24
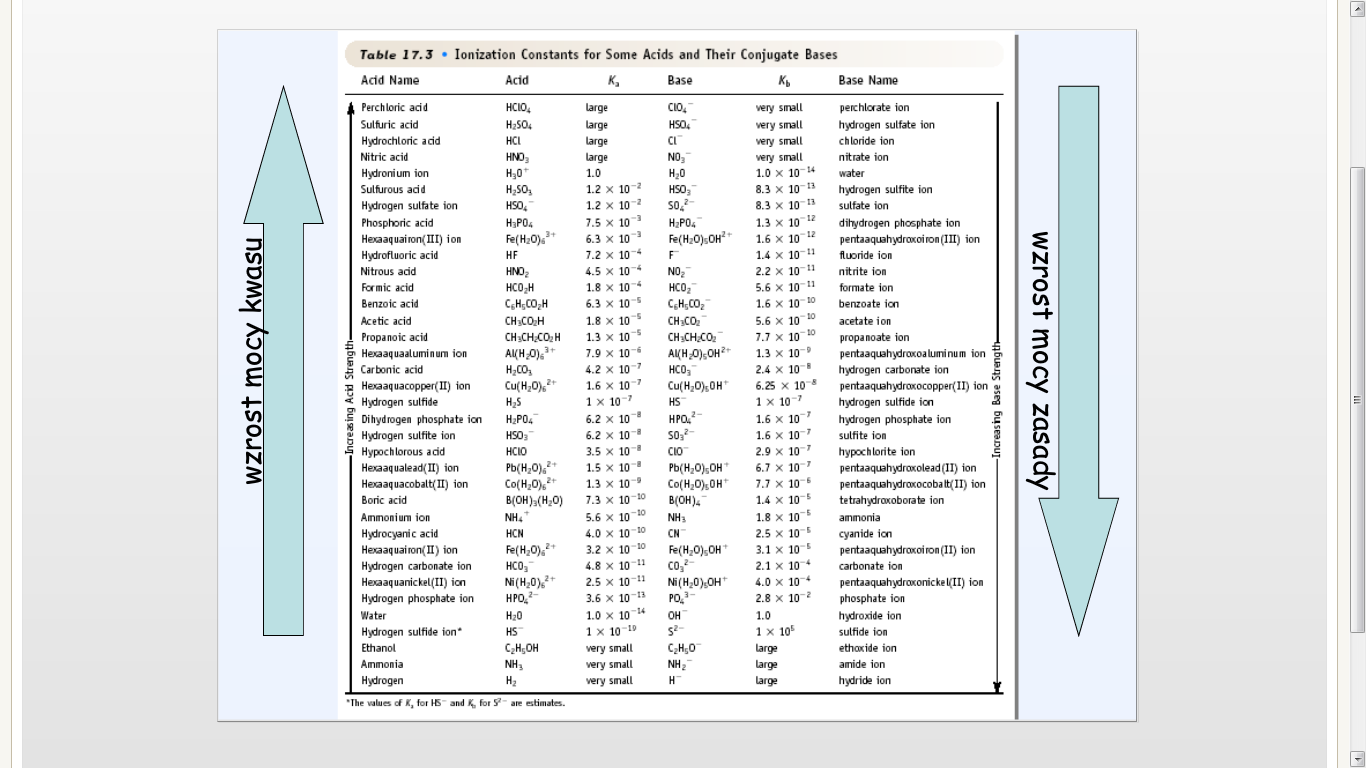

Table 17.3 • Ionization Constants for Sonie Acids and Their Conjugate Bases
|
Acld Name |
Acid |
Km |
Base |
*b |
Base Name | |
|
Perchloric acid |
HC10< |
large |
CłO*" |
very smali |
perchlccate ion | |
|
Sulhjrlc add |
H:SO< |
large |
HSOt |
very smali |
hydrogen sulfate ion | |
|
Hydrochloric add |
HCl |
large |
Cl |
wiy smali |
chloride ion | |
|
Nitric acid |
hnd3 |
large |
N03 ■ |
wry smali |
nitrate ion | |
|
Hydrom urn ion |
Ha(T |
1.0 |
H.,0 |
1.0 X 10 14 |
water | |
|
Sulfurous add |
H.SOj |
1.2 X 10 2 |
HSOj |
8.3 X 10 n |
hydrogen sulfite ion | |
|
Hydrogen sulfate ion |
HSO* |
1.2 X 10 2 |
so*2- |
8.3 X 10 11 |
sulfate ion | |
|
Phosphoric acid |
HiPO* |
7.5 X 10 3 |
H;P0* |
1.3 X 10 17 |
dihydrogen phosphate ion | |
|
Hexaaquairon<M) ion |
Fe(H:0)43' |
6.3 X 10 3 |
Fe(HiO)tJOH2 |
1.6 X 10 17 |
pentaaquahydrcwoiron(III) ion | |
|
Hydrofluoric acid |
HF |
7.2 X 10 * |
F~ |
1.4 X 10 11 |
fluoride ion | |
|
Nitrous add |
HMD; |
4.5 X 10 4 |
NO;" |
2.2 X 10 11 |
nitrite ion | |
|
Famie acid |
HC02H |
1.8 X 10 4 |
HC02 |
5.6 X 10 11 |
formate ion | |
|
Benzoic acid |
C*Hr,C02H |
6.5 X 10 5 |
c*h5co2 |
1.6 X 10 10 |
benzoate ion | |
|
Acetic acid |
CH1CO2H |
1.8 X 10 5 |
CHiCOj |
5.6 X 10 10 |
acetate ion | |
|
j Propanoic acid |
CHjCHiCOzH |
1.5 X 10 5 |
CHiCHiCOj ‘ |
7.7 X 10 10 |
propanoate ion | |
|
Hexaaquaaluminum ion |
7.9 X 10 4 |
Al^KOH2' |
1.3 X 10 9 |
pentaaquahydrcwoaluminum ion |
— CT> r | |
|
jS Catbonie acid |
h2cch |
4.2 X 10 7 |
HCO3 |
2.4 X 10 8 |
hydrogen carbonate ion |
•T -S |
|
u Hexaaquacopper(II) ion |
Cu(H20)62, |
1.6 X 10 7 |
Cu(H2OKOH |
6.25 X 10 8 |
pentaaquahydroxocopper(II) ion |
O* |
|
< Hydrogen sulfide |
h2s |
1 X 10"7 |
HS- |
1 X 10‘7 |
hydrogen sulfide icn |
<2 |
|
| Dihydrogen phosphate icn |
6.2 X 10 8 |
HPOu7 |
1.6 X 10 7 |
hydrogen phosphate ion |
rj< •C | |
|
Hydrogen sulfite ion |
HSO}“ |
6.2 X 10 8 |
SOb2' |
1.6 X 10 7 |
sulfite ion |
i w. |
|
Hypochlorous add |
HCIO |
3.5 X 10 8 |
CK) |
2.9 X 10 7 |
hypochlorite ion |
0 c M |
|
Hexaaqualead<II) ion |
Pb(H20)i‘ |
1.5 X 10 8 |
Pb(H20)rj0H |
