
Contents
lists
available
at
Resuscitation
j o
u
r n
a l
h o m
e p a g e
:
w w w . e l s e v i e r . c o m / l o c a t e / r e s u s c i t a t i o n
Experimental
paper
Pharmacokinetics
of
intraosseous
and
central
venous
drug
delivery
during
cardiopulmonary
resuscitation
夽,夽夽
Stephen
L.
Hoskins
,
Paulo
do
Nascimento
Jr.
,
Rodrigo
M.
Lima
,
Jonathan
M.
Espana-Tenorio
,
George
C.
Kramer
a
Resuscitation
Research
Laboratory,
Department
of
Anaesthesiology,
University
of
Texas
Medical
Branch,
301
University
Blvd,
Galveston,
TX
77555-0801,
United
States
b
Sao
Paulo
Medical
school,
Department
of
Anesthesiology,
Unesp,
Botucatu,
SP,
Brazil
a
r
t
i
c
l
e
i
n
f
o
Article
history:
Received
27
January
2011
Received
in
revised
form
20
July
2011
Accepted
26
July
2011
Keywords:
Intraosseous
Cardiopulmonary
resuscitation
CPR
Pharmacokinetics
Tracers
Drug
delivery
a
b
s
t
r
a
c
t
We
compared
the
pharmacokinetics
of
intraosseous
(IO)
drug
delivery
via
tibia
or
sternum,
with
central
venous
(CV)
drug
delivery
during
cardiopulmonary
resuscitation
(CPR).
Methods:
CPR
of
anesthetized
KCl
arrest
swine
was
initiated
8
min
post
arrest.
Evans
blue
and
indocyanine
green,
each
were
simultaneously
injected
as
a
bolus
with
adrenaline
through
IO
sternal
and
tibial
needles,
respectively,
n
=
7.
In
second
group
(n
=
6)
simultaneous
IO
sternal
and
IV
central
venous
(CV)
injections
were
made.
Results:
Peak
arterial
blood
concentrations
were
achieved
faster
for
sternal
IO
vs.
tibial
IO
administration
(53
±
11
s
vs.
107
±
27
s,
p
=
0.03).
Tibial
IO
dose
delivered
was
65%
of
sternal
administration
(p
=
0.003).
Time
to
peak
blood
concentration
was
similar
for
sternal
IO
and
CV
administration
(97
±
17
s
vs.
70
±
12
s,
respectively;
p
=
0.17)
with
total
dose
delivered
of
sternal
being
86%
of
the
dose
delivered
via
CV
(p
=
0.22).
Conclusions:
IO
drug
administrations
via
either
the
sternum
or
tibia
were
effective
during
CPR
in
anes-
thetized
swine.
However,
IO
drug
administration
via
the
sternum
was
significantly
faster
and
delivered
a
larger
dose.
© 2011 Elsevier Ireland Ltd. All rights reserved.
1.
Introduction
Survival
from
out-of-hospital
cardiac
arrest
depends
on
a
sequence
of
therapeutic
interventions
termed
the
“chain
of
sur-
vival”
by
the
American
Heart
Association
(AHA).
This
sequence
includes
rapid
access
to
emergency
medical
care,
cardiopul-
monary
resuscitation
(CPR),
defibrillation,
advanced
care,
and
post
resuscitation
techniques
such
as
hypothermia,
percutaneous
coro-
nary
interventions,
and
implantable
Unfortunately,
survival
rates
after
cardiac
arrests
are
dismal
(2.5–10.5%).
rapid
vascular
accesses
for
drug
delivery
dur-
ing
CPR
may
be
one
way
of
improving
survival.
Intravenous
access
during
CPR
can
be
difficult
even
for
an
experienced
caregiver.
In
one
study,
the
median
time
required
to
establish
an
intravenous
(IV)
line
by
well-trained
paramedics
夽 A
Spanish
translated
version
of
the
abstract
of
this
article
appears
as
Appendix
in
the
final
online
version
at
doi:10.1016/j.resuscitation.2011.07.041
.
夽夽 Financial
support:
American
Heart
Association
Texas
Affiliate
Grant-in-Aid
#0455157Y.
∗ Corresponding
author.
Tel.:
+1
409
772
3969;
fax:
+1
409
772
8895.
addresses:
(G.C.
Kramer).
in
the
field
was
2
min
for
first
attempts
and
5
min
when
further
attempts
were
required.
overall
success
rate
to
establish
an
IV
line
in
the
field
for
medical
emergencies
is
less
than
There
remains
a
need
for
more
rapid
vascular
accesses
for
drug
delivery
during
CPR
may
be
one
way
of
improving
survival.
Intra-
venous
access
during
cardiopulmonary
resuscitation
(CPR)
can
be
difficult
even
for
an
experienced
caregiver.
Intraosseous
vascular
(IO)
access
is
an
established
rapid,
safe,
and
effective
alternative
for
peripheral
intravenous
drug
delivery.
American
Heart
Association
and
the
European
Resuscitation
Council
Guidelines
for
Pediatric
Life
Support
recommend
IO
access
via
the
tibia
for
pedi-
atric
patients.
the
last
10
years,
several
large
bore
IO
needles
for
adult
patients
have
become
available
that
use
IO
access
via
the
sternum,
tibia
and
humerus.
These
devices
have
been
evaluated
in
both
patients
and
of
these
devices
provides
rapid
access
to
the
systemic
circulation
during
normovolemia.
However,
the
effectiveness
of
IO
drug
delivery
via
different
anatom-
ical
sites
during
CPR
has
been
under
evaluation.
We
used
a
swine
model
of
cardiac
arrest
to
determine
the
phar-
macokinetics
of
IO
delivery
of
a
double
dye
tracer
method
during
CPR
using
simultaneous
IO
injections
in
the
sternum
and
tibia.
We
also
compared
the
pharmacokinetics
of
tracer
administration
via
the
sternum
vs.
central
venous
IV
administration.
0300-9572/$
–
see
front
matter ©
2011 Elsevier Ireland Ltd. All rights reserved.
doi:

108
S.L.
Hoskins
et
al.
/
Resuscitation
83 (2012) 107–
112
2.
Methods
2.1.
Animal
preparation
The
study
protocol
was
approved
by
the
University
of
Texas
Medical
Branch’s
Institutional
Animal
Care
and
Use
Committee
(IACUC).
UTMB
animal
facilities
are
accredited
by
the
American
Association
for
the
Accreditation
of
Laboratory
Animal
Care.
The
experimental
model
was
Yorkshire
swine
(25–35
kg).
