
Vol. 10
FLAME RETARDANCY
21
FLAME RETARDANCY
Introduction
The retardants discussed herein are those believed to be in actual use or in ad-
vanced stage of development. Where no reference is cited, the information is prin-
cipally from the manufacturers’ literature.
The global usage of flame retardants was estimated in 2003 to be almost 1
million metric tons/year valued at $2.2 billion. On the basis of value, brominated
materials comprise about 35–37%, chlorine (and chlorine–phosphorus) about 5%,
nonhalogenated phosphorus about 25%, antimony oxide about 16%, alumina tri-
hydrate about 11%, and others (melamines, magnesium hydroxide, borates, etc)
about 6%. Both the entire industry and the brominated segment are growing at
about 4% annually (1,2).
In terms of usage, the main applications are construction, electrical equip-
ment, and motor vehicles. Other important areas are textiles, paper, and wood
products.
Inorganic Flame Retardants
Inorganic phosphorus compounds are described in the section, commercial
Phosphorus-Based Flame Retardants.
Oxides.
Alumina Trihydrate (Aluminum Hydroxide, Aluminum Trihydroxide,
ATH)[21645-51-2].
On a weight basis, ATH is the largest flame retardant in
current use http://www.manufacturing.net/pur/article/CA147910 (Nov. 1999). A
review of ATH and other metal hydroxides and hydroxycarbonates is available
(3). Much proprietary technology, involving either grinding (generally less costly)
or precipitation achieves the desired particle size distribution, morphology, surface
properties, and consistency (4–6). There are various surface-treated versions
where the treatment (such as silanation) improves the miscibility or the mechan-
ical properties of the final composition.
Major applications are vinyl and polyolefin cable jacket, unsaturated
polyester resin (sanitary ware, counter tops), cast artificial stone, elastomers such
as conveyer belts, and carpet backing. ATH tends to suppress smoke as well as
flame. The mode of action is believed to be attributable mainly to endothermic
dehydration at about 230
◦
C with 34.5% weight loss of water by 350
◦
C, consuming
Encyclopedia of Polymer Science and Technology. Copyright John Wiley & Sons, Inc. All rights reserved.

22
FLAME RETARDANCY
Vol. 10
1190 J/g of heat. This water loss acts to withdraw heat, which would other-
wise serve to pyrolyze the polymer, and the resultant emission of water vapor
also dilutes and cools combustible volatile fragments emitted from breakdown
of the polymer. The residual anhydrous alumina also serves as a thermal bar-
rier. Typical levels of addition are at least 50 parts by weight per 100 parts of
polymer.
Magnesium Hydroxide[1309-42-8].
An estimated 8000 ton (value about
$15 million) was used as flame and smoke suppressant in 2003. Its use appears
to be growing more rapidly than ATH despite higher cost per pound (3,7,8). In
contrast to ATH, magnesium hydroxide can be used in polymers with processing
temperatures up to about 340
◦
C, for example, in polypropylene or polyamides. It
is also less abrasive than ATH.
The largest-volume uses are wire and cable, and increasingly in thermoplas-
tic polyolefin roofing membranes (9). Grades in use range from the natural mineral
brucite to tailored particle morphologies such as Kyowa’s KISUMA. A typical less-
specialized and inexpensive grade is Martin Marietta’s MAGSHIELD, which can
be used at up to about 330–340
◦
C (8).
The mode of action is endothermic water release as in the case of ATH, but not
starting until about 340
◦
C completing itself with 30.9% weight loss and consuming
1300 J/g of heat by about 450
◦
C. The residual MgO serves as a highly reflective
thermal barrier. In some polymers, magnesium hydroxide favors charring to a
greater degree than ATH (8).
The main disadvantage is the high loading required; for example about 60%
Mg(OH)
2
is needed to reach V-0 in polypropylene. Some synergists have been
found to lower this level somewhat (10). The stiffening effect is less detrimental
in softer polymers such as polyolefin or ethylene vinyl acetate cable jacket or TPO
roofing membranes.
Antimony Oxides.
Antimony oxide is available both as the commodity anti-
mony trioxide (Sb
2
O
3
) [1309-64-4], and various specialty products such as colloidal
antimony pentoxide (Sb
2
O
5
) [1314-60-9] or sodium antimonate [15432-85-6].
There are also various proprietary combinations such as zinc/antimony oxide. An-
timony oxides are not primary flame retardants but are important synergists for
the halogen flame-retardant additives and halogen-containing plastics. Antimony
trioxide is sold by Albemarle, Great Lakes Chemical, Anzon, Atofina, Campine,
Harcros, Laurel, PQ, and others, and is largely supplied from China.
Antimony trioxide is a white powder, dusty unless oil-treated or pelletized. It
remains solid at all polymer-processing temperatures. The synergism with halo-
gen results from thermal formation of SbCl
3
or SbBr
3
, which inhibit flame by
scavenging hydrogen atoms and other radicals.
In contrast to antimony trioxide, which acts as a white pigment, the par-
ticle size of antimony pentoxide (colloidal), available as powders, dispersions, or
polymer concentrates from Nyacol Nano Technologies, is small enough to cause
minimal light scattering and thus able to produce translucent or transparent
polymer formulations. It is more costly than antimony trioxide but this may be
partially offset by lower loadings.
In acid-sensitive thermoplastic polyesters such as PET, PBT, or PC, sodium
antimonate is advantageous since it avoids some undesired catalytic action of the
oxide itself.

Vol. 10
FLAME RETARDANCY
23
Borates.
Boric Acid [10043-35-3].
This powdered solid, sold by U.S. Borax and oth-
ers, serves to prevent smoldering combustion of cotton batting (11), and is used
in mattresses and furniture, where it is mixed in mechanically, its binding to the
fibers aided by oil. Boric acid probably works by melting and coating the cellulose,
although some vapor action is also likely.
Sodium Borates (11).
These water-soluble salts, made by U.S. Borax and
others, have been known since the eighteenth century as nondurable flame retar-
dants for cotton, rope, canvas, and paper. The hydrated salts can act as endother-
mic heat sinks and moreover the salt fuses to a nonflammable glassy barrier layer.
Sodium borates, in particular borax (sodium tetraborate decahydrate [1303-96-4]),
are used in disposable paper and nonwoven cellulosic products, and as wood flame
retardants where their water solubility can be tolerated. The borate salts are ef-
fective on flaming combustion but allow severe afterglow; they are best used in
formulations with afterglow preventatives, and often used with boric acid.
Zinc Borates.
These are usually used as hydrates, one of the most common
being U.S. Borax’s FIREBRAKE ZB, which is 2ZnO
· 3B
2
O
3
· 3.5H
2
O [138265-88-0].
These hydrated salts have an endothermic action and also, at the elevated temper-
ature of burning, they can flux with or sinter with other minerals such as alumina
to form nonflammable barriers. For plastics processed at higher temperature, a
lower hydrate FIREBRAKE 415, 4ZnO
· B
2
O
3
· H
2
O [149749-62-2 or 1332-07-6] is
used. A main use of zinc borates is as smoke suppressants and flame-retardant
synergists in PVC or in other polymers with halogen additives. They can replace
all or part of the usual antimony oxide synergist, often with the result that the
smoke evolution during burning is lowered (12). The endothermic loss of water
from these hydrates is a substantial part of their mode of action; in the case of
PVC, they also induce dehydrochlorination at fire exposure temperatures.
Zinc borates, including the very thermally stable anhydrous 2ZnO
· 3B
2
O
3
[12767-90-7 or 1332-07-6], FIREBRAKE 500, have been found to act as syner-
gists with magnesium hydroxide or aluminum trihydroxide in polyolefin and other
thermoplastic formulations, by promoting char/ceramic barrier formation (13–15).
Several other zinc borates are available from R. J. Marshall Co., Barium metabo-
rate (Chapman Chemical’s FLAMEBLOC 550), calcium borates, and magnesium
borates are also used as flame retardants and smoke suppressants. These borates
can serve also as anticorrosives and preservatives, especially in coatings.
Other Inorganics.
Molybdates.
Calcium molybdates and zinc molybdates and various mod-
ifications thereof (15) are commercially available from Sherwin Williams as
KEMGARDS, and are principally used in PVC as smoke suppressants (15).
Ammonium octamolybdate (AOM), (NH
4
)
4
Mo
8
O
26
[12411-64-2], a slightly
water-soluble crystalline salt, is a smoke suppressant for PVC (15). It is used in
wire and cable, mass transit vehicle interiors, and carpet backing. It is believed
to work by forming a barrier and by modifying the decomposition of the PVC.
Stannates.
Zinc stannate [12036-37-2], Alcan’s FLAMTARD S, and zinc
hydroxystannate [12027-96-2], FLAMTARD HS, are both white water-insoluble
powders useful as smoke suppressants in PVC. They can partly replace antimony
oxide as flame-retardant synergists in halogen-containing flame-retardant formu-
lations, with reduced smoke (16,17).

24
FLAME RETARDANCY
Vol. 10
Zinc Sulfide [1314-98-3].
This stable white pigment shows synergistic
flame-retardant action in PVC and can be partially substituted for antimony oxide.
It can enhance other flame retardants in nylons and nylon blends (18).
Potassium Hexafluorozirconate [16923-95-8].
This water-soluble com-
pound is a flame retardant for wool by the “Zirpro” process. Applied in combi-
nation with formic acid and citric acid, the fluorozirconate exhausts onto wool and
after mild heating, provides a fairly wash-durable flame-retardant finish. Wool
upholstery for aircraft makes use of this treatment (19).
Layered Clays (“Nanoclays”) and Other Finely Divided Inorganic
Additives.
Layered clays such as montmorillonite or hectorite, when treated with
substances which can penetrate between the layers, such as quaternary ammo-
nium compounds, can be made to separate (exfoliate) into individual layers with
nanometer thicknesses. At loadings of only a few percent in many polymers, physi-
cal properties such as modulus and heat deflection temperature may be improved.
Moreover, the rate of heat release during burning as measured by cone calorimetry
can be greatly reduced (20,21). Generally, this effect is not enough to pass typi-
cal flame retardancy requirements except in combination with some other flame
retardant, and frequently faster ignition is an undesirable side-effect. However,
commercially useful flame retardancy improvements with nanoclays in combina-
tion with, for example, alumina trihydrate, can be achieved in polyolefin cable (22).
Synthetic platy silicates can be used in place of the montmorillonite (23). Besides
the layered clays, some other finely divided minerals such as attapulgite, kaolinite,
talc, or silica can have this beneficial effect, which may be related to heat-transfer-
or mass-transfer-barrier formation or barrier strengthening in cooperation with
char formation. In polycarbonates or polycarbonate blends, nanodimensioned ti-
tania or boehmite can be used in conjunction with other flame retardants (24).
At higher loadings, as are often used in elastomer formulations, nonlayered
clays such as kaolin clays and attapulgite serve to impart flame retardance by
dilution of the combustible material, and heat sink action enhanced by water loss
if the clays are not calcined.
Expandable Graphite Flake.
This product, which has long been known, is
made by treating graphite flake with nitric and sulfuric acid, which intercalate be-
tween the graphite layers. It is available commercially from Graftech and Nyacol
in the United States and from Asian manufacturers. When heated by fire expo-
sure to above about 160
◦
C, gases generated by the action of the oxidizing acids on
the graphite cause the flakes to swell up greatly to form elongated twisted “worm-
like” fibrils. This expanded material is an excellent heat insulator. Elastomeric
polymers, such as flexible foams and coatings, putties, and firestop products can
be flame retarded with expandable graphite, used by itself or compounded with
other flame retardants.
Halogenated Flame Retardants
The products containing both halogen and phosphorus are discussed under Phos-
phorus. Brominated products have their own section because they are the largest
category of flame retardants.
Vol. 10
FLAME RETARDANCY
25
The mode of action of halogenated flame retardants is often described as
proceeding with the production of hydrogen halide (or, in the presence of antimony
oxide, antimony trihalide), which enters the flame zone and serves as a flame
inhibitor. Part of this flame inhibition is generally believed to result from the
scavenging of free hydrogen atoms, which are responsible for the rate-controlling
chain branching steps in flame chemistry; however another part of the mode of
action appears to be physical dilution, heat capacity, and endothermicity (25,26).
Chlorine-Containing Flame Retardants.
Chloroparaffins.
These range from pourable liquids to waxes to rather high
melting solids, depending on the chain length of the paraffin and the level of
chlorination. The liquid lower chlorinated ones are mainly of interest in metal
cutting formulations. Some highly chlorinated liquid chloroparaffins are used in
PVC and elastomers to function as secondary plasticizers and as flame retardants.
A wide range of chloroparaffins is available from Dover Chemical Co.
At or above 70% chlorine content, the materials are solids with softening
temperatures from 103 to 160
◦
C. These are useful as flame-retardant additives
particularly for polyolefins, rubber, paints and adhesives. A typical formula for
meeting UL 94 V-0 with polyethylene uses 20–24% chloroparaffin and 8–10%
antimony oxide (27). Above the 13-carbon chain length, the toxicological charac-
teristics of the long-chain chloroparaffins [63449-39-8] is very favorable.
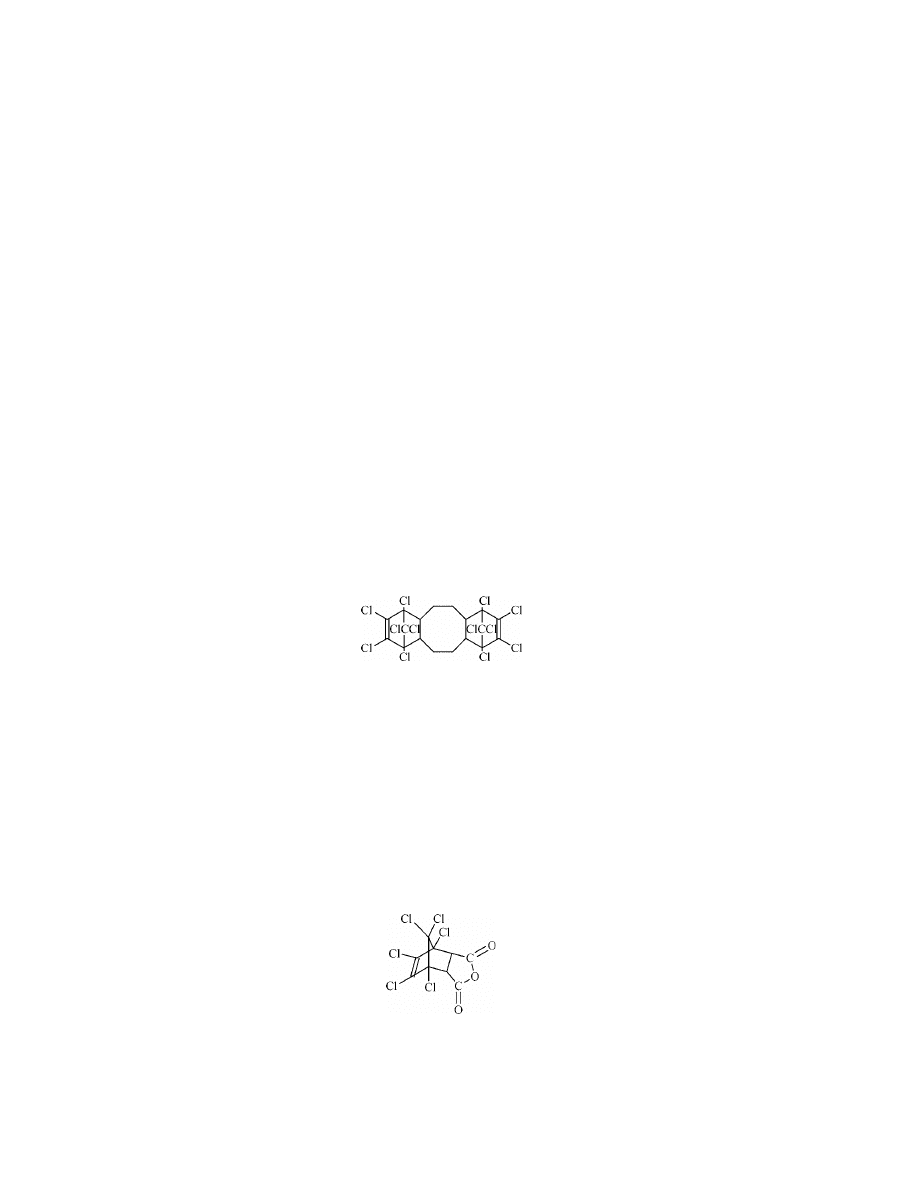
Polycyclic Chlorohydrocarbon (DECHLORANE PLUS) [13560-89-9].
There is only one commercial additive flame retardant in this group, Oxychem’s
DECHLORANE PLUS, which is a polycyclic chlorohydrocarbon of the structure
in which all of the chlorine atoms are so positioned that dehydrochlorination can-
not readily occur. Thus, the molecule is thermally very stable.
It was originally developed for polyethylene and other polyolefins, and is still
used to some extent in those applications, but its use in polyamide 6,6 has become
more important.
DECHLORANE PLUS can be synergized by antimony oxide, but better re-
sults including lower smoke levels are achieved by use of some iron oxide as syn-
ergist or as cosynergist with antimony oxide (28).
Chlorendic Anhydride (HET Anhydride) [115-27-5].
This compound is the
Diels–Alder adduct of hexachlorocyclopentadiene and maleic anhydride, having
the following structure:

