
O R I G I N A L R E S E A R C H P A P E R
Biotransformation of menthol and geraniol by hairy root
cultures of Anethum graveolens: effect on growth
and volatile components
Jorge M. S. Faria
Æ Ineˆs S. Nunes Æ
A. Cristina Figueiredo
Æ Luis G. Pedro Æ
Helena Trindade
Æ Jose´ G. Barroso
Received: 13 January 2009 / Revised: 20 January 2009 / Accepted: 23 January 2009 / Published online: 11 February 2009
Ó Springer Science+Business Media B.V. 2009
Abstract
Two oxygen-containing monoterpene sub-
strates, menthol or geraniol (25 mg l
-1
), were added
to Anethum graveolens hairy root cultures to evaluate
the influence of the biotransformation capacity on
growth and production of volatile compounds. Growth
was assessed by the dissimilation method and by fresh
and dry weight measurement. The volatiles were
analyzed by GC and GC–MS. The total constitutive
volatile component was composed, in more than 50%,
by falcarinol (17–52%), apiole (11–24%), palmitic
acid (7–16%), linoleic acid (4–9%), myristicin (4-8%)
and n-octanal (2-5%). Substrate addition had no
negative influence on growth. The relative amount of
menthol quickly decreased 48 h after addition, and the
biotransformation product menthyl acetate was con-
comitantly formed. Likewise, the added geraniol
quickly decreased over 48 h alongside with the pro-
duction of the biotransformation products. The added
geraniol was biotransformed in 10 new products,
the alcohols linalool, a-terpineol and citronellol, the
aldehydes neral and geranial, the esters citronellyl,
neryl and geranyl acetates and linalool and nerol oxides.
Keywords
Anethum graveolens
Biotransformation
Geraniol Hairy roots
Menthol
Volatiles
Introduction
Chemical synthesis is still the major source of
important phytochemicals used in the pharmaceutical
and food industries. Nevertheless, in the past few
years, a renewed interest in the use of natural
products has motivated various industries in attempt-
ing alternative production procedures, namely by
plant biotechnology.
Hairy root cultures offer a flexible and versatile
system that is a promising technology for large scale
production of valuable phytochemicals (Srivastava
and Srivastava
). Moreover, the production, in
these systems, can be qualitatively and/or quantita-
tively different from the whole plant (Santos et al.
). Phytochemical production by hairy roots can be
improved by substrate feeding which stimulates the
plant cell enzymatic machinery—mainly enzymes that
can undergo reactions such as reductions, oxidations,
methylations and, particularly, glycosylation, that are
very common in plant cells (Li et al.
).
Monoterpenes are of great importance in Apiaceae
and, in some cases, represent their dominant volatile
Electronic supplementary material
The online version of
this article (doi:
) contains
supplementary material, which is available to authorized users.
J. M. S. Faria
I. S. Nunes A. C. Figueiredo (
&)
L. G. Pedro
H. Trindade J. G. Barroso
Faculdade de Cieˆncias de Lisboa, Departamento de
Biologia Vegetal, Instituto de Biotecnologia e
Bioengenharia, Centro de Biotecnologia Vegetal,
Universidade de Lisboa, C2, Campo Grande,
1749-016 Lisbon, Portugal
e-mail: acsf@fc.ul.pt
123
Biotechnol Lett (2009) 31:897–903
DOI 10.1007/s10529-009-9934-3

components (Croteau
). In modern society,
terpene alcohols play an essential role in the food
industry, as flavours, and in the perfume industry, as
fragrances. Considering the importance of monoter-
pene alcohols and given the lack of information on the
biotransformation of these compounds by hairy root
cultures, this study evaluates the biotransformation
capacity of Anethum graveolens hairy root cultures by
studying the influence of the addition of two oxygen-
containing monoterpenes, menthol and of geraniol, on
growth and volatiles composition.
