POTASSIUM AMIDE
1
Potassium Amide
KNH
2
[17242-52-3]
H
2
KN
(MW 55.13)
InChI = 1/K.H2N/h;1H2/q+1;-1/rH2KN/c1-2/h2H2
InChIKey = FEMRXDWBWXQOGV-WQAKZGBJAR
(strong base and nucleophile; used for the generation and trapping
of arynes; has been used extensively to study the reactivity of
heterocyclic systems)
Solubility:
1.7 M in liquid ammonia;
1
1.3 × 10
−4
M in THF.
2
Preparative Methods:
a solution of potassium amide in liquid
ammonia is prepared by adding pieces of Potassium to liquid
Ammonia in an ordinary three-necked flask equipped with a
mechanical stirrer.
3
A piece of potassium is added to liquid
ammonia and after the appearance of a blue color, a few crystals
of iron(III) nitrate hydrate are added as catalyst. The remaining
pieces of potassium are added at a rate which maintains active
hydrogen evolution. Discharge of the deep blue color indicates
complete conversion to potassium amide. External cooling is
not required since the evaporation of ammonia will provide
ample cooling.
4
If the reaction must be maintained at −78
◦
C
then a dry ice–acetone condenser is necessary, otherwise an air
condenser is sufficient. The resulting opaque mixture contains
potassium amide which is mostly in solution. A more elabo-
rate two-flask assembly for the generation and transfer of a
potassium amide solution has also been described.
5
Handling, Storage, and Precautions:
potassium amide is flamm-
able and ignites on contact with moisture. Excess material is
destroyed by careful treatment with ethanol or isopropanol.
In the preparation of potassium amide the following precau-
tions should be noted. Potassium is a silvery gray metal but it
can form an explosive peroxide coating. If it acquires an or-
ange or red color or an appreciable oxide coating it should be
considered extremely hazardous. Extreme caution should be
exercised in any attempt to isolate potassium amide as it is sus-
pected to be shock sensitive following partial oxidation. An
explosion has been reported during the isolation of dry potas-
sium amide.
6
Reactions should be performed in a fume hood to
prevent exposure to ammonia. Hydrogen is evolved during the
generation of potassium amide. No ignition source should be
present.
Introduction. Potassium amide is both a strong base and a
