
Skin diseases in the alpaca (Vicugna pacos): a literature
review and retrospective analysis of 68 cases (Cornell
University 1997–2006)
Danny W. Scott*
,†
, Jeff W. Vogel*, Rebekah I.
Fleis
†
, William H. Miller Jr*
,†
and Mary C. Smith
‡
*Department of Clinical Sciences, College of Veterinary Medicine,
Cornell University, Ithaca, NY 14853, USA
†
Department of Biomedical Sciences, College of Veterinary
Medicine, Cornell University, Ithaca, NY 14853, USA
‡
Department of Population Medicine and Diagnostic Services,
College of Veterinary Medicine, Cornell University, Ithaca, NY 14853,
USA
Correspondence: Danny W. Scott, Department of Clinical Sciences,
College of Veterinary Medicine, Cornell University, Ithaca, NY 14853,
USA. E-mail: shb3@cornell.edu
Sources of Funding
This study is self-funded.
Conflict of Interest
No conflicts of interest have been declared.
Abstract
This retrospective study describes 68 alpacas with
skin diseases investigated from 1997 through 2006 at
Cornell University. During this time period, 40 of 715
(5.6%) alpacas presented to the university hospital
had dermatological diseases. In addition, skin-biopsy
specimens accounted for 86 of 353 (24.4%) of alpaca
biopsy specimens submitted to the diagnostic labo-
ratory, and of these 86 specimens, follow-up was
available for 28 cases. The following diseases were
most common: bacterial infections (22%); neoplasms,
cysts and hamartomas (19%); presumed immuno-
logical disorders (12%); and ectoparasitisms (10%).
Conditions described for the first time included
intertrigo, collagen and hair follicle hamartomas,
lymphoma, hybrid follicular cysts, melanocytoma,
anagen
defluxion,
telogen
defluxion,
presumed
insect-bite hypersensitivity, ichthyosis, and possible
hereditary bilateral aural haematomas and chondri-
tis. The results of the retrospective study are com-
pared and contrasted with the results of a literature
review.
Accepted 22 May 2010
Introduction
Alpacas (Vicugna pacos, formerly Lama pacos) are grow-
ing in popularity and are increasingly being presented for
veterinary care and often present with skin disorders that
provide diagnostic and therapeutic challenges for the
practicing veterinarian.
1–4
Reviews exclusively devoted to
alpaca skin conditions are not available in the literature.
Clinicians have to rely upon literature for the llama,
5,6
or extrapolations from mixed camelid reviews,
2,4,7–9
or
information from scattered case reports.
A postal survey was conducted in the UK between
2000 and 2001 by D’Alterio et al.
10
This survey indicated
that the percentage of alpaca ownership was increasing.
In 1993, alpacas accounted for 21% of the camelid popu-
lation, whereas in 1998 they accounted for 77% of the
camelid population. In the same survey, 51.1% (111 of
217) of respondents indicated that skin diseases were
seen at one time or another, and that up to 9% of all ani-
mals could be affected at a given time. Most respondents
reported that skin conditions were more prevalent in
summer, and that most affected animals were nonwhite
coloured.
The most common skin lesions reported in the UK
survey were alopecia, crusts, scales and pruritus. The
most commonly affected body sites were the nose, ears,
periorbital region, medial thighs, axillae, dorsum and
abdomen. The most common dermatological diagnoses
rendered by attending veterinarians, as reported by the
owners, were zinc-responsive dermatitis (23%), ectopar-
asitism (19%), fungal infection (3%), bacterial infection
(3%), allergy (3%), dermatophilosis (2%) and contagious
viral pustular dermatitis (‘orf’; 1%). This information must
be carefully interpreted, as it is not known how the diag-
noses were established.
The UK survey also indicated that many alpacas were
housed on farms with other domestic species, as follows:
horses and ponies, 66.7%; sheep, 55.9%; goats, 22.6%;
and cattle, 19%. This has obvious implications for the
infectious and ectoparasitic diseases that can affect
those domestic species and alpacas. It is not known if
similar percentages of commingling of species occur in
the USA, but the practice is undoubtedly common.
In this paper we firstly review the literature (predomi-
nantly English language) on alpaca skin conditions, sec-
ondly present the results of a retrospective study of
dermatological disorders in alpacas examined at the
Cornell University Hospital for Animals (CUHA), and
thirdly present the results of a retrospective study of
alpaca skin-biopsy specimens submitted to the Animal
Health Diagnostic Center (AHDC) at Cornell University.
Materials and methods
From January 1997 through December 2006 (10 years), 715 alpacas
were patients at the CUHA, and 40 of these (5.6%) had dermatologi-
cal diseases (Table 1). Follow-up information was available for 33
2
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
DOI: 10.1111/j.1365-3164.2010.00918.x

(82%) of these patients. The number of alpaca patients per year
increased from 18 in 1997 to 240 in 2006. The female:male ratios of
the total alpaca patient population and the dermatology cases were
3:1 and 4:1, respectively. During the same time period, 86 skin-
biopsy specimens from alpacas were submitted to the AHDC. Fol-
low-up information was available for 28 (33%) of these alpacas
(Table 1). Skin-biopsy specimens accounted for 24.4% (86 of 353)
alpaca biopsy specimens submitted during the study period.
Case results and literature review
Bacterial diseases
Bacterial infections (including dermatophilosis) accounted
for only 5% of the cutaneous diagnoses made in alpacas
by veterinarians in the UK postal survey.
10
However, bac-
terial infections were documented and
⁄ or diagnosed (by
cytological examination and response to antibacterial
therapy) in 22% of the alpacas in our retrospective study.
In general, there are no systemic antibiotics approved
for use in alpacas. Off-label antibiotics that are commonly
useful for treating skin diseases in alpacas include the fol-
lowing:
1
(i) procaine penicillin at 20 000–40 000 IU
⁄ kg,
twice daily, subcutaneously (s.c.) or intramuscularly (i.m.);
(ii) ceftiofur at 2.2 mg
⁄ kg, twice daily, s.c., i.m. or intra-
venously (i.v.); (iii) oxytetracycline at 20 mg
⁄ kg every
3 days, s.c. or i.m.; or (iv) enrofloxacin at 10 mg
⁄ kg, once
daily, per os (p.o.). The subcutaneous space of alpaca
skin is inelastic and small, and no more than 10 mL of
solution should be injected into a single site; the fold
of skin in the axilla or just cranial to the shoulder is
recommended.
2
Corynebacterium pseudotuberculosis infection
Corynebacterium pseudotuberculosis infections appear
to be common in alpacas.
2,11–13
Infection may occur by
wound contamination or by consuming infected milk.
13
Affected animals range from 22 days to 14 months of
age.
Lesions are solitary or multiple subcutaneous nodules
or abscesses. The head, submandibular and ventral cervi-
cal regions are most commonly affected. Early lesions are
characterized by pyogranulomatous inflammation with
central caseous necrosis (nodule), while older lesions are
characterized by liquefactive necrosis and peripheral fibro-
sis (abscess).
13
Diagnosis is confirmed by culture.
Traditional treatment consists of surgical drainage,
flushing and systemic antibiotics.
11
However, en bloc
excision may be the best way to control local infections,
as abscesses may recur following traditional treatment.
11
Furthermore, spontaneous or surgical drainage potentially
contaminates the environment.
11
Corynebacterium pseu-
dotuberculosis is considered a zoonotic risk; however,
C. pseudotuberculosis infection was not documented in
this retrospective study.
Tooth root abscesses
Tooth root abscesses present as firm mandibular swell-
ings, with or without draining tracts.
14,15
Mandibular teeth
are more commonly involved than maxillary teeth, and
molars and premolars are more commonly affected than
incisors. The median age at presentation is 5 years,
which correlates with the eruption of permanent teeth.
15
It has been postulated that infection occurs when the
deciduous caps are loose but the permanent teeth are
not fully mature, thus allowing for trauma from roughage
during mastication.
15
Actinomyces spp. and unidentified anaerobes were the
most common isolates in one large study.
15
Radiography
is recommended to confirm the diagnosis and to evaluate
the extent of osteolysis.
15
Treatment usually includes combined tooth extraction
and systemic antibiotics.
14,15
The two most successful
antibiotic regimens (in combination with tooth extraction)
were ceftiofur at 2.2 mg
⁄ kg once or twice daily, i.v. or
s.c., for a mean duration of 11.4 ± 5.5 days, or procaine
penicillin at 20 000–40 000 IU
⁄ kg once or twice daily,
i.m., for a mean duration of 12.9 ± 7.2 days.
15
We diagnosed a tooth root abscess in a 4-year-old
female alpaca with an abscess below the left eye
(Table 1, case 18). The animal also had symmetrical,
3–4 mm diameter, punched-out ulcers, crusts, scaling
and hair loss that followed blood vessels at the pinnal
margins as well as linear, healing areas of alopecia, and
scaling on the convex surface of the pinnal mid-line. The
pinnal lesions had appeared a few weeks after the
abscess had been noted and were neither pruritic nor
painful. Biopsies were not performed, and a presumptive
diagnosis of pinnal vasculitis, possibly secondary to the
tooth root abscess, was made. The animal was treated
with maxillary tooth extraction and ceftiofur, and both the
abscess and the presumed pinnal vasculitis resolved.
Dermatophilosis
Anecdotal reports indicate that dermatophilosis occurs in
alpacas.
2,4,7,8,10
The disease is said to occur most fre-
quently in hot, humid regions of the USA, and frequently
to present as thick crusts on the pinnae.
8
Although we
see dermatophilosis in horses, cattle and goats in our
practice area, we documented no cases in our retrospec-
tive study.
Bacterial folliculitis
Anecdotal reports indicate that bacterial (staphylococcal)
folliculitis occurs in alpacas.
2,4,8
We diagnosed bacterial
folliculitis in 13 alpacas in our retrospective study. In ten
animals, the folliculitis was idiopathic (cases 9–15 and
45–47), and in three animals it was associated with pre-
sumed insect-bite hypersensitivity (cases 29 and 31) or
contact dermatitis (case 44). Lesions consisted of ery-
thematous papules, pustules, brown-to-yellow crusts,
epidermal collarettes, and annular areas of alopecia and
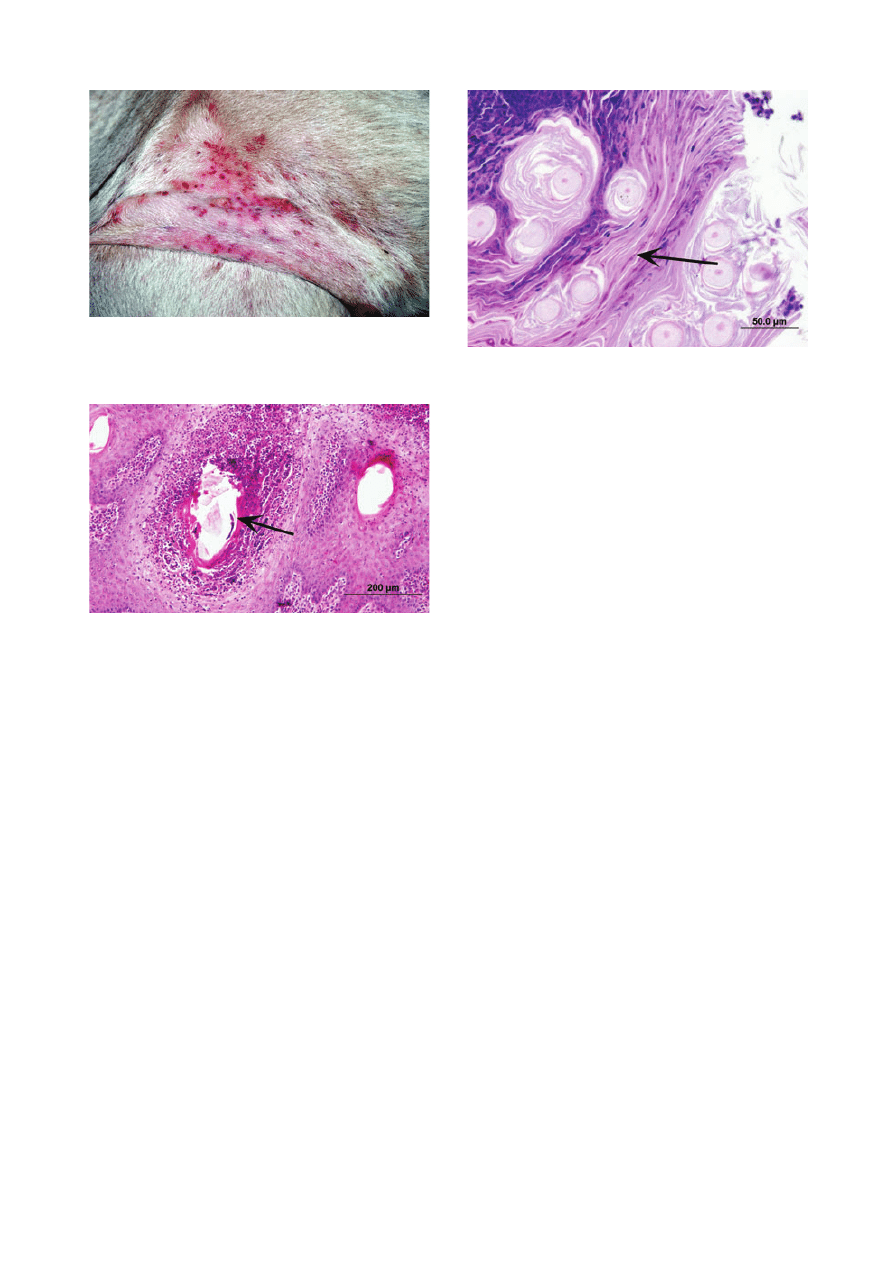
scaling (Figure 1). The muzzle, back, ventrum and distal
hindlegs were most commonly affected. Pruritus was
only reported in the three alpacas with concurrent pre-
sumed insect-bite hypersensitivity or contact dermatitis.
