
Biomaterials 23 (2002) 2863–2869
Evaluation of the TiMo
12
Zr
6
Fe
2
alloy for orthopaedic implants:
in vitro biocompatibility study by using primary human
fibroblasts and osteoblasts
L. Trentani
a
, F. Pelillo
a
, F.C. Pavesi
a
, L. Ceciliani
a
, G. Cetta
b
, A. Forlino
b,
*
a
Department of Morphologic, Eidologic and Clinical Sciences, Section of Orthopaedy and Traumatology, University of Pavia,
Piazzale C. Golgi 19, 27100 Pavia, Italy
b
Department of Biochemistry ‘A. Castellani’, University of Pavia, Via Taramelli 3B, 27100 Pavia, Italy
Received 5 July 2001; accepted 5 December 2001
Abstract
To reveal the biocompatibility of TiMo
12
Zr
6
Fe
2
(TMZF), a new titanium alloy used since 1998 for orthopaedic prosthesis, we
compared the behavior of primary human fibroblasts and osteoblasts grown on TMZF discs or on plastic tissue culture dishes, a
widely used material specifically treated by the manufacturer to enhance cell growth. Proliferation, differentiation, RNA and
collagen type I expression level of human cells were carried out. The analysis were performed over a period of 96 h. Fibroblasts
behaved at the same way on the two different supports after 48 h, their number increased after 96 h when cells were grown on the
alloy. Osteoblasts adhered and proliferated on the alloy discs as well as on plastic. RNA expression level was not affected.
Interestingly, cell number at each time point was higher for fibroblasts than for osteoblasts. The RNA expression level was higher
for the osteoblasts.
Both cell types cultured on the alloy revealed an increase in the amount of type I collagen and a similar electrophoretic pattern
was found for collagen produced by fibroblasts and osteoblasts grown on either supports.
These results indicate good biocompatibility of the TMZF alloy, which allowed adhesion and proliferation of both the examined
cell types and suggest that TMZF is a promising material for orthopaedic implants. r 2002 Elsevier Science Ltd. All rights reserved.
Keywords: Titanium alloy; TMZF; Orthopaedic implants; Biocompatibility; Osteoblasts; Fibroblasts
1. Introduction
In the last decades the increasing clinical application
of orthopaedic implants determined the development of
new biomaterials, in general metals or alloys, with
specific mechanical and physical features. Metals and
alloys are probably the most common materials used as
surgical implants since they are, as a rule, well tolerated
by the human body for several years though they always
involve a foreign body reaction. Simultaneously, the
interest of researchers on testing the biocompatibility of
these elements increased [1–8]. An ideal biomaterial for
bone and joint replacement should possess peculiar
characteristics as excellent fatigue and tensile strength,
superior corrosion and wear resistance, low modulus of
elasticity, good hardness and low density. Furthermore,
high biocompatibility is an important property at the
implant site. The necessity to study in vitro and in vivo
the cellular behavior at the interface with the different
materials used for orthopaedic implants is evident. The
animal models are essential in providing informations
on biological reactions to implant in bone, but the
results may be difficult to interpret and transfer to
cellular level because of the numerous and complex
events that occur into a bloody wound site [9,10]. In
vitro approaches bypass this limitation and represent a
valid system to analyze the response to cellular growth
on biomaterials. The use of differentiated human cells is
of great interest since they are representative of some
tissue reactions around the implant in vivo without the
complication of the whole body effects [11–14]. Two cell
types were chosen for our studies: fibroblasts and
*Corresponding author. Tel.: +39-0382-507-235; fax: +39-0382-
423-108.
E-mail address:
aforlino@unipv.it (A. Forlino).
0142-9612/02/$ - see front matter r 2002 Elsevier Science Ltd. All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 1 ) 0 0 4 1 3 - 6

osteoblasts, this last being directly in contact with the
orthopaedic implants surface [15]. The fibroblasts allow
us to study the basal cytocompatibility of a biomaterial,
which relates to the common cellular functions (attach-
ment, viability, proliferation, cellular properties, mem-
brane state). The osteoblasts allow the analysis of the
specific cytocompatibility, which refers to the specific
functions of cell type (morphology, specific enzyme and/
or protein synthesis) characterizing the cellular pheno-
type expression of the cells at the implant site.
The titanium alloys are extensively used as surgical
implant materials for the superior mechanical properties
as well as for their good biocompatibility. Numerous
studies reported in literature on the TiAl
6
V
4
alloy show
its good biocompatibility, higher than Cr–Co and
Cr–Co–Mo alloys and ceramics [16–19]. However, this
alloy have been proven to behave poorly in friction since
wear particles were often detected in tissues and organs
associated with titanium implanting [20]. Haynes et al.
[21] reported that titanium–aluminium–vanadium par-
ticles were likely to cause the release of high amounts of
inflammatory mediators implicated in osteolysis, which
does not bode well for the longevity of the prosthesis.