Materials and methods
Hairy root cultures
Anethum graveolens hairy roots, established as pre-
viously described by Santos et al. (
), were
maintained in SH medium (Schenk and Hildebrandt
) with 30 g sucrose l
-1
, in darkness at 24
°C on
orbital shakers (80 rpm). Routinely the cultures were
subcultured every three weeks.
Biotransformation
Erlenmeyer flasks with 100 ml SH medium were
aseptically inoculated with 1 g (fresh wt) of dill hairy
roots and maintained as above. Fifteen days following
subculture, 2% (v/v) or 2% (w/v) for geraniol or
menthol in methanol, respectively was added to each
culture flask, at 25 mg l
-1
. Growth and volatiles
production were evaluated after 1, 4, 8, 24, 32 and 48 h
and weekly during the following 4 weeks. Two
independent experiments were separately run, for each
substrate, and two replicates of each flask were used in
each experiment.
Control cultures (=without substrates) were grown
simultaneously. Substrate evaporation and decompo-
sition control experiments were performed by adding
the same amount of substrate to flasks containing only
basal culture medium, and keeping them in the
same conditions as the culture flasks throughout the
experiment.
Determination of hairy root growth
Hairy roots growth was measured, both in control and
substrate added cultures, by the dissimilation method,
and by fresh and dry weight determination, as
previously described (Santos et al.
Isolation of the volatile components
Volatiles were isolated from dill hairy roots by
distillation–extraction, for 3 h, using a Likens-Nick-
erson type apparatus (Likens and Nickerson
)
using distilled n-pentane as organic solvent. The
volatile oils were stored at -20
°C in the dark until
analysis.
GC and GC–MS
The isolated volatiles were analyzed by GC and GC–
MS as previously described (Costa et al.
Results and discussion
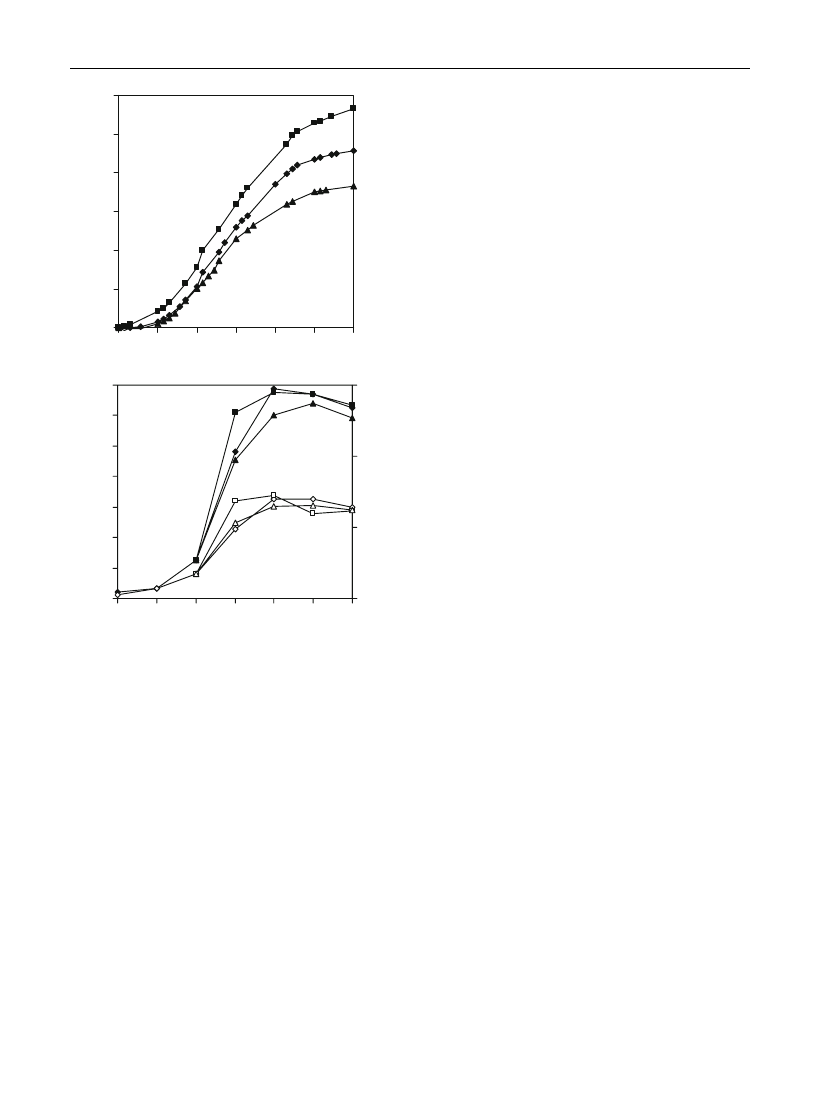
Hairy root growth
Independently of the growth evaluation procedure
(Fig.
