
Hormesis and synergy: pathways and mechanisms of quercetin
in cancer prevention and management
Ashley J Vargas and Randy Burd
nure_301
418..428
Quercetin is a unique dietary polyphenol because it can exert biphasic
dose-responses on cells depending on its concentration. Cancer preventative effects
of quercetin are observed at concentrations of approximately 1–40
mM and are likely
mediated by quercetin's antioxidant properties. Pro-oxidant effects are present at
cellular concentrations of 40–100
mM. However, at higher concentrations, many
novel pathways in addition to ROS contribute to its effects. The potent bioactivity of
quercetin has led to vigorous study of this compound and revealed numerous
pathways that could interact synergistically to prevent or treat cancer. The effect of
intake and concentration on emerging pathways and how they may interact are
discussed in this review.
© 2010 International Life Sciences Institute
INTRODUCTION
Quercetin is a dietary polyphenol that is readily found in
a variety of foods and is consumed daily. The tremendous
growth in the study of this bioactive compound has
revealed numerous pathways that could possibly interact
to prevent or treat diseases such as cancer, so a review of
the recent literature utilizing this compound is pertinent.
Quercetin also has a unique ability to act as an antioxi-
dant or a pro-oxidant depending on its concentration,
which is indicative of its hormetic properties.
1
For the
purpose of this review, hormesis is defined as a biphasic
dose-response whereby low doses of quercetin result in a
given effect (antioxidant properties) and higher doses
result in another effect (pro-oxidant properties). Given
this assumption, there are two fundamental factors that
impact quercetin’s bioactivity as either an oxidant or an
antioxidant. First, and arguably most important, is quer-
cetin’s tissue bioavailability and digestion process. Second
is the concentration and isoform or conjugate form of
quercetin in the target tissue. In this review, multiple
effects of quercetin are proposed that are concentration
dependent; this implies a dependence on the entire meta-
bolic process of this flavonoid. It is also proposed that
multiple pathways could interact to produce synergistic
effects, which again rely on the concentration of quercetin
at the tissue site. Quercetin’s hormetic nature makes it
well-suited to use in cancer prevention efforts; these
efforts would likely involve lower and long-term con-
sumption of quercertin-containing foods, and/or supple-
mentation or therapeutic administration in combination
with conventional therapies. Although this review focuses
on quercetin in relation to cancer, it is generally under-
stood that the same pathways could be applied to other
disease states as well.
CENTRAL ROLE OF REACTIVE OXYGEN SPECIES IN
QUERCETIN ACTIVITY IN CANCER
The biphasic oxidation properties of quercetin are likely
beneficial in cancer prevention and therapy because dif-
ferent concentrations of quercetin counter the transfor-
mation and growth processes of cancer.
1
Malignant
tumors result from uncontrolled cell growth due to muta-
tions. Mutations are a result of DNA damage, which is
commonly incurred through exposure to reactive oxygen
species (ROS). Quercetin is able to donate electrons
to ROS
2
and thereby reduce their ability to damage cellu-
lar DNA.
3
This is the primary mechanism by which
Affiliation: AJ Vargas and R Burd are with the Department of Nutritional Sciences at the University of Arizona, Tucson, Arizona, USA.
Correspondence: R Burd, Department of Nutritional Science, University of Arizona, Shantz Building, Room 301, 117 E. 4th Street, Tucson,
AZ 85721, USA. E-mail: rburd@u.arizona.edu, Phone:
+1-520-626-1863; Fax: +1-520-621-9446.
Key words: estrogen receptor, HSPs, P53, quercetin, ROS
Emerging Science
doi:10.1111/j.1753-4887.2010.00301.x
Nutrition Reviews® Vol. 68(7):418–428
418

quercetin exerts antioxidant and chemopreventive effects
on the cell.
3
Typically, this effect is seen at cellular quer-
cetin concentrations in the range of 1–40 mM, which
could likely be achieved by diet.
4
However, after a tumor
has formed, quercetin could continue to have beneficial
anti-tumor effects at higher doses by exerting cytotoxic
effects. Quercetin is able to increase oxidative stress and
cytotoxicity in tumor cells, usually at concentrations
greater than 40 mM; it is able to do this by becoming an
ROS itself and by increasing damage or apoptotic path-
ways in the transformed cell.
2,3
These benefits rely on ROS
and the quercetin concentration to produce either anti-
or pro-oxidant effects. There are various pathways and
mechanisms that can interact and these are described in
more detail below. In addition, because biomedical
research must be translatable to real-life situations, this
review begins with an assessment of the bioavailability
and metabolism of quercetin. Since much of the research
discussed in this review has only been conducted in vitro
or in cell culture, an attempt is made here to link these
studies with biologically relevant concentrations in
humans.
ABSORPTION AND METABOLISM OF QUERCETIN
Quercetin is consumed daily by millions of people
through nuts, teas, vegetables, and herbs in the diet.
3
It is
also available as a commercial dietary supplement, and it
is now being included in functional foods. Quercetin is
generally recognized as safe in oral dosages of 1,000 mg/
day or in intravenously administered dosages of 756 mg/
day.
5
Up to 60% of orally ingested quercetin is absorbed,
5
and the average dietary intake of quercetin is somewhere
between 6 and 31 mg daily (not including supplement/
intravenous use).
6
Quercetin is part of the flavanol family
and it is normally found in the glycosylated form.
7
Diges-
tion of most dietary quercetin, in the form of quercetin
glycosides, begins in the oral cavity with some cleavage of
the glycosides catalyzed by b-glycosidases (Figure 1).
7
Some of quercetin’s aglycoside form is absorbed in the
mouth as well.
7
There is some disagreement as to the exact post-oral
cavity metabolism of this substrate; however, a few pos-
sibilities exist (Figure 1). It is likely that the colonic micro-
flora hydrolyze the glycoside-form of quercetin to the
more active aglycone quercetin. Once aglycosylated, the
molecule becomes more lipophillic and can then be
absorbed into the epithelial cells of the colon.
8
Another
possibility is that some of the glycosidic quercetins are
absorbed directly, particularly those that are bound with
glucose.
8
It is also probable that colonic microflora
ferment quercetin into phenolic compounds and carbon
dioxide.
5
Both the carbon dioxide and the phenolic com-
pounds are then expelled from the body.
5
There are yet
other hypotheses, including the idea that some hydrolysis
occurs in the small intestines, via both b-glycosidase and
lactase phlorizen hydrolase (LPH).
9
Despite differences of
opinion, it is generally accepted that bioavailability
depends on the location and type of sugar group attached
to quercetin.
8
Most likely, the digestion and absorption
of quercetin occurs through a combination of the pro-
posed pathways, depending on which form the quercetin
is in at a given point in time.
Hydrolysis of quercetin by b-glycosidase results in
different metabolites of quercetin depending on what
the original glycoside was (i.e., where the glycosidic bond
was located and what type of sugar was attached). These
metabolites include not only free quercetin, but also con-
jugates such as glucuronides, O-methylated products, and
sulfate forms.
8
This conjugation of quercetin is reported
to occur throughout the processes of digestion and
absorption.
8
In animals, it appears that quercetin and its
metabolites are transported unevenly throughout the
body.
10
Animal studies have also shown that blood con-
tains mostly quercetin metabolites after quercetin inges-
tion,
8,10
whereas only the organs involved in quercetin
metabolism (i.e., kidney, liver, and intestines) can contain
significant amounts of free quercetin in addition to
methylated forms.
10
However, few studies have focused
on detecting quercetin concentrations at target tissues
and further research is greatly needed in this area. The
findings of one study conducted in pigs indicated that the
kidney, liver, and jejunum had concentrations of querce-
tin between approximately 2.0 and 6.0 mM/L.
10
Human
studies are not available to confirm this finding
4
; however,
both human and animal studies suggest that quercetin’s
distribution and absorption depend on its form.
4,10
Further, studies have shown that both the bioavailability
and other intestinal contents can affect absorption of
quercetin and its derivatives.
8
The reduction-oxidation potential of a quercetin
molecule is also dependent on quercetin’s form. For
example, non-catechol containing structures do not
chelate oxidative metals as well as those that do contain
catechol.
