
Recent Patents on Biotechnology 2010, 4, 23-29
23
1872-2083/10 $100.00+.00
© 2010 Bentham Science Publishers Ltd.
Secretory Production of Recombinant Proteins in Escherichia coli
Sung Ho Yoon
1
, Seong Keun Kim
1
and Jihyun F. Kim
1,2
*
1
Industrial Biotechnology and Bioenergy Research Center, Korea Research Institute of Bioscience and Biotechnology
(KRIBB), 111 Gwahangno, Yuseong, Daejeon 305-806, Republic of Korea,
2
Functional Genomics Program, School of
Science, University of Science and Technology, Yuseong, Daejeon 305-333, Republic of Korea
Received: July 31, 2009; Accepted: September 8, 2009; Revised: September 25, 2009
Abstract: Extracellular production of heterologous proteins using the Escherichia coli cell factory offers several
advantages over intracellular production and mammalian culture. Properly folded proteins can be rapidly accumulated in
the culture media, and downstream processes for isolation and purification can be much simplified. Efforts to enhance the
secretory production of target proteins can be largely classified as selection and modification of the signal peptide,
coexpression of proteins to assist translocation and folding, improvement of periplasmic release, and protection of target
proteins from degradation and contamination. Here, we review recent patents on the secretory production of recombinant
proteins in E. coli.
Keywords: Secretion, excretion, periplasm, extracellular production, signal peptide, recombinant protein, Escherichia coli.
INTRODUCTION
Inarguably,
Escherichia coli has been the most widely
used organism for mass-production of recombinant proteins
of pharmaceutical and industrial importance. Despite the
lack of post-translational modification and the existence of
endotoxin, this remarkable microorganism has numerous
desirable characteristics as a production host such as fast cell
growth, easy manipulation, straightforward high cell density
cultivation, and capacity to hold over 50% of foreign protein
in total protein expression [1].
Secretory production of recombinant proteins in E. coli
has been particularly useful for production of pharmaceutical
proteins [2,3]. Compared to cytoplasmic production,
targeting a protein of interest to the periplasmic space or the
culture medium enables downstream processing much easier
at a reduced process cost. Isolation and purification of the
over-expressed products can be much simplified due to
reduced contamination of various cellular components and
circumvention of proteolytic degradation by intracellular
proteases. Correct folding of eukaryotic proteins containing
multiple disulfide bonds is more likely to happen in the
oxidative environment of the periplasm. Secretory process
allows removal of the amino-terminal signal sequence,
which leads to the appearance of mature proteins as naturally
occurring sequences contain no N-terminal methionine
residue. In addition, laboratory and industrial strains of E.
coli normally do not secrete much extracellular proteins [4],
which further facilitates already simple purification steps.
E.
coli has various systems (Type I through V) for
transporting proteins from the cytosol into the periplasm or
the extracellular milieu [5]. In the type I mechanism, an ATP
binding cassette (ABC) transporter recognizes a C-
*Address correspondence to this author at the KRIBB, 111 Gwahangno,
Yuseong-gu, Daejeon 305-806, Republic of Korea; Tel: +82-42-860-4412;
Fax: +82-42-879-8595; E-mail: jfk@kribb.re.kr
terminal signal peptide, in most cases E. coli
–haemolysin
(HlyA), connected to a target protein, and transports it
directly from the cytoplasm to the medium. As the secreted
protein doesn’t experience a periplasmic intermediate, it
contains a signal sequence which should be cleaved off later
to result in the intact native protein. The type II secretion
system transports proteins in two steps, periplasmic
translocation and extracellular transport. Transport from the
cytoplasm to the periplasm is mediated by the Sec-dependent
pathway or the twin-arginine translocation (Tat) pathway. In
the Sec-dependent pathway, the cytosolic chaperone SecB or
the ribonucleoprotein signal recognition particle (SRP) binds
an unfolded preprotein harboring an N-terminal signal
peptide and moves them to inner membrane-bound SecA [6].
By contrast, the Tat system recognizes a folded preprotein by
TatBC complex. When the preprotein traverses the inner
membrane through SecYEG or TatA channel, the signal
peptide is removed by a signal peptidase. Extracellular
release of the periplasmic protein is carried out by a
concerted action of 12-16 proteins constituting a secreton.
Attempts to improve the secretion productivity have been
made on every step of the secretion process [2,3]. Trans-
portation of cytosolic recombinant proteins to the cyto-
plasmic membrane can be enhanced by proper selection and
modification of the signal peptide. In a next step where the
precursor protein is transported across the cytoplasmic
membrane to the periplasm, proteins constituting the
transport machinery can be co-expressed with a target
protein. Finally, the periplasmic protein can be secreted to
the culture medium by osmotic shock or periplasmic lea-
kage. E. coli host cells have been engineered to be a more
efficient cell factory for secretory production of foreign
proteins. For example, it can be made devoid of host-specific
proteases or genes controlling the regulation of proteases.
Despite remarkable progress in utilizing the E. coli cell
factory for secretory production of recombinant proteins, the
challenges of industrial production are still daunting. Target
24 Recent Patents on Biotechnology 2010, Vol. 4, No. 1
Yoon et al.
protein production is limited by insufficient capacity of the
transport machinery. Especially, secretory production into
the periplasm is physically limited by periplasmic volume
and normally results in increased cellular burden and
reduced cell growth. Also, foreign proteins can be degraded
by host proteases. High rate of target translation may lead to
accumulation inside the cell as an inclusion body.