), dill hairy roots showed an initial latent phase
of about seven days, followed by a short exponential
phase, subsequently a linear growth phase that
reached approximately the 30th day and then the
stationary phase. This growth profile is similar to that
obtained previously, with dill hairy root cultures
(Santos et al.
), indicating a stable growth
pattern for this in vitro system.
Menthol or geraniol addition, in the linear phase
(15th day), did not greatly affect A. graveolens hairy
roots growth, as shown by the growth curves similar
to those of the control cultures (Fig.
a, b). Similar
results were obtained with Achillea millefolium cell
suspension cultures (Figueiredo et al.
) and with
Levisticum officinale hairy root cultures (Nunes et al.
).
Constitutive volatile components
Forty-two components were identified in the constitu-
tive volatile fraction of dill hairy roots, for 6 weeks,
in a total relative amount [82% (Table
). Falcarinol
(17–52%), apiole (11–24%) and palmitic acid (7–16%)
were the dominant compounds. Other major compo-
nents were linoleic acid (4–9%), myristicin (4–8%) and
n-octanal (2–5%). Polyacetylenes constituted the major
898
Biotechnol Lett (2009) 31:897–903
123
group of volatile components, represented only by
falcarinol, also named panaxynol, which is a powerful
anti-fungal substance (Seigler
) and a potential
anti-cancer drug (Zheng et al.
). Phenylpropa-
noids (16-31%) and fatty acids (13-25%) were also
present in considerable amounts. The terpene fraction
was constituted only by monoterpenes and didn’t
exceed 8%. These results are in agreement with those
obtained previously by Santos et al. (
) with the
same in vitro system, suggesting that A. graveolens
hairy roots have a relatively stable production of
volatile compounds, as the cultures have been main-
tained for over twelve years with a routine subculture
every three weeks. The higher relative amount of fatty
acids, found in the present study, when compared to
that obtained by Santos et al. (
), may be due to the
different culture medium used, which may alter the
volatile composition as was found in L. officinale hairy
roots (Costa et al.
).
Biotransformation products
The detailed relative amounts of the substrates and
their biotransformation products during the time-
course study are given in Supplementary Table
and
a biosynthetic scheme showing the probable relation-
ship between these various compounds is given in
Supplementary Fig.
.
Menthol addition, 15 days after subculture, dras-
tically altered the constitutive volatiles composition
of A. graveolens hairy root cultures. Menthol was
quickly biotransformed into menthyl acetate, decreas-
ing from a maximum of 51% in the first hour, to 11%,
48 h after addition (Fig.
a). Concomitantly, menthyl
acetate increased from 4% in the 1st hour to a
maximum of 55%, 48 h after menthol addition. From
this point on both compounds slowly decreased
throughout the next 4 weeks.