26
FLAME RETARDANCY
Vol. 10
It has been used since the 1960s as a component of unsaturated polyester
resins, where it imparts, besides flame retardancy, an enhanced degree of corro-
sion resistance (solvent resistance) and thus resins made from it are useful for
industrial vessels and ducts.
Brominated Flame Retardants
This is the largest flame retardant category, in terms of economic value. At present,
the leading US sources are Great Lakes Chemical Co. and Albemarle, both of
whom have access to the warm bromine-rich brine found in Arkansas, and an
Israeli producer, Dead Sea Bromine Group (DSBG), using the bromine-rich Dead
Sea brine. Recently, these American producers have also begun to use bromine
from the Dead Sea. Overviews of the usage of brominated flame retardants and
the environmental aspects are available (29,30).
Additive Brominated Flame Retardants.
Hexabromocyclododecane [25637-99-4].
This product is a solid mixture
(31,32) of stereoisomers, mp about 175–195
◦
C. It is available from Great Lakes,
Albemarle, and DSBG. The main application is in expanded polystyrene foam. It
is generally used without antimony oxide, and allows for the melt-flow mode of ex-
tinguishment in addition to quenching the flame by evolved hydrogen bromide. It
may be synergized with a small amount of a peroxide or other free-radical genera-
tor which probably work by degrading the polymer under fire-exposure conditions,
thus aiding extinguishment by dripping. A heat-stabilized grade (32) can also be
used in polypropylene to reach UL 94 V-2 ratings such as for building/construction
applications in pipe, lamp sockets, and stadium seats. Hexabromocyclododecane is
also useful in textile flame-retardant backcoating, in combination with antimony
oxide and a binder. A recent review for the CPSC by a toxicology panel gave a
favorable report to this use (33).
Pentabromodiphenyl Ether [32534-81-9].
This is a very viscous liquid or
low melting solid, mp
<30
◦
C. It is made as Great Lakes Chemical’s DE-71 by
direct catalyzed bromination of diphenyl ether. It has been used in admixture
with a triaryl phosphate as a flame retardant for flexible polyurethane slab-stock
foam, the major advantage in that application being its stability and freedom
from causing “scorch” (discoloration and deterioration of the foam at the peak
temperature of water-initiated blowing). In this kind of mixture, it has been mar-
keted as Akzo Nobel’s FYROL PBR and Great Lakes Chemicals’s DE-60(F) or
DE-61.
In recent years, pentabromodiphenyl ether has been detected widely in the
environment such as in marine animals and the milk of nursing mothers. In view
of concern regarding possible chronic toxicity (34), it has been largely discontinued
and the only US producer is halting production in 2004.
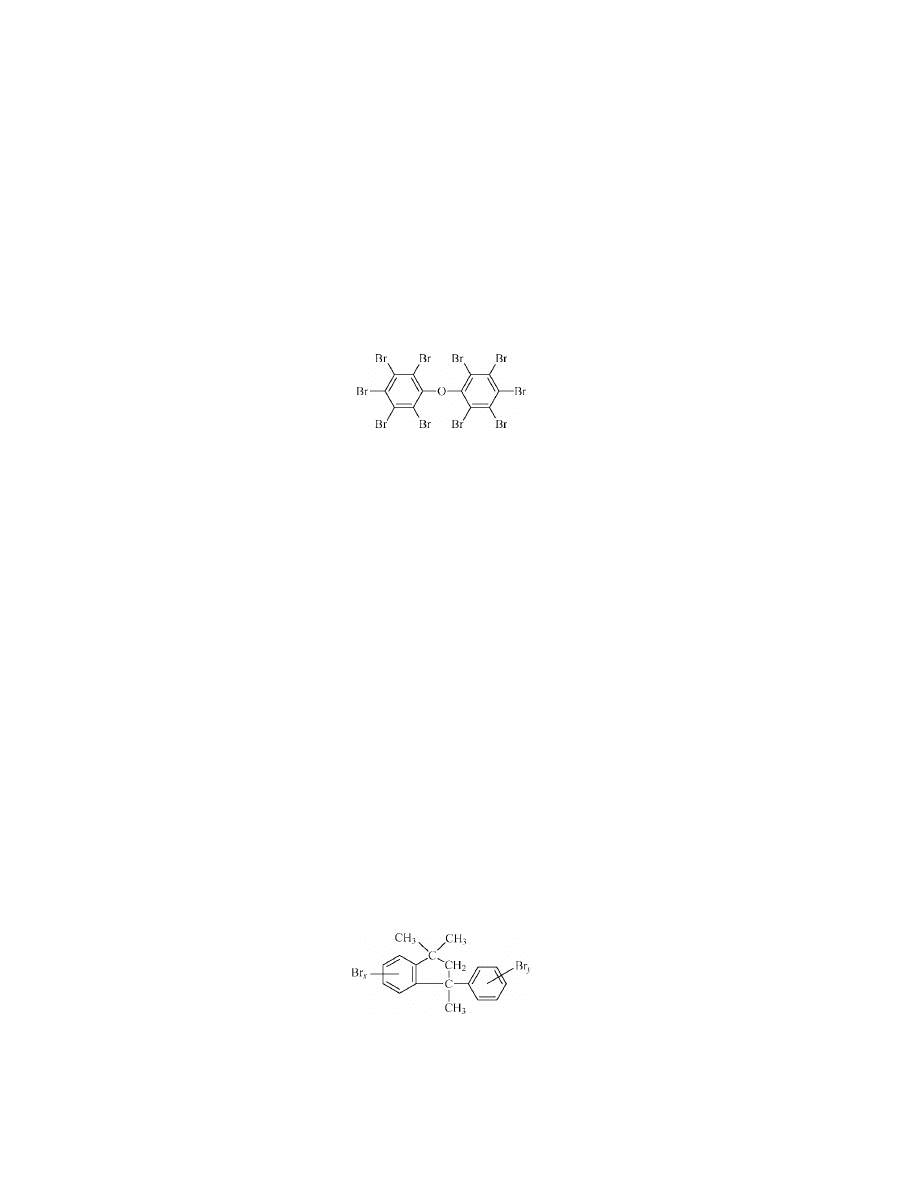
Octabromodiphenyl Ether[32536-52-0].
This is a solid melting in the
range 70–150
◦
C, comprising a mixture of several isomers of octa- with lower and
higher brominated diphenyl ethers. It has been a preferred flame-retardant addi-
tive for ABS, polyamides, or PBT because it does not reduce the impact strength
as badly as less soluble additives.
Vol. 10
FLAME RETARDANCY
27
Recently (in 2003), the EU required that the use of this product be discon-
tinued because of toxicological concerns. In 2003, it was banned in California
(effective as of 2007) but its use has already declined.
Decabromodiphenyl Ether[1163-19-5].
This is the second largest bromi-
nated flame retardant, and is used in HIPS, textile backcoating, and in polyolefins,
generally together with antimony oxide. It is made, as are the other members of
this series, by bromination of diphenyl ether and is commercially available by Albe-
marle as SAYTEX 102, Great Lakes Chemical as DE-83, and Dead Sea Bromine
(DSBG) as FR-1210. In view of the large volume and multiple producers, it is
priced favorably. The structure is as follows:
Decabromodiphenyl ether is a solid, melting at about 304–309
◦
C, substan-
tially insoluble in water, and with negligible vapor pressure. In contrast to the
lower brominated diphenyl ethers, it has only rarely been found as an environ-
mental pollutant and is low in toxicity. Risk studies conducted in the United States
and the European Union (33,35) indicate a low degree of risk in the use of this
flame retardant. It is the major flame retardant used in high impact polystyrene
(HIPS) with antimony oxide, and has substantial use in polyolefin wire and cable
as well as electrical parts made of other plastics such as polyamides and thermo-
plastic polyesters.
Tetradecabromodiphenoxybenzene [58965-66-5].
This product has an
advantage for use in polyamide 6,6 because it has less tendency to “bloom” (to
come to the surface) than decabromodiphenyl ether. It is available from Albemarle
as SAYTEX 120.
1,2-Bis(2,4,6-tribromophenoxy)ethane [37853-59-1].
This product, mp
223–225
◦
C, containing 70% Br, sold by Great Lakes as FIREMASTER 680, is used
mainly in ABS where it appears compatible and has only a moderate tendency to
bloom.
Decabromodiphenylethane (1,2-Bis[pentabromophenyl]ethane) [84852-
53-9].
This solid additive, Albemarle SAYTEX 8010, is an alternative to the
polybromodiphenyl ethers. It has 80% Br, a very high melting point (above
350
◦
C), excellent thermal stability (good recyclability), fair UV stability, and gives
performance similar to decabromodiphenyl ether in polyolefins and styrenics. An
important use is in HIPS for TV and other electrical or electronic enclosures.
Octabromo-1-phenyl-1,3,3-trimethylindane [155613-93-7].
This is a
solid 73% bromine additive, mp 240–255
◦
C, produced by DSBG as FR-1808, which
can also be substituted for decabromodiphenyl ether. The structure is as follows:
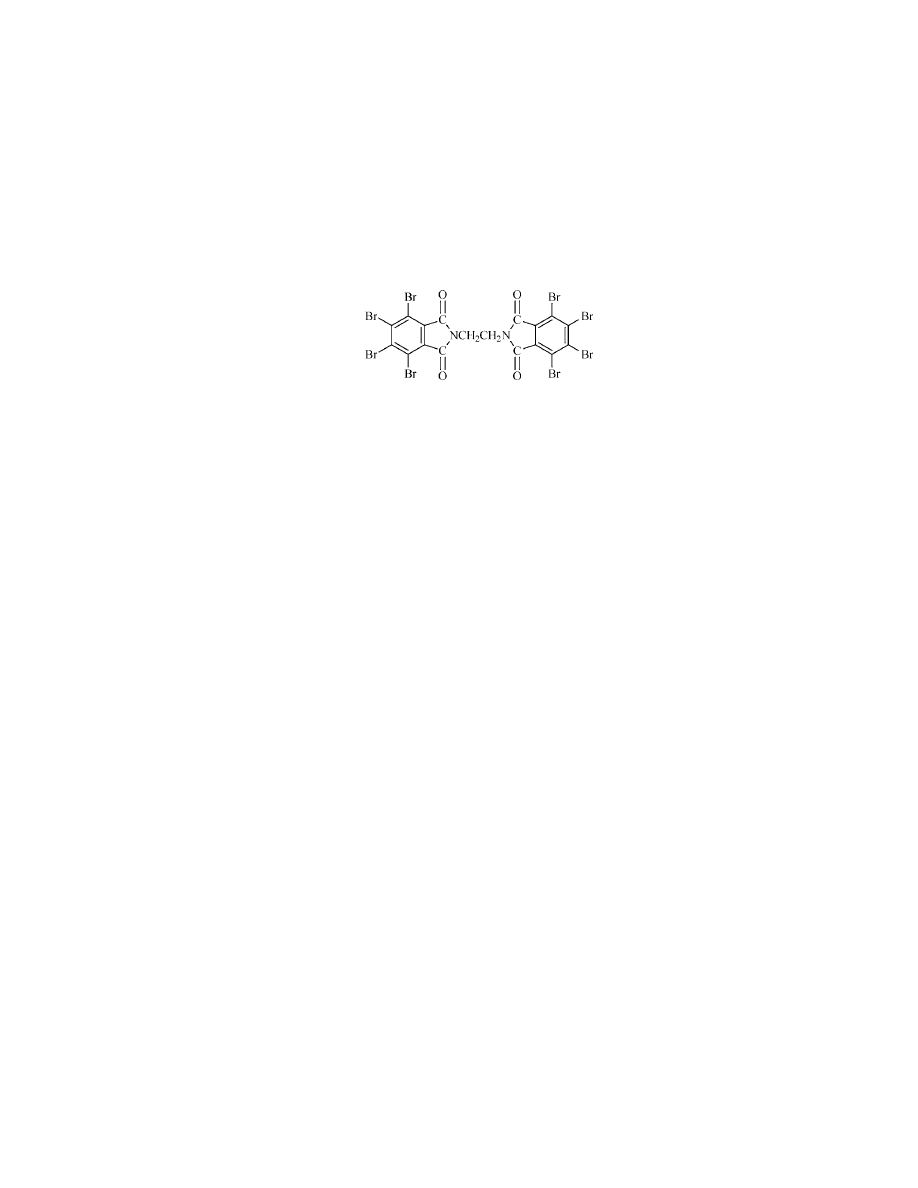
28
FLAME RETARDANCY
Vol. 10
In styrenics, polyamide 6,6, and engineering thermoplastics, it is effective
with antimony oxide and has good flow and impact properties even in filled for-
mulations (36).
Ethylenebis(tetrabromophthalimide) [32588-76-4].
This is a high melting
crystalline compound, mp 450–455
◦
C, made from tetrabromophthalic anhydride
and ethylenediamine. It is available from Albemarle as SAYTEX BT-93.
It has better sunlight stability than the polybrominated diphenyl ethers and
thus can be used in nonyellowing polyolefins and styrenics. By removal of a small
impurity, Albemarle has made available a grade SAYTEX BT-93W with even bet-
ter color photostability.
Tris(2,4,6-tribromophenoxy)-1,3,5-triazine [25713-60-4].
This is a newer
member of the bromine additive family made by DSBG as FR-245 from 2,4,6-
tribromophenol and cyanuric chloride. It is a white solid, mp 230
◦
C, containing
67% Br. It can be substituted for decabromodiphenyl ether in styrenics and their
alloys, and has melt flow advantages (permitting shorter cycle times and thinner
walls) as well as good impact and UV stability (36).
Tetrabromobisphenol A Bis(2,3-dibromopropyl) Ether [21850-44-2].
This is a rather low melting (
∼95–111
◦
C) colorless solid containing 68% bromine
which is effective as an economical flame retardant in polypropylene, olefin copoly-
mers, and HIPS. It is available from Albemarle as SAYTEX HP-800A, from DSBG
as FR-720, and from Tosoh as FLAMECUT 121K. Principal uses are in wire and
cable, and in electrical sockets to achieve a UL 94 V-2 rating.
Poly(2,6-dibromophenylene Oxide) [69882-11-7].
This is a light tan melt-
processable solid, softening range 210–240
◦
C, containing about 64% Br. It is used
in unfilled and reinforced PBT and PET, and polyamides 6 and 66. It allows good
chemical resistance and light stability. It is available from Great Lakes Chemical
as PO-64P and from Unitex as UNIPLEX FRP-64.
Tetrabromobisphenol A Polycarbonate Oligomer (Tribromophenyl End
Capped) [71342-77-3].
This is a thermally stable resinous solid made from
tetrabromobisphenol A and phosgene. It is principally used as a flame-retardant
additive for PBT, ABS, and polycarbonate for electrical and electronic apparatus
housing. It is available from Great Lakes Chemical as BC-58.
Brominated Epoxy Oligomers (BEO) [68928-70-1] as Additives.
The re-
action of tetrabromobisphenol A with epichlorohydrin can be conducted in a
ratio to make either diglycidyl ether or to make oligomers of reduced epoxy
functionality, or end capped with tribromophenol to avoid functionality. These
oligomers are mainly used as melt-blendable additives useful in many thermoplas-
tics, notably in ABS, HIPS, and polyamides. Processing and UV stability advan-
tages are often superior to those of decabromodiphenyl ether, and a good balance of
properties is obtained for use in ABS in business machine applications (37). These

