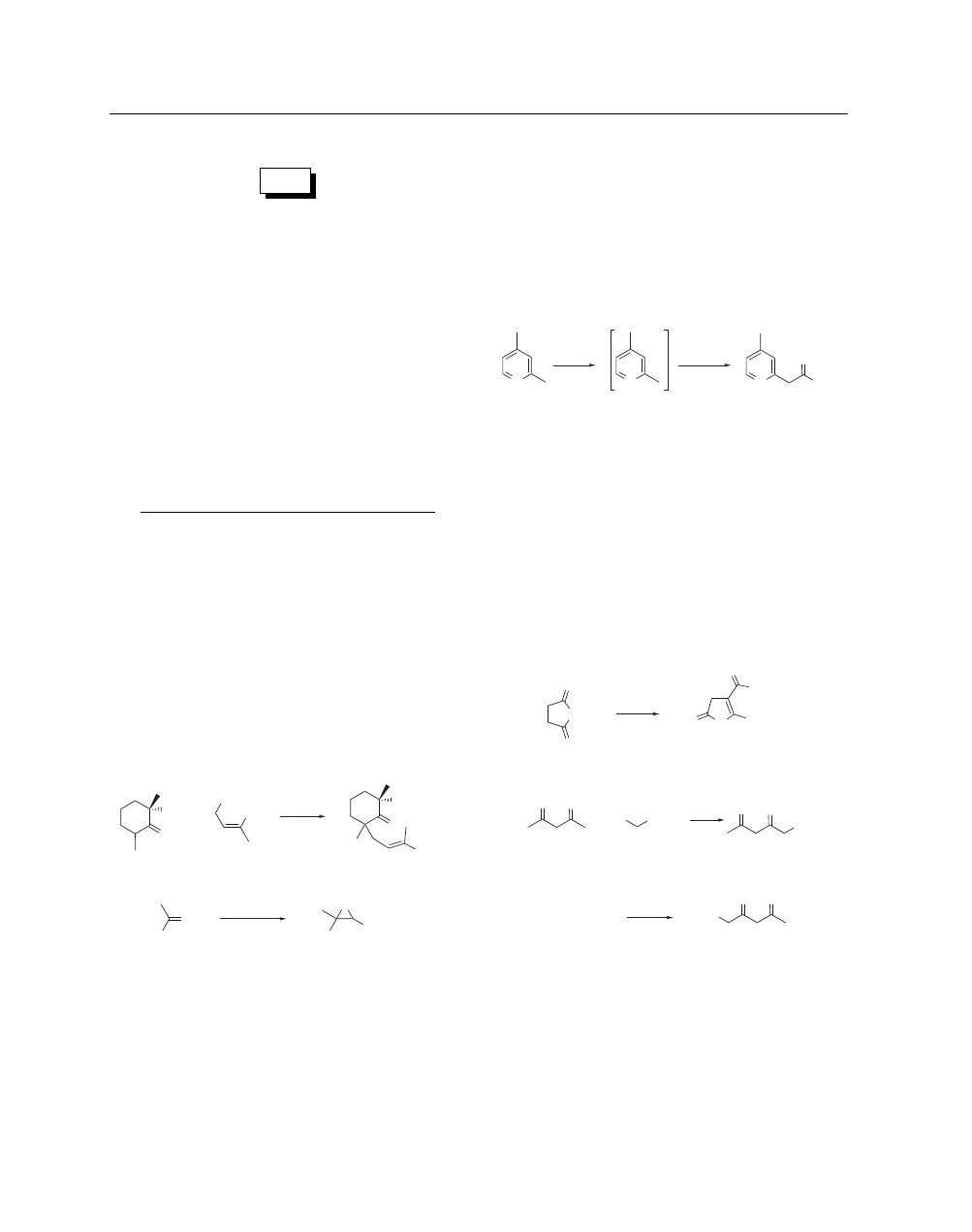
SODIUM AMIDE
1
Sodium Amide
NaNH
2
[7782-92-5]
H
2
NNa
(MW 39.02)
InChI = 1/H2N.Na/h1H2;/q-1;+1
InChIKey = ODZPKZBBUMBTMG-UHFFFAOYAF
(strong base;
3
strong nucleophile
38
)
Alternate Name:
sodamide.
Physical Data:
mp 210
◦
C; bp 400
◦
C/760 mmHg.
Solubility:
sol liq ammonia (∼1 mol L
−1
at −33
◦
C).
1
Form Supplied in:
commercially available as a powder; easily
prepared in the laboratory.
Preparative Methods:
combination of Ammonia, small quan-
tities of an iron(III) salt, and Sodium leads to formation of a
black catalyst, whereupon the remainder of the sodium is added.
Published procedures differ in details.
2
Handling, Storage, and Precautions:
flammable; corrosive; when
opened to air, decomposes and forms a potentially explosive
yellow byproduct.
1
Reaction as a Base. Sodamide often serves as a base to gen-
erate reactive anions.
3
In DMSO in the presence of various bases,
including sodamide, carbohydrates are benzylated in good yield
with Benzyl Chloride.