strong nucleophile and thus it has been used most effectively in
reactions which exploit both of these properties, such as the gen-
eration and trapping of arynes, the amination of aromatic systems,
and the rearrangement of various heterocyclic systems. Examples
of these types of transformations are described below. The reagent
has also been used simply as a strong base to induce deprotona-
tion or elimination reactions, provided there are no competing
nucleophilic reaction pathways available. This is currently a less
important feature of the reagent given the ready availability of
strong, nonnucleophilic bases, but a few examples are described
at the end of this section.
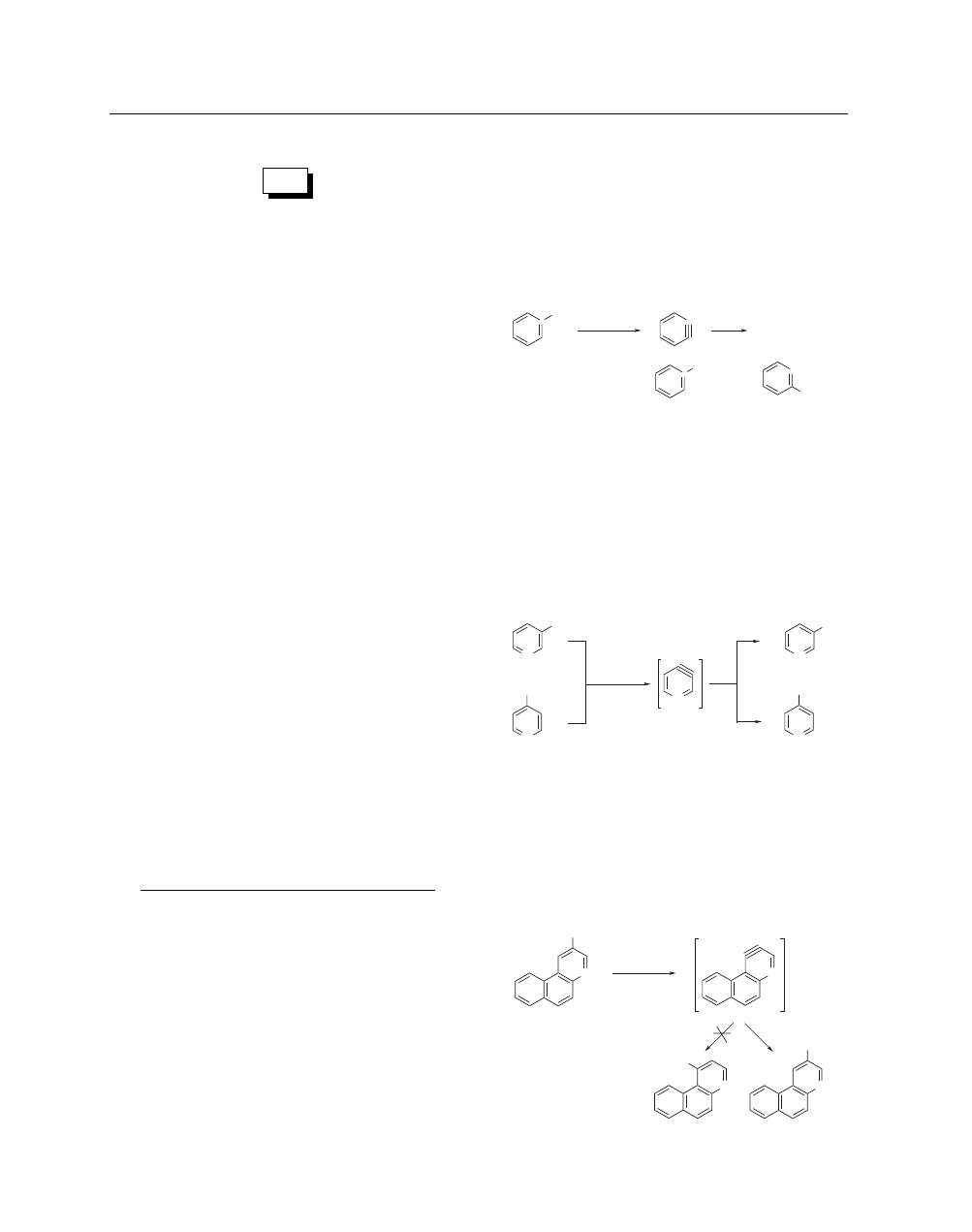
Aryne Formation. Potassium amide in liquid ammonia has
been used extensively in the generation and trapping of benzynes
via dehydrohalogenation of phenyl halides.
7
One of the definitive
experiments providing evidence for the existence of benzyne
involved the treatment of [1-
14
C]chlorobenzene with potassium
amide in liquid ammonia to provide equal amounts of [1-
14
C]-
aniline
and
[2-
14
C]aniline
(eq
1).
8
The
potassium
amide–ammonia system has since been used for the prepa-
ration of various substituted anilines.
9
•
Cl
•
•
NH
2
KNH
2
, NH
3
•
NH
3
NH
2
+
(1)
43%
Potassium amide has also been utilized extensively for the
generation and trapping of various six-membered hetarynes in-
cluding those generated from halogenated pyridines, diazines, iso-
quinolines, naphthyridines, and certain multicyclic systems.
10
Of
the various hetarynes, the evidence supporting the existence of
3,4-pyridyne is recognized as the most convincing. Treatment of
either 3- or 4-chloropyridine with potassium amide in liquid am-
monia provides a constant ratio of the isomeric amine products
(eq 2).
11
N
Cl
N
N
NH
2
N
or
70%
N
+
Cl
(2)
1/3
NH
2
KNH
2
, NH
3
2/3
Although mixtures of amines are usually formed in potassium
amide-induced aryne formations, in some cases selectivity for one
isomer can be achieved. For example, it was found that treatment
of a tricyclic bromobenzo[f]quinoline with potassium amide in
liquid ammonia provided only one product via its postulated het-
aryne intermediate (eq 3).
12
Steric interference by the angular ring
is presumed to block formation of the other isomer.