Thus, the use of titanium and its alloys for joint
prosthesis has always been viewed with apprehension.
In 1998 a new b titanium alloy: TiMo
12
Zr
6
Fe
2
(TMZF) has been introduced in the market of ortho-
paedic implants. TMZF alloy has mechanical and
physical properties similar to the TiAl
6
V
4
but specific
tests, to evaluate abrasion resistance, revealed that
TMZF alloy generates less debris than TiAl
6
V
4
. No
biocompatibility tests had been performed on TMZF
alloy. The aim of our research project was to analyze the
cellular behavior of human primary cultured fibroblasts
and osteoblasts grown on TMZF over a period of 96 h
to verify the biocompatibility of this new alloy. In
particular, we analyzed proliferation, differentiation,
total RNA expression level and synthesis of type I
collagen of fibroblasts and osteoblasts grown on TMZF
discs by in vitro reconstruction of bone–prosthesis
interface.
2. Materials and methods
2.1. Biomaterial
The biomaterial used in the experiments was a b
titanium alloy: TiMo
12
Zr
6
Fe
2
. The a titanium is present
in a close hexagonal crystalline structure below 8821C
and is modified in b titanium cube-shaped structure over
this temperature. On this titanium characteristic is based
the microstructural control and properties of all
titanium alloys. TMZF alloy keeps the b microstructure
during the cooling from the formation temperature
(7541C) to room temperature. The microstructure of b
phase gives to the TMZF alloy flexibility, strength and
resistance to the corrosion and ductility. TMZF is 25%
more flexible than the well studied TiAl
6
V
4
alloy. It is
resisting to the loading, to the intaglio, to the wear and
abrasion and it has high resistance to the traction, to the
fractures and to the physical exertions. Beside the
principal elements such as molybdenum, zirconium
and iron useful to stabilize the b phase, TMZF alloy
also contains a small percentage of oxygen, nitrogen and
carbonium, which give more stability (Table 1).
For the present study hand-polished TMZF discs
2 cm in diameter and 2 mm thick were provided by
Stryker Howmedica which tested the mechanical,
metallurgical and structural properties of the alloy.
The discs were sterilized in a steam autoclave at 1341C
for 10 min before using them as cellular growth support,
the tissue culture plastic dishes were received in sterile
packing.
2.2. Cell cultures
Human
dermal
fibroblasts
(2056.CRL
and
2127.CRL) were purchased from American Type
Culture Collection (ATCC; Bethesda, MD, USA). Cells
were grown in Dulbecco Modified Eagle’s Medium
(DMEM, Sigma) containing 10% fetal calf serum,
2 10
5
U/l penicillin and streptomycin, 16 mm HEPES,
112 mm NaHCO
3
and cultured at 371C in an atmosphere
of 5% CO
2
. For the experiments cells from 10th to 15th
passages were used.
Osteoblast primary cultures were established from
surgical bone chips obtained from cancellous bone.
Osteoblasts were released from the bone chips using
collagenase P digestion (5 mg/ml) in Jocklik medium
(Sigma) at 371C for 30 min, stirring. Following collage-
nase P digestion, bone chips were plated in 75 cm
2
flasks
in Iscove’s Modified Dulbecco Medium supplemented
with 10% fetal calf serum, 2.0 mm glutamine, 2 10
5
U/l
penicillin and streptomycin at 371C in an atmosphere of
8% CO
2
. Cells were grown at confluence. Bone chips
were plated three times; the osteoblasts were collected
each time and frozen at passage 0.
Table 1
Elements weight (%)
Element
Weight (%)
Titanium
Balance
Molybdenum
10–13
Zirconium
5–7
Iron
1.5–3.5
Oxygen
0.28
Carbonium
0.05
Hydrogen
0.02
Nitrogen
0.05
L. Trentani et al. / Biomaterials 23 (2002) 2863–2869
2864

Light microscopy appearance and alkaline phospha-
tase staining (Sigma) routinely verified the osteoblast
phenotype. Briefly, cells were plated on 35 mm Petri
dishes and grown for 24 h. Medium was removed; cells
were fixed with a citrate–acetone–formaldehyde fixative
solution and stained with FRV Alkaline Solution for
15 min at room temperature on the dark. Cells were
washed and counterstained with Hematoxylin Solution
for 2 min according to the manufacturer’s suggestion.
Only osteoblasts at passage 0 or 2nd were used in the
present study.
A total of 4 osteoblast primary lines were established:
OB1, from a 31 years old patient, OB2 from a 42 years
old patient and OB3 and OB4 from 11 years old patients
(Table 2).