Skin scrapings and trichography were negative for para-
sites and fungi. Cytological examination revealed degen-
erate and nondegenerate neutrophils and phagocytosed
cocci. Cultures were not performed. Histological exami-
nation of biopsy specimens confirmed suppurative lumi-
nal folliculitis in six animals (cases 11–13 and 45–47;
Figure 2). Eosinophils were rarely seen. Follow-up infor-
mation was available for eight treated animals, as follows:
two (cases 9 and 13) were cured with the topical applica-
tion of povidone-iodine; and six (cases 11, 12, 29 and
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
3
Skin diseases in the alpaca

Table 1. Data on 68 alpacas with dermatological disease (Cornell University 1997–2006)*
Case
Age (years)
Sex
Diagnosis(es)
Follow-up
period (months)
1
1
F
Bite wounds
24
2
1
F
Bite wounds
Died
3
1
F
Bite wounds
12
4
8
M
Bite wounds
Died
5
0.8
F
Bite wounds
Died
6
1.5
F
Bite wounds
Died
7
1
F
Bite wounds
12
8
0.7
F
Bite wounds
Died
9
Adult
F
Bacterial folliculitis
12
10
Adult
F
Bacterial folliculitis
0
11
7
F
Bacterial folliculitis
12
12
1.5
M
Bacterial folliculitis
20
13
Adult
F
Bacterial folliculitis
6
14
3
F
Bacterial folliculitis
0
15
3
F
Bacterial folliculitis
0
16
3
F
Bacterial intertrigo
24
17
7
F
Bacterial intertrigo
0
18
4
F
Tooth root abscess, pinnal vasculitis
†
12
19
4
F
Zinc-responsive dermatitis
12
20
3
F
Zinc-responsive dermatitis
12
21
3
F
Zinc-responsive dermatitis
6
22
4
F
Zinc-responsive dermatitis
12
23
1.5
M
Zinc-responsive dermatitis
12
24
0.8
F
Chorioptic mange
Euthanized
25
5.5
F
Chorioptic mange
0
26
5
M
Chorioptic mange
18
27
7
F
Psoroptic mange
†
12
28
0.8
M
Psoroptic mange
†
12
29
11
F
Insect-bite hypersensitivity
†
, bacterial folliculitis
24
30
1
M
Insect-bite hypersensitivity
†
24
31
1
M
Insect-bite hypersensitivity
†
, bacterial folliculitis
0
32
8
F
Insect-bite hypersensitivity
†
12
33
0.8
F
Contagious viral pustular dermatitis
Died
34
0.1
F
Anagen defluxion
6
35
Adult
F
Telogen defluxion
0
36
0.3
F
Ichthyosis
Euthanized
37
1
M
Sterile eosinophilic folliculitis and furunculosis
24
38
5
M
Adverse cutaneous drug reaction
†
12
39
8
F
Hair follicle hamartomas
12
40
5
F
Hair follicle hamartomas
12
41
6
F
Viral papillomas
6
42
4
M
Viral fibropapillomas
12
43
Adult
F
Dermatophytosis
9
44
Adult
M
Contact dermatitis
†
, bacterial folliculitis
18
45
2
F
Bacterial folliculitis
12
46
3
M
Bacterial folliculitis, abscess
6
47
0.7
M
Bacterial folliculitis, pyogranuloma
12
48
2
F
Psoroptic mange
6
49
Adult
F
Chorioptic mange
†
24
50
2
F
Idiopathic urticaria
72
51
5
F
Insect-bite hypersensitivity
†
Killed
52
4
F
Ichthyosis
24
53
1.5
M
Ichthyosis
Euthanized
54
3
F
Hybrid follicular cysts
6
55
2
M
Hybrid follicular cysts
24
56
Adult
M
Hybrid follicular cysts
24
57
11
F
Fibroma
24
58
Adult
F
Fibroma
36
59
6
F
Melanocytoma
48
60
14
M
Trichoepithelioma
24
61
1.5
F
Lymphoma
Euthanized
62
0.1
F
Bilateral aural haematomas and chondritis
36
63
0.1
M
Bilateral aural haematomas and chondritis
24
64
0.3
F
Bilateral aural haematomas and chondritis
36
65
0.2
F
Bilateral aural haematomas and chondritis
36
66
0.3
M
Bilateral aural haematomas and chondritis
24
67
0.1
F
Bilateral aural haematomas and chondritis
24
68
3
M
Collagenous hamartomas
12
*Cases 1–40 were patients at the Cornell University Hospital for Animals. Cases 41–68 had skin-biopsy specimens submitted to the Animal Health Diagnos-
tic Center at Cornell University.
†
Presumptive diagnosis.
F, female; M, male.
4
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
Scott et al.
45–47) were cured after the systemic administration of
ceftiofur for 10–21 days.
Intertrigo
Intertrigo (skin-fold dermatitis) is a frictional dermatitis
that occurs where two skin surfaces are intimately
apposed. We diagnosed intertrigo with secondary bacte-
rial infection in two alpacas (cases 16 and 17). In both
cases, cytological examination revealed pus and phagocy-
tosed cocci. The intertrigo and secondary infection were
perivulvar in case 17. In case 16, a hernia developed
in the left ventral flank area post-Caesarean section. The
intertrigo and bacterial infection developed in the apposed
skin of the left inguinal region and medial aspect of the
left thigh. The animal was cured after surgical repair of
the hernia and a course of systemic ceftiofur.
Miscellaneous bacterial infections
Bacterial
pseudomycetoma
(‘botryomycosis’)
was
reported in one alpaca in the USA.
8
The animal had multi-
ple abscesses and granulomas, 0.5–4 cm diameter, on
the medial thigh. Diagnosis was confirmed by histological
examination and culture (Staphylococcus aureus). Sur-
gery followed by 4 weeks of broad-spectrum antibiotic
therapy was reported to be successful. Further details
were not given.
Mycobacterium ulcerans was identified (by culture and
polymerase chain reaction) in a large ulcer on a leg of an
alpaca in southeastern Australia.
16
No details were given.
Cutaneous infection with Actinobacillus lignieresi was
reported in an alpaca in the UK.
2
No details were given.
Fungal diseases
Anecdotal reports indicate that dermatophytosis occurs in
alpacas.
2,4,7–10
Fungal infection accounted for 3% of the
dermatological diagnoses made by attending veterinari-
ans in the UK postal survey.
10
We documented only one
case of dermatophytosis in our retrospective study (1.5%
of the cases). An adult female alpaca (case 43) developed
an alopecic, crusted, hyperkeratotic area on the upper lip.
Histological examination revealed a suppurative luminal
folliculitis, with fungal hyphae and arthroconidia within fol-
licular keratin, but not within hair shafts (Figure 3). Fungal
culture was not performed. The condition was treated
with topical clotrimazole, twice daily, and resolved in
2 weeks.
Viral diseases
Contagious viral pustular dermatitis (‘orf’ or ‘contagious
ecthmya’) is a parapoxvirus infection that has been
reported in alpacas.
2,4,7–10,17
Affected animals are typi-
cally 2–4 months old. Thick crusts are present on the lips
and nostrils. Infected nursing cria may transmit the
disease to the teats of the dam. The disease is a zoonotic
risk. The condition is typically self-limiting, although one
author mentions that a chronic form lasting ‘months’ can
be seen.
8
Diagnosis is confirmed by viral isolation and
viral antigen detection techniques.
We diagnosed presumptive contagious viral pustular
dermatitis in a 9-month-old alpaca that died shortly after
being admitted to the CUHA (case 33). Thick crusts, ooz-
ing and ulceration were present on the nostrils, upper and
lower lips. Necropsy examination revealed pericardial
effusion and hepatic lipidosis. Histological examination of
skin specimens revealed ballooning degeneration of
epidermal keratinocytes and eosinophilic intracytoplasmic
inclusion bodies consistent with parapox infection.
Figure 1. Ventral abdominal area of an alpaca with bacterial folliculi-
tis. Note erythematous papules, pustules and crusts.
Figure 2. Photomicrograph of skin-biopsy specimen from an alpaca
with bacterial folliculitis. Note luminal suppurative folliculitis (arrow).
Haematoxylin and eosin; scale bar = 200 lm.
Figure 3. Photomicrograph of skin-biopsy specimen from an alpaca
with dermatophytosis. Dermatophyte hyphae are present in the kera-
tin surrounding hair shafts (arrow). Periodic acid–Schiff; scale
bar = 50 lm.
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
5
Skin diseases in the alpaca

Specific viral isolation and identification techniques were
not performed.
Ectoparasitic diseases
By far the most commonly reported skin diseases of
alpacas are caused by ectoparasites, especially mange
mites and lice.
2,4,8,10
Ectoparasitism accounted for 19%
of the dermatological diagnoses rendered by attending
veterinarians in the UK postal survey.
10
Ectoparasitism
accounted for 10% of the cases in our retrospective
study. Alpacas develop sarcoptic mange, psoroptic
mange and chorioptic mange; and all three mites have
been reported to simultaneously infect the same
animals.
18
In general, there are no ectoparasiticides approved for
use in alpacas. In addition, pharmacokinetic studies of
macrocyclic lactones (avermectins) in New World came-
lids are limited, and most have been conducted in
llamas.
19–22
These studies suggest that the absorption of
these compounds, irrespective of the route of administra-
tion, is somewhat lower than that in cattle and sheep.
Thus, higher doses (e.g. 0.4 mg
⁄ kg ivermectin, every
7 days, s.c.) may be necessary in alpacas.
2
Sterile
abscesses may be seen with the s.c. administration of
ivermectin, and inconspicuous injection sites (such as the
axilla) have been recommended.
1,2
It is common to inject
ivermectin s.c. in the neck just cranial to the shoulder
(much easier to do here; fibre coat makes any resulting
lumps inconspicuous).
An additional consideration is that alpaca fibre does not
contain lanolin, so that topical applications of insecticides
and acaricides used on other ruminants may not be as
effective in alpacas.
2
Sarcoptic mange
Sarcoptic mange is caused by Sarcoptes scabiei var.
auchinae. It has been reported from many countries in
the world, and is a significant cause of weight loss and
decreased
fibre
production.
