
196
THE JOURNAL OF BONE AND JOINT SURGERY
J. G. Burke, FRCS I, Specialist Registrar in Orthopaedics
R. W. G. Watson, PhD, Lecturer and Head of Laboratory
M. G. Walsh, MCh, FRCS I, Consultant Orthopaedic Surgeon
D. McCormack, MCh, FRCS Consultant Orthopaedic Surgeon
J. M. Fitzpatrick, MCh, FRCS I, Professor of Surgery
Mater Misericordiae Hospital, 47 Eccles Street, Dublin 7, Ireland.
F. E. Dowling, MCh, FRCS I, Consultant Orthopaedic Surgeon
Meath/Adelaide/National Children’s Hospital, Tallaght, Dublin, Ireland.
Correspondence should be sent to Mr J. G. Burke at 55 Greenlee Drive,
Little Benton, Newcastle Upon Tyne NE7 7GA, UK.
©2002 British Editorial Society of Bone and Joint Surgery
0301-620X/02/212511 $2.00
Intervertebral discs which cause low back
pain secrete high levels of proinflammatory
mediators
J. G. Burke, R. W. G. Watson, D. McCormack, F. E. Dowling,
M. G. Walsh, J. M. Fitzpatrick
From University College Dublin, the Mater Misericordiae Hospital and
Meath/Adelaide/National Children’s Hospital, Dublin, Ireland
H
erniated intervertebral disc tissue has been shown
to produce a number of proinflammatory
mediators and cytokines, but there have been no
similar studies using discs from patients with
discogenic low back pain.
We have compared the levels of production of
interleukin-6 (IL-6), interleukin-8 (IL-8) and
prostaglandin E
2
(PGE
2
) in disc tissue from patients
undergoing discectomy for sciatica (63) with that from
patients undergoing fusion for discogenic low back
pain (20) using an enzyme-linked immunoabsorbent
assay.
There was a statistically significant difference
between levels of production of IL-6 and IL-8 in the
sciatica and low back pain groups (p < 0.006 and
p < 0.003, respectively).
The high levels of proinflammatory mediator found
in disc tissue from patients undergoing fusion suggest
that production of proinflammatory mediators within
the nucleus pulposus may be a major factor in the
genesis of a painful lumbar disc.
J Bone Joint Surg [Br] 2002;84-B:196-201.
Received 8 June 2001; Accepted 3 August 2001
The pathophysiology of discogenic low back pain is incom-
pletely understood.
1
The changes which occur as a disc
degenerates are well documented, but are unhelpful in
determining whether a degenerate disc will cause pain.
2
It
is known that disc tissue from patients undergoing dis-
cectomy for sciatica synthesises proinflammatory mediators
and cytokines.
3-12
Sequestrated and extruded discs produce
higher levels of these mediators than specimens in which
the annulus is intact.
5,10,12,13
To date, there have been no studies of the production of
inflammatory mediators in disc tissue from patients under-
going operations for discogenic low back pain. It has been
shown, however, that degenerate discs in these patients
contain more nociceptive nerve endings in the endplates of
the disc and in the nucleus pulposus than do degenerate
discs which do not cause low back pain.
14,15
We have therefore compared levels of production of the
proinflammatory mediators tumour necrosis factor alpha
(TNF
␣), interleukin-1beta (IL-1), interleukin-6 (IL-6),
interleukin-8 (IL-8) and prostaglandin E
2
(PGE
2
), in disc
tissue from patients undergoing discectomy for sciatica
with those from patients undergoing fusion for discogenic
low back pain.
Patients and Methods
We obtained specimens of intervertebral disc from 63
patients undergoing primary lumbar discectomy for sciati-
ca. Intraoperative assessment of the morphology of the disc
herniation revealed 25 in which the annulus was intact (AI),
30 in which a nuclear extrusion was present (EXT) and
eight in which the nucleus was sequestrated (SEQ). The
mean ages were 42 years in the AI group, 39.5 years in the
EXT group and 42 years in the SEQ group. The male:
female ratio in the AI, EXT and SEQ groups was 17:8,
20:10 and 6:2, respectively. Three specimens were from the
L3/L4 level, 28 from the L4/L5 level and 32 from the L5/
S1 level.
We also obtained disc specimens from 20 patients under-
going primary lumbar interbody fusion for discogenic low
back pain, which had been confirmed by discography.
There were six men and 14 women with a mean age of 38.5
years. Twelve specimens were from the L4/L5 level and
eight from the L5/S1 level. Information regarding the
morphology of the disc was available for 13 specimens;
four AI and nine EXT.
We excluded patients with degenerative spinal stenosis,
tumours, infections, previous lumbar surgery and those
who had had an epidural injection of corticosteroids within
six months of operation.
