
Contents
lists
available
at
Vaccine
j
o u r n a
l
h o
m e
p a g
e :
w w w . e l s e v i e r . c o m / l o c a t e / v a c c i n e
Mucosal
immunization
with
Shigella
flexneri
outer
membrane
vesicles
induced
protection
in
mice
A.I.
Camacho
,
J.
de
Souza
,
S.
Sánchez-Gómez
,
M.
Pardo-Ros
,
J.M.
Irache
,
C.
Gamazo
a
Department
of
Microbiology,
University
of
Navarra,
31008
Pamplona,
Spain
b
Department
of
Pharmacy
and
Pharmaceutical
Technology,
University
of
Navarra,
31008
Pamplona,
Spain
a
r
t
i
c
l
e
i
n
f
o
Article
history:
Received
7
June
2011
Received
in
revised
form
25
August
2011
Accepted
30
August
2011
Available online 10 September 2011
Keywords:
Shigella
Outer
membrane
vesicles
Vaccine
Nanoparticles
Adjuvant
a
b
s
t
r
a
c
t
Vaccination
appears
to
be
the
only
rational
prophylactic
approach
to
control
shigellosis.
Unfortunately,
there
is
still
no
safe
and
efficacious
vaccine
available.
We
investigated
the
protection
conferred
by
a
new
vaccine
containing
outer
membrane
vesicles
(OMVs)
from
Shigella
flexneri
with
an
adjuvant
based
on
nanoparticles
in
an
experimental
model
of
shigellosis
in
mice.
OMVs
were
encapsulated
in
poly(anhydride)
nanoparticles
prepared
by
a
solvent
displacement
method
with
the
copolymer
PMV/MA.
OMVs
loaded
into
NPs
(NP-OMVs)
were
homogeneous
and
spherical
in
shape,
with
a
size
of
197
nm
(PdI
=
0.06).
BALB/c
mice
(females,
9-week-old,
20
±
1
g)
were
immunized
by
intradermal,
nasal,
ocular
(20
g)
or
oral
route
(100
g)
with
free
or
encapsulated
OMV.
Thirty-five
days
after
administration,
mice
were
infected
intranasally
with
a
lethal
dose
of
S.
flexneri
(1
× 10
7
CFU).
The
new
vaccine
was
able
to
protect
fully
against
infection
when
it
was
administered
via
mucosa.
By
intradermal
route
the
NP-OMVs
formulation
increased
the
protection
from
20%,
obtained
with
free
extract,
to
100%.
Interestingly,
both
OMVs
and
OMV-NP
induced
full
protection
when
administered
by
the
nasal
and
conjuntival
route.
A
strong
association
between
the
ratio
of
IL-12p40/IL-10
and
protection
was
found.
Moreover,
low
levels
of
IFN-
␥
correlate
with
protection.
Under
the
experimental
conditions
used,
the
adjuvant
did
not
induce
any
adverse
effects.
These
results
place
OMVs
among
promising
candidates
to
be
used
for
vaccination
against
Shigellosis.
© 2011 Elsevier Ltd. All rights reserved.
1.
Introduction
According
to
World
Health
Organization
(WHO),
approximately
2.5
billion
cases
of
diarrhea
occurred
worldwide
which
results
in
1.5
million
deaths
among
children
under
the
age
of
five.
It
is
a
common
cause
of
death
in
developing
countries
and
the
second
most
com-
mon
cause
of
infant
deaths.
Among
the
main
causes,
Shigellosis
is
responsible
of
more
than
165
million
cases
annually,
leading
to
1.2
million
deaths
many
cases
progress
into
serious
damages
in
their
intestinal
epithelium
that
will
limit
the
correct
nutrient
absorption
with
the
subsequent
sequel
for
life.
Shigella
spread
massively
within
the
community
and
from
person
to
per-
son,
and
hence,
prevention
relies
on
basic
sanitary
measures,
which
unfortunately
may
be
not
possible
applied
for
many
countries.
In
addition,
the
increasing
problem
of
antibiotic
resistance
alerts
on
the
urgent
need
of
protective
vaccines.
In
fact,
the
World
Health
Organization
has
made
the
development
of
a
safe
and
effective
vaccine
against
Shigella
a
high
priority
∗ Corresponding
author.
Tel.:
+34
9
48
42
56
88;
fax:
+34
9
48
42
56
49.
address:
(C.
Gamazo).
The
efforts
have
been
mainly
focussed
on
live
oral
vaccines
with
several
vaccine
candidates
on
clinical
trials
develop-
ment
of
such
safe
Shigella
vaccine
is
being
problematical,
and
no
vaccine
is
still
available
Currently,
most
vaccines
in
development
are
acellular
vaccines
which
comparison
to
live-attenuated
or
whole
inactivated
organism,
are
safer.
However,
these
prototypes
require
adjuvants
to
achieve
a
more
effective
immune
response.
The
challenge
is
the
designing
of
formulations
able
to
enhance
the
immunogenicity
of
associated
antigens,
through
the
right
activation
of
the
immune
system,
and
susceptible
to
be
administered
by
mucosal
routes.
Previous
studies
of
our
group
have
evaluated
the
adjuvant
capa-
bility
of
nanoparticles
made
from
the
copolymer
of
methyl
vinyl
ether
and
maleic
anhydride
(Gantrez
AN
®
).
These
nanoparticles
demonstrated
their
ability
to
initiate
a
strong
and
balanced
mucosal
immune
response
and
then,
to
efficiently
induce
Th-1
subset
In
addition,
these
nanoparticles
loaded
with
different
antigens
have
showed
to
be
effective
against
experimental
challenges
with
Salmonella
or
Brucella
In
this
work
we
propose
the
use
of
outer
membrane
vesicles
(OMVs)
from
Shigella
as
the
source
of
relevant
antigens
to
be
included
in
the
acellular
vaccine.
OMVs
are
secreted
from
the
outer
membrane
of
a
large
variety
of
Gram-negative
bacteria,
during
0264-410X/$
–
see
front
matter ©
2011 Elsevier Ltd. All rights reserved.
doi:

A.I.
Camacho
et
al.
/
Vaccine
29 (2011) 8222–
8229
8223
in
vitro
culture
and
during
infection
Currently,
there
have
been
described
many
functions
for
this
blebbing
process.
Functions
proposed
vary
from
facilitating
the
intracellular
bacterial
growth
within
phagocytes
to
the
delivery
of
effectors
molecules
critical
for
pathogen
dissemination
such
as
pathogen-associated
molecular
patterns
(PAMPs)
and
other
virulence
factors
to
host
cells
We
therefore
describe
here
the
preparation,
characterization
and
evaluation
of
Shigella
flexneri
outer
membrane
vesicles
in
order
to
be
used
in
vaccination.
We
obtained
the
OMVs
from
S.
flexneri
2a,
being
this
the
most
common
cause
of
shigellosis.
In
fact,
it’s
responsible
for
25–50%
of
all
cases
in
the
developing
world
The
protective
efficacy
of
OMVs
either
in
their
free
form
or
adju-
vanted
in
NP
were
tested
in
the
murine
pneumonia
model
after
immunization
with
one
single
dose
by
intradermal
or
mucosal
routes.
The
OMVs
formulations
obtained
and
characterized
here
were
found
to
induce
protection
in
mice
after
one
single
dose
against
a
lethal
dose
of
S.
flexneri
2a.
2.
Materials
and
methods
2.1.
Preparation
and
characterization
of
outer
membrane
vesicles
OMVs
were
obtained
from
S.
flexneri
2a
(clinical
isolate
from
Hospital
de
Navarra,
Pamplona,
Spain).
Vesicles
were
purified
from
a
method
adapted
from
Horstman
and
Kuehn
were
grown
in
LB
broth
overnight
to
early
stationary
phase.
Then,
bac-
teria
were
inactivated
with
a
solution
of
binary
ethylenimine
and
formaldehyde
(6
mM
BEI-0,
06%
FA,
6
h,
37
◦
C).
BEI
was
prepared
as
a
0.1
M
solution
by
cyclization
of
0.1
M
2-bromoethylamine
hydrobromide
(Sigma)
in
0.175
M
NaOH
solution
for
1
h
following
the
method
of
Bahnemann
were
removed
by
pelleting
(10,000
×
g,
10
min).
Supernatant
was
filtered
through
a
0.45
m
Durapore
PVDF
filter
(Millipore)
and
purified
by
ultradiafiltration
via
a
300-kDa
tangential
filtration
concentration
unit
(Millipore).
The
retentate
was
freezed
in
order
to
induce
larger
blebs
formed
through
reassociation
of
the
smaller
ones
into
multimicelles,
as
had
been
proposed
previously
product
was
recovered
by
centrifugation
at
40,000
×
g,
2
h.
Total
protein
content
was
deter-
mined
by
the
method
of
Lowry,
with
bovine
serum
albumin
as
standard.
