483
Clinical Science (1999) 96, 483ñ491 (Printed in Great Britain)
Acute effect of ephedrine on
24-h energy balance
John R. SHANNON, Keith GOTTESDIENER, Jens JORDAN, Kong CHEN,
Stacey FLATTERY, Patrick J. LARSON, Mari Rios CANDELORE, Barry GERTZ,
David ROBERTSON and Ming SUN
Autonomic Dysfunction Center, AA3228 MCN, Vanderbilt University, Nashville, TN 37232ñ2195 , U.S.A.
A
B
S
T
R
A
C
T
Ephedrine is used to help achieve weight control. Data on its true efficacy and mechanisms in
altering energy balance in human subjects are limited. We aimed to determine the acute effect
of ephedrine on 24-h energy expenditure, mechanical work and urinary catecholamines in a
double-blind, randomized, placebo-controlled, two-period crossover study. Ten healthy
volunteers were given ephedrine (50 mg) or placebo thrice daily during each of two 24-h periods
(ephedrine and placebo) in a whole-room indirect calorimeter, which accurately measures
minute-by-minute energy expenditure and mechanical work. Measurements were taken of 24-
h energy expenditure, mechanical work, urinary catecholamines and binding of (
p)ephedrine in
vitro to human
β
1
-,
β
2
- and
β
3
-adrenoreceptors. Twenty-four-hour energy expenditure was 3.6 %
greater (8965
p1301 versus 8648p1347 kJ, P
0.05) with ephedrine than with placebo, but
mechanical work was not different between the ephedrine and placebo periods. Noradrenaline
excretion was lower with ephedrine (0.032
p0.011 µg/mg creatinine) compared with placebo
(0.044
p0.012 µg/mg creatinine) (P
0.05). (
p)Ephedrine is a relatively weak partial agonist of
human
β
1
- and
β
2
-adrenoreceptors, and had no detectable activity at human
β
3
-adrenoreceptors.
Ephedrine (50 mg thrice daily) modestly increases energy expenditure in normal human
subjects. A lack of binding of ephedrine to
β
3
-adrenoreceptors and the observed decrease in
urinary noradrenaline during ephedrine treatment suggest that the thermogenic effect of
ephedrine results from direct
β
1
-/
β
2
-adrenoreceptor agonism. An indirect
β
3
-adrenergic effect
through the release of noradrenaline seems unlikely as urinary noradrenaline decreased
significantly with ephedrine.
INTRODUCTION
The prevalence of obesity in the United States continues
to increase despite a dramatic increase in the percentage
of the American population reporting consumption of a
low-fat diet [1]. Individual efforts to control obesity by
decreasing caloric intake and increasing physical exercise
are often ineffective. Furthermore, abnormalities in
sympathetic function [2–4] as well as mutations in the
β
$
-
adrenoreceptor may be associated with an increased risk
of obesity in some groups of humans [5–7]. The struggle
of individuals to lose weight and the evidence of possible
Key words :
β-adrenoreceptors, energy expenditure, ephedrine, mechanical work, noradrenaline.
Abbreviations : EE, energy expenditure ; MW, mechanical work ; TEF, thermic effect of food.
Correspondence : Dr M. Sun.
sympathetic dysfunction have contributed to the use of
sympathomimetic agents in the treatment of obesity.
One of the more extensively studied sympathomimetic
agents, ephedrine, has been shown to increase energy
expenditure in humans [8]. Long-term studies have not
necessarily translated to an increase in weight loss with
the use of ephedrine [9–12]. These mixed results may in
part be due to the complex actions of ephedrine, which
may include central sympathetic activation [13], per-
ipheral noradrenaline release [13,14], direct actions on
adrenoreceptors [13], a decrease in appetite [15] (which
could decrease caloric intake, causing a decrease in energy
# 1999 The Biochemical Society and the Medical Research Society
484
J. R. Shannon and others
expenditure [16]) and an increase in spontaneous physical
activity due to central sympathetic activation (which
would increase energy expenditure [17]).
Thus, the actions of ephedrine in human subjects are
quite complex. Questions remaining unresolved include :
(1) Exactly how much does ephedrine increase energy
expenditure ? (2) Is the increase in energy expenditure
with ephedrine mainly due to a direct effect on meta-
bolically active tissues or to an increase in spontaneous
physical activity ? (3) If the effect is direct on meta-
bolically active tissues, are these effects mediated in-
directly through sympathetic stimulation or directly
through interactions with adrenoreceptors ?
Accurately defining the effects of ephedrine in humans
is not an academic exercise. Ephedrine is widely available,
being contained in prescription and non-prescription
medications and in popular herbal products such as ma
huang. It is not a benign drug, and deaths have been
associated with its use [18]. This study was designed to
help answer some of these important questions.
METHODS
Subjects
Ten subjects (six males and four females) were recruited
from a pool of normal volunteers. Subjects were non-
smokers, and on no medication. None had actively
attempted to lose weight during the previous 6 weeks.
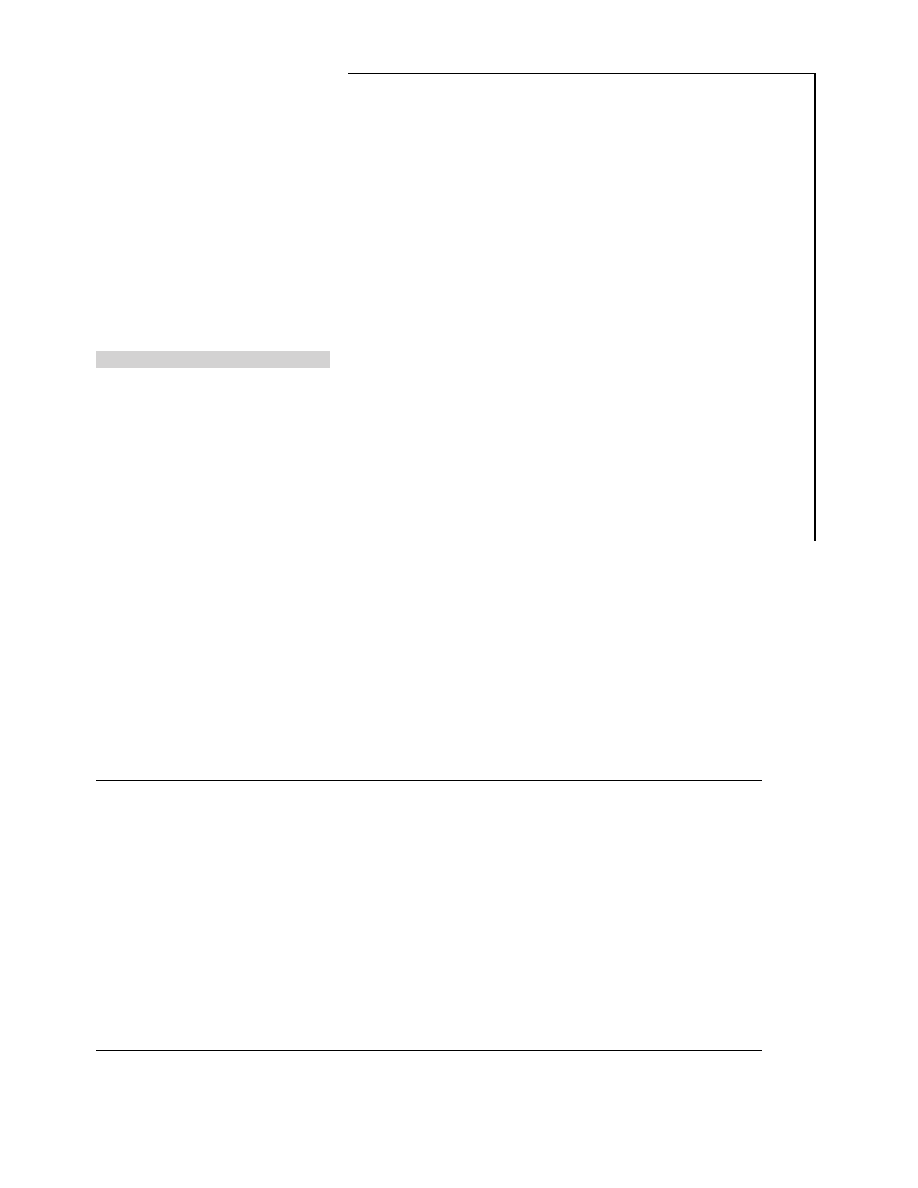
Figure 1
Study protocol
(Top) Overview. On days 1ñ6, 8ñ13 and 15ñ20, subjects maintained their usual diet. On days 8ñ13 and 15ñ20, subjects received placebo single-blinded (s.b.) thrice
daily. On day 7 (first chamber day), all subjects received placebo single-blinded (s.b.) thrice daily. On day 14 (second chamber day), subjects received either placebo
or ephedrine, 50 mg thrice daily, double-blinded (d.b.). On day 21 (third chamber day), subjects were crossed over. (Bottom) Chamber days (days 7, 14 and 21). Subjects