Vol. 10
FLAME RETARDANCY
29
additives are available from DSBG and a similar series from Resolution Perfor-
mance Products. The lower molecular weight members have enough epoxy func-
tionality for use as reactives in epoxy, phenolic, and unsaturated polyester resins.
Brominated Polystyrenes.
There are two main classes of brominated
polystyrenes. The older class, suitable for the principal polyamides, is made by
brominating a low molecular weight polystyrene, probably with bromine chloride
or a mixture of bromine and chlorine. A version with 68% Br and improved thermal
stability has been developed by Albemarle as SAYTEX HP-7010, particularly for
glass-filled engineering resins (38). The other class of brominated polystyrenes,
more thermally stable and preferred for use in higher temperature polyamides,
is made from brominated styrene monomers. A further variant is a copolymer
of dibromo- and tribromostyrene with some glycidyl methacrylate (Great Lakes
FIREMASTER CP-44HF which improves melt flow, compatibility, and mechanical
properties in engineering thermoplastics.
Bis(2-ethylhexyl) Tetrabromophthalate [20566-35-2][26040-51-7].
This
liquid ester with plasticizing character and containing 45% Br is available from
Great Lakes Chemical as DP-45, by Oxychem as PYRONYL 45, and by Unitex as
UNIPLEX FRP-45. It is mainly used in PVC wire and cable insulation, coatings,
and elastomers.
2-Ethylhexyl Tetrabromobenzoate [183658-27-7].
This liquid ester was
recently introduced by Great Lakes Chemical as FIREMASTER BZ-54 mainly for
use in combination with a triaryl phosphate as a nonscorching flame retardant
for water-blown flexible polyurethane foam slab stock.
Poly(pentabromobenzyl acrylate) [59447-57-3].
This polymeric additive,
DSBG’s FR-1025, is melt-blendable with polyolefins, and at 8.5% in polypropylene
produces a V-2-rated formulation. It is also useful in engineering thermoplastics
such as PBT and polyamides.
Disodium Tetrabromophthalate [25357-79-3].
This is a water-soluble salt,
available as Great Lakes FR-756, used in nondurable textile flame-retardant
finishing.
Reactive Bromine Compounds.
Tetrabromophthalic Anhydride [632-79-1].
This is a reactive aromatic
bromine compound, available from Albemarle as SAYTEX RB-49 and from Great
Lakes as PHT-4, with several diverse uses in flame retardancy. It can be reacted
with diols and, generally, maleic anhydride to make polyesters, which are then di-
luted with styrene to produce flame-retardant unsaturated polyester resins suit-
able for panels and bulkheads in buildings and public transport.
Tetrabromophthalic anhydride can be reacted with ethylenediamine to make
the bisphthalimide additive discussed above, and also converted to a diol, dis-
cussed below.
Hydroxethoxyethyl Hydroxypropyl Tetrabromophthalate [20566-35-2].
This diol, a viscous liquid, is available from Albemarle as SAYTEX RB-79 and
from Great Lakes Chemical as PHT4-DIOL. It has the following main component:
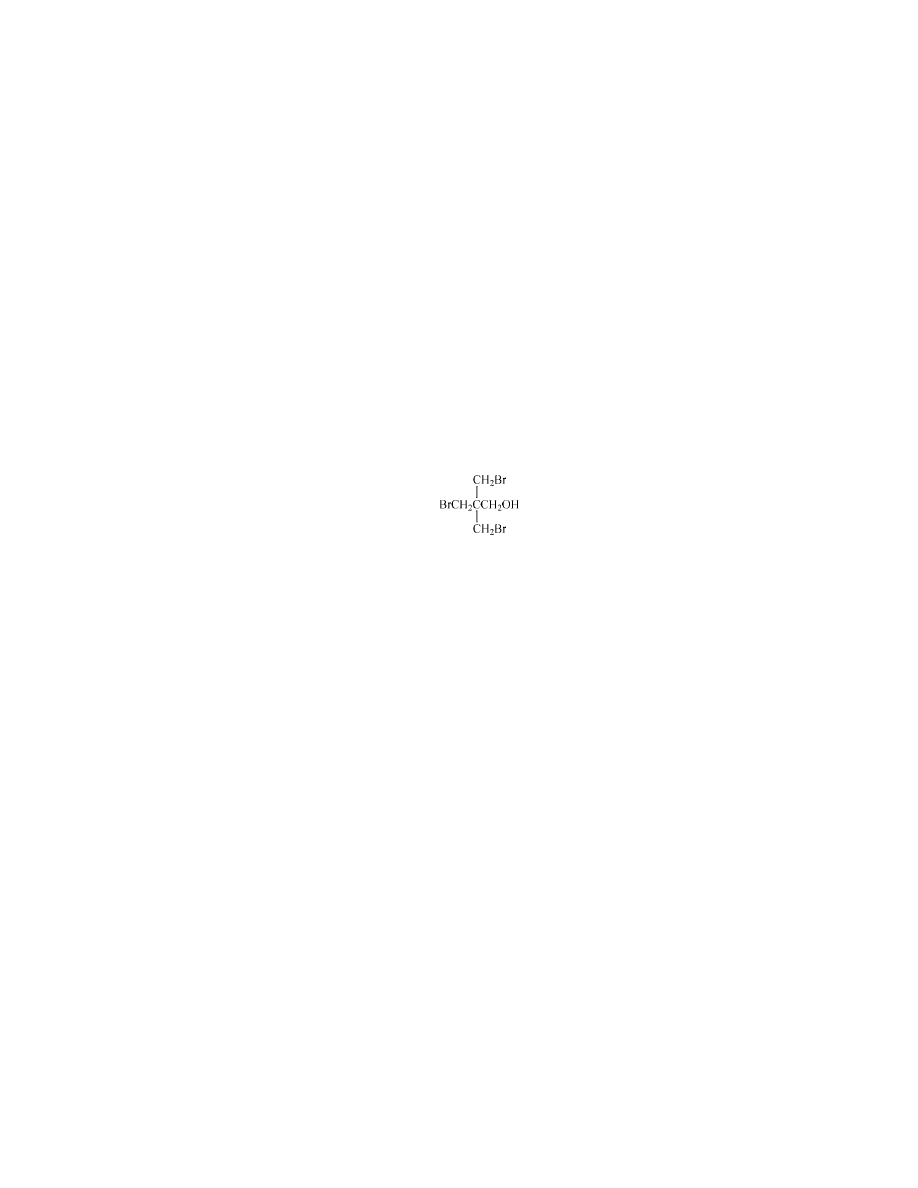
30
FLAME RETARDANCY
Vol. 10
It is useful for the preparation of flame-retardant flexible or rigid
polyurethane foams. In the foam applications, where it is often used as a blend
with a triaryl phosphate such as in SAYTEX RB 7980 (higher hydroxyl num-
ber) or RX 8500 (lower hydroxyl number). Its advantage is that it allows flexible
polyurethane foam to reach high temperatures in the water-blown foaming step
without “scorch” (thermal damage).
More recently, a more reactive analog was introduced by Great Lakes Chem-
ical as FIREMASTER 520. This probably has a hydroxyethyl group instead of
hydroxypropyl.
Dibromoneopentyl Glycol [3296-90-0].
This diol, available from DSBG
as FR-522, is made by reaction of pentaerythritol with hydrogen bromide. It is
a crystalline compound, mp 109–110
◦
C, and has been used in flame-retardant
unsaturated polyester resins and polyurethane foams.
Tribromoneopentyl Alcohol [1522-92-5].
This is also made by DSBG as
FR-513 by reaction of pentaerythritol with hydrogen bromide. It is a crystalline
compound, mp 62-67
◦
C, with the following structure:
It can be used by itself or more conveniently in a liquid mixture with a triaryl
phosphate as a reactive for the preparation of nonscorching flexible polyurethane
foams.
Another use for this alcohol is as a precursor of tris(tribromoneopentyl) phos-
phate, discussed below in the Phosphorus Flame Retardants section.
Tetrabromobisphenol A [79-94-7].
This compound, a solid, mp 179-181
◦
C
is the largest of the bromine-containing flame retardants. Tetrabromobisphenol A
is available from Albemarle as SAYTEX CP-2000, Dead Sea Bromine as FR-1524,
and Great Lakes Chemical as BA-59P.
The largest volume use is in epoxy circuit boards (printed wiring boards) to
meet the FR-4 standard in electronic equipment (computers, printers, communi-
cation equipment). The usual mode of usage is as a “chain extender,” wherein it
is reacted with a nonhalogenated epoxy resin.
A related use is to produce the diglycidyl ether of tetrabromobisphenol A,
which can be used as such in epoxy thermoset fabrication but is mainly reacted
with methacrylic acid to make flame-retardant vinyl ester resins. These are useful
for making fiber-reinforced corrosion resistant containers and ducts. The digly-
cidyl ether can also be chain extended with more tetrabromobisphenol A to make
a family of, mainly, additives for thermoplastics.
Another important mode of usage of tetrabromobisphenol A is as an additive
in styrenics, particularly in dark-pigmented ABS. It is typically used at 18–22%
plus 4–8% antimony oxide. This application is favored by the low cost of tetrabro-
mobisphenol A.
Pentabromobenzyl Acrylate[59447-55-1].
This monomer, available from
DSBG as FR-1025M, can be used in reactive extrusion to graft onto polypropylene

Vol. 10
FLAME RETARDANCY
31
and various other thermoplastics. Dyeable flame-retardant polypropylene fibers
can be produced. The homopolymer is discussed under Additives.
Vinyl Bromide[593-60-2].
This low boiling monomer (bp 15.8
◦
C) is mainly
used for copolymerization with other vinyl monomers to make modacrylic fibers,
for inherently flame-retardant textiles. Copolymers with vinyl chloride are also
useful in flame-retardant coatings.
Commercial Phosphorus-Based Flame Retardants
Phosphorus compounds comprise, value-wise, the second largest group of flame
retardants, and are probably the fastest growing segment as a result of environ-
mental concerns with various bromine–antimony retardants. An earlier review is
available (39).
Inorganic Phosphorus Compounds.
Red Phosphorus [7723-14-0].
This polymeric form of phosphorus is rela-
tively nontoxic, not spontaneously flammable, and stable up to
∼450
◦
C. In finely
divided, coated, and stabilized form it is available from Clariant, Rhodia, Ital-
match, and Tosoh. It has been found to be a powerful flame-retardant additive
(40) effective at relatively low loadings. In Europe, it is used in molded nylon
electrical parts in a coated and stabilized form. Handling hazards and color have
deterred usage in the United States. The development of masterbatches by Ital-
match overcomes the flammable dust problem, but evolution of trace phosphine
from reaction with water is still a concern (41).
Ammonium
Phosphates.
Monoammonium
Phosphate
[7722-76-1],
NH
4
H
2
PO
4
, and diammonium phosphate [7783-28-0], (NH
4
)
2
HPO
4
(or mixtures
of the two), have long been used for low cost flame retarding and afterglow
prevention of paper, textiles, disposable nonwoven cellulosic fabrics, and wood
products (42–44). Ammonium phosphate finishes are resistant to dry-cleaning
solvents but not to water.
Formulations of ammonium phosphates and ammonium bromide, sometimes
with wetting and softening agents, are sold for use on cellulosic–synthetic fiber
blends. A large-volume use in the United States for ammonium phosphate is in for-
est fire control, usually by aerial application and often combined with ammonium
sulfate.
Water-soluble ammonium pyrophosphates and lower molecular weight
(water-soluble) ammonium polyphosphates have uses on textile and paper simi-
larly to ammonium phosphates but have less tendency to crystallize and to impair
the texture.
Insoluble Ammonium Polyphosphate.
When ammonium phosphates are
heated in the presence of urea, relatively water-insoluble ammonium polyphos-
phate [68333-79-9] is produced (45) consisting of long chains of repeating
OP(O)(ONH
4
)
units.There are several crystal forms (type II is the least-
soluble), and the commercial products, available from a number of manufacturers,
differ in molecular weight, particle size, solubility, and surface coating (46). These
finely divided solids are principal ingredients of intumescent paint and mastics
(47). In such formulations, ammonium polyphosphate is considered to function
as a catalyst for charring an organic component such as pentaerythritol or an

32
FLAME RETARDANCY
Vol. 10
aminoplast resin (48,49). A blowing agent such as melamine or chlorowax is also
present to impart a foamed character to the char, thus forming a fire-resistant
insulating barrier. In addition, the intumescent formulations typically contain
resinous binders, pigments, ceramic components, and other fillers.
A series of compounded flame retardants, based on finely divided insolu-
ble ammonium polyphosphate together with char-forming nitrogenous resins,
has been developed for polyolefins, ethylene–vinyl acetate, and urethane elas-
tomers (50–56). Ammonium polyphosphate is also an efficient flame retardant in
rigid polyurethane foam, with minimal adverse effects on other foam properties
(57).
Additive Organic Phosphorus Flame Retardants.
Melamine Phosphates and Other Amine Phosphates.
Commercial
products
in
this
group
include
melamine
orthophosphate
[20208-95-1],
C
3
H
6
N
6
· H
3
O
4
P, and melamine pyrophosphate [15541-60-3], 2C
3
H
6
N
6
· H
4
O
7
P
2
.
The pyrophosphate, available from Cytec, Chemische Fabrik Budenheim, and
Broadview Technology, is less water-soluble (0.09 g/100 mL water) and also more
thermally stable. These are available as finely divided solids and used principally
in flame-retardant coatings (58), although plastic and elastomer applications also
show promise (59). A detailed study of the thermal decomposition of the these
compounds has been published (60).
A more recent member of this family is melamine polyphosphate (MELAPUR
200, originally from DSM, now from Ciba), which has a thermal stability advan-
tage for use in polyamide 6 and 66 (61) and in combination with char-assisting
additives, in polyolefins such as those used for stadium seating.
Ethylenediamine Salt of Phosphoric Acid.
This is a finely divided solid, mp
250
◦
C, slightly soluble in water. It was introduced by Albright & Wilson as Amgard
EDAP and is now available as Broadview Technology’s INTUMAX AC-3, mainly
as an additive for polyolefins, EVA, and PVC. Unlike ammonium polyphosphate,
it is self-intumescent and does not require a char-forming synergist (62). It is
synergized by melamine or melamine pyrophosphate, and is available as a blend
with these, respectively from Unitex Corp. and Broadview Corp.
Triethyl Phosphate.
Triethyl phosphate [78-40-0], C
6
H
15
O
4
P, is a colorless
liquid boiling at 209–218
◦
C containing 17 wt% phosphorus, made by Eastman from
diethyl ether and phosphorus pentoxide (63,64). Triethyl phosphate has been used
commercially as an additive for polyester laminates and in cellulosics. In polyester
resins, it functions as a viscosity depressant as well as a flame retardant, and
permits high loadings of alumina trihydrate (65).
Dimethyl Methylphosphonate (DMMP) [756-79-6].
This is a water-soluble
liquid, bp 185
◦
C, with the formula CH
3
P( O)(OCH
3
)
2
, contains 25% phosphorus,
which is near the maximum possible for a phosphorus ester, and therefore highly
efficient as a flame retardant. It is sold by Akzo Nobel as FYROL DMMP. Ap-
plications include use as a viscosity depressant and flame retardant in alumina
trihydrate (ATH)-filled polyester resins (66) such as used for bathtubs and shower
stalls. Some applications have been found in rigid polyurethane foams and as an
intermediate for making other flame retardants. In Europe, DMMP has an “R46”
(mutagen) classification, which deters its use.
Diethyl Ethylphosphonate (DEEP) [78-38-6].
This is a liquid compound
introduced by Bayer as LEVAGARD VP AC 4048 for applications similar to those

Vol. 10
FLAME RETARDANCY
33
of DMMP. It is less susceptible to undesirable interactions with blowing agents or
amine catalysts. It has a less toxic European rating than DMMP.
Dimethyl Propylphosphonate (DMPP) [18755-43-6].
This liquid additive
was recently introduced by Bayer as LEVAGARD DMPP, an alternative to the
chloroalkyl phosphates for flame-retarding rigid polyurethane foams.
Aluminum Diethylphosphinate.
This solid additive, available in combina-
tion with a melamine or inorganic synergist as Clariant’s EXOLIT OP 1311 and
1312, is a highly effective additive in polyamides, PBT, and epoxy (67–69).
Oligomeric Ethyl Phosphates.
An oligomeric ethyl phosphate additive con-
taining 19% phosphorus has been introduced for use in flexible polyurethane
foams by Akzo Nobel as FYROL PNX. Because of the high percentage of phospho-
rus, it is quite efficient and as little as 5 php is effective in passing the California
117 test and MVSS 302 in a 1.5–1.8 lb/cu.ft. foam. For automotive applications,
this low-volatility additive has the advantage of not causing windshield fogging.
A lower viscosity version is available as FYROL PNX-S.
Halogenated Aliphatic Phosphates and Phosphonates.
The princi-
pal use of these additives is in polyurethane foams; broader reviews of this impor-
tant flame retardant application area have been published (70,71).
2-Chloroethanol Phosphate (3:1).
Tris(2-chloroethyl) phosphate [115-96-
8], C
6
H
12
Cl
3
O
4
P [2-chloroethanol phosphate (3:1)], is a low viscosity liquid product
that has found widespread usage because of low cost, low odor, high percentage
of phosphorus, and compatibility with essentially all polymers containing polar
groups. Akzo Nobel’s FYROL CEF contains 10.8% phosphorus and 36.7% chlorine,
and is made from ethylene oxide and phosphorus oxychloride (72). This phosphate
is used in rigid polyurethane and polyisocyanurate foams, carpet backing, flame-
laminated and rebonded flexible foam, flame-retardant coatings, most classes of
thermosets, adhesives, cast acrylic sheet, and wood–resin composites such as par-
ticle board. It is used with melamine in flexible urethane foam cushions and in-
stitutional mattresses.
1-Chloro-2-Propanol Phosphate (3:1).
Tris(1-chloro-2-propyl) phosphate
[13674-84-5], C
9
H
18
Cl
3
O
4
P, is a liquid containing 33% chlorine and 9.5% phos-
phorus. It is produced by reaction of propylene oxide and phosphorus oxychlo-
ride. Because of the branchy structure, this phosphate is much lower in re-
activity to water and bases than the 2-chloroethyl homologue (73). It is sold
as Akzo Nobel’s FYROL PCF, Bayer’s LEVAGARD PP, or Albright & Wilson’s
(now Albemarle’s) ANTIBLAZE 80 or TMCP and is a preferred additive for rigid
urethane foams where good storage stability in the isocyanate or the polyol–
catalyst mixture is required. It is used in isocyanurate foam to reduce friability and
brittleness, and is also used on a large scale in flexible urethane foams, often with
melamine.
1,3-Dichloro-2-Propanol
Phosphate
(3:1).
Tris(1,3-dichloro-2-propyl)
phosphate [13674-87-8], C
9
H
15
Cl
6
O
4
P, sold by Akzo Nobel as FYROL FR-2 and
by Albright & Wilson (now Albemarle) as ANTIBLAZE 180 or 195, contains 49%
chlorine and 7.2% phosphorus. It is made from epichlorohydrin and phosphorus
oxychloride . The principal structure has 1,3-dichloro-2-propyl groups with a
minor percentage of 2,3-dichloro-1-propyl groups.
Compared to the foregoing chloroalkyl phosphates, this product has a greatly
reduced volatility, much lower water solubility, and high stability toward the