Menthol has been most thoroughly studied in
species of the genus Mentha, particularly in Mentha
9 piperita. In this species, Martinkus and Croteau
(
) have identified an acetyl-CoA:monoterpenol
acetyltransferase that is responsible for the transfor-
mation of l-menthol into menthyl acetate. According
to Lange et al. (
), studying the same species, the
enzyme menthol acetyltransferase was responsible for
menthol acetylation. Probably an enzyme of this
acetyltransferase family is active in A. graveolens
hairy root cultures that acetylates menthol, with no
detectable effects in growth. Nevertheless this capac-
ity seems to be species specific, as in Levisticum
officinale hairy root system this capacity was not
detected (Nunes et al.
) and in A. millefolium
cell suspension cultures (Figueiredo et al.
menthyl acetate was produced, though in trace
amounts.
The addition of geraniol to A. graveolens hairy
root cultures resulted in the formation of 10 new
0
2
4
6
8
10
12
0
14
21
28
35
42
Time (days)
D
is
s
imila
tio
n
(m
g
.ml
-1
)
(a)
0
5
10
15
20
25
30
35
0
14
21
28
35
42
Time (days)
Fresh weight (g)
0
1
2
3
Dry weight (g)
(b)
7
7
Fig. 1
Growth curves of dill hairy roots evaluated by the
dissimilation method (a) and by fresh and dry weight methods
(b). Dissimilation growth curves: control cultures [=without
substrates, r (standard deviation 0–2 mg l
-1
)], menthol [j
(standard deviation 0–3 mg l
-1
)] and geraniol [m (standard
deviation 0–1 mg l
-1
)] added cultures. Fresh and dry weight
growth curves: control [r (standard deviation 0–5 g), e
(standard deviation 0–1 g), respectively], menthol [j (standard
deviation 1–5 g), h (standard deviation \0.5 g)] and geraniol
[m (standard deviation 0–4 g), D (standard deviation \0.5 g)]
added cultures, respectively
Biotechnol Lett (2009) 31:897–903
899
123

Table 1
Percentage composition of the constitutive volatiles isolated from dill hairy roots maintained for 6 weeks in SH medium, in
darkness at 24
°C and 80 rpm
Components
RI
Anethum graveolens hairy root cultures
Time (days)
7
14
21
28
35
42
Benzaldehyde
927
0.1
0.3
0.9
0.3
0.3
0.6
a-Pinene
930
0.5
t
0.8
0.1
t
0.3
n-Heptanol
952
0.1
0.2
0.5
0.2
0.1
0.2
Sabinene
958
t
t
t
t
t
t
1-Octen-3-ol
961
t
t
t
t
t
t
b-Pinene
963
0.4
0.9
1.4
0.8
0.8
0.7
2-Octanone
967
0.2
0.1
0.2
0.1
0.2
0.1