received doses of medication at 07.20, 14.00 and 19.30 h. Meals were served at 11.00, 17.00 and 21.30 hours. Basal metabolic rate (BMR) was determined at
06.00 hours.
The mean (
pS.D.) age, weight, height and body mass
index of the subjects was 30.9
p4.9 years, 74.5p12.4 kg,
175.2
p9.1 cm and 24.1p2.49 kg\m
#
respectively. All
subjects underwent a thorough clinical examination,
ECG, and admission urinalysis and blood work. Written
informed consent was obtained before entry to the study.
All studies were approved by the institutional review
board.
Protocol
The study lasted a total of 21 days, three of which (days
7, 14 and 21) were spent in a whole-room indirect
calorimetry chamber (metabolic chamber). The protocol
for the study is illustrated in Figure 1. Subjects were
instructed to maintain their usual diet during the study
(i.e. between days in the chamber). Subjects maintained a
diet diary for 1 week before meeting with a dietician so a
diet could be prepared which reflected their usual intake.
The diet for each subject was identical on each of the 3
chamber days to reduce the influence of food intake on
energy expenditure. After each day in the chamber, meals
were examined by a dietician to determine the total
calories (expressed in kJ) consumed during that day.
All subjects underwent a 7-day baseline period, during
which they consumed their usual diet and became
accustomed to taking medication (placebo capsules thrice
daily, single-blinded). All subjects spent the last baseline
day (day 7) in the chamber, in order to familiarize
themselves with the chamber and procedures. Subjects
# 1999 The Biochemical Society and the Medical Research Society

485
Effect of ephedrine on 24-h energy balance
Figure 2
Diagram of activityñenergy measurement system
BMR, basal metabolic rate ; EE, energy expenditure ; RQ, respiratory quotient.
were then randomized to a two-period crossover se-
quence, receiving, in a double-blinded fashion, either
placebo or ephedrine, 50 mg thrice daily, on day 14 ;
on day 21, the subjects were crossed over. Subjects
continued their usual diet and took placebo capsules
(single-blinded, thrice daily) on days 8 to 13, and days
15 to 20. Medications were prepared by the investigational
drug service of Vanderbilt University. Both placebo and
ephedrine, 50 mg, were prepared as identical opaque
gelatin capsules.
On days 7, 14 and 21, 24-h energy expenditure was
determined in the metabolic chamber. On each of these
three days, subjects entered the chamber in an overnight-
fasted state at 07.00 hours. Urine collection for volume,
creatinine and catecholamine measurements was initiated
upon entering the chamber and terminated upon leaving.
Subjects were instructed to minimize physical activity
(i.e. not to exercise) while in the chamber. During the first
20 min, the subjects sat quietly to establish a baseline
metabolic rate before administration of the first medi-
cation dose. Test medication was given at 07.20, 14.00 and
19.30 hours. To help minimize interference between
ephedrine’s effect on energy expenditure and the thermic
effect of food, mealtimes were staggered with medication
doses. Meals were given at 11.00, 17.00 and 21.30 hours.
Subjects recorded the time they started and finished each
meal. The following morning, basal metabolic rate was
determined for 30 min after the subject awoke but while
still supine. While in the chamber, subjects kept a log of
side effects. Also, when they left the chamber the
following morning, they were asked about medication
side effects.
Determination of energy expenditure and
mechanical work
Energy expenditure was measured for 24 h by the
Vanderbilt activity–energy measurement system. This
system combines a whole-room indirect calorimeter with
a large force platform inside as the floor (see Figure 2).
The unit is housed in Vanderbilt’s General Clinical
Research Center and represents a highly accurate system
for measurement of energy expenditure and physical
activity [19–21]. The room calorimeter is an air-tight
room measuring 2.5
i3.4i2.4 m. Equipped with a desk,
chair, toilet, sink, telephone, television, video recorder,
audio system and bed, it provides the control of lab-
oratory conditions while mimicking free-living con-
ditions as much as possible. Oxygen consumption (V
O
#
)
and carbon dioxide production (V
CO
#
) are measured at 1-
min intervals and used to calculate minute-by-minute
energy expenditure with a system error of less than 1 %
[20]. The force platform, measuring 2.5
i2.5 m, covers
the entire living area inside the whole-room indirect
calorimeter and is supported by multiple precision force
transducers (LCD load cells, Omega Eng. Inc., Stamford,
CT, U.S.A.). During a subject’s stay, body position, dis-
placement and mechanical forces are measured by the
force platform 60 times per second with an accuracy of
# 1999 The Biochemical Society and the Medical Research Society

486
J. R. Shannon and others
at least 97 % [21]. Eight event buttons are used by
the subject to indicate periods of sleeping, meals and
television viewing. In addition, sensors installed inside
the television
\video recorder, underneath the sleeping
mattress, inside the chair, and at the airlock door where
the subject receives food, also monitor the subject’s
activities. The combination of the force platform, sensors
and event buttons allows precise determination of the
nature, duration and frequency of the subject’s physical
activities while simultaneously obtaining readings of
energy expenditure and mechanical work. The details of
the calorimeter have been reported previously [20,21].