34
FLAME RETARDANCY
Vol. 10
amine catalysts used in foam manufacture. It is a long-established and a lead-
ing additive for flexible urethane foams (74) and can be added to the isocyanate
or the polyol-catalyst mixture. This phosphate shows little tendency to scorch, ie
to cause discoloration and degradation, except in some low density water-blown
foams. Flexible foam formulations containing this and other haloalkyl phosphates
can be further stabilized against scorch by appropriate antioxidants and acid ac-
ceptors (75–78). Scorch is believed to be aggravated if the flame retardant can
alkylate an aminoaryl group resulting from hydrolysis of isocyanate (79).
Combinations of tris(dichloroisopropyl) phosphate or tris(1-chloro-2-propyl)
phosphate with melamine are used in cushioning formulations and are effective for
passing antismoldering requirements (80) as well as stringent British furniture
standards. Tris(dichloroisopropyl) phosphate is also useful as a flame retardant
in styrene–butadiene and acrylic lattices for textile backcoating and binding of
nonwovens.
The mode of action of tris(dichloroisopropyl) phosphate in flexible
polyurethane foams appears to be both physical and chemical (81).
Oligomeric 2-Chloroethyl 1-Ethylidine Phosphonate.
This is made from
the reaction of tris(2- chloroethyl) phosphite with acetaldehyde, and has the fol-
lowing structure:
This liquid oligomer, Clariant’s EXOLIT 5087, contains 27–28% Cl and 15%
P, and is useful as an additive flame retardant for transparent cast polymethyl
methacrylate, unsaturated polyester resins, or rigid polyurethane foams. It has
good light stability, but is not stable to heat above about 180
◦
C.
Aliphatic Diphosphates.
These have low volatility and good to fair ther-
mal stability, and are thus useful in those open-cell (flexible) foams which have
requirements for improved resistance to dry and humid aging.
A diphosphate introduced originally as Monsanto’s Phosgard 2XC20 [38051-
10-4], C
13
H
24
Cl
6
O
8
P
2
, is now available as Albemarle’s) ANTIBLAZE 100 or V6.
The compound has the following structure:
This compound has enhanced hydrolytic stability in addition to low volatility.
It is useful in many types of flexible foam, as well as in adhesives and epoxy- or
phenolic-based laminates.
Oligomeric 2-Chloroethyl Phosphate.
Akzo Nobel’s FYROL 99 [109640-
81-5] (82,83) is low in volatility and useful in resin-impregnated air filters, in
flexible urethane foam, rebonded foam, structural foam and flame-retardant
coatings.
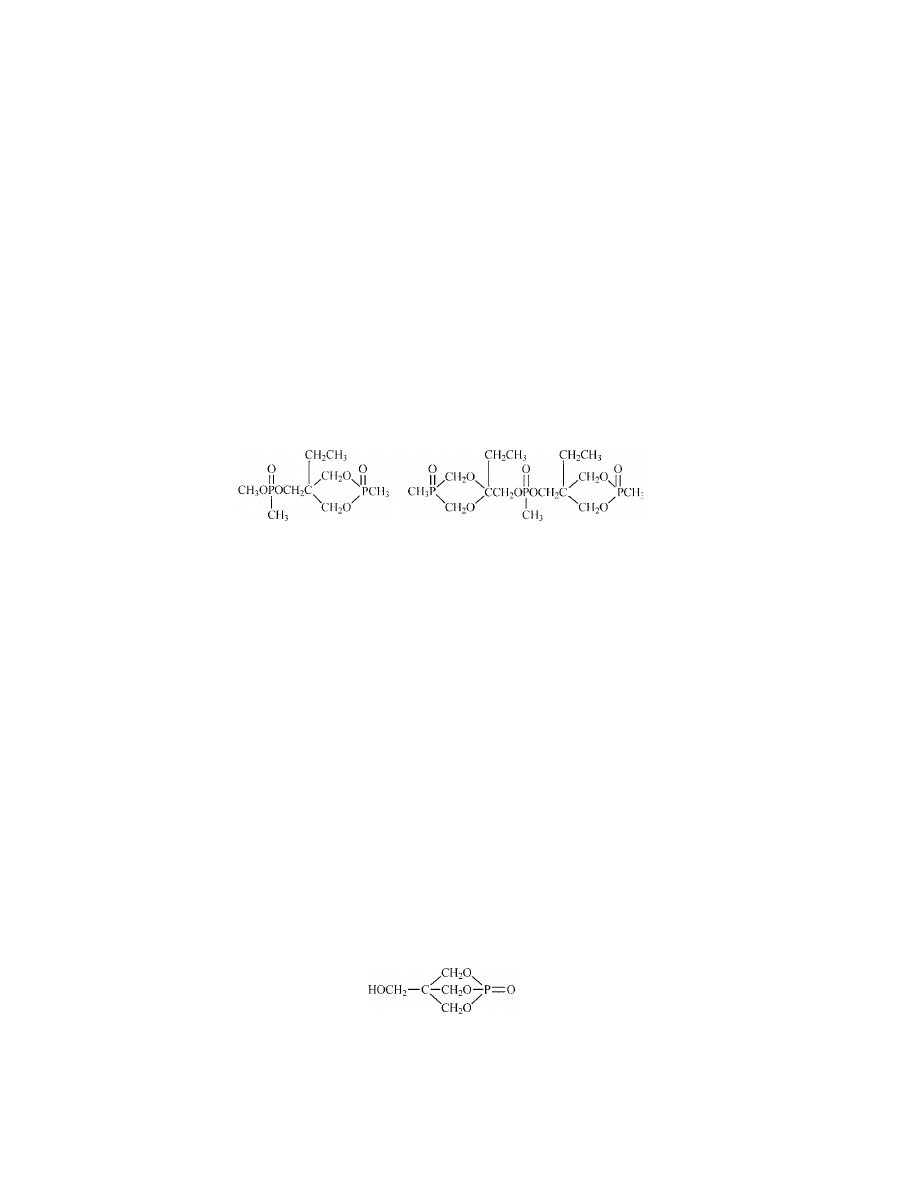
Vol. 10
FLAME RETARDANCY
35
Tris(tribromoneopentyl) Phosphate [19186-97-1].
This solid, mp 181
◦
C,
compound has stable but highly efficient bromine. It can be used as an additive,
with or without an antimony oxide synergist, in a wide range of thermoplas-
tics including polypropylene and HIPS (84). It is particularly advantageous in
polypropylene fiber, since it melts at the spinning temperature, does not require
high loadings, and can be used without antimony oxide. It has good light stabil-
ity and favorable toxicology. It is available from DSBG at FR-370 (special carpet
fiber grade as FR-372) and from Unitex as BAP-370. It can be synergized to favor
the melt-flow mode of extinguishment by small amounts of a hydrocarbon radical
generator (Akzo Nobel’s PERKADOX 30) (85).
Cycloaliphatic Phosphates and Phosphonates.
Oligomeric Cyclic Phosphonates.
AMGARD CU or CT, also Clariant’s EX-
OLIT OP 910, and AMGARD 1045 are mixtures of the diphosphonate and triphos-
phonate with the following structures (C
9
H
20
O
6
P
2
[41203-81-0], and C
15
H
31
O
9
P
3
[42595-49-9]).
These are made via the bicyclic phosphite from trimethylolpropane subjected
to the Arbuzov reaction with dimethyl methylphosphonate (86,87). The bicyclic
phosphite intermediate is highly neurotoxic, but, after the ring-opening step, the
product is low in toxicity. AMGARD CU (higher viscosity) or CT (lower viscosity)
is largely the diphosphonate. AMGARD 1045 (high viscosity) contains a larger
amount of the triphosphonate. Both materials are water-soluble, thermally stable
low volatility liquids having about 20–21 % phosphorus content and no halogen.
AMGARD CU or CT is used as a flame-retardant finish for polyester fabric by the
“thermosol” process. After the phosphonate is applied from an aqueous solution,
the fabric is heated to swell and soften the fibers, allowing the phosphonate to
be absorbed and strongly held after the fabric cools. Many formulated polyester
textile finishes are on the market based on this active ingredient.
AMGARD 1045 can advantageously be added to polyethylene terephthalate
melt before spinning. These products are also useful flame retardants for other
thermoplastics, thermoset polyester resins, polyurethanes, polycarbonates, nylon
6, and textile backcoating.
Pentaerythritol Phosphates.
These products take advantage of the excel-
lent char-forming ability of the pentaerythritol structure (88).
A bicyclic pentaerythritol phosphate, Great Lakes CN-1197 or Unitex UNI-
PLEX FRX 44-33, [5301-78-0], was introduced for use in thermosets, preferably in
combination with melamine or ammonium polyphosphate (89). It is a high melting
solid with the following structure:
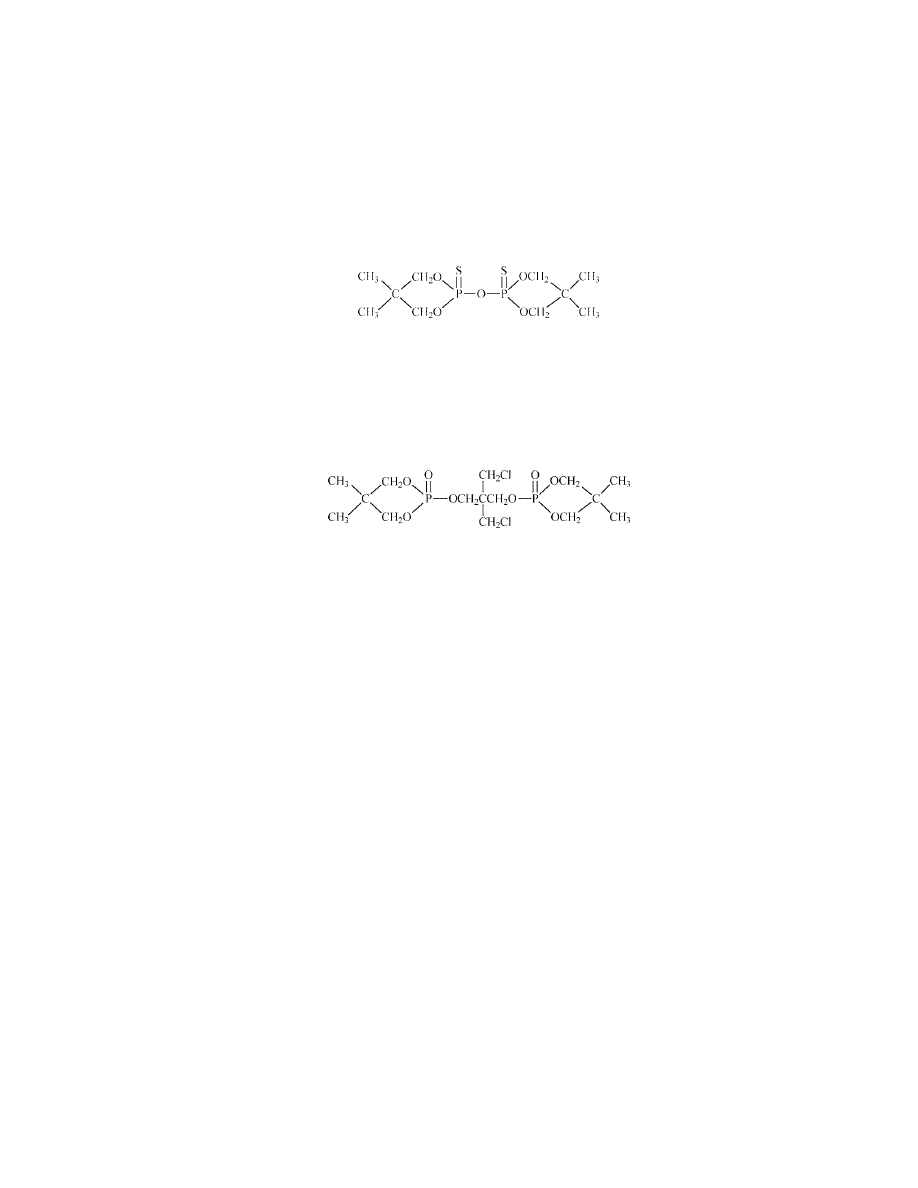
36
FLAME RETARDANCY
Vol. 10
Cyclic Neopentyl Thiophosphoric Anhydride.
This solid additive, SAND-
OFLAM 5060, was commercialized in Europe by Sandoz (sold now by Clariant
as EXOLIT 5060) for use in viscose rayon (regenerated cellulose) (90,91). It is
sold as an aqueous dispersion or wet filter cake, and has the following structure
[4090-50-1]:
Despite the anhydride structure, it is remarkably stable, surviving addition
to the highly alkaline viscose, the acidic coagulating bath, and also resisting mul-
tiple laundering of the rayon fabric. Typical levels of addition are 12–22%.
2,2-Bis(chloromethyl)propanediol Bis(cyclic neopentyl phosphate).
This
solid additive, originally commercialized by Sandoz (90,91), has been sold by Clari-
ant as EXOLIT 5085 (VP). It has the following structure:
The neopentyl structure increases thermal stability, so that this compound is
useful as a flame-retardant additive in extruded polymethyl methacrylate sheet.
Aryl Phosphates.
Aryl phosphates were introduced into commercial use
early in the twentieth century as plasticizers (qv) for flammable plastics such as
cellulose nitrate and later for cellulose acetate and PVC (92). Principal applica-
tions are in wire and cable insulation, connectors, automotive interiors, vinyl mois-
ture barriers, plastic greenhouses (Japan), furniture upholstery, conveyer belts
(especially in mining), and vinyl foams (93).
In vinyls, the aryl phosphates are frequently used in combinations with ph-
thalate plasticizers. The proportion of the more expensive phosphate is usually
chosen so as to permit the product to reliably pass the flammability specifications.
In plasticized vinyls used in automotive interiors, these phosphates are used to
pass the Federal Motor Vehicle Safety Standard 302.
Triaryl phosphates are also used on a large scale as flame-retardant hy-
draulic fluids, lubricants, and lubricant additives. Smaller amounts are used as
nonflammable dispersing media for peroxide catalysts.
Triaryl phosphates are produced from the corresponding phenols (usually
mixtures) by reaction with phosphorus oxychloride, usually in the presence of
a catalyst (94,95). Tricresyl phosphate was originally made from petroleum-
derived or coal-tar-derived cresylic acids (cresols). Discovery of the neurotoxicity
of the ortho-cresyl isomers led manufacturers to select cresols having very little
ortho-isomer.
In the 1960–1980 period, the use of more economical synthetic isopropyl- and
t-butylphenols as alternatives to cresols was developed (96,97). Commercial tri-
aryl phosphates such as FMC’s (now Great Lakes’) KRONITEX 100 and Akzo No-
bel’s PHOSFLEX 31P and 41B are based on partially isopropylated or t-butylated