2,4,8,10,18,23–30
Sarcoptic
mange has been reported to be responsible for up to
95% of the economic losses caused by ectoparasites in
alpacas, with up to 40% of alpacas being infested.
Alpacas with sarcoptic mange present with alopecia
and usually severe pruritus.
2,4,8,10,18,30
Early skin lesions
consist of erythema, papules and yellow-to-grey crusts.
Chronic changes include marked skin thickening, lichenifi-
cation and hyperpigmentation. The disease often begins
on the ventral abdomen and chest, axillae and groin, with
gradual extension to the medial thighs, prepuce, peri-
neum, legs, interdigital spaces, face and pinnae. Second-
ary bacterial infection can complicate the condition.
8
Sarcoptic mange in alpacas is a potential zoonosis.
2,4,8
Diagnosis of sarcoptic mange is confirmed by finding
mites in skin scrapings; however, negative skin scrapings
do not rule out the disease.
2,4
Histopathological findings
include a superficial eosinophilic interstitial dermatitis,
marked parakeratotic hyperkeratosis, and mites in the
surface keratin and crusts.
28
Ivermectin (0.2–0.4 mg
⁄ kg, every 7–14 days, s.c., for
two to four injections) has been reported to be an effec-
tive treatment.
2,4,18,27
It has been suggested that sarcop-
tic mange is rare in the USA because of the use of
ivermectin for routine deworming.
4–6
However, there
have been reports of treatment failures with ivermec-
tin,
2,29,30
as well as doramectin,
29,30
eprinomectin,
30
amitraz
29
and diazinon.
29
We diagnosed no cases of sarc-
optic mange in our retrospective study.
Psoroptic mange
Psoroptic mange is caused by a psoroptic mite previously
referred to as Psoroptes communis var. auchinae, P. au-
chinae, P. cuniculi and P. ovis.
31,32
In fact, the species of
Psoroptes mite that infests alpacas has not been officially
named, and recent authors use the name Psoroptes sp.
2
Alpacas with psoroptic mange most commonly present
with dermatitis on the head, face and pinnae.
2,4,8,10,18,31–33
In some individuals, only the ear canals are affected, and
the animals present for ear twitching, head shaking, large
dry flakes in the ear canals and occasional purulent dis-
charge due to secondary bacterial infection.
8,32
Skin
lesions include papules, crusts, exudation, alopecia and
pruritus. A more widespread distribution of lesions may
be seen: shoulders, back, rump, sides and perineum.
2,8,32
Psoroptic mange does not constitute a zoonotic risk.
Diagnosis is confirmed by finding mites in skin scrap-
ings. Ivermectin, as described previously for sarcoptic
mange, is usually effective for treatment.
2,4,18,31
We diagnosed psoroptic mange (positive skin scrap-
ings) in one alpaca (case 48) and suspected it in two others
(cases 27 and 28). All animals had crusts, scales, alopecia
and pruritus involving the pinnae and face. Skin scrapings
were negative in two cases, but cytological examination
revealed eosinophilic inflammation. Biopsy specimens
from two animals (cases 27 and 48) revealed superficial
and deep eosinophilic interstitial dermatitis with marked
parakeratotic hyperkeratosis. Mites were seen in surface
crust in one case (case 48). All three alpacas were cured
after a course of s.c. ivermectin injections.
Chorioptic mange
Chorioptic mange is caused by Chorioptes bovis. It has
been reported from many countries in the world, and
appears to be the most common mite infestation of alpa-
cas.
2,4,18,32,34–40
Alpacas with chorioptic mange often initially present
with scale, crusts and alopecia on the ventral tail, perineal
region, ventral abdomen and medial thighs.
2,8,32
Lesions
then spread to the axillae, tips and lateral surface of the
pinnae, interdigital spaces and distal limbs up to the fet-
locks. In some outbreaks, lesions are more commonly
seen on the pinnae, face, neck, dorsum and feet.
4,37,39,40
In severe cases, erosions, ulcers and lichenification can
be seen. Pruritus is usually absent or mild. Chorioptic
mange does not constitute a zoonotic risk.
Diagnosis is confirmed by finding mites in skin scrap-
ings. In a study on the prevalence of Chorioptes bovis
infestation in alpacas in the UK,
39
skin scrapings were
positive in 55% of the clinically normal in-contact animals,
but positive in only 28% of the animals with skin lesions.
Clinically normal, skin-scrape-positive alpacas tended to
be younger (<24 months old), whereas alpacas with skin
lesions tended to be older. It is not clear whether or not
all of the alpacas with skin lesions in the UK study had
chorioptic mange. However, other investigators have also
6
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
Scott et al.
indicated that heavily infested alpacas can be clinically
normal, and alpacas with extensive lesions can have
low numbers of mites.
4,36,40
Skin scrapings from the
axilla and interdigital space are the most commonly posi-
tive.
4,39
Histological examination of skin-biopsy speci-
mens is reported to reveal eosinophilic perivascular
dermatitis and eosinophilic epidermal microabscesses
and pustules.
37
Chorioptic mange in alpacas is a therapeutic challenge.
Repeated s.c. injections of ivermectin (0.2–0.4 mg
⁄ kg)
reduce mite numbers and lesion severity, but do not erad-
icate mites and any associated dermatitis.
2,18,37,38
Similar
results were obtained with repeated topical applications
of eprinomectin (0.5 mg
⁄ kg).
38,40
Anecdotal reports sug-
gest that topical applications of fipronil may be effective.
2
We diagnosed chorioptic mange in three alpacas (cases
24–26) in our retrospective study and suspected it in one
other (case 49). All four animals had nonpruritic crusts,
scales and alopecia on the tail, perineum, hindlegs and
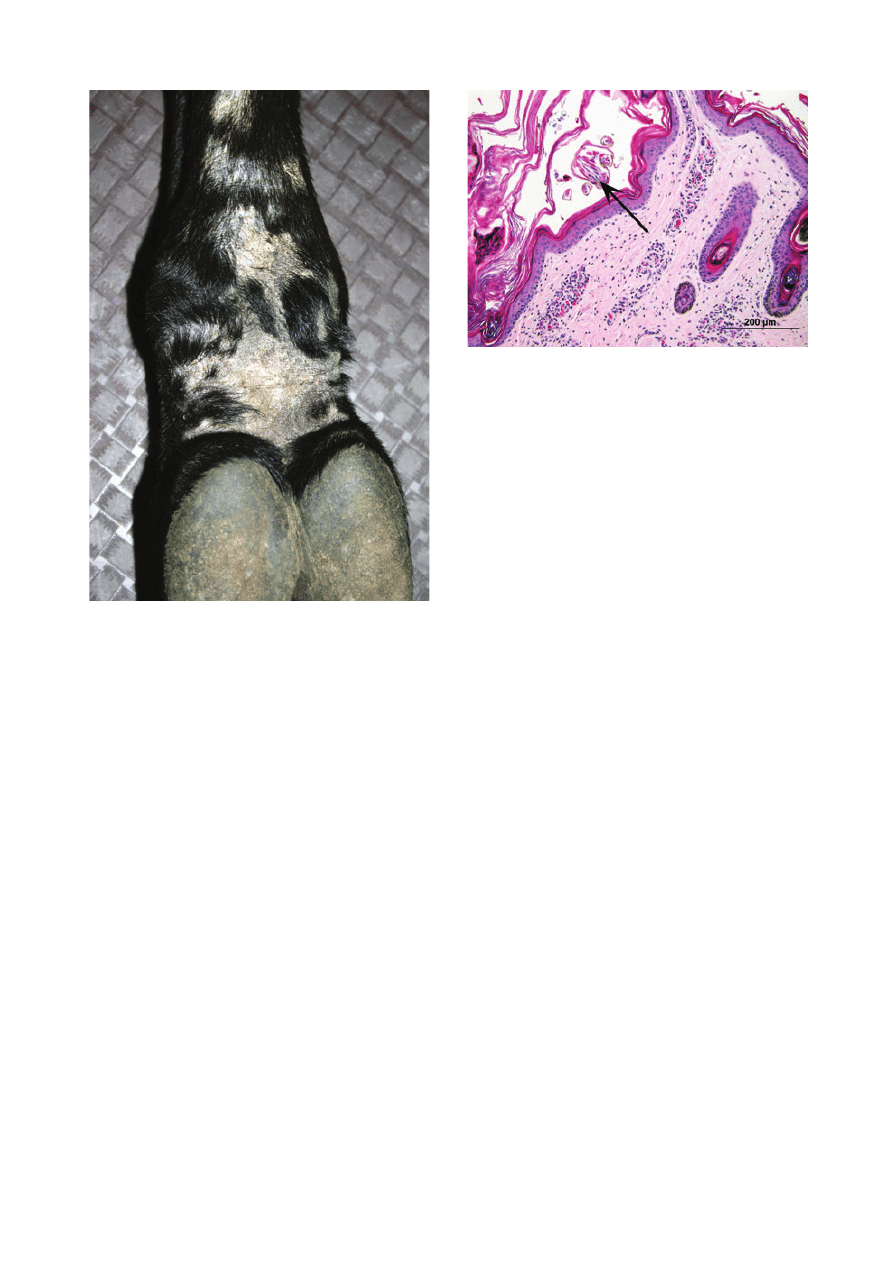
hindfeet (Figure 4). Two animals additionally had lesions
on the axillae and front feet. One animal also had lesions
on the pinnae. Skin scrapings were positive, and biopsy
specimens revealed eosinophilic interstitial dermatitis in
three alpacas (cases 24, 26 and 49). Mites were present
in surface crusts in case 24 (Figure 5). One animal (case
24) was euthanized due to endometritis. Two animals
(cases 26 and 49) were successfully treated with weekly
topical applications of 2% lime sulfur for 6–8 weeks.
Pediculosis
Pediculosis in alpacas is caused by two species of lice:
the chewing louse Bovicola (Lepikentron) breviceps and
the sucking louse Microthoracius mazzi (praelongiceps).
Louse infestations have been reported from many areas
of the world.
2,4,7–9,41–48
Pediculosis has been estimated
to produce annual net profit losses of 5–85%, with
weight loss and fibre damage resulting from disturbed
feeding and sleeping schedules and stress in association
with pruritus.
41,42,44
Heavy louse infestations cause alpacas to bite, rub and
kick their skin, resulting in variable degrees of traumatic
alopecia, excoriation and secondary bacterial infec-
tions.
2,4,7–9,41,42,44–47
Chewing lice may be more numer-
ous on the rump, dorsal trunk and neck, and sucking lice
on the head, neck and shoulder. Heavy infestations with
sucking lice may produce anaemia. Diagnosis is made by
parting the fleece in various areas and visualizing lice on
the skin surface and
⁄ or eggs (‘nits’) attached to the fibres
(often 5–10 mm above skin surface).
Treatment recommendations for pediculosis in alpacas
are often anecdotal and contradictory. Topical applications
have been reported usually to be ineffective.
46
Repeated
injections of ivermectin or moxidectin have been reported
to be effective,
41,44
or effective for sucking lice but not
for chewing lice.
7
A single alpaca was reported to be
cured
with
topical
applications
of
cypermethrin
(10 mg
⁄ kg), but no follow-up period was stated.
45
Eradi-
cation of B. breviceps from 25 alpacas in Australia was
achieved with two 8 min shower-dip applications of
spinosad (25 g
⁄ L) in combination with a surfactant wet-
ting agent (alcohol alkocylate at 1000 g
⁄ L) with a 17-day
interval.
47
If feasible, shearing is a useful prelude to the
use of antiparasitic agents. We did not diagnose pediculo-
sis in our retrospective study.
Miscellaneous ectoparasites
Anecdotal reports indicate that alpacas may be infested
with fleas; attacked by mosquitoes, black flies, tabanids
and ticks; and suffer from myiasis.
7–9
The same reports
indicate that Otobius megnini (spinose ear tick) can affect
alpacas in the western USA, causing head shaking and
Figure 4. Caudal pastern of an alpaca with chorioptic mange. Note
alopecia, scaling and crusts.