(3)
N
Br
N
N
H
2
N
N
NH
2
KNH
2
, NH
3
Avoid Skin Contact with All Reagents
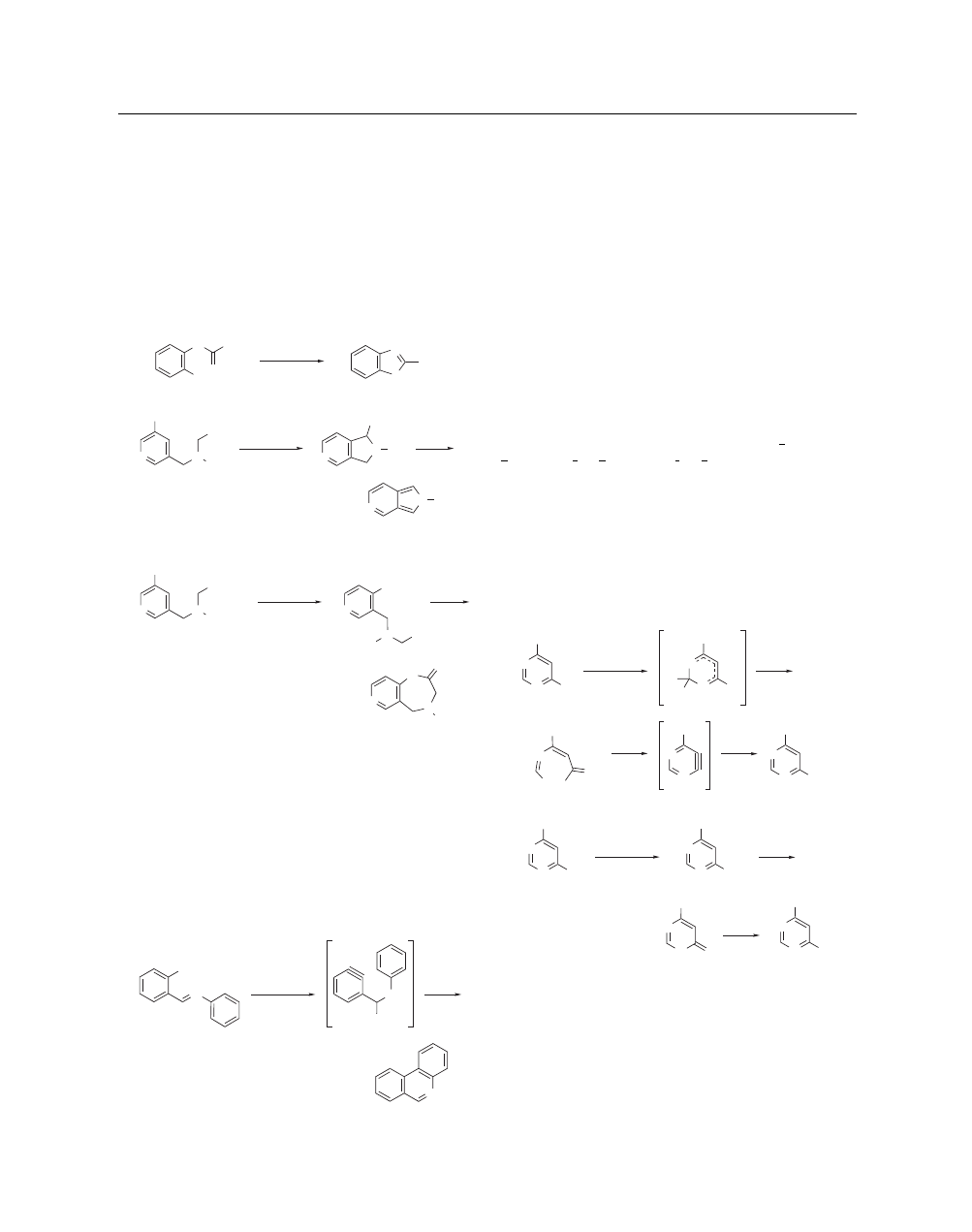
2
POTASSIUM AMIDE
Even in the presence of potassium amide, an intramolecular
nucleophile can often compete effectively with amide ion to trap
an aryne intermediate, resulting in ring closure. For example, 2-
phenylbenzothiazole has been prepared in 90% yield utilizing
this strategy (eq 4).
13
Intramolecular cyclization onto potassium
amide-generated benzyne derivatives has been achieved with car-
bon, nitrogen, oxygen, and sulfur nucleophiles.
14
The strategy has
also been successfully used with certain hetarynes, particularly 5-
substituted 3,4-pyridynes (eq 5).
15
In some cases, however, when
potassium amide is used as the aryne-generating base, competitive
amination can occur to a significant extent (eq 6).
