposed to
chlorine had 56 workers who had at least 3 episodes of chlorine exposure which
led to hospital admission. These workers were examined 5years after the last
exposure. Barret et al. noted Ôno increase in the frequency of clinical manifesta-
tions of pulmonary disease in the exposed subjects, nor was there any deterio-
ration in respiratory function.Õ They also reported that Ôpre-existing lung
conditions did not affect the occurrence of sequelae in patients exposed to
chlorine.Õ
As opposed to BarretÕs report of no deterioration, Enarson et al. [21] found
significant airflow obstruction in non-smokers who worked in the bleach plant of
a pulpmill in British Columbia. In a subsequent study from the same group,
Kennedy and co-workers [36] noted that pulpmill workers who reported being
‘‘gassed’’ were significantly more likely to report wheezing than were other
pulpmill workers and nonsmoking and formerly smoking pulpmill workers who
reported being ‘‘gassed’’ had a significantly lower average midmaximal flow rate
and FEV1/FVC ratio than did their counterparts in the remainder of the pulpmill
population.
Kennedy et al. [36] noted that in previous reports of acute, accidental
exposure to chlorine, persistent lung problems were rarely observed and that the
difference between accidentally exposed individuals and pulp mill workers was
that the pulp mill workers were repetitively exposed to low levels of chlorine,
while those accidentally exposed had isolated exposure to higher concentrations.
Kennedy hypothesized that an inflammatory reaction occurred in small airways
in response to the first accidental high exposure incident. Further, in workers
with ongoing accidental exposures the inflammatory reaction does not com-
pletely resolve as the stimulus to inflammation is either continually or repeatedly
present.
Henneberger et al. re-examined the cohort of Berlin, NH pulp mill workers in
1985[27] and noted that in their cohort, there was a 3-way interaction between
cumulative smoking, cumulative pulp mill exposure and gassings, leading to de-
crease in FEV1, FVC and FEV1/FVC ratio.
The association of smoking and repeated low level occupational chlorine
exposure with chronic obstructive pulmonary disease (COPD) and asthma has
been examined in more detail by MaloÕs group in Montreal. Gautrin and Malo
Chlorine: State of Art
163

et al. [23, 44] found that loss of lung function was associated with chlorine Ôgas-
sings,Õ and that this effect was most profound in heavy smokers, which they
defined as >20 pack years. For the workforce as a whole, there was no increase in
loss of pulmonary function in ÔgassedÕ vs. Ônon-gassedÕ workers. The authors noted
that this lack of effect had previously been found by Humerfelt [29] in a 20-year
follow-up study of men with known exposure to chlorine.
Time Course for Improvement in Asthma Following Chlorine Gassing
Most individuals who suffer single chlorine gassings recover normal pulmonary
function, even if the exposure is overwhelming. However, workers with ongoing
low level exposures can develop obstructive airways disease, including asthma.
Bherer et al. [8] found that 25
% of construction workers exposed to up to 20
chlorine gassings during a refit of a pulp mill were (based on exposure and onset
of symptoms) at moderate to high risk for developing RADS. In this group, 29
(41
%) workers were indeed methacholine positive 18-24 months following the
exposure. Malo et al. [44] then performed repeat methacholine tests and pulmo-
nary function tests on 19 of these 29 men 30-36 months after the gassings. In
MaloÕs group, there was 1 non-smoker, 8 ex-smokers and 11 smokers. All had
developed bronchial hyper-reactivity while five workers developed airways
obstruction, with an FEV1/FVC ratio of less than 85
% of predicted. One-third of
the 19 workers had significant improvement in airways hyper-responsiveness 30-
36 months after the gassings, as compared to 18-24 months after the gassings.
None of the 5men with airway obstruction, however, improved. MaloÕs finding of
improvement in bronchial reactivity in some patients 3 years after exposure ceased
is similar to the improvement in pulmonary function at 2-3 years after exposure
found by Kowitz et al. in 1967.