Figure 5. Photomicrograph of skin-biopsy specimen from an alpaca
with chorioptic mange. Note cross-section of mite (arrow) in surface
keratin. Haematoxylin and eosin; scale bar = 200 lm.
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
7
Skin diseases in the alpaca
otorrhoea.
7–9
Ivermectin injections (0.2 mg
⁄ kg) were
reported to be an effective treatment.
Neoplastic and non-neoplastic tumours
In a retrospective study of 368 alpaca specimens (biopsy
or necropsy) from the northwestern USA, neoplasia was
diagnosed in 18 animals (prevalence of 4.9%).
49
Cutane-
ous and mucocutaneous neoplasms accounted for 50%
of the cases. Eight of the nine skin neoplasms were
characterized as fibropapillomas or fibromas. Affected
animals ranged from 4 to 12 years old, and had single or
multiple lesions on the face, nose, lip or distal leg. Poly-
merase chain reaction studies to look for papillomavirus
DNA were not performed. One alpaca in this report had a
solitary fibrosarcoma on the lip.
Mucocutaneous fibropapillomas associated with a
unique camelid papillomavirus were reported in three
alpacas, all of which were 6 years old, from the northeast-
ern USA.
50
Lesions manifested as grey, hyperkeratotic,
nodular masses on lips or cheeks. Histologically, the
masses were similar to equine sarcoids. Polymerase
chain reaction testing revealed that all lesions were posi-
tive for papillomavirus DNA that had 73% homology with
bovine papillomavirus-1. In two animals, lesions were
surgically excised, with no recurrence after a 9-month
follow-up period.
Multiple trichoepitheliomas were reported in a 13-year-
old male alpaca.
51
The lesions were well-circumscribed,
ovoid, 1–4 cm diameter dermal masses distributed over
both sides of the body (especially neck, thorax and rump).
Neoplastic and non-neoplastic tumours accounted for
19% of the cases in our retrospective study (Table 1).
Two animals had multiple presumed viral papillomas
⁄
fibropapillomas (raised, hyperkeratotic, 0.2–2 cm diame-
ter) on the lips (case 41) or on three pasterns (case 42).
Histological findings were typical for papillomavirus infec-
tion (koilocytosis, irregularities in keratohyalin granule
morphology), but no specific tests were performed.
There had been no regression of nonexcised lesions after
6–12 months. Two animals (cases 57 and 58) had solitary
fibromas
(white,
alopecic,
firm,
2–3 cm
diameter)
excised from the lower lip with no recurrence after
24–36 months. A solitary dermal melanocytoma (black,
alopecic, firm, 4 mm diameter) was excised from the
right lower eyelid with no recurrence after 48 months
(case 59). A solitary trichoepithelioma (alopecic, firm,
ulcerated) was excised from the lateral neck region with
no recurrence after 24 months (case 60). One animal
(case 61) had multiple subcutaneous lymphomas widely
distributed over the body. The animal was euthanized,
and necropsy was not performed.
Three animals (cases 54–56) had multiple hybrid follicu-
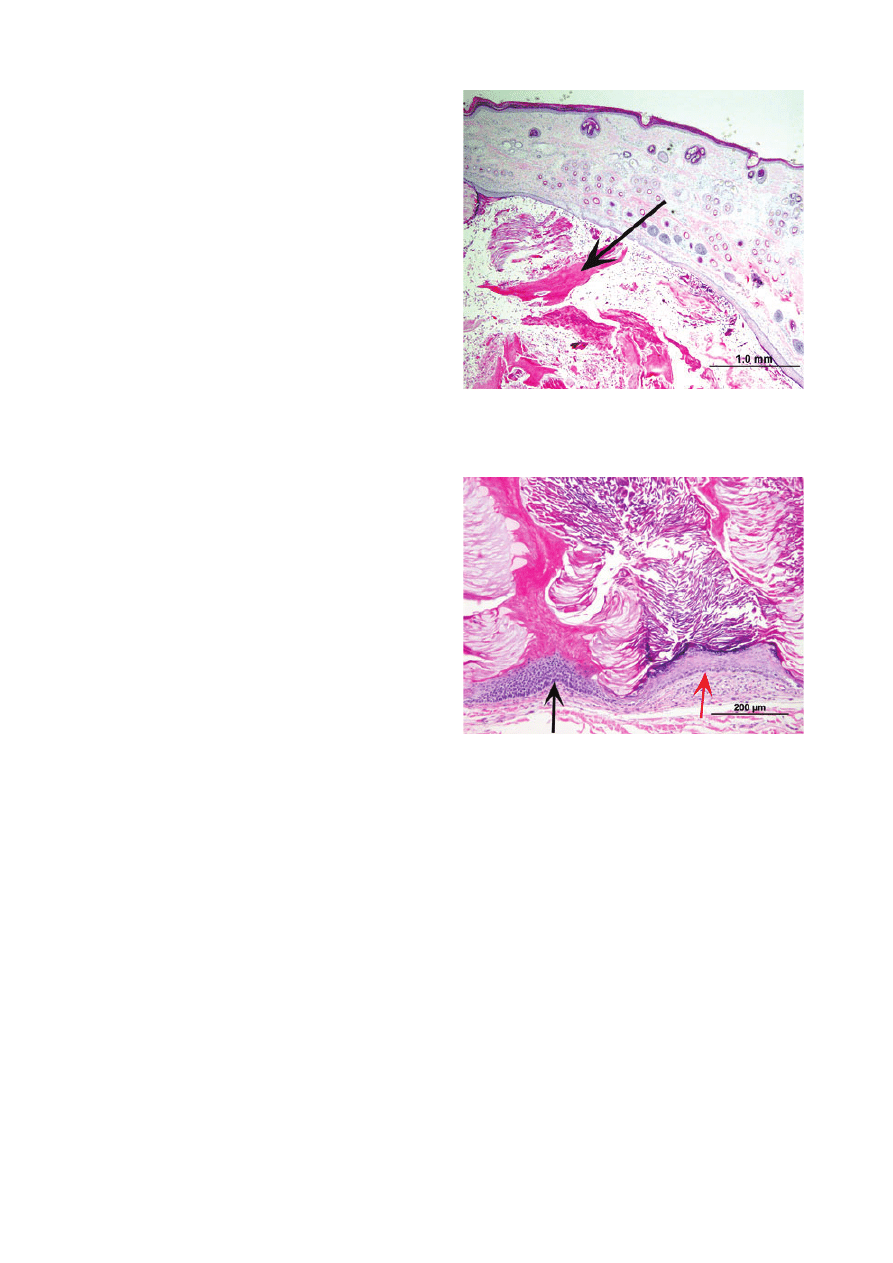
lar cysts (Figures 6 and 7; white, smooth, 1–3 cm diame-
ter, occasional spontaneous caseous black discharge)
widespread over the body, especially the trunk. Cytologi-
cal examination of the caseous discharge revealed
corneocytes and numerous melanin granules. Lesions
seemed to be stable during 6- to 24-month follow-up
periods.
Multiple hamartomas were diagnosed in three animals.
In case 68, the lesions (firm, smooth) were present on
eyelid, neck and foot. Histologically, these were collage-
nous hamartomas. The lesions seemed stable over a
12-month follow-up period. In two animals (cases 39 and
40), a mother and daughter, lesions were first noted at
about 3 years of age. The lesions were multiple, multifocal
(especially the trunk), firm, round-to-rectangular, slightly
elevated dermal plaques varying from 1 to 10 cm in diam-
eter. Fleece loss was minimal, and the skin overlying the
lesions was smooth to mildly hyperkeratotic. Lesions
were neither pruritic nor painful. Histologically, these
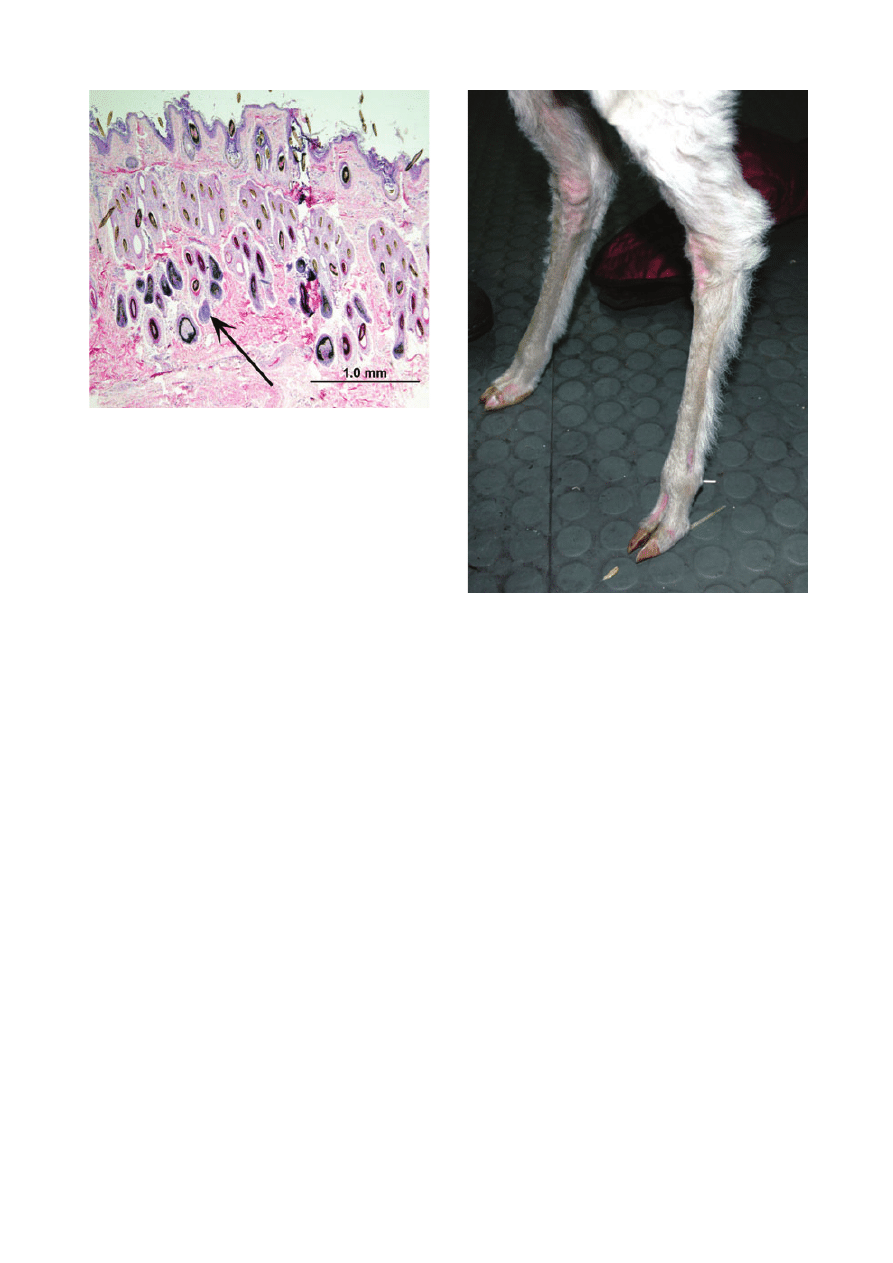
lesions were hair follicle hamartomas (Figure 8). Lesions
seemed to be stable after a 12-month follow-up period.
Environmental diseases
Traumatic wounds
Anecdotal reports indicate that lacerations and puncture
wounds are common in alpacas.
7,8
Lacerations (especially
to the pinnae, neck, caudal pelvic limbs and scrotum) are
often produced by barbed wire fencing or by aggressive
Figure 6. Photomicrograph of hybrid follicular cyst excised from an
alpaca. Cyst cavity (arrow) contains both lamellar and tricholemmal
keratin. Haematoxylin and eosin; scale bar = 1 mm.
Figure 7. Close-up
of
Figure 6.