Comparison of the baseline day to the randomized,
double-blind placebo day allowed for determination of
intra-individual variability with placebo. Comparisons of
the placebo day with the ephedrine day during the two-
period crossover portion of the study allowed for
determination of the effect of ephedrine treatment within
each subject and among all subjects on the various
parameters measured. These parameters included 24-h
energy expenditure, the basal metabolic rate, energy
expenditure during sleep, the thermic effect of food and
energy expenditure due to mechanical work during these
activities. Energy expenditure is expressed in kJ
\min and
is the average energy expenditure per minute during the
period measured. The sleep period was defined as the
period from 20 min after the subject lay down until
20 min before the subject awoke the next morning. The
energy expenditure measurements during any given
period were adjusted for mechanical work during that
period by the formula : EE
adj
l EE
total
kMWi15, where
EE
adj
l adjusted energy expenditure, EE
total
l total en-
ergy expenditure and MW
l mechanical work per-
formed on the force platform. Because the efficiency of
energy conversion to mechanical work is approximately
6.7 %, MW is multiplied by a factor of 15 to give the
amount of energy expended on spontaneous physical
activity [22].
The thermic effect of food (TEF) is defined as the
postprandial increase in energy expenditure above the
resting energy expenditure [23,24]. That is, EE
TEF
l
EE
PP
kEE
rest
, where EE
TEF
l energy expenditure due to
the thermic effect of food, EE
PP
l postprandial energy
expenditure and EE
rest
l resting energy expenditure.
From our experience with over 200 subjects, we have
found that the difference between sleeping energy ex-
penditure and resting energy expenditure in the morning
after fasting overnight is less than 3 %. Therefore, we
used the average energy expenditure during sleep (EE
sleep
)
as resting energy expenditure. Thus : EE
TEF
l EE
PP
k
EE
sleep
. Post-meal periods were evaluated between the
conclusion of each meal and either the time of the next
test drug dose or the time the subject went to bed (i.e.
approximately 11.30 am to 14.00 hours, 17.30 to
19.30 hours and 22.00 to approximately 23.30 hours).
These time blocks were designed to minimize any overlap
with effects due to drug or to sleep. A major difficulty in
determining the thermic effect of food is the interference
of body movement and its associated increase in energy
expenditure. We adjusted for this interference by using
the force platform system to remove this component.
Therefore, EE
TEF
l EE
PP−adj
kEE
sleep
, where EE
PP−adj
l energy expenditure during the postprandial period
adjusted for energy expenditure due to mechanical work
during that period.
Studies
in vitro
cAMP assay
Chinese hamster ovary cells expressing human
β
"
-,
β
#
- or
β
$
-adrenoreceptors were seeded 3 days before assay. The
receptors were expressed at receptor densities of 46–88
fmol
\mg (β
$
-adrenoreceptors) or 300–500 fmol
\mg (β
"
-
and
β
#
-adrenoreceptors). The human
β
$
-adrenoreceptor
was obtained from Dr J. Granneman (Wayne State
University) [25]. Cells were harvested using enzyme-free
dissociation media (Specialty Media). The cells were
resuspended in adenylate cyclase cell-based buffer
(75 mM Tris, pH 7.4, 250 mM sucrose, 12.5 mM MgCl
#
,
1.5 isobutylmethylxanthine) at a density of 200 000 cells
per 100
µl. Special dilutions of ligands were added to
the cells. The reaction mixture was incubated at room
temperature with shaking and terminated by placing in a
boiling water bath for 3 min. Levels of cAMP in response
to ligand were measured using a cAMP
"#&
I-SPA assay
kit from Amersham (RPA 556) according to the manu-
facturer’s instructions. In all experiments, isoproterenol
was used as a positive control.
Binding assay
Binding assays were performed by a method described
previously [26]. Essentially, 10–30
µg of membrane
protein were incubated with increasing concentrations of
ligands in a final volume of 250
µl. β
"
- and
β
#
-
adrenoreceptor membranes were incubated with 40 pM
[
"#&
I]iodocyanopindolol while the concentration of
radioactive tracer for the
β
$
-adrenoreceptor equilibrium
binding assays was 250 pM.
Analytical methods
Urine catecholamines were collected at a pH of 2 and
titrated to a pH of 8 at the time of assay. A 10-
µl aliquot
of urine was partially purified by batch alumina ex-
traction followed by reverse-phase liquid chroma-
tography for separation. Components were then quanti-
fied by electrochemical detection using a modification of
the technique of Goldstein et al. [27]. Recovery through
alumina extraction is approximately 75 % for both
noradrenaline and adrenaline. Catecholamine concen-
trations in each sample were corrected for recovery of a
known quantity of the internal standard dihydroxy-
benzalamine, which was run simultaneously then ad-
# 1999 The Biochemical Society and the Medical Research Society
487
Effect of ephedrine on 24-h energy balance
justed per mg of creatinine. The limits of detection of
noradrenaline and adrenaline are 5 and 15 ng
\ml re-
spectively.
Statistics
All data are expressed as means
pS.D. An ANOVA
model appropriate for a two-period crossover study was
used to compare the energy expenditure and mechanical
work parameters after ephedrine or placebo adminis-
tration. The ANOVA model contained effects for se-
quence, subject within sequence, period and treatment.
In general, no period effects were found in the two-
period crossover study ; thus, the baseline measurement
and placebo treatment were also examined to provide a
pure within-subject variability measurement using an
ANOVA model with factors for subject and time
(equivalent to a paired t-test). Urinary catecholamine
concentrations after ephedrine and placebo were com-
pared by a paired t-test. A P value
0.05 (two-tailed)
was considered to be statistically significant.
RESULTS
All subjects completed the study, and there were no
serious adverse events. With ephedrine, seven subjects
reported side effects including difficulty sleeping (n
l 5),
increased or stronger heartbeat (n
l 4) or decreased
appetite (n
l 3). Less frequently reported side effects
were skin tingling, coldness of hands and feet and mouth
dryness. With placebo, one subject reported heart
pounding and difficulty sleeping ; another reported a
brief period of palpitations and decreased appetite.