Summary
Chlorine is a highly toxic gas that has been associated with mortality and mor-
bidity since its discovery in 1772. Transportation and industrial accidents are rare,
but can be lethal. Pulmonary edema, obstructive disease, restrictive disease and
RADS have been reported in survivors. The variable outcome of individuals with
isolated high level exposure to chlorine relates to differences in intensity of
exposure, minute ventilation, and host characteristics such as pre-existent asthma
or ongoing smoking. As compared to single exposures to chlorine during acci-
dents, workers in the aluminum recycling and pulp mill industries are frequently
exposed to ÔpuffsÕ of chlorine. Such workers are at risk of developing obstructive
airways disease and/or asthma. The risk of developing obstructive airways disease
in repetitively ÔgassedÕ workers is increased in heavy smokers, while the risk of
development of asthma is dependent on number of ÔpuffsÕ, smoking history, prior
asthma and severity of exposure.
164
R. B. Evans

Full recovery is anticipated in nearly all those who survive. However, it may
take 2-3 years before improvement is seen.
References
1. Agabiti N, Ancona C, Forastiere F, et al.(2001) Short-term respiratory effects of acute exposure to
chlorine due to a swimming pool accident. Occup Env Med 58:399–404
2. Amoore JE, Hautala E (1983) Odor as an aid to chemical safety: odor thresholds compared with
threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J Appl
Tox 3:272–290
3. Baader C (1952) Chlorine anhydride poisoning (the Walsum Disaster). Med Deporte Trab
17:5252,5254,5256,5258–59
4. Barrow CS, Alarie Y, Warrick Jc. Stock MF (1977) Comparison of the sensory irritation response
in mice to chlorine and hydrogen chloride. Arch Environ Health. 32:68–76
5. Barret L, Faure J (1984) Chlorine Poisoning. Lancet 1 (8376):561–2
6. Bates DV, Christie RV (1964) Respiratory function in disease—an introduction to the integrated
study of the lung WB Saunders Co, Philadelphia, pp 398
7. Berghoff RS (1919) The more common gases—their effect on the respiratory tract. Observation on
two thousand cases. Arch Intern Med 24:678–84
8. Bherer L, Cushman R, Courteau JP, et al.(1994) Survey of construction workers repeatedly ex-
posed to chlorine over a three to six month period in a pulpmill: II. Followup of affected workers
by questionnaire, spirometry, and assessment of bronchial responsiveness 18 to 24 months after
exposure ended. Occup Environ Med. 51:225–8
9. Black JE, Glenny ET, McNee JW (1915) Observations on 685 cases of poisoning by noxious gases
used by the enemy. Br. Med. J 165
10. Borak J, Diller WF (2001) Phosgene exposure: mechanisms of injury and treatment strategies. J
Occup Environ Med. 43:110–9
11. Bradford JR, Elliott TR (1915) Cases of gas poisoning among the British troops in Flanders. Br J
Surg 3:234.
12. Brooks SM, Weiss MA, Bernstein IL (1985) Reactive airways dysfunction syndrome (RADS).
Persistent asthma syndrome after high level irritant exposures. Chest 88:376–84
13. Capodaglio E, Pezagno G, Bobbio GC, Cazzoli F (1970) Investigation of the respiratory function
of workers employed in electrolytic production of chlorine and soda. Med Lav 60:192–2021970
14. Charan NB, Lakshminarayan S, Myers GC, Smith DD (1985) Effects of accidental chlorine
inhalation on pulmonary function. West Med J 143:333–336
15. Chasis H, Zapp JA, Bannon JH, et al.(1947) Chlorine accident in Brooklyn. Occup Med 4; : 152–
176
16. Chester EH, Gillespie DG, Krause FD (1969) The prevalence of chronic obstructive pulmonary
disease in chlorine gas workers. Am Rev Resp Dis 99:365–371
17. DÕAlessandro A, Kuschner W, Wong H, Boushey HA, Blanc PD (1996) Exaggerated responses to
chlorine inhalation among persons with non-specific airways hyperreactivity. Chest. 109:331–37
18. Deschamps D, Soler P, Rosenberg N, Baud F, Gervais P (1994) Persistent asthma after inhalation
of a mixture of sodium hypochlorite and hydrochloric acid. Chest 105:1895–6
19. Donnelly SC, FitzGerald MX (1990) Reactive airways dysfunction syndrome (RADS) due to
chlorine gas exposure. Ir J Med Sci. 159:275–6
20. Eiserich JP, Cross CE, Jones AD, Halliwell B, van der Vliet A (1996) Formation of nitrating and
chlorinating species by reaction of nitrite with hypochlorous acid. A novel mechanism for nitric
oxide-mediated protein modification. J Biol Chem. 271:19199–208
21. Enarson DA, Johnson A, Block G, et al.(1984) Respiratory health at a pulpmill in British
Columbia. Arch Environ Health. 39:325–30
22. Ferris BG Jr, Burgess WA, Worcester J (1967) Prevalence of chronic respiratory disease in a pulp
mill and a paper mill in the United States. Br J Ind Med 24:26–37
23. Gautrin D, LeroyerC , Infante-Rivard C, et al.(1999) Longitudinal assessment of airway caliber
and responsiveness in workers exposed to chlorine. Am J Respir Grit Care Med. 160:1232–7
Chlorine: State of Art
165