2.3. Experimental procedures
The biomaterial discs were placed on the bottom of
35 mm Petri dishes. Fibroblasts and osteoblasts were
plated at 1 10
5
cells per well in 35 mm Petri dishes in
presence or in absence of the biomaterial with the
appropriate media described above. Osteoblasts were
counted in presence of Trypan Blue (1:1, v/v) directly
after thawing and plated. Cells were plated in a 100 ml
drop of media covering the upper surface of the discs
when they were present or covering about the same area
of the plastic wells. After 4–5 h incubation at 371C, to
allow cells attachment, the wells were filled upto 3 ml by
culture medium and incubated at 371C. Absence of cells
around the biomaterial discs was evaluated by light
microscopy.
2.4. Expression analysis
Fibroblasts and osteoblasts were grown for 48 and
96 h. Media supplemented with 100 mg/ml ascorbic acid
was changed daily. At each time point media was
removed and RNA and DNA were extracted using 1 ml
of TriReagent (Sigma) according to the manufacturer’s
suggestions.
Briefly, medium was removed and cells were lysated
for 5 min at room temperature in TriReagent solution,
RNA was separated in presence of chloroform and
precipitated with isopropanol. RNA was resuspended in
diethylpyrocarbonate treated water and spectrophoto-
metric measurements were performed at 260 nm.
DNA had been extracted from the same samples
using the initial organic phase after the chloroform
separation. DNA was precipitated in presence of linear
polyacrylamide carrier (40 mg) and ethanol. DNA was
resuspended in 40 mm NaOH and spectrophotometric
measurements were performed at 260 nm.
2.5. Osteoblast phenotype analysis
Osteoblasts (passage 0) were plated at 1 10
5
cells for
well in 35 mm Petri dishes in presence and in absence of
biomaterial discs as described above (passage I). Iscove’s
modified Dulbecco medium was used. Cells were grown
for 48 h. Osteoblasts from both wells were then
trypsinized and plated again in Petri dishes without
biomaterial discs (passage II). Alkaline phosphatase
staining was performed on the cells before and after
biomaterial or plastic contact, about 200–300 cells were
counted and the percentage of the positive stained cells
was calculated and compared.
2.6. Protein analysis
Fibroblasts and osteoblasts were plated at 1 10
5
in
35 mm Petri dishes in presence and absence of TMZF
alloy discs and grown for 72 h. Cells were labeled with
50 mCi of L-(2, 3 -
3
H) Proline for 24 h. Fibroblasts were
labeled in DMEM without fetal calf serum after
ascorbate stimulation (100 mg/ml). Osteoblasts were
labeled in the presence of DMEM supplemented with
2% FCS after stimulation with 100 mg/ml ascorbate [22].
Radiolabeled procollagen was harvested from media
and cell layer as previously described [23]. Collagen was
prepared from procollagen samples by pepsin digestion
(100 mg/ml) of procollagen samples for 4 h at 41C. Total
incorporated radioactivity (dpm) was measured in
medium and cell layer fractions by b counter (TRI-
CARB 2300TR, Packard). The samples were loaded on
6% SDS-Urea-PAGE.
2.7. Statistical analysis
Results are reported as mean
7SEM of n ¼ 426
measurements. Cell number and expression level data
were examined using two-way analysis of variance
(accounting for time and type of materials used for
plating) to determine differences among groups. Tukey’s
test was used for multiple comparison. Kruskal–Wallis
one-way analysis of variants on Ranks was used to
evaluate the differences between fibroblasts and osteo-
blasts cell number and expression level followed by
Dunn’s method for multiple comparison. A P value
o0.05 was considered statistically significant.
Table 2
Osteoblast cell lines
Osteoblast primary
cultures
Patients age
Biopsy location
OB1
31
First metatarsal
OB2
42
First metatarsal
OB3
11
Accessory tarsal scaphoid
OB4
11
Accessory tarsal scaphoid
L. Trentani et al. / Biomaterials 23 (2002) 2863–2869
2865
3. Results
To investigate the biocompatibilty of TMZF alloy,
fibroblast and osteoblast cells grown on TMZF discs or
polystyrene tissue culture dishes were analyzed at 48 and
96 h for differentiation, proliferation, expression activity
and synthesis of type I collagen, a major extracellular
matrix protein in both cell types.
3.1. Osteoblast characterization
Primary cultured cells, obtained from bone chips as
described in Materials and Methods, were identified as
osteoblasts on the basis of the following results: first of
all, a cube-shaped or polygonal morphology typical of
osteoblasts was observed by light microscopy analysis.
Staining for alkaline phosphatase showed extensive
cytoplasmatic dark purple areas and granules, which
indicated the presence of the enzyme confirming the
osteoblasts phenotype (data not shown).
3.2. Adhesion and proliferation study
Total cellular DNA was extracted and quantified.
Cells number was calculated assuming that the amount
of DNA per 10
6
diploid cells of human origin equals to
7.1 mg [24].