Tissue culture. The degenerate and control disc specimens
were freshly obtained at the time of surgery and stored in
normal saline solution at 4°C until transported to the
laboratory, within six hours. All specimens were prepared
for culture by the principal author (JGB). The specimens
were washed with normal saline to remove blood con-
taminants and, when possible, the nucleus pulposus was
identified and separated from the other disc tissues. Great
care was taken to exclude fragments of bone, cartilage and
granulation tissue from the cultures. Only tissue which
appeared morphologically to be nucleus pulposus was
cultured.
The tissue was diced and 200 mg specimens were incu-
bated in 3 ml of Neumann-Tytell serum free medium (Gib-
co, Cambridge, UK) at 37°C for 72 hours in a humidified
atmosphere of 5% CO
2
in air, which is a modification of the
method described by Kang et al.
3-5
Penicillin (100 units),
streptomycin (100
g) and amphotericin B (2.5 g) were
added to the medium as prophylaxis against microbial
infection (Sigma-Aldrich Co Ltd, Poole, UK). At the end of
the incubation period the medium was harvested, aliquoted
and stored at -80°C for biochemical analysis. Contamina-
tion of the medium by micro-organisms and cellular growth
from the disc tissue were outruled by light microscopy and
culture.
Biochemical analysis. Levels of TNF
␣, IL-1, IL-6 and
IL-8 in the media were determined by enzyme-linked
immunoabsorbent assay, using commercially available kits
(R and D Systems, Minneapolis, Minnesota), according to
the manufacturers’ instructions. Levels of PGE
2
were
measured using a commercially available competitive bind-
ing assay (R & D Systems). The TNF
␣, IL-1, IL-6, IL-8
and PGE
2
kits were sensitive to concentrations of 4.4, 1,
0.7, 10 and 36.2 pg/ml, respectively.
Statistical analysis. Statistical analysis of the data was
carried out using SPSS (SPSS Inc, Chertsey, UK) statistical
software for non-parametric analysis by the Mann-Whitney
U test. Significance was assumed at p < 0.05.
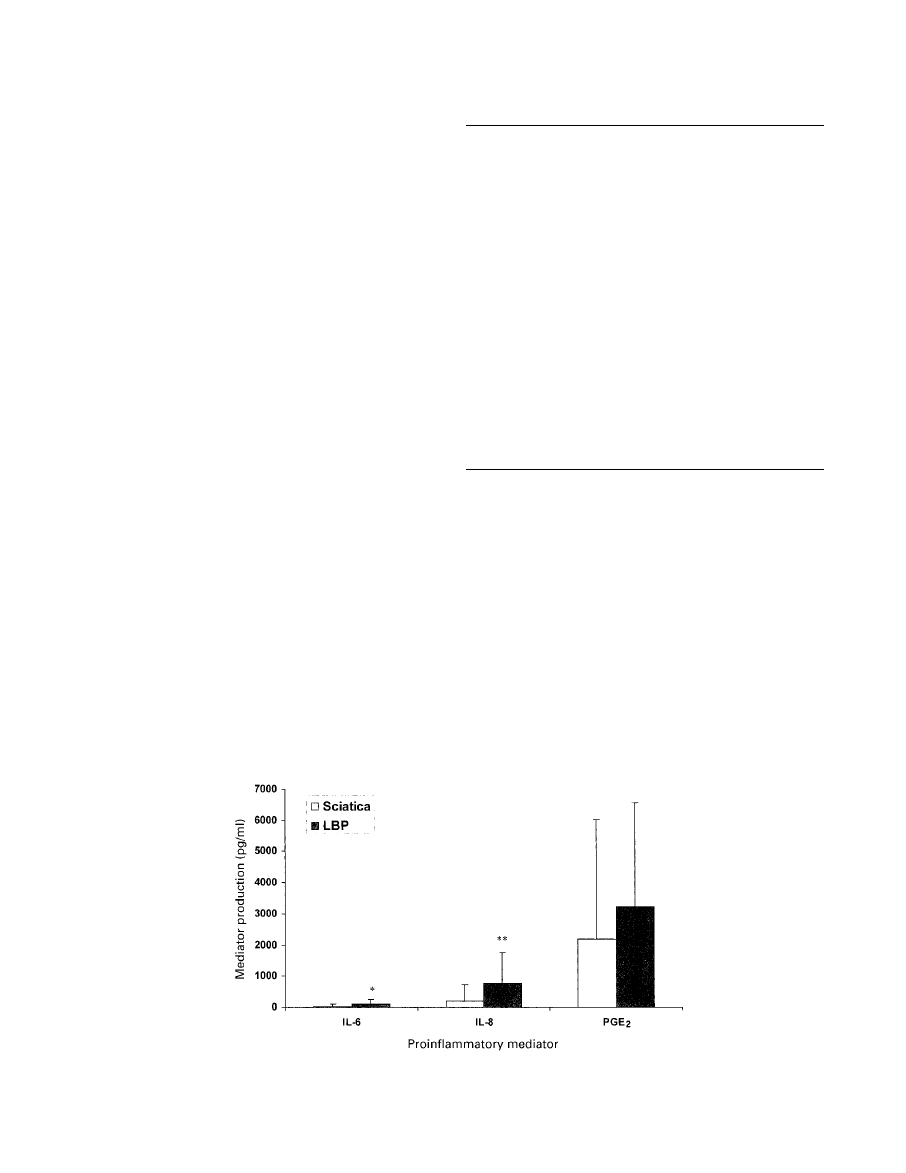
Results
Significant quantities of IL-6, IL-8 and PGE
2
were pro-
duced by both the sciatica and low back pain groups (Fig.