Vol. 10
FLAME RETARDANCY
37
phenol. The relative volatilities and oxidative stabilities of these phosphates have
been compared; the t-butylphenyl phosphates are the most oxidatively stable of
the alkylphenyl phosphates (98).
Triphenyl Phosphate [115-86-6].
This is a colorless solid, mp 48–49
◦
C,
usually produced in the form of flakes or shipped in heated vessels as a liquid.
It is available from Akzo Nobel as PHOSFLEX TPP. An early application was
as a flame retardant for cellulose acetate safety film. It is also used in cellulose
nitrate, various coatings, triacetate film and sheet, and rigid urethane foam. It
has been used as a flame-retardant additive for engineering thermoplastics such
as polyphenylene oxide–high impact polystyrene and ABS–polycarbonate blends.
Cresyl Diphenyl Phosphate [26444-49-5].
This is the most efficient plas-
ticizer of the liquid phosphates, but it is relatively volatile. It is used, especially
in Europe, in vinyls and in ABS–polycarbonate blends.
Tricresyl Phosphate [1330-78-5] and Trixylyl Phosphate [25155-23-1].
The commercial tricresyl phosphate has been for many years a mainly m,p-isomer
mixture to avoid the neurotoxicity caused by the o-cresyl isomer. Typical products
of this class are Akzo Nobel’s LINDOL [1330-78-5], C
21
H
21
O
4
P, or FMC’s (now
Great Lakes’) KRONITEX TCP [68952-35-2]. These are nearly colorless liquids,
bp about 260–275
◦
C at 1.3 kPa (10 mm Hg). Tricresyl phosphate is used as a
relatively flame-retardant plasticizer in flexible and semi-rigid PVC, cellulose ni-
trate, ethylcellulose coatings, and various rubbers. Typical applications are vinyl
tarpaulins, mine conveyer belts, air ducts, wire and cable insulation, and vinyl
films. Trixylenyl phosphate is a related product of lower volatility and less ex-
tractability, having advantages for wire and cable insulation.
Isopropylphenyl Diphenyl Phosphate [28108-99-8], [68937-41-7], [68782-
95-6].
The plasticizer efficiency is close to that of tricresyl phosphate. It is made
from the product of isopropylation of phenol by propylene. The phosphate is a
mixture of mainly o- and p-isomers and contains a distribution of different levels
of alkylation (99–101). It is sold by Akzo Nobel in the PHOSFLEX series and by
Great Lakes in the REOFOS series. Main uses are in PVC and as a component
of nonscorching or non-windshield-fogging formulations for flexible polyurethane
foams (97a). There are also functional fluid applications outside the scope of this
article.
tert-Butylphenyl Diphenyl Phosphate [56803-37-3], [68937-40-6].
This
is a slightly less efficient plasticizer than the isopropyl homolog although in
admixture with dialkyl phthalates the difference can be minimal and it can be
used in such formulations as a PVC plasticizer with reduced flammability. It has
been used as a flame retardant in engineering thermoplastics (102) and as a fire-
retardant hydraulic fluid. The products from different manufacturers (Akzo, Great
Lakes) are somewhat different in isomer distribution and alkylation levels.
Alkyl Diphenyl Phosphates.
These are products originally developed to
provide improved low temperature flexibility, a fault of triaryl phosphate plasticiz-
ers in PVC (103). These phosphates generally provide slightly less flame-retardant
efficacy but are generally superior to the triaryl phosphates in regard to smoke
when the vinyl formulation is burned. Two commercial products of this family are
2-ethylhexyl diphenyl phosphate [1241-94-7], C
20
H
27
O
4
P, Ferro’s SANTICIZER
141 or Akzo Nobel’s PHOSFLEX 362, and isodecyl diphenyl phosphate [29761-
21-5], C
22
H
31
O
4
P, Ferro’s SANTICIZER 148 or Akzo Nobel’s PHOSFLEX 390. A

38
FLAME RETARDANCY
Vol. 10
newer member of this group was introduced by Monsanto; it is believed to be a
slightly higher alkyl chain length and has improved low temperature properties
with reduced volatility (104).
Aromatic Diphosphates and Oligomeric Phosphates.
Tetraphenyl Resorcinol Diphosphate [57583-54-7].
This is the main com-
ponent of an oligomeric phosphate flame retardant, Akzo Nobel’s FYROLFLEX
RDP or Great Lakes Chemical’s REOFOS RDP, designed for use in engineer-
ing thermoplastics such as polyphenylene oxide blends (105,106), thermoplastic
polyesters, polyamides, polycarbonates, and ABS–polycarbonate blends. A major
use was in PPO–HIPS blends and later in ABS–polycarbonate blends (107). It is
a colorless to light-yellow liquid, viscosity 400–800 mPa
· s at 25
◦
C, and a pour
point of
−12
◦
C. It is less volatile than the triaryl phosphates and has a higher
percentage of phosphorus (11% P) than triphenyl phosphate.
A closely related diphosphate with 2,6-dimethylphenyl groups is available
from Daihachi (Japan), and has improved hydrolytic resistance but is higher in
price.
Tetraphenyl Bisphenol-A Diphosphate [5945-33-5].
This is the main com-
ponent of an oligomer, Akzo Nobel’s FYROLFLEX BDP, Great Lakes’ REOFOS
BAPP, or Albemarle’s NCendX P30, and is used for the same applications as the
RDP discussed above. RDP and BDP are mainly the diphosphates with small
amounts of the higher oligomers. Although BDP has a lower percentage of phos-
phorus (9%) than RDP (11%), it is lower in cost, and has a substantial hydrolytic
stability advantage (108), a factor of particular importance for the application in
polycarbonate–ABS blends.
Substantial development work has been done on other tetraaryl diphos-
phates and higher oligomers thereof, such as with aliphatic linking groups (109).
Reactive Organic Phosphorus Compounds.
Organophosphorus Monomers.
Many vinyl monomers containing phos-
phorus have been described in the literature (110,111), but few have gone beyond
the laboratory. Methacryloxyethyl acid phosphates are commercially available
but appear to be used in coatings as adhesion improvers, not as flame retardants.
Bis(2-chloroethyl) vinylphosphonate [115-98-0] and the corresponding acid are
available from Rhodia but do not seem to have commercial use as flame retar-
dants probably due to cost. These and related monomers have been reviewed and
their copolymerization behavior has been studied (110,111).
Phosphorus-Containing Diols and Polyols.
The commercial develop-
ment of several phosphorus-containing diols occurred in response to the need to
flame retard rigid urethane foam insulation used in transportation and construc-
tion. There are a large number of references to phosphorus polyols (112) but only
a few of these have been used commercially.
Albright & Wilson’s (now Albemarle’s) VIRCOL 82 is a mixed diol mixture
containing bis(hydroxypropyl) dibutyl propanediol diphosphates (113). The neu-
tral liquid has an OH number of 205 mg KOH/g and contains 11.3% phosphorus.
It is used in rigid foam.
Diethyl N,N-bis(2-hydroxyethyl)aminomethylphosphonate [2781-11-5].
Over several decades, commercial usage (limited by cost) has been made of this
phosphonate diol (Akzo Nobel’s FYROL 6, Bayer’s LEVAGARD 4090N) (114),
(HOCH
2
CH
2
)
2
NCH
2
P(O)(OC
2
H
5
)
2
.

Vol. 10
FLAME RETARDANCY
39
The product contains 12.6% phosphorus and has an OH number in the 450 mg
KOH/g range. FYROL 6 is used to impart a permanent Class II E-84 flame-spread
rating to rigid foam for insulating walls and roofs. Particular advantages are
low viscosity, stability in polyol-catalyst mixtures, and outstanding humid aging
resistance. FYROL 6 is used in spray foam, froth, pour-in-place, and slab stock.
Its mode of action is believed to be increased char formation (115).
Oligomeric Ethyl Ethylene Phosphate Diol.
This halogen-free phosphorus-
containing diol was developed by Hoechst for rigid or flexible foams and is now mar-
keted as EXOLIT 550 by Clariant (116). The product is a hydroxyethyl-terminated
ethyl ethyleneglycol phosphate oligomer with about 17% phosphorus content and
is said to be useful for rigid and flexible foams, especially molded or high density
slabstock.
Oligomeric Phosphate—Phosphonate.
A commercially used reactive
oligomeric alcohol, Akzo Nobel’s FYROL 51 [70715-06-9], is a mixture of
methylphosphonate units and methyl phosphate units with mainly hydroxy end
groups. A typical component has the structure as follows (110):
FYROL 51 is a water-soluble liquid containing about 21% phosphorus. The
end groups are principally primary hydroxyl and the compound can thus be incor-
porated chemically into aminoplasts, phenolic resins, and polyurethanes. FYROL
51, or 58 (diluted) is used with amino resins to produce a flame-retardant resin
finish on paper used for automotive air filters, or for backcoating of upholstery
fabric to pass the British or California flammability standards. Under the trade
name FYROLTEX HP (Akzo Nobel), it has been in development for cotton textile
finishing, in combination with an amino resin (117).
Combinations with FYROL 6 permit the OH number to be adjusted to typical
values used in flexible foam, urethane coating, or reaction injection molding (RIM)
applications (118,119).
Reactive Organophosphorus Compounds in Textile Finishing.
The
flame retarding of cotton requires the application of a textile finish. Markets for
finishes have been in military goods, industrial protective clothing, curtains, hos-
pital goods, and children’s sleepwear. An extensive review is available (120).
Tetrakis(hydroxymethyl)phosphonium Salts.
The reaction of formalde-
hyde and phosphine in aqueous hydrochloric or sulfuric acid yields tetrakis-
(hydroxymethyl)phosphonium chloride [124-62-1 or the sulfate [55566-30-8].
These are made by Rhodia, Cytex, and Chinese producers. The salts have the
general structure (HOCH
2
)
4
P
+
X
−
. They are water-soluble crystalline compounds
sold as concentrated aqueous solutions. The methylol groups are highly reactive
(121–125) and capable of being cured on the fabric by reaction with ammonia or
amino compounds and post-oxidized to form durable cross-linked phosphine oxide
structures.
Finishes based on these reagents are durable to numerous launderings. This
finish is primarily used on work clothing. This finishing process, developed by
Albright & Wilson (now marketed by Rhodia), is known as the PROBAN process,
which employs a precondensate of the phosphonium compound with urea (125,
126).
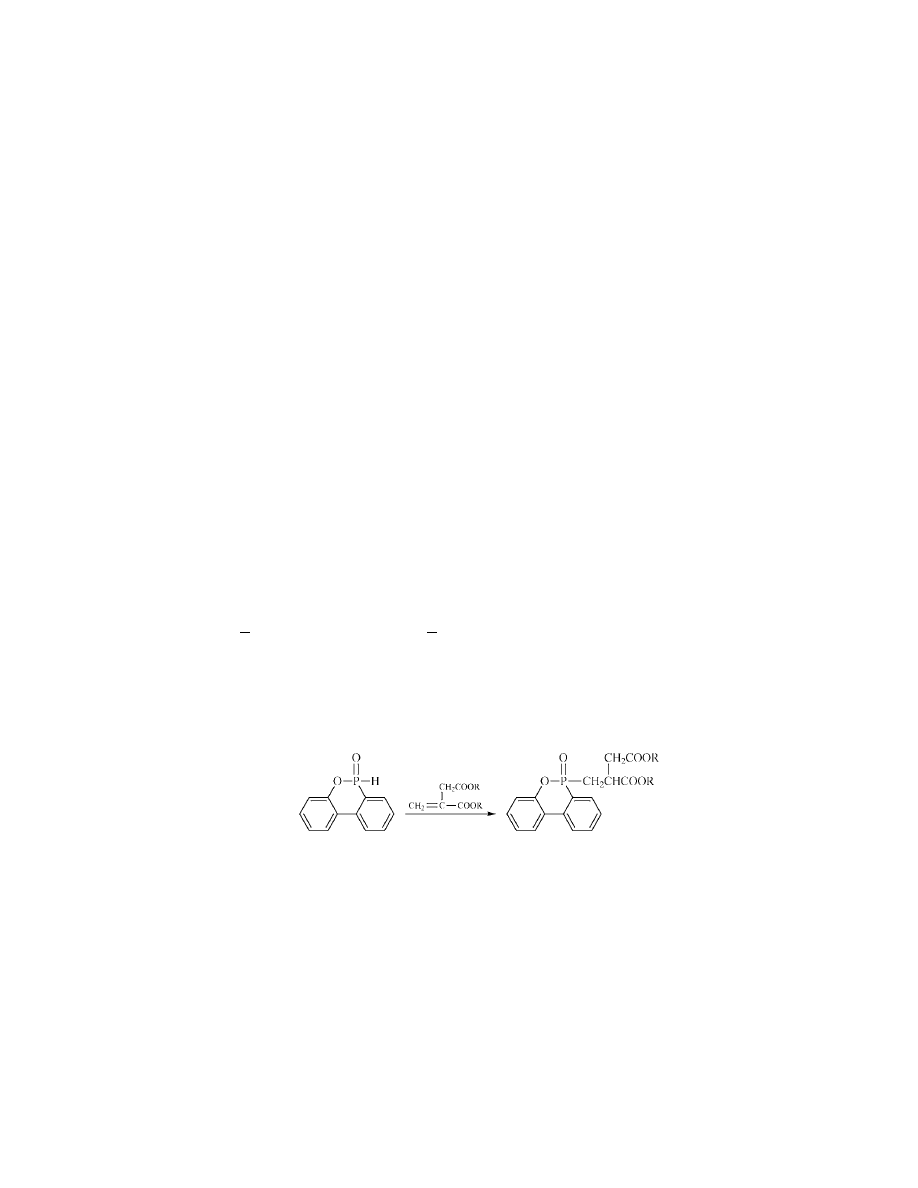
40
FLAME RETARDANCY
Vol. 10
Dimethyl N-Methylolphosphonopropionamide or 3-([Hydroxymethyl]-
Amino)-3-oxopropyl)Phosphonic Acid, Dimethyl Ester.
This cotton finish,
Ciba’s PYROVATEX CP, was introduced by Geigy (later Ciba) in the 1970s
especially for children’s sleepwear and upholstery. It has the structure
(CH
3
O)
2
P(O)CH
2
CH
2
C(O)NHCH
2
OH with some related compounds (127). The
methylol group is the reactive part of the molecule. PYROVATEX CP coreacts on
cellulose with an amino resin such as a methylolmelamine in the presence of a
phosphoric acid catalyst or latent acid catalyst, to produce finishes durable to as
many as 50 washes (128,129). A higher assay version, PYROVATEX CP New, has
been introduced. An apparently similar product, PEKOFLAM DPN, is available
from Clariant. This finish is practiced in Europe and the Far East but very lit-
tle in North America. Recent efforts have been made to lower the formaldehyde
emissions from this system (130).
Phosphorus-Containing Polymers as Textile Fibers.
A large number
of addition and condensation polymers having phosphorus built in have been de-
scribed, but few have been commercialized (131). No general statement seems
warranted regarding the efficacy of built-in vs additive phosphorus (132). How-
ever, in textile fibers, there is greater assurance of permanency.
Polyester Fibers Containing Phosphorus.
Numerous patents describe
poly(ethylene terephthalate) (PET) flame retarded with phosphorus-containing
difunctional reactants. At least two of these appear to be commercial (see
P
OLYESTERS
, F
IBERS
).
Hoechst-Celanese’s TREVIRA 271 (now practiced by KoSa) appears to be
based on a cyclic phosphinic carboxylic anhydride [15171-48-9] which is re-
acted with glycol and then introduced into the PET backbone to produce a
flame-retardant polyester [82690-14-0], having phosphinate units of the struc-
ture
OP(O)(CH
3
)CH
2
CH
2
COO
(133,134). FR TREVIRA 271 is used for
children’s sleepwear, work clothing, and home furnishings. A phosphorus content
as low as 0.6% is reported to be sufficient to pass drapery and upholstery tests.
Toyobo’s HEIM II (former GH) is apparently based on the following chemistry
via a phosphinate intermediate dihydrooxaphosphaphenanthrene oxide (DOPO)
[63562-31-2] produced from o-phenylphenol and phosphorus trichloride (135):
This dicarboxylic ester is then copolycondensed with the other reactants
in PET manufacture to produce a flame-retardant polyester [63745-01-7]. The
advantage of this rather unusual phosphinate structure is its high thermal and
hydrolytic stability. The fabric is probably used mainly for furnishings in public
buildings in Japan.
Phosphorus-Containing Printed Wiring Boards.
This same phos-
phinate intermediate, dihydrooxaphosphaphenanthrene oxide (DOPO), is also
the subject of substantial efforts in the Far East and Europe to react it into
epoxy printed wiring boards and encapsulated electronic circuit elements as a

Vol. 10
FLAME RETARDANCY
41
hypothetically “green” alternative to the established use of tetrabromobisphenol
A (136). A number of other approaches to use of reacted-in phosphorus structures
have been proposed and are in field trials at present (136).
Sulfur-Containing Flame Retardants
Ammonium Sulfate [7723-20-2].
This is a very inexpensive water-
soluble salt used as a paper or wood flame retardant. In combination with ammo-
nium phosphates, it is used in fighting forest fires from the air. In cellulosics, its
acidity can cause discoloration and strength loss, so it is usually used in disposable
items such as temporary decorations.
Ammonium Sulfamate [7773-06-0].
This salt is similar to ammonium
sulfate but somewhat less acidic and corrosive. It is applied as an aqueous solu-
tion to paper or cellulosic fabric to obtain a flame retardant finish nondurable to
washing. It has fairly good afterglow retardation.
Potassium Perfluorobutylsulfonate [29420-49-3] (3M’s FR-2025),
Potassium Diphenylsulfonesulfonate [63336-43-8] (Sloss Industries and
Seal Sands, Ltd.), and Sodium Trichlorophenylsulfonate [53423-65-7]
(Sloss Industries).
These substantially neutral salts are used at levels below
1% as flame retardants in polycarbonate, often in combination with other retar-
dants. These sulfonates permit clear formulations. At levels as low as 0.2%, the
sulfonates accelerate carbon dioxide evolution and the breakdown of the polymer
to phenolic fragments, thence to char (137).
Nitrogen-Based Flame Retardants
Melamine [108-78-1].
This inexpensive solid melts with vaporization at
about 350
◦
C and, if evaporation is constrained, decomposition also occurs to am-
monia and nonvolatile condensation products—melam, melem, and eventually
melone. It is occasionally used by itself as a flame retardant but more often used
as a blowing agent and flame-retardant adjuvant in intumescent coatings, elas-
tomers, and plastic formulations (138) as well as in flexible polyurethane foams
(139,140).
Melamine Cyanurate [37640-57-6].
This 1:1 adduct (salt) of melamine
and cyanuric acid, decomposition temperature
∼413
◦
C, is a leading flame retar-
dant for unfilled polyamide 6, where it is typically used at 5–10% by weight, but
it does not work very well in most glass-filled polyamide 6. It is also less efficient
in polyamide 6,6. It is available from Ciba and several other producers. The mode
of action has been studied (141–143).
Hindered Amine Ethers.
This unusual new family of flame retardants
(or synergists) was discovered by Ciba, which had been exploring the light sta-
bilizer family of 2,2,6,6-tetramethylpiperidine derivatives (see UV S
TABILIZERS
).
The N-alkoxy or cycloalkoxy derivatives appear to have flame-retardant action
particularly in polyolefins. One member of this group, where the N-cyclohexyloxy-
2,2,6,6-tetramethylpiperidinyl group is attached to an oligomeric polymer chain,
has been in market development as Ciba’s NOR 116 (144).