3a
Reaction of (1) and (2) in the presence
3b
of sodamide gives (3) (eq 1). Sodamide is effective in generat-
ing the acetonitrile anion for reaction with sulfines.
4
Deprotona-
tion of phenylacetic esters in the presence of sodamide allows
aldol reaction with benzaldehyde derivatives to afford 2,3-diaryl-
3-hydroxypropionic acids.
5
Similarly, reaction of acetophenone
and ethyl chloroacetate (eq 2) gives the Darzens’ product (4).
6
Treatment of primary anilines and cyanopyridines with sodamide
leads to good yields of carboxamidines.
7
Oxygenation of hindered
4-alkylphenols in the presence of sodamide provides a convenient
source of quinols.
8
Ph
O
Cl
Cl
Ph
O
Cl
+
(1)
(1)
(2)
(3)
69%
NaNH
2
O
Ph
O
CO
2
Et
Ph
(2)
(4)
NaNH
2
62–64%
ClCH
2
CO
2
Et
Sodamide in THF with boric acid neutralization has proven
effective for the deconjugation of conjugated unsaturated
steroids.
9
The presence of sodamide in liquid ammonia at low
temperature facilitates interconversion of 1,4- and 1,3-cyclo-
hexadienes.
10
Deprotonation of 2-bromothiophenes and 2-
halothianaphthalenes affords the 3-halo isomers via a series of
complex equilibria.
11
Cyclopropenes, which possess an acidity
comparable to alkynes, are rapidly metalated by sodamide (and
other alkali amides) to produce reactive intermediates for alkyl-
ation.
12
Selective deprotonation occurs with a wide variety of
acidic methyl, methylene, and methine hydrogens adjacent to car-
bonyls or attached to heterocycles. For example, 2,4-lutidine (5)
undergoes deprotonation (eq 3) to (6) followed by reaction with
ethyl benzoate to yield (7).
13a
Deprotonation followed by reac-
tion with electrophiles is a powerful method for generating com-
plex carbon skeletons.
13
Examination of the role of bases, includ-
ing sodamide, on the stereochemistry (including isomerization)
of products formed in the Michael reaction has been reported.
14
In the racemization of the single stereogenic center in nicotine,
sodamide was inferior to Potassium tert-Butoxide.
15
N
NaNH
2
N
N
Ph
O
–
(6)
(3)
(7)
(5)
85%
PhCO
2
Et
Dianion Generation.
Numerous early investigations into
dianion chemistry.
16
employed sodamide as the base. Conversion
of the simple heterocycle (8) into the corresponding dianion with
sodamide in liquid ammonia followed by reaction with benzoni-
trile (eq 4) led to an interesting rearrangement product (9).
17
β
-Dicarbonyl dianions are routinely prepared by reaction with
sodamide. These strongly nucleophilic species undergo regios-
elective alkylation (eq 5) by reaction of disodioacetylacetone
(10) (much more soluble in liquid ammonia than its dipotassium
counterpart
16a
) with 11-bromoundecanoic acid to give (11)
18
and
reaction of (10) (eq 6) with diphenyliodonium chloride to yield
(12).
19
NH
O
O
PhCN
N
H
O
NH
2
O
Ph
(4)
(8)
(9)
NaNH
2
58%
O
O
Br
CO
2
Li
Na
+
Na
+
O
O
CO
2
H
–
–
+
(5)
(10)
(11)
( )
11
( )
10
82%
H
+
(6)
O
O
Ph
(10)
+
Ph
2
ICl
H
+
(12)
60–64%
Elimination Reactions. Sodamide’s utility as a reagent for
elimination reactions is illustrated by the following selected ex-
amples. Methiodide (13) undergoes facile loss of HI and diethyl-
methylamine to generate methyl vinyl ketone.
20
Five isomeric
alkenes and a cyclopropane result from treatment of 2-benzyl-3-
phenylpropyltrimethylammonium iodide with sodamide.
21
Upon
reaction with sodamide, various thioamides eliminate hydrogen
sulfide to form ynamines in fair yield.