In this review, we summarize various attempts to inc-
rease secretory production of recombinant proteins and
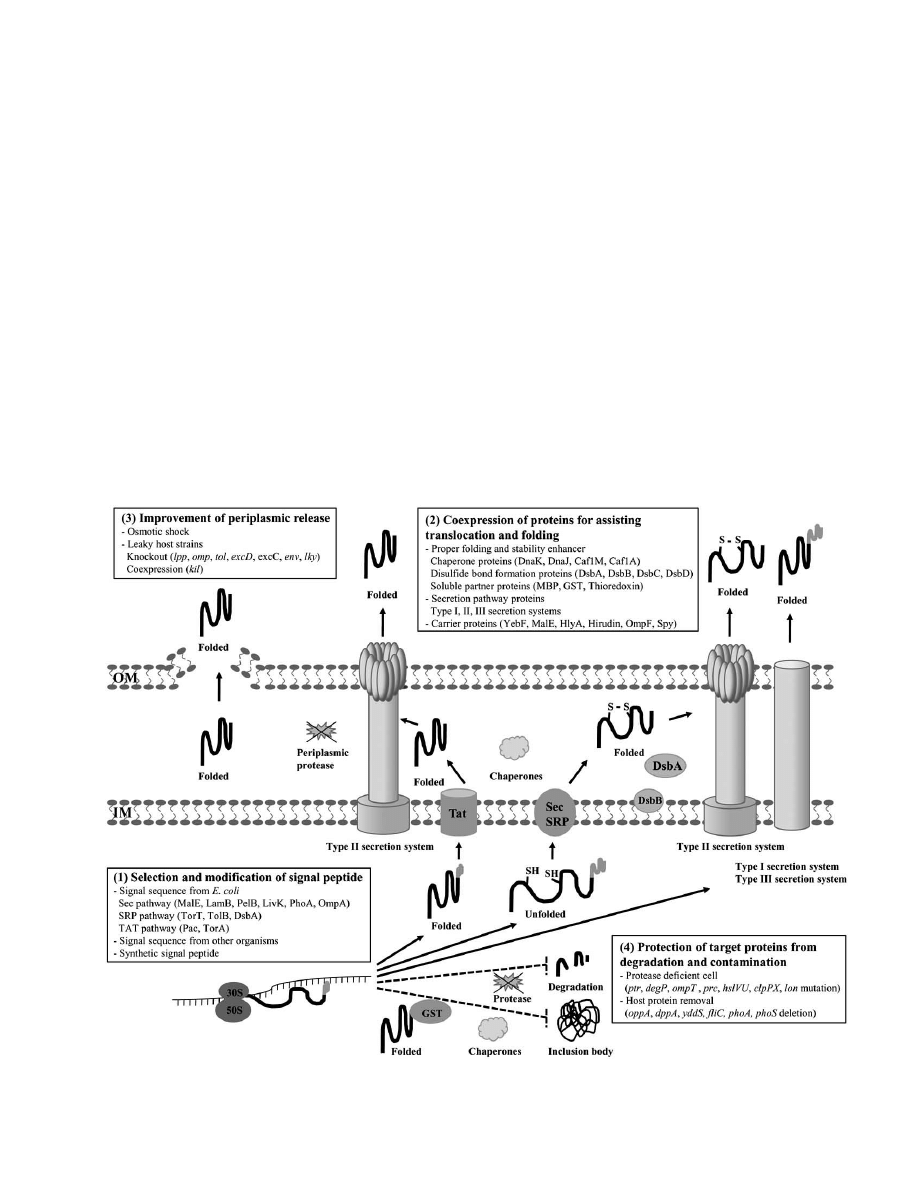
applications recently patented (Fig. (1)).
1. SELECTION AND MODIFICATION OF THE
SIGNAL PEPTIDE
Selection of a signal peptide is the primary choice for
efficient secretion of a recombinant protein. The majority of
the signal peptides developed are for secretory production
through the Sec-dependent system. In E. coli, signal
sequences typically consist of a positively charged n-region,
a nonpolar hydrophobic h-region, and a neutral polar c-
region which has a cleavage site for the signal peptidase [2].
By pattern match or sequence homology searches with
known ones, for example BLAST searches into public seq-
uence databases such as GenBank, Swiss-Prot or a sequen-
ced genome, signal sequences can be readily identified.
Also, machine learning approaches such as a neural net-work
or hidden Markov model can be used for the computational
prediction of signal peptides and the cleavage site [7].