(4)
Br
S
H
N
Ph
S
N
Ph
KNH
2
, NH
3
90%
N
N Me
N
N
CN
Me
Br
CN
N
N Me (5)
KNH
2
, NH
3
69%
N
N
N
CO
2
Et
Me
Br
NH
2
N
Me
CO
2
Et
KNH
2
, NH
3
N
N
H
N
O
Me
(6)
15%
Potassium amide-generated arynes can also be trapped intra-
molecularly by a sufficiently nucleophilic phenyl ring. For exam-
ple, appendage of a negatively charged atom to an aromatic ring
can confer sufficient nucleophilicity to the ortho and para po-
sitions for this type of reaction.
16
An example of this strategy is
shown in the synthesis of phenanthridines from haloanils (eq 7).
17
In this case, potassium amide is used not only to generate the req-
uisite benzyne but also to activate the system to ring closure via
addition of amide ion to the azomethine linkage. For the reaction
(7)
Cl
N
N
–
NH
2
N
KNH
2
, NH
3
>90%
to succeed, the nucleophilic addition of the amide ion must be very
fast relative to the formation of the benzyne. This phenanthridine
synthesis tolerates a variety of substituents in the aniline ring, with
the exception of hydroxy and nitro groups which are thought to
slow the amide addition to the azomethine group significantly.
18
Rearrangement of Heterocyclic Systems.
The generation
of pyridyne utilizing potassium amide prompted the investiga-
tion of the reaction of potassium amide with other heterocyclic
azine systems in a search for other hetarynes. However, the halo-
genated precursors to hetarynes are often more reactive toward
nonaryne reactions and in some cases alternative mechanisms for
amination are involved. Although it was initially believed that 4-
bromo-6-phenylpyrimidine reacted with potassium amide via a
6-substituted 4,5-didehydro intermediate, extensive examinations
of the reaction of potassium amide with halopyrimidines led to
the elucidation of another mechanism for nucleophilic substitu-
tion which proceeds through a ring opened intermediate (eq 8).
19
This mechanism is referred to as S
N
(ANRORC) for addition of the
nucleophile, ring opening, and ring closure. The evidence accu-
mulated to support its existence has been reviewed.
20
In particular,
labeling studies have been used to demonstrate that the amide an-
ion nitrogen becomes incorporated into the ring system (eq 9).
This mechanism has been shown to be operative (to a greater or
lesser extent) in the reaction of potassium amide with a variety
of halogen-substituted heterocyclic systems in addition to pyrim-
idines, including quinazolines, triazines, and purines. A review
of the extensive literature in the area, categorized by ring type, is
available.
21
N
N
Ph
Br
N
N
Ph
Br
H
H
2
N
N
N
Ph
NH
2
N
N
Ph
N
H
2
N Br
Ph
NH
KNH
2
, NH
3
–
(8)
*N
N
*
Ph
Br
*N
N
Ph
NH
2
KNH
2
, NH
3
*N
N
Ph
Br
*N
N
H
Ph
O
*
(9)
Under the conditions of potassium amide in liquid ammonia,
many other examples of skeletal rearrangements of heterocyclic
systems are known.
22
For example, ring contractions have been
documented, such as that of 2-chloropyrazine to 2-cyanoimidazole
(eq 10).
23
In this system, as in many cases, multiple rearrangement
pathways lead to multiple products. The details of these potassium
amide-induced heterocyclic ring transformation studies are avail-
able in a monograph.