The values of energy expenditure and mechanical work
for the 3 days in the metabolic chamber are given in Table
1. The total 24-h energy expenditure observed on the
randomized placebo day (6.008
p0.937 kJ\min for
1440 min
\24 h) represents 8648p1347 kJ\day, which is
consistent with the low level of activity while in the
metabolic chamber. Mean 24-h energy expenditure in-
creased to 6.226
p0.904 kJ\min (8965p1301 kJ\24 h)
with ephedrine, an increase of 317 kJ
\24 h or 3.6%
compared with placebo (P
0.05). On an individual
basis, 24-h energy expenditure increased in seven sub-
jects, did not change in two subjects and actually
Table 1
Mean average energy expenditure (EE) and mechanical work (MW) during the total 24-h period and during sleep at
baseline, with placebo and with ephedrine
P (baseline
P (placebo
Baseline
Placebo
Ephedrine
versus placebo)
versus ephedrine)
EE, 24 h (kJ/min)
6.146
p0.904
6.008
p0.937
6.226
p0.912
0.203
0.047
MW, 24 h (kJ/min)
0.050
p0.029
0.050
p0.017
0.050
p0.025
0.984
0.728
EE, sleep (kJ/min)
4.904
p0.941
4.753
p0.845
5.155
p0.895
0.103
0.001
MW, sleep (kJ/min)
0.004
p0.004
0.004
p0.004
0.008
p0.008
0.485
0.048
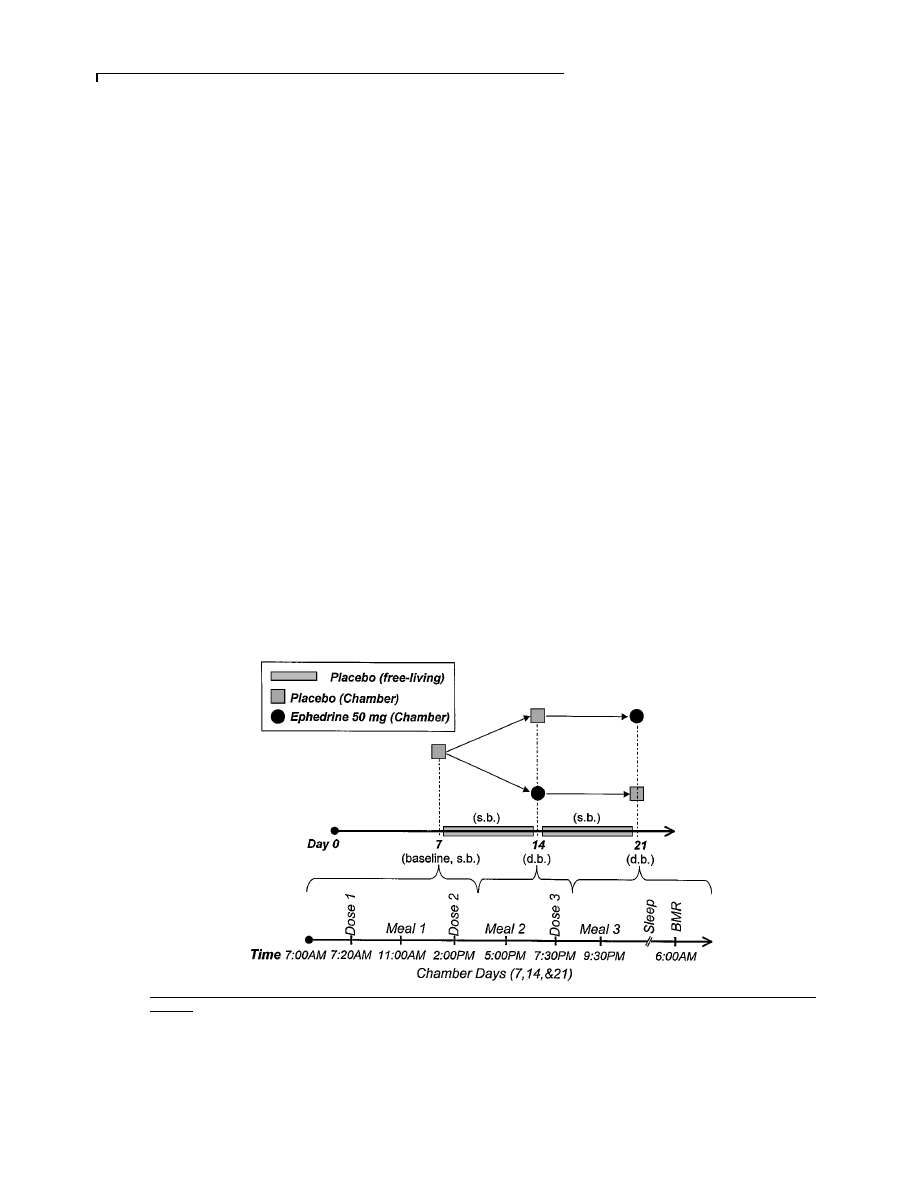
Figure 3
Twenty-four-hour energy expenditure (EE) with
placebo and ephedrine in 10 subjects on days 14 and 21
Energy expenditure increased in seven, did not change in two and decreased in
one subject. 1 kcal
l 4.184 kJ.
decreased in one subject (Figure 3). There was no period
effect over the two-period crossover portion of the study
(i.e. whether the second placebo day was on day 14 or on
day 21 made no difference).
Over 50 % of the additional calories burned with
ephedrine treatment were expended during the period
defined as sleep time (approximately 23.30 to 06.30
hours). Ephedrine increased energy expenditure by 8.4 %
(from 4.753
p0.920 kJ\min with placebo to 5.155p
0.895 kJ
\min with ephedrine) (P l 0.001) during this
period. An increase in energy expenditure during the
night with ephedrine was observed in all 10 subjects.
Basal metabolic rate, determined between 06.30 and
07.00 hours, was not significantly different between
baseline and placebo days (4.945
p0.879 kJ\min versus
4.841
p0.950 kJ\min) (P l 0.5), or between placebo and
ephedrine (4.841
p0.950 kJ\min versus 5.092p0.661
kJ
\min) (P l 0.097). However, the trend for an increased
basal metabolic rate after ephedrine is consistent with the
increase in energy expenditure during sleep described
above.
Mechanical work measurements during the ephedrine
and placebo days were not significantly different
(0.050
p0.025 and 0.050p0.017 kJ\min respectively).