Tricholemmal
differentiation
(black arrow) and associated tricholemmal keratinization alternates
with epidermal differentiation (red arrow) and associated lamellar
keratinization. Haematoxylin and eosin; scale bar = 200 lm.
8
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
Scott et al.
males interacting with other males or attacking females.
Dogs (individually or in packs) can inflict horrible wounds
that can be lethal.
We diagnosed dog bite wounds in eight alpacas (cases
1–8). Seven of the eight animals were approximately
1 year old. Bite wounds most commonly involved the
neck and hindlimbs, and cellulitis and subcutaneous
emphysema were present in six and four cases, respec-
tively. All animals received aggressive emergency and
supportive care, but five (62%) died.
Contact dermatitis
Anecdotal reports indicate the contact dermatitis can
be seen in alpacas.
7,8
Incriminated contactants include
plants, chemicals and povidone-iodine. Anecdotal reports
indicate that shearing alpacas with clippers that are too
hot can result in thermal burns, which may result in
sloughing of severely damaged skin several weeks later.
We made a presumptive diagnosis of contact dermatitis
and secondary bacterial folliculitis in one alpaca (case 44)
in our retrospective study. The animal had a chronic his-
tory of pruritic dermatitis affecting the entire ventrum.
Histological findings included suppurative luminal folliculi-
tis and a superficial perivascular-to-interstitial lymphoplas-
macytic dermatitis. Eosinophils were rarely seen. Pruritic
dermatitis continued after the bacterial infection was trea-
ted with ceftiofur. Prednisone (1 mg
⁄ kg once daily) was
administered p.o. for 14 days, the animal’s access to the
outdoor environment was restricted, and the pruritic
dermatitis completely resolved. Provocative exposure
was not attempted. No relapse was seen over an 18-
month follow-up period.
Snake bite
Rattlesnake envenomation has been reported in the
southwestern USA.
52
Most bites occur in the period from
spring to summer, especially at night or early morning,
and most commonly involve the face. Bite wounds and
swelling are present, and concurrent systemic signs
(especially tachypnoea, respiratory distress and hyper-
thermia) may be seen in alpacas.
Miscellaneous diseases
Noninflammatory alopecia
Shedding in alpacas may be imperceptible or occur as pat-
chy alopecia, especially over the neck.
7
It is occasionally
mistaken for a sign of disease.
Anecdotal reports indicate that large patches of hair
may be lost with no apparent inciting cause.
8
Often the
hairs appear broken off, and analogous to ‘wool break’
(anagen defluxion) in sheep. We diagnosed anagen
defluxion
53
in a cria (case 34) that acutely developed mul-
tiple areas of hair loss over the face, trunk and legs 7 days
after the onset of a diarrhoeal disorder (Salmonella sp.
cultured from faeces) and antibiotic therapy (Figure 9).
The skin appeared normal, and short, broken-off hairs
could be seen and palpated in the hypotrichotic areas.
Trichographic findings were consistent with anagen
defluxion. We also diagnosed telogen defluxion
53
in an
alpaca (case 35) presented for the acute onset of hair loss
over the trunk and face several weeks after a poorly
defined illness (Figure 10). The skin appeared normal, and
hair was totally absent in alopecic areas. Trichographic
findings were consistent with telogen defluxion.
Figure 8. Photomicrograph of hair follicle hamartoma excised
from an alpaca. The middle and deep dermis are filled with hyper-
plastic hair follicles (arrow). Haematoxylin and eosin; scale bar =
1 mm.
Figure 9. Anagen defluxion in an alpaca cria in association with a
diarrhoeal disorder. Note noninflammatory hair loss on the legs.
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
9
Skin diseases in the alpaca
Immunological diseases
Anecdotal reports indicate that pemphigus-like diseases,
adverse cutaneous drug reactions, cutaneous vasculitis,
and food and environmental allergies may occur in alpa-
cas.
2,10
We diagnosed presumed pinnal vasculitis associ-
ated with a tooth root abscess in one alpaca (see
discussion of case 18 with tooth root abscess).
We diagnosed a presumed adverse cutaneous drug
reaction in a 5-year-old male alpaca (case 38) with an
acute history of skin and systemic disease following an
injection of ivermectin. The animal first developed
‘bumps’ on the pinnae, which rapidly progressed to ulcers
and general pinnal swelling (Figure 11). The alpaca
became febrile, depressed and inappetent, and skin
lesions appeared on the perineum and ventral abdomen.
When the animal was examined at the CUHA, additional
findings included numerous pustules on the ventral abdo-
men, prepuce, perineum, and inguinal and axillary areas
(Figure 12). Skin scrapings and trichography were nega-
tive. Cytological examination of pus revealed eosinophils
and nondegenerate neutrophils, and no microorganisms.
A pustule was cultured and was negative for bacteria and
fungi. Results of routine haematology and serum bio-
chemistry panel were unremarkable. Histological findings
of multiple skin biopsies were characterized by eosino-
philic and neutrophilic epidermitis and luminal folliculitis
and furunculosis (Figure 13), and special stains for micro-
organisms were negative. The alpaca was treated with
methylprednisolone (1 mg
⁄ kg given once daily p.o.) and
Figure 11. Presumed adverse cutaneous drug reaction due to iver-
mectin in an alpaca. Note linear ulcer on convex (dorsal) surface of
pinna.
Figure 12. Same alpaca as in Figure 11. Note multiple pustules
(arrow) on ventral abdomen.
Figure 10. Telogen defluxion in an adult alpaca following a poorly
defined illness. Note noninflammatory hair loss on face.
Figure 13. Photomicrograph of skin-biopsy specimen from the
alpaca in Figures 11 and 12. Large pustule (large arrow) is formed by
coalescence of furunculosis of three pilosebaceous units (small
arrows). Haematoxylin and eosin; scale bar = 1 mm.
10
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
Scott et al.
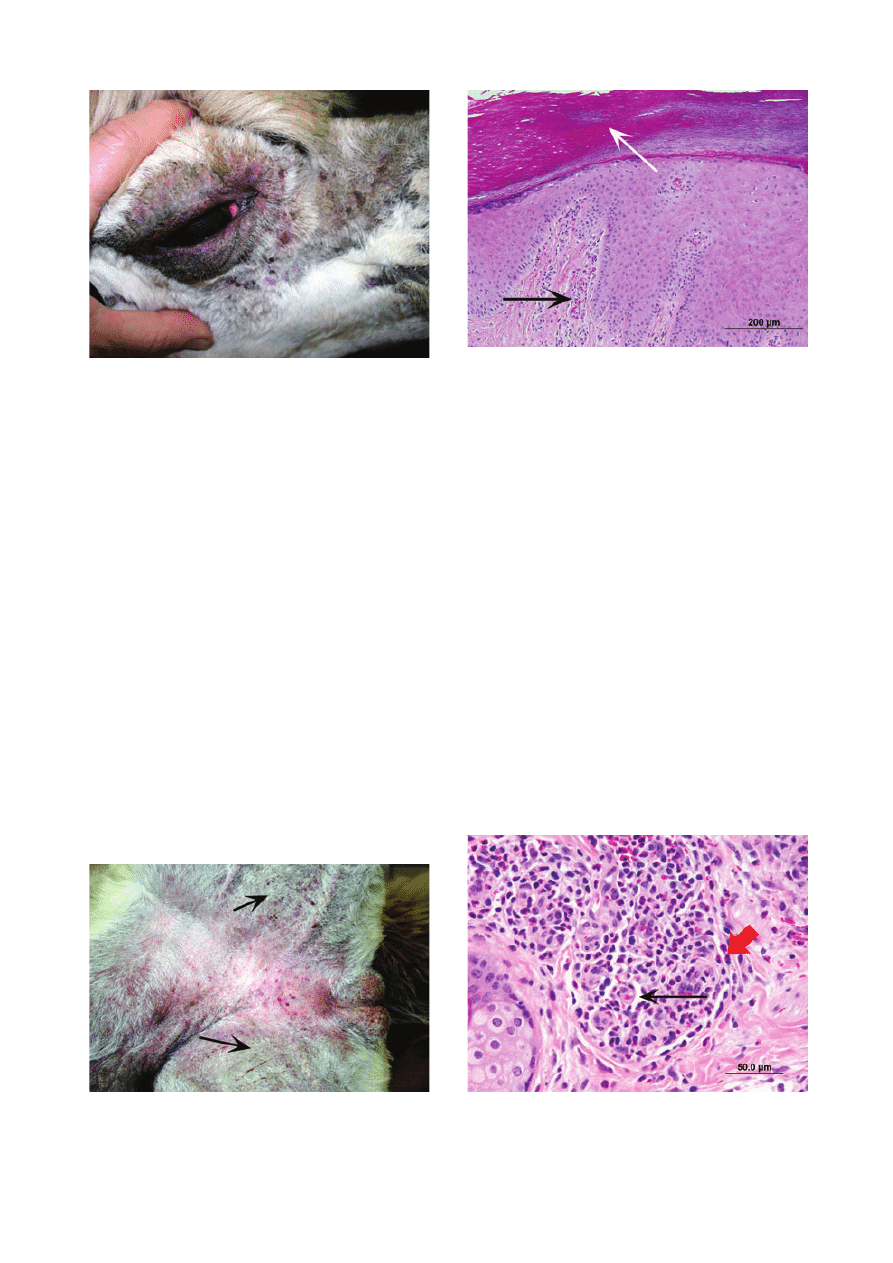
completely recovered. Ivermectin has not been readmin-
istered, and the alpaca remains normal after a 12-month
follow-up period.
Idiopathic urticaria was diagnosed in one alpaca (case
50) in our retrospective study. The animal had recurring
nonpruritic wheals over the neck and trunk. No triggering
agents were identified. Histological findings included
pure superficial perivascular lymphoeosinophilic derma-
titis with moderate superficial dermal oedema. After a
several week course of lesions, the condition disappeared
for a 72-month follow-up period.
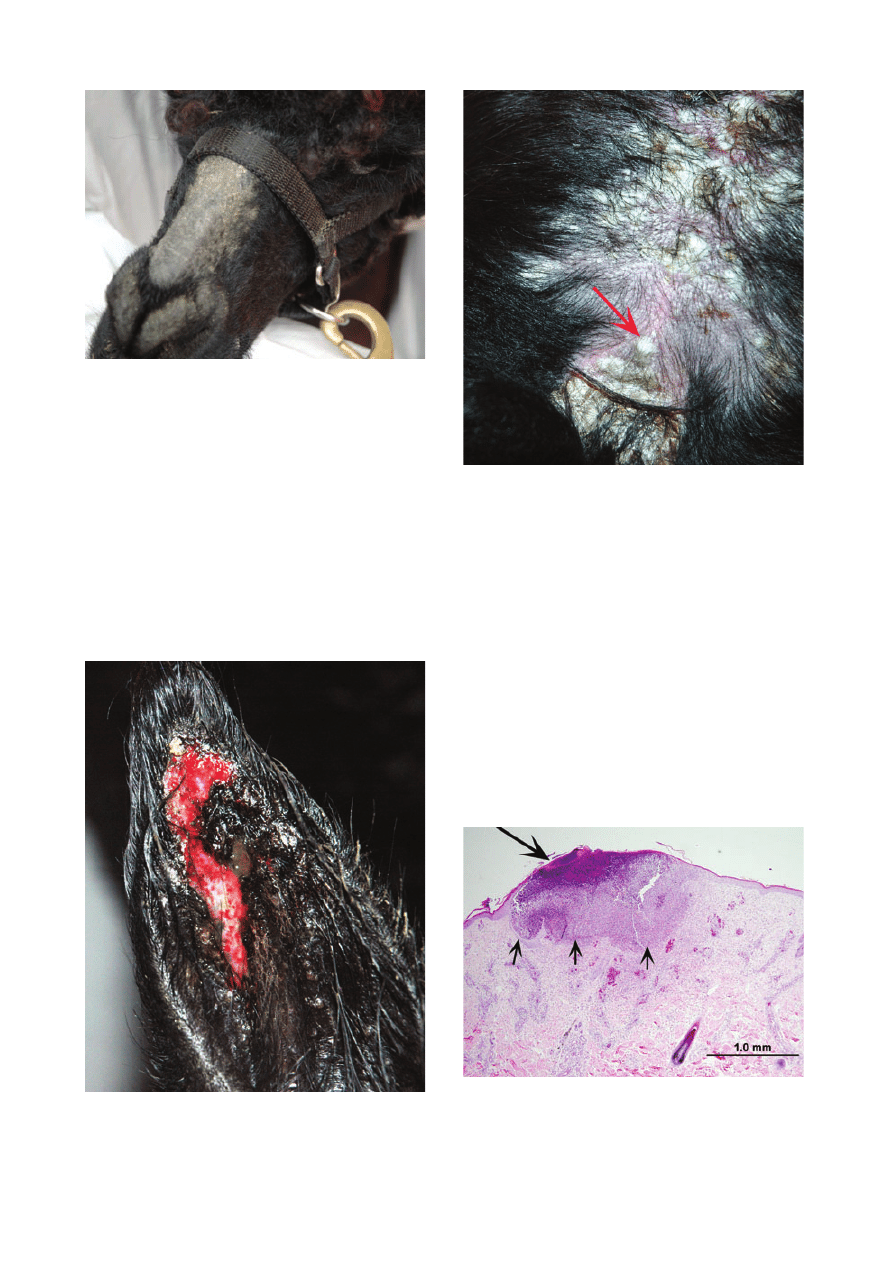
Presumed insect-bite hypersensitivity (probably associ-
ated with Culicoides spp. gnats) was diagnosed in five
alpacas (cases 29–32 and 51) in our retrospective study.