Lypopolysaccharide
(LPS)
content
was
determined
by
Purpald
assay
Briefly,
to
50
L
of
LPS
samples
or
stan-
dards
each
of
the
duplicate
wells
in
a
96-well
tissue
culture
plate,
50
L
of
32
mM
NaIO
4
was
added,
and
the
plate
was
incu-
bated
for
25
min
followed
by
addition
of
50
L
of
136
mM
purpald
reagent
in
2
N
NaOH.
After
further
incubation
for
20
min,
50
L
of
64
mM
NaIO
4
was
added,
and
the
plate
was
incubated
for
another
20
min.
The
foam
in
each
well
can
be
eliminated
by
addition
of
20
L
2-propanol.
The
absorbance
of
each
well
was
measured
by
a
plate
reader
at
550
nm.
Finally,
extract
was
resuspended
in
sample
buffer
1
×
and
analyzed
by
SDS-PAGE
and
immunoblotting,
using
polyclonal
pool
sera
from
patient
infected
with
S.
flexneri
(Clínica
Universidad
de
Navarra)
or
anti
IpaC
mAb
(kindly
provided
by
A.
Phalipon,
Institut
Pasteur).
The
morphology
of
the
vesicles
was
examined
by
Field
Emission
Scanning
Electron
Microscope.
2.1.1.
Outer
membrane
proteins
(OMPs)
Outer
membrane
proteins
(OMPs)
from
S.
flexneri
were
prepared
by
sequential
detergent
extraction
of
cell
envelopes
after
the
disruption
of
cells
by
sonication
(4
pulses
×
5
min,
power
2,
Branson
Sonifier
450),
whole
bacteria
were
removed
by
cen-
trifugation
at
6000
×
g,
15
min.
Cell
envelopes
were
recovered
from
supernatant
by
centrifugation
(40,000
× g,
1
h).
Pellet
was
resus-
pended
in
1%
Sarkosyl
(N-Lauryl
sarcosine,
Sigma
Chemical
Co.,
St.
Louis,
USA),
incubated
for
30
min
and
further
centrifuged
at
40,000
×
g,
1
h,
twice.
The
enriched
sediment
in
outer
membrane
proteins
was
suspended
in
0.5
M
Tris–HCl
(pH
6.8)
with
10%
SDS
(Lauryl
sulfate,
Sigma)
and
boiled
for
15
min
and
finally,
centrifuged
(20,000
×
g;
30
min).
The
OMPs
of
S.
flexneri
were
present
in
the
final
supernatant.
2.1.2.
Ipa
(invasion
plasmid
antigens)
proteins
secretion
assay
Secretion
of
Ipa
proteins
through
the
TTSS
(Type
three
secre-
tion
system)
was
induced
using
a
Congo
Red
secretion
assay
Exponential-phase
bacteria
were
harvested,
resuspended
in
10
M
Congo
Red/PBS,
and
incubated
at
37
◦
C
for
30
min.
Following
incu-
bation,
bacteria
were
pelleted
by
centrifugation,
and
supernatants
were
collected
and
passed
through
a
0.22
m-pore
filter.
Proteins
in
the
supernatants,
which
represent
proteins
secreted
through
the
TTSS,
were
then
concentrated
by
tricholoroacetic
acid
precip-
itation.
Finally,
extract
was
resuspended
in
sample
buffer
1
×
and
analyzed
by
SDS-PAGE
and
immunoblotting
using
anti-IpaB
or
-
IpaC
mAb
(kindly
provided
by
A.
Phalipon,
Institut
Pasteur).
2.2.
Preparation
and
characterization
of
nanoparticles
Poly(anhydride)
nanoparticles
were
prepared
by
a
modification
of
the
solvent
displacement
method
100
mg
of
the
copolymer
of
methyl
vinyl
ether
and
maleic
anhydride
(PVM/MA)
(Gantrez
®
AN
119;
M.W.
200
kDa)
was
dissolved
in
5
mL
acetone
under
magnetic
stirring
at
room
temperature.
On
the
other
hand,
5
mg
OMVs
were
dispersed
by
ultrasonication
with
a
probe
Micro-
son
TM
(Misonix
Inc.,
New
York,
USA)
in
10
mL
water
for
1
min.
After
dispersion,
nanoparticles
were
formed
by
addition
of
this
water
phase
containing
OMVs.
The
agitation
was
maintained
dur-
ing
15
min
in
order
to
allow
the
stabilization
of
the
system.
Organic
solvents
were
removed
under
reduced
pressure
(Büchi
R-144,
Switzerland).
The
obtained
nanoparticles
were
collected
by
cen-
trifugation
(27,000
×
g,
20
min,
4
◦
C)
and
washed
with
water
twice.
Finally,
particles
were
freeze-dried
using
sucrose
5%
as
crioprotec-
tor.
The
preparation
of
empty
nanoparticles
was
performed
in
the
same
way
in
the
absence
of
OMVs.
2.2.1.
Characterization
of
nanoparticles
The
particle
size
and
the
zeta
potential
of
nanoparticles
were
determined
by
photon
correlation
spectroscopy
(PCS)
and
electrophoretic
laser
Doppler
anemometry,
respectively,
using
a
Zetamaster
analyzer
system
(Malvern
Instruments
Ltd.,
Worces-
tershire,
UK).
The
diameter
of
the
nanoparticles
was
determined
after
dispersion
in
ultrapure
water
(1/10)
and
measured
at
25
◦
C
by
dynamic
light
scattering
angle
of
90
◦
C.
The
zeta
potential
was
determined
as
follows:
200
L
of
the
samples
was
diluted
in
2
mL
of
a
0.1
mM
KCl
solution
adjusted
to
pH
7.4.
The
morphology
of
the
vesicles
was
examined
by
Field
Emission
Scanning
Elec-
tron
Microscope
(Carl
Zeiss,
model
Ultra
Plus).
For
this
purpose
freeze-dried
formulations
were
resuspended
in
ultrapure
water
and
centrifuged
at
27,000
× g
for
20
min
at
4
◦
C.
Then,
supernatants
were
rejected
and
the
obtained
pellets
were
mounted
on
TEM
grids.
The
yield
of
the
nanoparticles
preparation
process
was
determined
by
gravimetry
as
described
previously
poly(anhydride)
nanoparticles,
freshly
prepared,
were
freeze-dried.
Then,
the
yield
was
calculated
as
the
difference
between
the
initial
amount
of
the
polymer
used
to
prepare
nanoparticles
and
the
weight
of
the
freeze-
dried
carriers.
2.2.2.
Loading
capacity
of
nanoparticles
The
yield
of
nanoparticles
was
calculated
from
the
difference
between
the
initial
amount
of
the
polymer
used
to
prepare
the

8224
A.I.
Camacho
et
al.
/
Vaccine
29 (2011) 8222–
8229
particles
and
the
weight
of
the
freeze-dried
samples.
The
abil-
ity
of
PVM/MA
nanoparticles
to
entrap
the
complex
antigen
was
directly
determined
after
degradation
of
loaded
nanoparticles
with
NaOH.
Briefly,
OMVs-loaded
Gantrez
nanoparticles
(15
mg)
were
dispersed
in
water
vortexing
1
min.
After
centrifugation
(27,000
×
g,
15
min)
pellet
was
resupended
in
NaOH
0.1
M
soni-
cated
(Microson
TM
Ultrasonic
cell
disruptor)
and
incubated
for
1
h
to
assess
the
total
delivery
of
the
associated
antigen.
After
this
time,
the
amount
of
antigen
released
from
the
nanoparticles
was
determined
using
microbicin
choninic
acid
(microBCA)
protein
assay
(Pierce,
Rockford,
CA,
USA).
In
order
to
avoid
interferences
of
the
process,
calibration
curves
were
made
with
degraded
blank
nanoparticles,
and
all
measurements
were
performed
in
triplicate.
2.2.3.
Determination
of
the
structural
integrity
and
antigenity
of
OMVs
Western
blot
analysis
was
used
as
a
qualitative
tool
to
exam-
ine
the
structure
of
the
antigens,
complementing
the
quantification
performed
by
microBCA.
To
accomplish
this
analysis,
the
protocol
for
nanoparticle
degradation
was
modified
in
order
to
avoid
any
interference
of
the
enzyme.
In
this
case,
after
nanoparticle
isolation,
15
mg
of
loaded
nanoparticles
were
dispersed
in
water
vortexing
1
min.
After
centrifugation
(27,000
×
g,
15
min)
pellet
was
resu-
pended
in
2
mL
of
a
mixed
of
dimethilformamide:acetone
(1:3)
(
−80
◦
C,
1
h).
After
centrifugation,
pellet
was
resuspended
in
ace-
tone
(
−80
◦
C,
30
min).
Finally,
extract
was
resuspended
in
sample
buffer
1
×
and
analyzed
by
SDS-PAGE
and
immunoblotting
using
polyclonal
sera
from
hyperimmunized
rabbit
with
S.
flexneri
2.3.
