ubiq
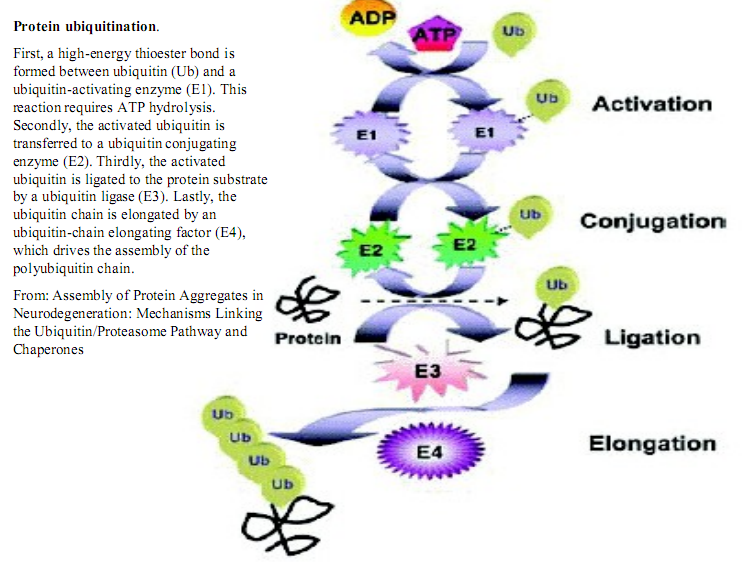
Protein ubiquitination.
First, a high-energy thioester bond is formed between ubiąuitin (Ub) and a ubiquitin-activating enzyme (El). This reaction requires ATP hydrolysis. Secondly, the activated ubiquitin is transferred to a ubiquitin conjugating enzyme (E2). Thirdly, the activated ubiquitin is ligated to the protein substrate by a ubiquitin ligase (E3). Lastly, the ubiquitin chain is elongated by an ubiquitin-chain elongating factor (E4), which drives the assembly of the polyubiquitin chain.
From: Assembly of Protein Aggregates in Neurodegeneration: Mechanisms Linking the L'biquitin/Proteasome Pathway and Cha peron es
Protein
ATP
Ub
% 3
E2

Conjugation
Ub

Ligatfion
Elongation
Wyszukiwarka
Podobne podstrony:
Protein ubiq uitination. ADP First, a high-energy thioester bond is formed between ubiąuitin (Ub) an
jak elektrony są przenoszone M two high-energy olectrons from sugar oxidation H / H OIb
Supernova explosions^ (SNe) are high energy star e _ Tvpe I: no hydrogen - Type II: there is
center for weird studies Home of the High Energy Cheese lab, the Bozon Control Project, and other th
UGH ENERGY *111* 1P-P0P7 TD0217 n"- Hm VłuW. flttnw,VNłcht. HIGH ENERGYVA
Hughes, Susanna Stephanie (SB) Om: High! sh*. dnami about fom. She dnom! of lu and passión abstra
S5003982 i 0 Daphne Nash 1987 First published 1987 Ty peset by Servis Filmsetting Ltd., Manchester a
mbs 068 MY BREATHING SYSTEM I think I was the first author who prescribed exactly, in each single cx
S5003982 i 0 Daphne Nash 1987 First published 1987 Ty peset by Servis Filmsetting Ltd., Manchester a
PROTEINENPORTIE Ok. tocgogcvon. Dc/o bruin witto puree is niot do meoit ie*y sloobcr van do bendo. M
WK* Guitar Pro needs to be activated upon first use. BK/ Please enter the license information (USER
htdctmw 045 Look, the first thing a fledgling artist needs is self-confidence. And here’s the way to
II Recenzje i omówieniaSUMMARY Linguistics today Linguistics today, the first decade of XXI century,
więcej podobnych podstron