42
FLAME RETARDANCY
Vol. 10
Health, Safety and Environmental Aspects
Several factors should be considered: the toxicity of the compounds themselves, the
effect of the flame retardants on combustion product toxicity and visible smoke,
and environmental effects.
Broad Studies of Flame Retardants.
Several broad studies of health,
safety, and environmental factors of flame retardants have been published by pub-
lic agencies. A critical review by a US government–appointed toxicology panel was
conducted to facilitate CPSC regulations on flammability of furniture upholstery
(145). The panel found ammonium polyphosphate, alumina trihydrate, zinc bo-
rate, hexabromocyclododecane, decabromodiphenyl ether (oxide), PYROVATEX
CP, and THPC to be usable with minimum risk on residential furniture even
with worst-case assumptions. Antimony trioxide, several organophosphates, chlo-
rinated paraffins, and molybdate salts were said to need more exposure studies.
A study at the University of Surrey sponsored by the UK Department of
Trade and Industry weighed health risks (toxicity and exposure) of a wide range
of flame retardants vs their benefits (146). This study encompassed alumina trihy-
drate, antimony trioxide, decabromodiphenyl ether, tetrabromobisphenol A, and
tris(chloroisopropyl) phosphate, and in general found that the benefits outweighed
the risks.
An overview of the health, exposure, and environmental aspects of flame re-
tardants was issued in 1997 by the International Programme on Chemical Safety
of the World Health Organization, International Labor Organization, and UN
Environment Programme (147).
Toxicity, exposure, and environmental data on many of the commercial flame
retardants can be found conveniently in an Australian database (148), which is
supplemented as new products are imported. Ongoing assessments of flame re-
tardants in use, particularly the brominated ones, and possible alternatives are
published by the Danish Environmental Department (149).
Comparative fire tests with a variety of flame-retarded items such as might
be found in a typical room vs the same set of items without flame retardants have
been run under the auspices of the National Institute of Standards and Technology.
These tests showed that the room with the flame-retardant objects afforded 15-
fold better escape time, lower material consumption, less heat, and less carbon
monoxide (150).
A number of risk assessments are being done on flame retardants under EU
auspices, and reasonably current status reports are available (151,152). Prioriti-
zation factors for the assessments, including tonnage, recognized aquatic hazards,
bioamplification and persistence have been summarized as of 2004 (153).
Broad studies of the emissions of brominated and phosphorus flame retar-
dants from consumer products and building materials show, as expected, that the
more volatile ones such as tris(1-chloro-2-propyl) phosphate, triphenyl phosphate,
and the brominated additives up to 6 bromine atoms are detectible in indoor air
(154,155).
Organohalogens.
Much attention has been directed to organohalogen
products, in view of the legacy of environmental concern over such materials as
DDT insecticide, PCB transformer fluid, and PBB (a flame retardant which in one
incident contaminated cattle feed).

Vol. 10
FLAME RETARDANCY
43
Some halogenated flame retardants, such as the polychlorinated polycyclic
hydrocarbon DECHLORANE PLUS, have escaped adverse reports. Chloroparaf-
fins have been found to be of toxicological concern at the low molecular weight end
of the series (IARC 2B rating: possible carcinogen) but above C13, the chloroparaf-
fins are rated as noncarcinogenic (156,157). Likewise, the aquatic toxicity favors
the long-chain chloroparaffins.
Many workshops and conferences on the bromine compounds have been held
under auspices of government/nongovernmental organizations and industry (ex-
amples: References 158–160). Concern has lately focused on the lower brominated
diphenyl ethers (such as penta-) which are detected widely in the environment.
In a review, Dutch researchers conclude that the toxicity mechanism of poly-
brominated diphenyl ethers is the same as that of the dioxins, and, although
the present concentrations are not a large risk, these ethers are accumulative.
Decabromodiphenyl oxide is less toxic than the less-brominated congeners, and
its absorption from the gastrointestinal tract is low.
At the present time, pentabromodiphenyl ether and octabromodiphenyl ether
have been judged by authorities in California and the European Union to have
enough environmental presence and health risk to require a ban (161). Decabro-
modiphenyl ether, which is of large commercial importance as a flame retardant,
seems much less of an environmental contamination problem, and its toxicology
per se elicits less concern. However, its photolytic and biological debromination to
lower brominated diphenyl ethers (162,163) is a factor which is being weighed by
the EU and California authorities. A final decision as to its regulatory status is
not available at the time of writing.
Risk analyses are underway (2004) in the European Union on hexabromocy-
clododecane.
Militating against a ban to include all of the polybrominated additives is
the evidence that the high thermal and hydrolytic stability of decabromodiphenyl
ether and related polyhalogenated flame retardants makes it feasible to recy-
cle the flame-retarded plastics, which is more difficult with the halogen-free
flame retardants (164). An overview, from an industry standpoint, of the ef-
fects of regulations on the bromine-containing flame retardants is available
as of early 2004 (165). Recyclability of these plastics appears to provide an
advantage.
Antimony Oxide.
Antimony oxide is used very often as a synergist with
halogenated flame retardants. There are some toxicological concerns about anti-
mony oxide because of some equivocal carcinogenicity data, but, on balance, the
risk is deemed acceptable (particularly since it is extremely water-insoluble and
poorly bioavailable). It is still used in large amounts (166,167). A World Health
Organization toxicology critique and drinking water guideline is available (2003)
as a draft (168).
Phosphorus Flame Retardants.
The original version of tricresyl phos-
phate was found in the 1930–1940 period to cause paralysis on ingestion as a
food or liquor adulterant. However, as a result of basic studies, it was shown
that the neurotoxicity characteristic was related to the o-cresyl isomer and the
manufacturers thereafter have avoided using o-cresol to make triaryl phosphates
(169). The newer isopropylphenyl and t-butylphenyl phosphates have little or no
neurotoxicity (170).

44
FLAME RETARDANCY
Vol. 10
The structure–toxicity relationships of organophosphorus compounds have
been extensively researched and are relatively well understood (169–174).
A critical review of the toxicity of the haloalkyl phosphates and the poten-
tial metabolic products is available (175). The flame retardant “tris” (tris-2,3-
dibromopropyl) phosphate was found to be mutagenic in laboratory tests; its pro-
duction and use were discontinued in the 1970s. Tris(2-chloroethyl) phosphate and
tris(dichloroisopropyl) phosphate have voluntary “R40” labels (limited evidence of
carcinogenicity in animal tests). Tris(chloroisopropyl) phosphate does not require
special classification. The chloroalkyl phosphates are undergoing (as of 2004) an
EU risk assessment. They have been detected at various locales in municipal
waste water (176), indoor dust, or indoor air (177,178).
Analytical data indicates the emission of trace vapors of triphenyl phosphate,
which is controversially claimed to have some allergenic properties, from warm
HIPS TV cabinetry (179). However, this emission results in air concentrations
far below those which might be considered hazardous (180). The higher molec-
ular weight diphosphates and oligomers appear to avoid toxicological problems
(181).
Effect of Flame Retardants on Combustion Products.
There appears
to be no documented case of any type of fire retardant contributing to human fire
casualties. There is ample evidence that carbon monoxide is the main acute tox-
icant in flames, although oxygen depletion and other toxic gases may contribute.
This topic has been studied intensively (182–185).
It appears that in a large fire (which cause most of the casualties), the car-
bon monoxide yield and the overall smoke toxicity are almost independent of the
material burning; this belief has had a dampening effect on combustion toxicology
studies (186–188).
Although hydrogen halides (from halogen-containing plastics) are toxic, it
is difficult to find cases of fire deaths attributable to them. Using quantitative
assessment methods for the fire gases, the Federal Aviation Authority (189,190)
model does not ascribe a heavy contribution by HCl and HBr compared to CO and
HCN.
The concern about halogens in combustion often focuses on the formation, en-
vironmental contamination, and chronic (not acute) toxicity of halogenated diben-
zodioxin (“dioxin”) or halogenated dibenzofurans, particularly during disposal of
halogen-containing plastics by incineration. Much study has been done on the
dioxins by analytical chemists, toxicologists, and epidemiologists, a copious lit-
erature including books has been produced (191,192), and major international
conferences are held annually (159) (A thorough overview of the chemistry, bio-
chemistry, toxicology, environmental occurrence, and risk aspects can be found in
Reference 193.). Some but not all authorities consider the dioxins to be carcino-
genic; they may be cocarcinogens. The linkage of human health problems to incin-
eration products is tenuous and the link to dioxins even more so. It has been shown
that dioxins are produced by all chlorine- or bromine-containing fuels, and pre-
date the era of plastics. EPA data shows that present-day government-approved
incinerator–scrubber installations (waste-to-energy facilities) have reduced dioxin
emissions to very low levels (194,195). Certain metal smelters such as Boliden in
Sweden have also been found to handle halogen-containing waste with acceptably
minimal dioxin emissions (165).

Vol. 10
FLAME RETARDANCY
45
A survey of data from small-scale combustion or pyrolysis experiments re-
vealed no consistent pattern of decrease or increase in the yields of toxic gases
(CO, HCN) when phosphorus flame retardants were present (196). Experimental
conditions (such as burning temperature) seem to dominate over the composition
of the material being burned (197).
Effects on Visible Smoke.
Smoke is a main impediment to egress from
a burning building. In general, the halogen–antimony systems tend to increase
the smoke yield relative to the mass burned, but when successful as retardants,
they may reduce the total smoke by lessening the mass burned (198,199).
A comparative study of fire performance of halogenated and nonhalogenated
cables discusses the smoke performance (200).
Chlorine–antimony systems or chlorine–bromine–antimony systems usually
tend to be less smoky than bromine–antimony (201), and it is also possible to lower
smoke in some plastics by use of further additives such as zinc borate, molybdates,
or iron oxides. In general, the use of ATH or Mg(OH)
2
as part of the flame-retardant
system results in lower smoke with most polymers (202,203).
Although some examples are known where specific phosphorus flame retar-
dants increased visible smoke in small-scale tests, other instances are reported
where the presence of the retardant reduced smoke. The effect appears to be a
complex function of burning conditions and of other ingredients in the formu-
lation (204,205). In one careful study, ammonium phosphate was found to raise
or lower visible smoke from wood depending on the pyrolysis temperature (206).
Where the phosphorus flame retardant functions by char enhancement, lower
smoke levels are likely to be observed.
Environmental Considerations.
The halogenated flame retardants dif-
fer greatly in environmental contamination. As mentioned already, pentabro-
modiphenyl ether has become widely dispersed, but decabromodiphenyl ether
much less so due to its negligible solubility and vapor pressure. Antimony tri-
oxide also is nonmigratory because of its very low solubility and negligible vapor
pressure but nonetheless is under close scrutiny from the regulatory authorities
(166,207).
The newer families of polymeric or oligomeric or reacted-in bromine, as well
as very high molecular weight chlorine- or bromine-containing flame retardants
are well designed to avoid migration and to be innocuous environmentally, since
they have essentially no water solubility or vapor pressure.
The phosphate flame retardants have come under close environmental
scrutiny. Results published to date on acute toxicity to aquatic algae, inverte-
brates, and fish indicate substantial differences between the various aryl phos-
phates (208–212). It has been found that the higher molecular weight phosphorus
compounds are much better in terms of toxicity to aquatic organisms (213,214).
Tests in pure water, river water, and activated sludge showed that commer-
cial triaryl phosphates and alkyl diphenyl phosphates undergo reasonably facile
degradation by hydrolysis and biodegradation (215,216), the chloroalkyl phos-
phates less so.
Several risk–benefit analyses have favored the use of the flame retardants,
since fire and smoke cause severe environmental injury besides the direct losses of
life and property. For example, the polycyclic aromatic hydrocarbons which occur
in copious amounts in soot have been indicated to be orders of magnitude more

46
FLAME RETARDANCY
Vol. 10
serious than the dioxins, as was revealed in life-cycle assessments of TV sets
(217) and furniture (218) with and without flame retardants. The fact that fire is,
itself, a severe environmental pollutant, in the absence of a flame retardant, is
reemphasized (219).
Nevertheless, environmental pressures continue to impact the flame retar-
dant field, in some cases reinforced by “green” marketing strategies, “eco labels”
and misleading generalizations about entire families of chemicals. These pres-
sures have stimulated serious effort, some already successful, to find flame re-
tardants less subject to criticism. Current research and development efforts are
in the direction of halogen-free phosphorus compounds, inorganics, high molec-
ular weight halogen compounds, polymeric additives, reactives, flame-retardant
polymer modifications and blends.
BIBLIOGRAPHY
“Fire Retardancy” in EPST 1st ed., Vol. 7, pp. 1–64, by Raymond B. Hindersinn and George
Wagner; Suppl. Vol. 2, pp. 270–339, by Raymond Hindersinn, Hooker Research Corp.;
“Flammability” in EPSE 2nd ed., Vol. 7, pp. 154–210, by Richard G. Gann and Richard
A. Dipert, U.S. National Bureau of Standards, and Michael J. Drews, Clemson University.
1. A. H. Tullo, Chem. Eng. News 43 (Nov. 7, 2003).
2. F. Gastrock (of BRG Townsend), personal communication, Feb. 2004.
3. W. E. Horn Jr., in A. F. Grand and C. A. Wilkie, eds., Fire Retardancy of Polymeric
Materials, Marcel Dekker, Inc., New York, 2000, pp. 285–352.
4. T. J. Lynch, T. Chen, and D. Riley, Reinf. Plast. 44–46 (Sept. 2003).
5. T. Chen, in 52nd International Wire and Cable Symposium/Focus, Nov. 16–20, 2003.
6. R. Sauerwein, in Flame Retardants ’04, Conference Proceedings, Jan. 2004, Inter-
science Communications, London, 2004.
7. R. Rothon and B. Ritchie, Flame Retardants 2000, Proceedings of the 9th Conference,
Interscience Communications, London, 2000, pp. 113–120.
8. J. Innes, A. W. Cox, and M. W. Walter, Proc. Intl. Conf. Fire Sci. 25, 303–314.
9. H. C. Ashton, T. Chen, and T. J. Lynch, in Polyolefins 2003, SPE, Houston, 2003,
pp. 395–406.
10. E. D. Weil, M. Lewin, and H.-S. Lin, J. Fire Sci. 16, 383–404 (1998).
11. L. T. Arthur and K. Quill, in Plastics & Rubber Institute, eds., Flame Retardants ’92,
Elsevier Science, London, 1992, pp. 223–237.
12. K. Shen, in Recent Adv. Flame Retard. Polym. Mater. 11, 213–221 (2000).
13. K. Shen and R. M. Leeuwendahl, in 14th Annual BCC Conference on Flame Retar-
dancy, Stamford, Conn., June 2–4, 2003.
14. S. Bourbigot, R. Leeuwendal, K. Shen, and D. Schubert, Polym. Degrad. Stabil. 64,
419–425 (1999).
15. L. L. Musselman, F. W. Moore, and W. J. Kennelly, “Flame-Retarding and Smoke-
Suppressing Molybdates and Borates,” in J. Edenbaum, ed., Plastics Additives and
Modifiers Handbook, Kluwer Academic Publishers, Dordrecht, the Netherlands, 1992.
16. D. Chaplin and S. C. Brown, Flame Retardants ’90, British Plastics Federation, Else-
vier Applied Science, London, 1990, pp. 114–125.
17. P. Cusack, Flame Retardants ’90, British Plastics Federation, Elsevier Applied Sci-
ence, London, 1990, pp. 176–190.
18. E. D. Weil, M. Lewin, and V. Barinov, in 13th Annual BCC Conference on Flame
Retardancy, Stamford, Conn., June 3–5, 2002.