SDS-PAGE
and
immunoblotting
SDS-PAGE
was
performed
in
12%
acrylamide
slabs
(Criterion
XT,
Bio
Rad
Laboratories,
CA)
with
the
discontinuous
buffer
sys-
tem
of
Laemmli
and
gels
stained
with
Coomassie
blue
or
silver
staining.
After
electrophoresis,
gels
were
electroblotted
to
a
PVDF
(polyvinylidene
fluoride)
membrane
at
0.8
mA/cm
2
for
30
min.
Then,
membranes
were
soaked
overnight
in
a
blocking
solution
containing
3%
(w/v)
of
non-fat
milk
and
then
incubated
in
the
pres-
ence
of
different
sera,
described
above.
After
the
incubation,
the
membranes
were
washed
five
times;
the
anti-rabbit
or
human
Ig-
alkaline
phosphatase
conjugate
was
added,
followed
by
incubation
for
an
additional
hour.
The
membranes
were
exhaustively
washed
and
the
antibody–antigen
complexes
were
visualized
after
addition
of
the
substrate/chromogen
solution
(H
2
O
2
/cloronaftol).
2.4.
Active
immunization
and
challenge
All
mice
were
treated
in
accordance
with
institutional
guide-
lines
for
treatment
of
animals
(Protocol
087/06
of
animal
treatment,
approved
in
1
October
2007
by
the
Ethical
Comity
for
the
Animal
Experimentation,
CEEA,
of
the
University
of
Navarra).
Nine-week-
old
BALB/c
mice
(20
±
1
g)
were
separated
in
randomized
groups
of
6
animals
and
immunized
with
OMVs
either
free
or
encapsulated
in
PVM/MA
NPs
by
intradermal,
nasal,
ocular
(20
g
of
extract)
or
oral
route
(100
g
of
extract).
The
scheme
of
administration
and
doses
are
summarized
in
Challenge
infection
was
performed
on
day
35
intranasally
with
a
lethal
dose
of
1
×
10
7
UFC/Mouse
of
S.
flexneri
2a
(clinical
isolate)
grown
to
logarithmic
phase
and
suspended
in
20
L
of
prewarmed
PBS.
The
number
of
dead
mice
after
challenge
was
recorded
daily.
2.5.
Measurement
of
immune
response
in
the
mouse
Blood
samples
were
collected
from
the
reto-orbital
plexures
of
anesthetized
mice.
2.5.1.
ELISA
The
antibody
response
was
measured
by
an
enzyme-linked
immunosorbent
assay
(ELISA).
In
brief,
96-well
microtiter
plates
(MaxiSorb;
Nunc,
Wiesbaden,
Germany)
were
coated
with
100
L
of
10
g/mL
OMVs
in
coating
buffer
(60
mM
carbonate
buffer,
pH
9.6).
Afterwards,
unspecific
binding
sites
were
saturated
with
3%
bovine
serum
albumin
(BSA)
in
PBS
for
1
h
at
RT.
Sera
from
mice
were
serially
diluted
in
PBS
with
1%
BSA
and
incubated
overnight
at
RT.
After
intense
washing
with
PBS
Tween
20
(PBS-T)
buffer,
the
alkaline
phosphatase
(AP)-conjugated
detection
antibody,
class-
specific
goat
anti-mouse
IgG/IgA
(Sigma)
for
sera,
was
added
for
1
h
at
37
◦
C.
The
detection
reaction
was
performed
by
incubating
the
sample
with
ABTS
substrate
for
20
min
at
room
temperature.
Absorbance
was
measured
with
an
ELISA
reader
(Sunrise
remote;
Tecan-Austria,
Groeding,
Austria)
at
a
wavelength
of
405
nm.
2.5.2.
Quantification
of
cytokines
from
sera
Cytokines
(IL-2,
IL-4,
IL-5,
IL-6,
IL-10,
IL-12(p40),
IL-12(p70),
IL-
13,
IL17,
IFN-
␥,
and
tumor
necrosis
factor)
were
quantified
from
serum
by
luminex-based
multiplex
assay
(Milliplex;
Millipore,
Bil-
lerica,
MA)
using
a
Bioplex
analyzer
(Bio-Rad,
Hercules,
CA).
2.6.
Statistics
Statistical
analyses
were
performed
using
GraphPad
Prism
5
for
Mac
OS
X.
All
experiments
were
performed
with
n
=
6.
Statis-
tical
comparisons
between
antibody
serum
levels
were
performed
using
Kruskal–Wallis
test,
followed
by
Dunn’s
post
hoc
test.
The
statistical
significance
was
set
at
P
<
0.05.
For
cytokine
levels,
it
was
performed
using
single-factor
analysis
of
variance,
followed
by
Turkey’s
post
hoc
test.
The
statistical
significance
was
set
at
P
<
0.001.
The
Kaplan–Meyer
curves
were
used
for
analysis
of
the
protection
experiment.
3.
Results
3.1.
Isolation
and
characterization
of
S.
flexneri
OMVs
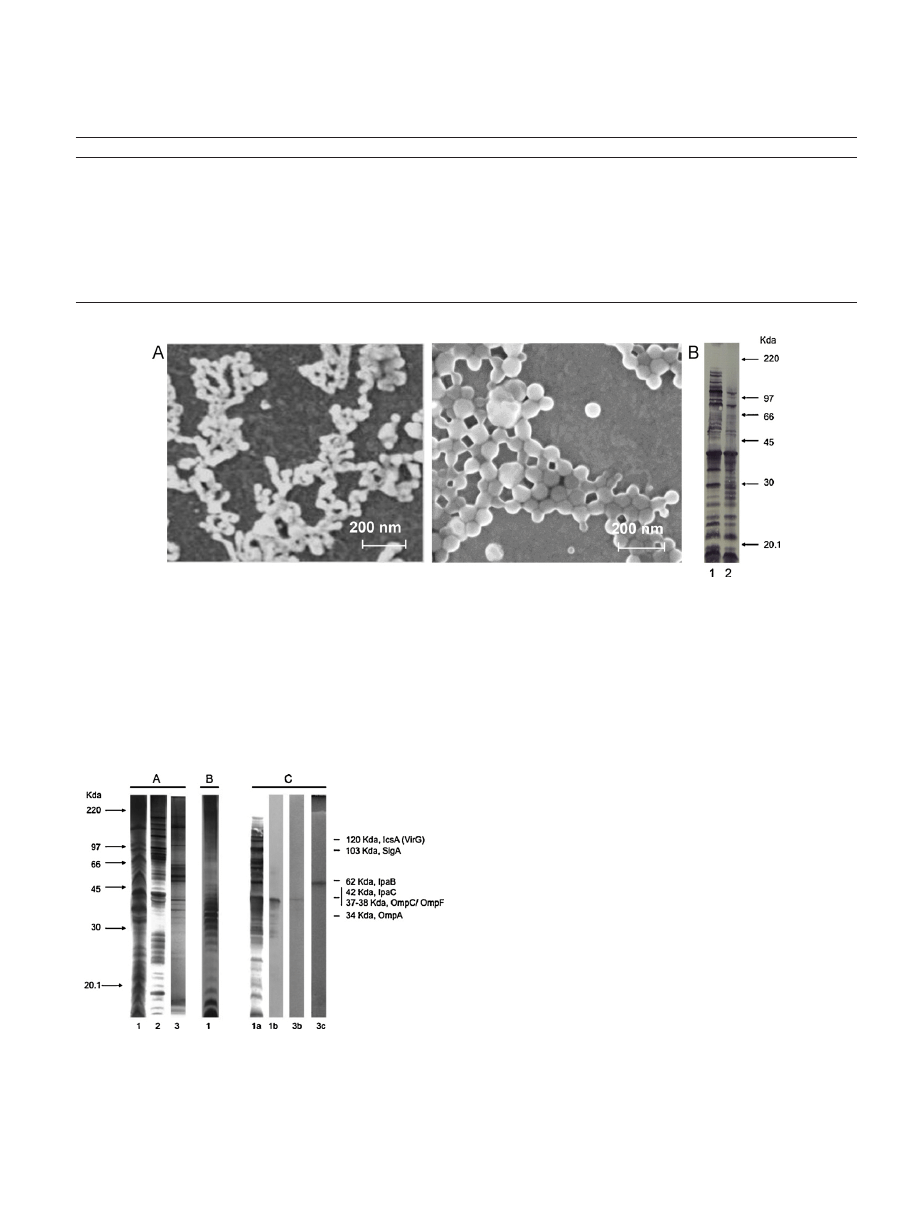
The
scanning
electron
microscopy
showed
that
the
OMVs
secreted
in
vitro
by
S.
flexneri
were
spherical,
with
an
average
diam-
eter
of
50
nm
(
The
yield
obtained
was
18
±
0,
04
g/mg
determined
after
lyophilisation
and
referred
to
the
original
cell
culture
dry
weight.