8
Given the immense variability in quercetin
metabolism, it is tremendously complicated to assess
quercetin’s direct ability to exert pro-oxidant and antioxi-
dant affects in the body. Additionally, there are too many
factors to provide a complete comprehensive review of
the literature involving quercetin metabolism. Thus, this
review focuses on studies that have examined oral supple-
mentation and/or dietary intake of quercetin versus
blood concentrations or tissue concentrations. Since it is
conceivable that long-term consumption and chronic
quercetin blood concentrations will eventually infiltrate
tissues, a general assumption is made that it may be pos-
sible to achieve levels of quercetin in tissues and tumors
that are somewhat near those measured in blood. Also,
Nutrition Reviews® Vol. 68(7):418–428
419
given that limited data are available on quercetin concen-
tration and form in human organs or tissue and that
many of the conjugate forms of quercetin are converted
back to the parental compound by cellular processes, only
those mechanisms involved in free quercetin action at the
target tissue will be examined.
11
It should be noted that
when discussing the high concentrations of quercetin
that would be required for therapeutic effect, it is likely
that high-dose supplementation or more direct forms of
administration would be required. While this is an over-
simplification, the diverse nature of the polyphenols
makes it necessary to focus on the mechanisms of the
parental compound in order to to further understanding
of its conjugates and how all factors combined will ulti-
mately impact the outcome of using quercetin for cancer
prevention and treatment in humans.
There are very few human studies that have evalu-
ated the absorption of quercetin, and most of them were
performed with low doses that could be achieved through
diet. Egert et al.
4
supplemented 18 men and 18 women for
2 weeks with various levels of the oral quercetin aglycone.
The study participants had no difficulty absorbing
dosages of up to 150 mg/day but the researchers did find
variations in individual blood serum concentrations that
were independent of fat mass or sex.
4
The blood plasma
measurements for average total quercetin levels from
50 mg/day, 100 mg/day, and 150 mg/day supplementa-
tion were 145 nmol/L, 217 nmol/L, and 380 nmol/L,
respectively, after only 2 weeks of daily ingestion.
4
Two
well-known, specific quercetin metabolites (isorham-
metin and tamarixetin) were increased to between 9 and
23 nmol/L after treatment with the various dosages of the
oral quercetin aglycone.
4
These findings agree with those
from other human quercetin absorption studies.
4,12
It
should be noted, however, that the measurement of total
quercetin includes all detectable conjugates, which can
then be converted to free quercetin in the cell.
11
Because
the preventative effects of free quercetin are seen in vitro
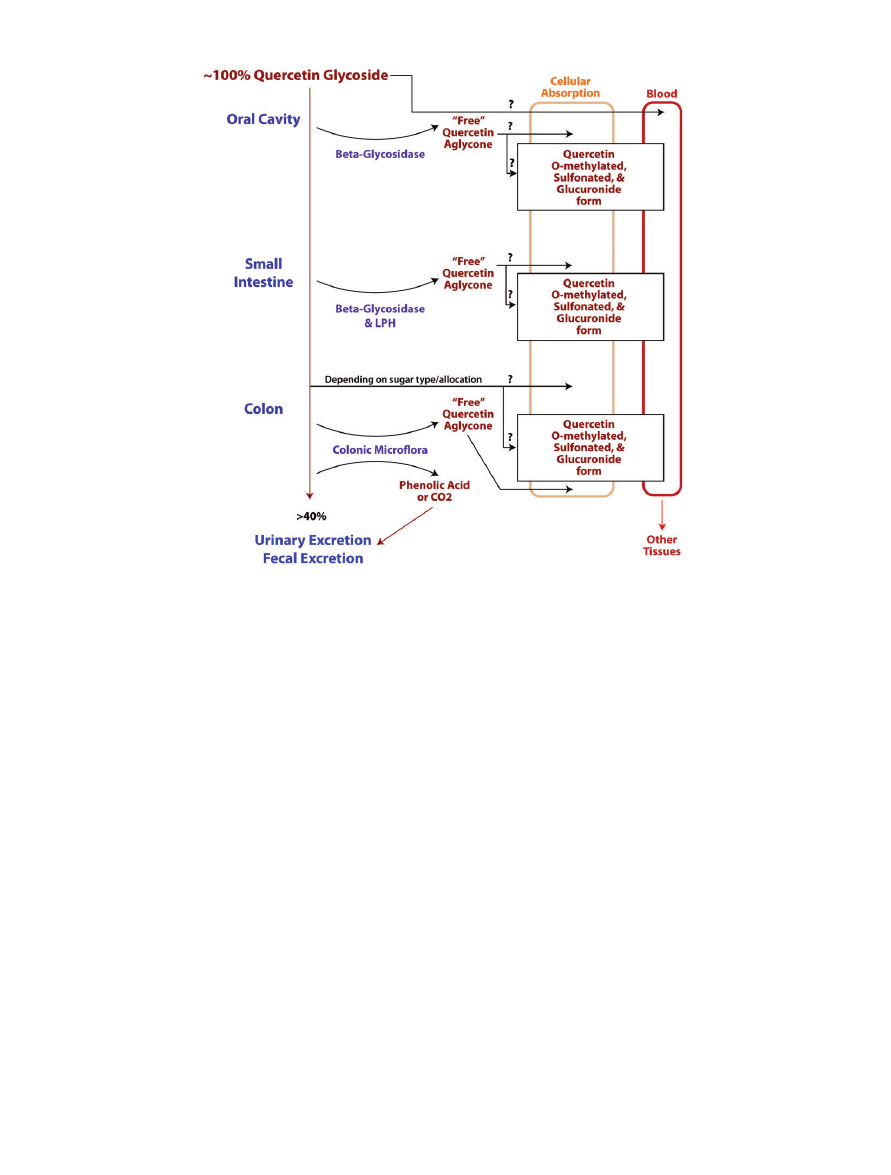
Figure 1 Schematic of possible pathways by which quercetin is digested, absorbed, metabolized, and excreted in the
human body. Typically, quercetin glycoside is ingested orally and is then probably partially digested in the oral cavity. Surplus
quercetin is then digested and absorbed at multiple sites along the gastrointestinal tract. During absorption, or shortly
thereafter, quercetin undergoes modification and then enters the circulatory system in a conjugate form. The circulatory system
delivers quercetin to other tissues in mostly conjugated forms, and once quercetin reaches the target tissues it can likely be
converted back into the parental compound.
Nutrition Reviews® Vol. 68(7):418–428
420

at approximately 1,000–40,000 nmol/L (1–40 mM), for
antioxidant effects, it is likely that these concentrations
could be achieved through diet or, more likely, dietary
supplementation of quercetin.
4
However, the cancer-
treating pro-oxidant effects are not commonly seen
until cellular concentrations reach 40,000 to above
100,000 nmol/L (40–
>100 mM). There are animal studies
that support the possibility of reaching higher concentra-
tions in vivo. Silberberg et al.
13
found that the combined
plasma concentration in rats, after oral consumption of
45–47 mg/day for 2 weeks, was approximately 60 mM.
Another possibility is to administer quercetin intrave-
nously.
14
A phase I clinical study found that individuals
with a cancer diagnosis could tolerate acute serum levels
of 200–400 mM.
14
Although more research is needed, it
appears to be physiologically possible to meet the ranges
required to both prevent and potentially treat carcino-
genesis with quercetin.
ANTIOXIDANT MECHANISMS OF QUERCETIN
Quercetin is able to react with ROS and chelate ROS-
producing metal ions, both of which allow for decreased
oxidative DNA damage.
8
Preventing this DNA damage is
believed to be the general mechanism by which quercetin
is able to prevent tumorigenesis.
8
In particular, it is
known that quercetin’s hydroxyl groups have electron-
accepting capacity when they are in the semiquinone state
and that its catechol group is the structure that confers the
ability to chelate metal ions.
8
The addition of sugar mol-
ecules to form quercetin glycosides can obstruct both of
its antioxidant activities. Therefore, the aglycosylated
form is usually of higher antioxidant potency than the
glycoside form, depending on where the sugar molecule is
attached.
8
In this review, references to quercetin indicate
the free, aglyconated form.