1). None of the specimens produced TNF
␣ or IL-1. There
was no significant difference in age- or gender-matching of
the groups, but there was a predominance of men in those
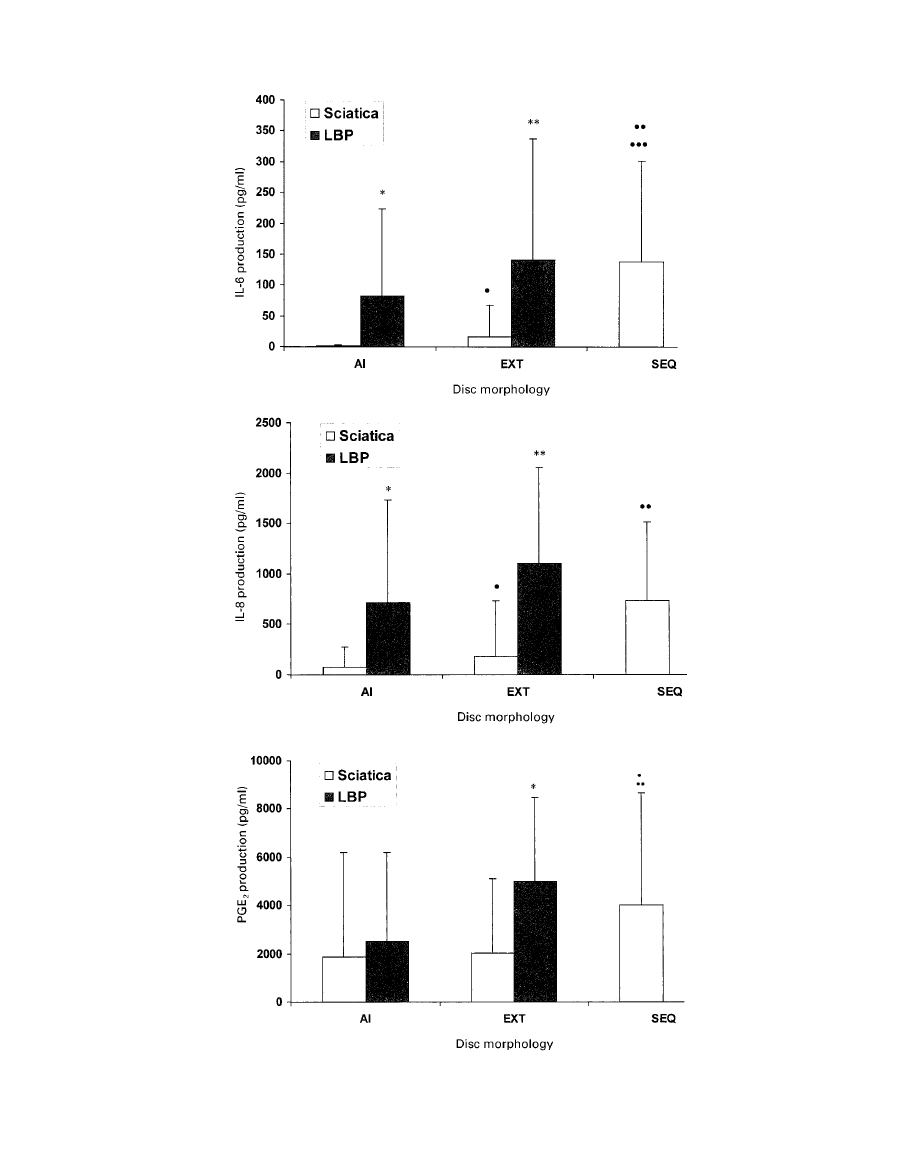
with sciatica. Figure 2 and Table I show and compare the
production of mediator according to the morphology of the
disc herniation in the two groups. Figure 3 shows the
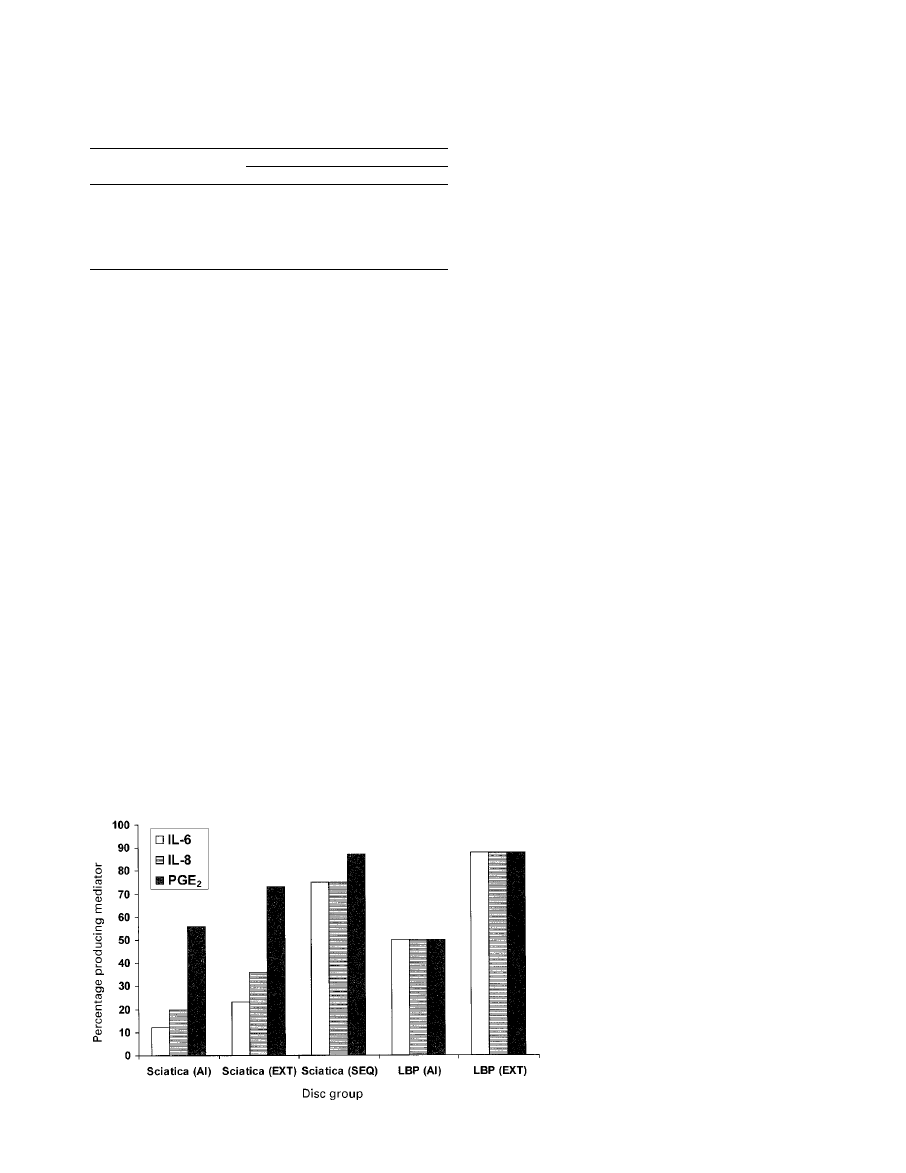
percentage of disc specimens in each group which pro-
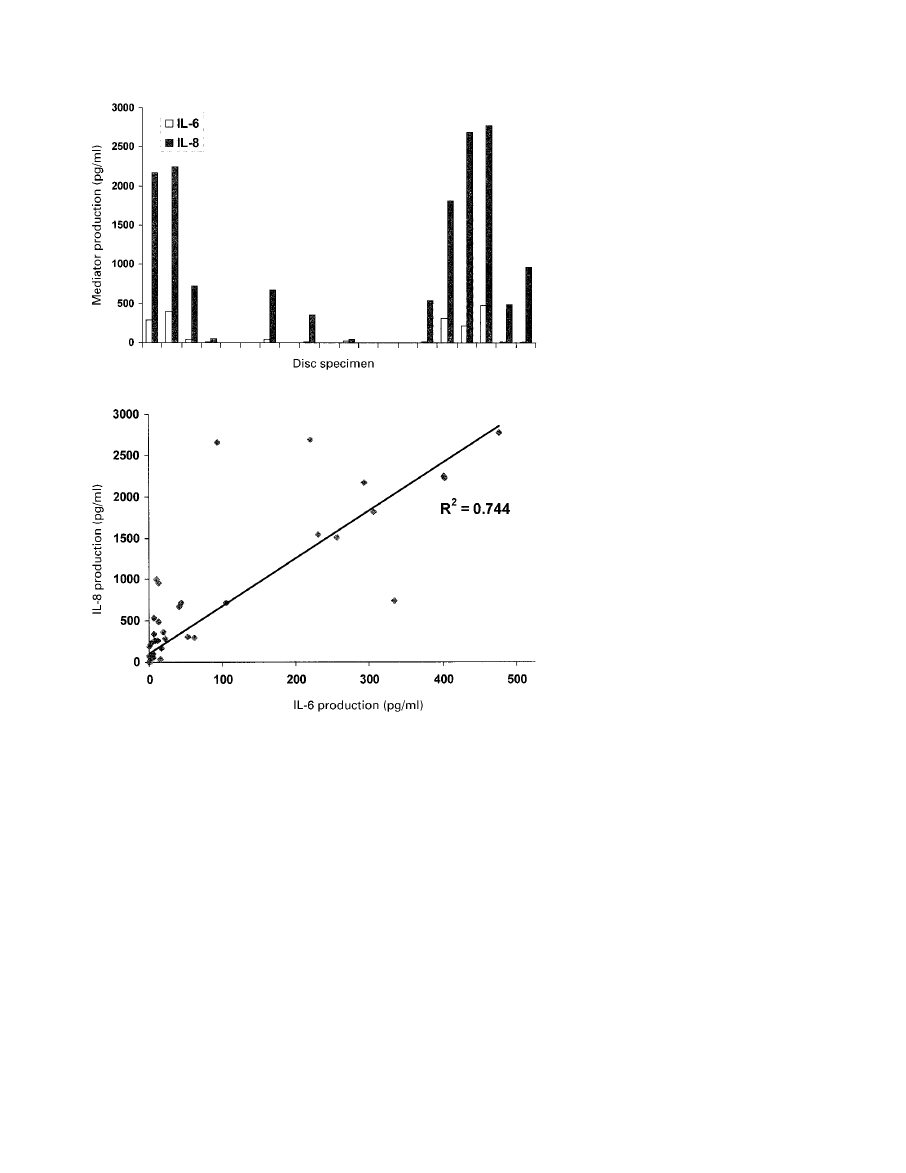
duced each mediator. Figure 4 shows the production of IL-6
and IL-8 in the individual disc specimens from the group
with low back pain. There was a linear relationship
between the production of IL-6 and IL-8 (Pearson correla-
tion coefficient 0.744; Fig. 5). The Pearson correlation
coefficients for IL-6 and PGE
2
and IL-8 and PGE
2
were
0.24 and 0.3, respectively.
Discussion
In recent years, attention has begun to focus on the cellular