Although mechanical work during sleep was quite low
# 1999 The Biochemical Society and the Medical Research Society
488
J. R. Shannon and others
Table 2
Caloric intake over 24 h and dietary composition
during the placebo day compared with the ephedrine day
Placebo
Ephedrine
Energy intake (kJ/day)
9406
p3196
9142
p3084
Protein (g/day)
83
p40
80
p39
Carbohydrates (g/day)
307
p93
298
p90
Fat (g/day)
81
p37
78
p34
Fibre (g/day)
18
p12
17
p12
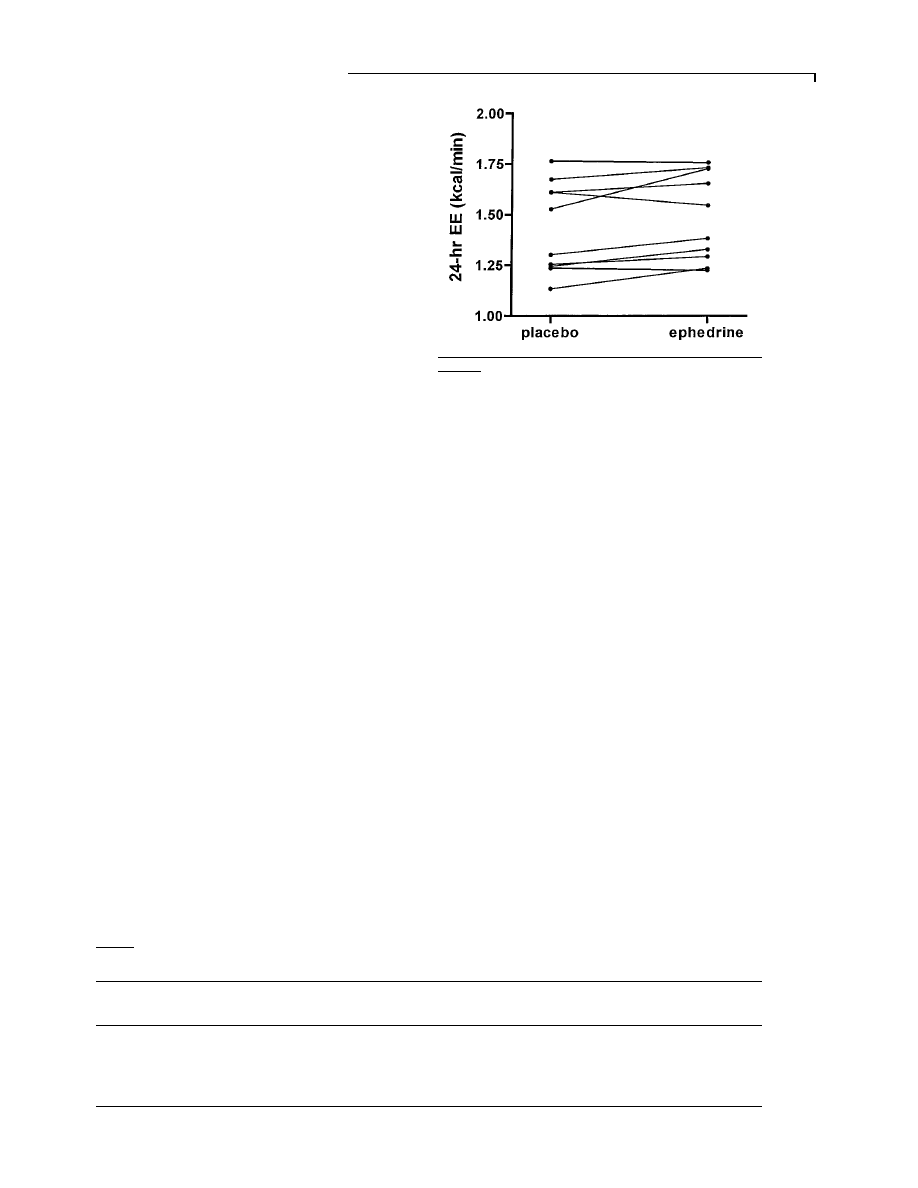
Figure 4
Twenty-four-hour urinary noradrenaline (NE) and
adrenaline (Epi) after placebo (plac) and ephedrine (eph)
treatment
Values are means
pS.D. and are adjusted per mole of creatinine.
with both placebo (0.004
p0.004 kJ\min) and ephedrine
(0.008
p0.008 kJ\min), the change with ephedrine treat-
ment was significant (P
l 0.048). When adjusted for
energy expenditure due to mechanical work, total energy
expenditure during the night was 7.3 % greater with
ephedrine (5.017
p0.879 kJ\min) than with placebo
(4.673
p0.816 kJ\min) (P
0.005).
Caloric intake and dietary composition during the
placebo day and ephedrine day were not significantly
different (Table 2). The thermic effect of food with
Table 3
Activity of ephedrine on cloned human
β-adrenoreceptors, determined by its ability
to increase cAMP (EC
50
), and its ability to inhibit binding of [
125
I]iodocyanopindolol (IC
50
)
Percentage activity was calculated based on the maximal isoproterenol activation. ND
l none detectable (EC
50
values could
not be determined because of the low level of activation). *Percentage activation at a ligand concentration of 10
µM.
n
(
k) Ephedrine
(
j) Ephedrine
(
p) Ephedrine
β
1
3
EC
50
(nM)
1310
p221
ND
9130
p1510
% activity
16
p5
5
p2*
16
p3
3
IC
50
(nM)
49 367
p44252
70 000
p36000
100000
β
2
3
EC
50
(nM)
1274
p534
ND
3630
p1281
% activity
61
p5
6
p2*
69
p4
3
IC
50
(nM)
10000
63 333
p47000
10000
β
3
3
EC
50
(nM)
ND
ND
ND
% activity
5
p3*
8
p1*
2
p3*
3
IC
50
(nM)
10000
10000
10000
placebo (1.368
p0.391 kJ\min) was significantly greater
than with ephedrine (0.966
p0.318 kJ\min) (P
0.05).
However, this apparent increase in the thermic effect of
food was mostly accounted for by the different baselines
(i.e. resting energy expenditure, or EE
sleep
) for each
period. Energy expenditure after meals, adjusted for
mechanical work, was not significantly different between
placebo and ephedrine (6.11
p1.063 versus 6.15p
1.109 kJ
\min).
The values for urinary noradrenaline and adrenaline
are shown in Figure 4. Noradrenaline excretion was
significantly lower with ephedrine [21
p7.4 µmol\mol
creatinine (0.032
p0.011 µg\mg creatinine)] compared
with placebo [29
p8.0 µmol\mol creatinine (0.044p
0.012
µg\mg creatinine)] (P
0.05). Adrenaline excre-
tion
was
9.9
p6.8 µmol\mol creatinine (0.016p
0.011
µg\mg creatinine) with placebo and 6.2p
6.2
µmol\mol creatinine (0.010p0.010 µg\mg creati-
nine) with ephedrine (P
l 0.12).
The results of studies in vitro are presented in Table 3.
Binding studies demonstrated that (
p)ephedrine is a
relatively weak partial agonist of human
β
"
- and
β
#
-
adrenoreceptors. The EC
&!
for
β
"
-adrenoreceptors was
9130
p1510 nM (approximately 18% of the activity of
isoproterenol). For
β
#
-adrenoreceptors, the EC
&!
was
3630
p1281 nM (approximately 75% of the activity of
isoproterenol). (
p)Ephedrine had no detectable activity
at human
β
$
-adrenoreceptors.
DISCUSSION
Activity of the sympathetic nervous system and energy
expenditure are intimately related [4,28–30]. Infusion of
catecholamines increases energy expenditure in animals
[31] and in humans [32,33]. In humans, plasma noradren-
aline levels correlate with energy expenditure [34], and
are inversely related to percentage body fat [2]. Although
ephedrine has been considered to exert a sympathomi-
metic action through several mechanisms, its efficacy in
# 1999 The Biochemical Society and the Medical Research Society

489
Effect of ephedrine on 24-h energy balance
increasing energy expenditure acutely remains unclear.
For example, a previous study showed that 20 mg of
ephedrine taken orally failed to produce a significant
effect over the subsequent 3 h, whereas 30 mg orally
increased energy expenditure by 6.6 % [35]. In another
study, 10 mg of ephedrine orally increased energy ex-
penditure over 3 h by 92.9
p36.0 kJ, which represents an
increase of approximately 10 % [36]. In our study, 150 mg
of ephedrine in a 24-h period increased mean 24-h
expenditure by 3.6 %.