6.7 X 10 7 |
pentaaquahydroxolead(JI) ion | |
|
Hexaaquacobalt(II) ion |
Co(H20)fc2t |
1.5 X 10 9 |
Co(H20)t0H |
7.7 X 10 4 |
perrtaaqu3hydroxocobatt(II) ion | |
|
Boric acid |
B(0H)3(H20) |
7.5 X 10 10 |
B(OH)r |
1.4 X 10 4 |
tetrahydrewoborate ion | |
|
Ammonium ion |
NH*ł |
5.6 X 10 10 |
NHj |
1.8 X 10 Ł |
ammonia | |
|
Hydrocyanic acid |
HCN |
4.0 X 10 10 |
CH- |
2.5 X 10 1 |
cyanide icn | |
|
Hexaaquairon<II) ion |
tyHiO)**' |
3.2 X 10 10 |
Fe(H20)rOH |
3.1 X 10 1 |
pentaaquahydroxoiron(ll) ion | |
|
Hydrogen carbonate ion |
HCOj |
4.8 X 10 11 |
co32- |
2.1 X 10 4 |
carbonate ion | |
|
Hexaaquanickel(II) icn |
Ni(H,0)fc2" |
2.5 X 10 11 |
Ni(H20)ŁOH |
4.0 X 10 4 |
pentaaquahydroxonickel/II) ion | |
|
Hydrogen phosphate ion |
HPO*2- |
3.6 X 10 11 |
P043- |
2.8 X 10 2 |
phosphate ion | |
|
Water |
HjO |
1.0 X 10 W |
OH |
1.0 |
hydroride ion | |
|
Hydrogen sulfide ion* |
HS |
1 X 10 19 |
s2- |
1 X 10l |
sulfide ion | |
|
Ethanol |
C2Hr,OH |
v«ry smali |
C,H,0 |
larg? |
ethewide ion | |
|
Ammonia |
NHS |
very smali |
NH2- |
larg? |
amide ion | |
|
Hydrogen |
H; |
very smali |
H |
larg? |
hydr id? ion |
*lhe k-jlun cł K, fot 15 and K,, fot S7 łte eitinuln.
Wyszukiwarka
Podobne podstrony:
Such boys, due to constant impersonation of women and their habits, adopt ąuite a good amount of eff
skanowanie0014 Odczyn zasadowy takiego roztworu rośnie ze wzrostem stężenia soli i ze spadkiem mocy
19540 shoes&pattens 8 98 Shoes and Pałtens Table 17. The wood used for patten soles. Alnus sp. ce
Image110 Bramka pobiera tylko około 2 mW mocy. Wzrost mocy traconej w funkcji częstotliwości w bramc
00168 Bacb53e24cc766a3f8c5490a3082d66 169 Economic Control Chart Models with Cycle Duration Constra
00201 ?4588c1cb831ecdbfa0f0e40ba1f7ac 203 Strategies for Statistical Monitoring of Integral Control
00208 ?1906ab0bcf2542dac76203983810dc 210 Messina, Montgomery, Keats & Runger Table 4. Average
img043 (3) Czynniki Modyfikujące Krzywe Przeżycia: Moc dawki Wzrost Mocy Dawki prowadzi do wzrostu
17 * f r / / / ) / / t 4 I Bcnois’ set for Giselle: the style was fairy-tale, the colors
więcej podobnych podstron