In general, eukaryotic signal peptides do not function
efficiently in a prokaryotic host and has been applied for
prokaryotic expression with limited success [8]. A number of
signal peptides from bacterial proteins have been linked to
recombinant proteins. A full-length immunoglobulin G (IgG)
antibody having a complex structure was secreted to the
culture media by being attached to signal sequences from
bacterial proteins which are translocated via various secre-
tion pathways – the Sec pathway (maltose-binding protein
subunit, MalE; maltoporin, LamB; pectate lyase subunit,
PelB; leucine binding protein subunit LivK; alkaline phos-
phatase subunit, PhoA; outer membrane protein, OmpA), the
SRP pathway (periplasmic sensory protein, TorT; subunit of
the Tol-Pal cell envelope complex, TolB; disulfide
oxidoreductase, DsbA), and the Tat pathway (penicillin
acylase, Pac; subunit of trimethylamine N-oxide reductase I,
TorA) [9]. Heterologous signal sequences can come from
pullulanases (derived from Klebsiella, Thermoanaero-
bacter, and Thermotoga), penicillinase (Staphylococcus,
Fig. (1). Strategies for enhancing the secretory production in Escherichia coli

Secretory Production in E. coli
Recent Patents on Biotechnology 2010, Vol. 4, No. 1 25
Klebsiella, and Bacillus), cyclomaltodextrin glucano-
transferase (Klebsiellla oxytoca), or PelB (Erwinia and Xan-
thomonas). Also, the signal sequence of the bacteriophage
M13 major coat protein can be used for antibody production
[10] and that of the Bacillus endoxylanase for human granu-
locyte colony stimulating factor (hG-CSF) [11]. US020080-
193974A1 [12] claimed a variety of signal sequences for a
mutant phosphate binding protein (Pdb), disulfide
oxidoreductase (DsbA), protein disulfide isomerase (DsbC),
Bce, CupA2, CupB2, CupC2, NikA, FlgI, a tetratricopeptide
repeat family protein (ORF5550), a toluene tolerance protein
(Ttg2C), and a methyl accepting chemotaxis protein (ORF-
8124). Until now, only a limited number of bacterial signal
peptides such as those of OmpA, LamB, PelB, PhoA, and
thermostable enterotoxin II (STII) have been widely used in
recombinant E. coli.
The signal sequence has been artificially modified and
optimized. It is reported that the positive charge of the N-
terminal region and the hydrophobicity of the central
hydrophobic region are important for secretion efficiency
[13,14]. Bacterial signal sequences such as STII have been
modified [15,16]. A novel synthetic signal peptide can be
designed based on a rule-based approach. EP00000-
1290197B1 [17] describes synthetic features of a signal
peptide – (i) two or more positively charged amino acids
close to the N terminus, (ii) a region of between 7 and 16
consecutive hydrophobic amino acid residues, (iii) one or
more amino acids which may act as an alpha helix disrupter,
and (iv) at the C-terminus, the sequence Z-X-Z, wherein Z is
an amino acid having a small side chain and X is any amino
acid. Various modifications to the native signal peptide have
been made, too. OmpA signal sequence was modified to
ensure production of the nerve growth factor without N-
terminal methionine [18]. Basic amino acid-rich polypeptide
was added after the signal peptide [19]. A secretional
enhancer consisting of hydrophilic amino acids was linked to
the basic n-region alone or the basic n-region and the
hydrophobic h-region [20]. To ensure that the signal is
appropriately expressed and cleaved from the expressed
protein, alanine-phenylalanine-alanine was added just before
the cleavage site [21]. Along with the synthetic approach,
experimental methods to screen for a new signal peptide
have been developed [22].
2. COEXPRESSION OF PROTEINS FOR ASSISTING
TRANSLOCATION AND FOLDING
Coexpression of helper proteins with target proteins has
been exploited for a proper folding and translocation through
the cytosolic membrane. They come in separate expression
vectors or are fused with the target protein.
2.1. Coexpression of Proteins that Enhance a Proper
Folding and Solubility
Expression of many heterologous proteins in E. coli often
leads to intracellular aggregates due to their incorrect folding
[23]. Although inclusion body formation allows target pro-
teins to be highly resistant to proteolysis and to be expressed
in large quantity, it should be followed by a sophisticated
downstream processing such as isolation, renaturation, and
refolding to obtain functionally active proteins [24].
Two types of proteins, molecular chaperones and enzy-
mes involved in disulfide bond formation, have been
coexpressed for assisting in correct folding of the recom-
binant proteins. Coexpression of DnaK and DnaJ chaperones
remarkably increased the amount of granulocyte colony
stimulating factor (G-CSF) produced [25]. To increase
periplasmic folding and extracellular secretion of human
Interleukin-1 (hIL-1) or human granulocyte-macrophage
colony-stimulating factor (hGM-CSF), periplasmic cha-
perone (Caf1M) and usher protein (Caf1A) from Yersinia
pestis were coexpressed [26]. To translocate overexpressed
chaperones into the periplasm, an OmpA signal sequence
was fused to DnaJ, the N-terminal fragment of DnaJ, or a
small heat shock protein [27]. Genes encoding inclusion
body-associated proteins, IbpA and IbpB, were deleted from
the E. coli host to reduce inclusion body formation and to
enhance secretory production [28]. Conversely, those genes
were amplified to produce a target protein as an inclusion
body.
Most mammalian proteins contain multiple disulfide
bonds that require correct oxidation and pairing between the
two partner cysteines for their folding. Disulfide bond
formation in the cytoplasm is catalyzed by an oxidized form
of thioredoxins (TrxA and TrxC) and glutaredoxins [29,30].
However, in the cytosol of E. coli, these enzymes are mostly
in a reduced form by the action of thioredoxin reductase
(encoded by trxB),
-glutamylcysteine synthetase (gshA),
glutathione synthetase (gshB), and glutathione oxidore-
ductase (gor). US000007410788B2 [31] describes a method
for changing the reductive cytoplasm into a more oxidative
environment by introducing a null mutation to trxB, gshA,
gshB and/or gor. In order to increase the reducing capacity of
the cytoplasm enough to support the cell growth, the mutant
strain was further engineered to have a mutated form of
AhpC, subunit of alkyl hydroperoxidase. As for increasing
disulfide bond formation in the periplasm, E. coli disulfide
isomerases (DsbC or DsbG) [32] or human disulfide iso-
merase [33] have been coexpressed. Also, plasmids con-
taining cell envelope proteins involved in disulfide bond
formation (DsbA, DsbB, DsbC, and DsbD) have been
constructed [34, 35]. The periplasmic protein folding can be
improved simply by adding L-arginine [36] or a reducing
thiol reagent such as reduced glutathione [27].