22a
A list of General Abbreviations appears on the front Endpapers
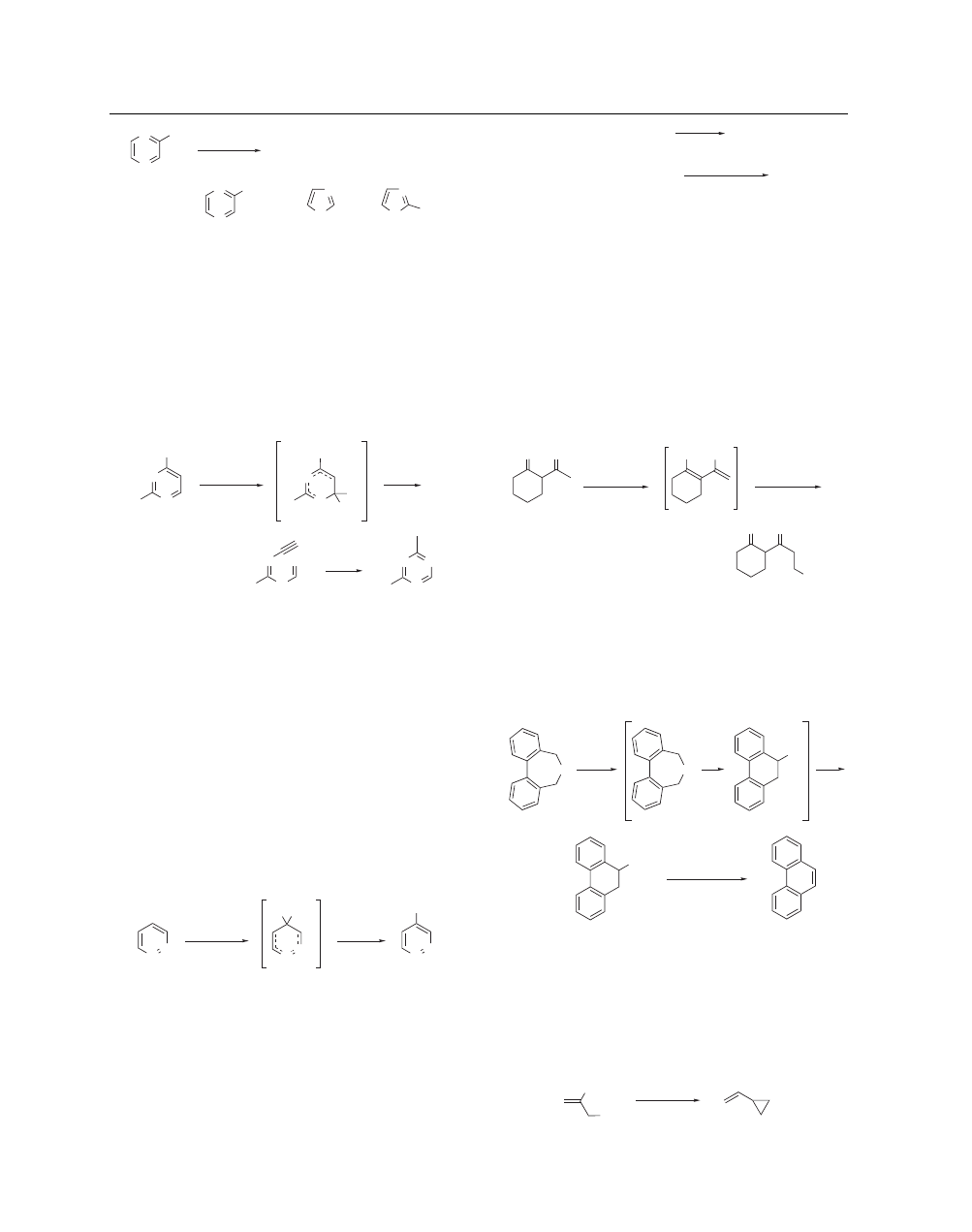
POTASSIUM AMIDE
3
N
N
Cl
N
N
NH
2
N
N
H
N
N
H
KNH
2
, NH
3
+
CN
(10)
+
13–15%
13–15%
35%
–65 °C
A particularly useful transformation is the rearrangement of
2-substituted 4-halopyrimidines into s-triazines via a ring-opened
intermediate (eq 11).
24
This reaction allows for the preparation
of unsymmetrically substituted s-triazines which are difficult to
obtain by other methods. Competing formation of the 4-amino-
pyrimidine is minimized by utilizing the chloro derivatives
(X = Cl).
N
N
KNH
2
, NH
3
R
1
X
N
N
R
1
X
NH
2
H
N
N
H
R
1
NH
N
N
N
R
1
(11)
R
1
= alkyl, aryl
R
1
= R
2
2
N
–
<40%
80–90%
Direct Amination of Aromatic Rings. Potassium amide can
be used to induce a direct nucleophilic substitution of amide an-
ion for hydrogen in certain azines.
25
Potassium amide in liquid
ammonia adds readily to pyrazine, pyrimidine, and pyridazine to
give anionic σ-adducts which can be oxidized by Potassium Per-
manganate to give the corresponding aminoaza heterocycle in
good yield (eq 12).
26
More highly electron deficient systems, like
pteridines and nitroaza aromatics, are able to add ammonia itself
to give neutral σ-adducts which are also oxidized by KMnO
4
to
heteroarylamines.