We did not find statistical significant difference in cell
proliferation for fibroblasts plated on plastic tissue
culture dishes or TMZF alloy discs at 48 h. Difference
was found at 96 h; cells grown on the alloy increased in
number with respect to the ones grown on plastic dishes
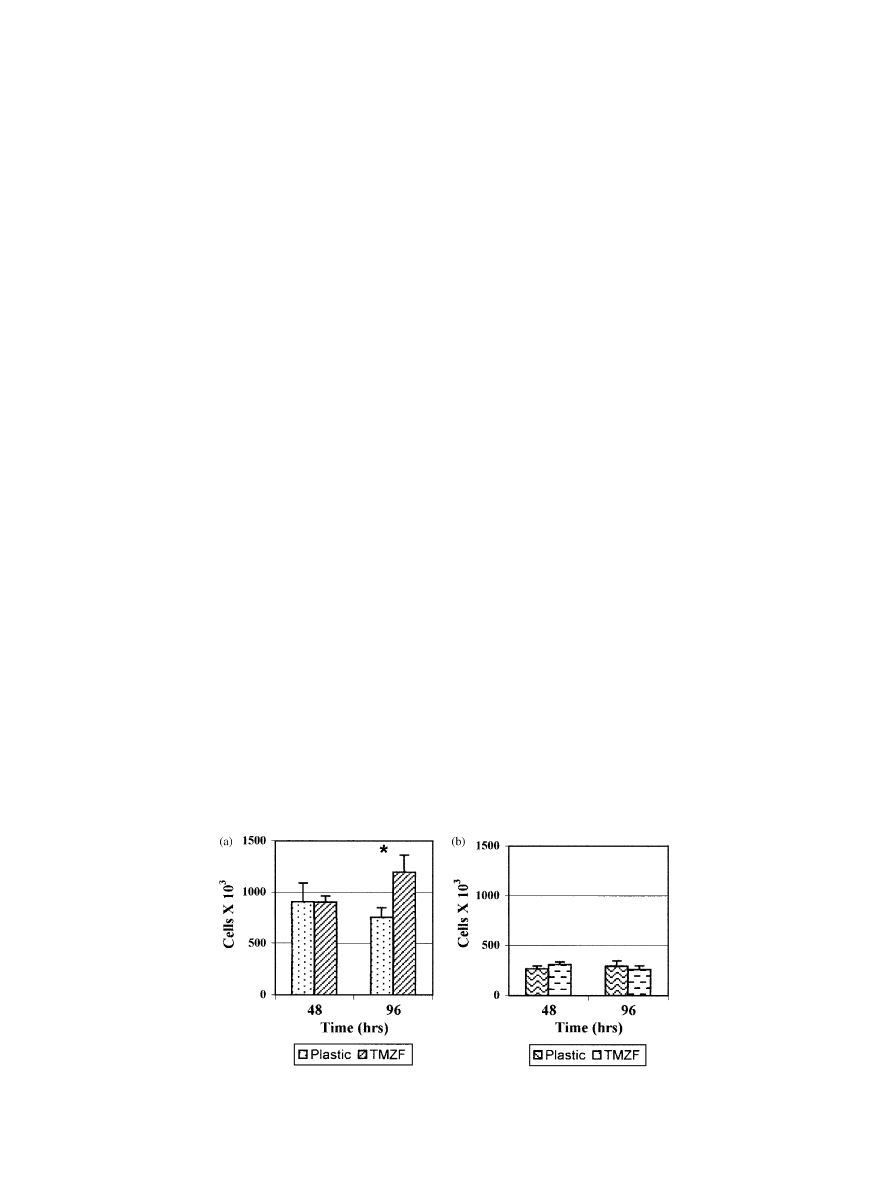
(P ¼ 0:0343) (Fig. 1a).
No differences were reported for the osteoblasts
plated at 1 10
5
on plastic tissue culture dishes or
TMZF alloy discs at 48 and 96 h (Fig. 1b).
Interestingly, we found a meaningful difference in cell
growth between fibroblasts and osteoblasts. Fibroblast
cells proliferated more than osteoblasts in culture at
each examined time point either on alloy discs or on
plastic tissue culture dishes. The difference in the median
values among fibroblasts and osteoblasts cell number
was significantly different (P
o0:001).
3.3. Expression analysis
The expression level of fibroblasts and osteoblasts was
calculated by quantitation of total cellular RNA
normalized to total cellular DNA [25].
Two-way ANOVA test was performed to evaluate
statistical significant difference on expression level of
fibroblasts and osteoblasts accounting for the different
time point and the presence or absence of the alloy discs.