22
In the presence of
sodamide, cis-1,4-dichloro-2-butene (14) yields mainly trans-
Avoid Skin Contact with All Reagents
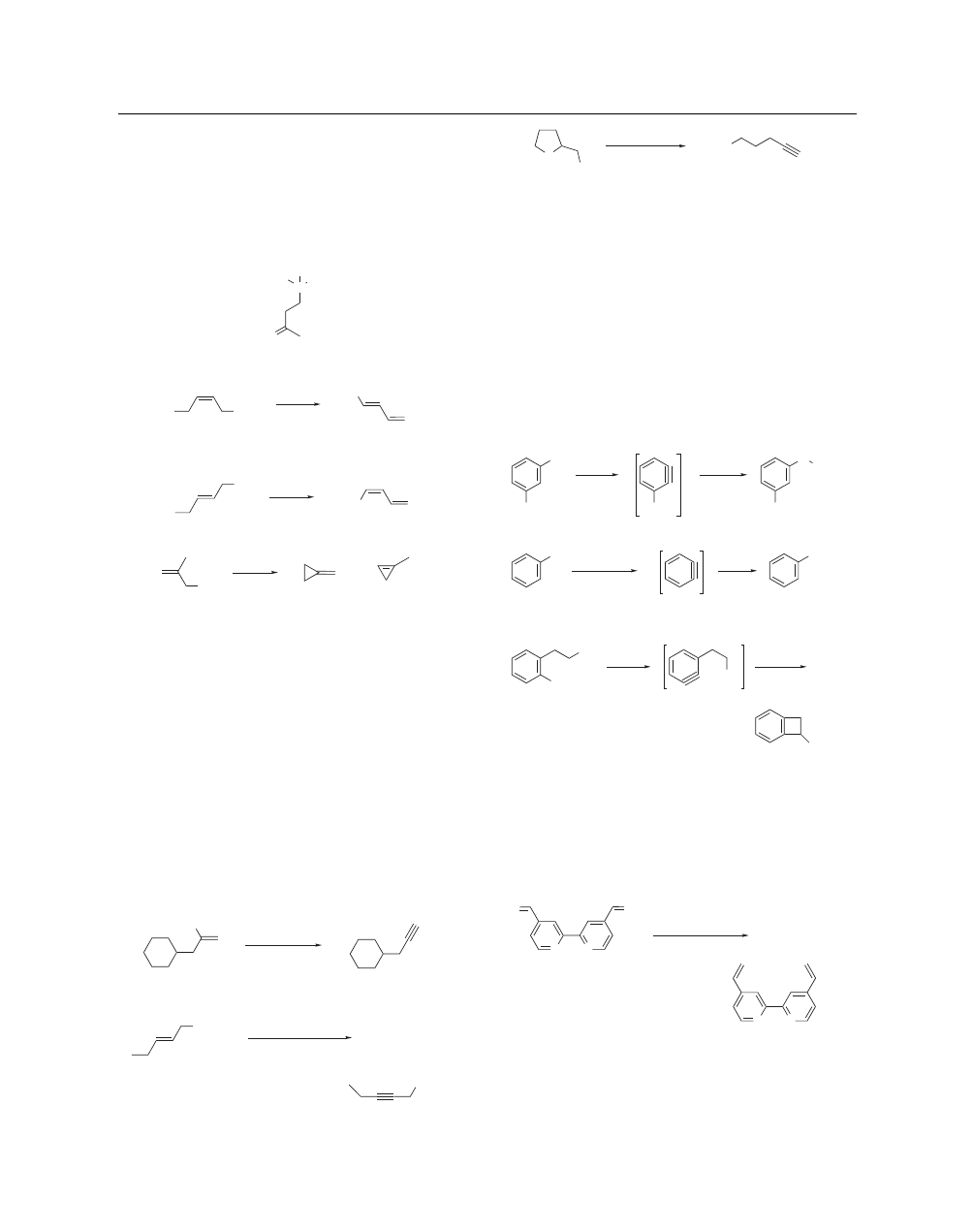
2
SODIUM AMIDE
1-chloro-1,3-butadiene (15) (eq 7) while trans-1,4-dichloro-2-
butene gives a preponderence of cis-1-chloro-1,3-butadiene (16)
(eq 8).
23
Upon warming a mixture of methallyl chloride (17) and
sodamide (eq 9), there is formed methylenecyclopropane (18) and
1-methylcyclopropene (19)
24
Sodamide, Sodium Hydride, and
Sodium Methoxide all have utility in the Bamford–Stevens reac-
tion for the conversion of tosylhydrazones into alkenes.
25
(13)
O
N
Me
Et
Et
+
I
–
(7)
Cl
Cl
Cl
(14)
(15)
52%
NaNH
2
(8)
(16)
Cl
Cl
Cl
72%
NaNH
2
(9)
(19)
Cl
+
(18)
(17)
72%
NaNH
2
Preparation of Alkynes. Sodamide-mediated elimination of
one or two moles of HX from a suitable substrate is a classical
method for the synthesis of alkynes. For example, β-bromostyrene
with sodamide in liquid ammonia provides an excellent source of
phenylacetylene.
26
Cyclohexylpropyne (21) can be generated by
reaction (eq 10) of vinyl bromide (20) with 3 equiv (excess) of
sodamide.
27
Oleic acid (22) can be transformed into stearolic acid
(23) by a straightforward sequence (eq 11) involving bromination
followed by reaction with excess sodamide.
28
Similar method-
ology has been employed to synthesize many other alkynes.
29
Dehydrohalogenation with concomitant ether cleavage provides
an efficient route to complex alkynes. For example, reaction of
(24) with sodamide (eq 12) provides the hydroxylic terminal pen-
tyne (25)
29j
Alkyne–allene isomerization has been accomplished
with sodamide.
30
(10)
3 equiv NaNH
2
(21)
(20)
Br
66%
( )
7
( )
7
( )
7
1. Br
2
CO
2
H
(23)
(11)
(22)
CO
2
H
( )
7
2. 3 equiv NaNH
2
, H
+
42–52%
(12)
(25)
(24)
O
Cl
HO
3.5 equiv NaNH
2
NH
3
, NH
4
Cl
75–85%
Aryne Chemistry. Among the many existing methods for the
generation of arynes,.