27
This modified Chichibabin reaction has been
reviewed elsewhere.
28
KNH
2
, NH
3
N
N
N
N
KMnO
4
–
NH
2
H
N
N
NH
2
(12)
91%
Potassium amide can also displace a suitably situated halide
on a heteroaromatic ring to provide the aromatic amine, but this
reaction is often accompanied by other products from competing
rearrangement pathways (eq 10). Simple phenols can be converted
to the corresponding aniline in a two-step process involving treat-
ment of the aryl diethyl phosphate ester with potassium amide and
potassium metal in liquid ammonia (eq 13).
29
KNH
2
, K in NH
3
(13)
ArOH
+
NaOH
+
(EtO)
2
POCl
80–90%
ArOPO(OEt)
2
ArNH
2
56–78%
Anion Generation. Potassium amide is a strong base which
can be used in simple deprotonation reactions for the generation
of various anions. In enolate chemistry, potassium amide in liquid
ammonia has been used to generate the dianions of β-diketones
and β-ketoaldehydes.
30
These species can then be regioselectively
alkylated at the γ-position. In unsymmetrical β-diketones the sec-
ond deprotonation occurs at the less substituted γ-position (eq 14).
The scope of this reaction, including a tabular survey of known ex-
amples, has been carefully reviewed.
30
Potassium amide in liquid
ammonia has also been used for the preparation of 1,3-dinitro-2-
keto derivatives from the reaction of cycloalkanones with alkyl
nitrates.
31
O
O
OK
OK
O
O
Ph
KNH
2
, NH
3
1. PhCH
2
Cl
2. H
3
O
+
(14)
58%
Deprotonation of benzyl ethers by potassium amide in liquid
ammonia has been used to effect the Wittig rearrangement.
32
In
a preparation of phenanthrene, use of potassium amide accom-
plished the rearrangement to the carbinol in 90% yield after 1 h,
whereas Phenyllithium required 1 week (eq 15).
33
(15)
O
O
O
–
KNH
2
NH
3
–
OH
90%
MeCOCl, reflux
1 h
85%
Examples of large-scale preparations utilizing potassium amide