and molecular activity of intervertebral disc tissue in the
search for an understanding of the pathophysiology of
sciatica and discogenic low back pain.
3-12
It is clear from
imaging studies that radicular pain is not simply a mechan-
ical phenomenon.
16,17
It has been shown that degenerate
disc tissue from patients with sciatica synthesises IL-6 and
PGE
2
3,4,6
and that the quantities of these substances
increase with increasing exposure of the nucleus.
5,10,12,13
Our study confirms these findings. We have recently shown
that human nucleus pulposus also produces IL-8.
18
IL-1

and TNF
␣ have been isolated from homogenates of human
disc material by Takahashi et al.
10
We have found no
evidence of production by the disc of either of these
mediators, even in those specimens producing high levels
of other proinflammatory mediators. There are no previous
197
INTERVERTEBRAL DISCS WHICH CAUSE LOW BACK PAIN SECRETE PROINFLAMMATORY MEDIATOR
VOL. 84-B, N
O
. 2, MARCH 2002
Fig. 1
Graph showing production of IL-6, IL-8 and PGE
2
in the sciatica and low back pain
groups.
198
J. G. BURKE, R. W. G. WATSON, D. MCCORMACK, F. E. DOWLING, M. G. WALSH, J. M. FITZPATRICK
THE JOURNAL OF BONE AND JOINT SURGERY
Fig. 2a
Fig. 2b
Fig. 2c
Graphs showing production of a) IL-6, b) IL-8 and c) PGE
2
according to the morphology of
the disc.
studies in the literature comparing the levels of production
of inflammatory mediators in degenerate discs which cause
sciatica with those which cause low back pain.
Our study has shown that significantly more IL-6, IL-8
and PGE
2
are produced by discs from patients with low
back pain compared with discs from patients with sciatica.
There was a trend towards less exposure of the nucleus
pulposus in the group with low back pain only compared
with those with sciatica introducing a bias towards higher
levels of mediator production in the latter.