The disparity between the findings in previous studies
and in this study could be due to differences in the
method of gas collection for analysis, the duration of
measurements and the limitations inherent in the as-
sessment of spontaneous physical activity. Metabolic
hoods or mouthpieces, commonly used for gas collection
in metabolic studies [33,37], have limitations. First, they
allow for the measurement of energy expenditure only
for several hours at a time and not during sleep. Perhaps
more importantly, the apparatus itself may affect sym-
pathetic tone during the actual time of data collection.
Determination of energy expenditure shortly after a
single dose may overestimate the effect, or it may
underestimate possible delayed effects.
Prolonged measurement of energy expenditure to
include measurements during sleep in a relatively com-
fortable environment can be obtained using metabolic
chambers. Metabolic chambers equipped with motion
detectors can detect the presence or absence of move-
ment, but cannot quantify the energy expenditure
associated with such movements. The metabolic chamber
equipped with the force platform used in this study is
unique in that it allows for prolonged measurement of
energy expenditure and the accurate quantification of
mechanical work due to physical activity [21]. In this
study, mechanical work was not different with ephedrine
compared with placebo. Therefore, the increase in mean
24-h energy expenditure with ephedrine must be due to
its metabolic effects and not to an increase in physical
activity.
Twenty-four-hour measurement showed that the ma-
jority of the increase in energy expenditure occurred
during the night. Ephedrine, 50 mg orally, gives maximal
plasma concentrations in approximately 2 h and has an
elimination half-life of approximately 9 h [38]. The effects
of a 50 mg oral dose of ephedrine on blood pressure and
heart rate are sustained for at least 7.5 h [39]. Therefore,
a dose accumulation effect may, in part, explain the
increase in energy expenditure during the night. Al-
though blood pressure and heart rate were not monitored
while subjects were in the chamber, cardiovascular
stimulation could have accounted for a component of the
observed increase in energy expenditure after ephedrine.
At the end of the 24-h period (10.5 h after one third
medication dose) when basal metabolic rate was de-
termined, the effect of ephedrine on energy expenditure
was less, and no longer significantly different from
placebo. The increase in mechanical work with ephedrine
during the night could be attributed to increased physical
activity during sleep or to increased activity by subjects
unable to sleep, but the magnitude of the effect was small.
Since the increases in mean 24-h energy expenditure
with ephedrine cannot be explained by an increase in
mechanical work, the mechanism of ephedrine’s thermo-
genic effect must be due to activation of the sympathetic
nervous system. The influence of the sympathetic ner-
vous system on energy expenditure is mediated through
stimulation of
β-adrenoreceptors [40]. Theoretically,
ephedrine could directly activate
β-adrenoreceptors, or
activate them indirectly by causing the release of nor-
adrenaline. In humans, by administering ephedrine with
and without a
β
"
-
\β
#
-adrenoreceptor blocking agent
(nadolol), it has been estimated that approximately 60 %
of the increase in energy expenditure with ephedrine
appears to be due to stimulation of
β
"
- and
\or β
#
-
adrenoreceptors, and 40 % due to
β
$
-adrenoreceptor
agonism [35]. Ephedrine’s lack of binding to human
β
$
-
adrenoreceptors in vitro, at concentrations much greater
than the plasma concentrations achieved with 50 mg of
ephedrine taken orally [38], indicates that the observed
metabolic effects must be due to direct effects on
β
"
-
and
\or β
#
-adrenoreceptors, or to indirect effects on
β
"
-,
β
#
- and
β
$
-adrenoreceptors through the release of nor-
adrenaline. It is remarkable that urinary noradrenaline
decreased by 27 % with ephedrine. The decrease in
urinary noradrenaline with ephedrine in this study could
be explained by a baroreflex-mediated decrease in sym-
pathetic tone due to direct
α
"
-adrenergic stimulation.
Conceivably, a centrally mediated reduction in sym-
pathetic tone could occur due to direct stimulation of
central
α
#
-adrenoreceptors [41] masked by an increase in
blood pressure and heart rate due to peripheral
α
"
- and
β
"
-adrenoreceptor activation. An action to alter nor-
adrenaline handling in the kidney is also possible. Thus,
the direct effect on
α- and β-adrenoreceptors may be
relatively more important, and the indirect nor-
adrenaline-releasing effect less important, than has been
previously recognized.
The lack of binding of ephedrine to the human
β
$
-
adrenoreceptor may also contribute to its relatively
modest acute efficacy in increasing energy expenditure.
In our subjects, the increase in energy expenditure with
ephedrine was 314 kJ
\day. This amount of energy is
roughly equivalent to one slice of white bread or a 14-min
walk on a paved road [42]. A sustained increase in energy
expenditure of 314 kJ
\day for 1 year (if food intake and
physical activity remain constant) would translate to a
3 kg weight loss.
The long-term effect of ephedrine on weight reduction
was not addressed in this study. Other studies that have
done so have shown that ephedrine alone (50 mg thrice
daily) in combination with a low-calorie diet does not
# 1999 The Biochemical Society and the Medical Research Society

490
J. R. Shannon and others
promote weight loss any more than a low-calorie diet
plus placebo [12]. Long-term studies using a combination
of ephedrine with caffeine and
\or aspirin [11,43] have
shown a significant decrease in body mass index. How-
ever, the risks associated with the use of a sympathomi-
metic agent in an obese population already at increased
risk of cardiovascular morbidity and mortality raises
concerns about the long-term use of agents such as
ephedrine for the management of obesity. While eph-
edrine is not readily available in its pure form in the
United States, it is contained in herbal products (e.g. ma
huang) in pharmacological doses. Several compounds
chemically related to ephedrine such as phenylpropanol-
amine and pseudoephedrine are widely available over-
the-counter in the United States. Since 1993, the FDA has
received more than 800 reports of illnesses or injuries
associated with the use of dietary supplements containing
ephedrine [18]. Most of these adverse events seem to be
due to cardiovascular sympathomimetic effects or to
central nervous system effects. Deaths have been
reported, although no cause–effect relationship with
ephedrine has been established. The toxicity of sym-
pathomimetic agents is exacerbated by physical exercise,
dehydration and increases in body temperature. Hence,
the use of sympathomimetic drugs by recreational and
competitive athletes [44] attempting to improve per-
formance, decrease body fat and increase lean body mass
is of particular concern. Medical conditions such as
hyperthyroidism may also augment the risk of serious
adverse events with sympathomimetic agents. Further-
more, although not widely recognized, ephedrine can
produce a euphoria similar to that produced by amphet-
amine [15,44], increasing the potential for abuse.
Considering the relatively low efficacy of the sym-
pathomimetic agents currently available for the treatment
of obesity and their cardiovascular and central nervous
system side effects, non-pharmacological management of
obesity should continue to be the mainstay of treatment.
Recent advances hold promise for the development of
pharmacological agents. Selective
β
$
-adrenergic agonists
may be useful in the treatment of obesity with fewer
cardiovascular side effects. The importance of the role of
the
β
$
-adrenoreceptor in regulation of metabolism is
becoming increasingly apparent. Genetically obese mice
have decreased
β
$
-adrenoreceptor RNA and blunted
responses to selective
β
$
-adrenoreceptor agonists com-
pared with normal mice [45]. In humans, mutations of the
β
$
-adrenoreceptor have been linked to obesity in some
populations [5–7]. Therefore, the development of selec-
tive
β
$
-adrenergic agonists may allow pharmacological
manipulation of the metabolic rate with fewer cardio-
vascular and central nervous system side effects.