Solubility of an insoluble polypeptide can be increased
by expressing a highly soluble partner such as maltose-
binding protein, glutathione S-transferase (GST), or thiore-
doxin [37]. Probability of protein solubility was calculated
based on the amino residues constituting the protein, and
aggregate-prone human interleukin-3 (hIL-3) was expressed
substantially in the soluble form when fused with NusA,
GrpE, YjgD-YjgD, or BFR [38]. Fusion expression systems
have been devised using the omega leader sequence of
tobacco mosaic virus [39], a lysyl tRNA synthetase [40], and
a domain of p26, SicA, or alpha crystalline type proteins
[41].
A method for monitoring folding and solubility of target
proteins in the periplasm has been devised using a reporter
gene. EP000001407052B1 [42] reported an on-line method
based on the promoter of the major periplasmic protease
DegP fused to the luciferase gene. WO002008089132A2

26 Recent Patents on Biotechnology 2010, Vol. 4, No. 1
Yoon et al.
[43] describes TEM-I -lactamase secreted through the SRP
dependent pathway. The N-terminal signal sequence from
proteins which can be recognized by the SRP dependent
pathway (e.g. DsbA, TotT, SfmC, TolB, YraI, CcmH, FocC,
NikA, and FlgI) was fused to the target protein sequence,
where -lactamase was attached as a reporter. These
monitoring methods would be useful in screening of protein
variants that fold correctly in the periplasm.
2.2. Coexpression of Proteins Involved in the Secretion
Pathways
If translocation into the periplasm is a rate-determining
step, coexpression of proteins involved in membrane
transport can be considered. The type II secretion pathway is
mostly used for industrial secretory production. As for Sec-
dependent secretion, SecY and/or SecE of E. coli were
overexpressed for enhancing the secretory production of
interleukin 6 (IL-6) [44]. In US5824502A [45], various
plasmids containing one or two sec genes (secB, secD/F,
secG and secE/Y) and the human growth hormone (hGH)
gene were constructed and transformed into various E. coli
strains. The accumulation of hGH in the periplasm varied
depending on the selection of the sec gene and the host
strain, and was affected by the expression level of sec.
Secretion pathways other than type II secretion can be
used for the production of heterologous proteins, too.
Although transport via the Tat pathway is less efficient and
slower than the Sec pathway, it has an outstanding feature of
transporting fully-folded cytosolic proteins through the
cytosolic membrane [46]. The Tat pathway can be used for
secreting proteins which fold slowly or incorrectly in the
periplasm and are often degraded before being getting
secreted into the medium. Weiner and coworkers reported a
paper and applied a patent on proteins which are involved in
the Tat pathway of E. coli and are encoded by the mttABC
operon [47,48]. Later, the mttA gene was found to be two
genes and the operon was renamed as tatABCD [49]. A
functional Tat pathway was also identified in Gram-positive
Bacillus subtilis, and expression of Bacillus tat genes was
required for the secretion of a Bacillus protein in E. coli [50].
Secretory production of the lipase or the protease of
Pseudomonas fluorescens in E. coli was accomplished by
expressing an ABC transporter gene cluster from P.
fluorescens [51]. E. coli harboring the type III secretion gene
(hrp) cluster of Erwinia chryanthemi was constructed for
expression of foreign proteins [52]. The strain also can be
used to screen effector proteins of the type III pathway
which are potential virulence factors of bacterial pathogens.
On the other hand, the oligopeptide permease (opp)
operon or the dipeptide permease (dpp) operon has been
disrupted to reduce the peptide uptake rate, ending up with
increased peptide secretion [53].
2.3. Coexpression of Carrier Proteins
Proteins directing the secretion of target proteins to the
periplasm or culture media can be used. Fusion system was
disclosed for the YebF putative lipoprotein [54], MalE [55],
and HlyA [56]. US000007202059B2 [57] discloses an
expression vector consisting of sequences for, in order,
hirudin, arginine, and the target protein. Hirudin drives the
target protein into the media and the fusion protein is cleaved
off at the arginine site by digestion with trypsin. The
secretion capacity can be further improved by adding a
signal peptide.
With the observation of a large accumulation of outer
membrane porin (OmpF) in culture media during high cell
density cultivation of E. coli BL21(DE3) [58], OmpF was
fused with the N-terminal region of
-endorphin [59].
Recently, an expression system in which OmpF is co-
expressed, but not fused to the target protein, was developed
and used for the production of epithelial cell growth factor
(EGF) and human leptin in a pure form [60]. Spy, a small
periplasmic protein having no cysteine residue can be used
as a carrier protein [61]. Oftentimes, the carrier protein
enables an affinity purification of the fusion protein.
3. IMPROVEMENT OF PERIPLASMIC RELEASE
Proteins accumulated in the periplasm can be made
secreted into the culture media through various strategies [3].
An apparatus and a method for subjecting cells to osmotic
shock were devised [62]. Leaky strains which are defective
in the outer membrane were constructed by introducing a
mutation in the coding region or in the promoter of the outer
membrane lipoprotein gene (lpp) [63]. Using various
mutants of omp, tol, excD, excC, lpp, env, and lky, full-
length antibodies were released to the culture media [64].