31
reaction of a halobenzene derivative with
sodamide (as in the example (eq 13) of (26) going to (27)
32a
) is
a commonly employed procedure.
32
The highly reactive interme-
diate arynes can be made to undergo reaction with nucleophiles
other than amide anion. Thus bromobenzene (28) is converted
(eq 14) into aryl sulfide (29).
33a
Sodamide-generated arynes have
also been reacted with more complex species,
34
as illustrated by
the transformation (eq 15) of (30) into (31) followed by cycliza-
tion to (32)
34a
Intramolecular benzyne reactions involving so-
damide have been used successfully in the synthesis of aporphine
alkaloids.
35
Br
OMe
NaNH
2
OMe
H
N
OMe
R
(26)
(13)
(27)
68–85%
RNH
2
Br
NaNH
2
SEt
(28)
(14)
(29)
52%
HMPA–THF
EtSH
(15)
(30)
(31)
Cl
CN
CN
CN
–
64–66%
(32)
NaNH
2
Generation of Ylides. Sodamide is a common base for the
generation of ylides in the Wittig reaction.
36
The commercially
available instant ylide consists
37a
of a 1:1 stoichiometric mixture
of Methyltriphenylphosphonium Bromide and sodium amide
(eq 16).
37b
N
N
O
O
N
N
(16)
THF
54%
PPh
3
MeBr•NaNH
2
Reaction as a Nucleophile. Nucleophilic addition reactions
are a major feature of sodamide chemistry. Addition followed
by intramolecular attack provides a convenient methodology
for the construction of unusual adducts.
38
Sodamide, sodamide/
A list of General Abbreviations appears on the front Endpapers
SODIUM AMIDE
3
potassamide mixtures, and other alkali metal amides have been
found to catalyze the amination of alkenes.
39
The Chichibabin
reaction and its variants
40
provide a useful route to numerous
substituted heterocycles. The addition–elimination reaction of so-
damide on a heterocyclic substrate is nicely illustrated by the
transformation (eq 17) of (33) into 6-methylisocytosine (34)
41
Nucleophilic addition reactions to nitro-substituted aromatic sub-
strates have been observed.
42
Also intriguing are the various reac-
tion pathways observed for heterocycles containing an appended
trifluoromethyl group.
43
Photochemically assisted additions of
sodamide have been reported (eq 18).
44
Sodamide is also an
effective reagent for accomplishing N-dealkylations (eq 19)
45a
and N-deacylations.
45b
HN
N
O
MeO
HN
N
O
H
2
N
(17)
(33)
(34)
52%
NaNH
2
(18)
H
2
N
hν
, NaNH
2
NH
3
, Et
2
O
57%
N
N
O
Et
HN
N
O
(19)
NH
3
97%
NaNH
2
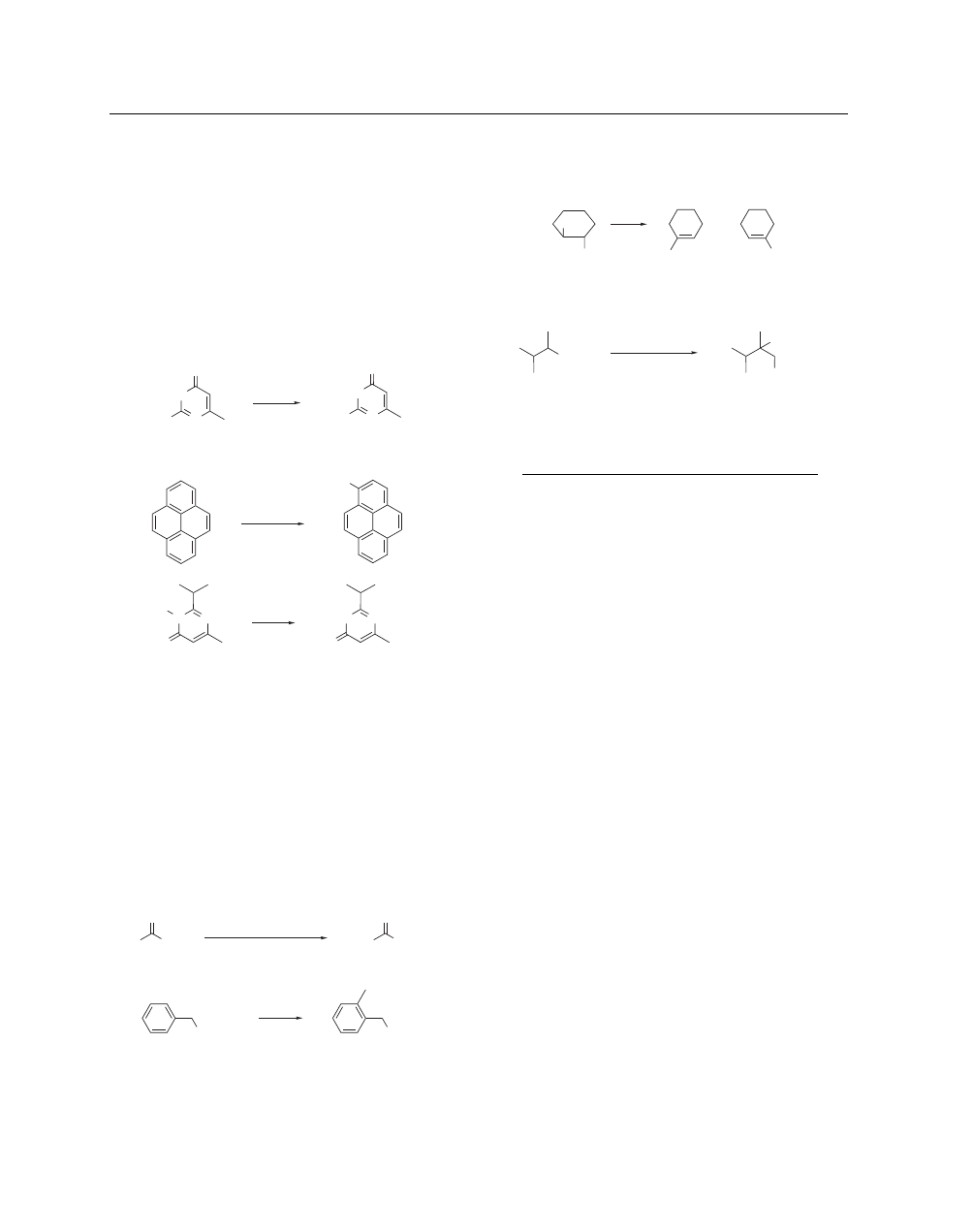
Cleavage and Rearrangement.
