Many poly(phenylenevinylene), or PPV,
derivatives have been evaluated for
light-emitting diode (LED) applications,
but few possess the necessary high
photoluminescence efficiencies.
Now, Kung-Hwa Wei and coworkers at
Taiwan’s National Chiao Tung
University have synthesized highly
fluorescent polymers that contain
dendritic phenyl side chains (DENPPV)
[Dinakaran
et al., Macromolecules
(2005) 38, 10429].
DENPPV homopolymers with a
molecular weight of 300 000 Da have
very low solubility in toluene and
dimethylformamide, making it
unfeasible to prepare thin films using
spin coating. This can be overcome by
preparing copolymers of DENPPV and
2-methoxy-5-(2-ethylhexyloxy)-1,4-
phenylenevinylene (MEHPPV). A
copolymer consisting of 75% DENPPV
and 25% MEHPPV displays excellent
photoluminescence with quantum
yields as high as 82% in solution and
the solid state, making the copolymers
potentially useful for use LEDs.
The researchers were able to make
electroluminescent devices by spin-
coating the DENPPV-MEHPPV
copolymer onto a device and adding a
top electrode.
The thermal stability and glass
transition temperature of the dendritic
copolymer is greater than those
reported for other PPV polymers
because the bulky, rigid dendritic
phenyl groups provide restricted
polymer chain mobility and resistance
to thermal oxidation.
Without altering the electronic
properties of PPV, the dendritic side
chains result in better
electroluminescence efficiency by
minimizing self-quenching and
preventing polymer self-aggregation.
John K. Borchardt
Dendritic side
groups for high
fluorescence
POLYMERS
Controlling cell adhesion to polymer surfaces is essential for
the success of biomaterial implants in many medical
applications.
The tripeptide sequence Arg-Gly-Asp, or RGD, binds to protein
receptors called integrins on cell surfaces, and can be used to
promote cell adhesion to substrate surfaces.
While coating polymer surfaces with RGD peptides is well
known, researchers at the Technical University of Munich,
Germany have developed a method of controlling cell
adhesion by changing the distance and orientation of RGD
peptides relative to a polymer surface [Auernheimer
et al.,
J. Am. Chem. Soc. (2005) 127, 16107].
Horst Kessler and coworkers synthesized a set of peptides
containing a photoswitchable spacer between the surface
anchoring group and the RGD peptide. The
photoisomerization of the spacer changes the distance
between the anchor and the RGD protein. The researchers
coated poly(methyl methacrylate), or PMMA, surfaces with
these peptides.
Irradiation of the surfaces with 366 nm wavelength light
results in a shorter distance between the RGD peptide and
the surface. The switching in the conformation of the peptide
coating can be reversed using 450 nm radiation.
The expanded geometry provides stronger cell adhesion to the
PMMA surfaces. The shorter spacer configuration reduces the
cell adhesion to almost the same level as that of uncoated
PMMA.
This control of the spacer geometry could provide a means of
generating patterns of cell-adhesive and cell-repulsive areas