2-Pentyl furan
973
t
0.1
0.2
0.7
0.1
t
n-Octanal
973
2.6
3.8
3.2
1.9
3.5
4.5
Benzene acetaldehyde
1002
0.3
0.2
0.7
0.2
0.3
0.3
p-Cymene
1003
0.3
0.2
0.8
t
t
t
Limonene
1009
0.3
0.8
1.1
0.4
0.3
1.1
n-Octanol
1045
0.1
t
0.5
t
0.1
t
2-Nonanone
1058
0.3
0.8
0.6
0.3
0.4
0.5
Terpinolene
1064
0.3
0.1
0.2
t
t
t
2-Hexyl furan
1064
t
t
t
t
t
t
Phenyl ethyl alcohol
1064
t
t
t
t
t
t
Nonanal
1073
0.4
1.3
1.0
0.5
0.9
0.9
Vertocitral C*
1077
0.3
0.1
0.2
0.2
0.2
0.3
trans-Tagetone
1116
0.3
0.5
1.1
0.8
0.9
1.5
cis-Tagetone
1123
t
t
0.2
t
t
0.4
2- trans -Nonen-1-al
1114
0.9
0.9
1.2
1.1
1.2
2.1
Decanal
1180
0.4
0.3
0.2
0.3
0.2
0.4
cis-Ocimenone
1200
0.7
0.1
0.6
0.3
0.3
0.5
trans-Ocimenone
1207
t
t
0.1
t
t
0.1
2-trans-Decenal
1224
0.5
1.0
0.7
0.5
0.5
0.8
2 trans-4-cis-Decadienal
1242
0.1
0.1
0.1
0.2
0.1
0.2
2-Undecanone
1271
0.4
0.1
0.1
0.3
0.3
0.5
Carvacrol
1286
0.7
1.1
1.0
2.2
2.5
2.3
2 trans-4 trans-Decadienal
1286
0.5
0.2
t
t
0.2
0.5
trans-2-Undecenal
1323
t
0.8
0.2
0.6
0.2
0.4
trans-4-Undecenal*
1402
0.6
0.6
0.3
0.5
1.1
1.1
Myristicin
1493
4.2
7.5
7.9
3.5
4.8
3.7
c-Undecalactone*
1535
t
0.6
t
t
t
t
2,4-Dimethoxyacetophenone
1544
t
0.1
t
t
0.2
t
Dill apiole
1587
2.6
1.8
1.3
1.2
1.7
1.7
Apiole
1640
23.7
20.5
15.2
11.2
13.0
17.6
Myristic acid
1723
0.5
0.2
t
0.2
0.5
0.1
Pentadecanoic acid*
1778
t
1.0
0.6
0.6
0.8
1.4
Palmitic acid
1908
16.4
14.6
14.7
6.7
8.1
12.3
Falcarinol
2002
17.2
23.8
27.4
51.6
42.6
25.1
900
Biotechnol Lett (2009) 31:897–903
123
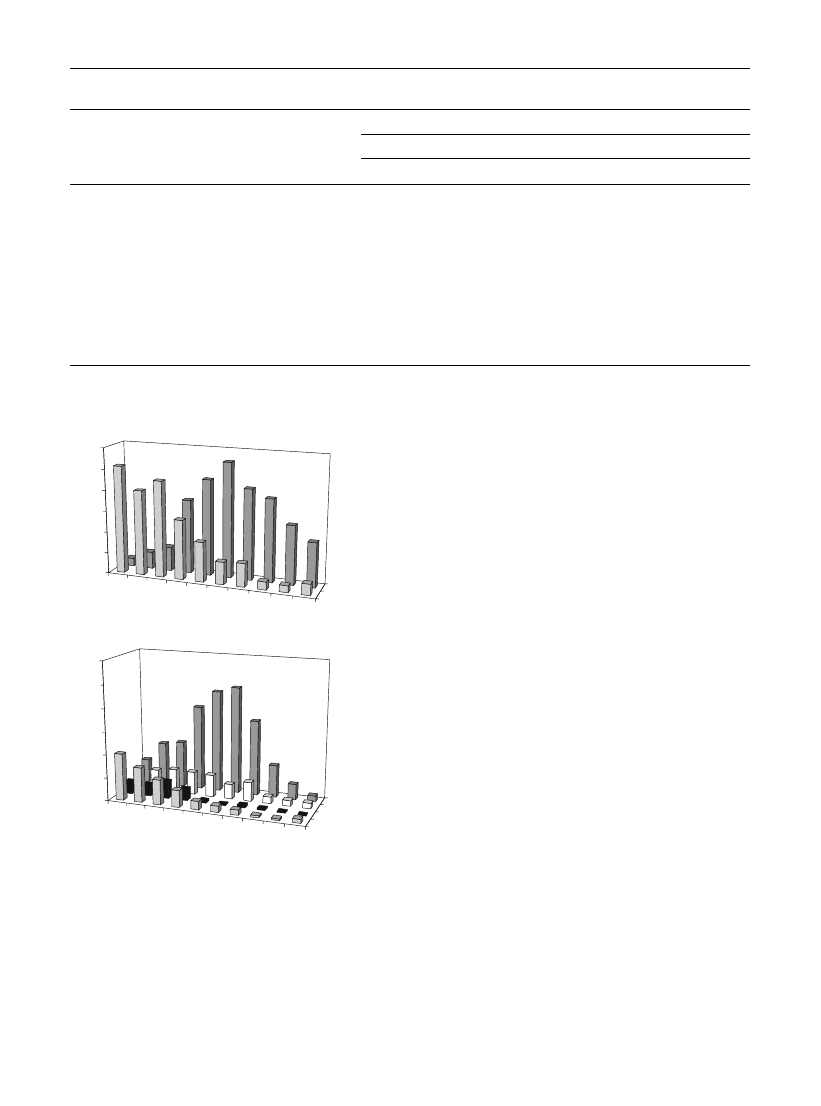
biotransformation products. These were the alcohols
linalool, a-terpineol and citronellol, the aldehydes
neral and geranial, the esters citronellyl, neryl and
geranyl acetates and, in traces, linalool and nerol
oxides. Geranial and neral occurred in similar relative
amounts throughout the time-course study. L. officin-
alle (Apiaceae) hairy root cultures, grown under
similar conditions, were able to biotransform geraniol
into nerol and neral but not into geranial (Nunes et al.
). According to several authors (in Iijima et al.
), it is not yet established if citral (mixture of
geranial and citral) is formed from geraniol by the
action of an alcohol dehydrogenase or by an oxidase,
not even if geraniol is the only substrate in the
formation of citral, since nerol can also be a precursor.