Quantitative
analysis
showed
that
protein
con-
tent
was
54.52
±
3.2%,
whereas
the
LPS
content
was
37.6
±
4.8%.
A
comparative
SDS-PAGE
analysis
of
the
OMVs
revealed
that
con-
tained
proteins
corresponded
to
the
OmpA,
34
kDa;
OmpC/OmpF,
38/42
kDa;
VirG,
120
kDa
already
described
by
other
authors
as
the
main
inmunodominant
antigenic
proteins
As
expected,
the
outer
membrane
protein
enriched
fraction
and
the
purified
OMVs
showed
a
similar
profile.
Furthermore,
OMVs
con-
tained
bands
at
62
kDa,
42
kDa
and
38
kDa
that
correspond
with
IpaB,
IpaC
and
IpaD
respectively
(
Immunoblot
assay
using
a
monoclonal
antibody
specific
to
IpaB
or
IpaC
demonstrated
that
these
proteins
were
located
on
vesicles
(
confirming
the
observation
of
Kadurugamuwa
and
Beveridge
3.2.
Characterization
of
OMVs-containig
nanoparticles
The
yield
of
the
OMV
antigen-loaded
NPs
manufactured
in
rela-
tion
to
the
initial
amount
of
polymer
employed
was
consistent
(89%).
Vaccine
formulations
were
homogeneous
and
spherically
shaped
The
average
size
of
NP-OMV
was
197
nm
with
a
polydispersity
index
of
0.06.
The
Z
potential
of
NP
was
tested
before
and
after
OMV
encap-
sulation.
Results
suggest
that
OMV
is
at
least
partially
bound
on
the
NP
surface,
indicated
by
the
change
in
Z
of
NP.
Zeta
potential
of
A.I.
Camacho
et
al.
/
Vaccine
29 (2011) 8222–
8229
8225
Table
1
Immunization
protocol
and
administration
route
strategy.
OMV:
free
outer
membrane
vesicles
extract
from
Shigella
flexneri
2a.
NP-OMVs:
OMVs
loaded
nanoparticles
(PBS:
phosphate
buffered
saline).
OMVs
NP-OMVs
Intradermal
Dosage
(administered
1
×)
20
g
2.5
mg
NP-OMVs
(eqv.
20
g
OMVs)
Volume
(50
L
PBS/dose)
Nasal
Dosage
(administered
3
×,
8
h
interval)
3
g/nostril
416
g
NP-OMVs
(eqv.
3
g
OMVs)
Volume
(3
L
PBS/nostril)
Ocular
Dosage
(administered
3
×,
8
h
interval)
3
g/nostril
416
g
NP-OMVs
(eqv.
3
g
OMVs)
Volume
(3
L
PBS/eye)
Oral
Dosage
(administered
1
×)
100
g
12.5
mg
NP-OMVs
(eqv.
100
g
OMVs)
Volume
(200
L
PBS/dose)
Fig.
1.
(A)
Scanning
electron
micrograph
images
of
outer
membrane
vesicles
(OMVs)
from
Shigella
flexneri
2a
(up),
or
loaded
in
nanoparticles
(NP-OMVs)
(down).
Scale
bar
indicates
200
nm.
(B)
Integrity
and
antigenicity
of
the
outer
membrane
vesicles
components
antigenic
components
after
encapsulation
into
nanoparticles.
Panel
shows
the
immunoblotting
developed
with
a
pool
of
sera
from
rabbit
hyperimmunized
with
whole
cells
from
Shigella
flexneri:
lanes
correspond
with
the
following
samples:
(1)
free
OMVs
and
(2)
OMVs
released
from
OMV-loaded
NPs.
free
OMVs
was
−14.1
±
3
mV.
The
encapsulation
of
the
extract
in
nanoparticles
resulted
in
a
change
of
Z
potential
from
−44
±
4
mV
to
−27
±
4
mV
when
OMVs
were
loaded
into
PVM/MA
nanoparticles.
To
further
confirm
OMV
encapsulation
into
NPs,
BCA
pro-
tein
determination
and
SDS-PAGE/immunoblotting
were
also
Fig.
2.
Comparative
analysis
of
Shigella
flexneri
outer
membrane
vesicles.
SDS-PAGE
with
silver
staining
for
proteins
(A)
or
for
LPS
(B),
and
immunoblotting
(C)
of
(1)
outer
membrane
vesicles
(OMVs),
(2)
extract
enrich
in
outer
membrane
proteins
(OMPs),
and
(3)
extract
enrich
in
Ipa
proteins.
Immunoblots
were
developed
with
polyclonal
antibodies
from
a
patient
infected
with
S.
flexneri
(lane
a),
anti-IpaC
mAb
(lane
b)
and
anti-IpaB
mAb
(lane
c).
Molecular
weight
markers
and
identity
of
some
bands
are
indicated.
performed.
The
procedure
involved
the
use
of
a
purification
step
in
order
to
discard
unbound
OMV.
S.
flexneri
OMVs
were
efficiently
associated
with
PVM/MA
nanoparticles,
as
they
showed
a
loading
encapsulation
of
20
g
OMVs/mg
of
polymer.
Besides,
an
immunoblotting
was
carried
out
using
sera
from
rabbit
hyper-
immunized
with
S.
flexneri.
Results
indicate
that
entrapment
in
nanoparticles
did
not
alter
its
antigenic
properties
(
3.3.
Evaluation
of
the
immunogenicity
and
protection
conferred
by
OMVs
vaccine
Groups
of
6
mice
were
immunized
once
by
intradermal
or
mucosal
routes
with
OMVs
(20
g/mouse),
either
free
or
encap-
sulated
in
NPs.
A
control
group
of
non-immunized
mice
was
also
included.
All
animals
immunized
by
nasal
or
ocular
routes
remained
in
good
health,
exhibiting
no
respiratory
difficulties,
changes
in
body
temperature,
or
abnormal
behaviour.
Oral
immu-
nized
mice
showed
a
transient
abdominal
swelling
a
few
hours
after
immunization.
By
contrast,
mice
immunized
intradermally
exper-
imented
sweating
and
lethargy
during
2
days
post-immunization,
which
disappeared
thereafter.
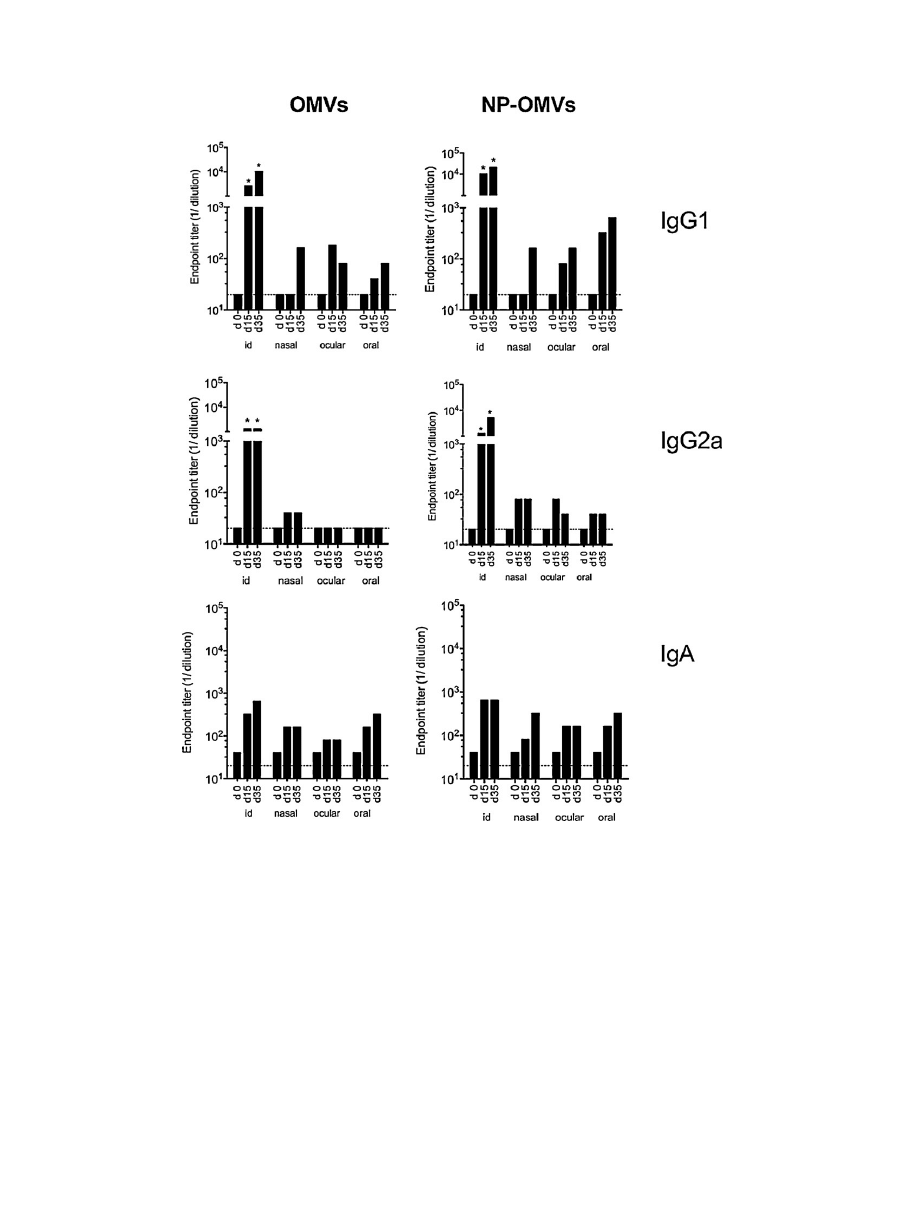
Specific
IgG2a
and
IgG1
against
OMVs
antigens
were
deter-
mined
by
indirect-ELISA
at
days
0,
15
and
35
post-immunization
Results
expressed
that
the
OMV
immunization
by
either
route
elicited
significant
levels
of
serum
IgG1
and
IgG2a
with
respect
control
mice
Higher
levels
of
IgG
were
found
in
groups
immunized
intradermally.