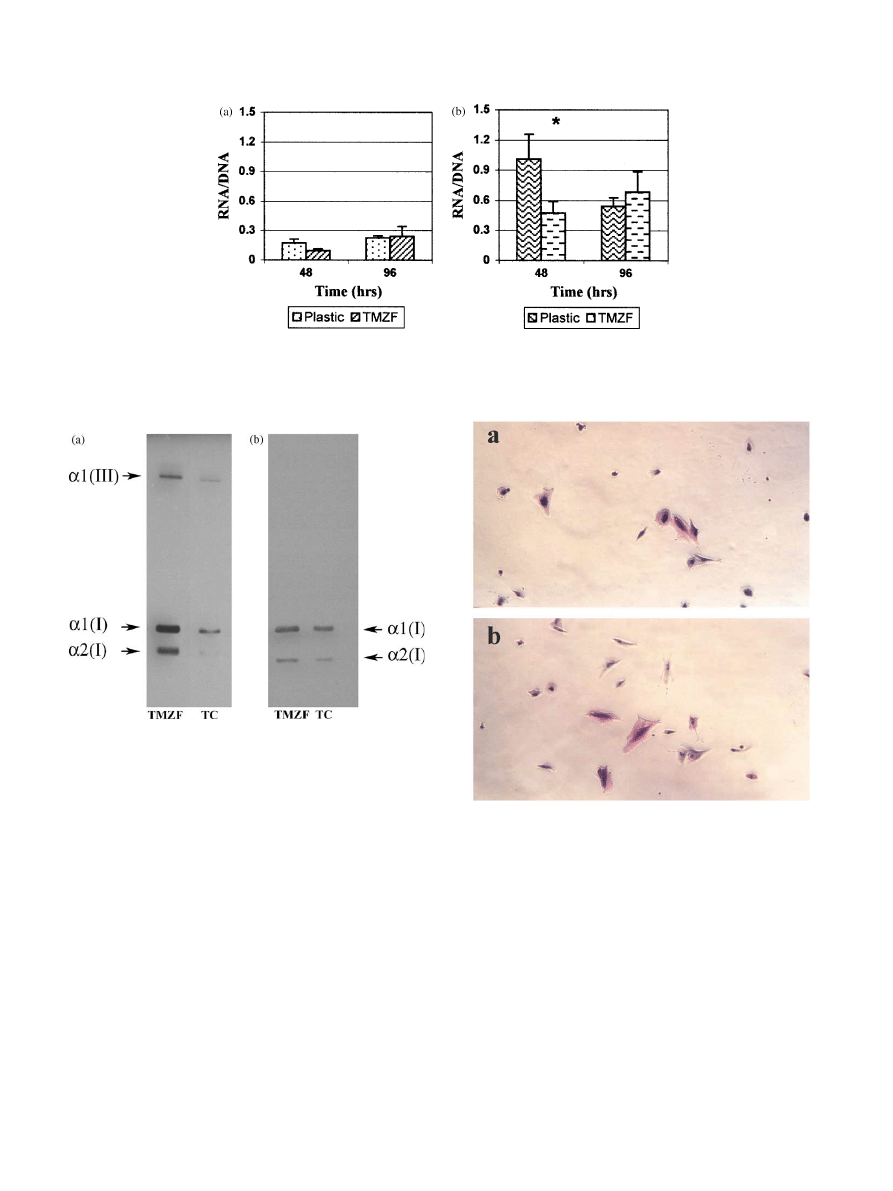
No differences were detected for fibroblasts (Fig. 2a).
We found at 48 h a higher expression for osteoblasts
plated on tissue culture dishes (P ¼ 0:046). The differ-
ence disappeared at 96 h suggesting only a temporal
delay at short time on the RNA expression level
(Fig. 2b). Significant difference was found between
fibroblasts and osteoblasts expression level both in
presence and absence of TMZF alloy, the osteoblasts
showed a higher level of expression than fibroblasts at
each examined time point (P
o0:001). The osteoblasts
even after 96 h from plating were still not confluent as
suggested by the number of cells counted, whereas
fibroblasts even at 48 h revealed a higher confluence
state and probably contact inhibition influenced their
expression level.
3.4. Collagen synthesis in fibroblasts and osteoblasts
Labeled collagen synthesized by fibroblasts and
osteoblasts grown either on TMZF discs or plastic was
collected as described in Materials and Methods. We
extracted types I and III collagen with the described
procedure, type I collagen represents the major fraction
Fig. 1. Proliferation of (a) fibroblasts grown on plastic tissue culture dishes and TMZF alloy discs and (b) osteoblasts grown on plastic tissue culture
dishes and TMZF alloy discs at the indicated time points. The asterisk indicates statistically significant difference (P
o0:05).
L. Trentani et al. / Biomaterials 23 (2002) 2863–2869
2866
for fibroblasts and the almost complete fraction for
osteoblasts, which synthesize only a small amount of
type III collagen. Total incorporated radioactivity was
calculated by b counter measurements of the samples.
For fibroblasts the total dpm value was higher for cells
grown on TMZF discs than on plastic, 1.9 and 1.8 times,
respectively.
Similarly,
osteoblasts
showed
higher
amount of dpm in both medium and cell layer fractions
when they were grown on b titanium alloy, 2.9 and 2.6
times, respectively.
Medium and cell layer samples were run on SDS-
Urea-PAGE and similar electrophoretic pattern was
found both for fibroblasts (Fig. 3a) and osteoblasts
grown on either supports (Fig. 3b).