All the new biotransformation products, with the
exception of linalool and nerol oxides, were detected
1 h after
geraniol
addition
(Fig.
b).
Geraniol
decreased quickly from 20%, in the first hour, to
\3%, 48 h after addition. The new alcohols formed,
didn’t exceed 10%, throughout the time-course study
(Fig.
b). Citral and citronellol reached a maximum
8 h after substrate addition and linalool attained a
maximum only 32 h after geraniol addition.
Studying Vitis vinifera L., grape berry mesocarp,
Luan et al. (
) were able to identify a stereose-
lective geraniol reductase responsible for citronellol
production after labelled geraniol addition. Never-
theless they were unable to clarify its origin since
geraniol also yielded its isomer neral, which can also
be converted to citronellol. These authors have also
reported the production, although in small amounts,
of geranial and neral which requires the presence of
an oxidase and/or a dehydrogenase.
Table 1
continued
Components
RI
Anethum graveolens hairy root cultures
Time (days)
7
14
21
28
35
42
Linoleic Acid
2125
5.8
8.8
5.8
5.0
4.3
4.2
% Identification
81.7
93.5
91.0
92.5
90.7
86.4
Grouped components
Monoterpene hydrocarbons
1.8
2.0
4.3
1.3
1.1
2.1
Oxygen-containing monoterpenes
2.0
1.8
3.2
3.5
3.9
5.1
Polyacetylenes
17.2
23.8
27.4
51.6
42.6
25.1
Phenylpropanoids
30.5
29.8
24.4
15.9
19.5
23.0
Fatty acids
22.7
24.6
21.1
12.5
13.7
18.0
Others
7.5
11.5
10.6
7.7
9.9
13.1
RI Retention index relative to C
9
–C
22
n-alkanes on the DB-1 column, t traces (\0.1%)
* Identification based on mass spectra only
1
4
8
24
32
48
168 336
504 672
Menthol
Menthyl acetate
0
10
20
30
40
50
60
Relative amount (%)
Time after substrate addition (h)
(a)
1
4
8
24
32
48
168 336
504 672
Geraniol
Aldehydes
Alcohols
Esters
0
10
20
30
40
50
60
Relative amount (%)
Time after substrate addition (h)
(b)
Fig. 2
Time-course study of the relative amounts of substrates
and of their biotransformation products. a Menthol and the
biotransformation product, menthyl acetate. b Geraniol and the
biotransformation products, esters, alcohols and aldehydes
Biotechnol Lett (2009) 31:897–903
901
123

In comparison with the other A. graveolens hairy
root geraniol biotransformation products, the ester
acetates attained higher relative amounts (47%) but
were slower in reaching their peaks (Fig.