Overall
the
levels
of
IgG2a
(Th1
response)
were
higher
than
that
those
of
IgG1
(Th2).
An
adjuvant
effect
after
encapsulation
was
observed
on
the
immunogenicity
8226
A.I.
Camacho
et
al.
/
Vaccine
29 (2011) 8222–
8229
Fig.
3.
Antibody
immune
response
induced
after
vaccination
of
BALB/c
mice.
Serum
IgG1,
IgG2a
and
IgA
titers
in
vaccinated
mice
(n
=
6/group)
with
either
free
extract
(OMVs)
or
loaded
in
nanoparticles
(NP-OMVs)
at
weeks
0,
2
and
5
after
immunization.
Broken
line
indicates
first
dilution
tested.
Data
are
mean
value
(*P
<
0.05
for
immunized
mice
vs.
control).
(global
specific
antibody
response)
especially
after
oral
immuniza-
tion.
There
were
not
found
significant
differences
in
the
mucosal
levels
of
the
IgA
elicited
after
intradermal
or
mucosal
deliveries.
Levels
of
serum
cytokines
were
determined
at
day
15
post-
immunization
The
encapsulation
of
OMVs
in
NPs
induced
an
increase
in
the
level
of
IL-12
(p40)
and
a
decrease
of
IL-10
with
respect
to
the
free
form,
by
intradermal
or
oral
delivery.
In
con-
trast,
after
ocular
or
nasal
immunization,
the
inverse
switching
phenomenon
was
observed.
At
day
35
after
immunization,
mice
were
challenged
with
S.
flexneri
via
intranasal
route
and
monitored
for
survival
over
30
days
(n
=
6
mice/group)
Nasal
or
ocular
immunizations
with
free
OMVs
provided
complete
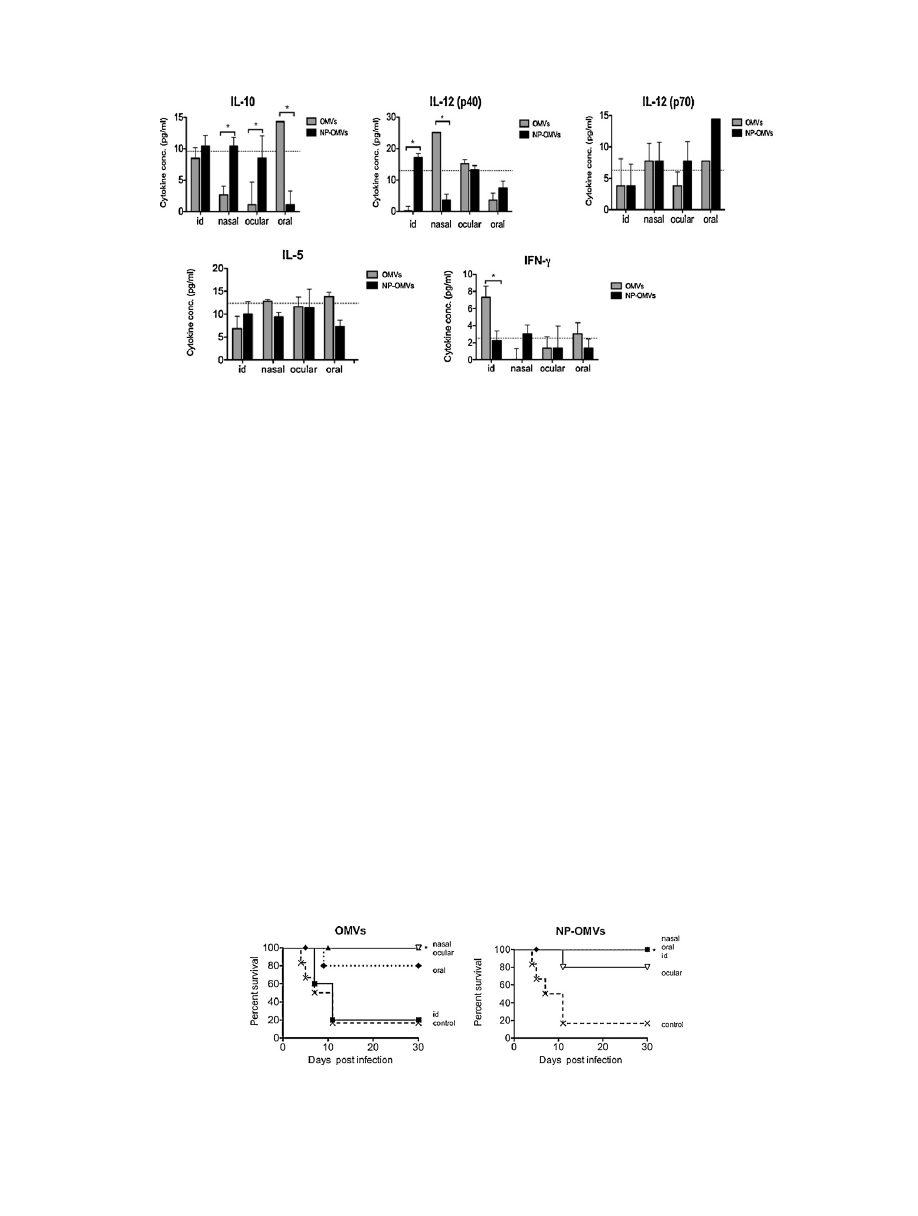
protection.
Non-significant
differences
were
found
between
OMV
free
or
nano-
encapsulated
in
groups
immunized
by
nasal,
ocular
or
oral
route.
In
contrast,
the
intradermally
delivery
of
free
OMVs
was
not
protective,
while
the
encapsulated
extract
conferred
full
protec-
tion.
4.
Discussion
Currently,
live
vaccines
provide
better
protection
as
compared
to
the
inactivated
vaccines,
including
the
acellular
ones
However,
it
is
always
difficult
to
properly
calibrate
attenuation
to
achieve
the
minimum
of
toxicity
with
the
optimal
immuno-
genicity.
Besides,
the
use
of
live
Shigella
vaccines
is
questionable
since
this
pathogen
is
able
to
strongly
interfere
with
the
immune
response,
by
inducing
an
immunosuppressive
condition
that
favors
infective
process.
In
our
present
experimental
study,
we
support
the
use
of
mucosal
immunization
with
acellular
vaccines.
Results
A.I.
Camacho
et
al.
/
Vaccine
29 (2011) 8222–
8229
8227
Fig.
4.
Immune
response
induced
after
vaccination
of
BALB/c
mice.
Cytokines
serum
level
(IL-10,
IL-12
(p40),
IL-12
(p70),
IL-5,
and
IFN-
␥)
detected
at
day
15
after
immunization
with
either
free
outer
membrane
vesicles
(OMVs)
(gray
bars)
or
loaded
in
nanoparticles
(NP-OMVs)
(black
bars).
Broken
line
indicates
serum
level
before
immunization.
Data
are
mean
value
(*P
<
0.001).
demonstrated
a
significant
efficacy
and
no
reactogenicity
in
the
mice
pulmonary
model.
The
best
prophylactic
measure
probably
would
be
to
prevent
bacterial
invasion
by
neutralizing
key
surface
virulence
factors.
The
outer
membrane
(OM)
of
Shigella
contains
several
main
vir-
ulence
factors,
including
outer
membrane
proteins
(OMP),
protein
adhesins,
the
highly
conserved
virulence-plasmid-encoded
Ipa
pro-
teins
well
as
LPS.
