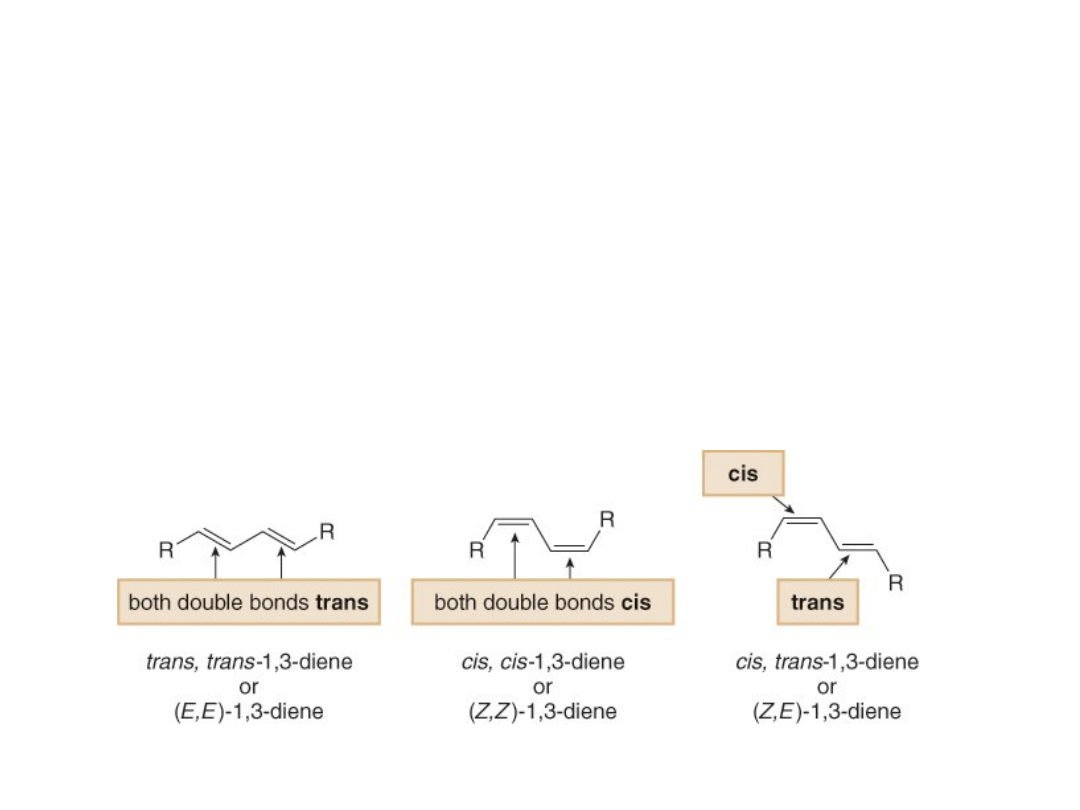
Conjugated Dienes
•Conjugated dienes are compounds having two
double bonds joined by one bond.
•Conjugated dienes are also called 1,3-dienes.
•1,3-Butadiene (CH
2
=CH-CH=CH
2
) is the simplest
conjugated diene.
•Three stereoisomers are possible for 1,3-dienes
with alkyl groups bonded to each end carbon of
the diene.
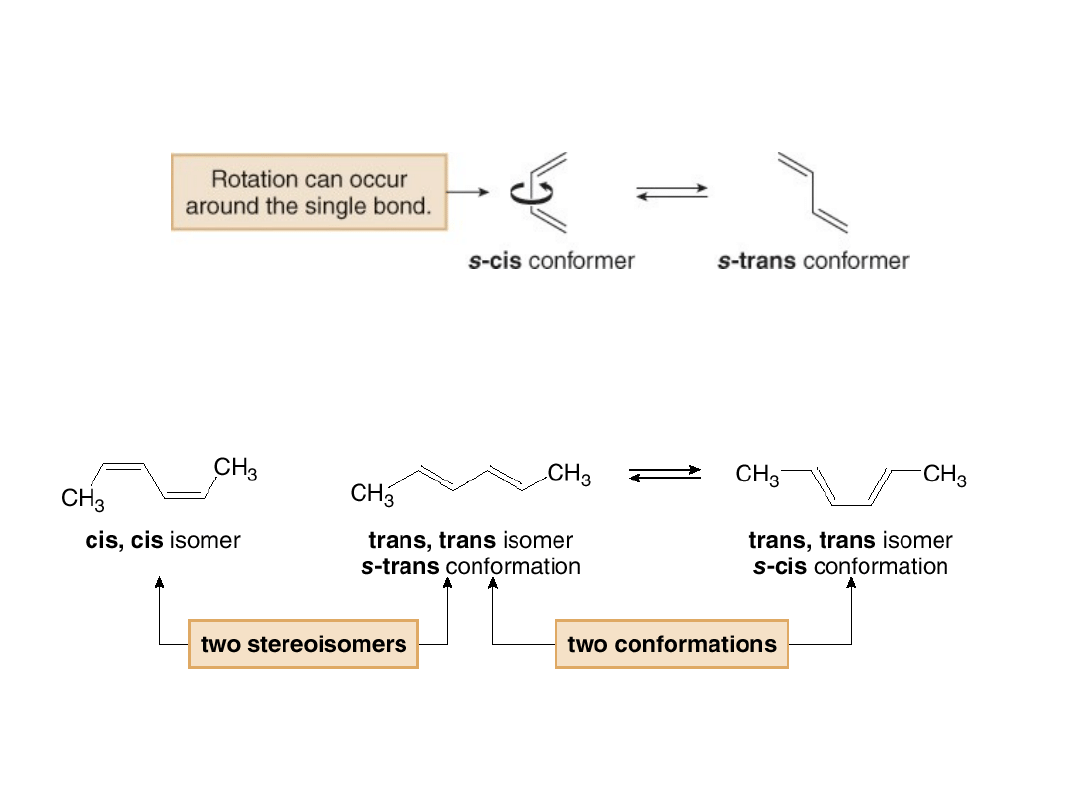
•Two possible conformations result from rotation
around the C—C bond that joins the two double
bonds.
•Note
that
stereoisomers
are
discrete
molecules, whereas conformations interconvert.