Sodamide is involved in
many cleavage and rearrangement reactions. Cleavage re-
actions,
46
with specific reference to the Haller–Bauer reaction,
47
exemplified by (35) going to (36) (eq 20),
47d
are a convenient
synthetic transform. It is significant that the addition of 1,4-
Diazabicyclo[2.2.2]octane (DABCO) permits the Haller–Bauer
reaction to be performed with commercial sodamide.
47d
Rear-
rangement reactions involving sodamide are well-known,
48
with
several being common name reactions such as the Truce–Stiles,
49
the Sommelet–Hauser,
50a
and the Stevens
50a
reactions. A typi-
cal Sommelet–Hauser rearrangement is illustrated by (37) going
to (38) (eq 21).
50b
Vinylpyridines undergo polymerization in
sodamide/liquid ammonia.
51
(20)
O
Ph
Ph
O
Ph
NH
2
3 equiv NaNH
2
(35)
(36)
3 equiv DABCO, PhH, heat
73%
NMe
3
I
–
NMe
2
+
(37)
(38)
(21)
NH
3
97%
NaNH
2
In recent years, sodamide has been combined with other
bases (especially with alkali metal t-butoxides) to create a whole
family of so-called complex bases with exceptional properties (see
Sodium Amide–Sodium tert-Butoxide).
52
Typical applications of
these bases are in the syn elimination depicted
52d
by (39) going to
(40) and (41) (eq 22) and the carbanion alkylation involving the
conversion of (42) to (43) (eq 23).
52f
(22)
Br
Cl
Br
Cl
+
(40)
(41)
(39)
65:35
3:97
NaNH
2
, t-BuONa, 87%
NaNH
2
, t-BuONa, 15-crown-5, 76%
(23)
(42)
(43)
CHO
CHO
Ph
NaNH
2
, t-BuONa
PhCH
2
Br
84%
Related Reagents. Lithium Amide; Potassium Amide; Potas-
sium t-Butoxide; Sodium Amide–Sodium t-Butoxide; Sodium–
Ammonia; Sodium Hydride.
1.
Fieser & Fieser 1967
, 1, 1034.
2.
(a) Vaughn, T. H.; Vogt, R. R.; Nieuwland, J. A., J. Am. Chem. Soc. 1934,
56
, 2120. (b) Hauser, C. R.; Adams, J. T.; Levine, R., Org. Synth., Coll.
Vol. 1955
, 3, 291. (c) Hauser, C. R.; Dunnavant, W. R., Org. Synth. 1960,
40
, 38. (d) Jones, E. R. H.; Eglinton, G.; Whiting, M. C.; Shaw, B. L
Org. Synth., Coll. Vol. 1963
, 4, 404. (e) Khan, N. A.; Deatherage, F. E.;
Brown, J. B., Org. Synth., Coll. Vol. 1963, 4, 851. (f) Greenlee, K. W.;
Henne, A. L., Inorg. Synth. 1946, 2, 128.
3.
(a) Iwashige, T.; Saeki, H., Chem. Pharm. Bull. 1967, 15, 1803.
(b) Ireland, R. E.; Kierstead, R. C., J. Org. Chem. 1966, 31, 2543.
4.
Loontjes, J. A.; van der Leij, M.; Zwanenberg, B., Recl. Trav. Chim.
Pays-Bas 1980
, 99, 39.
5.
Kratchanov, C. G.; Kirtchev, N. A., Synthesis 1971, 317.
6.
Allen, C. F. H.; VanAllan, J., Org. Synth., Coll. Vol. 1955, 3, 727.
7.
Hisano, T.; Tasaki, M.; Tsumoto, K.; Matsuoka, T.; Ichikawa, M., Chem.