These
are
essential
components
in
the
invasion
process,
and
can
alter
the
course
of
infection
and
the
host
responses,
and
therefore
their
neutralization
for
the
host
will
suc-
ceed
in
protective
immunity
Outer
membrane
vesicles
(OMVs)
consist
of
OM
and
solu-
ble
periplasmic
components
shed
from
Gram-negative
bacteria.
This
blebbing
process
is
considered
as
a
peculiar
bacterial
extra-
cellular
secretion
system
than
enable
bacterial
colonization
and
impairs
host
immune
response
Therefore,
it
is
plausible
to
think
on
Shigella
OMVs
as
ideal
candidates
for
an
acellular
vac-
cine.
The
capacity
of
OMV-based
vaccines
to
stimulate
a
protective
immune
response
has
already
been
exploited
against
several
bac-
terial
pathogens,
such
as
Brucella
ovis
S.
typhimurium
Flavobacterium
Neisseria
meningitides
B,
with
over
55
million
doses
administered
to
date
of
the
former
As
many
Gram-negative
bacteria,
Shigella
bleb
off
membrane
vesicles
during
normal
growth.
Kadurugamuwa
and
Beveridge
already
obtained
and
characterized
membrane
vesicles
from
S.
flexneri
order
to
obtain
this
material
massively,
we
devel-
oped
an
extraction
protocol
that
also
maximize
OMV
purity.
Vesicles
were
isolated
from
concentrated,
cell-free
culture
super-
natant
leading
to
an
appropriate
antigenic
profile
as
well
as
high
purity
grade.
Besides,
final
product
was
ultradiafiltered
in
order
to
avoid
interferences
in
the
encapsulation
process.
OMV
used
here
contain
key
alarm
signals
such
as
LPS,
OMPs
and
Ipa
recognized
by
the
innate
immune
system,
including
epthe-
lial
cells,
MALT
and
antigen
presenting
cells
therefore
have
the
capacity
to
either
enhance
bacterial
clearance
or
cause
host
tissue
damage
by
activating
an
inflammatory
response.
It
is
interesting
to
note
that
these
components
provide
a
prolonged
stimulation
of
the
inflammatory
response
that,
at
first
instance,
facilitates
bacterial
survival
in
the
tissues.
However,
this
fact
will
lead
to
the
bacteria
elimination
by
the
host
immune
system
In
fact,
our
results
indicate
that
a
single
dose
of
non-adjuvated
OMVs
delivered
by
mucosal
routes
is
able
to
protect
against
a
lethal
challenge
with
S.
flexneri.
Vaccines
that
stimulate
protec-
tive
mucosal
immune
responses
often
need
an
adjuvant
for
proper
delivery
and
presentation
to
the
mucosal
immune
tissues.
The
mechanisms
underlying
the
effectiveness
of
free
OMV
without
external
adjuvant
may
be
explained
by
the
nature
of
some
individ-
ual
components
contained
within
this
“proteoliposome”
or/and
by
the
biophysical
properties
of
these
vesicles
Ipa
con-
taining
OMVs
may
contribute
to
its
adjuvanticity
by
their
ability
to
interact
with
host
cell
receptors
which
facilitate
OMVs
transcyto-
sis
across
mucosal
epithelial
barriers
the
other
hand,
the
amphipatic
properties
of
OMVs
may
facilitate
its
own
movement
through
mucosal
tissues,
enhancing
antigen
presentation
to
drive
a
protective
response.
In
this
study,
we
measured
the
levels
of
cytokines
in
OMVs
vac-
cinated
mice
2
weeks
after
the
immunization.
Then,
we
analyzed
their
association
with
the
challenge
outcome.
A
strong
association
Fig.
5.
Protection
study
against
Shigella
flexneri.
BALB/c
mice
(20
±
1
g)
were
immunized
with
20
g
of
outer
membrane
vesicles
either
free
(OMVs)
or
loaded
into
nanoparticles
of
PVM/MA
(NP-OMVs)
by
intradermal
(
),
nasal
(
),
ocular
(
)
or
oral
(
),
routes.
An
extra
group
was
included
as
non-immunized
control
(
×).
At
day
35
after
immunization,
all
groups
received
an
intranasal
lethal
challenge
of
10
7
UFC/mouse
of
Shigella
flexneri
2a
(clinical
isolate).
Graphs
indicate
the
percentage
of
mice
that
survived
the
infective
challenge
at
the
indicated
days
after
immunization
(*P
<
0.01,
Logrank
test).

8228
A.I.
Camacho
et
al.
/
Vaccine
29 (2011) 8222–
8229
between
the
ratio
of
IL-12p40/IL-10
and
protection
was
found.
Moreover,
low
levels
of
IFN-
␥
correlated
with
protection.
However,
conclusions
from
these
particular
data
must
be
taken
with
caution
since
cytokine
levels
were
measured
directly
from
serum.
At
this
point,
further
studies
are
being
carried
out
to
really
establish
a
correlation
of
these
parameters
and
protection.
After
oral
administration,
under
steady-state
conditions,
some
factors
released
by
enterocytes,
such
as
retinoic
acid,
thymic
stromal
lymphopoietin
and
TGF-
,
will
“condition”
non-activated
resident
DCs
to
elicit
a
Th2
or
regulatory
responses
following
an
inflammatory
stimulus,
a
recruitment
of
DC
express-
ing
CX3CR1
to
the
mucosal
tissues
is
observed,
increasing
the
number
of
DC
extending
dendrites
into
intestinal
lumen.
Under
this
state
of
high
activation,
DC-expressing
massively
co-stimulatory
molecules,
present
the
antigenic
determinant
to
the
specific
T
naïve
cells
in
the
T
area
MALT.
The
substantial
distinctive
release
of
IL-12
from
those
DCs
will
also
contribute
to
the
further
differentiation
of
naïve
cells
to
Th1/Th2/Th17,
linked
to
an
inflammatory
response.
Actually,
our
results
would
support
it
since
OMVs
adjuvanted
into
NPs
induced
increasing
levels
of
IL-12
(p40)
and
decreasing
IL-10
with
respect
to
the
free
form,
either
by
intradermal
or
oral
delivery.
NPs
can
enhance
the
delivery
of
the
loaded
antigen
to
the
gut
lym-
phoid
cells
due
to
their
ability
to
be
captured
and
internalized
by
cells
of
the
gut-associated
lymphoid
tissue
(GALT),
and
to
induced
maturation
of
DCs
with
a
significant
upregulation
of
CD40,
CD80,
and
CD86
and
a
Th1
response
in
animal
models.
The
mechanisms
responsible
for
DC
maturation
may
be
related
to
TLR-NP
specific
interaction
On
the
other
hand,
the
encapsulation
of
OMVs
in
NPs
induced
an
increase
in
the
level
of
IL-10
and
a
decrease
of
IL-12
(p40)
with
respect
to
the
free
form,
by
ocular
or
nasal
routes,
which
is
char-
acteristic
of
mucosal
adjuvants
that
usually
stimulate
a
Th2
T-cell
response
by
increased
secretory
IgA,
high
proportions
of
antigen-specific
serum
IgG1,
and
the
stimulation
and
synthesis
of
IL-4,
IL-5,
and
IL-10.
The
specific
immune
mechanisms
that
mediate
resistance
to
Shigella
infection
have
not
been
clearly
defined
and
are
currently
being
debated.
Thus,
in
humans,
up
regulation
of
both
proinflam-
matory
and
anti-inflammatory
are
observed
during
the
first
stages
of
infection.
Later,
in
relation
with
the
convalescent
stage
of
shigel-
losis,
an
increase
in
IFN-
␥
is
observed.
Summing
up,
although
Th1
is
effective
to
control
infection,
a
Th2
response
may
be
also
as
effective
but
shorter-lasting.
Concerning
the
antibody
response
elicited
after
OMV
immu-
nization,
we
cannot
establish
a
relation
between
antibody
levels
and
protection.
Serum
and
mucosal
antibodies
to
LPS
and
the
Ipa
proteins
have
been
demonstrated
during
human
shigellosis
However,
it
has
not
been
established
the
role
of
these
antibodies
to
limit
the
spread
or
severity
of
the
infection.
The
apparent
inconsis-
tency
between
IgG
subclass
response
and
cytokine
profile
may
be
due
to
immune
cells
other
than
T
helper
cells.
The
ultimate
goal
for
vaccination
is
to
stimulate
long-lasting
protective
immunological
memory.
Toll-like
receptors
ally
promote
adaptive
immune
responses
indirectly
by
activating
innate
immune
cells.
It
has
been
recently
shown
that
the
use
of
multiple
TLR-agonists
carried
by
nanoparticles
influence
in
the
induction
of
long-term
memory
cells
Recent
studies
report
that
in
a
murine
model
of
acute
bacte-
rial
infection
with
S.
flexneri
the
T
cell
response
is
dominated
by
the
induction
of
long-term
memory
Shigella-specific
Th17
cells
that
contribute
to
mediate
protective
immunity
against
reinfection
Now,
new
research
shows
an
unexpected
direct
role
for
TLR2
signalling
in
T
cells
themselves,
promoting
the
differentiation
and
proliferation
of
T
helper
17
(T
H
17)
cells
into
account
these
data
and
together
with
previous
results
from
our
own
group
about
the
high
ability
of
PVM/MA
to
stimulate
TLR2
that
these
nanoparticles
are
good
adjuvant
candidate
for
further
investigation.