on biomaterial surfaces. Such patterns could be generated
using photolithography techniques.
John K. Borchardt
Switching cell adhesion on and off
BIOMATERIALS
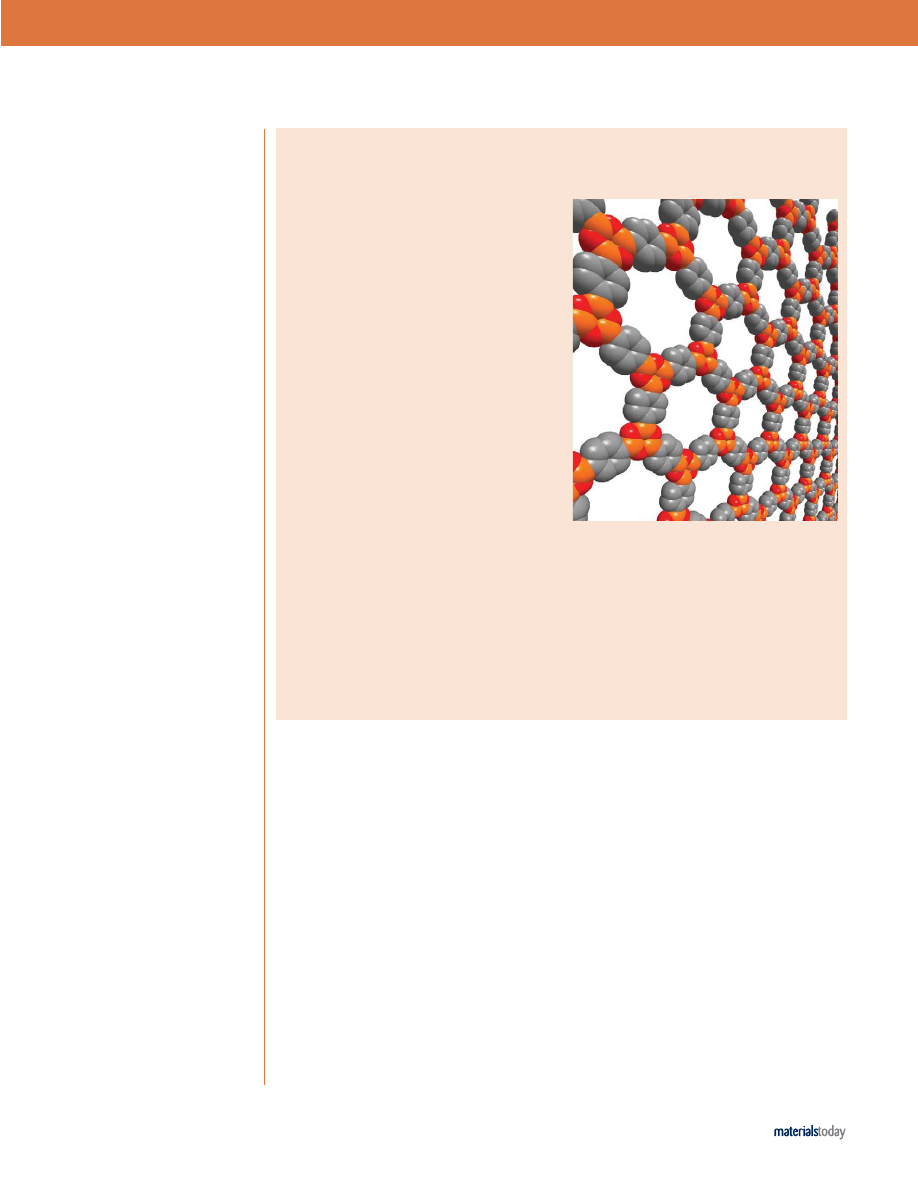
All-organic frameworks are light, rigid, and stable
POROUS MATERIALS
Chemists at the University of Michigan, Ann Arbor and
Arizona State University have synthesized highly
porous structures that consist of common organic
building blocks covalently bonded into extended,
crystalline frameworks [Côté
et al., Science (2005) 310,
1166]. The covalent organic frameworks (COFs) have
rigid structures, exceptional thermal stability, and low
densities as a result of the strong bonds between
B, C, and O atoms. With specific surface areas
surpassing those of well-known zeolites and porous
silicates, the team led by Omar M. Yaghi is hopeful
that the new lightweight materials can be optimized
for gas storage, photonic, and catalytic applications.
Yaghi’s group have previously produced porous
materials called metal-organic frameworks (MOFs), in
which organic molecules are held in a three-
dimensional lattice through metal oxide linkers. By
modifying the components, the porosity and
functionality of MOFs can be tailored. The team take a
similar approach for making COFs, suggesting similar
modifications could be incorporated.
The general strategy for synthesizing COFs is based on
a simple one-step condensation reaction that has a
high yield and uses mild reaction conditions in which
three boronic acid molecules converge to form a cyclic,
planar boroxine anhydride molecule with the loss of
three water molecules. By using 1,4-benzenediboronic
acid (BDBA) instead, an extended hexagonal sheet is
formed. Heating BDBA at 120°C for 72 hours under a
mesitylene dioxane solution allows the reaction to
proceed slowly. A crystalline material, COF-1, is
isolated in 71% yield with a graphite-like structure of
stacked two-dimensional hexagonal sheets. COF-1 has
pores of 6-12 Å in size and a surface area of 640 m
2
g
-1
.
Another compound, COF-5, was constructed using an
analogous condensation reaction between BDBA and
hexahydroxyl triphenylene under similar conditions.
COF-5 has a larger pore width of 27 Å and a surface
area of 1590 m
2
g
-1
.
Jonathan Wood
The covalently bonded, crystalline sheets of COF-1.
(Courtesy of Adrien Côté.)
JAN-FEB 2006 | VOLUME 9 | NUMBER 1-2
1 5
RESEARCH NEWS
Wyszukiwarka
Podobne podstrony:
#0810 – Switching Cell Phone Plans
Page153 Model 2491 2492 2493 Digital Switchboard meter c
Baumer Inductive proximity switch IFFM 08P17A6 KS35L
BODY SWITCH
5 3 3 5 Packet Tracer Configure Layer 3 Switches Instructions
1830 switch datasheet
Bacterial cell shape
MikroTik jako zarządzany switch
Instrukcja SWITCH
61 STEERING COLUMN SWITCHES
0 0 0 1 Lab Initializing and Reloading a Router and Switch
Cisco 1900 Catalyst Switch Commands
Inventor2011 Classic UI Switch
[2006] Application of Magnetic Energy Recovery Switch (MERS) to Improve Output Power of Wind Turbine
Instrukcja SWITCH2
więcej podobnych podstron