Pharm. Bull. 1983
, 31, 2484.
8.
Nishinaga, A.; Itahara, T.; Matsuura, T., Bull. Chem. Soc. Jpn. 1975, 48,
1683.
9.
Shapiro, E. L.; Leggatt, T.; Weber, L.; Olivetto, E. P.; Tanabe, M.; Crowe,
D. F., Steroids 1964, 3, 183.
10.
Rabideau, P. W.; Huser, D. L., J. Org. Chem. 1983, 48, 4266.
11.
(a) Reinecke, M. G.; Hollingworth, T. A., J. Org. Chem. 1972, 37, 4257.
(b) Brandsma, L.; de Jong, R. L. P., Synth. Commun. 1990, 20, 1697.
12.
(a) Schipperijn, A. J.; Smael, P., Recl. Trav. Chim. Pays-Bas 1973, 92,
1121. (b) Schipperijn, A. J.; Smael, P., Recl. Trav. Chim. Pays-Bas 1973,
92
, 1159.
13.
(a) Levine, R.; Dimmig, D. A.; Kadunce, W. M., J. Org. Chem. 1974,
39
, 3834. (b) Yamamoto, M.; Sugiyama, N., Bull. Chem. Soc. Jpn. 1975,
48
, 508. (c) Kaiser, E. M.; Bartling, G. J.; Thomas, W. R.; Nichols, S.
B.; Nash, D. R., J. Org. Chem. 1973, 38, 71. (d) Harris, T. M.; Harris, C.
M.; Wachter, M. P., Tetrahedron 1968, 24, 6897. (e) Vanderwerf, C. A.;
Lemmermann, L. V., Org. Synth., Coll. Vol. 1955, 3, 44. (f) Coffman, D.
D., Org. Synth., Coll. Vol. 1955, 3, 320. (g) Hauser, C. R.; Adams, J. T.;
Levine, R., Org. Synth., Coll. Vol. 1955, 3, 291. (h) Potts, K. T.; Saxton,
J. E., Org. Synth. 1960, 40, 68. (i) Kaiser, E. M.; Bartling, G. J., J. Org.
Chem. 1972
, 37, 490. (j) Rash, F. H.; Boatman, S.; Hauser, C. R., J. Org.
Chem. 1967
, 32, 372.
14.
(a) Gospodova, T. S.; Stefanovsky, Y. N., Monatsh. Chem. 1990, 121,
275. (b) Viteva, L. Z.; Stefanovsky, Y. N., Monatsh. Chem. 1982, 113,
181.
Avoid Skin Contact with All Reagents

4
SODIUM AMIDE
15.
Tsujino, Y.; Shibata, S.; Katsuyama, A.; Kisaki, T.; Kaneko, H.,
Heterocycles 1982
, 19, 2151.
16.
(a) Harris, T. M.; Harris, C. M., Org. React. 1969, 17, 155. (b) Harris, T.
M.; Harris, C. M., J. Org. Chem. 1966, 31, 1032.
17.
Kashima, C.; Yammamoto, M.; Kobayashi, S.; Sugiyama, N., Bull.
Chem. Soc. Jpn. 1974
, 47, 1805.
18.
Pendarvis, R. O.; Hampton, K. G., J. Org. Chem. 1974, 39, 2289.
19.
Hampton, K. G.; Harris, T. M.; Hauser, C. R., Org. Synth. 1971, 51, 128.
20.
(a) duFeu, E. C.; McQuillin, F. J.; Robinson, R., J. Chem. Soc. 1937, 53.
(b) Cornforth, J. W.; Robinson, R., J. Chem. Soc. 1949, 1855.
21.
Bumgardner, C. L.; Iwerks, H., J. Am. Chem. Soc. 1966, 88, 5518.
22.
Halleux, A.; Reimlinger, H.; Viehe, H. G., Tetrahedron Lett. 1970, 3141.
23.
Heasley, V. L.; Lais, B. R., J. Org. Chem. 1968, 33, 2571.
24.
(a) Fisher, F.; Applequist, D. E., J. Org. Chem. 1965, 30, 2089. (b) Salaun,
J. R.; Conia, J. M., J. Chem. Soc., Chem. Commun. 1971, 1579. (c) Koster,
R.; Arora, S.; Binger, P., Synthesis 1971, 322. (d) Arora, S.; Binger,
P.; Koster, R., Synthesis 1973, 146. (e) Fitjer, L.; Conia, J.-M., Angew.
Chem., Int. Ed. Engl. 1973
, 12, 332.
25.
Kirmse, W.; von Bullow, B.-G.; Schepp, H., Justus Liebigs Ann. Chem.
1966, 691, 41.
26.
Vaughan, T. H.; Vogt, R. R.; Nieuwland, J. A., J. Am. Chem. Soc. 1934,
56
, 2120.
27.
Lespieau, R.; Bourguel, M., Org. Synth., Coll. Vol. 1941, 1, 191.
28.
Khan, N. A.; Deatherage, F. E.; Brown, J. E., Org. Synth., Coll. Vol. 1963,
4
, 851.
29.