Four animals (cases 29, 31, 32 and 51) had a recurrent or
seasonal (spring to autumn) pruritic dermatitis for ‘several
years’, which spontaneously resolved without treatment
every winter. All five alpacas were the only affected ani-
mals in their respective herds. Treatment with ivermectin
had been ineffective. Lesions had the following character-
istics: (i) they were more-or-less symmetrical; (ii) they
most commonly affected the pinnae, periocular area,
bridge of the nose, axillae, groin, ventral mid-line and dis-
tal legs; and (iii) they consisted of alopecia, crusts, licheni-
fication and occasionally papules (Figures 14 and 15).
Skin scrapings and trichography were negative in all
cases. Cytological examination revealed eosinophilic
inflammation in two animals. Two animals (cases 29 and
31) had concurrent bacterial folliculitis (see earlier discus-
sion). Histological findings in biopsy specimens from two
alpacas (cases 29 and 51) included eosinophilic interstitial
dermatitis, eosinophilic epidermal microabscesses, eosin-
ophilic infiltrative mural and luminal folliculitis, and com-
pact orthokeratotic hyperkeratosis (Figures 16 and 17).
The latter finding is consistent with chronic rubbing,
scratching and chewing in response to pruritus. Three ani-
mals (cases 29, 30 and 32) improved markedly with pro-
tective housing (dusk to dawn) and permethrin-containing
sprays.
Congenital diseases
Ichthyosis was diagnosed in three alpacas (cases 36, 52
and 53). The animals had a generalized keratinization dis-
order since birth or shortly thereafter. Fine, nonadherent
white scales covered nearly the entire body surface and
were scattered throughout the fleece. In one of the ani-
Figure 14. Presumed insect-bite hypersensitivity in an alpaca. Note
alopecia, erythema and multiple crusts on face.
Figure 15. Same alpaca as in Figure 14. Note alopecia, erythema,
papules and crusts on ventral abdomen, medial thighs (arrows) and
perineum.
Figure 16. Photomicrograph of skin-biopsy specimen from an alpaca
with presumed insect-bite hypersensitivity. Note hyperplastic peri-
vascular dermatitis (black arrow) with compact orthokeratotic hyper-
keratosis (white arrow) consistent with chronic rubbing, scratching
and chewing in response to pruritus. Haematoxylin and eosin; scale
bar = 200 lm.
Figure 17. Close-up of Figure 16. Note perivascular (black arrow
indicates segment of blood vessel) accumulation of eosinophils (red
arrow). Haematoxylin and eosin; scale bar = 50 lm.
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
11
Skin diseases in the alpaca

mals, multiple areas of erythema were present on the
abdomen. Pruritus was absent, and the animals appeared
to be otherwise healthy. Parents and relatives of these
alpacas were reported to be unaffected. Histological find-
ings in skin-biopsy specimens revealed diffuse orthokera-
totic hyperkeratosis (epidermis and hair follicles) and
minimal to no inflammation. Eosinophils were not seen.
Treatment was not attempted, and two animals were
euthanized (no necropsy performed).
Bilateral aural haematomas and chondritis occurred in
six crias (cases 62–67) on the same farm in our retrospec-
tive study. All cria were affected at birth. There was no
history of prior trauma to the pinna. The same sire and
three different dams were involved. The sire had been
used on other farms and had reportedly produced other
affected cria. Multiple small, fluid-filled, flat plaques
occurred on the lateral surface of both pinnae. Signs of
infection (heat, discoloration, wounds and draining tracts)
were not present. Pruritus and otitis were not present,
and the crias were otherwise healthy. Skin-biopsy speci-
mens were taken with a 6-mm biopsy punch when the
haematomas were drained. Pinnal lesions healed with
scarring and deformation (Figure 18). Histological findings
included variable combinations of dermal oedema and
haemorrhage, and necrotic cartilage which was dissected
by neutrophils.
Zinc-responsive dermatitis
Zinc-responsive dermatitis accounted for 23% of the der-
matological diagnoses made by attending veterinarians in
the UK postal survey,
10
but only 8% of the cases in our
retrospective study.
There is limited peer-reviewed literature on the subject
of zinc-responsive dermatitis in alpacas.
2–5,8–10
Lesions
consist of scales and papules and plaques with thick,
tightly-adherent crusts, which are most commonly seen
in relatively hairless areas such as the perineum, ventral
abdomen, groin, medial thighs, axillae and medial fore-
legs. The bridge of the nose, muzzle and periocular region
may also be affected. Pruritus is absent or mild. It has
been suggested that young animals (1–2 years old),
males, and animals with dark fleeces may be more fre-
quently affected.
5,8
Typically, only a few individuals in a
herd are affected.
In a study conducted on a German farm,
54
the zinc sta-
tus of alpacas was evaluated. The animals were supple-
mented with a commercial camelid feed, but their hay
had an inadequate mineral content. Twenty-five per cent
of the animals in the herd had lesions on the bridge of the
nose and the pinnae. Only females were affected, and all
had given birth that year or were pregnant. Animals with
nonwhite fleeces were more likely to have skin lesions.
There was no influence of sex, fleece colour or breed (suri
versus huacaya) on serum zinc or copper concentrations.
It was not possible to distinguish affected from nonaffect-
ed alpacas based on serum mineral concentrations. The
authors of this study made the following suggestions: (i)
that skin lesions are most likely to occur first in breeding
females when the mineral content of the diet is marginal;
(ii) that dark fleeces, which contain more zinc and copper
than white fleeces, are likely to exert higher demands on
mineral metabolism; and (iii) that serum zinc concentra-
tions may not reflect total body zinc levels. Histological
findings are reported to include diffuse orthokeratotic
hyperkeratosis (epidermis and hair follicles) and a mild to
moderate perivascular dermatitis containing lympho-
cytes, macrophages, plasma cells and occasional eosin-
ophils.
2–5,8,54
Diagnosis is based on history, physical examination,
ruling out other differential diagnoses, and response to
zinc supplementation. Animals are given 1–2 g ZnSO
4
or
2–4 g zinc methionine, once daily, p.o., with improvement
being seen within 30–90 days.
2,4,8,54
We diagnosed zinc-responsive dermatitis in five alpa-
cas (cases 19–23) presenting with chronic histories of
nonpruritic dermatitis consisting of alopecia, adherent
scales, hair casts, thick crusts and occasional hyperkera-
tosis. Lesions most commonly occurred on the face
(muzzle, periocular region), pinnae, neck, ventral abdo-
men, medial thighs and medial forelegs (Figure 19). The
feet and distal legs were less commonly affected. The
animals were on good diets with no mineral supplements
Figure 18. Bilateral aural haematomas and chondritis in a cria. Note
scarring and disfigurement of both pinnae.
Figure 19. Zinc-responsive dermatitis in an alpaca. Thick scale and
crusts on top of head and dorsal neck.
12
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
Scott et al.

and were generally the only individual in the herd
affected. Case 19 was one of a herd of 52 alpacas, two
others of which had ‘a similar skin problem’. Skin biopsies
were performed in three animals (cases 19, 20 and 22)
and revealed lymphoeosinophilic superficial perivascular
dermatitis with papillated epidermal hyperplasia, papillo-
matosis, and predominantly lamellar orthokeratotic hyper-
keratosis of the epidermis and hair follicle infundibula
(Figure 20). Occasional papillae were ‘capped’ with
parakeratotic hyperkeratosis. The skin lesions in all five
alpacas were reported to have resolved with zinc methio-
nine (2 g
⁄ day p.o.). Zinc supplementation was continued,
and all five alpacas were in remission following periods of
6–12 months.
Focal sterile eosinophilic and neutrophilic folliculitis and
furunculosis
A 1-year-old male alpaca (case 37) presented to the CUHA
with a 1-month history of dermatitis and pruritus around
the right eye. The other animals in the herd were unaf-
fected. Previous treatment with topical miconazole and
oral enrofloxacin was unsuccessful. The right periocular
area was alopecic, erythematous and oedematous with a
few pustules and crusts (Figure 21). Skin scrapings and
trichography were negative. Cytological examination
revealed eosinophilic inflammation. Histological findings
included eosinophilic folliculitis and furunculosis, and spe-
cial stains were negative for microorganisms. Prednisone
administered p.o. (1 mg
⁄ kg once daily for 2 weeks) was
curative. This condition is similar to the sterile eosinophilic
folliculitis and furunculosis that occurs in dogs
55
and
horses,
56
presumably associated with insect envenoma-
tion.
Idiopathic nasal
⁄ perioral hyperkeratotic dermatosis
Idiopathic nasal
⁄ perioral hyperkeratotic dermatosis (‘mun-
ge’) is anecdotally reported to occur in alpacas.
2–5,7,8
This
condition is very poorly understood and may, in fact, be
nothing more than a reaction pattern in the skin caused
by many different factors.
The dermatosis usually begins at 6–24 months of age.
The nasal and periocular areas become covered with thick
crusts that occasionally obstruct the nostrils. Lesions
may occasionally occur on the bridge of the nose and peri-
ocular region. Pruritus is usually absent or mild.
Histological findings are reported to include various
combinations of epidermal oedema, hyperplasia and
necrosis; palisaded crusts (orthokeratotic
⁄ parakeratotic
hyperkeratosis and pus); and a mixed perivascular derma-
titis.
2–5,8
Treatment
observations
are
consistent
with
the
aforementioned idea that many different conditions are
being called ‘munge’. Cases are reported to respond to
topical and
⁄ or systemic antibiotics, topical and ⁄ or sys-
temic glucocorticoids, oral zinc, or to regress sponta-
neously.
2–5,8
We
presently
believe
that
idiopathic
nasal
⁄ perioral hyperkeratotic dermatosis is a cutaneous
reaction pattern of alpacas possibly provoked by disor-
ders such as bacterial folliculitis, dermatophilosis, dermat-
ophytosis, contagious viral pustular dermatitis, chorioptic
mange, fly bites, viral papillomas
⁄ fibropapillomas, contact
dermatitis and zinc-responsive dermatitis. The authors
are not aware of any laboratory tests or therapeutic
interventions that allow one to diagnose ‘munge’. Such a
multifactorial aetiology could explain the anecdotal suc-
cesses of popular topical concoctions such as ‘witches
brew’, which contains gentamicin, ivermectin, dimethyl
sulfoxide and mineral oil.
57
Discussion
The popularity and numbers of alpacas have increased in
many parts of the world.