3.5. Analysis of the osteoblast phenotype
Osteoblast cells at passage 0 were plated either on
TMZF discs or plastic tissue culture dishes; after 48 h
cells were trypsinized, plated on plastic dishes and
stained for alkaline phosphatase (Fig. 4a and b). The
percentage of the cells positive for the alkaline
phosphate staining was basically identical to the
percentage of the original frozen cells demonstrating
that the osteoblast phenotype was conserved (Table 3).
Fig. 3. SDS-Urea-PAGE of type I collagen extracted from the
medium of cultured (a) fibroblasts and (b) osteoblasts. The cells
cultured in vitro for 72 h on TMZF discs (TMZF) or plastic tissue
culture dishes (TC) were labeled with
3
H-proline for 24 h in the
presence of ascorbic acid. Collagen had been run on 6% polyacryla-
mide gel.
Fig. 2. Expression level of (a) fibroblasts grown on plastic tissue culture dishes and TMZF alloy discs and (b) osteoblasts grown on plastic tissue
culture dishes and TMZF alloy discs at the indicated times. Values are expressed as ratio of total RNA and total DNA. The asterisk indicates
statistically significant difference (P
o0:05).
Fig. 4. Alkaline phosphatase staining. Osteoblasts were plated on (a)
TMZF discs or (b) plastic tissue culture dishes, grown for 48 h,
trypsinized and plated again on Petri dishes. Alkaline phosphatase
staining followed.
L. Trentani et al. / Biomaterials 23 (2002) 2863–2869
2867

4. Discussion
The interface between implant and bone is of vital
importance in prosthesis and ingrowth of bone improves
stability and helps to minimize loosening [1]. We studied
the cellular behavior at this location by mimic in vitro
the bone–prosthesis contact area. We used in vitro
fibroblast and osteoblast primary cultures. The choice of
the cell types was determined first of all by the fact that
fibroblasts and osteoblasts are the type of cells which
orthopedic implants will be in contact with during
clinical use [17]. Furthermore, the initial contact of
osteoblasts with implant surfaces is an important event
for osteointegration of implants [26,27]. It is important
to ascertain biomaterial effect on primary cultured cells
since immortalization of cells may provide us with a
ready source of human materials for biocompatibility
studies, but those cells could have change their wild-type
cellular behavior.
This work represents the first study demonstrating the
biocompatibility of the TiMo
12
Zr
6
Fe
2
(TMZF) alloy, a
b titanium alloy with improved mechanical and physical
features with respect the more diffuse TiAl
6
V
4
.
The present work demonstrates that the b titanium
alloy TMZF supports fibroblasts and osteoblasts cell
attachment, proliferation and RNA synthesis as well as
plastic tissue culture dishes, a widely used material
which is specifically treated by the manufacturer to
enhance cell growth. Fibroblasts and osteoblasts plated
at 1 10
5
were grown for 48 and 96 h and cell number
was extrapolated from the amount of extracted DNA.
No difference was detected at any of the examined time
points with the exception of an increased number of
fibroblasts after 96 h of growth on the alloy discs,
suggesting a stimulatory effect on cell growth due to the
implant material. Osteoblasts in our culture conditions
grew slower than fibroblasts. Plated at identical cell
density the proliferation rate was significantly reduced in
osteoblasts compared to fibroblasts. These data are in
agreement with those published in literature. Bordji et al.
[16] reported that final cell density measured on titanium
alloy samples were always higher with fibroblasts than
with osteoblasts, reflecting slower proliferation capacity
and maybe higher sensitivity of the osseous callus.
No difference was detected on the expression level for
fibroblasts grown on TMZF discs at the different
examined time points, expression was evaluated as ratio
between total RNA and DNA. At 48 h osteoblasts
expression level was higher for cells grown on tissue
culture dishes, although this difference disappears at the
latest time point (96 h). This observation suggests that
after short time the b titanium alloy inhibits osteoblasts
expression level while this effect disappears after longer
growth time. Interestingly, a different level of expression
was detected between fibroblasts and osteoblasts. The
expression was higher in osteoblasts. It seemed that
increased expression level was associated to low cell
density, indicating a higher metabolic activity for cells in
the growth phase.
Type I collagen is the most abundant protein present
in different connective tissues especially in skin and
bone. A number of studies have demonstrated the
pivotal role of collagen in modulating cell growth and
differentiation [28]. Collagen type I serves as a basis for
the mineral scaffold [29]. Our protein studies revealed
that the collagen amount was higher for both fibroblasts
and osteoblasts grown on the alloy discs. Collagen
glycosylation can be analyzed on the basis of electro-
phoretic migration; overglycosylated collagen could
appear as broad or double band due to the delay in
the migration of the abnormal chains. No difference was
detected on electrophoretic pattern for both cell types
grown either on TMZF discs or plastic tissue culture
dishes excluding any influence of the b titanium alloy in
the glycosylation pattern.