We conclude that a relatively high dose of ephedrine
(50 mg thrice daily) acutely increased 24-h energy ex-
penditure (by 3.5 %, corresponding to approximately
314 kJ
\day) with noticeable side effects in most subjects.
This increase in energy expenditure could not be
explained by an increase in physical activity. A lack of
binding of ephedrine to
β
$
-adrenoreceptors and the
observed decrease in urinary noradrenaline during eph-
edrine treatment suggests that the thermogenic effect of
ephedrine results from direct
β
"
-
\β
#
-adrenoreceptor
agonism. An indirect
β
$
-adrenergic effect through the
release of noradrenaline seems unlikely as urinary nor-
adrenaline decreased significantly with ephedrine.
ACKNOWLEDGMENT
This work was supported in part by NIH grants
RR00095, PO1 HL 56693, 1U01 NS33460, National
Aeronautics and Space Administration grant NAS
9–19483, grants from the International Life Sciences
Institute, a grant from Merck Research Laboratories and
the Nathan Blaser Shy–Drager Research Program. J. J. is
supported by the Deutsche Forschungsgemeinschaft. A
preliminary report of this study has appeared in abstract
form [Clin. Auton. Res. (1997) 7, 260].
REFERENCES
1 Heini, A. F. and Weinsier, R. L. (1997) Divergent trends in
obesity and fat intake patterns : the American paradox.
Am. J. Med. 102, 259–264
2 Peterson, H. R., Rothschild, M., Weinberg, C. R., Fell,
R. D., McLeish, K. R. and Pfeifer, M. A. (1988) Body fat
and the activity of the autonomic nervous system. N.
Engl. J. Med. 318, 1077–1083
3 Saad, M. F., Alger, S. A., Zurlo, F., Young, J. B., Bogardus,
C. and Ravussin, E. (1991) Ethnic differences in
sympathetic nervous system-mediated energy expenditure.
Am. J. Physiol. 261, E789–E794
4 Spraul, M., Ravussin, E., Fontvieille, A. M., Rising, R.,
Larson, D. E. and Anderson, E. A. (1993) Reduced
sympathetic nervous activity. A potential mechanism
predisposing to body weight gain. J. Clin. Invest. 92,
1730–1735
5 Widen, E., Lehto, M., Kanninen, T., Walston, J., Shuldiner,
A. R. and Groop, L. C. (1995) Association of a
polymorphism in the beta 3-adrenergic-receptor gene with
features of the insulin resistance syndrome in Finns. N.
Engl. J. Med. 333, 348–351
6 Walston, J., Silver, K., Bogardus, C. et al. (1995) Time of
onset of non-insulin-dependent diabetes mellitus and
genetic variation in the beta 3-adrenergic-receptor gene
[see comments]. N. Engl. J. Med. 333, 343–347
7 Clement, K., Vaisse, C., Manning, B. S. et al. (1995)
Genetic variation in the beta 3-adrenergic receptor and an
increased capacity to gain weight in patients with morbid
obesity [see comments]. N. Engl. J. Med. 333, 352–354
8 Evans, E. and Miller, D. S. (1977) The effect of ephedrine
on the oxygen consumption of fed and fasted subjects.
Proc. Nutr. Soc. 36, 136A
9 Pasquali, R., Casimirri, F., Melchionda, N. et al. (1992)
Effects of chronic administration of ephedrine during
very-low-calorie diets on energy expenditure, protein
metabolism and hormone levels in obese subjects. Clin.
Sci. 82, 85–92
10 Astrup, A., Breum, L., Toubro, S., Hein, P. and Quaade,
F. (1992) The effect and safety of an ephedrine
\caffeine
compound compared to ephedrine, caffeine and placebo in
obese subjects on an energy restricted diet. A double blind
trial. Int. J. Obes. Relat. Metab. Disord. 16, 269–277
# 1999 The Biochemical Society and the Medical Research Society

491
Effect of ephedrine on 24-h energy balance
11 Daly, P. A., Krieger, D. R., Dulloo, A. G., Young, J. B.
and Landsberg, L. (1993) Ephedrine, caffeine and aspirin :
safety and efficacy for treatment of human obesity. Int. J.
Obes. 17 (Suppl. 1), S73–S78
12 Pasquali, R., Baraldi, G., Cesari, M. P. et al. (1985) A
controlled trial using ephedrine in the treatment of obesity.
Int. J. Obes. 9, 93–98
13 Hoffman, B. B. and Lefkowitz, R. J. (1996)
Catecholamines, sympathomimetic drugs, and adrenergic
receptor antagonists. In Goodman and Gilman’s The
Pharmacological Basis of Therapeutics (Hardman, J. G.,
Limbird, L. E., Molinoff, P. B., Ruddon, R. W. and
Gilman, A. G., eds.), pp. 199–248, McGraw-Hill, New
York
14 Boobis, A. R., Burley, D., Davies, D. M., Davies, D. S.,
Harrison, P. I., Orme, M. L., Park, B. K. and Goldberg, L.
I. (1991) Ephedrine (hydrochloride). In Therapeutic Drugs
(Dollery, C., ed.), pp. E26–E29, Churchill Livingstone,
New York
15 Martin, W. R., Sloan, J. W., Sapira, J. D. and Jasinski, D. R.
(1971) Physiologic, subjective, and behavioral effects of
amphetamine, methamphetamine, ephedrine,
phenmetrazine, and methylphenidate in man. Clin.
Pharmacol. Ther. 12, 245–258
16 Halaas, J. L., Boozer, C., Blair-West, J., Fidhusein, N.,
Denton, D. A. and Freidman, J. M. (1997) Physiological
response to long-term peripheral and central leptin
infusion in lean and obese mice. Proc. Natl. Acad. Sci.
U.S.A. 94, 8878–8883
17 Levitsky, D. A. and Strupp, B. J. (1985) Direct and indirect
thermogenic effects of anorectic drugs [Review]. Adv.
Nutr. Res. 7, 187–201
18 FDA. (1996) Docket No. 95N-0304 : Food advisory
committee meeting : clinical summaries of adverse event
reports on dietary supplements that may contain ephedrine
alkaloids.
19 Sharp, T. A., Reed, G. W., Sun, M., Abumrad, N. H. and
Hill, J. O. (1992) Relationship between aerobic fitness level
and daily energy expenditure in weight-stable humans.