24. Gilchrist HI, Matz PB (1933) The residual effects of warfare gases–the use of chlorine gas, with
report of cases. Med Bull Vet Admin 9:229–70
25. Gunn JA(1920) The action of chlorine, etc., on the bronchi. J. Med. 13:121.
26. Hasan FM, Gehshan A, Fuleihan FJ (1983) Resolution of pulmonary dysfunction following acute
chlorine exposure. Arch Environ Health 38:76–80
27. Henneberger PK, Ferris BG Jr, Sheehe PR (1993) Accidental gassing incidents and the pulmonary
function of pulp mill workers. Am Rev Respir Dis. 148:63–7
28. Hoveid P(1956) The chlorine gas accident in Mjondalen 26 January 1940. A post-investigation.
Nord Hyg Tidskr 37:59–66
29. Humerfelt S, Gulsvik A, Skjaerven R, et al.(1993) Decline in FEV1 and airflow limitation related
to occupational exposures in men of an urban community. Eur Respir J. 6:1095–1103
30. Jones AT (1952) Noxious gases and fumes. Proc. Roc. Society Med. 45 : 609–610
31. Jones RN, Hughes JM, Glindmeyer H, Weill H (1986) Lung function after acute chlorine expo-
sure. ARRD 134:1190–1195
32. Joy RJT (1997) Textbook of Military Medicine: Medical Aspects of Chemical and Biological
Warfare: Chapter 3 Historical Aspects of Medical Defense Against Chemical Warfare. Office of
the Surgeon General, Department of the Army US Government Printing Office, Washington, DC,
pp 87–109
33. Joyner RE, Durel EG (1962) Accidental liquid chlorine spill in a rural community. J Occup Med
4:152–54
34. Kales SN, Polyhronopoulos GN, Castro MJ, Goldman RH, Christiani DC (1997) Mechanisms of
and facility types involved in hazardous materials incidents. Environ Health Perspect. 105:998–
1001
35. Kaufman J, Burkons D (1971) Clinical, roentgenologic, and physiologic effects of acute chlorine
exposure. Arch Environ Health 23:29–34
36. Kennedy SM, Enarson DA, Janssen RG, Chan-Yeung M (1991) Lung health consequences of
reported accidental chlorine gas exposures among pulpmill workers. Am Rev Respir Dis. 143:74–9
37. Kowitz TA, Reba RC, Parker RT, Spicer WS Jr (1967) Effects of chlorine gas upon respiratory
function. Arch Env Health 14:545–558
38. Laciak J, Sipa K (1958) The importance of testing the olfaction of workers in some branches of the
chemical industry. Med Pr 9:85–90
39. Lane DA, Thomson BA (1981) Monitoring a chlorine spill from a train derailment. J Air Pollut
Control Assoc. 31:122–127
40. Lawson J (1981) Chlorine exposure: a challenge to the physician. Am Fam Phys 23:135–138
41. Leduc D, Gris P, Lheureux P, et al.(1992) Acute and long-term respiratory damage following
inhalation of ammonia. Thorax 47:755–7
42. Leroyer C, Malo JL, Infante-Rivard C, Dufour JG, Gautrin D (1998) Changes in airway function
and bronchial responsiveness after acute occupational exposure to chlorine leading to treatment in