5,10,12,13
Figure 2
illustrates the difference between the two groups. The effect
of increasing exposure of the nucleus pulposus on the
production of mediators is not significant in the group with
low back pain, but marked in those with sciatica. Within
each category of abnormality of the disc there are sig-
nificant differences in the production of mediators between
the two groups. Figure 3 shows the number of disc speci-
mens in each group producing each mediator. The rates of
production of IL-6 and IL-8 in the AI and EXT categories
of discs in low back pain are much higher than those found
in those with sciatica, further underlining the differences
between them. These findings suggest that degenerate discs
which cause low back pain differ at a cellular and molec-
ular level from those which cause sciatica. Specimens of
sequestrated disc from the sciatica group produced similar
quantities of inflammatory mediators to those with low
back pain. These, however, are known to be infiltrated with
macrophages and T-cells, which may contribute to the
levels of production of mediators.
13,19-22
The disc material
in sequestrated herniations is also in a different anatomical
location to the contained or semicontained specimens in the
group with low back pain only.
The reasons for increased
production of inflammatory mediators by the nucleus pul-
posus in patients with discogenic low back pain are
unknown. A recent study has shown that few inflammatory
cells are found in these discs
23
and therefore the source of
the mediators must be cells from the nucleus pulposus
itself. It is known that such tissue can produce a range of
proinflammatory cytokines.
3-12
We suggest that as some
discs degenerate the cells of the nucleus pulposus may be
exposed to a proinflammatory stimulus leading to a form of
inflammatory degeneration which gives rise to low back
pain. The nature of this stimulus is currently unknown.
Discs which cause low back pain have higher concentra-
tions of sensory nerves than are seen in those which do not
cause such pain.
14,15
The sensory nerves in the former are
found in the endplates and in the nucleus pulposus and lose
their normal relationship with blood vessels. The ingrowth
of nerves into degenerate discs which cause low back pain
may be mediated by chemotactic substances released by the
degenerating disc.
24
A combination of the innervation of
the nucleus pulposus and increased production of pro-
inflammatory mediators suggests that the mechanism for
discogenic low back pain may be the induction of hyper-
algesia in the newly innervated degenerating nucleus pul-
posus. Both IL-8 and PGE
2
are known to induce
hyperalgesia.
25
Micromovement may occur between the vertebral bod-
ies, anteriorly, in the presence of a solid posterior fusion.
Weatherley, Prickett and O’Brien
26
have published a series
in which discogenic pain persisted postoperatively despite a
solid posterior fusion. These patients were cured by the
addition of an anterior fusion. Butterman et al
27
confirmed
these findings and correlated the failure of posterior fusion
alone with the presence of Modic changes
28
(inflammatory
199
INTERVERTEBRAL DISCS WHICH CAUSE LOW BACK PAIN SECRETE PROINFLAMMATORY MEDIATOR
VOL. 84-B, N
O
. 2, MARCH 2002
Table I.
p values of comparisons of mediator production by inter-
vertebral discs from the different patient groups (sciatica and low back
pain (LBP)) and the different morphology groups (AI, EXT and SEQ)
Mediator
Group comparisons
IL-6
IL-8
PGE
2
Sciatica v LBP
<0.006
<0.003
AI sciatica v AI LBP
<0.003
<0.005
EXT sciatica v EXT LBP
<0.003
<0.0007
<0.02
AI sciatica v EXT sciatica
<0.02
<0.05
AI sciatica v SEQ sciatica
<0.001
<0.01
<0.05
EXT sciatica v SEQ sciatica
<0.05
<0.05
<0.05
Fig. 3
Graph showing the percentage of disc specimens in
each group which produced each mediator.
bone marrow changes adjacent to a degenerate disc) at the
symptomatic level. When micromovement is sufficient to
cause pain which responds to excision and fusion of a disc,
the mechanism of the generation of the pain cannot be
attributed to instability, but is consistent with hyperalgesia
induced in an innervated nucleus pulposus by inflammatory
mediators.
Figure 4 shows the production of mediators by individual
discs in low back pain. Only 65% of these discs produced
mediators and therefore this is not a homogenous group. It
is possible that the 35% of discs in low back pain which did
not produce mediators may produce pain by some other
mechanism. Alternatively, the diagnosis of discogenic pain
in these patients may be incorrect, or the culture process
may not have detected an inflammatory region of the disc.
However, the production of relatively high levels of media-
tor is a strong argument in favour of the occurrence of an
inflammatory form of disc degeneration which causes the
pain.
The linear correlation between the production of IL-6
and IL-8 (Fig. 5) supports the theory that individual discs
can produce an inflammatory response and suggests that the
stimulus provoking production of these mediators is the
same. The rather poor correlation between the production
of PGE
2
and that of IL-6 and IL-8 suggests that a different
stimulus may be responsible for the former. This, combined
with the smaller differences between levels of production
of PGE
2
in the group with sciatica and those with low back
pain only may indicate that it is not of major importance in
defining the different disc pathologies at a cellular and
molecular level.