Vol. 10
FLAME RETARDANCY
47
19. S. A. Hasselbrack, in Improved Fire- and Smoke-Resistant Materials for Commercial
Aircraft Interiors, National Materials Advisory Board, National Academies Press,
Washington, 1995, pp. 165–174.
20. J. Markarian, Plast. Add. Compdg. Nov.–Dec. 2003, 32–36.
21. T. Kashiwagi, J. R. Shields, R. H. Harris Jr., and W. H. Awad, in 14th Annual BCC Con-
ference on Recent Advances in Flame Retardancy of Polymeric Materials, Stamford,
Conn., June 2–4, 2003.
22. G. Beyer, Plast. Add. Compdg. 4(10), 22–28 (2002).
23. J. M. Cogen, T. S. Lin, A. B. Morgan, and J. M. Garc´es, in Proceedings of the 52nd
International Wire and Cable Symposium, Nov. 17–20, 2003.
24. U.S. Pat. 5,849,827 (1998), M. B¨odiger, T. Eckel, D. Wittmann, and H. Alberts (to
Bayer).
25. E. Larsen, in Kirk–Othmer Encyclopedia of Chemical Technology, 2nd ed., Vol. 10,
John Wiley & Sons, Inc., New York, pp. 373–379.
26. E. D. Weil and M. Lewin, in A. R. Horrocks and D. Price, eds., Fire Retardant Materials,
Woodhead Publishing Ltd., Cambridge, U.K., 2001, pp. 31–68.
27. D. Stein and D. Stevenson, in Polyolefins 2003, SPE International Conference on Poly-
olefins, Houston, Tex., Feb. 23–26, 2003, pp. 601–624.
28. R. Markezich, in Proceedings of 51st International Wire and Cable Symposium 2000,
pp. 413–417.
29. “Brominated Flame Retardants, Substance Flow Analysis and Assessment
of
Alternatives,”
Danish
Environmental
Protection
Agency,
June
1999.
http://www.mst.dk/19908pubs/87-7909-416-3/default.htm.
30. 2nd International Workshop on Brominated Flame Retardants, May 14–18, 2001,
sponsored by Swedish Ministry of the Environment, Swedish Academy of Science,
and World Health Organization.
31. E. R. Larsen and E. L. Ecker, J. Fire Sci. 4, 261–276 (1986); J. Fire Sci. 6(2), 139–159
(1988).
32. B. M. Valange, S. E. Calewarts, G. A. Bonner, F. A. Pettigrew, and R. A. Schliefstein,
in Flame Retardants ’90 [Proc.], Elsevier, London, 1990, pp. 67–77.
33. National Academy of Science, Toxicological Risks of Selected Flame Retardant Chem-
icals, National Academy Press, Washington, D.C., 2000.
34. P. O. Darnerud, Environ. Int. 29, 841–853 (2003).
35. “Bis(pentabromophenyl) Ether,” European Union Risk Assessment Report, EUR
20402, First Priority List, Vol. 17, 2002.
36. P. Georlette, Plast. Add. Compdg. 3(4), 28–33 (2001).
37. F. Heine, R. Braibant, A. Fonze, B. Plaitin, and J. Rivi`ere, in World Compounding
Congress 2000, Dec. 5–7, 2000, Neuss/D ¨
usseldorf, Germany.
38. D. De Schryver, S. D. Landry, and J. S. Reed, Polym. Degrad. Stabil. 64, 471–477
(1999).
39. E. D. Weil, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons,
Inc., New York, 2001. (online)
40. E. N. Peters, J. Appl. Polym. Sci. 24, 1457–1464 (1979).
41. E. Weil, in 11th Annual BCC Conference on Recent Advances in Flame Retardancy of
Polymeric Materials, Stamford, Conn., May 22–24, 2000.
42. M. Lewin, in M. Lewin and S. Sello, eds., Handbook of Fiber Science and Technology:
Chemical Processing of Fibers and Fabrics, Vol. 2, Marcel Dekker, Inc., New York,
1984, Part B, pp. 1–141.
43. R. H. Barker and J. E. Hendrix, in W. C. Kuryla and A. J. Papa, eds., Flame Retardancy
of Polymeric Materials, Vol. 5, Marcel Dekker, Inc., New York, 1979, pp. 1–65.
44. S. LeVan, in R. Rowell, ed., The Chemistry of Solid Wood, American Chemical Society,
Adv. Chem. Ser. 207, Washington, D.C., 1984, pp. 531–574.

48
FLAME RETARDANCY
Vol. 10
45. C. Y. Shen, N. E. Stahlheber, and D. R. Dyroff, J. Am. Chem. Soc. 91, 62–67 (1969).
46. O. Schacker, Plast. Add. Compdg. 4(4), 28–30, 32–33 (2002).
47. H. J. Vandersall, J. Fire Flamm. 2, 97–140 (1971).
48. G. Camino and L. Costa, Rev. Inorg. Chem. 8(1–2), 69–100 (1986).
49. D. Scharf, in Fire Retardant Engineering Polymers & Alloys, Fire Retardant Chemicals
Association, Lancaster, Pa., 1989, pp. 183–202.
50. R. Mount, in International Conference on Fire Safety, Fire Retardant Chemicals As-
sociation, New Orleans, Mar. 25–28, 1990, pp. 253–267.
51. Ger. Pat. Appl. 3,720,094 (Mar. 3, 1988), H. Staendeke and D. Scharf (to Hoechst
Celanese).
52. U.S. Pat. 4,579,894 (Apr. 1, 1986), G. Bertelli and R. Locatelli (to Montedison SpA).
53. U.S. Pat. 4,336,182 (June 22, 1982), G. Landoni, S. Fontani, and O. Cicchetti (to Monte-
dison SpA).
54. G. Montaudo, E. Scamporrino, and D. Vitalini, J. Polym. Sci. 21, 3321–3331,
3361–3371 (1983).
55. U.S. Pat. 4,504,610 (Mar. 12, 1985), R. Fontanelli, G. Landoni, and G. Legnani (to
Montedison SpA).
56. M. Le Bras, G. Camino, S. Bourbigot, and R. Delobel, eds., Fire Retardancy of Polymers:
The Use of Intumescence, Royal Society of Chemistry Special Publication 224, Springer
Verlag, Berlin, 1998.
57. R. Walz, C. Schuetz, and M. Sicken, Kunststoffe Plast. Europe 86(2), pp. 34–36 (1996).
58. E. D. Weil and B. McSwigan, J. Coat. Technol. 66(839), 75–82 (1994).
59. E. Weil and B. McSwigan, Plast. Compdg. 31–39, May–June 1994.
60. L. Costa, G. Camino, and M. P. Luda di Cortemiglia, in G. Nelson, ed., Fire & Poly-
mers, American Chemical Society Symposium Series, No. 425, Washington, D.C., 1990,
pp. 211–238.
61. R. Grabner, in Addcon World 99 (5th), Prague, Czech Republic, Oct. 27–28, 1999,
RAPRA Conference Proceedings 1999.
62. C. L. Goin and M. T. Huggard, in M. Lewin and G. S. Kirshenbaum, eds., Recent Ad-
vances in Flame Retardancy of Polymeric Materials, Vol. II, Business Communication
Co., Inc., Norwalk, Conn., 1992, pp. 94–101.
63. U.S. Pat. 2,430,569 (Nov. 11, 1947) D. C. Hull and A. H. Agett (to Eastman Kodak Co.).
64. U.S. Pat. 2,407,279 (Sept. 10, 1946), D. C. Hull and J. R. Snodgrass (to Eastman Kodak
Co.).
65. U.S. Pat. 3,909,484 (Sept. 30, 1975), A. N. Beavon (to Universal-Rundle Corp.).
66. P. V. Bonsignore and J. H. Manhart, Proc. 29th Annu. Conf. Reinf. Plast. Compos. Inst.
SPE 23C, 1–8 (1974).
67. U.S. Pat. 6,255,371 (2001), E. Schlosser, B. Nass, and W. Wanzke (to Clariant GmbH).
68. U.S. Pat. 6,547,992 (2003), E. Schlosser, B. Nass, and W. Wanzke (to Clariant GmbH).
69. O. Schacker, W. Wanzke, and D. Scharf, in New Century of FR Compounding, Fire
Retardant Chemicals Association Fall Conference, Cleveland, Ohio, Oct. 20–22, 2002,
pp. 101–111.
70. E. D. Weil and S. Levchik, J. Fire Sci. 22, 183–209 (2004).
71. E. D. Weil and S. Levchik, Polym. Int. (in press).
72. U.S. Pat. 3,100,220 (Aug. 6, 1963), A. L. Smith (to Celanese Corp.).
73. J. W. Crook and G. A. Haggis, J. Cell. Plast. 119–122, Mar./Apr. 1969.
74. U.S. Pat. 3,041,293 (June 26, 1962), R. Polacek (to Celanese Corp.).
75. U.S. Pat. 4,477,600 (Oct. 16, 1984), G. Fesman (to Stauffer Chemical Co.).
76. U.S. Pat. 4,794,126 (Dec. 27, 1988), G. Fesman, B. Jacobs, and B. Williams (to Akzo
America Inc.).
77. S. Andrews, in J. Kresta, ed., 60 Years of Polyurethanes, Technomics Publishing Co.,
Lancaster, Pa., 1998.

Vol. 10
FLAME RETARDANCY
49
78. R. L. Gray and R. L. Lee, in G. Pritchard, ed., Plastics Additives, Chapman Hall,
London, pp. 567–575.
79. M. L. Luda, P. Bracco, L. Costa, and S. V. Levchik, Polym. Degrad. Stabil. 82, 215–220
(2004).
80. O. M. Grace and co-workers, in Proceedings of the Polyurethanes World Congress, Sept.
29–Oct. 2, 1987, Aachen, Germany.
81. M. Ravey, I. Keidar, E. D. Weil, and E. M. Pearce, J. Appl. Polym. Sci. 68, 217–228,
231–254 (1998).
82. U.S. Pat. 3,896,187 (July 22, 1975), E. D. Weil (to Stauffer Chemical Co.).
83. U.S. Pats. 4,382,042 (May 3, 1983) and
4,458,035 (July 3, 1984), T. A. Hardy and
F. Jaffe (both to Stauffer Chemical Co.).
84. U.S. Pat. 6,150,442 (Nov. 21, 2001), D. Chundusry and R. Sanford (to Ferro Corp.).
85. Eur. Pat. Appl. 1,239,005 (Nov. 9, 2002), Y. Bar Yakov and S. Hini (to Bromine Com-
pounds, Ltd.).
86. U.S. Pat. 3,789,091 (Jan. 29, 1974), J. J. Anderson, J. G. Camacho, and R. E. Kinney
(to Mobil Oil Corp.).
87. U.S. Pat. 3,849,368 (Nov. 19, 1974), J. J. Anderson, J. G. Camacho, and R. E. Kinney
(to Mobil Oil Corp.).
88. Y. Halpern, M. Mott, and R. H. Niswander, Ind. Eng. Chem. Prod. Res. Dev. 23,
233–238 (1984).
89. E. Termine and K. G. Taylor, in Additive Approaches to Polymer Modification, SPE
RETEC Conference Papers, Toronto, Ontario, Canada, Sept. 1989.
90. U.S. Pat. 4,220,472 (Sept. 2, 1980), C. Mauric and R. Wolf (to Sandoz Ltd.).
91. R. Wolf, Ind. Eng. Chem., Prod. Res. Dev. 20, 413–420 (1981).
92. D. L. Buszard, Chem. Ind. (London) 16, 610–615 (1978).
93. L. G. Krauskopf, in L. I. Nass and C. A. Heiberger, eds., Encyclopedia of PVC, 2nd
ed., Vol. 2, Marcel Dekker, Inc., New York, 1988, pp. 143–261.
94. T. W. Lapp, Report NTIS PB-251678, Midwest Research Institute, Feb. 1976.
95. F. A. Lowenheim and M. K. Moran, Faith, Keyes and Clark’s Industrial Chemicals,
4th ed., John Wiley & Sons, Inc., New York, 1975, pp. 849–853.
96. J. M. Heaps, Plastics (London) 33, 410–413 (1969).
97. U.S. Pat. 3,919,158 (Nov. 11, 1975), D. R. Randell and W. Pickles (to CIBA-GEIGY
AG).
98. S. G. Shankwalkar and D. G. Placek, Ind. Eng. Chem. Res. 31, 1810–1813 (1992).
99. A. J. Duke, Chimia 32, 457–463 (1978).
100. E. R. Nobile, S. W. Page, and P. Lombardo, Bull. Environ. Contam. Toxicol. 25, 755–761
(1980).
101. J. Andrews, A. Noonan, L. Timberlake, and R. Rose, in 11th International Flame
Retardancy Conference, London, Jan. 27–28, 2004.
102. U.S. Pat. 6,140,399 (2000), S. Munro (to Great Lakes Chemical).
103. J. R. Darby and J. K. Sears, in N. M. Bikales, ed., Encyclopedia of Polymer Science
and Technology, Wiley-Interscience, New York, 1975, pp. 229–306.
104. D. H. Paul, in Fire Retardant Chemicals Association, Coronado, Calif., Oct. 20–23,
1991, pp. 79–108.
105. Eur. Pat. Appl. 356,633 (Mar. 7, 1990), C. A. A. Claesen and J. H. G. Lohmeijer (to
General Electric Co.).
106. D. Bright, S. Dashevsky, P. Moy, and K.-M. Tu, Annu. Tech. Conf. Soc. Plast. Eng. 56,
2875–2879 (1998).
107. E. A. Murashko, G. F. Levchik, S. V. Levchik, D. A. Bright, and S. Dashevsky, J. Fire
Sci. 16, 233–249, 278–296 (1998).
108. S. V. Levchik, D. A. Bright, G. Alessio, and S. Dashevsky, J. Vinyl Add. Technol. 7(2),
98–103 (2001).