Along with a knock-out strategy, a membrane opening
system can be made by expression of the bacteriocin release
protein gene (kil). High expression of the kil gene causes cell
lysis, and thus, regulating the expression level and the
induction time is important. In the production of strepto-
kinase which is derived from Streptococcus equisimilis and
used in the treatment of heart diseases, bacteriocin release
protein (BRP) was induced to form permeable zones in the
cell envelope [65]. The kil gene was fused with a stationary
phase promoter and the target
-glucanase was released into
the surrounding media [66]. However, in general, those
leaky strains do not provide robustness enough for high-
density cultivation.
4. PROTECTION OF TARGET PROTEINS FROM
DEGRADATION AND CONTAMINATION
Preventing target proteins from degradation and conta-
mination can make the purification process much easier.
Protease-deficient cells are particularly suitable for the
production of proteolytically sensitive proteins. Georgiou et
al. [67] constructed many hosts deficient in ptr coding for
protease III, degP (periplasmic serine protease, HtrA), ompT
(outer membrane protease), and/or prc (periplasmic protease)
combined with the rpoH (heat shock sigma factor) mutation,
which resulted in a significantly increased yield in the
production of proteolytically sensitive peptides. Multiple
deletion mutations in the HslVU protease genes (hslVU) and
major ATP-dependent proteases genes of E. coli (clpPX and
lon) were constructed [68]. Gene and putative genes
encoding aminoamidases that cleave N-terminal amino acid
residues from some recombinant proteins such as human
growth hormone were all eliminated from the chromosome
[69]. To compensate for the growth and stability of the cells,
Secretory Production in E. coli
Recent Patents on Biotechnology 2010, Vol. 4, No. 1 27
the spr gene whose product suppresses cell growth exhibited
by prc mutants was further mutated [70].
To enhance the degree of purity of the produced proteins,
host proteins to be secreted to the culture media (OppA,
DppA, YddS, FliC, PhoA, and PhoS) were identified and the
coding genes were deleted from the host chromosome [71].
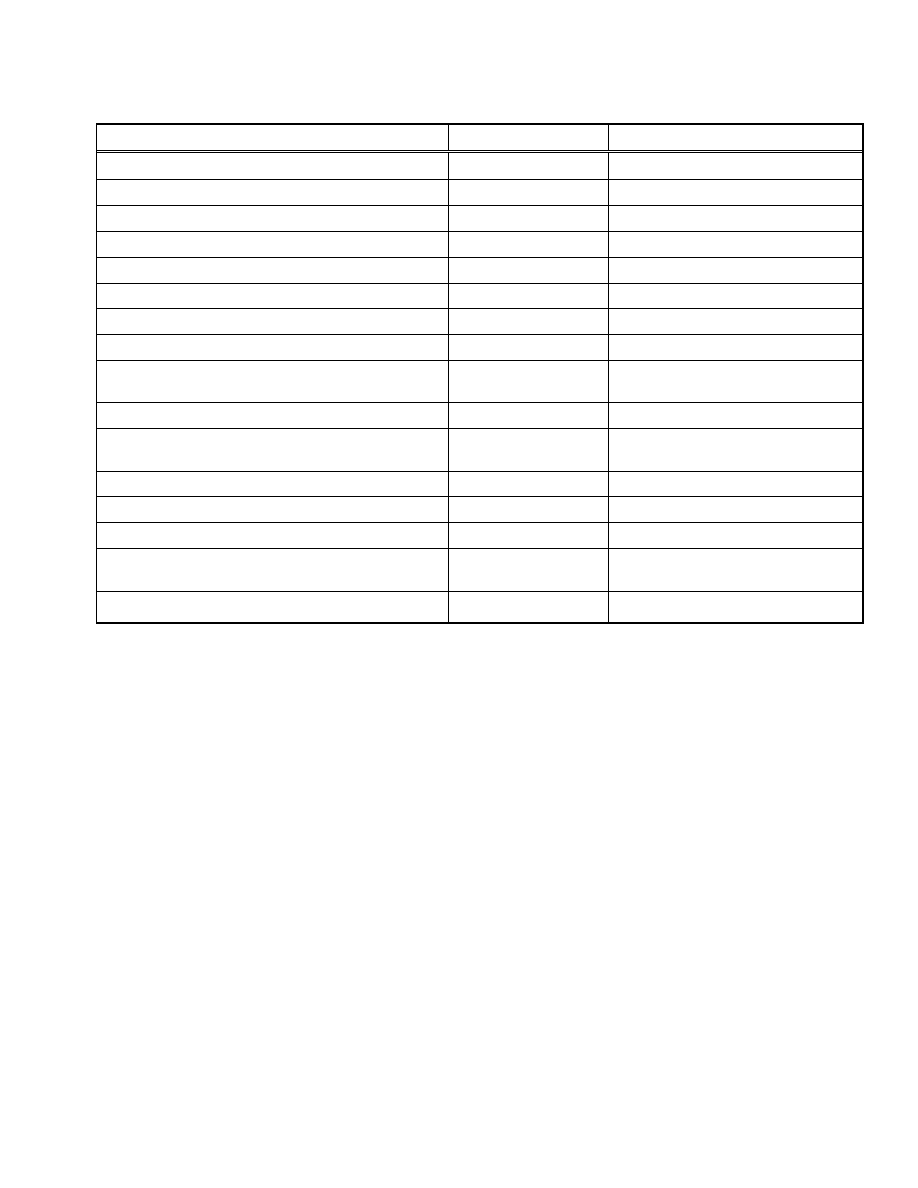
5. CURRENT & FUTURE DEVELOPMENTS
Past decades have witnessed remarkable advances in
understanding the membrane transport mechanism and
secretory production of foreign proteins using E. coli. A
number of aforementioned patents are now available in com-
mercially marketed systems (Table 1). However, industrial
application has been fraught with technical challenges. An
optimum signal peptide should be found by trial and error
because secretion efficiency largely depends on the
combination of the signal peptide, the target protein, and the
host strain, which is hard to predict. Coexpression of the
helper proteins can cause a severe metabolic burden to the
host cell and reduce the capacity to hold target proteins. In
addition, current understanding of function and regulation of
heat shock proteins is far from complete due to their comple-
xity. There remains to be issues regarding coordinated
activities of chaperones and proteases that supervise protein
folding and degradation, regulation of rpoH primarily at the
translational level, and possibility of the existence of another
protease [72]. Genetic mutations are normally accompanied
by growth defects, making such host strains of little
commercial value.