in liquid ammonia for the benzylic deprotonation of lutidine and
diphenylacetonitrile have been published.
3,34
Allylic deprotona-
tion of 2-chloromethyl-1-butene with potassium amide in THF
at 65
◦
C has been used in an effective preparation of vinylcyclo-
propane (eq 16).
35
(16)
Et
Cl
KNH
2
, THF
65 °C
ca. 60%
Avoid Skin Contact with All Reagents
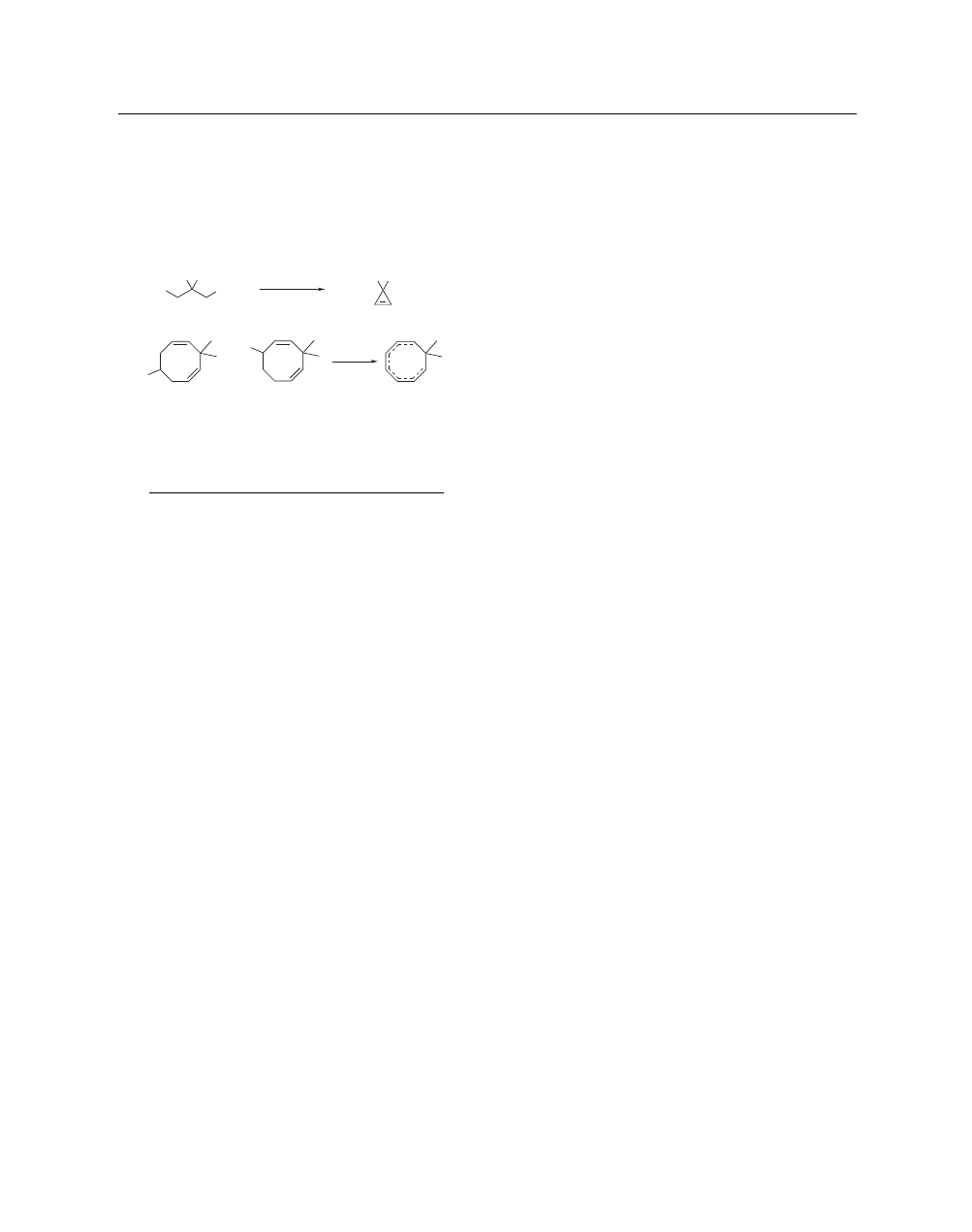
4
POTASSIUM AMIDE
Elimination Reactions.
Potassium amide in liquid ammo-
nia can be used as a base to induce elimination reactions.
36
For
example, this reagent has been used in a preparation of dimethoxy-
cyclopropene via an intramolecular alkylation followed by elimi-
nation (eq 17).
37
Potassium amide-induced elimination followed
by an additional deprotonation has also been used to generate
8,8-dimethylcyclooctatrienyl anion (eq 18).
38
(17)
OMe
MeO
Br
Cl
OMe
MeO
KNH
2
, NH
3
40–50%
(18)
Cl
Cl
–
+
KNH
2
NH
3
Related Reagents.
Lithium Amide; Lithium Diisopropyl-
amide; Potassium 3-Aminopropylamide; Potassium Hexamethyl-
disilazide; Potassium Diisopropylamide; Sodium Amide.
1.
Biehl, E. R.; Stewart, W.; Marks, A.; Reeves, P. C., J. Org. Chem. 1979,
44
, 3674.
2.
Buncel, E.; Menon, B., J. Organomet. Chem. 1977, 141, 1.
3.
Hauser, C. R.; Dunnavant, W. R., Org. Synth., Coll. Vol. 1963, 4, 962.
4.
Fieser & Fieser 1967
, 1, 907.
5.
Bunnett, J. F.; Hrutfiord, B. F.; Williamson, S. M., Org. Synth., Coll. Vol.
1973, 5, 12.
6.
Sanders, D. R., Chem. Eng. News 1986, 64 (21), 2.
7.
Hoffmann, R. W., Dehydrobenzene and Cycloalkynes; Academic: New
York, 1967; Chapter 1 and references therein.
8.
Roberts, J. D.; Simmons, H. E., Jr.; Carlsmith, L. A.; Vaughan, C. W., J.
Am. Chem. Soc. 1953
, 75, 3290.
9.
Hoffmann, R. W. Dehydrobenzene and Cycloalkynes; Academic: New
York, 1967; pp 115.
10.
Reinecke, M. G., Tetrahedron 1982, 38, 427.
11.
Pieterse, M. J.; den Hertog, H. J., Recl. Trav. Chim. Pays-Bas 1961, 80,
1376.