OMVs
are
safe
and
protective
in
mice,
therefore,
the
use
of
OMVs
adjuvanted
into
NP
to
trigger
mucosal
immunity
and
effectively
neutralize
Shigella
infection
open
the
door
to
safely
deals
with
vaccination,
especially
critical
when
young
children
are
the
primary
target.
Acknowledgments
This
research
was
financially
supported
by
Health
Department
of
“Gobierno
de
Navarra”
(28/2007),
“Instituto
de
Salud
Carlos
III”
(PS09/01083
and
PI070326),
from
Spain.
Ana
Camacho
was
also
financially
supported
by
“Instituto
de
Salud
Carlos
III”
(FI08/00432).
References
[1]
Kotloff
KL,
Winickoff
JP,
Ivanoff
B,
Clemens
JD,
Swerdlow
DL,
Sansonetti
PJ,
et
al.
Global
burden
of
Shigella
infections:
implications
for
vaccine
devel-
opment
and
implementation
of
control
strategies.
Bull
World
Health
Organ
1999;77(8):651–66.
[2]
Levine
MM,
Kotloff
KL,
Barry
EM,
Pasetti
MF,
Sztein
MB.
Clinical
trials
of
Shigella
vaccines:
two
steps
forward
and
one
step
back
on
a
long,
hard
road.
Nat
Rev
Microbiol
2007;5(July
(7)):540–53.
[3] Kweon
MN.
Shigellosis:
the
current
status
of
vaccine
development.
Curr
Opin
Infect
Dis
2008;21(June
(3)):313–8.
[4]
Kaminski
RW,
Oaks
EV.
Inactivated
and
subunit
vaccines
to
prevent
shigellosis.
Expert
Rev
Vaccines
2009;8(December
(12)):1693–704.
[5] Tamayo
I,
Irache
JM,
Mansilla
C,
Ochoa-Reparaz
J,
Lasarte
JJ,
Gamazo
C.
Poly(anhydride)
nanoparticles
act
as
active
Th1
adjuvants
through
Toll-like
receptor
exploitation.
Clin
Vaccine
Immunol
2010;17(September
(9)):1356–62.
[6] Ochoa
J,
Irache
JM,
Tamayo
I,
Walz
A,
DelVecchio
VG,
Gamazo
C.
Protective
immunity
of
biodegradable
nanoparticle-based
vaccine
against
an
experi-
mental
challenge
with
Salmonella
Enteritidis
in
mice.
Vaccine
2007;25(May
(22)):4410–9.
[7] Gomez
S,
Gamazo
C,
San
RB,
Vauthier
C,
Ferrer
M,
Irachel
JM.
Development
of
a
novel
vaccine
delivery
system
based
on
Gantrez
nanoparticles.
J
Nanosci
Nanotechnol
2006;6(September
(9–10)):3283–9.
[8]
Salman
HH,
Gamazo
C,
Campanero
MA,
Irache
JM.
Salmonella-like
bioadhesive
nanoparticles.
J
Control
Release
2005;106(August
(1–2)):1–13.
[9] Gomez
S,
Gamazo
C,
Roman
BS,
Ferrer
M,
Sanz
ML,
Irache
JM.
Gantrez
AN
nanoparticles
as
an
adjuvant
for
oral
immunotherapy
with
allergens.
Vaccine
2007;25(July
(29)):5263–71.
[10] Kulp
A,
Kuehn
MJ.
Biological
functions
and
biogenesis
of
secreted
bacterial
outer
membrane
vesicles.
Annu
Rev
Microbiol
2010;64(October
(13)):163–84.
[11]
Fernandez-Moreira
E,
Helbig
JH,
Swanson
MS.
Membrane
vesicles
shed
by
Legionella
pneumophila
inhibit
fusion
of
phagosomes
with
lysosomes.
Infect
Immun
2006;74(June
(6)):3285–95.
[12]
Kesty
NC,
Mason
KM,
Reedy
M,
Miller
SE,
Kuehn
MJ.
Enterotoxigenic
Escherichia
coli
vesicles
target
toxin
delivery
into
mammalian
cells.
EMBO
J
2004;23(November
(23)):4538–49.
[13] Kadurugamuwa
JL,
Beveridge
TJ.
Virulence
factors
are
released
from
Pseu-
domonas
aeruginosa
in
association
with
membrane
vesicles
during
normal
growth
and
exposure
to
gentamicin:
a
novel
mechanism
of
enzyme
secretion.
J
Bacteriol
1995;177(July
(14)):3998–4008.
[14]
Wai
SN,
Lindmark
B,
Söderblom
T,
Takade
A,
Westermark
M,
Oscarsson
J,
et
al.
Vesicle-mediated
export
and
assembly
of
pore-forming
oligomers
of
the
enter-
obacterial
ClyA
cytotoxin.
Cell
2003;115(October
(1)):25–35.
[15]
Mallett
CP,
Hale
TL,
Kaminski
RW,
Larsen
T,
Orr
N,
Cohen
D,
et
al.
Intransal
or
intragastric
immunization
with
proteosome-Shigella
lipopolysaccharide
vac-
cines
protects
against
lethal
pneumonia
in
a
murine
model
of
Shigella
infection.
Infect
Immun
1995;63(June
(6)):2382–6.
[16]
Horstman
AL,
Kuehn
MJ.
Enterotoxigenic
Escherichia
coli
secretes
active
heat-
labile
enterotoxin
via
outer
membrane
vesicles.
J
Biol
Chem
2000;275(April
(17)):12489–96.
[17]
Bahnemann
HG.
Inactivation
of
viral
antigens
for
vaccine
preparation
with
particular
reference
to
the
application
of
binary
ethylenimine.
Vaccine
1990;8(August
(4)):299–303.
[18] Gamazo
C,
Winter
AJ,
Moriyon
I,
Riezu-Boj
JI,
Blasco
JM,
Diaz
R.
Comparative
analyses
of
proteins
extracted
by
hot
saline
or
released
spontaneously
into
outer
membrane
blebs
from
field
strains
of
Brucella
ovis
and
Brucella
melitensis.
Infect
Immun
1989;57(May
(5)):1419–26.
[19]
Marolda
CL,
Lahiry
P,
Vines
E,
Saldias
S,
Valvano
MA.
Micromethods
for
the
char-
acterization
of
lipid
A-core
and
O-antigen
lipopolysaccharide.
Methods
Mol
Biol
2006;347:237–52.
[20] Lee
CH,
Tsai
CM.
Quantification
of
bacterial
lipopolysaccharides
by
the
pur-
pald
assay:
measuring
formaldehyde
generated
from
2-keto-3-deoxyoctonate
and
heptose
at
the
inner
core
by
periodate
oxidation.
Anal
Biochem
1999;267(February
(1)):161–8.

A.I.
Camacho
et
al.
/
Vaccine
29 (2011) 8222–
8229
8229
[21]
Horstman
AL,
Bauman
SJ,
Kuehn
MJ.
Lipopolysaccharide
3-deoxy-D-manno-
octulosonic
acid
(Kdo)
core
determines
bacterial
association
of
secreted
toxins.
J
Biol
Chem
2004;279(February
(9)):8070–5.
[22]
Bahrani
FK,
Sansonetti
PJ,
Parsot
C.
Secretion
of
Ipa
proteins
by
Shigella
flexneri:
inducer
molecules
and
kinetics
of
activation.
Infect
Immun
1997;65(October
(10)):4005–10.
[23]
Arbos
P,
Wirth
M,
Arangoa
MA,
Gabor
F,
Irache
JM,
Gantrez.
AN
as
a
new
poly-
mer
for
the
preparation
of
ligand-nanoparticle
conjugates.
J
Control
Release
2002;83(October
(3)):321–30.
[24] Diaz
R,
Bosseray
N.
Study
of
the
antigenic
relations
between
Yersina
entero-
colitica
serotype
9
and
other
Gram
negative
bacterial
strains.
Microbiol
Esp
1974;27(January
(1)):1–14.
[25]
Mukhopadhaya
A,
Mahalanabis
D,
Chakrabarti
MK.
Role
of
Shigella
flexneri
2a
34
kDa
outer
membrane
protein
in
induction
of
protective
immune
response.
Vaccine
2006;24(August
(33–34)):6028–36.
[26] Al-Hasani
K,
Navarro-Garcia
F,
Huerta
J,
Sakellaris
H,
Adler
B.