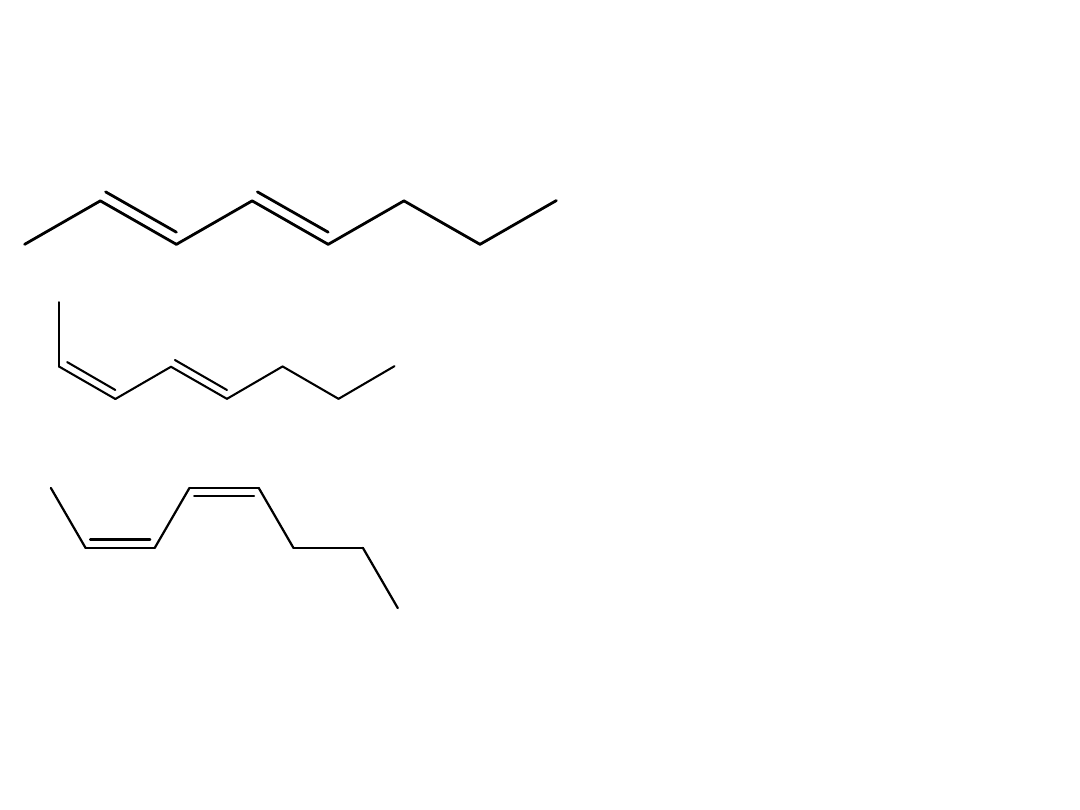
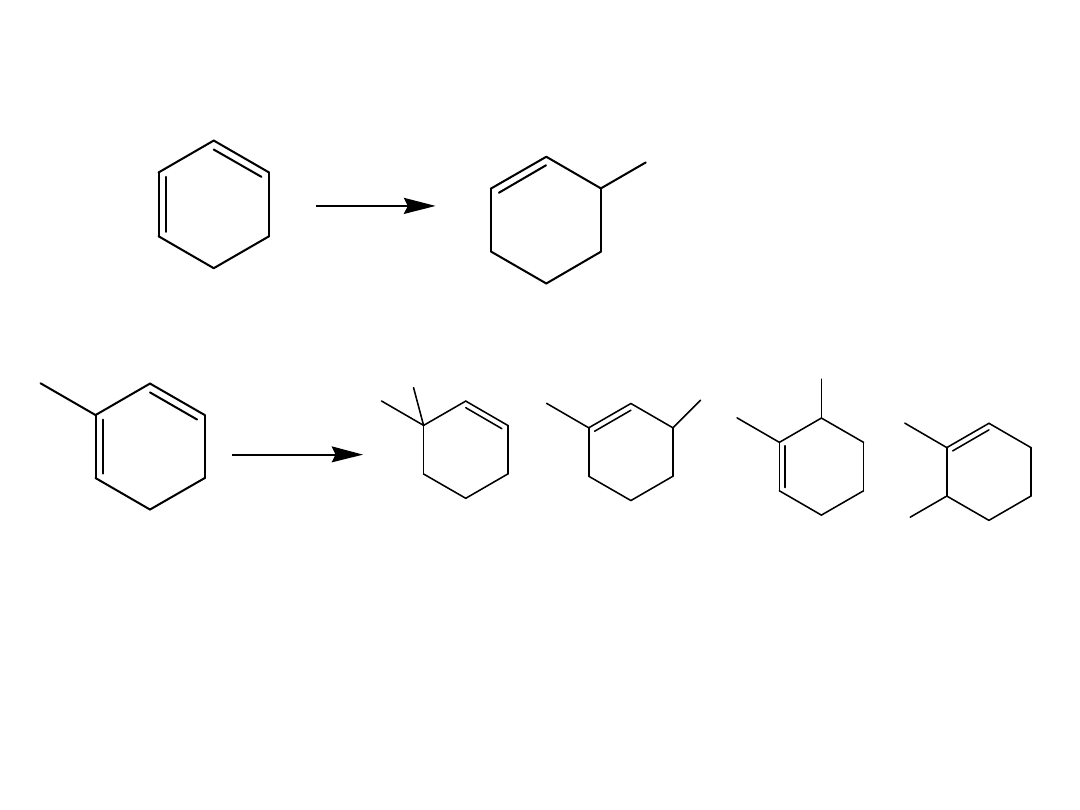
Draw the three possible stereoisomers of 2,4-
octadiene. Pick which one is (2E,4E) 2,4-octadiene.
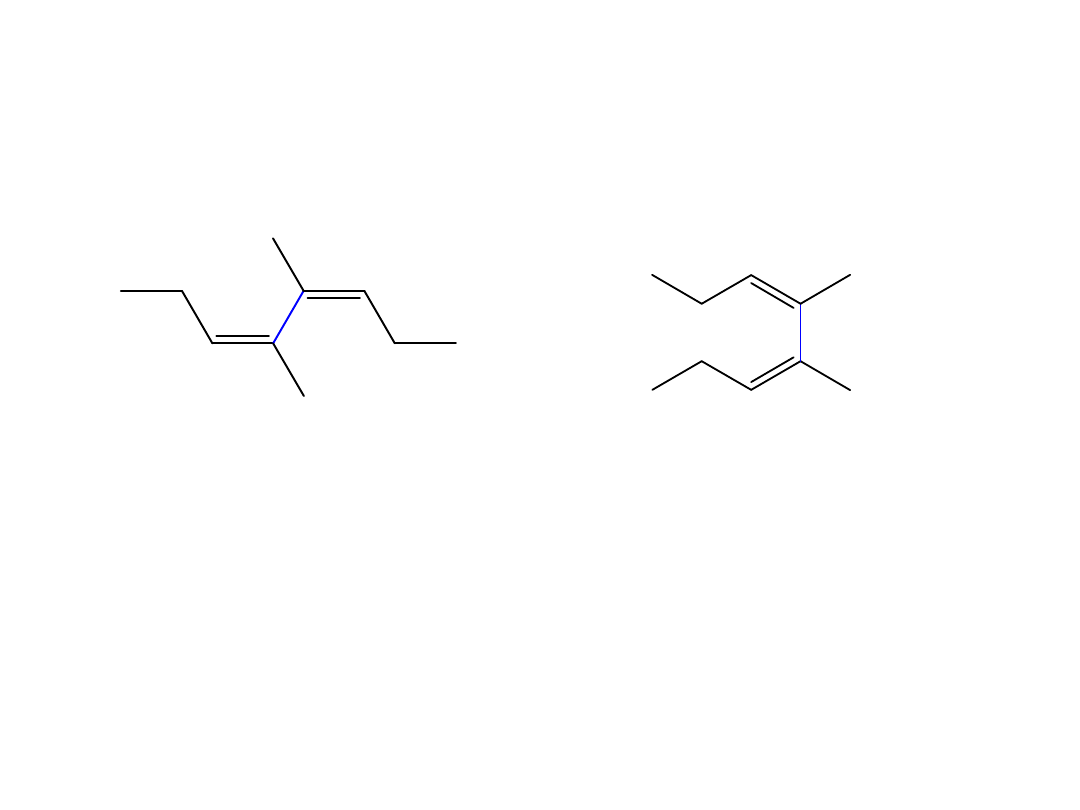
Draw the s-cis and s-trans conformations of (3Z,5Z)-
4,5-dimethyl -3,5-octadiene
s-
trans
s-cis
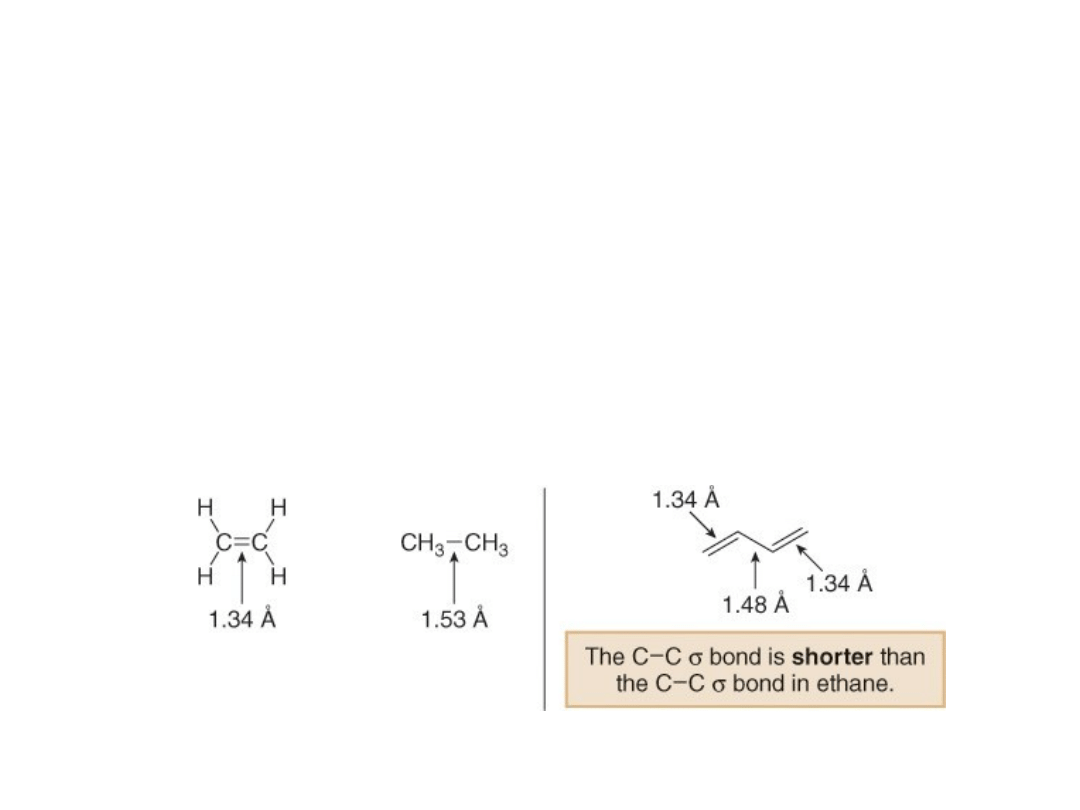
The Carbon—Carbon Bond Length in 1,3-
Butadiene
Four features distinguish conjugated dienes from
isolated dienes.