Traditional biotechnology based on the case-by-case
approach is turning into “systems biotechnology” which can
develop an efficient industrial process based on compu-
tational modeling and simulation that utilize high-throughput
omics data [73]. This transition is being accelerated by rapid
development of genome engineering tools [74] and synthetic
genomics. Recently, the genome sequence of BL21(DE3),
the most popular E. coli strain as an industrial host, along
with those of two BL21(DE3) derivatives and REL606
another B strain used in a long-term evolution experiment
have been determined and their omics based systems study
has begun [75-77]. Such advances will make it possible to
have the E. coli bioengine customized to secrete correctly
folded recombinant proteins into the culture medium in high
yield.
ACKNOWLEDGEMENTS
We thank Soon-Kyeong Kwon and Choong Hoon Lee for
critical reading of the manuscript. This work was financially
supported by the KRIBB Research Initiative Program and
the 21C Frontier Microbial Genomics and Applications
Center Program, Ministry of Science and Technology,
Republic of Korea.
Table 1.
Examples of Commercialized Protein Secretion Systems
Feature Company
Product
Sec, SRP, TAT pathway signal sequence
AthenaES
®
The
ACES
TM
Signal Sequence Kit
Sec pathway signal sequence (PelB)
Progen
pOPE 101 vector
Sec pathway signal sequence (PelB, OmpT)
Novagen
®
pET-12, 20, 22, 25, 26, 27 vectors
Sec pathway signal sequence (OmpA)
IBA
pASK-IBA2, 4, 6, 12, 14, 16, 32, 44 vectors
Sec pathway signal sequence (OmpA)
SIGMA
pFLAG-ATS
TM
, pFLAG-CTS
TM
vector
Leader peptide from the bacteriophage fd gene III protein (gIII)
Invitrogen
pBAD/gIII vector
Coexpression of chaperone proteins
TAKARA BIO INC.
Chaperone Plasmid Set
Enhanced cytoplasmic disulfide formation (trxB, gor mutant strain)
Novagen
®
Orgami
TM
, Orgami B series
Enhanced cytoplasmic disulfide formation (trxB, gor, ahpC mutant
and DsbC expression strain)
NEW ENGLAND BioLabs
®
INC.
SHuffle
TM
strains
Enhanced disulfide formation fusion (DsbA, DsbC)
Novagen
®
pET-39, 40 vectors
Soluble protein fusion (MBP)
NEW ENGLAND BioLabs
®
INC.
pMAL
TM
Protein Fusion and Purification System
Soluble protein fusion (Trx, GST, NusA)
Novagen
®
pET-32, 41, 42, 43.1 vectors
Soluble protein fusion (GST)
GE Healthcare
pGEX vectors
Carrier protein fusion (YebF)
AthenaES
®
The
ACES
TM
YebF Protein Export Kit
Carrier protein fusion (MBP fused with M13 pIII leader sequence)
NEW ENGLAND BioLabs
®
INC.
pMal-pIII vector
Enhanced secretion (Modified outer membrane strain)
Wacker Chemie AG
The WACKER secretion system

28 Recent Patents on Biotechnology 2010, Vol. 4, No. 1
Yoon et al.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
[1]
Choi JH, Keum KC, Lee SY. Production of recombinant proteins
by high cell density culture of Escherichia coli. Chem Eng Sci
2006; 61: 876-885.
[2]
Choi JH, Lee SY. Secretory and extracellular production of
recombinant proteins using Escherichia coli. Appl Microbiol
Biotechnol 2004; 64: 625-635.
[3]
Mergulhao FJ, Summers DK, Monteiro GA. Recombinant protein
secretion in Escherichia coli. Biotechnol Adv 2005; 23: 177-202.
[4]
Francetic O, Belin D, Badaut C, Pugsley AP. Expression of the
endogenous type II secretion pathway in Escherichia coli leads to
chitinase secretion. EMBO J 2000; 19: 6697-6703.
[5]
Thanassi DG, Hultgren SJ. Multiple pathways allow protein
secretion across the bacterial outer membrane. Curr Opin Cell Biol
2000; 12: 420-430.
[6]
van der Does C, Nouwen N, Driessen AJM. The SEC translocase.
Protein Secretion Pathways in Bacteria. In: Oudega B, Ed. Springer
USA 2003; 23-50.