A recent study looking at quercetin’s antioxidant
mechanism in colorectal adenocarcinoma cells (Caco2)
found that treatments consisting of 1 mM concentrations
of quercetin led to decreased double-stranded DNA
breaks, but that higher concentrations of quercetin
increased double-stranded DNA breakage.
15
Recall that
double-stranded DNA breakage is a major source of
mutagenesis and subsequent malignancies in cells.
15
However, increased double-stranded breaks can also lead
to increased apoptosis, as described later in this review.
Congruently, this group found decreased hydrogen
peroxide-induced single-strand DNA breakage in Caco2
cells pretreated with low-dose quercetin as compared to
cells that were untreated.
15
Additionally, it was deter-
mined that both low (1 mM) and high (100 mM) quercetin
treatments led to increased expression of human
8-oxyguanine DNA glycosylase (hOGG1). The hOGG1
protein is involved in repairing DNA.
15
This suggests that
quercetin is able to prevent oxidative DNA damage and
increase DNA repair at lower dosages.
Quercetin also has the ability to work synergistically
with other antioxidant systems in the body in order to
decrease oxidative stress.
16
When quercetin exerts its anti-
oxidant power, it can advance to the semiquinone or even
the 0-quinone state.
16,17
In these highly oxidized states,
quercetin is potentially damaging to the cell and activates
another antioxidant pathway involving glutathione
(GSH).
17
Kim et al.
17
recently examined the relationship
between oxidized quercetin and GSH in a human
hepatoma cell line (HepG2).
17
Their findings indicate that
10 and 100 mM doses of quercetin led to antioxidant
affects, but that exposure to 100 mM quercetin for longer
than 30 min led to pro-oxidant/pro-apoptotic effects.
17
More specifically, the data indicated that quercetin is able
to chelate reactive metal ions that produce ROS, react
with hydrogen peroxide to reduce ROS, and use GSH-
mediated reduction in order to return ROS to their
reduced states.
17
This cooperativity with GSH is likely one
mechanism by which quercetin can protect the cell from
mutagenesis. On the other hand, quercetin may be able to
cause cellular damage when it is administered in a long-
term high dose.
Animal models are frequently the vehicle for mea-
suring overall antioxidant status after treatment with
quercetin. Santos et al.
18
fed mice 4.2 mg of quercetin
daily for 3 weeks and then measured blood values of
quercetin metabolites against control mice. The primary
metabolites found in the blood were glucuronide sulfate
conjugates of isorhamnetin at a concentration of
4.2 mM.
18
The chemical structures of these conjugates
inhibit some of the antioxidant capacity as compared to
the parent compound. Thus, the bioactivity of quercetin
conjugates is lower than that of the parent compound.
18
This decrease in bioactivity was supported by the
unchanged antioxidant activity when the experimental
and control blood samples were compared.
18
Similar null
results were found in humans when serum quercetin
metabolite concentrations reached 1.031 mM after
onion consumption.
19
Like in the murine study,
18
the
researchers in the human study also attributed their null
results to the blood concentration probably being lower
than the threshold needed to significantly change anti-
oxidant biomarkers.
19
However, different results were
obtained when higher dosages, approximately 20 mg
quercetin, were administered to mice intragastrically.
18,20
These acutely exposed mice achieved a 13.2 mM serum
concentration of quercetin metabolites, which was
expectantly higher than the concentration in the previ-
ously discussed lower-dose studies.
20
The higher concen-
tration was enough to increase the antioxidant capacity
of the treated mice, at 119 nmol Trolox equivalents/mL
plasma, relative to the control, at 48 nmol Trolox
Nutrition Reviews® Vol. 68(7):418–428
421
equivalents/mL plasma.
20
These studies illustrate how
dependent quercetin’s antioxidant capacity is on both the
concentration and form of quercetin in the blood and,
presumably, target tissues.
PRO-OXIDANT MECHANISMS OF QUERCETIN
As described above, quercetin is not only an antioxi-
dant
1
; it can also become a pro-oxidant at high concen-
trations or for longer incubations at the greater
concentration. The present review of the literature indi-
cates that, in general, quercetin is able to act as a pro-
oxidant at concentrations greater than 40 mM, which is
in agreement with Watjen et al.
1
Although cytotoxicity
may not be a desirable outcome in healthy cells, it would
be greatly beneficial in tumor cells. Thus, if quercetin
was supplemented at high does or administered intrave-
nously, like other chemotherapeutic drugs, it may be
possible to use this pro-oxidative tendency in order to
initiate apoptosis in humans with cancer. Therefore,
quercetin could likely be used as an adjuvant to current
chemotherapies, and if quercetin is activated (oxidized)
by enzymes in tumor cells, the dose needed for the pro-
oxidant or anti-tumor responses could be considerably
lower.
21,22
Recently discovered mechanisms by which
quercetin is able to bring about advantageous cell death
are discussed below.
MITOCHONDRIAL APOPTOTIC PATHWAY
(P53-DEPENDENT AND -INDEPENDENT)
The mitochondrial apoptotic pathway is initiated via Bcl-
2-associated X protein (Bax) and/or Bcl-2 homologous
antagonist/killer (Bak) proteins that bring about an
increase in the mitochondria outer-membrane pore
size. This allows for cytochrome C, among other pro-
apoptotic proteins, to leak out into the cytoplasm. When
cytochrome C is freed into the cytoplasm, it is able to
combine with apoptotic protease activating-factor 1
(APAF-1) and undergo a conformational change, thus
forming the apoptosome. The apoptosome then enlists
caspase-9 in order to activate the so-called executioner
proteins, caspase-7 and caspase-3. Cell death is subse-
quently carried out by these caspase proteins (Figure 2).
23
Quercetin is a known inducer of apoptosis in multiple
cancer cell lines when administered in doses of 40–50 mM
or greater concentrations.
24–26
Larger doses of quercetin
and longer exposure times lead to decreased cancer cell
viability. It has been proposed that the mitochondrial-
mediated cell-death pathway is a mechanism used by
quercetin in order to induce apoptosis.
25
Examples of quercetin’s antiproliferative effect are
largely documented as being mediated through the
induction of P53.
24–26
This tumor-suppressor protein can
activate Bax and initiate cell death.
27
Recently, Tan et al.
24
investigated protein expression and cell status of a human
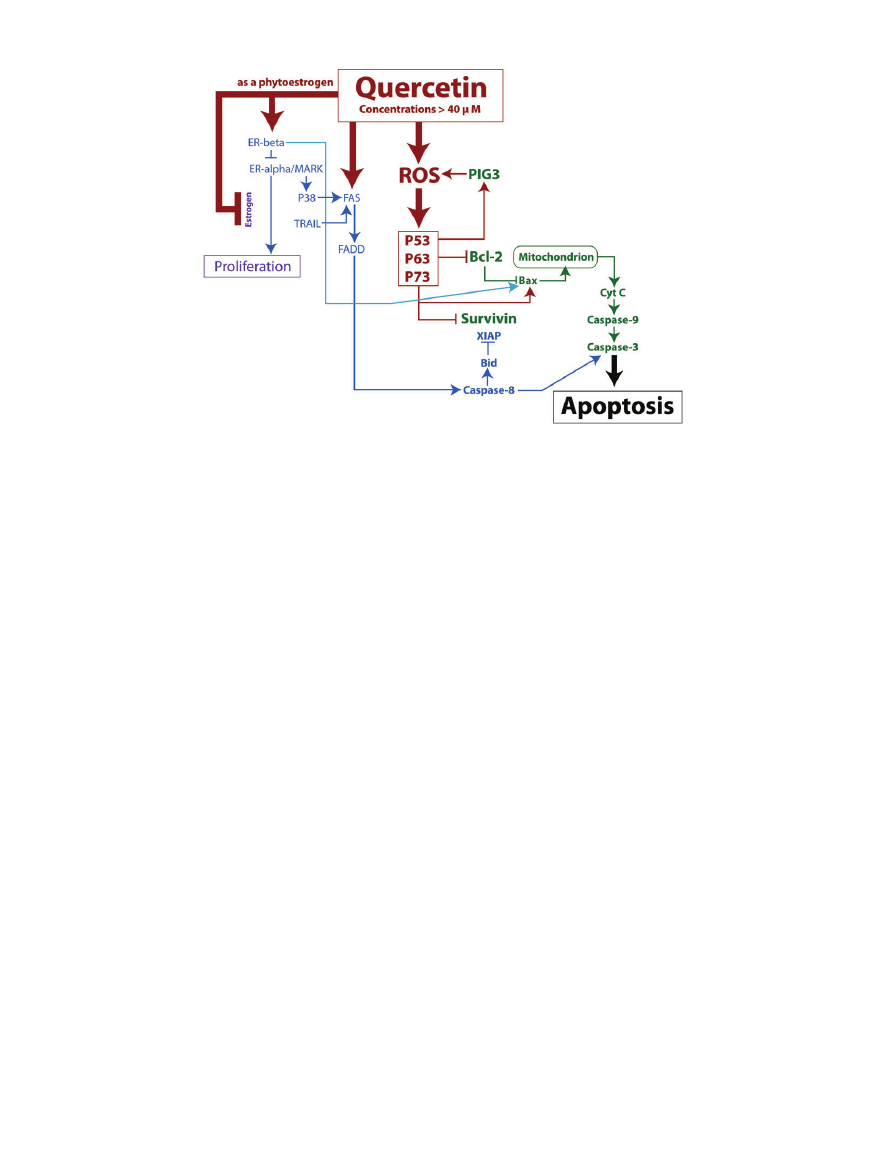
Figure 2 Map of several pro-apoptotic pathways triggered by quercetin concentrations greater than 40
mM. Quercetin
can generate increased cellular ROS, which then increases tumor suppressor proteins and leads to cell death via the mitochon-
drial pathway. Quercetin can also initiate cell death via the death domain pathways. Lastly, quercetin contributes to the
inhibition of proteins that encourage proliferation. Note: Arrows do not always indicate a direct mechanism of action.