The
night
before
the
experiment
food
was
withheld
from
the
animals,
though
they
had
free
access
to
water.
Pre
sedation
was
induced
the
day
of
the
experiment
by
an
intramuscular
injection
of
telazol,
ketamine,
and
xylazine.
A
22
gauge
peripheral
intravenous
catheter
was
placed
in
the
ear
vein
to
deliver
fluids
and
alpha
chloralose.
The
animals
were
anesthetized
for
the
surgical
prep
with
2–4%
isoflu-
rane
by
facial
mask
and
then
intubated
orotracheally
using
direct
laryngoscopy.
Animals
were
placed
supine
on
a
heating
blanket
to
maintain
body
temperature
between
38
and
39
◦
C.
Surgical
areas
were
scrubbed
and
covered
with
sterile
surgical
drapes.
Mechani-
cal
ventilation
was
established
at
a
tidal
volume
of
15–20
ml/kg
and
a
ventilatory
rate
of
12–16
breaths/min
to
maintain
end
tidal
car-
bon
dioxide
between
30
and
40
mmHg.
Thereafter,
isoflurane
was
discontinued
and
anesthesia
was
maintained
with
an
IV
infusion
of
1%
alpha
chloralose
via
the
catheter
in
the
ear,
administered
as
an
initial
bolus
of
50
mg/kg
and
sustained
with
a
continuous
infusion
at
10
mg/kg/h.
The
carotid
artery
was
cannulated
for
arterial
blood
sampling
via
an
incision
of
the
right
side
of
the
neck.
A
central
venous
catheter
was
placed
via
the
external
jugular
vein
to
provide
dye
tracer
administration
into
the
central
venous
circulation.
Catheters
were
placed
into
the
aorta,
via
right
femoral
artery,
and
femoral
vein
for
acute
monitoring
and
recording
of
mean
arterial
pres-
sures
and
drug
delivery
by
sampling
arterial
blood,
respectively.
IO
needles
Jamshidi
(Baxter,
Deerfield,
IL)
or
EZ-IO
®
(VidaCare,
San
Antonio,
TX)
were
placed
in
the
manubrium
5
cm
caudal
of
the
ster-
nal
notch,
and
at
3
cm
distal
of
the
tibial
tuberosity,
respectively.
Correct
placement
was
confirmed
by
cross
section
at
necropsy.
Lac-
tated
Ringer’s
solution
was
administered
at
a
rate
of
15
ml/kg/h
during
surgery.
Standard
hemodynamics
were
monitored
(Hewlett
Packard,
Andover,
MA)
throughout
the
experiments.
Data
were
recorded
via
a
multi
channel
analog-digital
data
acquisition
pro-
gram
using
PowerLab
(AD
Instruments,
UK).
2.2.
Protocol
Two
protocols
were
employed
with
simultaneous
injections;
both
of
them
were
terminal
studies.
Protocol
I
(sternum
vs.
tibia)
compared
the
pharmacokinetics
of
two
different
dye
tracers
administered
intraosseously
and
simultaneously
via
the
sternum
and
the
tibia,
respectively.
Protocol
II
(sternum
vs.
central
venous
IV)
compared
the
pharmacokinetics
of
IO
administration
of
dye
tracers
via
the
sternum
with
a
simultaneous
administration
via
central
venous
IV.
A
60-min
baseline
time
period
was
established
after
completion
of
instrumentation.
Lactate
and
blood
gas
vari-
ables
were
monitored
to
ensure
that
the
animals
had
sufficiently
recovered
from
the
surgical
procedure
and
reached
a
physio-
logic
baseline
before
experimental
data
was
collected.
Heparin,
10,000
units
was
administered
IV
prior
to
the
induction
of
cardiac
arrest.
During
low
flow
states
such
as
cardiac
arrest,
blood
sampling
can
be
difficult
if
the
lines
become
clotted.
Prior
to
the
induction
of
cardiac
arrest,
the
animals
were
administered
a
ketamine
bolus
(30
mg/kg)
to
achieve
a
deeper
anesthesia
plane
and
avoid
any
dis-
tress
during
the
cardiac
arrest
and
resuscitation.
Cardiac
arrest
was
induced
by
rapid
IV
administration
of
10
ml
of
saturated
potassium
chloride
(KCl)
(Hospira
Inc.,
Lake
Forest,
IL)
solution
via
central
venous
catheter
followed
by
a
10
ml
saline
flush.
Immediately
following
the
injection
of
KCl
the
electrocardiogram
(EKG)
displayed
a
typical
ventricular
fibrillation
(VF)
waveform.
Ventilator
support
was
terminated
at
this
time.
Cardiac
arrest
was
followed
by
an
8-min
period
of
untreated
ventricular
fibrillation.
CPR
was
then
initiated
and
delivered
by
a
mechanical
chest
com-
pression
device
(Thumper
®
Michigan
Instruments,
Grand
Rapids,
MI)
at
100
compressions
per
min
(without
supplemental
O
2
)
and
at
duty
cycle
rate
of
50%.
A
compression
depth
was
set
at
2-in.
and
chest
compressions
were
delivered
in
an
anterior/posterior
posi-
tion
centered
on
the
sternal
body.
After
1-min
of
CPR
pre-tracer
arterial
blood
samples
were
taken.
The
volume
of
solution
utilized
was
1.5
ml
followed
by
a
1.0
ml
of
saline
flush.
2.3.
Tracers
Evans
blue
(EB)
(Sigma–Aldrich,
St.
Louis,
MO)
5.0
mg/ml,
and
indocyanine
green
(ICG)
(Alkorn,
Buffalo
Grove,
IL)
2.5
mg/ml
were
used
randomly
in
each
site
for
the
consecutive
experiments
as
trac-
ers
to
determine
the
relative
arterial
appearance
times
and
dose
delivered
from
the
IO
and
central
venous
routes.
Both
ICG
and
EB
dyes
are
inert
and
have
no
known
biological
activity.
Each
bolus
of
tracer
contained
0.014
mg/kg
of
adrenaline
(epinephrine).
At
2-min
post
CPR
(0
time
point)
the
tracers
EB
and
ICG
were
co-administered
simultaneously
to
the
designated
two
paired
sites
in
Protocol
I
(sternal
IO
and
tibial
IO)
and
in
Protocol
II
(central
venous
IV
and
sternal
IO).
Rapid
injection
of
the
2–3
ml
of
tracer
solution
was
immediately
followed
by
a
1
ml
flush
to
clear
the
needle.