[7]
Bendtsen JD, Nielsen H, Von Heijne G, Brunak S. Improved
prediction of signal peptides: SignalP 3.0. J Mol Biol 2004;
340:783-795.
[8]
Gray, J., Buechler, J., Veeramallu, U.K.: US20067094579B2
(2006).
[9]
Ostendorp, R., Popp, A., Fischer, M.: WO2009021548A1 (2009).
[10]
Humphreys, D.P.: EP1402036B1 (2004).
[11]
Lee, S.Y., Jeong, K.J.: US20067070989B2 (2006).
[12]
Coleman, R.J., Retallack, D., Schneider, J.C., Ramseier, T.M.,
Hershberger, C.D., Lee, S., Resnick, S.M.: US20080193974A1
(2008).
[13]
Andersson H, Von Heijne G. A 30-residue-long "export initiation
domain" adjacent to the signal sequence is critical for protein
translocation across the inner membrane of Escherichia coli. Proc
Natl Acad Sci USA 1991; 88: 9751-9754.
[14]
Hikita C, Mizushima S. Effects of total hydrophobicity and length
of the hydrophobic domain of a signal peptide on in vitro
translocation efficiency. J Biol Chem 1992; 267: 4882-4888.
[15]
Kwon, S.C., Jung, S.Y., Choi, K.D., Kim, C.S., Bae, S.M., Lee,
G.S.: US20067052867B2 (2006).
[16]
Kwon, S.C., Jung, S.Y., Shin, H., Choi, J.D., Choi, K.D., Lee, G.S.:
US20036605697B1 (2003).
[17]
Chen, T.-H.T., Schmidt, B.: EP1290197B1 (2003).
[18]
Meng, S.-Y., Morris, C.F., Tsai, L.B.: US5470719A (1995).
[19]
Inouye, S.: US20070287171A1 (2007).
[20]
Lee, S.J., Kim, Y.O., Nam, B.H.: US20090011995A1 (2009).
[21] Leonhartsberger, S., Candussio, A., Schmid, G.:
US20080076157A1 (2008).
[22]
Cannon, J.P., Haire, R.N., Litman, G.W.: US20067112434B2
(2006).
[23]
Kane JF, Hartley DL. Formation of recombinant protein inclusion
bodies in Escherichia coli. Trends Biotechnol 1988; 6: 95-101.
[24]
Chung BH, Choi YJ, Yoon SH, Lee SY, Lee YI. Process
development for production of recombinant human insulin-like
growth factor-I in Escherichia coli. J Ind Microbiol Biotechnol
2000; 24: 94-99.
[25]
Perez-Perez J, Martinez-Caja C, Barbero JL, Gutierrez J.
DNAK/DNAJ supplementation improves the periplasmic produc-
tion of human granulocyte-colony stimulating factor in Escherichia
coli. Biochem Biophys Res Commun 1995; 210: 524-529.
[26]
Korpela, T., Macintyreayane, S., Zavialov, A., Battchikova, N.,
Petrovskaya, L., Zav'yalov, V., Korobko, G.P.: US20056919198B1
(2005).
[27]
Ambrosius, D., Rudolph, R., Schaeffner, J., Schwarz, E.:
US026455279B1 (2002).
[28]
Lee, S.Y., Han, M.J., Park, S.J.: US20077291325B2 (2007).
[29]
Rietsch A, Beckwith J. The genetics of disulfide bond metabolism.
Annu Rev Genet 1998; 32: 163-184.
[30]
Stewart EJ, Aslund F, Beckwith J. Disulfide bond formation in the
Escherichia coli cytoplasm: an in vivo role reversal for the
thioredoxins. EMBO J 1998; 17: 5543-5550.
[31]
Beckwith, J., Aslund, F., Bessette, P.H., Georgiou, G., Ritz, D.,
Lim, J.E.-A.: US20087410788B2 (2008).
[32]
Georgiou, G., Oiu, J., Bessette, P., Swartz, J.: US006083715A
(2000).
[33]
Udaka, S., Sato, S., Kudo, T., Oka, S., Higashikuni, N., Kondo, M.:
EP1270730A1 (2003).
[34]
Joly, J.C., Swartz, J.R.: EP786009B1 (1997).
[35]
Kurokawa, Y., Yanagi, H., Yura, T.: US20046673569B1 (2004).
[36]
Ambrosius, D., Rudolph, R., Schaeffner, J., Schwarz, E.:
US016309861B1 (2001).
[37]
Kapust RB, Waugh DS. Escherichia coli maltose-binding protein is
uncommonly effective at promoting the solubility of polypeptides
to which it is fused. Protein Sci 1999; 8: 1668-1674.
[38]
Harrison, R.G., Davis, G.D.: US5989868A (1999).
[39]
Hua, Z., Zhang, H.: US20067056732B2 (2006).
[40]
Choi, S.I., Seong, B.L.: US20056852512B2 (2005).
[41]
Sanders, M.C.: US20056861403B2 (2005).
[42]
Horn, U., Strittmatter, W., Riesenberg, U.: EP1407052B1 (2004).
[43]
Delisa, M.P., Mansell, T.J., Fisher, A.C.: WO2008089132A2
(2008).
[44]
Perez-Perez J, Marquez G, Barbero JL, Gutierrez J. Increasing the
efficiency of protein export in Escherichia coli. Biotechnology
1994; 12: 178-180.
[45]
Honjo, M., Naito, N., Uchida, H.: US5824502A (1998).