a first aid unit. Occup Envir Med 55:356–359
43. Leube G, Kreiter H (1971) Acute chlorine gas-observations on 90 patients with acute intoxication.
Med Klin 66:354–57
44. Malo JL, Cartier A, Boulet LP, et al.(1994) Bronchial hyperresponsiveness can improve while
spirometry plateaus two to three years after repeated exposure to chlorine causing respiratory
symptoms. Am J Respir Grit Care Med 150:1142–5
45. Martin JG, Campbell HR, lijima H, et al.(2003) Chlorine-induced injury to the airways in mice.
Am J Respir Grit Care Med. 168:568–74
46. McCord CP (1926) Industrial poisoning from low concentrations of chlorine gas. JAMA 86:1687–
88
47. Meakins JC:(1919) after-effects of chlorine gas poisoning. Can Med Assoc J 9:968–74
48. Moore BB, Sherman M (1991) Chronic reactive airway disease following acute chlorine gas
exposure in an asymptomatic atopic patient. Chest 100:855–6
49. Moulick ND, Banavali S, Abhyankar AD, et al.(1992) Acute accidental exposure to chlorine
fumes—a study of 82 cases. Indian J Chest Dis Allied Sci 34:85–9
50. Nodelman V, Ultman JS (1999) Longitudinal distribution of chlorine absorption in human air-
ways: a comparison of nasal and oral quiet breathing. J Appl Physiol. 86:1984–93
166
R. B. Evans

51. Nodelman V, Ultman JS (1999) Longitudinal distribution of chlorine absorption in human air-
ways: a comparison to ozone absorption. J Appl Physiol 87:2073–80
52. Olin AC, Ljungkvist G, Bake B, Hagberg S, Henriksson L, Toren K (1999) Exhaled nitric oxide
among pulpmill workers reporting gassing incidents involving ozone and chlorine dioxide. Eur
Respir J 14:828–831
53. Patil LR, Smith RG, Vorwald AJ, Mooney TF Jr (1970) The health of diaphragm cell workers
exposed to chlorine. Am Ind Hyg Assoc J. 31:678–86
54. Phillip R, Shepperd C, Fawthrop F, Poulsom B (1985) Domestic chlorine poisoning. Lancet 2:495.
55. Ploysongsang Y, Beach BC, DiLisio RE (1982) Pulmonary function changes after acute inhalation
of chlorine gas. So. Med. J. 75:23–26
56. Romcke O, Evensen OK (1940) The chlorine poisoning in Mjondalen. Nord Med 7:1224–26
57. Rotman HH, Fliegelman MJ, Moore T, et al.(1983) Effects of low concentrations of chlorine on
pulmonary function in humans. J Appl Physiol 54:1120–1124
58. Sandall TE (1922) The later effects of gas poisoning. Lancet 2:857–858
59. Schins RP, Emmen H, Hoogendijk L, Borm PJ (2000) Nasal inflammatory and respiratory
parameters in human volunteers during and after repeated exposure to chlorine. Eur Respir J.
16:626–32
60. Schonhofer B, Voshaar T, Kohler D (1996) Long-term lung sequelae following accidental chlorine
gas exposure. Respiration 63:155–9
61. Schwartz DA, Smith DD, Lakshminarayan S (1990) The pulmonary sequelae associated with
accidental inhalation of chlorine gas. Chest 97:820–5
62. Sessa T, Peccora L, Vecchione G, Mole R (1970) Cardiorespiratory function in bronchopneum-
opathies caused by irritant gases. Poumon Coeur 26:1097–1107
63. Toren K, Blanc P (1997) The history of pulp and paper bleaching: respiratory health effects.
Lancet 348:1316–1318
64. Weill H (1969) Late evaluation of pulmonary function after acute exposure to chlorine gas. Am
Rev Resp Dis 99:374–379
65. Winder C(2001) The toxicology of chlorine. Environ Res Sect A. 85:105–114
66. Wolf DC, Morgan KT, Gross EA, et al.(1995) Two-year inhalation exposure of female and male
B6C3F1 mice and F344 rats to chlorine gas induces lesions confined to the nose. Fundam Appl
Toxicol 24:111–131
Accepted for publication: November 2, 2004
Chlorine: State of Art
167
Wyszukiwarka
Podobne podstrony:
Cl2 Osc anal i cyf
zad 1 takie same jak na pierwszym terminie ale dla Cl2
Cl2 Osc anal
cl2
EdPsych Modules word boh7850x CL2
Vivaldi Sinfonia cl2
Mendelssohn Spring Song Cl2
Halberstadt CL2 Schlasta23b
więcej podobnych podstron