(a) Khan, N. A., Org. Synth., Coll. Vol. 1963, 4, 969. (b) Ashworth, P. J.;
Mansfield, G. H.; Whiting, M. C., Org. Synth., Coll. Vol. 1963, 4, 128.
(c) Messeguer, A.; Serratosa, F.; Rivera, J., Tetrahedron Lett.. 1973, 2895.
(d) Armitage, J. B.; Jones, E. R. H.; Whiting, M. C., J. Chem. Soc. 1953,
3317. (e) Bohlmann, F., Chem. Ber. 1951, 84, 545. (f) Jones, E. R. H.;
Eglinton, G.; Whiting, M. C. Shaw, B. L., Org. Synth., Coll. Vol. 1963,
4
, 404. (g) Wasserman, H. H.; Wharton, P. S., J. Am. Chem. Soc. 1960,
82
, 661. (h) Newman, M. S.; Geib, J. R.; Stalick, W. M., Org. Prep.
Proced. Int. 1972
, 4, 89. (i) Brandsma, L.; Harryvan, E.; Arens, J. F.,
Recl. Trav. Chim. Pays-Bas 1968
, 87, 1238. (j) Jones, E. R. H.; Eglinton,
G.; Whiting, M. C., Org. Synth., Coll. Vol. 1963, 4, 755.
30.
(a) Carr, M. D.; Gan, L. H.; Reid, I., J. Chem. Soc., Perkin Trans. 2 1973,
672. (b) Montijn, P. P.; Kupecz, A.; Brandsma, L.; Arens, J. F., Recl.
Trav. Chim. Pays-Bas 1969
, 88, 958.
31.
Hoffmann, R. W., Dehydrobenzene and Cycloalkynes; Academic: New
York, 1967.
32.
(a) Biehl, E. R.; Patrizi, R.; Reeves, P. C., J. Org. Chem. 1971, 36, 3252.
(b) Biehl, E. R.; Stewart, W.; Marks, A.; Reeves, P. C., J. Org. Chem.
1979, 44, 3674. (c) Levine, R.; Biehl, E. R., J. Org. Chem. 1975, 40,
1835. (d) Biehl, E. R.; Smith, S. M.; Reeves, P. C., J. Org. Chem. 1971,
36
, 1841. (e) Biehl, E. R.; Nieh, E.; Hsu, K. C., J. Org. Chem. 1969, 34,
3595. (f) Biehl, E. R.; Hsu, K. C.; Nieh, E., J. Org. Chem. 1970, 35, 2454.
(g) Kraakman, P. A.; Valk, J.-M.; Niederländer, H. A. G.; Brower, D. B.
E.; Bickelhaupt, F. M.; de Wolf, W. H.; Bickelhaupt, F.; Stam, C. H., J.
Am. Chem. Soc. 1990
, 112, 6638. (h) Apeloig, Y.; Arad, D.; Halton, B.;
Randall, C. J., J. Am. Chem. Soc. 1986, 108, 4932.
33.
(a) Caubere, P., Bull. Soc. Chem. Fr. 1967, 3446, 3451. (b) Carre, M.
C.; Ezzinadi, A. S.; Zouaoui, M. A.; Geoffroy, P.; Caubere, P., Synth.
Commun. 1989
, 19, 3323.
34.
(a) Skorcz, J. A.; Kaminski, F. E., Org. Synth. 1968, 48, 53. (b)
Carre, M.-C.; Gregoire, B.; Caubere, P., J. Org. Chem. 1984, 49, 2050.
(c) Loubinoux, B.; Caubere, P., Synthesis 1974, 201. (d) Buske, G. R.;
Ford, W. T., J. Org. Chem. 1976, 41, 1995.
35.
Kametani, T.; Fukumoto, K.; Nakano, T., J. Heterocycl. Chem. 1972, 9,
1363.
36.
(a) Moiseenkov, A. M.; Schaub, B.; Margot, C.; Schlosser, M.,
Tetrahedron Lett. 1985
, 26, 305. (b) Schaub, B.; Blaser, G.; Schlosser,
M., Tetrahedron Lett. 1985, 26, 307. (c) Schlosser, M.; Schaub, B.; de
Oliveira-Neto, J.; Jeganathan, S., Chimia 1986, 40, 244. (d) Schaub, B.;
Jeganathan, S.; Schlosser, M., Chimia 1986, 40, 246. (e) Dauphin, G.;
David, L.; Duprat, P.; Kergomard, A.; Veshambre, H., Synthesis 1973,
149. (f) Takahashi, H.; Fujiwara, K.; Ohta, M., Bull. Chem. Soc. Jpn.
1962, 35, 1498. (g) Yamamoto, Y.; Schimidbaur, H., J. Chem. Soc., Chem.
Commun. 1975
, 668. (h) Quast, H.; Jakobi, H., Chem. Ber. 1991, 124,
1619.
37.
(a) Schlosser, M.; Schaub, B., Chimia 1982, 36, 396. (b) Ciana, L.
D.; Dressick, W. J.; von Zelewsky, A., J. Heterocycl. Chem. 1990, 27,
163.
38.