Arterial
blood
samples
were
taken
every
10-s
for
5
1/2
min
and
then
at
every
30-s
for
the
remainder
of
the
8-min
time
period.
After
completion
of
the
study
CPR
was
stopped
and
the
animal
was
euthanized
with
a
high
dose
of
ketamine
and
KCl.
Plasma
tracer
concentrations
in
arterial
blood
were
determined
spectrophotometrically
(Beckman
Coulter
DU
800
spectropho-
tometer,
Brea,
CA)
using
absorbance
wavelengths
of
805
nm
for
ICG
and
620
nm
for
EB.
Calibration
standards
of
EB
and
ICG
were
pre-
pared
in
plasma
and
used
to
calculate
the
concentrations
of
EB
and
ICG
from
arterial
blood
samples.
The
area
under
the
curve
(AUC)
of
arterial
tracer
concentration
divided
by
the
tracer
dose
was
used
as
a
measure
of
the
drug
delivered
to
the
systemic
circulation
during
the
first
8
min
after
drug
injection
(0–480
s).
The
ratio
of
the
AUC
for
both
tracers
was
used
as
a
measure
of
the
relative
drug
delivery.
2.4.
Statistics
Summary
data
are
expressed
as
means
±
standard
error
of
the
mean
(SEM).
To
test
for
differences
of
appearance
times
a
paired
Student’s
t-test
was
conducted.
Correlation
coefficients
for
the
rela-
tionship
of
mean
arterial
pressure
(MAP)
to
appearance
time
were
calculated
utilizing
Sigma
plot
software
(Systat
Software
Inc.,
Ver-
sion
11,
San
Jose,
CA).
A
two-sided
alpha
level
of
significance
of
<0.05
was
used
for
assessing
statistical
significance.
3.
Results
Data
on
appearance
time
and
dose
delivered
for
all
individual
animals
and
groups
are
presented
in
figures
and
tables.
3.1.
Appearance
times
and
C)
and
data
for
each
experiment
of
appearance
times
calculated
in
seconds,
between
injection
and
time
to
peak
tracer
concentration,
in
Protocol
I—sternal
IO
and
tibial
IO
injections
(n
=
7).
Mean
time
to
maximum
concentration
was
53
±
11
s
for
the
sternal
injection
compared
to
107
±
27
s
the
tibial
injection.
The
range
was
from
20
to
90
s
and
40
to
240
s
for
the
sternal
and
tibial
routes,
respectively
(p
=
0.03).
Time
to
half
(50%)
S.L.
Hoskins
et
al.
/
Resuscitation
83 (2012) 107–
112
109
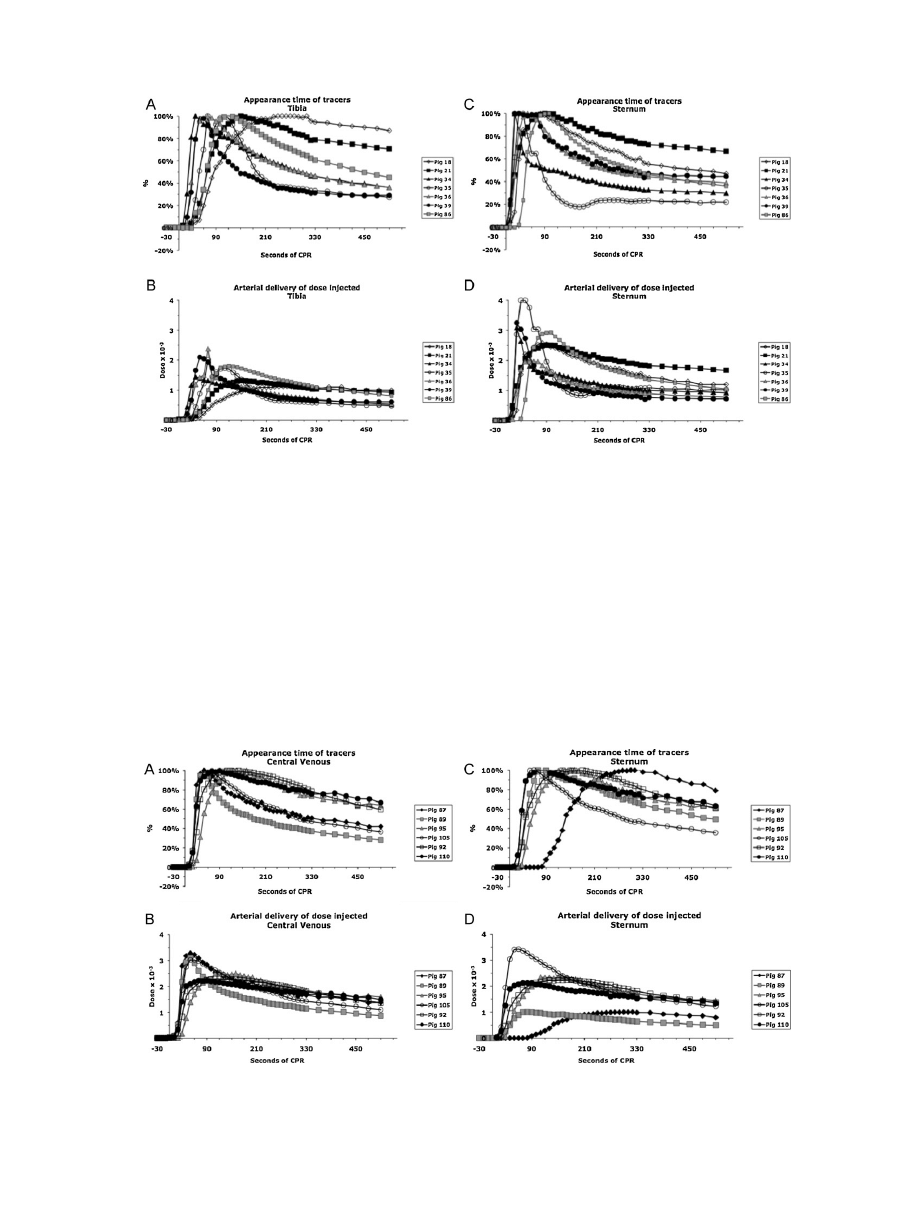
Fig.
1.
The
two
upper
graphs
show
appearance
times
of
tracers
vs.
time:
Protocol-I
(tibial
IO
vs.
the
sternal
IO):
appearance
times
of
tracers
tibia
(Graph-A)
vs.
sternum
(Graph-C).