b). While
geranyl and neryl acetates attained their maximum
after 48 h, citronellyl acetate reached the highest
relative amount only one week after substrate addi-
tion. This delay may indicate that this compound can
be formed directly from citronellol, through acetyla-
tion, or from the reduction of geranyl acetate. Such
secondary transformations are common and were
reported by King and Dickinson (
) in a study of
monoterpene alcohols biotransformation using yeast
cells. In that study, geraniol addition resulted in the
formation of, among others, linalool and a-terpineol,
and the addition of linalool resulted in the formation
of a-terpineol. This suggests that within the new
biotransformation products obtained, with A. graveo-
lens hairy roots, some may derive from secondary
reactions of geraniol primary biotransformation prod-
ucts. Geranyl acetate was also obtained from geraniol
biotransformation in lovage hairy root cultures under
the same conditions used in the present work (Nunes
et al.
The relative amounts of all biotransformation
products slowly decreased with culture time, which
may reflect their loss due to high volatility (King
and Dickinson
), or their incorporation into
non-volatile hairy roots components, by glycosyla-
tion, as it was found in other in vitro plant cul-
ture systems (Figueiredo et al.
; Nunes et al.
).
Everitt and Lockwood (
) reported the bio-
transformation
capacity
of
A.
graveolens
cell
suspension cultures. In that study, geraniol, added in
10, 20, 30, 50 and 100 mg l
-1
, was readily converted
into nerol. No other product was detected and both
geraniol and nerol were reduced to trace amounts after
48 h. Our results suggest that, for the same species,
biotransformation products greatly depend on the in
vitro system used.
In conclusion
, Anethum graveolens hairy root
cultures show biotransformation ability related to a
group of biocatalysts, namely redutases, isomerases
and transacetylases, among others, that readily trans-
form geraniol and menthol into different biotransfor-
mation products and with no visible negative impact
on growth.
References
Costa MM, Figueiredo AC, Barroso JG, Pedro LG, Deans SG,
Scheffer JJC (2008) Nitrogen stress induction on Levisti-
cum officinale hairy roots grown in darkness and under
photoperiod conditions: effect on growth and volatile
components. Biotechnol Lett 30:1265–1270
Croteau R (1980) The biosynthesis of terpene compounds. In:
Croteau R (ed) Fragrance and flavor substances. D&PS
Verlag, Germany, pp 13–36
Everitt ZM, Lockwood GB (1995) Anethum graveolens L.
(Dill). In vitro culture and metabolism of volatile con-
stituents. In: Bajaj YPS (ed) Biotechnology in agriculture
and forestry, vol 33, medicinal and aromatic plants III.
Springer-Verlag, Berlin, pp 21–34
Figueiredo AC, Almendra MJ, Barroso JG, Scheffer JC (1996)
Biotransformation of monoterpenes and sesquiterpenes by
cell suspension cultures of Achillea millefolium L. ssp.
millefolium. Biotechnol Lett 18:863–868
Iijima Y, Gang DR, Fridman E, Lewinsohn E, Pichersky E
(2004) Characterization of geraniol synthase from the
peltate glands of sweet basil. Plant Physiol 134:370–379
King A, Dickinson JR (2000) Biotransformation of monoter-
pene alcohols by Saccharomyces cerevisiae, Torulaspora
delbrueckii and Kluyveromyces lactis. Yeast 16:499–506
Lange BM, Wildung MR, Stauber EJ, Sanchez C, Pouchnik D,
Croteau R (2000) Probing essential oil biosynthesis and
secretion by functional evaluation of expressed sequence
tags from mint glandular trichomes. Proc Natl Acad Sci
USA 97:2934–2939
Li W, Koike K, Asada Y, Yoshikawa T, Nikaido T (2003)
Biotransformation of low molecular-weight alcohols by
Coleus forskohlii hairy root cultures. Carbohyd Res
338:729–731
Likens ST, Nickerson GB (1964) Detection of certain Hop oil
constituents in brewing products. Am Soc Brew Chem
Proc 5:13–19
Luan F, Mosandl A, Mu¨nch A, Wu¨st M (2005) Metabolism of
geraniol in grape berry mesocarp of Vitis vinifera L. cv.