Provocative discography is currently the method of
choice for diagnosing discogenic low back pain. It is a
subjective test relying on the radiologists’ and patients’
perceptions to determine the result.
29-36
Many patients with
such complaints have associated psychological or psychiat-
ric disturbances which may or may not be associated with
medicolegal factors. All of these decrease their ability to
give an accurate opinion as to whether the pain produced at
discography is that of which they are complaining.
29-36
200
J. G. BURKE, R. W. G. WATSON, D. MCCORMACK, F. E. DOWLING, M. G. WALSH, J. M. FITZPATRICK
THE JOURNAL OF BONE AND JOINT SURGERY
Fig. 4
Graph showing the level of production of IL-6 and IL-8
by each specimen of disc from patients with low back
pain.
Fig. 5
Graph showing the linear relationship between the pro-
duction of IL-6 and IL-8 in discs.

There clearly remains a need for an objective diagnostic
test for discogenic low back pain. Our study has indicated
that there are differences between degenerate discs which
cause such pain and those which cause sciatica. It may be
possible to exploit these differences to develop an objective
diagnostic test for discogenic low back pain and to manip-
ulate the biology of the degenerate disc to develop non-
surgical treatments for inflammatory discogenic pain.
This work was funded by a Cappagh Trust Grant for Postgraduate
Research and Education from Cappagh Orthopaedic Hospital.
No benefits in any form have been received or will be received from a
commercial party related directly or indirectly to the subject of this
article.
References
1. Mooney V. Presidential Address International Society for the Study of
the Lumbar Spine, Dallas, 1986: Where is the pain coming from?
Spine 1987;12:754-9.
2. Buckwalter JA. Aging and degeneration of the human intervertebral
disc. Spine 1995;20:1307-14.
3. Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M,
Evans CH. Herniated cervical intervertebral discs spontaneously pro-
duce matrix metalloproteinases, nitric oxide, interleukin-6, and prosta-
glandin E
2
. Spine 1995;20:2373-8.
4. Kang JD, Georgescu HI, McIntyre-Larkin L, et al. Herniated
lumbar intervertebral discs spontaneously produce matrix metallopro-
teinases, nitric oxide, interleukin-6, and prostaglandin E
2
. Spine
1996;21:271-7.
5. Kang JD, Stefanovic-Racic M, McIntyre LA, Georgescu HI, Evans
CH. Toward a biochemical understanding of human intervertebral disc
degeneration and herniation: contributions of nitric oxide, interleukins,
prostaglandin E
2
, and matrix metalloproteinases. Spine
1997;22:1065-73.
6. O’Donnell JL, O’Donnell AL. Prostaglandin E
2
content in herniated
lumbar disc disease. Spine 1996;21:1653-5.
7. Nagano T, Yonenobu K, Miyamoto S, Tohyama M, Ono K.
Distribution of the basic fibroblast growth factor and its receptor gene
expression in normal and degenerated rat intervertebral discs. Spine
1995;20:1972-8.
8. Rand N, Reichert F, Floman Y, Rotshenker S. Murine nucleus
pulposus-derived cells secrete interleukins-1-beta, -6, and -10 and
granulocyte-macrophage colony-stimulating factor in cell culture.
Spine 1997;22:2598-601.
9. Saal JS, Franson RC, Dobrow R, et al. High levels of inflammatory
phospholipase A2 activity in lumbar disc herniations. Spine
1990;15:674-8.
10. Takahashi H, Suguro T, Okazime Y, et al. Inflammatory cytokines in
the herniated disc of the lumbar spine. Spine 1996;21:218-24.
11. Tolonen J, Gronblad M, Virri J, et al. Basic fibroblast growth factor
immunoreactivity in blood vessels and cells of disc herniations. Spine
1995;20:271-6.
12. Nygaard OP, Mellgren SI, Osterud B. The inflammatory properties
of contained and noncontained lumbar disc herniation. Spine
1997;22:2484-8.
13. Doita M, Kanatani T, Harada T, Mizuno K. Immunohistologic
study of the ruptured intervertebral disc of the lumbar spine. Spine
1996;21:235-41.
14. Coppes M, Marani E, Thomeer R, Groen RJ. Innervation of
‘painful’ lumbar discs. Spine 1997;22:2342-9.
15. Brown M, Hukkanen M, McCarthy I, et al. Sensory and sym-
pathetic innervation of the vertebral endplate in patients with degener-
ative disc disease. J Bone Joint Surg [Br] 1997;79-B:147-53.
16. Thelander U, Fagerlund M, Friberg S, Larsson S. Describing the
size of lumbar disc herniations using computed tomography: a com-
parison of different size index calculations and their relation to
sciatica. Spine 1994;19:1979-84.