Concentrations
were
normalized
in
this
figure
to
the
maximal
concentration
in
order
to
better
visualize
time
differences
to
peak
concentration.
The
two
lower
graphs
show
dose
delivered
to
the
arterial
blood
calculated
as
dose
injected
(mg)
by
aortic
blood
concentration
(
g/ml)
for
the
same
protocol
tibia
(Graph-B)
and
sternum
(Graph-D).
maximum
concentration
was
22
±
3
s
using
the
sternal
route
and
50
±
8
s
for
the
tibial
route
(p
=
0.006).
and
C)
and
the
appearance
times
of
trac-
ers
for
Protocol
II,
sternal
IO
and
central
venous
IV
injections
(n
=
6).
Mean
peak
time
to
the
maximum
tracer
concentrations
after
simul-
taneous
injections,
via
IO
and
central
vein
were
not
significantly
different
97
±
17
s
and
70
±
12
s,
respectively
(p
=
0.17).
Times
for
tracers
to
reach
their
50%
maximal
concentrations
were
36
±
4
s
for
sternal
IO
and
30
±
4
s
for
the
central
vein
routes
(p
=
0.06).
3.2.
Dose
delivered
Dose
delivered
was
determined
by
using
an
area
under
the
curve
analysis
(AUC)
for
aortic
concentration
divided
by
injected
dose.
and
D)
and
the
doses
of
tracer
delivery
to
the
aortic
blood,
for
each
animal
of
Protocol
I,
calculated
as
AUC.
The
ratio
of
the
AUC
between
Protocol
I
(tibial
IO
vs.
sternal
IO)
is
a
measure
of
the
relative
effectiveness
of
dose
delivery
via
the
two
routes.
The
tibial
IO
route
delivered
less
dose
to
the
arterial
blood
or
Fig.
2.
The
two
upper
graphs
show
appearance
times
of
tracers
vs.
time:
Protocol-II
(sternal
IO
vs.
central
venous
IV):
appearance
times
of
tracers
central
venous
(Graph-A)
vs.
sternum
(Graph-C).
Concentrations
were
normalized
in
this
figure
to
the
maximal
concentration
in
order
to
better
visualize
time
differences
to
peak
concentration.
The
two
lower
graphs
show
dose
delivered
to
the
arterial
blood
calculated
as
dose
injected
(mg)
by
aortic
blood
concentration
(
g/ml)
for
the
same
protocol
central
venous
(Graph-B)
and
sternum
(Graph-D).

110
S.L.
Hoskins
et
al.
/
Resuscitation
83 (2012) 107–
112
Table
1
Appearance
times
in
seconds
from
injection
to
maximum
tracer
concentrations
and
half
(50%)
maximal
concentration.
Tibial
IO
vs.
sternal
IO
injection
Animal
(n
=
7)
Peak
concentration
50%Peak
concentration
Sternum
Tibia
Sternum
Tibia
86
80
110
36
57
21
90
150
22
68
18
80
240
25
85
34
20
40
15
25
35
30
100
18
50
39
20
50
22
33
36
50
60
13
35
Mean
53
107
22
50
SEM
11
27
3
8
CI
30–75
55–158
16–27
34–65
CI,
confidence
interval
(confidence
level
=
95%);
SEM,
standard
error
of
the
mean.
*
p
=
0.03
–
peak
concentration
–
tibia
vs.
sternum.
§
p
=
0.006
–
50%
peak
concentration
–
tibia
vs.
sternum.
65
±
5%
as
compared
with
the
sternal
route,
mean
AUC’s
difference
was
statically
significant
(p
=
0.003).
and
D)
and
the
actual
values
and
ratio
of
the
AUC
between
Protocol
II
(sternal
IO
vs.
central
venous
IV).
The
ster-
nal
IO
route
was
86
±
10%
as
effective
as
the
central
venous
route
in
tracer
delivery,
although
the
mean
AUCs
were
not
significantly
different
(p
=
0.22).
4.
Discussion
To
the
best
of
our
knowledge
the
present
study
is
the
first
to
use
a
double
tracer
technique
to
assess
effectiveness
of
simultaneous
drug
delivery,
during
CPR
into
two
IO
sites.
Overall
the
study
demonstrated
that
the
intraosseous
(IO)
route
is
an
effective
means
of
delivering
drugs
during
CPR
for
tibia
and
sternum
IO
sites.
Peripheral
IV
lines
are
the
most
commonly
used
routes
for
drug
delivery
by
EMS
personnel.
An
absence
of
venous
blood
flow
and
low
pressure
during
cardiac
arrest
can
lengthen
the
time
to
obtain
peripheral
IV
access
and
delay
critically
needed
drug
ther-
apy.
Experienced
medics
can
achieve
IV
access
rapidly
under
ideal
conditions.
However,
prehospital
conditions
in
the
field
transport
to
hospital,
and
the
skill
levels
of
medics
can
vary
widely.
Clini-
cal
studies
have
shown
that
peripheral
IV
access
times
can
range
from
2
to
49
success
rate
for
establishing
periph-
eral
IV
access
after
cardiac
arrest
and
difficult
IV
is
variable
and
ranges
broadly
between
30
and
75%
in
adult
with
lower
Table
2
Appearance
times
in
seconds
from
injection
to
maximum
tracer
concentrations
and
half
(50%)
maximal
concentration.
Sternal
IO
vs.
central
venous
IV
injection
Animal
(n
=
6)
Peak
concentration
50%Peak
concentration
Sternum
IV
Sternum
IV
87
100
50
36.4
24
89
70
50
34
23
105
60
50
29
28
95
110
110
52
48
110
70
90
28
27
92
170
110
38
36
Mean
97
70
36
30
SEM
17
12
4
4
CI
64–129
45–94
28–42
22–37
p
=
0.17
–
peak
concentration
–
sternum
vs.
central
venous
infusion.
p
=
0.06
– 50%
peak
concentration
–
sternum
vs.
central
venous
infusion.
CI,
confidence
interval
(confidence
level
=
95%);
SEM,
standard
error
of
the
mean.
Table
3
Dose
delivered
for
tibial
vs.
sternal
IO
injections
calculated
as
area
under
the
curve
for
aortic
concentration
g/ml
divided
by
dose
injected
(mg)
over
480
s
after
injec-
tion.
The
relative
effectiveness
of
the
two
routes
is
shown
as
a
ratio
of
the
area
under
the
curve
(AUC),
tibial
IO
divided
by
sternal
IO.