1–4,10,40
This naturally has led to
increasing demands for veterinary care. The number of
alpaca patients at our university practice increased
approximately 13-fold over a 10-year period. During this
period of time, dermatological diseases were docu-
mented in 5.6% of the alpacas, which is similar to previ-
ously reported data on our equine patients (4.1% of all
horses over a 21 year period).
58
Skin-biopsy specimens
accounted for 24.4% of all alpaca biopsy specimens
received over the 10 year period, which is again similar to
previously reported data on our equine population (23.4%
of all biopsy specimens).
58
Figure 20. Photomicrograph of skin-biopsy specimen from an alpaca
with zinc-responsive dermatitis. Note papillated epidermal hyperpla-
sia, papillomatosis (arrows) and orthokeratotic hyperkeratosis. Hae-
matoxylin and eosin; scale bar = 1 mm.
Figure 21. Presumed insect-bite reaction in an alpaca. Periocular
alopecia, erythema, oedema, pustules and crusts.
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
13
Skin diseases in the alpaca

To our knowledge, this literature review and retrospec-
tive case study is the first one entirely devoted to alpacas.
While some of our experiences are similar to those
reported in peer-reviewed and anecdotal literature, there
were some notable differences. For instance, while bac-
terial and neoplastic dermatoses accounted for only 5%
and 0%, respectively, of the conditions diagnosed in a UK
postal survey,
10
these two categories accounted for 22%
and 19%, respectively, of the dermatological diagnoses
in our retrospective study conducted in the northeastern
USA. Anecdotal literature (veterinary and lay) suggests
that dermatophilosis and dermatophytosis are routinely
diagnosed in alpacas. However, in our study, we con-
firmed no cases of the former and only one case of the
latter. Our case of dermatophytosis had no histological
evidence of hair shaft invasion, similar to what is seen
with Microsporum persicolor and Trichophyton mentagro-
phytes var. erinacei infections in dogs.
59,60
In such cases,
plucking hairs for fungal culture usually produces negative
results. We documented no cases of sarcoptic mange or
pediculosis.
In our study, we have also documented the clinicopath-
ological details of several cases of bacterial folliculitis, as
well as several previously unreported conditions, to
include intertrigo, collagenous and hair follicle hamarto-
mas, melanocytoma, lymphoma, hybrid follicular cysts,
anagen defluxion, telogen defluxion, a presumed adverse
cutaneous drug reaction to ivermectin, a presumed focal
insect-bite reaction and a unique, possibly hereditary
syndrome of bilateral aural haematomas and chondritis.
Ichthyosis had previously been reported in a llama,
61
and
we report three cases in the alpaca. The three alpacas
with multiple hamartomas precipitated a discussion of
the syndrome of multiple collagenous hamartomas (‘nod-
ular dermatofibrosis’) in association with renal disease as
recognized in dogs.
62
However, the owners declined
ultrasound examinations for their animals.
To our knowledge, we provide the original description of
alpacas with presumed insect-bite hypersensitivity. In our
practice area, the seasonality, environmental exposure
and clinicopathological features of the condition are con-
sistent with documented or presumed Culicoides hyper-
sensitivity in horses,
63
sheep,
64–66
goats
64
and cattle.
64,65
Skin-biopsy specimens were procured from three of
five alpacas with zinc-responsive dermatitis. The keratini-
zation abnormality in these three specimens was diffuse
and predominantly orthokeratotic. This is unlike the dif-
fuse parakeratotic hyperkeratosis classically reported in
swine,
67
cattle
68
and dogs.
69
In sheep
70–72
and goats,
73,74
however, the keratinization abnormality in zinc-responsive
dermatoses has been reported to be parakeratotic, ortho-
keratotic or a combination of both of these.
It has been suggested that eosinophils are commonly
present in chronic inflammatory dermatoses of alpacas
and are of little diagnostic significance.
2
In our study,
eosinophils were consistently numerous in presumed
insect-bite hypersensitivity, chorioptic mange, psoroptic
mange, zinc-responsive dermatitis, and in single cases
of urticaria, presumed focal insect-bite reaction and
presumed adverse cutaneous drug reaction. However,
eosinophils were rarely seen in cases of bacterial folliculi-
tis, dermatophytosis, contagious viral pustular dermatitis,
presumed
contact
dermatitis,
ichthyosis
and
aural
haematomas with chondritis.
In conclusion, skin disease is commonly seen in alpa-
cas, and the possible causes are many. We hope this
article will encourage practitioners and specialists to
investigate alpaca dermatoses in greater depth and with
enthusiasm and to report their findings. There is much to
be learned.
References
1. D’Alterio GL. Introduction to the alpaca and its veterinary care in
the UK. In Practice 2006; 28: 404–11.
2. Foster A, Jackson A, D’Alterio GL. Skin diseases of South Ameri-
can camelids. In Practice 2007; 29: 216–23.
3. Zanolari P, Meylan M, Roosje P. Dermatologie bei Neuweltka-
melidin. Teil 1: Propa¨deutik und dermatologische Diagnostik.
Tiera¨rztliche Praxis 2008; 36: 304–8.
4. Plant JD. Update on camelid dermatology. Proceedings of the
International Camelid Health Conference. Oregon State Univer-
sity, Corvallis, OR, 2007. pp. 127–30.
5. Rosychuk RAW. Llama dermatology. Veterinary Clinics of North
America – Food Animal Practice 1989; 5: 203–14.
6. Rosychuk RAW. Llama dermatology. Veterinary Clinics of North
America – Food Animal Practice 1994; 10: 228–39.
7. Fowler ME. Skin problems in llamas and alpacas. Llama 1989; 3:
45–8.
8. Fowler ME. Medicine and Surgery of South American Camelids.
Llama, Alpaca, Vicun˜a, Guanaco II. Ames, IA: Iowa State Univer-
sity Press, 1998. pp. 148–209.
9. Fowler ME, Cubas ZS. Biology, Medicine, and Surgery of South
American Wild Animals. Ames, IA: Iowa State University Press,
2001. pp. 392–401.
10. D’Alterio GL, Knowles TG, Eknaes EI et al. Postal survey of the
population of South American camelids in the United Kingdom in
2000
⁄ 01. Veterinary Record 2006; 158: 86–90.
11. Anderson DE, Ring DM, Kowalski J. Infection with Corynebacte-
rium pseudotuberculosis in 5 alpacas. Journal of the American
Veterinary Medical Association 2004; 225: 1743–7.
12. Braga WU, Chavera A, Gonzalez A. Corynebacterium pseudotu-
berculosis infection in highland alpacas (Lama pacos) in Peru.
Veterinary Record 2006; 159: 23–4.
13. Braga WU, Chavera AE, Gonza´lez AE. Clinical, humoral, and
pathologic findings in adult alpacas with experimentally induced
Corynebacterium
pseudotuberculosis
infection.
American
Journal of Veterinary Research 2006; 67: 1570–4.
14. Cebra ML, Cebra CK, Garry FB. Tooth root abscess in New
World camelids: 23 cases (1972–1994). Journal of the American
Veterinary Medical Association 1996; 209: 819–22.
15. Niehaus AJ, Anderson DE. Tooth root abscesses in llamas and
alpacas: 123 cases (1994–2005). Journal of the American Veteri-
nary Medical Association 2007; 231: 284–9.
16. Portaels F, Chemlal K, Elsen P et al. Mycobacterium ulcerans
in wild animals. Revue Scientifique et Technique 2001; 20:
252–64.
17. Preston Smith H. Ectima de los animales del Peru, dermatitis
pustular contagiosa. Ganaderia (Peru) 1947; 1: 27–32.
18. Geurden T, Deprez P, Vecruysse J. Treatment of sarcoptic, psor-
optic, and chorioptic mange in a Belgian alpaca herd. Veterinary
Record 2003; 153: 331–2.
19. Jarvinen JA, Miller JA, Oehler DD. Pharmacokinetics of ivermec-
tin in llamas (Lama glama). Veterinary Record 2002; 150: 344–6.
20. Hunter RP, Isaza R, Koch DE et al. The pharmacokinetics of topi-
cal doramectin in llamas (Lama glama) and alpacas (Lama pacos).
Journal of Veterinary Pharmacology and Therapeutics 2004; 27:
187–9.
21. Hunter RP, Isaza R, Koch DE et al. Moxidectin plasma concen-
trations following topical administration to llamas (Lama glama).
Small Ruminant Research 2004; 52: 275–9.
14
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
Scott et al.

22. Burkholder TH, Jensen J, Chen H et al. Plasma evaluation for
ivermectin in llamas (Lama glama) after standard subcutaneous
dosing. Journal of Zoo and Wildlife Medicine 2004; 35: 395–6.
23. Mellanby K. Sarcoptic mange in the alpaca. Transactions of the
Royal Society of Tropical Medicine and Hygiene 1946; 40: 359.
24. Alverado J, Astrom RG, Heath GBS. An investigation into reme-
dies of sarna (sarcoptic mange) of alpacas in Peru. Experimental
Agriculture 1966; 2: 245–54.
25. Chuvela J, Guerrero Diaz CA. Evaluacion de metodos en el
tratamiento de la sarna de alpacas. Revista de Investigaciones
Pecuarias 1973; 2: 23–8.
26. Leguia G. The epidemiology and economic impact of llama para-
sites. Parasitology Today 1991; 7: 54–5.
27. Ramos-Acunatt H, Catrejon-Valdez M, Valencia-Mamani N et
al. Control of sarcoptic mange (Sarcoptes scabiei var auche-
niae) in alpacas (Lama pacos) in Peru, with injectable long-
acting 1% W
⁄ W ivermectin. Veterinaria Argentina 2001; 17:
570–7.
28. McKenna PB, Hill FI, Gillett R. Sarcoptes scabiei infection on an
alpaca (Lama pacos). New Zealand Veterinary Journal 2005; 53:
213.
29. Borgsteede FHM, Timmerman A, Harmsen MM. Een geval van
emstige Sarcoptes – schurft bij alpacas (Lama pacos). Tijdschrift
voor Diergeneeskunde 2006; 131: 282–3.
30. Lau P, Hill PB, Rybnı´cˇek J et al. Sarcoptic mange in three alpacas
treated successfully with amitraz. Veterinary Dermatology 2007;
18: 272–7.
31. D’Alterio GL, Batty A, Laxon K et al. Psoroptes species in alpa-
cas. Veterinary Record 2001; 149: 96.
32. Bates P, Duff P, Windsor R et al. Mange mite species affecting
camelids in the UK. Veterinary Record 2001; 149: 463–4.
33. Frame NW, Frame RKA. Psoroptes species in alpacas. Veteri-
nary Record 2001; 149: 128.
34. Cremers HJ. The incidence of Chorioptes bovis (Acarina: Psorop-
tidae) in domesticated ungulates. Tropical and Geographical
Medicine 1983; 36: 105.
35. Cremers HJWM. Chorioptes bovis (Acarina: Psoroptidae) in
some camelids from Dutch Zoo. Veterinary Quarterly 1985; 7:
198–9.
36. Young E. Chorioptic mange in the alpaca, Lama pacos. Journal of
the South African Veterinary Medical Association 1996; 47: 474–
5.
37. Petrikowski M. Chorioptic mange in an alpaca herd. In: Kwochka
KW, Willemse T, von Tscharner C, eds. Advances in Veterinary
Dermatology, Vol. 3. Boston, MA: Butterworth-Heinemann,
1998:450–1.
38. D’Alterio GL, Jackson AP, Knowles TG et al. Comparative
study of the efficacy of eprinomectin versus ivermectin, and
field efficacy of eprinomectin, for the treatment of chorioptic
mange in alpacas. Veterinary Parasitology 2005; 130: 267–
75.
39. D’Alterio GL, Callaghan C, Just C et al. Prevalence of Chorioptes
sp. mite infestation in alpaca (Lama pacos) in the Southwest of
England: implications for skin health. Small Ruminant Research
2005; 57: 221–8.