17. Saal JS. The role of inflammation in lumbar pain. Spine 1995;
20:1821-7.
18. Burke JG, Watson RWG, McCormack D, et al. Spontaneous disc
herniation resorption may be mediated by chemokines. Proceedings of
the North American Spine Society, 14th Annual Meeting, Chicago,
1999; 130-1.
19. Gronblad M, Virri J, Tolonen J, et al. A controlled immuno-
histochemical study of inflammatory cells in disc herniation tissue.
Spine 1994;19:2744-51.
20. Haro H, Shinomiya K, Komori H, et al. Upregulated expression of
chemokines in herniated nucleus pulposus resorption. Spine
1996;21:1647-52.
21. Ikeda T, Nakamura T, Kikuchi T, et al. Pathomechanism of sponta-
neous regression of the herniated lumbar disc: histologic and immuno-
histochemical study. J Spinal Disord 1996;9:136-40.
22. Ito T, Yamada M, Ikuta F, et al. Histologic evidence of absorption of
sequestrain-type herniated disc. Spine 1996;21:230-4.
23. Roberts S, Menage J, Evans EH, et al. Inflammation of the inter-
vertebral disc: an uncommon finding in discs associated with dis-
cogenic back pain. J Bone Joint Surg [Br] 2000;82-B:Supp II:98.
24. Tessier-Lavigne M, Placzek M. Target attraction: are developing
axons guided by chemotropism? Trends Neurosci 1991;14:303-10.
25. Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated
inflammatory hyperalgesia limited by interleukin-1 receptor antago-
nist. Br J Pharmacol 2000;130:1418-24.
26. Weatherley C, Prickett C, O’Brien J. Discogenic pain persisting
despite solid posterior fusion. J Bone Joint Surg [Br] 1986;68-B:142-
3.
27. Buttermann GR, Heithoff KB, Ogilvie JW, Transfeldt EE, Cohen
M. Vertebral body MRI related to lumbar fusion results. Eur Spine J
1997;6:115-20.
28. Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease:
assessment of changes in vertebral body marrow with MR imaging.
Radiology 1988;166:193-9.
29. Colhoun E, McCall IW, Williams L, Cassar Pullicino VN. Provoca-
tion discography as a guide to planning operations on the spine. J
Bone Joint Surg [Br] 1988;70-B:267-71.
30. Collins CD, Stack JP, O’Connell DJ, et al. The role of discography
in lumbar disc disease: a comparative study of magnetic resonance
imaging and discography. Clin Radiol 1990;42:252-7.
31. Antti-Poika I, Soini J, Tallroth K, Yrjonen T, Konttinen YT.
Clinical relevance of discography combined with CT scanning: a
study of 100 patients. J Bone Joint Surg [Br] 1990;72-B:480-5.
32. Bogduk N, Modic MT. Lumbar discography. Spine 1996;21:402-4.
33. Brodsky AE, Binder WF. Lumbar discography: its value in diagnosis
and treatment of lumbar disc lesions. Spine 1979;4:110-20.
34. Derby R, Howard MW, Grant JM, et al. The ability of pressure-
controlled discography to predict surgical and nonsurgical outcomes.
Spine 1999;23:364-71.
35. Guyer RD, Ohnmeiss DD. Lumbar discography: position statement
from the North American Spine Society Diagnostic and Therapeutic
Committee. Spine 1995;20:2048-59.
36. Mooney V. Lumbar discography. Spine 1996;21:1479.
201
INTERVERTEBRAL DISCS WHICH CAUSE LOW BACK PAIN SECRETE PROINFLAMMATORY MEDIATOR
VOL. 84-B, N
O
. 2, MARCH 2002
Wyszukiwarka
Podobne podstrony:
low back pain
Serum cytokine levels in patients with chronic low back pain due to herniated disc
(IV)Interexaminer reliability of low back pain assessment using the McKenzie method
(IV)The McKenzie approach to evaluating and treating low back pain
(IV)Intertester reliability of the McKenzie evaluation in assessing patients with mechanical low bac
Association of low back pain
Categorizing patients with occupational low back pain by use of the Quebec Task Force Classification
Treating Non Specific Chronic Low Back Pain Through the Pilates Method
(IV)A Preliminary Report on the Use of the McKenzie Protocol versus Williams Protocol in the Treatme
The relationship of Lumbar Flexion to disability in patients with low back pain
Manage Back Pain
(IV)Relative therapeutic efficacy of the Williams and McKenzie protocols in back pain management
(IV)The diagnostic utility of McKenzie clinical assessment for lower back pain
(IV)McKenzie method and functional training in back pain rehabilitation A brief review including res
Flo Rida?at T Pain Low PL
Hine P Knack and Back Chaos
Biochemia TZ wyklad 12 integracja metabolizmu low
więcej podobnych podstron