Relative
dose
delivered
of
tracers
(Tibial
IO
vs.
sternal
IO
injection—AUC
0–480
s
)
Animal
g
s/ml)
Ratio
Sternum
Tibia
Tibia/sternum
21
912
450
0.49
18
776
382
0.49
34
601
400
0.67
35
645
368
0.57
39
509
423
0.83
36
511
418
0.82
86
783
545
0.70
Mean
677
427
0.65
SEM
57
22
0.05
CI
564–789
383–470
0.6–0.7
CI,
confidence
interval
(confidence
level
=
95%);
SEM,
standard
error
of
the
mean.
*
p
=
0.003
– comparison
between
AUC
0–480
– tibia
vs.
sternum.
success
rates
for
the
pediatric
patient
population
18–65%.
prospective
study
of
successful
prehospital
IV
placement
in
583
patients
showed
that
the
success
rate
at
first
attempt
was
74%
(368
patients).
Physicians
have
long
sought
alternate
routes
for
the
rapid
administration
of
drugs
during
cardiac
emergencies,
circulatory
shock,
and
low
flow
states.
The
endotracheal
route
is
often
used
as
a
convenient
and
rapid
alternative
for
IV
delivery
of
selected
drugs.
However,
efficacy
of
endotracheal
delivery
of
drugs
is
controversial.
IO
route
provides
access
to
systemic
circu-
lation
via
the
bone
marrow
cavity
which
provides
a
noncollapsible
delivery
point
into
the
central
circulation
for
emergency
infu-
sions
and
for
drug
delivery
in
the
operation
room
setting.
Current
American
Heart
Association
guidelines
and
the
Interna-
tional
Resuscitation
Council
Guidelines
recommend
the
IO
route
as
first
vascular
access
in
pediatric
emergencies
such
a
cardiac
adult
cardiac
arrest
IO
is
the
first
alternative
when
intravenous
access
is
delayed
or
impossible.
success
rate
when
IO
access
is
used
is
the
time
to
establish
a
IO
line
varies
between
20
s
and
1.5
most
common
adverse
effect
associated
with
IO
infusion
is
extravasation
and
this
complication
has
been
reported
in
12%
of
syndrome,
osteomyelitis,
and
tibial
fracture
are
rare,
but
have
been
reported.
Table
4
Dose
delivered
for
sternal
IO
versus
central
venous
IV
injections
calculated
as
area
under
the
curve
for
aortic
concentration
g/ml
divided
by
dose
injected
(mg)
over
480
seconds
after
injection.
The
relative
effectiveness
of
the
two
routes
is
shown
as
a
ratio
of
the
area
under
the
curve
(AUC),
sternal
IO
divided
by
central
venous
IV.
Relative
dose
delivered
of
tracers
(sternal
IO
vs.
central
venous
IV
injection—AUC
0–480
s
)
Animal
AUC
g
s/ml
Ratio
IV
Sternum
Sternum/IV
89
694
589
0.85
105
855
939
1.10
95
879
805
0.92
110
854
783
0.92
92
956
923
0.97
87
934
385
0.41
Mean
862
737
0.86
SEM
38
87
0.10
CI
788–935
566–907
0.7–1.0
p
=
0.22
– comparison
between
AUC
0–480
–
sternum
vs.
central
venous
infusion.
CI,
confidence
interval
(confidence
level
=
95%);
SEM,
standard
error
of
the
mean.

S.L.
Hoskins
et
al.
/
Resuscitation
83 (2012) 107–
112
111
Voelckel
et
al.
showed
that
bone
marrow
blood
flow
was
reduced
by
70–80%
after
hemorrhage.
CPR
the
bone
mar-
row
flow
is
expected
to
be
lower
than
in
hemorrhagic
shock.
Sato
et
al.
and
Del
Guercio
et
al.
showed
in
dogs
and
humans,
respec-
tively
that
during
CPR
the
cardiac
output
is
only
approximately
20–30%
of
our
study
mean
aortic
appearance
times
to
the
peak
concentration
of
the
tracer
was
97
±
17
s
for
the
sternal
IO
route
which
was
not
statistically
significant
(p
=
0.17)
compared
to
70
±
12
s
for
central
venous
route.
Barsan
et
al.
showed
similar
result
in
dogs
with
mean
time
to
peak
times
for
central
venous
infusion
of
84
s
with
range
between
53
and
100
s.
et
al.
showed
that
the
peak
concentration
of
dye
obtained
with
central
venous
injection
of
indocyanine
green
during
CPR
in
humans
was
at
30
s.
However,
only
three
patients
were
included
on
the
study.
Emerman
et
al.
demonstrated
in
dogs
that
the
interval
of
central
venous
injection
to
first
appearance
of
the
indocyanine
green
dur-
ing
CPR
was
37
±
17
et
al.
showed
mean
time
from
adrenaline
injection
to
peak
coronary
perfusion
of
60
±
6
s
when
the
drug
was
delivered
via
IO
vs.
43
±
4
after
IV
injection
during
CPR.
results
are
similar
to
our
finding
of
time
to
the
50%
peak
concentration,
i.e.
central
venous
(30
s),
sternal
(22
s—Protocol
I;
36
s—Protocol
II),
and
tibia
(50
s).
Some
factors
can
affect
the
appearance
times
and
the
dose
delivery
in
this
study.
One
is
that
sternum
is
located
closer
to
the
central
circulation
when
compared
with
the
tibia
location,
which
may
facilitate
the
faster
appearance
of
the
drug
on
the
systemic
circulation
when
the
drug
is
delivered
into
the
ster-
num.
Second,
there
is
a
difference
of
blood
perfusion
between
the
two
bones.
It
is
likely
that
the
sternum
perfusion
is
better
than
the
tibia
perfusion
and
this
may
facilitate
the
absorption
of
the
drug
to
the
systemic
circulation.
Gross
et
al.
showed
a
wide
heterogeneity
of
bone
blood
flow
comparing
hematopoietic
can-
cellous
bones
(red
marrow)
such
as
sternum,
rib,
ilium,
and
femur
epiphysis
(24
ml
min
−1
100
g
−1
)
vs.
nonhematopoietic
bones
(yel-
low
marrow)
such
as
tibia
and
mandible
(2
ml
min
−1
100
g
−1
).
The
authors
also
described
a
significant
decrease
in
blood
flow
and
an
increase
in
vascular
resistance
in
bone
during
hemorrhagic
A
key
point
during
the
CPR
maneuvers
is
the
quality
of
the
chest
compressions.