Nutrition Reviews® Vol. 68(7):418–428
422

hepatocellular carcinoma cell line after treatment with
40–120 mM dosages of quercetin. Tan et al.
24
found that
quercetin induced increases in P53, while decreasing the
antiapoptotic Survivin and Bcl-2 proteins.
24
Survivin acts
at the caspase level to prevent apoptosis while the ability
of Bcl-2 to prevent mitochondria-directed apoptosis is
dependent on the relative amounts of Bax present.
Further, the researchers found amplified caspase-9 and its
downstream proapoptotic substrate, caspase-3, activity.
24
Given that Bcl-2 is a negative regulator of apoptosis, and
that caspase-3 is a positive regulator of apoptosis,
24
one
can conclude that the P53-dependent induction of the
mitochondria-mediated pathway is what allows quercetin
to induce cell death in this cell line.
24,26
In a similar study performed in human breast cancer
cells (MDA-MB-231), P53 and the mitochondria-
mediated cell-death mechanism were also implicated as
the mechanism by which quercetin is able to induce
apoptosis. Chein et al.
28
observed an increase in P53,
caspase-9 activation, caspase-3, cytochrome c, and apop-
tosis in MDA-MB-231 cells treated with 200–250 mM
quercetin in vitro. In addition, this group measured and
found a decrease in mitochondrial membrane potential
after treatment with quercetin.
28
A decrease in membrane
potential would be consistent with the “leaky mitochon-
drion” evident in mitochondria-mediated apoptosis.
28
Even in the absence of P53, quercetin is able to exert
its mitochondria-mediated cell death via the presence of
P63 and P73.
26
Both P63 and P73 are similar enough in
structure to P53 that they are able to increase transcrip-
tion of Bax.
26
Recently, Zhang et al.
26
demonstrated the
dose-dependent cytotoxicity of quercetin to human
esophageal squamous cell carcinoma cell line (KYSE-510)
that is p53 mutated in vitro. The direct mechanism for
apoptosis was provided by the observed increased cleav-
age of procaspase-9 and caspase-3 after KYSE-510 cells
were treated with 80 mM quercetin.
26
Quercetin is there-
fore able to initiate apoptosis via the mitochondrial
pathway involving activation of caspase-3 downstream
from caspase-9, as long as a functioning p53-like protein
is activatable.
26
Of interest was another finding in this
same experiment involving the quercetin-provoked
increase in expression of p53-inducible gene 3 (PIG3).
26
PIG3 is quinone oxidoreductase and is responsible for the
NADP-dependent reduction of quinones, like quercetin.
It is thought that PIG3 induces cell death by enzymatically
upregulating ROS, but the exact mechanism has not been
elucidated fully.
29
SYNERGISTIC EFFECTS OF QUERCETIN IN APOPTOSIS
Higher dosages of quercetin can trigger apoptotic cas-
cades by multiple mechanisms and via both the mito-
chondrial and death-domain pathways in various cell
lines. The death-domain pathway involves activation of
FAS receptor, then FAS-associated death domain, and
subsequently
caspase-8.
30
Caspase-8
then
induces
caspase-3, which triggers cell death.
30
Quercetin can work
alone or in conjunction with other molecules in order to
bring about cell death (Figure 2).
31
Quercetin even has
some ability to differentiate between normal versus
malignant cells.
31
Below are some examples of novel
mechanisms by which quercetin can bring about tumor
cell death.
In addition to elucidating quercetin’s involvement in
the mitochondrial pathway, Chein et al.
28
found evidence
that quercetin may also induce a separate, p53-
independent apoptotic pathway known as the death-
receptor or death-domain pathway. In MDA-MD-231
cells, Chein et al.
28
found an increase in FAS and
caspase-8 activation after treatment with 250 mM of quer-
cetin. This indicates that high doses of quercetin likely
provoke cell death through the cell-death-receptor path-
ways, in addition to the mitochondria-dependent cell-
death pathway in the same cell line.
Quercetin may also work synergistically (Figure 2)
with other death-domain stimulators, like tumor necrosis
factor a (TNF-a)-related apoptosis-inducing ligand
(TRAIL), to bring about cancerous cell death.
31
Siegelin
et al.
31
recently found that the coadministration of 100 or
200 mM of quercetin will lead to the sensitization of
glioma cells (U87-MG, A172, U251, U373, and LN229) to
TRAIL and, consequently, apoptosis. Glioma cells are
notoriously resistant to TRAIL-induced apoptosis, and
this group found that neither quercetin nor TRAIL alone
caused significant cell death in their cell lines at doses
below 300 mM.
31
Combinations of quercetin and TRAIL,
however, produced apoptosis, as measured by the pres-
ence of cleaved poly(ADP-ribose) polymerase and flow
cytometry in all examined gliomas, except U373. This is
significant given that TRAIL selectively kills only cancer-
ous cells while leaving healthy tissue alive.
31,32
To confirm
the death-domain-pathway activation, Sieglin et al.
31
measured and found an increased active caspase-8 cleav-
age product in U87-MG, A172, and U251 but not in the
other cell lines. This indicates the death domain pathway
is an active player in the apoptosis seen in these cells.
Additionally, this study measured caspase-level inhibitors
of apoptosis. X-link inhibitor of apoptosis proteins
(XIAP) is similar to survivin in that it is antiapoptotic and
that both are upregulated in resistant glioma cells.
31,32
XIAP, along with survivin, were decreased after the cells
were exposed to both quercetin and TRAIL, again except
in the U373 cells.
31
As stated previously, both mitochondria- and death-
domain-directed apoptosis has been noted to occur in
the same cell line simultaneously. In addition to the
classic death-receptor-mediated pathway induced by
Nutrition Reviews® Vol. 68(7):418–428
423

TRAIL, Sieglin et al.
31
found evidence implicating the
mitochondrial pathway as well. The Bcl-2-interacting
domain (Bid) is involved in the mitochondrial apoptotic
pathway and leads to the inhibition of the downstream
caspase-inhibiting
survivin
and
XIAP
proteins.
32
Upregulation of Bid was seen in U87-MG and A172,
indicating that, at least in those cell lines, both types of
apoptosis are involved in quercetin/TRAIL-induced cell
death.
31
In addition to TRAIL, quercetin has been shown to
work with the estrogen receptor a (ER-a) in order to
induce cytotoxicity in some cervical cancer cell lines.