1. The C—C single bond joining the two double bonds is
unusually short.
2. Conjugated dienes are more stable than similar
isolated dienes.
3. Some reactions of conjugated dienes are different
than reactions of isolated double bonds.
4. Conjugated dienes absorb longer wavelengths of
ultraviolet light.
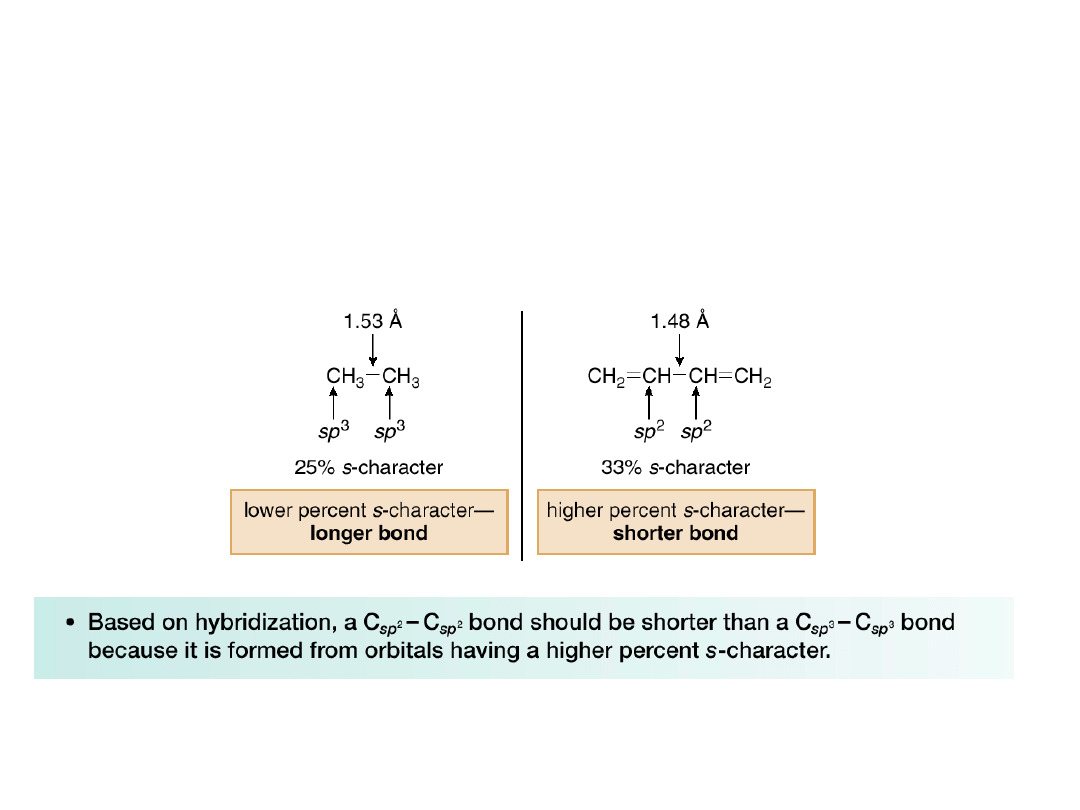
The Carbon—Carbon Bond Length in 1,3-
Butadiene
The observed bond distances can be explained
by looking at hybridization.