These data suggest a good biocompatibility of the
TMZF alloy and the presence of a stimulatory effect of
the alloy on collagen synthesis.
Since proper phenotype is of fundamental importance
for the correct interpretation of the obtained results, we
had special care in characterizing osteoblasts primary
cultures. The TMZF alloy did not cause in osteoblasts
the lost of the alkaline phosphatase activity, specific
property of this cell type, and did not change their
morphology, as demonstrated by light microscopy.
The results presented in this paper concern biomater-
ial/cell interactions on a scale of days. This time period
is crucial since it match with cell attachment, spreading
and growth on a substrate. Anyway long-term events
such as mineralization are of particular interest. We plan
to further investigate in this direction paying particular
attention to extracellular matrix synthesis and interac-
tion by use of biochemical, molecular and receptor
analysis techniques. A better understanding of these
later processes will contribute to improve quality and
long-term success of biomaterial implants.
Acknowledgements
We thank Mr. A. Gallanti for tissue culture work,
Mrs. E. Campari and Mr. M. Bellaviti for technical
support. This work was supported by MURST Cofin.
Table 3
% of ALP positive cells before and after biomaterial plating
Osteoblasts
Passage 0
Passage I
Passage II
Passage II
on plastic
no TMZF
after TMZF
contact
OB3+OB4
a
67.5
67
66
67
a
OB3+OB4 is the mean of OB3 and OB4.
L. Trentani et al. / Biomaterials 23 (2002) 2863–2869
2868

98, University of Pavia, Pavia; Target Project Biotech-
nology, CNR, Roma and IRCCS Grant, University of
Pavia, Pavia.
References
[1] Anselme K. Osteoblast adhesion on biomaterials. Biomaterials
2000;21:667–81.
[2] Evans EJ, Thomas IT. The in vitro toxicity of cobalt–chrome–
molybdenum alloy and its constituent metals. Biomaterials
1986;7:25–9.
[3] Pohler OE. Unalloyed titanium for implants in bone surgery.
Injury 2000;31(Suppl.):7–13.
[4] Schmidt C, Ignatius AA, Claes LE. Proliferation and differentia-
tion parameters of human osteoblasts and titanium and steel
surfaces. J Biomed Mater Res 2001;54:209–15.
[5] Krause A, Cowles EA, Gronowicz G. Integrin-mediated signaling
in osteoblasts on titanium implant materials. J Biomed Mater Res
2000;52:738–47.
[6] Thierry B, Tabriziam M, Savadogo O, Yahia L. Effects of
sterilization processes on NiTi alloy surface characterization.
J Biomed Mater Res 2000;49:88–98.
[7] Assad M, Lemieux N, Rivard CH, Yahia LH. Comparative in
vitro biocompatibility of nickel titanium, pure nickel, pure
titanium, and stainless steel: genotoxicity and atomic absorption
evaluation. Biomed Mater Eng 1999;9:1–12.
[8] Howlett CR, Zreiqat H, Wu Y, McFall DW, Mckenzie DR.
Effect of ion modification of commonly used orthopedic materials
on the attachment of human bone-derived cells. J Biomed Mater
Res 1999;45:345–54.
[9] Josset Y, Oum’Hamed Z, Zarrinpour A, Lorenzato M, Adnet JJ,
Laurent-Maquin D. In vitro reactions of human osteoblasts in
culture with zircania and alumina ceramics. J Biomed Mater Res
1999;47:481–93.
[10] Shanbhag AS, Jacobs JJ, Nlack J, Galante JO, Glant TT.
Macrophage/particle interactions: effect of size, composition and
surface area. J Biomed Mater Res 1994;28:81–90.
[11] Cooper LF, Masuda T, Yliheikkila PK, Felton DA. General-
izations regarding the process and phenomenon of osseointegra-
tion Part II. In vitro studies. Int J Oral Maxillofac Implants
1998;13:163–74.
[12] Ziats NP, Miller KM, Anderson JM. In vitro and in vivo
interactions of cells with biomaterials. Biomaterials 1998;9:5–13.
[13] Jauregui HO. Cell adhesion to biomaterials. The role of several
extracellular matrix components in the attachment of non-
transfected fibroblasts and parenchymal cells. ASALO Trans
1987;33:66–74.
[14] Harmand MF, Bordenave L, Duphil R, Jeandot R, Ducassou D.
Human differentiated cell cultures: in vitro models for character-
izations of cell/biomaterials interface. In: Christel P et al., editors.
Biological and biochemical performances of biomaterials. Am-
sterdam: Elsevier, 1986. p. 361–6.
[15] Ysander M, Branemark R, Olmarker K, Myers RR. Intramedul-
lary osseointegration: development of a rodent model and study
of histology and neuropeptide changes around titanium implants.
J Rehabil Res Dev 2001;38:183–90.
[16] Evans EJ. Cell damage in vitro following direct contact with fine
particles of titanium alloy and CoCrMb alloy. Biomaterials
1994;9:713–7.