33
In a recent study by Galluzzo et al.,
33
doses as low as
1 mM, and up to 100 mM, were shown to decrease the
number of cells in the human cervix epitheloid carci-
noma cell line (HeLa) that were ER-a-positive but not
the number of HeLa cells that were ER-a-delete. Due to
evidence from research on a related bioflavonoid, nar-
ingen, Galluzo et al.
33
hypothesized the apoptosis was a
result of quercetin stimulating the ER-a-P38/mitogen-
activated protein kinase apoptotic pathway. Although
this pathway only seems to profoundly affect certain cell
lines, it leads to increased FAS (pro-death domain
pathway) and increased Bax (pro-mitochondrial-cell-
death pathway).
34
ER-a can initiate both estrogen-
triggered proliferative and pro-apoptotic pathways.
33
Since quercetin is considered a phytoestrogen, it is likely
that this estrogen-mimicking ability is able to prevent
the ER-a from initiating prosurvival pathways by com-
peting with estrogen for binding at the receptor.
However, the non-estrogen-dependent phosphorylation
of p38 it still able to proceed and initiate the proapop-
totic pathways.
33
Despite quercetin’s ability to interact with ER-a, it
preferentially favors binding to estrogen receptor b
(ER-b) over ER-a.
35
Sotoca et al.
35
recently explored the
impact that quercetin’s receptor affinity has on apoptosis
in breast cancer (T47D-ER-a) and osteosarcoma (U2OS-
ER-a and -ER-b) cell lines. Upon administration of
ascorbate-stabilized quercetin, this group saw increased
quercetin-ER-b binding and apoptosis at concentrations
greater than 50 mM. A proliferative effect was noted at
lower concentrations. Additionally, they observed that
increased presence of ER-b independently contributed to
apoptosis.
35
Presumably, this is due to ER-b’s apoptotic
abilities when bound by a ligand and to its ability to
decrease ER-a’s proliferative effect.
36,37
ER-b is able to
induce apoptosis by increasing intracellular pH via
modulation of the cells’ Na
+
/H
+
exchanger, thus inducing
Bax and mitochondria-directed apoptosis.
36
This ability is
increasingly effective when ER-a’s proliferative activity is
also decreased by ER-b.
36
It is via this pathway that quer-
cetin is indirectly able to increase Bax and apoptosis in
some ER-b-positive cell lines.
QUERCETIN AND PROTEIN CHAPERONE INHIBITION
Quercetin promotes apoptosis by interfering with prolif-
eration and cell maintenance pathways as discussed above
(i.e., Bcl-2, survivin, and XIAP inhibition). Specifically,
however, emerging science is indicating that quercetin-
directed protein chaperone inhibition may play a large
role in the stimulation of cell death.
38,39
Protein chaper-
ones are responsible for the correct folding and mainte-
nance of proteins in the body. When protein chaperones
are unable to perform their duties, cell functionality is
decreased and cell death is plausible.
39
Heat shock protein
(HSP) chaperones, specifically, are unregulated in some
tumor cells and initiated by ionizing radiation.
39
Querce-
tin is able to inactivate these protein chaperones, seem-
ingly by its ability to inhibit the kinases that aid in HSP
induction (Figure 3).
39
This ability is currently being
explored as an anti-cancer mechanism and is discussed
below.
Expression of HSP70 is stimulated by radiation-
induced heat in tumor cells. The heat induces the phos-
phorylation of heat shock transcription factor 1 (HSF1)
by either of two kinases: casein kinase 2 (CK2) and
calcium/calmodulin kinase II (CamKII). Once phospho-
rylated, these kinases activate HSF1, which catalyzes the
transcription of HSP70. A novel experiment by Wang
et al.
39
in Jurkat cells (immortalized T-lymphocytic cells)
demonstrated that quercetin is able to inhibit the kinase
activity of CK2 and CamKII, subsequently decreasing
HSP70 expression and increasing tumor sensitivity to
radiation.
39
This effect, however, was not seen in human
HeLa cells.
39
Confounding the potential of quercetin’s
ability to inhibit HSPs is also the finding in this same
experiment that quercetin somehow contributes to the
phosphorylation and undesired activation of HSP27.
39
This finding is consistent with other findings that quer-
cetin’s actions are cell-type dependent, and Wang et al.
were resolute enough to elucidate two quercetin deriva-
tives that both inhibited HSP70 expression while not acti-
vating HSP27.
39
Quercetin has also been shown to decrease HSP90
expression in prostate cells.
38
HSP90 is a chaperone
protein that aids in the maintenance of oncoproteins,
such as human epidermal growth factor 2 (HER2) and
insulin-like growth factor binding protein-2 (IGFBP-2).
One can speculate that decreased HSP90 in the cell would
likely lead to diminished oncoprotein functionality and
subsequently decrease cancerous growth. HSP90 expres-
sion is positively correlated with the degree of aggression
of prostate cancer cells and is overexpressed in malignant
prostate cells.
38
Aalinkeel et al.
38
found that the levels of
HSP90, HER2, and IGFBP-2 were reduced as the concen-
tration (0–100 mM) of quercetin increased in prostate
cancer cells (LNCaP and PC-3). Conversely, they found
Nutrition Reviews® Vol. 68(7):418–428
424
that quercetin treatment in healthy prostate cells did not
show this affect.
38
Quercetin’s selective effect in this cell
line may be due to its propensity to target HSP90, which
is abundant in tumor cells but not in healthy cells. This
feature of quercetin action, which has also been seen in
other cell lines,
38
makes it a viable substrate for use in
anti-cancer therapy.
QUERCETIN AND ENDOPLASMIC RETICULUM STRESS
Very little research has been done on quercetin’s affect on
endoplasmic reticulum stress. However, a link can clearly
be made. Recall that the endoplasmic reticulum is the
cellular organelle responsible for the packaging and syn-
thesis of many nutrients, among other functions. Endo-
plasmic reticulum stress is also known as the unfolded
protein response, as an accumulation of misshapen pro-
teins increases endoplasmic reticulum stress in cells.
40
Heat shock proteins prevent endoplasmic reticulum
stress by catalyzing refolding of proteins in the cell. Spe-
cifically, inhibition of HSP90 has been shown to induce
endoplasmic reticulum stress and the subsequent endo-
plasmic reticulum stress proapoptotic pathways.
41
Endo-
plasmic reticulum stress has been proposed to initiate
mitochondria-mediated apoptosis by increasing intermi-
tochondrial calcium concentration.
41
The increased
calcium concentration leads to increased recruitment of
Bax,
41
decreased mitochondrial membrane potential, and
subsequently cytochrome C release from the mitochon-
dria. This release triggers the activation of caspase-9 and
-3, and then cell death.
41
As mentioned previously, Aakin-
keel et al.
38
found that quercetin treatment was able to
decrease HSP90 levels in the cell. Further, they found that
the amount of caspase-9 and caspase-3 activity increases
in a dose-dependent manner with the concentration of
quercetin added (Figure 3).
38
Although further research
needs to be done, it is conceivable that quercetin is able to
induce apoptosis via endoplasmic reticulum stress in
some cell lines.
HSP70 is also involved in alleviating endoplasmic
reticulum stress. As mentioned previously, quercetin
decreases HSP70 expression in cells.
39,40
Recently, MCF-7,
T47D, and MDA-MB-435 cell lines have demonstrated
that when HSP70 is inhibited by quercetin treatments of
100 mM, there is subsequent initiation of the unfolded
protein response.
40
This is problematic since this endo-
plasmic reticulum stress pathway initiates an increased
expression of glucose-regulated protein 78 (GRP78).
40
GRP78 functions to protect cells against chemotherapy
and to increase cell survival.
40,42
This provides a mecha-
nism for cells to resist quercetin-induced apoptosis.
However, it was recently established that when GRP78 is
inhibited, there is increased quercetin-mediated apopto-
sis. This provides evidence that quercetin may be able to
work cooperatively with other compounds in order to
mediate cell death via the endoplasmic reticulum stress
pathway.