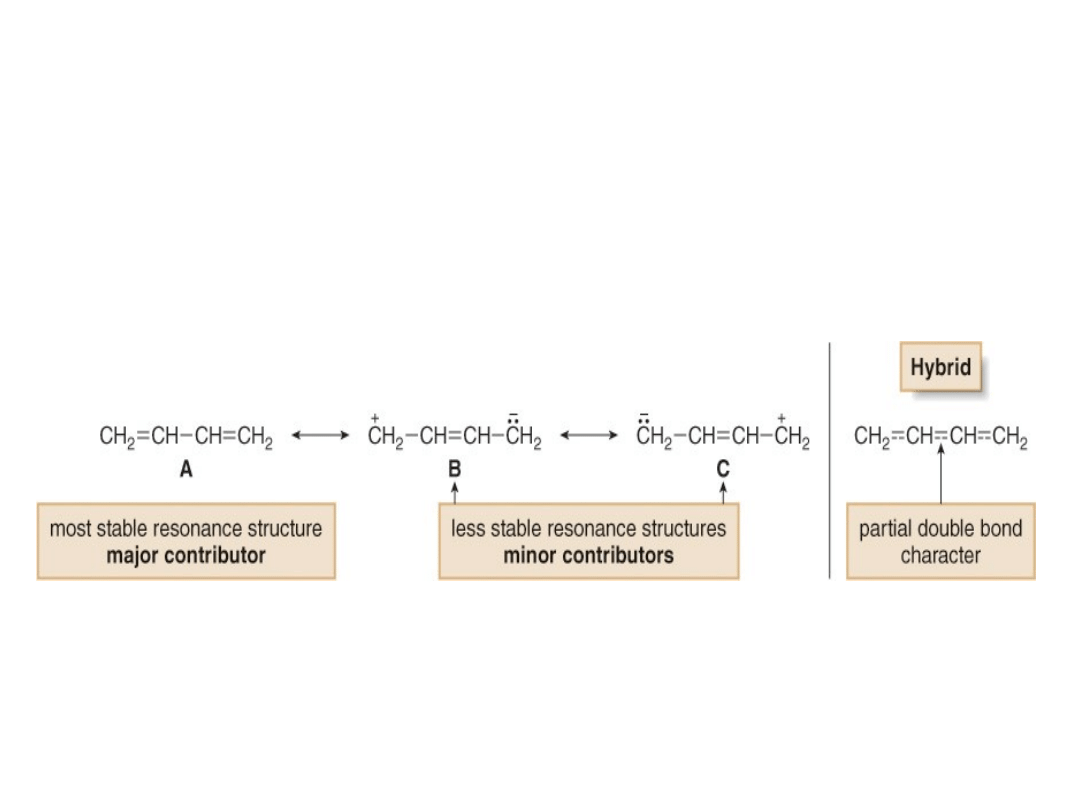
A resonance argument can also be used to explain
the shorter C—C bond length in 1,3-butadiene.
•Based on resonance, the central C—C bond in 1,3-
butadiene is shorter because it has partial double
bond character.
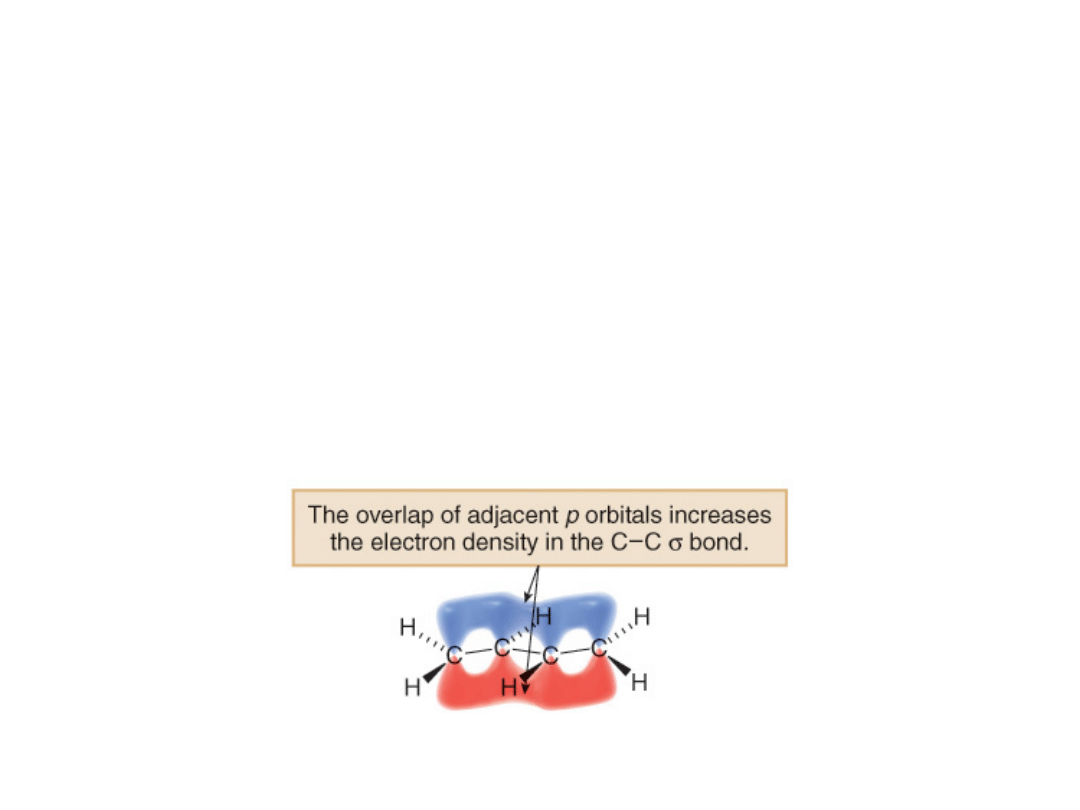
•Finally, 1,3-butadiene is a conjugated molecule
with four overlapping p orbitals on adjacent
atoms.
•Consequently, the electrons are not localized
between the carbon atoms of the double bonds,
but rather delocalized over four atoms.
•This places more electron density between the
central two carbon atoms of 1,3-butadiene than
would normally be present.
•This shortens the bond.
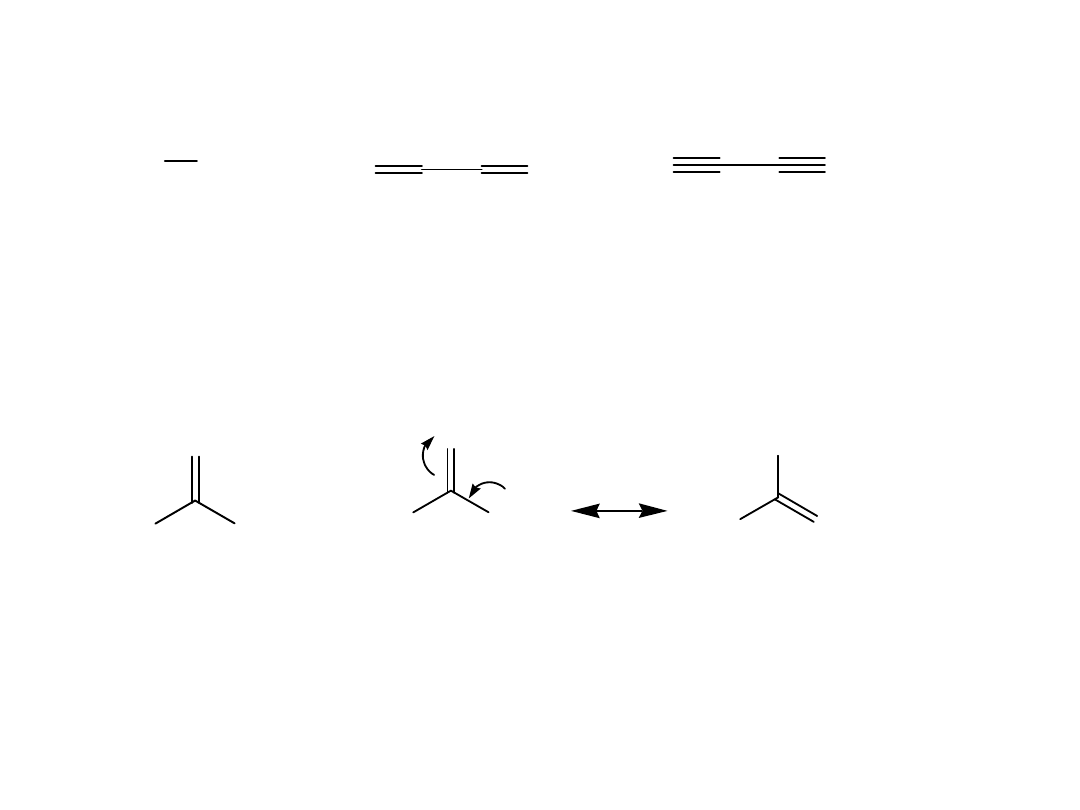
Using hybridization, compare the C-C bonds of the
following three compounds.