50
FLAME RETARDANCY
Vol. 10
109. PCT Intl. Appl. WO 03 55,940 (July 10, 2003), B. A. Williams, D. A. Bright, and
E. Pinzoni (to Akzo Nobel NV).
110. E. D. Weil, R. B. Fearing, and F. Jaffe, J. Fire Retard. Chem. 9(1), 39–49 (1982).
111. R. Gallagher and J. C. H. Hwa, J. Polym. Sci., Polym. Symp. 64, 329–337 (1978).
112. A. J. Papa, Ind. Eng. Chem. Prod. Res. Dev. 9, 478–496 (1970).
113. Br. Pat. 954,792 (Apr. 8, 1964) (to Virginia-Carolina Chem. Co.).
114. U.S. Pat. 3,235,517 (Feb. 16, 1966), T. M. Beck and E. N. Walsh (to Stauffer Chemical
Co.).
115. X. L. Wang, K. K. Yang, and Y. Z. Wang, J. Appl. Polym. Sci. 82, 276–282 (2001).
116. M. Sicken, C. Schutz, and S. Jung, in Polyurethanes Expo ’96, Las Vegas, Nev., Oct.
20–23, 1996, pp. 460–466.
117. C. Q. Yang, W. Wu, and J. Stowell, in 14th Annual BCC Conference on Recent Advances
in Flame Retardancy of Polymeric Materials, Stamford, Conn., June 2–4, 2003.
118. Eur. Pat. Publ. 138,204 (Apr. 24, 1985), G. Fesman, R. Y. Lin, and R. A. Raeder (to
Stauffer Chemical Co.).
119. Eur. Pat. Appl. 255,381 (Feb. 3, 1988), D. M. Konkus and P. Kraft (to Stauffer Chemical
Co.).
120. A. R. Horrocks, Rev. Prog. Coloration 16, 62–101 (1986).
121. S. L. Vail, D. J. Daigle, and A. W. Frank, Text. Res. J. 52, 678–692 (1982).
122. A. W. Frank, D. J. Daigle, and S. L. Vail, Text. Res. J. 52, 738–750 (1982).
123. D. J. Daigle and A. W. Frank, Text. Res. J. 52, 751–755 (1982).
124. W. A. Reeves, G. Drake, J. Beninate, and R. Perkins, Text. Chem. Color. 2, 283–285
(1969).
125. Br. Pat. 1,439,607 (1976), R. Cole and J. Stephenson (to Albright and Wilson Ltd.).
126. U.S. Pat. 4,311, 855 (1976), R. Cole and J. Stephenson (to Albright and Wilson Ltd.).
127. A. Kapura, J. Fire Sci. 12(1), 3–13 (1994); J. Fire Sci. 14(3), 169–185 (1996).
128. R. Aenishanslin and co-workers, Text. Res. J. 39, 375–381 (1969).
129. R. Aenishanslin and N. Bigler, Textilveredlung 3, 467–474 (1968).
130. R. Horrocks and D. Roberts, Exotextile ’98: Sustainable Development, Bolton, U.K.,
Apr. 7–8, 1998, Woodhead Publ. Ltd., Cambridge, U.K., 1999.
131. E. D. Weil, in J. I. Kroschwitz, ed., Encyclopedia of Polymer Science and Engineering,
Vol. 11, John Wiley & Sons, Inc., New York, 1988, pp. 96–126.
132. R. Stackman, in C. H. Carraher and M. Tsuda, eds., Modification of Polymers,
American Chemical Society Symposium Series, No. 121, Washington, D.C., 1980,
pp. 425–434.
133. U.S. Pat. 3,941,752 (Mar. 2, 1976), H. J. Kleiner, M. Finke, U. Bollert, and W. Herwig
(to Hoechst AG).
134. U.S. Pat. 4,033,936 (July 5, 1977), U. Bollert, E. Lohmar, and A. Ohorodnik (to Hoechst
AG).
135. U.S. Pat. 4,127,590 (Nov. 28, 1978), S. Endo, T. Kashihara, A. Osako, T. Shizuki, and
T. Ikegami (to Toyo Boseki KK).
136. E. D. Weil and S. Levchik, J. Fire Sci. 22, 25–39 (2004).
137. A. Ballistreri, G. Montaudo, E. Scamporrino, C. Puglisi, and D. Vitalini, J. Polym. Sci.,
Part A.: Polym. Chem 26, 2113–2127 (1988).
138. E. D. Weil and V. Choudhary, J. Fire Sci. 13, 104–126 (1995).
139. O. M. Grace, R. E. Mericle, and J. D. Taylor, J. Cell. Plast. 21, 311–317 (1985).
140. B. Bastin, R. Paleja, and J. Lefebvre, in Polyurethanes Expo 2002 Proceedings, Boston,
Oct. 13–16, 2002, API, pp. 244–254.
141. J. Kersjes and C. Furst, in Interflam ’99, Proceedings of the 8th International Confer-
ence, Vol. 2 Interscience Communications Ltd., London, 1999, pp. 1211–1216.
142. A. Casu, G. Camino, M. De Giorgi, D. Flath, V. Morone, and R. Zenoni, Polym. Degrad.
Stabil. 58, 297–302 (1997).

Vol. 10
FLAME RETARDANCY
51
143. G. Stern and H. Horacek, Int. J. Polym. Mater. 25, 255–268 (1994).
144. R. Srinivasan and B. Rotzinger, in Polyolefins 2000, International Conference on Poly-
olefins, Houston, Tex., Feb. 27-Mar. 1, 2000, SPE, pp. 571–581.
145. “Toxicological Risks of Selected Flame Retardant Chemicals,” Subcommittee on Flame
Retardant Chemicals, Committee on Toxicology, Board on Environmental Studies and
Toxicology, National Research Council, 2000, National Academy Press, Washington,
D.C., 2000. Full text at http://books.nap.edu/catalog/9841.html?onpi.newsdoc042700.
146. G. C. Stevens and A. H. Mann, “Risks and Benefits in the Use of Flame Retardants
in Consumer Products: A Report for the Department of Trade and Industry,” Poly-
mer Research Centre, School of Physical Sciences and School of Biological Sciences,
University of Surrey, Guildford, Surrey GU2 5XH, U.K., Jan. 1999.
147. “Flame Retardants: A General Introduction,” Environmental Health Criteria 192,
International Programme on Chemical Safety, World Health Organization, Inter-
national Labor Organization and UN Environment Programme, 1992. Full text at
http://www.inchem.org/documents/ehc/ehc/ehc192.htm.
148. Public Chemical Assessment Reports, National Industrial Chemical Notification and
Assessment Scheme (NICNAS), Australian Department of Environment and Her-
itage. Index at http://www.nicnas.gov.au/publications/car/pec/pecindex.htm.
149. “Brominated Flame Retardants in Widespread Use,” Danish Environment Agency,
2002; http://www.mst.dk/project/NyViden/2000/05130000.htm for more recent up-
date.
150. V. Babrauskas, R. H. Harris Jr., R. G. Gann, B. C. Levin, B. T. Lee, R. D. Peacock,
M. Paabo, W. Twilley, M. F. Yoklavich, and H. M. Clark, “Fire Hazard Comparison
of Fire-Retarded and Non-Fire-Retarded Products,” NBS Special Publication, No.
749, U.S. Dept. of Commerce, Washington, July 1988. Available on the Internet at
http://fire.nist.gov/bfrlpubs/fire88/art003.html.
151. “Summaries
of
EU
Risk
Assessments
Carried
Out
for
Flame
Retar-
dants,
and
Status
of
EU
Risk
Assessments
Underway,”
http://www.cefic-
efra.org/pdf/1202/EFRA˙sum˙Risk˙2002.pdf.
152. V. Steukers and S. Kroon, in Flame Retardants, ’04, Conference Proceedings, Jan.
2004, Interscience Communications, London, 2004.
153. S. Dungey, in Flame Retardants, ’04, Conference Proceedings, Jan. 2004, Interscience
Communications, London, 2004.
154. O. Hahn, S. Kemmlein, O. Jann, S. Kalus, and D. Stolle, in Flame Retardants,
’04, Conference Proceedings, Jan. 2004, Interscience Communications, London,
2004.
155. G. C. Stevens, R. Ghanem, J. L. Thomas, A. R. Horrocks, and B. Kandola, in Flame
Retardants. ’04, Conference Proceedings, Jan. 2004, Interscience Communications,
London, 2004.
156. “Long Chain Chlorinated Paraffin,” U.K. Environment Agency Risk Assessment, 2001
(draft).
157. “Updated Risk Assessment of Alkanes, C10–C13 Chloro-,” U.K. Environment Agency,
2003 (draft).
158. Brominated Flame Retardants and Foam Furniture Conference and Roundtable, Apr.
29–30, 2003, Environmental Finance Center, EPA Region 9, San Francisco, CA. Text
of most papers at http://www.greenstart.org/efc9/2003˙Conf.htm.
159. Dioxin 2003, 23rd International Symposium on Halogenated Organic and Persis-
tent Organic Pollutants, Boston, Mass., Aug. 24–29. Proceedings are reported in
Organohalogen Compd. 61–65 (2003). Papers specifically dealing with the brominated
flame retardants are reported in http://www.dioxin2003.org/BFR%20Highlights.pdf.
160. K. Moss, “Toxicology of Flame Retardants,” lecture at Workshop on Toxic Use Reduc-
tion at Toxic Use Reduction Institute, Amherst, Mass., Dec. 12, 2002.

52
FLAME RETARDANCY
Vol. 10
161. C. Hogue, Chem. Eng. News 81(46), 49–51 (2003).
162. G. Soderstrom, U. Sellstrom, C. A. de Wit, and M. Tysklind, Environ. Sci. Technol.
38(1), 127–132 (2004).
163. H. M. Stapleton, M. Alaee, R. J. Letcher, and J. E. Baker, Environ. Sci. Technol. 38(1),
112–119 (2004).
164. T. Imai, S. Hamm, and K. Rothenbacher, Environ. Sci. Technol. 37, 652–656
(2003).
165. D. Drohmann and L. Tange, in Flame Retardants. ’04, Conference Proceedings, Jan.
2004, Interscience Communication, London, 2004.
166. K. Van de Velde, in E & E Conference, Brussels, Belgium, Mar. 5–6, 2003. Updates
available at IAOIA, contact Karine.vandevelde@campine.be.
167. T. Long, in Flame Retardants in Our New World, Papers Presented at Fire Retar-
dant Chemicals Association [Spring Conference], San Antonio, Tex., Mar. 10–13, 2002,
pp. 57–58.
168. Revised Document for WHO Drinking Water Guidelines, 3rd ed. Draft avail-
able as: http://www.who.int/docstore/water˙sanitation˙health/GDWQ/draftchemicals/
antimony2003.pdf.
169. D. C. G. Muir, in O. Hutzinger, ed., Anthropogenic Compounds, Handbook of Environ-
mental Chemistry, Vol. 3, Springer-Verlag, Berlin, 1984, Part C, pp. 41–66.
170. Environmental Protection Agency, Fed. Regist. 57, 2138–2159 (1992).
171. M. B. Abou-Donia, in K. Blum and L. Manzo, eds., Neurotoxicology, Marcel Dekker,
Inc., New York, 1985, pp. 423–444.
172. M. B. Abou-Donia and D. M. Lapadula, Annu. Rev. Pharmacol. Toxicol. 30, 405–440
(1990).
173. M. K. Johnson, Toxicol. Appl. Pharmacol. 102, 385–399 (1990).
174. M. L. Weiner and B. S. Jortner, Neurotoxicology 20, 653–674 (1999).
175. J. W. Holleman, Health Effects of Haloalkyl Phosphate Flame Retardants and Potential
Metabolic Products, Oak Ridge National Laboratory, Tenn., 1984, DOE/NBM-4006848
(DE84 006848),
176. J. Prosch, W. Puchert, and M. Gluschke, Vom Wasser 95, 87–95 (2000); Chem. Abstr.
137: 388853s (2002).
177. G. Ingerowski, A. Friedle, and J. Thumella, Indoor Air 11(3), 145–149 (2001).
178. T. Salthammer, F. Fuhrmann, and E. Uhde, Indoor Air 13(1), 49–52 (2003).
179. H. Carlsson, U. Nilsson, and C. Ostman, Environ. Sci. Technol. 34, 3885–3889 (2000).
180. European Flame Retardant Association, “Position Paper Triphenyl Phosphate,” 2001.
Available at http://www.cefic-efra.org/pdf/DOC-01-00%20Revised.pdf.
181. R. Henrich, R. I. Freudenthal, B. M. Ryan, R. L. Sherwood, and co-workers, Intl. J.
Toxicol. 19, 223–276 (2000).
182. M. M. Hirschler, S. M. Debanne, J. B. Larsen, and G. L. Nelson, eds., Carbon Monoxide
and Human Lethality: Fire and Non-Fire Studies, Elsevier Science Publishers, Ltd.,
London, 1993.
183. B. C. Levin, Toxicology 115, 89–106 (1996).
184. D. Purser, in A. F. Grand and C. A. Wilkie, eds., Fire Retardancy of Polymeric Materials,
Marcel Dekker, New York, 2000, pp. 450–499.
185. R. G. Gann and co-workers, International Study of the Sublethal Effects of Fire Smoke
on Survivability and Health (SEFS): Phase I Final Report, NIST Technical Note 1439,
National Institute of Standards and Technology, U.S. Dept. of Commerce, Gaithers-
burg, Md., Aug. 2001.
186. M. M. Hirschler, in BCC Flame Retardancy Conference, Stamford, Con., May 23–25,
1995.
187. J. R. Hall Jr., Fire Technology 32, 351–371 (1996).
188. G. Hartzell, Toxicology 115, 7–23 (1996).

Vol. 10
FLAME RETARDANCY
53
189. L. C. Speitel, Final Report, July 1995, DOT/FAA/AR-95/5, Office of Aviation Research,
Washington, D.C.
190. L. C. Speitel, Toxicology 115, 167–177 (1996).
191. W. B. Crummett, Decades of Dioxin, Xlibris Corp., Princeton, N.J., 2002.
192. “Dioxins and Dioxin-Like Compounds in the Food Supply: Strategies to Decrease
Exposure,” Committee on the Implications of Dioxin in the Food Supply, Na-
tional Research Council, National Academies Press, 2003. Full text on line at
http://www.nap.edu/catalog/10763.html.
193. A. Schechter, ed., Dioxins and Health, Plenum Press, New York, 1994, pp. 625.
194. N. J. Themelis, Waste Management World, 2003–2004 Review Issue, July–Aug.
2003, pp. 40–47. Full text at http://www.seas.columbia.edu/earth/papers/global waste
to energy.html.
195. P. Deriziotis and N. J. Themelis, in North American Waste-to-Energy Conference,
ASME Intl., Tampa, Fla., Apr. 2003.
196. E. D. Weil and A. M. Aaronson, in Proceedings of the 1st European Conference on
Flammability and Fire Retardants, Brussels, BE, July 1977, Technomic Publishing
Co., Westport, Conn., 1978.
197. C. Herpol, Fire Mater. 1(1), 29–35 (1976).
198. F. B. Clarke, Fire Mater. 23(3), 109–116 (1999).
199. A. Heijboer, L. Utevskii, I. B. Yaakov, I. Finberg, and P. Georlette, in Advances in
Plastics Technology APT ’97, International Conference, Katowice, Poland, Dec. 9–11,
1997, pp. 7/1–7/16.
200. M. A. Barnes, P. J. Briggs, M. M. Hirschler, A. F. Matheson, and T. J. O’Neill, Fire
Mater. 20(1), 1–37 (1996).
201. R. L. Markezich, R. F. Mundhenke, and D. G. Aschbacher, in Flame Retardants ’94
[Proc. Conf.], 6th, Interscience Communications, London, 1994, pp. 203–211.
202. M. Rai and S. Brown, in Flame Retardants ’98 [Proc. Conf.], 8th, Interscience Com-
munications, London, 1998, pp. 83–91.
203. D. H. Park, M. J. Ahn, S. C. Kim, and G. J. Lee, in Proceedings of International Wire
& Cable Symposium, 50th, 2001, pp. 409–414.
204. T. C. Mathis and J. D. Hinchen, in 31st Annual Technical Conference of SPE, Technical
Papers, Vol. 19, 1973, pp. 343–348.
205. U.S. Pat. 3,869,420 (1975), T. C. Mathis and J. D. Hinchen (to Monsanto Co.)
206. Y. Uehara and Y. Ogawa, in V. M. Bhatnagar, ed., Advances in Fire Retardant Textiles,
Prog. Fire Ret. Series, Vol. 5, Technomic Publishing Co., Westport, Conn., 1975, pp.
507–599.
207. T. L. Long, in Papers Presented at Fire Retardant Chemicals Association [Spring Con-
ference], San Antonio, Tex, Mar. 10–13, 2002, Fire Retardant Chemicals Association,
Lancaster, Pa., 2002, pp. 57–58.
208. L. Cleveland, F. L. Mayer, D. R. Buckler, and D. U. Pulaski, Environ. Toxicol. Chem.
5, 273–282 (1986).
209. D. C. G. Muir, in O. Hutzinger, ed., Handbook of Environmental Chemistry, Vol. 3,
Springer, Berlin, 1984, Issue Part C, pp. 41–66.
210. J. E. Chambers and P. E. Levi, eds., Organophosphates, Chemistry, Fate and Effects,
Academic Press, New York, 1992.
211. B.-E. Bengtsson and co-workers, Environ. Toxicol. Chem. 5, 853–861 (1986).
212. R. S. Boethling and J. C. Cooper, Residue Rev. 94, 49–99 (1985).
213. R. T. Henrigh, R. L. Freudenthal and co-workers, Int. J. Toxicol. 19, 223–275 (2000).
214. Akzo Nobel Functional Chemicals LLC, Material Safety Data Sheet on FYROLFLEX
BDP.
215. Y. Kawagoshi, S. Nakamura, and I. Fukunaga, Chemosphere 48, 219–225 (2002).
216. W. Mabey and T. Mill, J. Phys. Chem. Ref. Data 7, 383–415 (1978).

54
FLAME RETARDANCY
Vol. 10
217. M. Simonson, C. Tullin, and H. Stripple, Chemosphere 46, 737–744 (2002).
218. P. Andersson, M. Simonson, and H. Stripple, SP Report 2003:22, Swedish National
Testing and Research Institute and IVL Swedish Environmental Research Institute,
2003.
219. J. Troitzsch, Polym. Degrad. Stabil. 64, 557–560 (1999).
E
DWARD
D. W
EIL
Polymer Research Institute, Polytechnic University
Wyszukiwarka
Podobne podstrony:
retardacja
Conan Pastiche ??mp, L Sprague The Flame Knife
combust flame 152 2008 604
Cleaning Flame Ionization Detectors When and How
retardacja
MCRP 3 37C Flame, Riot Contol Agents and Herbicide Ops
Zasady działania retardera
Retard ou Trouble Arige COQUET
wynik kontroli pierwszychf pytań z retardów
Retardery
eternal flame The Bangles
SMeyer EP0101761A3 Controlled Hydrogen Gas Flame
474 The Bangles Eternal flame
Estudos Biblicos Retardadores de bençãos
56652798 Keeper of the Flame
więcej podobnych podstron