In an indirect fashion, previous discoveries have pro-
vided researchers with more evidence that quercetin is
able to involve endoplasmic reticulum stress pathways in
order to decrease cell viability. Eukaryotic initiation
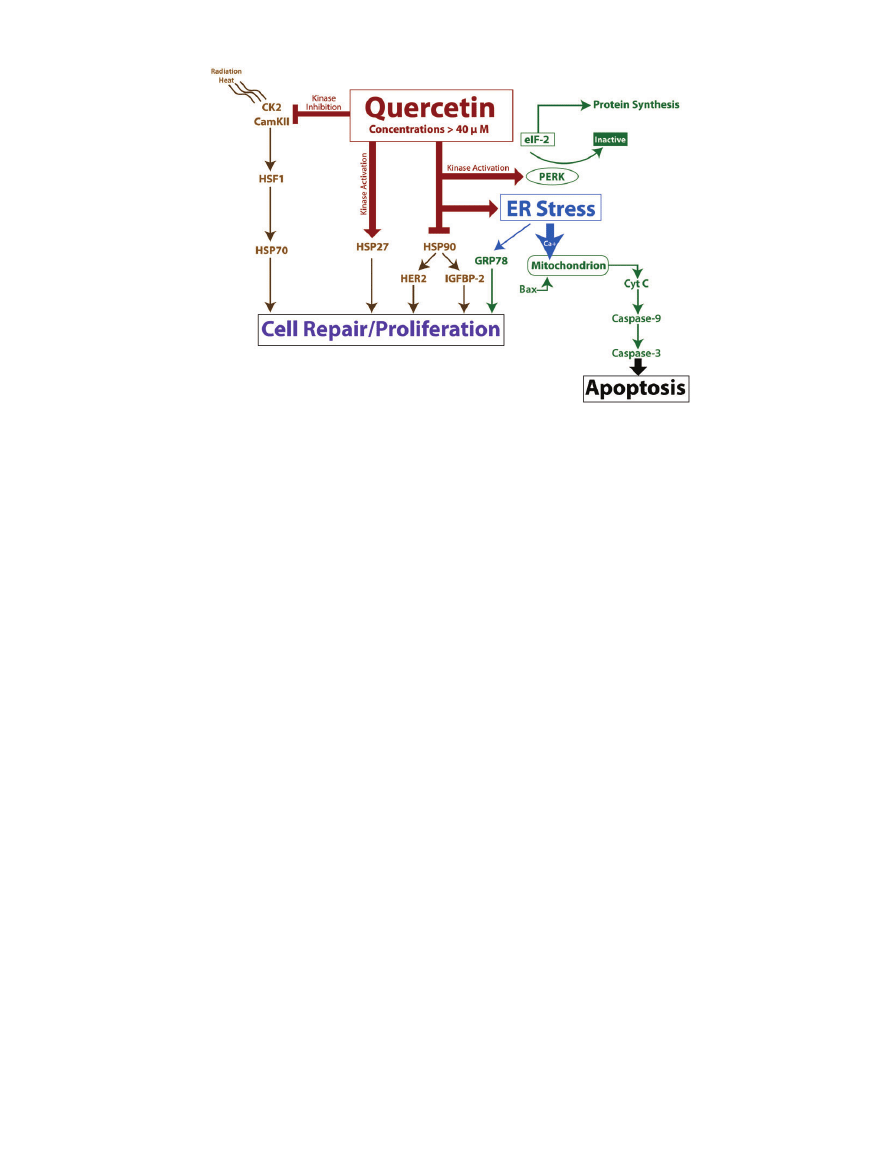
Figure 3 Diagram of several pro-apoptotic pathways triggered by quercetin concentrations greater than 40
mM. Quer-
cetin can initiate cell death via induction of ER stress. Quercetin can also modulate HSP activity, which leads to alterations in cell
repair and proliferation. Note: Arrows do not always indicate a direct mechanism of action.
Nutrition Reviews® Vol. 68(7):418–428
425
factor-2 (eIF-2) is responsible for regulating protein syn-
thesis in vivo.
43
PKR-like endoplasmic reticulum kinase
(PERK) is a protein located on the endoplasmic reticu-
lum. PERK is responsible for the phosphorylation of the
a-subunit of the eIF-2 and is activated under endoplas-
mic reticulum stress.
43
When the eIF-2’s a-subunit is
phosphorylated it is unable to dissociate from another
initiation factor, which essentially prevents mRNA and
protein synthesis.
43
Ito et al. observed that a 100 mM quer-
cetin treatment was able to increase eIF-2 a-subunit
phosphorylation and decrease protein synthesis in mul-
tiple mouse and human lymphoma and leukemia cell
lines. Upon further investigation, this group found that
quercetin was able to stimulate PERK activity, along with
two other eIF kinases, in order to catalyze phosphoryla-
tion of eIF-2 and decrease protein synthesis.
43
Decreased
protein synthesis generally leads to decreased cell growth,
repair, and viability. It is thought that this is further evi-
dence that quercetin uses endoplasmic reticulum stress as
a mechanism to induce eventual apoptosis and cell cycle
arrest in tumors.
43
INTERACTION OF PATHWAYS
Low levels of quercetin could likely be achieved in the
diet for long periods without supplementation, so inves-
tigating the effects of dietary quercetin on cancer preven-
tion is important, while investigating higher levels for
therapeutic purposes would likely require supplementa-
tion or infusion during a therapy. However, because the
exact cellular concentrations of quercetin and its accu-
mulation in cells have yet to be determined, the cellular
concentrations could be higher than currently supposed.
Also, many of the mechanisms and pathways described in
this review could be minimally activated at what would be
considered low-dose exposure and then combined to act
synergistically. For example, as discussed above, the mito-
chondrial and death-domain pathways commonly act
together to induce apoptosis. Specifically, both TRAIL
and mitogen-activated protein kinase pathways intersect
and activate FAS, leading to cell death (Figure 2). In addi-
tion, the inhibition of multiple HSP pathways that con-
verge to protect the cell could also result in a combinatory
effect (Figure 3).
Studies on cancer prevention in mice also focus on
factors other than ROS as a mechanism of cancer preven-
tion, such as the modulation of various signaling path-
ways. For example, recent studies by Ma et al.,
44
Moon
et al.,
45
and Miyamoto et al.
46
indicated that quercetin at
dietary concentrations inhibited proliferation and led to
chemoprevention in mice. Therefore, it is also conceiv-
able that pathways initiated by low and high doses could
interact for therapeutic purposes. Many tumors outgrow
their blood supply and are poorly perfused, giving way
to areas of hypoxia.
47
In this case, highly perfused areas of
a tumor would likely achieve higher concentrations
of quercetin compared to poorly perfused regions
(Figure 4). Therefore, the study of quercetin requires
knowledge of the effects of the conjugates, tissue concen-
trations, and the duration of exposure. Microenviron-
mental factors such as perfusion, pH, and hypoxia may
also play a role.
CONCLUSION
This review presents some of the most recent data
regarding the pathways involved in the quercetin
response. It is proposed that quercetin could be used in
both the prevention and treatment of cancer and that
diet would likely fulfill the concentration requirements
for prevention, but supplementation or another form of
delivery could be necessary for therapeutic responses.