To
give
effective
chest
compression
is
impor-
tant
that
the
rescuers
or
the
devices
used
to
perform
the
CPR
push
hard
(
≥5
cm)
and
fast
(
≥100/min).
chest
should
be
allowed
to
recoil
freely
after
each
compression.
Besides,
approximately
equal
compressions
and
relaxation
times
should
be
used
and
interrup-
tions
in
chest
compressions
should
be
minimized.
If
these
chest
compressions
are
not
effective
all
the
circulatory
blood
flow
can
be
affected
including
the
bone
marrow
anatomic
dif-
ference
between
the
animals
or
any
other
factor
that
impair
the
dynamic
of
the
chest
compressions
might
result
in
differences
in
cardiac
output
during
this
period,
which
might
consequently
delay
the
appearance
time
of
tracers
on
the
systemic
circulation.
The
dose
delivered
of
tracer
via
the
IO
route
was
similar
to
that
delivered
by
central
venous
route.
The
sternal
IO
route
delivered
86%
of
the
tracer
to
the
aorta
compared
with
central
vein
drug
deliv-
ery.
However,
in
one
animal,
the
ratio
between
sternum/central
venous
infusions
was
0.41
When
we
exclude
this
outlier
data
point
from
the
analysis,
the
resultant
sternum
dose
delivered
via
the
route
was
95%
that
of
the
central
venous.
The
effectiveness
of
the
IO
sternal
route
for
drug
delivery
during
CPR
may
be
due
to
one
or
more
factors.
The
red
bone
marrow
of
the
sternum
could
pro-
vide
sufficient
blood
flow
for
rapid
delivery
of
drugs
to
the
great
veins.
Further,
chest
compressions
may
facilitate
the
drug
egress
out
of
the
marrow
and
into
the
the
IO
delivery
of
tracer
may
be
independent
of
marrow
blood
flow.
It
may
be
that
a
1.5
ml
bolus
of
tracer
followed
by
the
1
ml
flush
used
in
our
study
is
sufficient
volume
to
advance
most
of
the
tracer
through
the
marrow,
out
of
the
injection
site
and
into
the
venous
circulation.
The
mean
dose
delivered
via
the
tibial
route
was
65%
and
53%
of
the
drug
delivery
via
the
sternum
and
central
venous
route,
respectively.
However,
even
for
the
tibial
route
the
half
maxi-
mal
concentrations
were
achieved
in
less
than
1
min.
Andropoulos
et
al.
used
HPLC
analysis
for
the
determination
of
tibial
adrenaline
delivery
during
CPR
in
lambs.
The
authors
determined
that
the
maximum
arterial
plasma
adrenaline
concentrations
were
similar
between
central
venous
and
tibial
IO
delivery.
However,
they
noted
reduced
appearance
time,
after
central
venous
administration
com-
pared
to
tibial
IO
injection
after
adrenaline
Our
measurements
of
appearance
times
and
doses
delivered,
coupled
with
an
additional
one
or
more
minutes
for
establishing
a
peripheral
IV,
suggest
that
even
when
using
the
slower
tibial
IO
route,
one
would
effectively
deliver
drugs
into
the
arterial
cir-
culation
during
CPR
in
a
shorter
time
than
the
time
needed
to
successfully
start
a
peripheral
IV.
As
such,
the
tibial
IO
route
is
both
an
efficacious
and
rapid
means
of
delivering
drug
therapy
dur-
ing
CPR.
The
size
of
the
saline
bolus
after
the
drug
infusion
may
also
have
an
important
role
on
the
time
for
maximum
concentra-
tion
of
the
dye.
If
we
had
used
a
larger
flush
the
effectiveness
of
the
IO
tibial
delivery
may
have
increased.
Wenzel
et
al.
demon-
strated
comparable
vasopressin
plasma
level
and
hemodynamic
variables
when
the
drug
was
delivered
both
by
the
intravenous
and
the
tibial
IO
routes
during
CPR.
However,
the
authors
infused
20
ml
of
saline
bolus
compared
with
1.0
ml
used
in
the
present
study.
Based
on
the
present
data,
we
recommend
that
sternal
IO
route
be
considered
as
the
first
choice
of
drug
delivery
during
CPR
when
IV
access
has
not
been
established,
and
that
the
tibial
IO
route
is
also
justified
as
second
choice.
The
practical
choices
of
which
route
to
use
in
adults
also
depend
on
which
IO
devices
are
avail-
able.
There
are
currently
6
adult
IO
devices
allowed
for
marketing
by
the
Food
and
Drug
Administration
(FDA).
This
includes
two
IO
devices
for
adult
sternal
access
(FAST1
(Pyng
Medical
Corp.,
Rich-
mond,
BC,
Canada)
and
Sternal
EZ-IO
(Vidacare
Corp.,
San
Antonio,
TX))
and
four
IO
devices
for
tibial
access
(SurFast
(Cook
Criti-
cal
Care,
Bloomington,
IN),
Jamishidi
(Baxter
Allegiance,
McGraw
Park),
Bone
Injection
Gun
(B.I.G.,
Waismed,
Houston,
TX),
EZ-IO
(Vidacare
Corp.,
San
Antonio,
pediatric
patients,
stan-
dard
butterfly
needle,
spinal
needle,
and
pediatric
versions
of
adult
IO
needles
can
be
used.
Most
recently
the
humerus
has
been
sug-
gested
as
a
route
for
IO
delivery.
Further
work
will
be
required
to
assess
the
relative
success
of
this
route
vs.
the
sternal
and
the
tibial
route.
There
are
limitations
to
our
study.
First,
swine
are
not
humans
and
conclusive
extrapolation
to
human
patient
responses
cannot
be
made.
The
shape
of
the
pig
thorax
is
different
from
the
human
thorax.
In
pigs,
the
ventricles
are
positioned
in
the
center
of
the
tho-
racic
cavity,
surrounded
by
lung
tissues
on
all
sides.
In
humans,
the
right
ventricle
is
positioned
just
under
the
sternum.
This
anatomic
difference
makes
it
more
difficult
to
get
a
compression
effect
on
the
heart
of
pigs.
Chest
compressions
in
pigs
increase
intratho-
racic
pressure
(thoracic
pump
mechanism),
which
in
turns
affects
the
heart.
In
humans
we
have
not
only
the
thoracic
pump
effect
but
also
the
direct
heart
pump
mechanism
affecting
the
heart
by
chest
compression.
we
did
not
measure
the
plasma
concentrations
of
adrenaline.
We
used
dye
tracers
as
a
surrogate
of
drug
delivery
in
place
of
the
biologically
active
drug.