H
3
C
CH
3
H
2
C
CH
2
HC
CH
sp
3
25% s
character
sp
2
33% s
character
sp
50% s
character
H
3
C
O
O
-
Using resonance, why are the two C—O bonds the
same length?
H
3
C
O
O
-
H
3
C
O
O
-
The two resonance structures show how the
electron density is delocalized over 3 atoms.
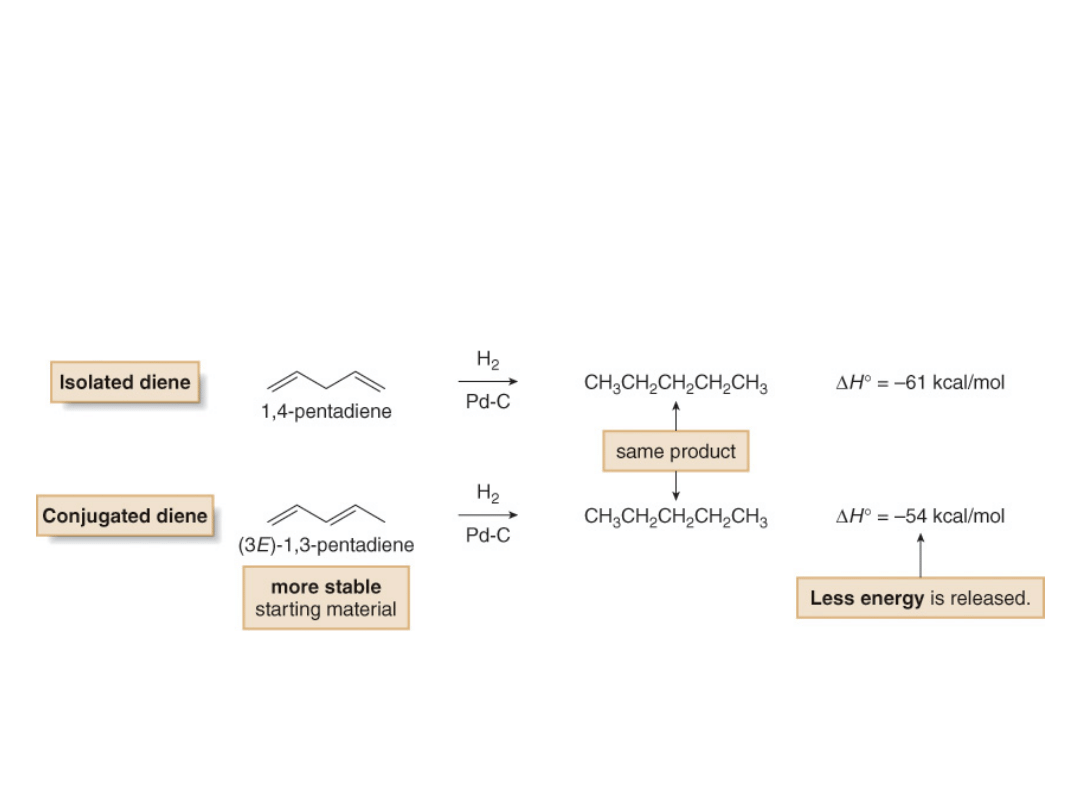
Stability of Conjugated Dienes
When hydrogenation gives the same alkane
from two dienes, the more stable diene has the
smaller heat of hydrogenation.
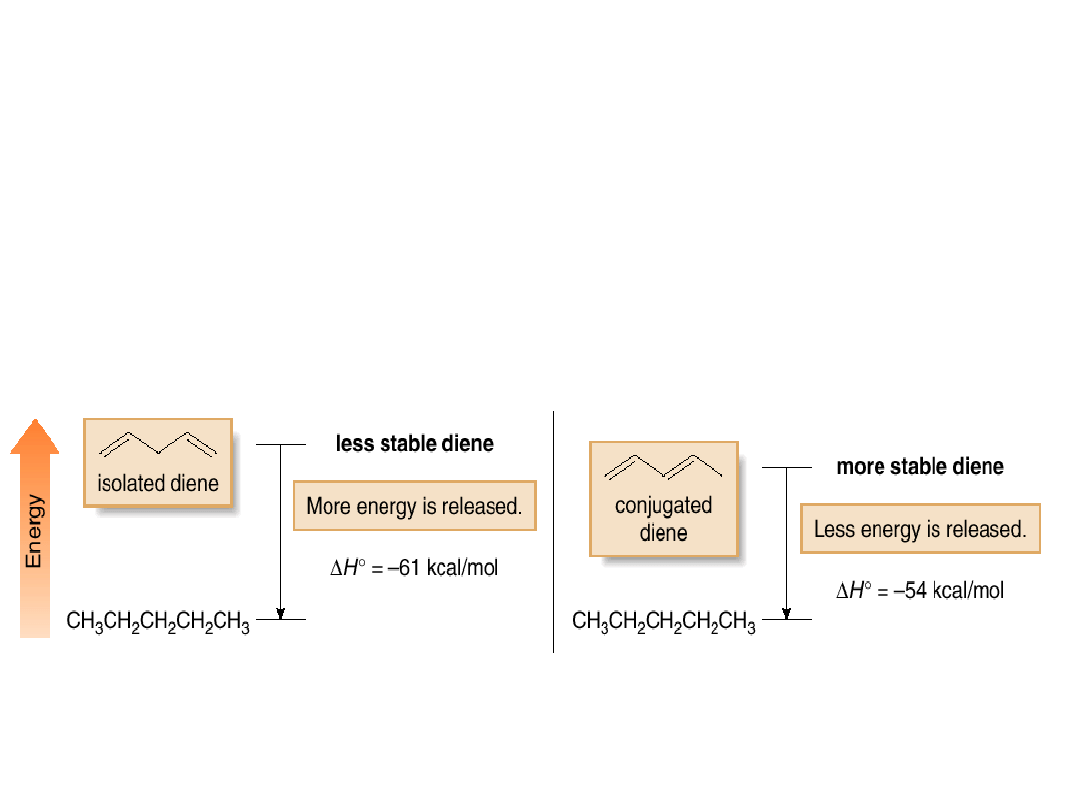
A conjugated diene has a smaller heat of
hydrogenation and is more stable than a similar
isolated diene.