Scheurebe: demonstration of stereoselective reduction,
E/Z-isomerization, oxidation and glycosylation. Phyto-
chemistry 66:295–303
Martinkus C, Croteau R (1981) Metabolism of monoterpenes.
Plant Physiol 68:99–106
Nunes IS, Faria JMS, Figueiredo AC, Pedro LG, Trindade H,
Barroso JG (2009) Menthol and geraniol biotransforma-
tion and glycosylation capacity of Levisticum officinale
hairy roots. Planta Medica (in press)
Santos PAG, Figueiredo AC, Lourenc¸o PML, Barroso JG,
Pedro LG, Oliveira MM, Schripsema J, Deans SG,
Scheffer JJC (2002) Hairy root cultures of Anethum
graveolens (dill): establishment, growth, time-course
study of their essential oil and its comparison with parent
plant oils. Biotechnol Lett 24:1031–1036
Schenk UR, Hildebrandt AC (1972) Medium and techniques
for induction and growth of monocotyledonous and
dicotyledonous plant cell cultures. Can J Bot 50:199–204
Seigler DS (1998) Plant secondary metabolism. Kluwer Aca-
demic Publishers, Massachussets, EUA
902
Biotechnol Lett (2009) 31:897–903
123

Srivastava S, Srivastava AK (2007) Hairy root culture for
mass-production of high-value secondary metabolites.
Crit Rev Biotechnol 27:29–43
Zheng G, Lu W, Aisa HA, Cai J (1999) Absolute configuration
of falcarinol, a potent antitumor agent commonly occur-
ring in plants. Tetrahedron Lett 40:2181–2182
Biotechnol Lett (2009) 31:897–903
903
123
Document Outline
Wyszukiwarka
Podobne podstrony:
Master Wonhyo An Overview of His Life and Teachings by Byeong Jo Jeong (2010)
Labyrinth13 True Tales of Occult Crime and Conspiracy by Curt Rowlett 3rd edn (2013)
Film Noir Films of Trust and Betrayal (by Paul Duncan) (2006)
A Matter of Life and Death By Derdriu oFaolain
The Secret of Ella and Micha (#1) by Jessica Sorensen
Legends of Babylon and Egypt by L W King
Gods of Air and Darkness by Richard Mooney
(wydrukowane)Removal of heavy metals from soil components and soils by na
Masonry and its Symbols in the Light of Thinking and Destiny by Harold Waldwin Percival
9 11 Was Planned and Realized by CIA and Mossad with Help of Zionists
Ghostly Encounters True Stories of America s Haunted Inns and Hotels by Frances Kermeen (2002)
In Pursuit of Gold Alchemy Today in Theory and Practice by Lapidus Additions and Extractions by St
Contemporary Macrobiotics Visions of Planetary Health and Peace by Edward Esko
Bridgewater Renewable fuels and chemicals by thermal processing of biomass
The Mystic Sciences The First Complete Handbook of Occult Wisdom Compiled and Ed by Margaret Waite
The Crack in the Cosmic Egg New Constructs of Mind and Reality by Joseph Chilton Pearce Fw by Thom
Of Kith and Kin by Chicklette
Book of the Ancients (A Guide to Dark Magick and Mythology) by Rev Xul
więcej podobnych podstron