However,
measurement
of
adrenaline
would
preclude
comparison
of
simulta-
neous
injections.
The
significant
variability
of
cardiac
output
during
CPR
results
in
an
animal
to
animal
variability
of
time
to
peak
con-
centration
and
dose
delivered;
while
simultaneous
2
tracer
paired
studies
provides
for
great
precision
for
comparing
differences.
Fur-
ther,
high
background
levels
of
endogenous
adrenaline
during
CPR

112
S.L.
Hoskins
et
al.
/
Resuscitation
83 (2012) 107–
112
make
precise
assessment
exogenous
drug
epinephrine
impossible.
Our
study
suggests
that
either
bone
marrow
blood
flow
or
the
vol-
ume
of
injectate,
or
both,
are
sufficient
for
tracer
delivery
through
the
emissary
veins
to
the
superior
vena
cava.
We
studied
young
pigs
with
healthy
hearts
and
peripheral
vessels,
while
clinical
ventric-
ular
fibrillation
occurs
largely
in
older
patients
with
some
amount
of
peripheral
artery
disease.
The
pig
is
the
most
often
used
animal
model
of
cardiac
arrest
and
data
on
tibial
IO
injec-
tions
in
swine
with
their
short
legs
may
not
be
comparable
to
that
of
adult
humans
with
longer
legs
farther
from
the
heart.
Blood
flow
in
the
leg
and
bone
marrow
cavities
below
the
diaphragm
could
be
less
in
humans
than
in
pigs
during
CPR.
5.
Conclusions
Both
tibial
and
sternal
IO
routes
are
an
effective
means
of
deliv-
ering
life
saving
drugs
during
CPR.
Dye
tracers
delivered
via
tibial
IO
or
sternal
IO
routes
of
anesthetized
swine
reached
maximal
con-
centrations
in
the
arterial
blood
during
CPR
in
less
than
2
min
with
both,
a
faster
and
a
greater
dose
delivered
using
the
sternum
route
than
with
the
tibial
route.
Sternal
IO
and
central
venous
routes
are
not
different
considering
pharmacokinetics
of
tracers
during
CPR
in
swine.
Conflict
of
interest
Dr.
Kramer
is
an
inventor
on
patents
for
intraosseous
technolo-
gies
and
a
compensated
consultant
to
Vidacare
2007–2010.
References
1.
Cummins
RO,
Ornato
JP,
Thies
WH,
Pepe
PE.
Improving
survival
from
sudden
cardiac
arrest:
the
“chain
of
survival”
concept.
Circulation
1991;85:1832–47.
2.
Nichol
G,
Aufderheide
TP,
Eigel
B,
et
al.
Regional
systems
care
for
out-of-hospital
cardiac
arrest.
Circulation
2010;121:709–29.
3.
Hollenberg
J,
Bang
A,
Lindqvist
J,
et
al.
Difference
in
survival
rates
after
out-of-
hospital
cardiac
arrest
between
the
two
largest
cities
in
Sweden:
a
matter
of
time?
J
Intern
Med
2005;257:247–54.
4.
Rea
TD,
Cook
AJ,
Stiell
IG,
et
al.
Predicting
survival
after
out-of-hospital
cardiac
arrest:
role
of
Utstein
data
elements.
Ann
Emerg
Med
2010;55:249–57.
5. Olasveengen
TM,
Sunde
K,
Brunborg
C,
Thowsen
J,
Steen
PA,
Wik
L.
Intra-
venous
drug
administration
during
out-of-hospital
cardiac
arrest.
JAMA
2009;302:2222–9.
6. Lapostolle
F,
Catineau
J,
Garrigue
B,
et
al.
Prospective
evaluation
of
peripheral
venous
access
difficulty
in
emergency
care.
Intensive
Care
Med
2007;33:1452–7.
7.
Constantino
T,
Parikh
A,
Satz
WA,
Fojtik
JP.
Ultrasonography-guided
periph-
eral
intravenous
access
versus
traditional
approaches
in
patients
with
difficult
intravenous
access.
Ann
Emerg
Med
2005;46:456–61.
8.
Paxton
JH,
Knuth
TE,
Klausner
HA.
Proximal
humerus
intraosseous
infusion:
a
preferred
emergency
venous
access.
J
Trauma
2009;67:606–11.
9.
Buck
ML,
Wiggins
BS,
Sesler
JM.
Intraosseous
drug
administration
in
chil-
dren
and
adults
during
cardiopulmonary
resuscitation.
Ann
Pharmacother
2007;41:1679–86.
10.
Ong
MEH,
Chan
YH,
Oh
JJ,
Ngo
ASY.
An
observational,
prospective
study
com-
paring
tibial
and
humeral
intraosseous
access
using
the
EZ-IO.
Am
J
Emerg
Med
2009;27:8–15.
11.
Shavit
I,
Hoffmann
Y,
Galbraith
R,
Waisman
Y.
Comparison
of
two
mechanical
intraosseous
infusion
devices:
a
pilot,
randomized
crossover
trial.
Resuscitation
2009;80:1029–33.
12.
Pediatric
advanced
life
support:
2010
American
Heart
Association
guideline
for
cardiopulmonary
resuscitation
and
emergency
cardiovascular
care.
Pediatrics
2010;126:e1361–99.
13.
Pediatric
basic
and
advanced
life
support
2010
International
Consensus
on
Car-
diopulmonary
Resuscitation
and
Emergency
Cardiovascular
Care
Science
with
Treatment
Recommendations—Part-10.
Resuscitation
2010;81:e213–59.
14.
Von
Hoff
DD,
Kuhn
JG,
Burris
HA,
Miller
LJ.
Does
intraosseous
equal
intravenous?
A
pharmacokinetic
study.
Am
J
Emerg
Med
2008;26:31–8.
15.
Stein
J,
George
B,
River
G,
Hebig
A,
McDermott
D.
Ultrasonograpically
guided
peripheral
intravenous
cannulation
in
emergency
department
patients
with
difficult
intravenous
access:
a
randomized
trial.
Ann
Emerg
Med
2009;54:33–40.
16.
Doninger
SJ,
Ishimine
P,
Fox
JC,
Kanegaye
JT.
Randomized
controlled
trial
of
ultrasound-guided
peripheral
intravenous
catheter
placement
versus
tra-
ditional
techniques
in
difficult-access
pediatric
patients.
Pediatr
Emerg
Care
2009;25:154–9.
17.
Brunette
D,
Fischer
R.
Intravascular
access
in
pediatric
cardiac
arrest.
Am
J
Emerg
Med
1988;6:577–9.