Figure 16.5
Relative energies
of an isolated and
conjugated diene
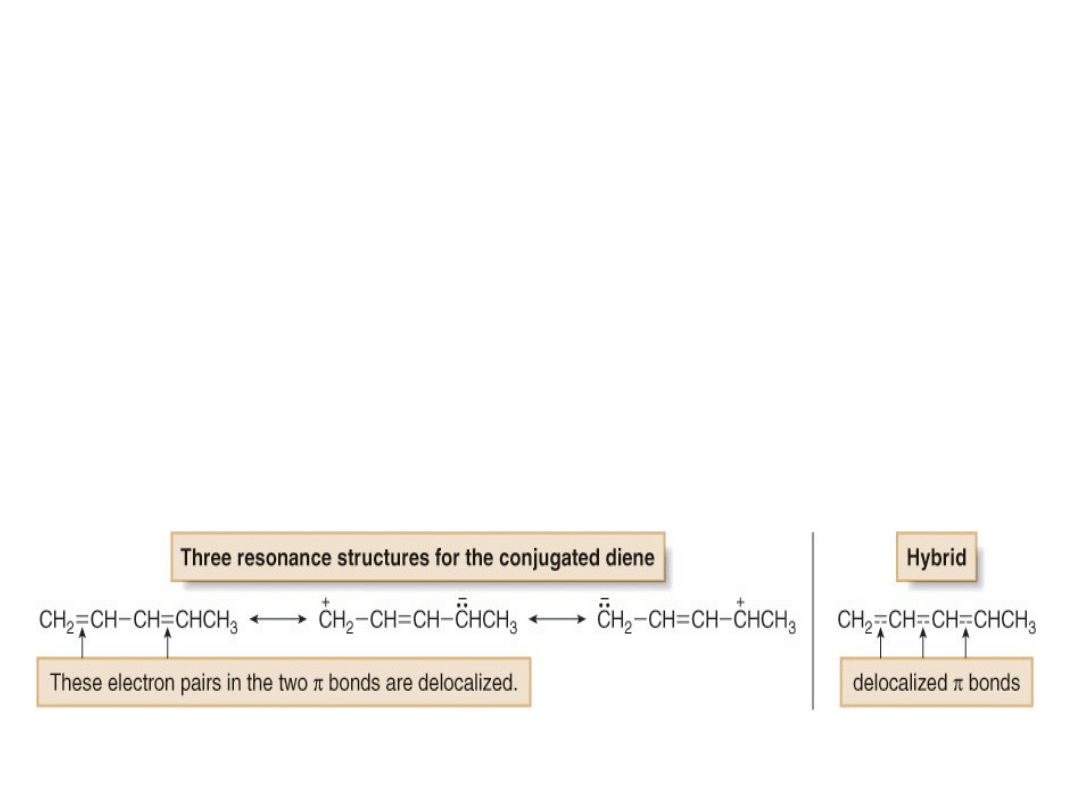
•A conjugated diene is more stable than an
isolated diene because a conjugated diene has
overlapping p orbitals on four adjacent atoms.
Thus, its electrons are delocalized over four
atoms.
•This delocalization, which cannot occur in an
isolated diene is illustrated by drawing
resonance
structures.
For
example,
no
resonance structures can be drawn for 1,4-
pentadiene, but three can be drawn for (3E)-1,3-
pentadiene (or any other conjugated diene).
Electrophilic Addition: 1,2- Versus 1,4-
Addition
• The bonds in conjugated dienes undergo
addition reactions that differ in two ways from
the addition reactions of isolated double
bonds.
1. Electrophilic addition in conjugated dienes gives a
mixture of products.
2. Conjugated dienes undergo a unique addition
reaction not seen in alkenes or isolated dienes.
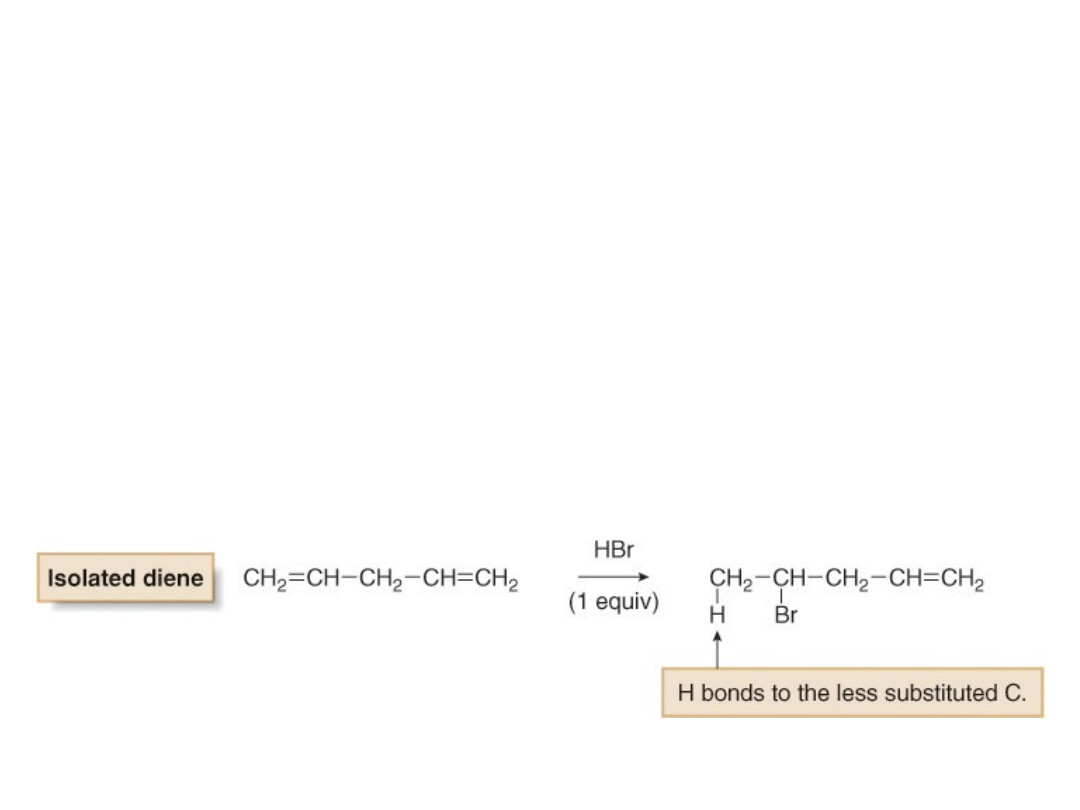
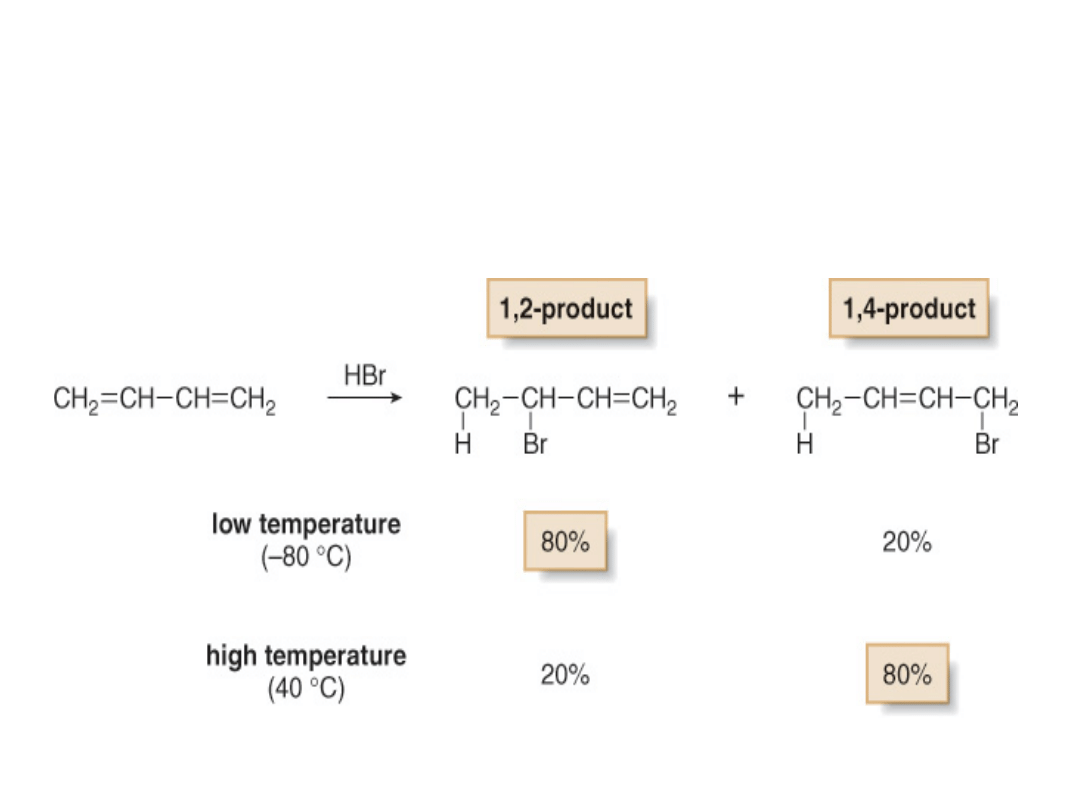
• Recall that electrophilic addition of one
equivalent of HBr to an isolated diene yields one
product and Markovnikov’s rule is followed.