Am. J. Physiol. 263, E121–E128
20 Sun, M., Reed, G. W. and Hill, J. O. (1994) Modification
of a whole room indirect calorimeter for measurement of
rapid changes in energy expenditure. J. Appl. Physiol. 76,
2686–2691
21 Sun, M. and Hill, J. O. (1993) A method for measuring
mechanical work and work efficiency during human
activities. J. Biomech. 26, 229–241
22 Stainsby, W. N., Gladden, L. B., Barclay, J. K. and Wilson,
B. A. (1980) Exercise efficiency : validity of base-line
subtractions. J. Appl. Physiol. 48, 518–522
23 Flatt, J. P. (1992) The biochemistry of energy expenditure.
In Obesity (Bjorntorp, P. and Brodhoff, B. N., eds.), pp.
100–116, J. G. Lippincott, Philadelphia
24 Tataranni, P. A., Larson, D. E., Snitker, S. and Ravussin, E.
(1995) Thermic effect of food in humans : methods and
results from use of a respiratory chamber. Am. J. Clin.
Nutr. 61, 1013–1019
25 Granneman, J. G., Lahners, K. N. and Rao, D. D. (1992)
Rodent and human beta 3-adrenergic receptor genes
contain an intron within the protein-coding block. Mol.
Pharmacol. 42, 964–970
26 Candelore, M. R., Deng, L., Tota, L. M., Kelly, L. J.,
Cascieri, M. A. and Strader, C. D. (1996) Pharmacological
characterization of a recently described human beta 3-
adrenergic receptor mutant. Endocrinology (Baltimore)
137, 2638–2641
27 Goldstein, D. S., Eisenhofer, G., Stull, R., Folio, C. J.,
Keiser, H. R. and Kopin, I. J. (1988) Plasma
Received 3 June 1998/9 December 1998; accepted 17 December 1998
dihydroxyphenylglycol and the intraneuronal disposition
of norepinephrine in humans. J. Clin. Invest. 81, 213–220
28 Halaas, J. L., Gajiwala, K. S., Maffei, M. et al. (1995)
Weight-reducing effects of the plasma protein encoded by
the obese gene. Science (Washington DC) 269, 534–549
29 Blaak, E. E., Sans, W. H. and Baak, M. A. (1997)
Adrenoreceptor subtypes mediating catecholamine-
induced thermogenesis in man. Int. J. Obes. Relat. Metab.
Disord. 17, S78–S81
30 Jansky, L. (1995) Humoral thermogenesis and its role in
maintaining energy balance. Physiol. Rev. 75, 237–259
31 Hsieh, A. and Wang, J. C. (1971) Calorigenic responses to
cold of rats after prolonged infusion of norepinephrine.
Am. J. Physiol. 221, 335–337
32 Jequier, E., Munger, R. and Felber, J. P. (1992)
Thermogenic effects of various
β-adrenoreceptor agonists
in humans : their potential usefulness in the treatment of
obesity. Am. J. Clin. Nutr. 55, 249S–251S
33 Blaak, E. E., van, B. A., Kester, A. D. and Saris, W. H.
(1995) Beta-adrenergically mediated thermogenic and heart
rate responses : effect of obesity and weight loss.
Metabolism 44, 520–524
34 Toth, M. J. and Poehlman, E. T. (1994) Sympathetic
nervous system activity and resting metabolic rate in
vegetarians. Metabolism 43, 621–625
35 Liu, Y. L., Toubro, G., Astrup, A. and Stock, M. J. (1995)
Contribution of beta 3-adrenoreceptor activation to
ephedrine-induced thermogenesis in humans. Int. J. Obes.
Relat. Metab. Disord. 19, 678–685
36 Astrup, A., Toubro, S., Cannon, S., Hein, P. and Madsen,
J. (1991) Thermogenic synergism between ephedrine and
caffeine in healthy volunteers : a double-blind, placebo-
controlled study. Metabolism 40, 323–329
37 Welle, S., Lilavivathana, U. and Campbell, R. G. (1980)
Increased plasma norepinephrine concentrations and
metabolic rates following glucose ingestion in man.
Metabolism 29, 806–809
38 Stromberg, C., Vanakoski, J., Olkkola, K. T., Lindqvist,
A., Seppala, T. and Laitinen, L. A. (1992) Exercise alters
the pharmacokinetics of midazolam. Clin. Pharmacol.
Ther. 51, 527–532
39 Bye, C., Dewsbury, D. and Peck, A. W. (1974) Effects on
the human central nervous system of the two isomers of
ephedrine and triprolidine, and their interaction. Br. J.
Pharmacol. 1, 71–78
40 Bukowiecki, L., Jahjah, L. and Follea, N. (1982)
Ephedrine, a potential slimming drug, directly stimulates
thermogenesis in brown adipocytes via beta-
adrenoreceptors. Int. J. Obes. 6, 343–350
41 Kawasuji, T., Koike, K. and Saito, H. (1996) Effects of
optical isomers of ephedrine and methylephedrine on the
twitch response in the isolated rat vas deferens and the
involvement of alpha 2-adrenoceptors. J. Smooth Muscle
Res. 32, 155–163
42 McArdle, W. D., Katch, F. I. and Katch, V. L. (1991)
Appendix D. In Exercise Physiology : Energy, Nutrition
and Human Performance (McArdle, W. D., Katch, F. I.
and Katch, V. L., eds.), pp. 804–811, Lea and Febiger,
Philadelphia
43 Breum, L., Pedersen, J. K., Ahlstrom, F. and Frimodt-
Moller, J. (1994) Comparison of an ephedrine
\caffeine
combination and dexfenfluramine in the treatment of
obesity. A double-blind multi-centre trial in general
practice. Int. J. Obes. Relat. Metab. Disord. 18, 99–103
44 Catlin, D. H. and Hatton, C. K. (1991) Use and abuse of
anabolic and other drugs for athletic enhancement. Adv.
Int. Med. 36, 399–424
45 Collins, S., Daniel, K. W., Rohlfs, E. M., Ramkumar, V.,
Taylor, I. L. and Gettys, T. W. (1994) Impaired expression
and functional activity of the beta 3- and beta 1-adrenergic
receptors in adipose tissue of congenitally obese
(C57BL
\6J ob\ob) mice. Mol. Endocrinol. 8, 518–527
# 1999 The Biochemical Society and the Medical Research Society
Wyszukiwarka
Podobne podstrony:
PROGRAM NA 24 GODZINY
BILANS ENERGETYCZNY ORGANIZMU CZŁOWIEKA, Farmacja
bilans energetyczny
12 Bilans energetycznyid 13235
Ćwiczenia 4 Masai skład ciała. Przemiana materii i bilans energetyczny, Medyczne, Studia pielęgniars
Suma wartości czasu przekraczająca 24 godziny, excel
Bilans energetyczny
Bilans energetyczny
Bilans energetyczny oddychania tlenowego
Składniki pokarmowe, bilans energetyczny
Bilans energetyczny, Dietetyka, Żywienie i dietetyka, Żywienie w Sporcie
bilans energetyczny 2019
12. Bilans energetyczny
bilans energetyczny 2019
Bilans energetyczny UE i Europa stan aktualny i perspektywy zmian(1)
Poznaj C++ w 24 godziny cz 2 (od 251 strony)
Bilans energetyczny
bilans energetyczny, Studia, Mechanika, mechanika
24 godzinna dieta owocowa
więcej podobnych podstron