18.
Orlowski
JP,
Gallagher
JM,
Porembka
DT.
Endotracheal
epinephrine
is
unreliable.
Resuscitation
1990;19:103–13.
19.
Caen
AR,
Reis
A,
Bhutta
A.
Vascular
access
and
drug
therapy
in
pediatric
resus-
citation.
Ped
Clin
N
Am
2005;55:909–27.
20.
Joseph
G,
Tobias
JD.
The
use
of
intraosseous
infusions
in
the
operating
room.
J
Clin
Anesth
2008;20:469–73.
21. Pediatric
advanced
cardiovascular
life
support:
2010
American
Heart
Associa-
tion
guidelines
for
cardiopulmonary
resuscitation
and
emergency
cardiovascu-
lar
care
Part
14.
Circulation
2010;122:S876–908.
22.
Adult
advanced
cardiovascular
life
support:
2010
American
Heart
Association
guidelines
for
cardiopulmonary
resuscitation
and
emergency
cardiovascular
care
Part
8.
Circulation
2010;122:S729–67.
23.
Lamhaut
L,
Dagron
C,
Aprotesei
R,
et
al.
Comparison
intravenous
and
intraosseous
access
by
pre-hospital
medical
emergency
personnel
with
and
without
CBRN
protective
equipment.
Resuscitation
2010;81:65–8.
24.
Fiorito
BA,
Mirza
F,
Doran
TM,
et
al.
Intraosseous
access
in
the
setting
of
pediatric
critical
care
transport.
Pediatr
Crit
Care
Med
2005;6:50–3.
25. Rosetti
VA,
Thompson
BM,
Miller
J,
Mateer
JR,
Aprahamian
C.
Intraosseous
infu-
sion:
an
alternative
route
for
pediatric
intravascular
access.
Ann
Emerg
Med
1985;14:885–8.
26.
Voelckel
W,
Lurie
K,
McKnite
S,
et
al.
Comparison
of
epinephrine
with
vaso-
pressin
on
bone
marrow
blood
flow
in
an
animal
model
of
hypovolemic
shock
and
subsequent
cardiac
arrest.
Crit
Care
Med
2001;29:1578–92.
27.
Sato
S,
Okubo
N,
Satsumae
T,
et
al.
Arteriovenous
differences
in
PCO2
and
cardiac
output
during
CPR
in
the
dog.
Resuscitation
1994;27:255–9.
28. Del
Guercio
LMR,
Coomaraswany
R,
State
D.
Cardiac
output
and
other
hemody-
namic
variables
during
external
massage
in
man.
N
Engl
J
Med
1963;269:1398.
29.
Barsan
WG,
Levy
RC,
Weir
H.
Lidocaina
levels
during
CPR.
Ann
Emerg
Med
1981;10:73–8.
30. Kuhn
GJ,
White
BC,
Swetneam
RE,
et
al.
Peripheral
vs
central
circulation
times
during
CPR:
a
pilot
study.
Ann
Emerg
Med
1981;10:417–9.
31.
Emerman
CL,
Pinchak
AC,
Hagen
JF,
Hancock
DE.
Dye
circulation
times
during
cardiac
arrest.
Resuscitation
1990;19:53–60.
32. Zuercher
M,
Kern
KB,
Indik
JH,
et
al.
Epinephrine
improves
24-hour
survival
in
a
swine
model
of
prolonged
ventricular
fibrillation
demonstratins
that
early
intraosseous
is
superior
to
delayed
intravenous
administration.
Anesth
Analg
2011;112:884–90.
33. Gross
PM,
Heistad
DD,
Marcus
ML.
Neurohumoral
regulation
of
blood
flow
to
bones
and
marrow.
Am
J
Physiol
1979;237:h440–8.
34.
Liao
Q,
Sjoberg
T,
Paskevicius
A,
Wolfart
B,
Steen
S.
Manual
versus
mechanical
cardiopulmonary
resuscitation.
An
experimental
study
in
pigs.
BMC
Cardiovasc
Disord
2010;10:53.
35.
Warren
DW,
Kissoan
N,
Mattar
A,
Morrissey
G,
Gravelle
D,
Rieder
M.
Pharma-
cokinetics
from
multiple
intraosseous
and
peripheral
intravenous
site
injections
in
normovolemic
and
hypovolemic
pigs.
Crit
Care
Med
1994;22:838–43.
36.
Andropoulos
DB,
Soifer
SJ,
Schreiber
MD.
Plasma
epinephrine
concentrations
after
intraosseous
and
central
venous
injection
during
cardiopulmonary
resus-
citation
in
the
lamb.
J
Pediatr
1990;116:312–5.
37.
Wenzel
V,
Lindner
KH,
Augenstein
S,
et
al.
Intraosseous
vasopressin
improves
coronary
perfusion
pressure
rapidly
during
cardiopulmonary
resuscitation
in
pigs.
Crit
Care
Med
1999;27:1565–9.
38.
Calkins
MD,
Fitzgerald
G,
Bentley
TB,
Burris
D.
Intraosseous
infusion
devices:
a
comparison
for
potential
use
in
special
operations.
J
Trauma-Inj
Infect
Crit
Care
2000;48:1068–74.
39.
Tobias
JD,
Ross
AK.
Intraosseous
infusions:
a
review
for
the
anesthesiologist
with
focus
on
pediatric
use.
Anesth
Analg
2010;110:391–401.
Document Outline
Wyszukiwarka
Podobne podstrony:
Pharmacokinetics of intraosseous and?ntral venous drug?livery during?rdiopulmonary resuscitation
Resuscitation- The use of intraosseous devices during cardiopulmonary resuscitation, MEDYCYNA, RATOW
The exploitation of carnivores and other fur bearing mammals during
ABC Of Arterial and Venous Disease
ABC Of Arterial and Venous Disease
Aspects of the development of casting and froging techniques from the copper age of Eastern Central
the illict preparation of morphine and heroin from pharmaceutical products containing codeine homeba
social capital strategic relatedness and the formation of intraorganizational likages
Historia gry Heroes of Might and Magic
Overview of Exploration and Production
Ayurvedic Pharmacopoeia of India API Vol 3
Ayurvedic Pharmacopoeia of India API Vol 2
Blanchard European Unemployment The Evolution of Facts and Ideas
Magnetic Treatment of Water and its application to agriculture
68 979 990 Increasing of Lifetime of Aluminium and Magnesium Pressure Die Casting Moulds by Arc Ion
ABC Of Occupational and Environmental Medicine
więcej podobnych podstron