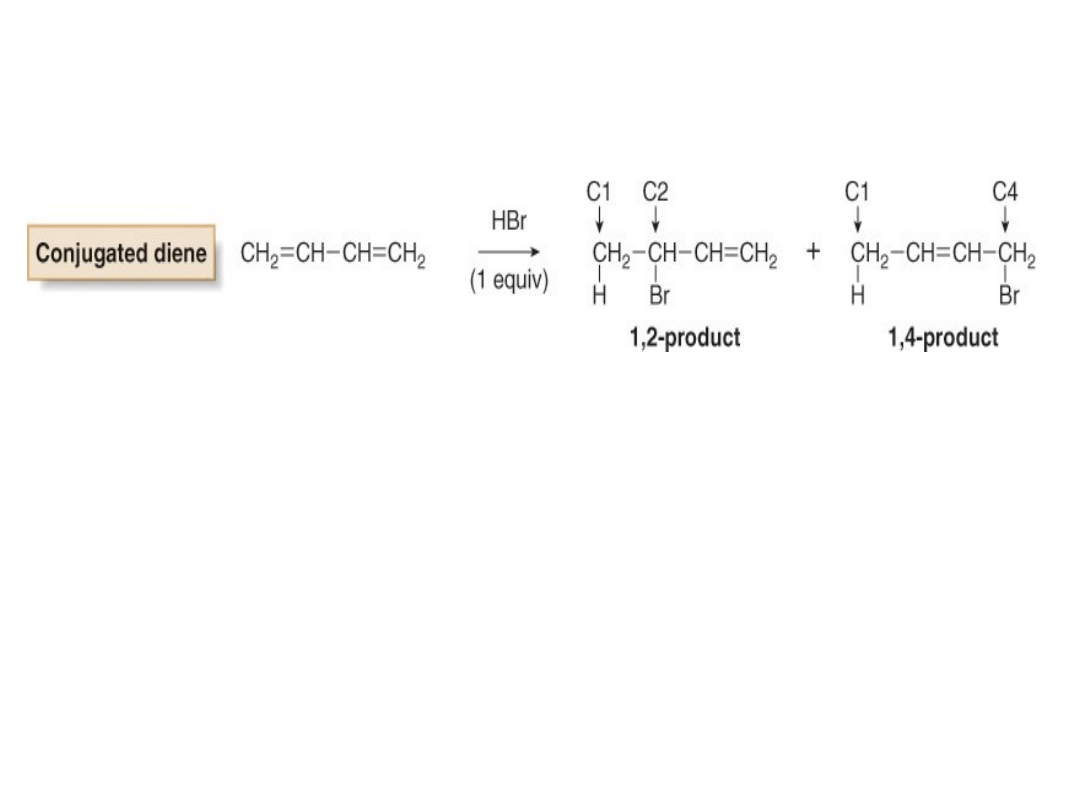
•With a conjugated diene, electrophilic addition of
one equivalent of HBr affords two products.
•The 1,2-addition product results from Markovnikov
addition of HBr across two adjacent carbon atoms
(C1 and C2) of the diene.
•The 1,4-addition product results from addition of
HBr to the two end carbons (C1 and C4) of the
diene. 1,4-Addition is also called
conjugate addition
.
•The ends of the 1,3-diene are called C1 and C4
arbitrarily, without regard to IUPAC numbering.
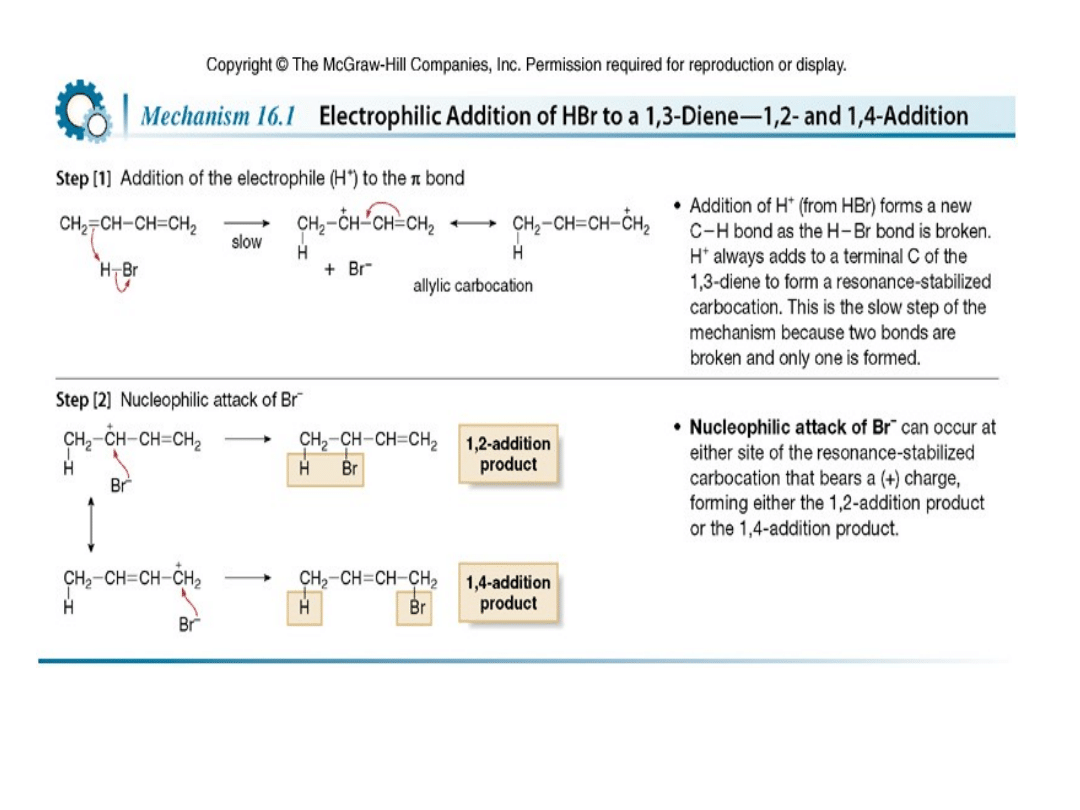
Addition of HX to a conjugated diene forms 1,2- and
1,4-products because of the resonance-stabilized
allylic carbocation intermediate.