[46]
Bruser T. The twin-arginine translocation system and its capability
for protein secretion in biotechnological protein production. Appl
Microbiol Biotechnol 2007; 76: 35-45.
[47]
Weiner JH, Bilous PT, Shaw GM, et al. A novel and ubiquitous
system for membrane targeting and secretion of cofactor-
containing proteins. Cell 1998; 93: 93-101.
[48]
Weiner, J.H., Turner, R.J.: EP1068339B1 (2001).
[49]
Sargent F, Bogsch EG, Stanley NR, et al. Overlapping functions of
components of a bacterial Sec-independent protein export pathway.
EMBO J 1998; 17: 3640-3650.
[50]
Bron, S., Jongbloed, J.D.H., Muller, J., Van Dijl, J.M.:
US20080166757A1 (2008).
[51]
Rhee, J.S., Pan, J.G., Ahn, J.H.: US016329172B1 (2001).
[52]
Bauer, D.W., Beer, S.V., Bogdanove, A.J., Collmer, A., Ham, J.H.:
US036596509B1 (2003).
[53]
Diaz-torres, M.R.: US20036642027B2 (2003).
[54]
Weiner, J.H., Zhang, G.: WO2006017929A1 (2006).
[55]
Lubitz, W., Resch, S.: US20036596510B1 (2003).
[56]
De Lorenzo, P.V., Fernandez Herrero, L.A.: US20080280346A1
(2008).
[57]
Habermann, P., Ertl, J.: US20077202059B2 (2007).
[58]
Jeong KJ, Lee SY. Excretion of human beta-endorphin into culture
medium by using outer membrane protein F as a fusion partner in
recombinant Escherichia coli. Appl Environ Microbiol 2002;
68:4979-4985.
[59]
Lee, S.Y., Jeong, K.J.: US20097491528B2 (2009).
[60]
Lee, S.Y., Choi, J.H.: US20080206814A1 (2008).
[61] Schloesser, T., Dassler, T., Pfeiffer-Schwiesow, K.:
US20080166764A1 (2008).
[62]
Patkar, A.Y., Sen, S., Chappell, M.L.: US20080182295A1 (2008).
[63]
Dassler, T., Wich, G., Schmid, G.: US20080254511A1 (2008).
[64]
Wich, G., Dassler, T.: US20080206818A1 (2008).
[65]
Kuppusamy, M., Srinivas, V.K., Lahiri, S., Ella, K., Khatri, G.S.:
US20067105327B1 (2006).
[66]
Miksch, G., Flaschel, E., Breves, R., Maurer, K.-H., Kleist, S.:
US20040005695A1 (2004).
[67]
Georgiou, G., Baneyx, F.: US5508192A (1996).
[68]
Georgiou, G., Baneyx, F.: EP866132A2 (1996).
[69]
Joly, J.C.: US6921659B2 (2005).
[70]
Chen, C.Y.-C.: US6828121B2 (2004).
[71]
Leonhartsberger, S., Wich, G.: US20080064062A1 (2008).
[72] Bross
CA.
Escherichia coli and Salmonella typhimurium cellular
and molecular biology. In: Neidhardt FC, Ed. ASM Press: USA
1996; 1382-1399.
[73]
Lee SY, Lee DY, Kim TY. Systems biotechnology for strain
improvement. Trends Biotechnol 2005; 23: 349-358.

Secretory Production in E. coli
Recent Patents on Biotechnology 2010, Vol. 4, No. 1 29
[74]
Blattner, F.R., Posfai, G., Herring, C.D., Plunkett III, G., Glasner,
J.D.: US20060270043A1 (2006).
[75]
Yoon SH, Jeong H, Kwon SK, Kim JF. Genomics, biological
features, and biotechnological applications of Escherichia coli B:
“Is B for better?!” Systems biology and biotechnology of E. coli.
In: Lee SY, Ed.: Springer: USA 2009; 1-17.
[76]
Jeong H, Barbe V, Lee CH, et al. Genome sequences of
Escherichia coli B strains REL606 and BL21(DE3). J Mol Biol
2009; 394: (in press) doi:101016/j.jmb.2009.09.052.
[77]
Yoon SH, Han MJ, Jeong H, et al. Comparative multi-omics
systems analysis of closely related Escherichia coli strains.
submitted.
Wyszukiwarka
Podobne podstrony:
Production of recombinant proteins in E coli
Solube expression of recombinant proteins in the cytoplasma of E coli
Method for enhancing solubility of the expressed recombinant protein in E coli
(gardening) Production of Therapeutic Proteins in Plants
Tuning different expression parametres to achive solube recombinant proteins in E coli
Expression of correctly folded proteins in E coli
Making recombinant proteins in animals
Refolding of recombinant protein
Rapid and efficient purification and refolding of a (His) tagged recombinant protein produced in E c
Advanced genetic strategies for recombinant protein expression in E coli
Producing proteins in transgenic plants and animals
Strategies to maximize heterologous protein expression in E coli
Formation of active inclusion bodies in E coli
Molecular chaperones involved in heterologous protein expression in E coli
Strategies for optimizing heterologous protein expression in E coli
An analysis of energy efficiency in the production of oilseed crops
Wiktorek Smagur, Aneta i inni Green Way of Biomedicine – How to Force Plants to Produce New Importa
LIST OF COLORANTS ALLOWED IN COSMETIC PRODUCTS
Production of benzaldehyde, a case study in a possible industrial application of phase transfer cata
więcej podobnych podstron