Enzymatic modification of quercetin could further lower
the threshold necessary for anti-tumor activity. It cannot
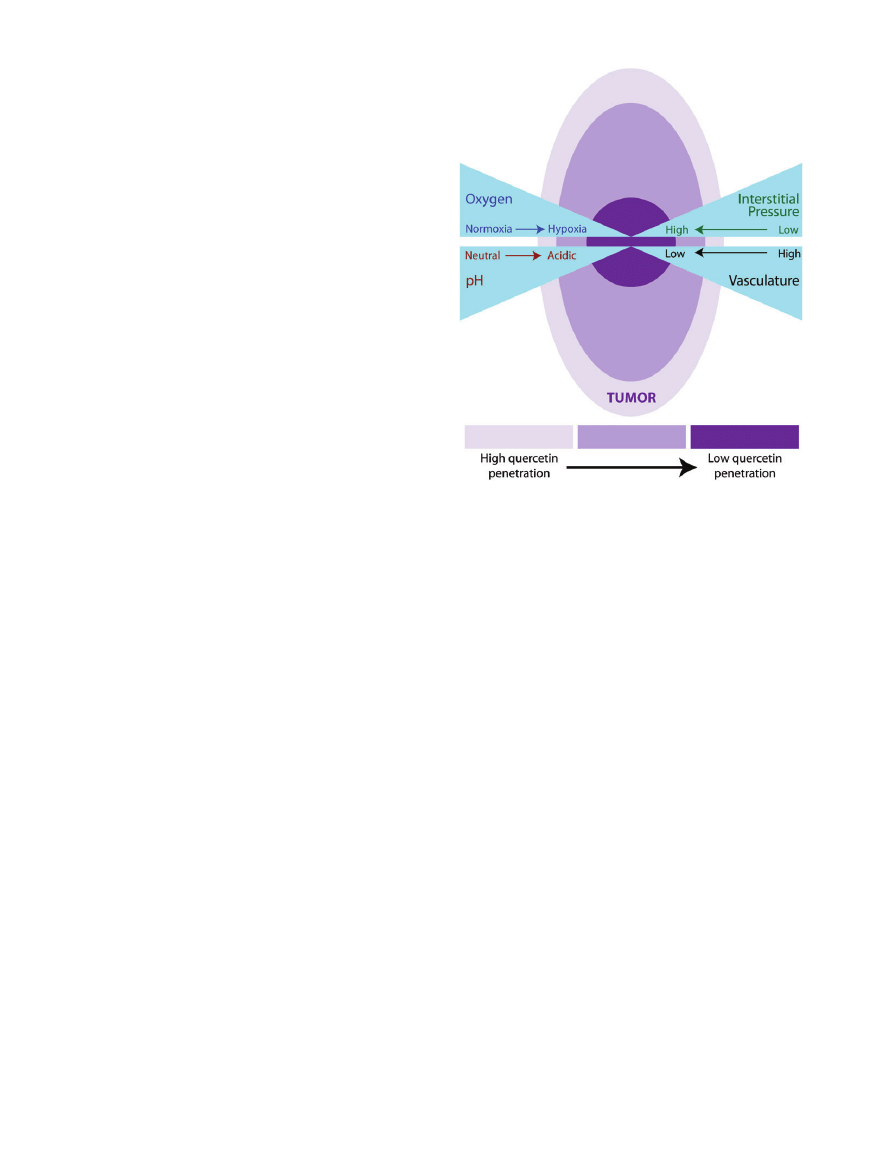
Figure 4 Depiction of theoretical quercetin penetration/
accumulation in a carcinogenic tumor. Quercetin is better
able to accumulate near the well-vascularized areas of a
tumor. The poorly vascularized areas of a tumor tend to have
decreased perfusion resulting in micronutrient deficiencies.
Additionally, in areas of decreased blood supply, there is
increased interstitial tumor pressure and other microenvi-
ronmental factors that make delivery of molecules such as
quercetin to these tumor regions a challenge.
Nutrition Reviews® Vol. 68(7):418–428
426

be ruled out that greater understanding of these path-
ways could lead to the promotion of quercetin in con-
ventional therapies and its use with other drugs in order
to interact and produce a therapeutic effect at lower con-
centrations. Quercetin’s ability to interact with electrons
at higher concentrations plays a central role in its
mechanism of action, mainly by the activation of pro-
teins and DNA damage leading to the induction of many
downstream pathways. Key challenges remain for the
study of quercetin, including the determination of quer-
cetin’s activity and concentration at the tissue site, the
intracellular concentration achievable, and the effect of
conjugates on the pathways. Microenvironmental factors
may also play a role. Further study is also needed to
explain the observed discrepancies between cancer risk
and
quercetin-containing
food
intake
in
larger
population-based studies.
48
Undoubtedly, measuring any
micronutrient in foodstuff is challenging because the
amount differs depending on the growth conditions,
varietals, and food-preparation methods to name a few.
48
Studies collecting dietary data are also well characterized
to have participant reporting bias that may skew out-
comes.
48
Given the confounding factors of epidemiologi-
cal studies and that basic science research has produced
ample proof that quercetin exerts anti-cancer properties,
this molecular gem appears worthy of more attention
and time in the limelight.
Acknowledgments
Declaration of interest. The authors have no relevant
interests to declare.
REFERENCES
1.
Watjen W, Michels G, Steffan B, et al. Low concentrations of
flavonoids are protective in rat H4IIE cells whereas high con-
centrations cause DNA damage and apoptosis. J Nutr.
2005;135:525–531.
2.
Awad HM, Boersma MG, Vervoort J, Rietjens IM. Peroxidase-
catalyzed formation of quercetin quinone methide-
glutathione adducts. Arch Biochem Biophys. 2000;378:
224–233.
3.
Metodiewa D, Jaiswal AK, Cenas N, Dickancaite E,
Segura-Aguilar J. Quercetin may act as a cytotoxic prooxidant
after its metabolic activation to semiquinone and quinoidal
product. Free Radic Biol Med. 1999;26:107–116.
4.
Egert S, Wolffram S, Bosy-Westphal A, et al. Daily quercetin
supplementation
dose-dependently
increases
plasma
quercetin concentrations in healthy humans. J Nutr.
2008;138:1615–1621.
5.
Harwood M, Nielewska-Nikiel B, Borzelleca JF, et al. A critical
review of the data related to the safety of quercetin and lack
of evidence of in vivo toxicity, including lack of genotoxic/
carcinogenic properties. Food Chem Toxicol. 2007;45:2179–
2205.
6.
Alia M, Mateos R, Ramos S, et al. Influence of quercetin and
rutin on growth and antioxidant defense system of a human
hepatoma cell line HepG2. Eur J Nutr. 2006;45:19–28.
7.
Walle T, Browning AM, Steed LL, Reed SG, Walle UK. Flavonoid
glucosides are hydrolyzed and thus activated in the oral
cavity in humans. J Nutr. 2005;135:48–52.
8.
Murota K, Terao J. Antioxidative flavonoid quercetin: implica-
tion of its intestinal absorption and metabolism. Arch
Biochem Biophys. 2003;417:12–17.
9.
Day AJ, Gee JM, DuPont MS, Johnson IT, Williamson G.
Absorption of quercetin-3-glucoside and quercetin-4
′-
glucoside in the rat small intestine: the role of lactase phlo-
rizin
hydrolase
and
the
sodium-dependent
glucose
transporter. Biochem Pharmacol. 2003;65:1199–1206.
10.
Bieger J, Cermak R, Blank R, et al. Tissue distribution of quer-
cetin in pigs after long-term dietary supplementation. J Nutr.
2008;138:1417–1420.
11.
Spencer JP, Kuhnle GG, Williams RJ, Rice-Evans C. Intracellular
metabolism and bioactivity of quercetin and its in vivo
metabolites. Biochem J. 2003;372:173–181.
12.
Manach C, Williamson G, Morand C, Scalbert A, Remesy C.
Bioavailability and bioefficacy of polyphenols in humans. I.
Review of 97 bioavailability studies. Am J Clin Nutr.
2005;81(Suppl):S230–S242.
13.
Silberberg M, Morand C, Manach C, Scalbert A, Remesy C.
Co-administration of quercetin and catechin in rats alters
their absorption but not their metabolism. Life Sci.
2005;77:3156–3167.
14.
Ferry DR, Smith A, Malkhandi J, et al. Phase I clinical trial of
the flavonoid quercetin: pharmacokinetics and evidence for
in vivo tyrosine kinase inhibition. Clin Cancer Res.
1996;2:659–668.
15.
Min K, Ebeler SE. Quercetin inhibits hydrogen peroxide-
induced DNA damage and enhances DNA repair in Caco-2
cells. Food Chem Toxicol. 2009;47:2716–2722.
16.
Boots AW, Kubben N, Haenen GR, Bast A. Oxidized quercetin
reacts with thiols rather than with ascorbate: implication for
quercetin supplementation. Biochem Biophys Res Commun.
2003;308:560–565.
17.
Kim GN, Jang HD. Protective mechanism of quercetin and
rutin using glutathione metabolism on HO-induced oxidative
stress in HepG2 cells. Ann N Y Acad Sci. 2009;1171:530–537.
18.