12.
Reinecke, M. G., Tetrahedron 1982, 38, 485.
13.
Hrutford, B. F.; Bunnett, J. F., J. Am. Chem. Soc. 1958, 80, 2021.
14.
Hoffmann, R. W., Dehydrobenzene and Cycloalkynes; Academic: New
York, 1967; pp 150.
15.
Ahmed, I.; Cheeseman, G. W. H.; Jaques, B., Tetrahedron 1979, 35,
1145.
16.
Kessar, S. V., Acc. Chem. Res. 1978, 11, 283.
17.
Kessar, S. V.; Gopal, R.; Singh, M., Tetrahedron 1973, 29, 167.
18.
Kessar, S. V.; Pal, D.; Singh, M., Tetrahedron 1973, 29, 177.
19.
de Valk, J.; van der Plas, H. C., Recl. Trav. Chim. Pays-Bas 1971, 90,
1239.
20.
van der Plas, H. C., Acc. Chem. Res. 1978, 11, 462.
21.
van der Plas, H. C., Tetrahedron 1985, 41, 237.
22.
(a)For examples see: van der Plas, H. C. Ring Transformations of
Heterocycles
; Academic: New York, 1973; 2. (b) Rykowski, A.; van
der Plas, H. C., J. Org. Chem. 1987, 52, 71. (c) Nagel, A.; van der Plas,
H. C.; Geurtsen, G.; van der, A., J. Heterocycl. Chem. 1979, 16, 305.
23.
Lont, P. J.; van der Plas, H. C.; Koudijs, A., Recl. Trav. Chim. Pays-Bas
1971, 90, 207.
24.
van der Plas, H. C. Ring Transformations of Heterocycles; Academic:
New York, 1973; Vol. 2, p 135.
25.
The general area of nucleophilic substitution (including KNH
2
) of
hydrogen in azines has been reviewed: Chupakhin, O. N.; Charushin,
V. N.; van der Plas, H. C., Tetrahedron 1988, 44, 1.
26.
Hara, H.; van der Plas, H. C., J. Heterocycl. Chem. 1982, 19, 1285.
27.
(a) Hara, H.; van der Plas, H. C., J. Heterocycl. Chem. 1982, 19, 1527.
(b) Wozniak, M.; van der Plas, H. C.; van Veldhuizen, B., J. Heterocycl.
Chem. 1983
, 20, 9.
28.
van der Plas, H. C.; Wozniak, M., Croat. Chem. Acta 1986, 59, 33.
29.
Rossi, R. A.; Bunnett, J. F., J. Org. Chem. 1972, 37, 3570.
30.
Harris, T. M.; Harris, C. M., Org. React. 1969, 17, 155.
31.
Feuer, H.; Hall, A. M.; Golden, S.; Reitz, R. L., J. Org. Chem. 1968, 33,
3622.
32.
Hauser, C. R.; Kantor, S. W., J. Am. Chem. Soc. 1951, 73, 1437.
33.
Weinheimer, A. J.; Kantor, S. W.; Hauser, C. R., J. Org. Chem. 1953, 18,
801.
34.
Kofron, W. G.; Baclawski, L. M., Org. Synth., Coll. Vol. 1988, 6, 611.
35.
Arora, S.; Binger, P.; Köster, R., Synthesis 1973, 146.
36.
Hauser, C. R.; Skell, P. S.; Bright, R. D.; Renfrow, W. B., J. Am. Chem.
Soc. 1947
, 69, 589.
37.
Baucom, K. B.; Butler, G. B., J. Org. Chem. 1972, 37, 1730.
38.
Staley, S. W.; Pearl, N. J., J. Am. Chem. Soc. 1973, 95, 2731.
Katherine S. Takaki
Bristol-Myers Squibb Company Wallingford, CT, USA
A list of General Abbreviations appears on the front Endpapers
Wyszukiwarka
Podobne podstrony:
potassium permanganate eros rp244
potassium carbonate eros rp205
potassium bromate eros rp197
potassium ferrate eros rp212
potassium permanganate eros rp244
sodium amide eros rs041
potassium hydroxide 18 crown 6 eros rp230
potassium carbonate 18 crown 6 eros rp206
potassium on alumina eros rp192
potassium permanganate copper II sulfate eros rp245
potassium hydroxide dimethyl sulfoxide eros rp231
więcej podobnych podstron