[17] Bordji K, Jouzeau JY, Mainard D, Payan E, Netter P, Rie KT,
Stucky T, Hage-Ali M. Cytocompatibility of Ti–6Al–4 V and
Ti–5Al–2.5Fe alloys according to three surface treatments,
using human fibroblasts and osteoblasts. Biomaterials 1996;
9:713–7.
[18] Morrison C, Macnair R, MacDonald C, Wykman A, Goldie I,
Grant MH. In vitro biocompatibility testing of polymers for
orthopaedic implants using cultured fibroblasts and osteoblasts.
Biomaterials 1995;16:987–92.
[19] Najy A, Harmand LA. Study of the effect of the surface state on
the cytocompatibility of Co–Cr alloy using human osteoblasts
and fibroblasts. J Biomed Mater Res 1990;24:861–71.
[20] Yao J, Cs-Szabo’ G, Jacobs JJ, Kuettner KE, Glant TT.
Suppression of osteoblast function by titanium particles. J Bone
Jt Surg Am 1997;79:107–12.
[21] Haynes DR, Rogers SD, Hay S, Pearcy MJ, Howie DW. The
differences in toxicity and release of bone resorbing mediators
induced by titanium and cobalt–chromium-alloy wear particles. J
Bone Jt Surg Am 1993;7:825–34.
[22] Sarafova AP, Choi H, Forlino A, Gajko A, Cabral WA, Tosi L,
Reing CM, Marini JC. Three novel type I collagen mutations in
osteogenesis imperfecta type IV probands are associated with
discrepancies between electrophoretic migration of osteoblast and
fibroblast collagen. Hum Mutat 1998;11:395–403.
[23] Valli M, Mottes M, Tenni R, Sangalli SA, Gomez-Lira M, Rossi
A, Antoniazzi F, Cetta G, Pignatti PF. A de novo G to T
transversion in a pro-alpha I (I) collagen gene for a moderate case
of osteogenesis imperfecta Substitution of cysteine for glycine 178
in the triple helical domain. J Biol Chem 1991;266:1872–8.
[24] Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG,
Smith JA, Struhl K. Appendix 1 in current protocols in molecular
biology, vol. 12. Wiley: New York, 1990. p. A.1.5.
[25] Zhou BS, Wu RS, Randall DJ, Lam PK. Bioenergetics and RNA/
DNA ratios in the common carp(Cyprinus carpio) under hypoxia.
J CompPhysiol 2001;171:49–57.
[26] Coelho
MJ,
Cabral
AT,
Fernandes
MH.
Human
bone
cell cultures in biocompatibility testing. Part I: osteoblastic
differentiation of serially passaged human bone marrow cells
cultured
in
alpha-MEM
and
in
DMEM.
Biomaterials
2000;21:1087–94.
[27] Coelho MJ, Fernandes MH. Human bone cell cultures in
biocompatibility testing. Part II: effect of ascorbic acid, beta-
glycerophosphate and dexametasone on osteoblastic differentia-
tion. Biomaterials 2000;21:1095–102.
[28] Roehlecke C, Witt M, Kasper M, Schulze E, Wolf C, Hofer A,
Funk RW. Synergistic effect of titanium alloy and collagen type I
on cell adhesion, proliferation and osteoblast-like cells. Cells
Tissues Organs 2001;168:178–87.
[29] Geissler U, Hempel U, Wolf C, Schamweber D, Worch H, Wenzel
KW. Collagen type I-coating of Ti6Al4 V promotes adhesion of
osteoblasts. J Biomed Mater Res 2000;51:752–60.
L. Trentani et al. / Biomaterials 23 (2002) 2863–2869
2869
Document Outline
- Evaluation of the TiMo12Zr6Fe2 alloy for orthopaedic implants: in™vitro biocompatibility study by using primary human fibroblas
Wyszukiwarka
Podobne podstrony:
51 721 736 Evaluation of the Cyclic Behaviour During High Temperature Fatique of Hot Works
Evaluation of the french pictogram JUliette Guillemont
Evaluation of the role of Finnish ataxia telangiectasia mutations in hereditary predisposition to br
Mechanical evaluation of the resistance and elastance of post burn scars after topical treatment wit
Evaluation of the riparian forest habitat
Security Evaluation of the OpenBSD Operating System
Borderline Pathology and the Personality Assessment Inventory (PAI) An Evaluation of Criterion and
Evaluating The Use Of The Web For Tourism Marketing
Evaluation of HS SPME for the analysis of volatile carbonyl
(IV)Intertester reliability of the McKenzie evaluation in assessing patients with mechanical low bac
Evaluation of biomass quality of selected woody species depending on the method of soil enrichment a
Spyra, Piotr Bot Wothes Mo Iwysse Ther Ware On the Nightmarish Bliss of the Pearl Vision (2009)
Validity of selected spatial attributes in the evaluation of 5 channel mic tech
więcej podobnych podstron