(a) Barnard, I. F.; Elvidge, J. A., J. Chem. Soc., Perkin Trans. 1
1983, 1813. (b) Yamagouchi, K., Bull. Chem. Soc. Jpn. 1976, 49,
1366.
39.
Pez, G. P.; Galle, J. E., Pure Appl. Chem. 1985, 57, 1917.
40.
Vorbruggen, H., Adv. Heterocycl. Chem. 1990, 49, 117.
41.
Botta, M.; De Angelis, F.; Finizia, G.; Gambacorta, A.; Nicoletti, R.,
Synth. Commun. 1985
, 15, 27.
42.
Gandhi, S. S.; Gibson, M. S.; Kaldas, M. L.; Vines, S. M., J. Org. Chem.
1979, 44, 4705.
43.
(a) Kobayashi, Y.; Kumadaki, I.; Taguchi, S.; Hanzawa, Y., Tetrahedron
Lett. 1970
, 3901. (b) Kobayashi, Y.; Kumadaki, I.; Hanzawa, Y.; Minura,
M., Chem. Pharm. Bull. 1975, 23, 2044. (c) Kobayashi, Y.; Kumadaki,
I.; Hanzawa, Y.; Mimura, M., Chem. Pharm. Bull. 1975, 23, 636.
(d) Kobayashi, Y.; Kumudaki, I.; Taguchi, S.; Hanzawa, Y., Chem.
Pharm. Bull. 1972
, 20, 1047.
44.
Tintel, C.; Rietmeyer, F. J.; Cornelisse, J., Recl. Trav. Chim. Pays-Bas
1983, 102, 224.
45.
(a) Hirai, Y.; Egawa, H.; Yamada, S.; Yamazaki, T., Heterocycles 1983,
20
, 1243. (b) Fraenkel, G.; Cooper, J. W., J. Am. Chem. Soc. 1971, 93,
7228.
46.
(a) Furukawa, N.; Tanaka, H.; Oae, S., Bull. Chem. Soc. Jpn. 1968,
41
, 1463. (b) Shiotani, S.; Kometani, T., Chem. Pharm. Bull. 1973, 21,
1160.
47.
(a) Hamlin, K. E.; Weston, A. W., Org. React. 1957, 9, 1. (b) Alexander, E.
C.; Tom, T., Tetrahedron Lett. 1978, 1741. (c) Paquette, L. A.; Maynard,
G. D., J. Org. Chem. 1989, 54, 5054. (d) Kaiser, E. M.; Warner, C. D.,
Synthesis 1975
, 395.
48.
(a) Mason, J. G.; Youssef, A. K.; Ogliaruso, M. A., J. Org. Chem. 1975,
40
, 3015. (b) Youssef, A. K.; Ogliaruso, M. A., J. Org. Chem. 1973, 38,
3998. (c) Sarel, S.; Klug, J. T.; Taube, A., J. Org. Chem. 1970, 35, 1850.
(d) Klein, K. P.; Hauser, C. R., J. Org. Chem. 1966, 31, 4275.
49.
Crowther, G. P.; Hauser, C. R., J. Org. Chem. 1968, 33, 2228.
50.
(a) Pine, S. H., Org. React. 1970, 18, 403. (b) Kantor, S. W.; Hauser, C.
R., J. Am. Chem. Soc. 1951, 73, 4122. (c) Giumanini, A. G.; Trombini,
C.; Lercker, G.; Lepley, A. R., J. Org. Chem. 1976, 41, 2187.
51.
Laurin, D.; Parravano, G., J. Polym. Sci. Part A-1, Polym. Chem. Ed.
1968, 6, 1047.
52.
(a) Caubere, P., Acc. Chem. Res. 1974, 7, 301. (b) Caubere, P., Top.
Curr. Chem. 1978
, 73, 49. (c) Ndebeka, G.; Raynal, S.; Caubere, P., J.
Org. Chem. 1980
, 45, 5394. (d) Croft, A. P.; Bartsch, R. A., J. Org.
Chem. 1983
, 48, 876. (e) Croft, A. P.; Bartsch, R. A., Tetrahedron Lett.
1983, 24, 2737. (f) Carre, M. C.; Ndebeka, G.; Riondel, A.; Bourgasser,
P.; Caubere, P., Tetrahedron Lett. 1984, 25, 1551. (g) Raynal, S., Eur.
Polym. J. 1986
, 22, 559.
John L. Belletire & R. Jeffery Rauh
The University of Cincinnati, Cincinnati, OH, USA
A list of General Abbreviations appears on the front Endpapers
Wyszukiwarka
Podobne podstrony:
potassium amide eros rp193
sodium perborate eros rs094
sodium borohydride eros rs052
benzyl chloride eros rb050
hydrobromic acid eros rh031
chloroform eros rc105
magnesium eros rm001
oxalyl chloride eros ro015
potassium permanganate eros rp244
peracetic acid eros rp034
p toluenesulfonic acid eros rt134
hexamethylenetetramine eros rh019
copper II chloride eros rc214
glyoxylic acid eros rg009
Sodium hydroxide
p methoxybenzaldehyde eros rm081
Rozdział V Eros
więcej podobnych podstron