Santos MR, Rodriguez-Gomez MJ, Justino GC, et al. Influence
of the metabolic profile on the in vivo antioxidant activity of
quercetin under a low dosage oral regimen in rats. Br J Phar-
macol. 2008;153:1750–1761.
19.
Murota K, Hotta A, Ido H, et al. Antioxidant capacity of
albumin-bound quercetin metabolites after onion consump-
tion in humans. J Med Invest. 2007;54:370–374.
20.
Justino GC, Santos MR, Canario S, et al. Plasma quercetin
metabolites: structure-antioxidant activity relationships.
Arch Biochem Biophys. 2004;432:109–121.
21.
Thangasamy T, Sittadjody S, Lanza-Jacoby S, et al. Quercetin
selectively inhibits bioreduction and enhances apoptosis in
melanoma cells that overexpress tyrosinase. Nutr Cancer.
2007;59:258–268.
22.
Thangasamy T, Sittadjody S, Limesand KH, Burd R. Tyrosinase
overexpression promotes ATM-dependent p53 phosphoryla-
tion by quercetin and sensitizes melanoma cells to dacarba-
zine. Cell Oncol. 2008;30:371–387.
23.
Hao Z, Duncan GS, Chang CC, et al. Specific ablation of the
apoptotic functions of cytochrome C reveals a differential
requirement for cytochrome C and Apaf-1 in apoptosis. Cell.
2005;121:579–591.
Nutrition Reviews® Vol. 68(7):418–428
427

24.
Tan J, Wang B, Zhu L. Regulation of survivin and Bcl-2 in
HepG2 cell apoptosis induced by quercetin. Chem Biodivers.
2009;6:1101–1110.
25.
Zhang Q, Zhao XH, Wang ZJ. Flavones and flavonols exert
cytotoxic effects on a human oesophageal adenocarcinoma
cell line OE33 by causing G2/M arrest and inducing apoptosis.
Food Chem Toxicol. 2008;46:2042–2053.
26.
Zhang Q, Zhao XH, Wang ZJ. Cytotoxicity of flavones and
flavonols to a human esophageal squamous cell carcinoma
cell line KYSE-510 by induction of G2/M arrest and apoptosis.
Toxicol In Vitro. 2009;23:797–807.
27.
Roos WP, Kaina B. DNA damage-induced cell death by apop-
tosis. Trends Mol Med. 2006;12:440–450.
28.
Chien SY, Wu YC, Chung JG, et al. Quercetin-induced apopto-
sis acts through mitochondrial- and caspase-3-dependent
pathways in human breast cancer MDA-MB-231 cells. Hum
Exp Toxicol. 2009;28:493–503.
29.
Porte S, Valencia E, Yakovtseva EA, et al. Three-dimensional
structure and enzymatic function of proapoptotic human
p53-inducible quinone oxidoreductase PIG3. J Biol Chem.
2009;284:17194–17205.
30.
Wajant H. The Fas signaling pathway: more than a paradigm.
Science. 2002;296:1635–1636.
31.
Siegelin MD, Reuss DE, Habel A, Rami A, von Deimling A.
Quercetin promotes degradation of survivin and thereby
enhances death-receptor-mediated apoptosis in glioma cells.
Neuro Oncol. 2009;11:122–131.
32.
Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-
dependent mitochondrial release of Smac/DIABLO. Genes
Dev. 2002;16:33–45.
33.
Galluzzo P, Martini C, Bulzomi P, et al. Quercetin-induced
apoptotic cascade in cancer cells: antioxidant versus estrogen
receptor alpha-dependent mechanisms. Mol Nutr Food Res.
2009;53:699–708.
34.
Porras A, Zuluaga S, Black E, et al. P38 alpha mitogen-
activated protein kinase sensitizes cells to apoptosis induced
by different stimuli. Mol Biol Cell. 2004;15:922–933.
35.
Sotoca AM, Ratman D, van der SP, et al. Phytoestrogen-
mediated inhibition of proliferation of the human T47D
breast cancer cells depends on the ERalpha/ERbeta ratio. J
Steroid Biochem Mol Biol. 2008;112:171–178.
36.
Subramanian M, Shaha C. Estrogen modulates human
macrophage apoptosis via differential signaling through
estrogen receptor-alpha and beta. J Cell Mol Med. 2009;13:
2317–2329.
37.
Williams C, Edvardsson K, Lewandowski SA, Strom A,
Gustafsson JA. A genome-wide study of the repressive effects
of estrogen receptor beta on estrogen receptor alpha signal-
ing in breast cancer cells. Oncogene. 2008;27:1019–1032.
38.
Aalinkeel R, Bindukumar B, Reynolds JL, et al. The dietary
bioflavonoid, quercetin, selectively induces apoptosis of
prostate cancer cells by down-regulating the expression of
heat shock protein 90. Prostate. 2008;68:1773–1789.
39.
Wang RE, Kao JL, Hilliard CA, et al. Inhibition of heat shock
induction of heat shock protein 70 and enhancement of heat
shock protein 27 phosphorylation by quercetin derivatives.
J Med Chem. 2009;52:1912–1921.
40.
Li M, Wang J, Jin J, et al. Synergistic promotion of breast
cancer cells death by targeting molecular chaperone GRP78
and heat shock protein 70. J Cell Mol Med. 2008;13:4540–
4550.
41.
Taiyab A, Sreedhar AS, Rao C. Hsp90 inhibitors, GA and
17AAG, lead to ER stress-induced apoptosis in rat histiocy-
toma. Biochem Pharmacol. 2009;78:142–152.
42.
Lee E, Nichols P, Spicer D, et al. GRP78 as a novel predictor of
responsiveness to chemotherapy in breast cancer. Cancer
Res. 2006;66:7849–7853.
43.
Ito T, Warnken SP, May WS. Protein synthesis inhibition by
flavonoids: roles of eukaryotic initiation factor 2alpha
kinases. Biochem Biophys Res Commun. 1999;265:589–594.
44.
Ma ZS, Huynh TH, Ng CP, et al. Reduction of CWR22 prostate
tumor xenograft growth by combined tamoxifen-quercetin
treatment is associated with inhibition of angiogenesis and
cellular proliferation. Int J Oncol. 2004;24:1297–1304.
45.
Moon YJ, Shin BS, An G, Morris ME. Biochanin A inhibits breast
cancer tumor growth in a murine xenograft model. Pharm
Res. 2008;25:2158–2163.
46.
Miyamoto S, Yasui Y, Ohigashi H, Tanaka T, Murakami A.
Dietary flavonoids suppress azoxymethane-induced colonic
preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem
Biol Interact. 2009;183:276–283.
47.
Wachsberger PR, Burd R, Marero N, et al. Effect of the tumor
vascular-damaging agent, ZD6126, on the radioresponse of
U87 glioblastoma. Clin Cancer Res. 2005;11:835–842.
48.
Wang L, Lee IM, Zhang SM, et al. Dietary intake of selected
flavonols, flavones, and flavonoid-rich foods and risk of
cancer in middle-aged and older women. Am J Clin Nutr.
2009;89:905–912.
Nutrition Reviews® Vol. 68(7):418–428
428
Wyszukiwarka
Podobne podstrony:
Hypothesized Mechanisms of Change in Cognitive Therapy for Borderline Personality Disorder
19 Mechanisms of Change in Grammaticization The Role of Frequency
Tea polyphenols and their role in cancer prevention and chemotherapy
The role of antioxidant versus por oxidant effects of green tea polyphenols in cancer prevention
8 95 111 Investigation of Friction and Wear Mechanism of Hot Forging Steels
CONTROL AND THE MECHANICS OF START CHANGE AND STOP
[EBOOK MECHANICS] Mechanics of Materials M4 Bending 1 Shear And Bending Moment Diagrams
Flavonoids a review of propable mechanisms of action and potential aplications
US Patent 613,809 Method Of And Apparatus For Controlling Mechanism Of Moving Vessels Or Vehicles
Polyphenols and human health prevention od diseas and mechanisms of action
Seahra The Classical and Quantum Mechanics of
Guide to the properties and uses of detergents in biology and biochemistry
Political Thought of the Age of Enlightenment in France Voltaire, Diderot, Rousseau and Montesquieu
więcej podobnych podstron