The
immuno-
genic
SigA
enterotoxin
of
Shigella
flexneri
2a
binds
to
HEp-2
cells
and
induces
fodrin
redistribution
in
intoxicated
epithelial
cells.
PLoS
One
2009;4(12):
e8223.
[27] Parsot
C,
Menard
R,
Gounon
P,
Sansonetti
PJ.
Enhanced
secretion
through
the
Shigella
flexneri
Mxi-Spa
translocon
leads
to
assembly
of
extracellular
proteins
into
macromolecular
structures.
Mol
Microbiol
1995;16(April
(2)):291–300.
[28]
Kadurugamuwa
JL,
Beveridge
TJ.
Delivery
of
the
non-membrane-permeative
antibiotic
gentamicin
into
mammalian
cells
by
using
Shigella
flexneri
membrane
vesicles.
Antimicrob
Agents
Chemother
1998;42(June
(6)):1476–83.
[29]
Levine
MM.
Immunogenicity
and
efficacy
of
oral
vaccines
in
developing
coun-
tries:
lessons
from
a
live
cholera
vaccine.
BMC
Biol
2010;8:129.
[30]
Canh
DG,
Lin
FY,
Thiem
VD,
Trach
DD,
Trong
ND,
Mao
ND,
et
al.
Effect
of
dosage
on
immunogenicity
of
a
Vi
conjugate
vaccine
injected
twice
into
2-
to
5-year-
old
Vietnamese
children.
Infect
Immun
2004;72(November
(11)):6586–8.
[31]
Jennison
AV,
Verma
NK.
Shigella
flexneri
infection:
pathogenesis
and
vaccine
development.
FEMS
Microbiol
Rev
2004;28(February
(1)):43–58.
[32]
Sansonetti
PJ,
Tran
Van
NG,
Egile
C.
Rupture
of
the
intestinal
epithelial
bar-
rier
and
mucosal
invasion
by
Shigella
flexneri.
Clin
Infect
Dis
1999;28(March
(3)):466–75.
[33] Li
A,
Rong
ZC,
Ekwall
E,
Forsum
U,
Lindberg
AA.
Serum
antibody
responses
against
shigella
lipopolysaccharides
and
invasion
plasmid-coded
antigens
in
shigella
infected
Swedish
patients.
Scand
J
Infect
Dis
1993;25(5):569–77.
[34]
Ellis
TN,
Kuehn
MJ.
Virulence
and
immunomodulatory
roles
of
bacterial
outer
membrane
vesicles.
Microbiol
Mol
Biol
Rev
2010;74(March
(1)):81–94.
[35]
Alaniz
RC,
Deatherage
BL,
Lara
JC,
Cookson
BT.
Membrane
vesicles
are
immuno-
genic
facsimiles
of
Salmonella
typhimurium
that
potently
activate
dendritic
cells,
prime
B
and
T
cell
responses,
and
stimulate
protective
immunity
in
vivo.
J
Immunol
2007;179(December
(11)):7692–701.
[36] Aoki
M,
Kondo
M,
Nakatsuka
Y,
Kawai
K,
Oshima
S.
Stationary
phase
culture
supernatant
containing
membrane
vesicles
induced
immunity
to
rainbow
trout
Oncorhynchus
mykiss
fry
syndrome.
Vaccine
2007;25(January
(3)):561–9.
[37]
Zhang
T,
Hashizume
T,
Kurita-Ochiai
T,
Yamamoto
M.
Sublingual
vaccination
with
outer
membrane
protein
of
Porphyromonas
gingivalis
and
Flt3
ligand
elicits
protective
immunity
in
the
oral
cavity.
Biochem
Biophys
Res
Commun
2009;390(December
(3)):937–41.
[38]
Holst
J,
Martin
D,
Arnold
R,
Huergo
CC,
Oster
P,
O’Hallahan
J,
et
al.
Properties
and
clinical
performance
of
vaccines
containing
outer
membrane
vesicles
from
Neisseria
meningitidis.
Vaccine
2009;27(June
(Suppl
2)):B3–12.
[39] Schroeder
GN,
Hilbi
H.
Molecular
pathogenesis
of
Shigella
spp.:
controlling
host
cell
signaling,
invasion,
and
death
by
type
III
secretion.
Clin
Microbiol
Rev
2008;21(January
(1)):134–56.
[40]
Biswas
A,
Banerjee
P,
Mukherjee
G,
Biswas
T.
Porin
of
Shigella
dysenteriae
activates
mouse
peritoneal
macrophage
through
Toll-like
receptors
2
and
6
to
induce
polarized
type
I
response.
Mol
Immunol
2007;44(February
(5)):812–20.
[41] Phalipon
A,
Sansonetti
PJ.
Shigella’s
ways
of
manipulating
the
host
intestinal
innate
and
adaptive
immune
system:
a
tool
box
for
survival?
Immunol
Cell
Biol
2007;85(February
(2)):119–29.
[42]
Sanders
H,
Feavers
IM.
Adjuvant
properties
of
meningococcal
outer
membrane
vesicles
and
the
use
of
adjuvants
in
Neisseria
meningitidis
protein
vaccines.
Expert
Rev
Vaccines
2011;10(March
(3)):323–34.
[43] Rimoldi
M,
Chieppa
M,
Salucci
V,
Avogadri
F,
Sonzogni
A,
Sampietro
GM,
et
al.
Intestinal
immune
homeostasis
is
regulated
by
the
crosstalk
between
epithelial
cells
and
dendritic
cells.
Nat
Immunol
2005;6(May
(5)):507–14.
[44]
Petrovsky
N,
Aguilar
JC.
Vaccine
adjuvants:
current
state
and
future
trends.
Immunol
Cell
Biol
2004;82(October
(5)):488–96.
[45]
Lindblad
EB.
Aluminium
compounds
for
use
in
vaccines.
Immunol
Cell
Biol
2004;82(October
(5)):497–505.
[46]
Bungener
L,
Geeraedts
F,
Ter
VW,
Medema
J,
Wilschut
J,
Huckriede
A.
Alum
boosts
TH2-type
antibody
responses
to
whole-inactivated
virus
influenza
vac-
cine
in
mice
but
does
not
confer
superior
protection.
Vaccine
2008;26(May
(19)):2350–9.
[47]
Coster
TS,
Hoge
CW,
VanDeVerg
LL,
Hartman
AB,
Oaks
EV,
Venkatesan
MM,
et
al.
Vaccination
against
shigellosis
with
attenuated
Shigella
flexneri
2a
strain
SC602.
Infect
Immun
1999;67(July
(7)):3437–43.
[48]
Oberhelman
RA,
Kopecko
DJ,
Salazar-Lindo
E,
Gotuzzo
E,
Buysse
JM,
Venkate-
san
MM,
et
al.
Prospective
study
of
systemic
and
mucosal
immune
responses
in
dysenteric
patients
to
specific
Shigella
invasion
plasmid
antigens
and
lipopolysaccharides.
Infect
Immun
1991;59(July
(7)):2341–50.
[49]
Bird
L.
T
cells:
TLRs
deliver
a
direct
hit
to
TH17
cells.
Nat
Rev
Immunol
2010;10(June
(6)):384.
[50] Kasturi
SP,
Skountzou
I,
Albrecht
RA,
Koutsonanos
D,
Hua
T,
Nakaya
HI,
et
al.
Programming
the
magnitude
and
persistence
of
antibody
responses
with
innate
immunity.
Nature
2011;470(February
(7335)):543–7.
[51]
Sellge
G,
Magalhaes
JG,
Konradt
C,
Fritz
JH,
Salgado-Pabon
W,
Eberl
G,
et
al.
Th17
cells
are
the
dominant
T
cell
subtype
primed
by
Shigella
flexneri
mediating
protective
immunity.
J
Immunol
2010;184(February
(4)):2076–85.
Document Outline
- Mucosal immunization with Shigella flexneri outer membrane vesicles induced protection in mice
- 1 Introduction
- 2 Materials and methods
- 3 Results
- 4 Discussion
- Acknowledgments
- References
Wyszukiwarka
Podobne podstrony:
Proteomics in gram negative bacterial outer membrane vesicles
Proteomics in gram negative bacterial outer membrane vesicles
Outer membrane permeability and antibiotic resistance
Outer membrane proteins key players for bacterial adaptation
A proteogenomic analysis of Sh flexneri
Molecular bases of epithelial cell invasion by Sh Flexneri
The pathogenesis of Sh flexneri infection lessons from in vitro and in vivo studies
Exposure of Sh flexneri to acid stress and heat shock enhances acid tolerance
Beta barrel proteins form bacterial outer membranes
Improving virus protection with an efficient secure architecture with memory encryption, integrity a
Differences in mucosal gene expression in the colon of two inbred mouse strains after colonization w
Image Processing with Matlab 33
Przywileje i immunitety dyplomatyczne 11b
L 5590 Short Sleeved Dress With Zipper Closure
M 5190 Long dress with a contrast finishing work
więcej podobnych podstron