40. Plant JD, Kutzler MA, Cebra CK. Efficacy of topical eprinomectin
in the treatment of Chorioptes sp. infestation in alpacas and lla-
mas. Veterinary Dermatology 2007; 18: 59–62.
41. Windsor RHS, Teran M, Windsor RS. Effects of parasitic infesta-
tion on the productivity of alpacas (Lama pacos). Tropical Animal
Health Production 1992; 24: 57–62.
42. Windsor RS, Windsor RHS, Teran M. Economic benefits of con-
trolling internal and external parasites in South American came-
lids. Annals of the New York Academy of Sciences 1992; 653:
398–405.
43. Rojas C, Lobato MY, Montalvo VM. Fauna parasitaria de cami-
lides sudamericanos y ovinos en pequen˜os reban˜os mixtos
familiares. Revista de Pecuaires Investigaciones Peru 1993; 6:
22–7.
44. Cicchino AC, Mun˜oz Coben˜as ME, Bulman GM et al. Identifica-
tion of Microthoracius mazzai (Phthiraptera: Anoplura) as an eco-
nomically important parasite of alpacas. Journal of Medical
Entomology 1998; 35: 922–30.
45. Vaughan JL, Carmichael I. Control of the camelid biting
louse, Bovicola breviceps in Australia. Alpacas Australia 1999;
27: 24–8.
46. Levot G. Resistance and the control of lice in humans and pro-
duction animals. International Journal for Parasitology 2000; 30:
291–7.
47. Vaughan JL. Eradication of the camelid biting louse, Bovicola
breviceps. Australian Veterinary Journal 2004; 82: 216–7.
48. Palma RL, McKenna PB. Confirmation of the occurrence of the
chewing louse Bovicola (Lepikentron) breviceps (Insecta: Phthi-
raptera: Trichodectidae) on alpacas (Lama pacos) in New
Zealand. New Zealand Veterinary Journal 2006; 54: 253–4.
49. Valentine BA, Martin JM. Prevalence of neoplasia in llamas and
alpacas (Oregon State University, 2001–2006). Journal of Veteri-
nary Diagnostic Investigation 2007; 19: 202–4.
50. Schulman FY, Krafft AE, Janczewski T et al. Camelid mucocuta-
neous fibropapillomas: clinicopathologic findings and association
with papillomavirus. Veterinary Pathology 2003; 40: 103–7.
51. Suedmeyer WK, Williams F. Multiple trichoepitheliomas in an
alpaca (Lama pacos). Journal of Zoo and Wildlife Medicine 2005;
36: 706–8.
52. Dukgraaf S, Pusterla N, Van Hoogmoed LM. Rattlesnake enven-
omation in 12 New World camelids. Journal of Veterinary Inter-
nal Medicine 2006; 20: 998–1002.
53. Scott DW, Miller WH Jr, Griffin CE. Muller & Kirk’s Small Animal
Dermatology, 6th edn. Philadelphia, PA: Saunders, 2001. pp.
893–4.
54. Clauss M, Lendl C, Schramel P et al. Skin lesions in alpacas and
llamas with low zinc and copper status – a preliminary report.
Veterinary Journal 2004; 67: 302–5.
55. Scott DW, Miller WH Jr, Griffin CE. Muller & Kirk’s Small Animal
Dermatology, 6th edn. Philadelphia, PA: W.B. Saunders, 2001.
pp. 641–2.
56. Scott DW, Miller WH Jr. Equine Dermatology. Philadelphia, PA:
W.B. Saunders, 2003. pp. 658–9.
57. http://www.pacapooalpacas.com/NewSite/Articles/animal-hus-
bandry-safley.htm. Accessed July 29, 2010.
58. Scott DW, Miller WH Jr. Equine Dermatology. Philadelphia, PA:
W.B. Saunders, 2003. pp. v–ix, Preface and Acknowledge-
ments.
59. Bond R, Middleton DJ, Scarff DH et al. Chronic dermatophytosis
due to Microsporum persicolor infection in three dogs. Journal
of Small Animal Practice 1992; 33: 571–6.
60. Fairley RA. The histological lesions of Trichophyton mentagro-
phytes var erinacei infection in dogs. Veterinary Dermatology
2001; 12: 119–22.
61. Belknap EB, Dunstan RW. Congenital ichthyosis in a llama. Jour-
nal of the American Veterinary Medical Association 1990; 197:
764–7.
62. Castellana MC, Idiart JR. Multifocal renal cystadenocarcinoma
and nodular dermatofibrosis in dogs. Compendium of Conti-
nuing Education for the Practicing Veterinarian 2005; 27: 846–
53.
63. Scott DW, Miller WH Jr. Equine Dermatology. Philadelphia, PA:
W.B. Saunders, 2003. pp. 458–67.
64. Scott DW. Color Atlas of Farm Animal Dermatology. Ames, IA:
Blackwell Publishing, 2007. pp. 56, 133, 175.
65. Yeruham I, Braverman Y, Orgad U. Field observations in
Israel on hypersensitivity in cattle, sheep, and donkeys
caused by Culicoides. Australian Veterinary Journal 1993; 70:
348–52.
66. Yeruham I, Perl S, Branerman Y. Seasonal allergic dermatitis in
sheep associated with Ctenocephalides and Culicoides bites.
Veterinary Dermatology 2004; 15: 377–80.
67. Norrdin RW, Krook L, Pond WG et al. Experimental zinc defi-
ciency in weanling pigs on high and low calcium diets. Cornell
Veterinarian 1973; 63: 264–90.
68. Singh AP, Netra PR, Vashistha MS et al. Zinc deficiency in cattle.
Indian Journal of Animal Science 1994; 64: 35–40.
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
15
Skin diseases in the alpaca

69. White SD, Bourdeau P, Rosychuk RAW et al. Zinc-responsive
dermatoses in dogs: 41 cases and literature review. Veterinary
Dermatology 2001; 12: 101–9.
70. Ott EA, Smith WH, Stob M et al. Zinc deficiency in the young
lamb. Journal of Nutrition 1964; 82: 41–50.
71. Masters DG, Chapman RE, Vaughan JD. Effects of zinc defi-
ciency on the wool growth, skin, and wool follicles of pre-rumi-
nant lambs. Australian Journal of Biological Science 1985; 38:
355–64.
72. Suliman HB, Abdelrahim AI, Zakia AM et al. Zinc deficiency in
sheep: field cases. Tropical Animal Health and Production 1988;
20: 47–51.
73. Miller WJ, Pitts WJ, Clifton CM et al. Experimentally produced
zinc deficiency in the goat. Journal of Dairy Science 1964; 47:
556–9.
74. Krumetter-Froetscher R, Hauser S, Baumgartner W. Zinc-respon-
sive dermatosis in goats suggestive of hereditary malabsorption:
two field cases. Veterinary Dermatology 2005; 16: 269–75.
Re´sume´ Cette e´tude re´trospective de´crit 68 cas de dermatose d’alpagas diagnostique´s entre 1997 et
2006 a` l’Universite´ de Cornell. Au cours de cette pe´riode, 40 des 715 (5.6%) alpagas vus en consultation a`
l’hoˆpital universitaire pre´sentaient des le´sions dermatologiques. De plus, les biopsies cutane´es repre´senta-
ient 86 des 353 (24.4%) biopsies d’alpaga soumises au laboratoire de diagnostic. Sur ces 86 e´chantillons,
28 ont pu eˆtre suivis. Les maladies les plus fre´quentes regroupaient : infections bacte´riennes (22%); ne´o-
plasmes, kystes et hamartomes (19%); de´sordres immunologiques pre´sume´s (12%); et ectoparasitisme
(10%). Les atteintes de´crites pour la premie`re fois comprenaient intertrigo, hamartomes collage´nique et
folliculaire, lymphome, kystes folliculaires hybrides, me´lanocytomes, effluvium anage`ne, effluvium te´lo-
ge`ne, hypersensibilite´ aux piquˆres d’insectes suppose´e, ichtyose et probablement chondrite et he´matome
auriculaire bilate´raux. Les re´sultats de cette e´tude re´trospective sont compare´s et mis en relief avec les
re´sultats d’une revue de la litte´rature.
Resumen Este estudio retrospectivo describe 68 alpacas con enfermedades de la piel investigadas des-
de 1997 hasta el an˜o 2006 en la Universidad de Cornell. Durante este periodo 40 de 715 alpacas (5,6%)
que llegaron al hospital universitario tenı´an enfermedades de la piel. Adema´s biopsias de piel representaron
86 de 353 (24,4%) de las biopsias de alpacas remitidas al laboratorio de diagnostico y de esos 86 especim-
enes seguimiento solo fue obtenido para 28 casos. Las enfermedades mas comunes fueron: infecciones
bacterianas (22%); neoplasias, quistes y hamartomas (19%); presuntos desordenes inmunolo´gicos (12%);
y ectoparasitismo (10%). Las condiciones descritas por primera vez incluyeron intertrigo, hamartomas col-
a´genos y de folı´culos pilosos, linfoma, quistes foliculares hı´bridos, melanocitoma, defluxio´n anage´nica, def-
luxio´n teloge´nica, presunta hipersensibilidad a picadura de insecto, ictiosis, y posibles hematomas aurales
bilaterales y condritis. Los resultados de este estudio retrospectivo se comparan y contrastan con los re-
sultados de una revisio´n bibliogra´fica.
Zusammenfassung Diese retrospektive Studie beschreibt 68 Alpakas mit einer Hauterkrankung, die
zwischen 1997 und 2006 an der Cornell Universita¨t untersucht wurden. Wa¨hrend dieser Zeit hatten 40 von
715 (5,6%) Alpakas, die an der Universita¨tsklinik vorgestellt wurden, dermatologische Erkrankungen.
Zusa¨tzlich standen 86 von 353 (24,4%) der Alpaka Biopsien, die an das diagnostische Labor gesendet
wurden, zur Verfu¨gung und von diesen 86 Proben gab es in 28 Fa¨llen einen Follow-Up. Die ha¨ufigsten Kran-
kheiten waren: bakterielle Infektionen (22%); Neoplasmen, Zysten und Hamartome (19%); vermeintliche
immunologische Erkrankungen (12%); und Ektoparasiten (10%). Vera¨nderungen, die zum ersten Mal besch-
rieben wurden, waren: Intertrigo, Kollagen- und Haarfollikelhamartome, Lymphome, Hybrid-Follikelzysten,
Melanozytome, anagene Defluxion, telogene Defluxion, vermeintliche Insektenstich-Hypersensibilita¨t,
Ichthyose und mo¨gliche heredita¨re bilaterale Ohr-Ha¨matome und Chondritis. Die Ergebnisse dieser retro-
spektiven Studie werden verglichen und den Ergebnissen der Literatur-Review gegenu¨bergestellt.
16
ª 2010 The Authors. Journal compilation ª 2010 ESVD and ACVD, Veterinary Dermatology, 22, 2–16.
Scott et al.
Wyszukiwarka
Podobne podstrony:
DERMA zebrane B.G. (2), Choroby skórne i weneryczne, Dermatologia, giełdy
program nauczania choroby skorne i weneryczne, IV rok Lekarski CM UMK, Dermatologia, Gablota, Sekret
upgrade, Choroby skórne i weneryczne, Dermatologia, giełdy
Pytania egzaminacyjne - Uzupelnione v1, Choroby skórne i weneryczne, Dermatologia, giełdy
moje (2), Choroby skórne i weneryczne, Dermatologia, giełdy
CHOROBY SKÓRNE
opr pyt egz, Choroby skórne i weneryczne, Dermatologia, giełdy
DERMA zebrane B.G, Choroby skórne i weneryczne, Dermatologia, giełdy
pyytania derma, Choroby skórne i weneryczne, Dermatologia, giełdy
Zespol Sweeta, Choroby skórne i weneryczne, Dermatologia, giełdy
Egzamin 2007, Choroby skórne i weneryczne, Dermatologia, giełdy
gielda 2010, Choroby skórne i weneryczne, Dermatologia, giełdy
pyt nasza grupa, Choroby skórne i weneryczne, Dermatologia, giełdy
więcej podobnych podstron