H
3
C
CH
3
HCl
H
3
C
CH
3
Cl
+
H
3
C
CH
3
Cl
HCl
Cl
HCl
Cl
HCl
Cl
+
Cl
Cl
+
+
Cl
Kinetic Versus Thermodynamic Products
•The amount of 1,2- and 1,4-addition products
formed in electrophilic addition reactions of
conjugated dienes depends greatly on the
reaction conditions.
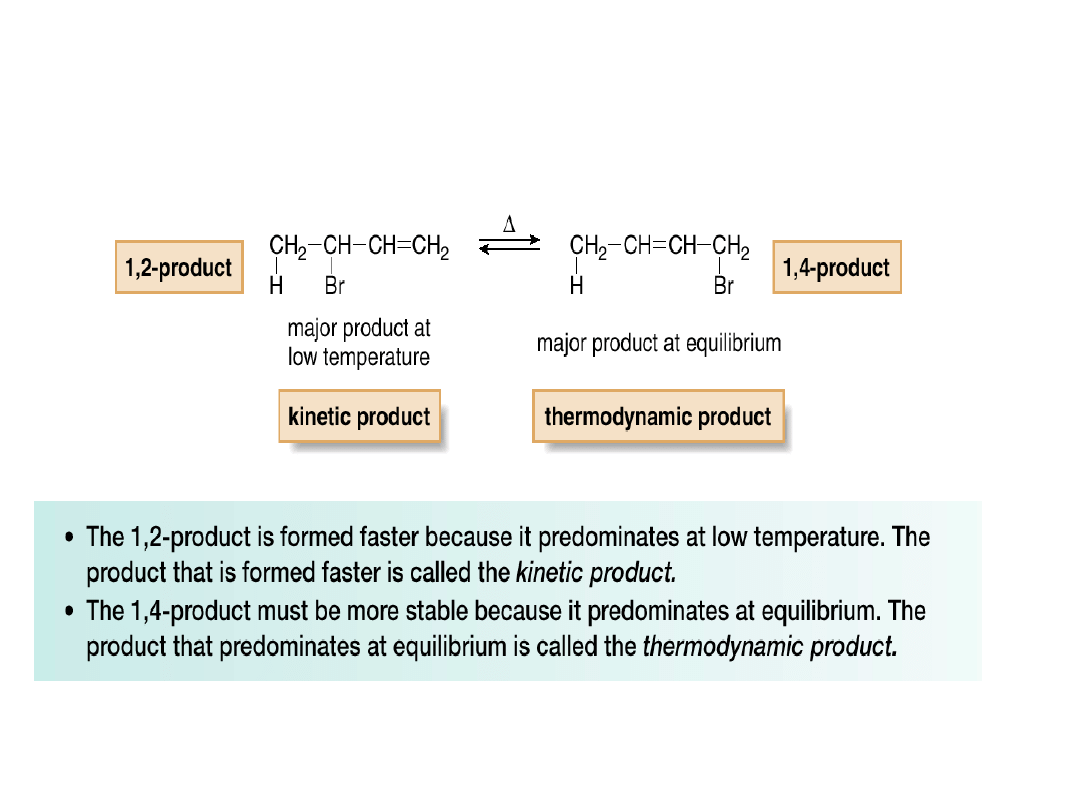
•When a mixture containing predominantly the
1,2-product is heated, the 1,4-addition product
becomes the major product at equilibrium.

•In the reactions we have learned thus far, the
more stable product is formed faster—i.e., the
kinetic and thermodynamic products are the
same.
•The electrophilic addition of HBr to 1,3-
butadiene is different in that the kinetic and
thermodynamic products are different—i.e.,
the more stable product is formed more slowly.
•Why is the more stable product formed more
slowly in this case?
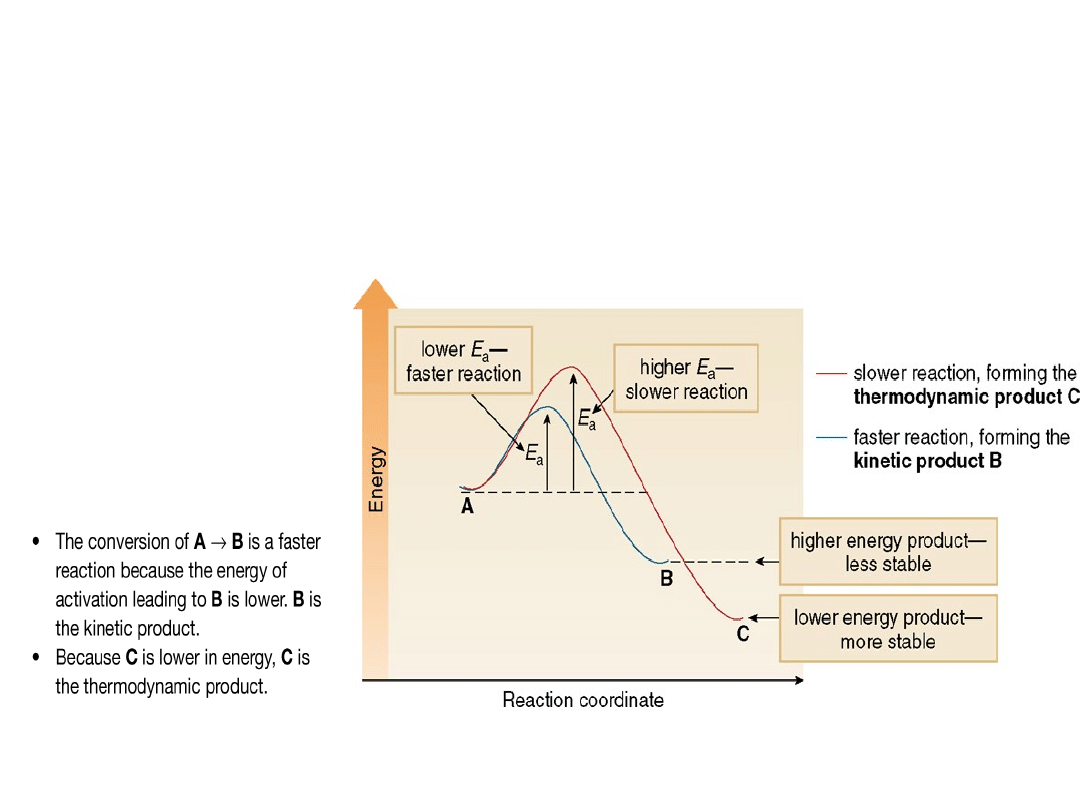
•Recall that the rate of a reaction is
determined by its energy of activation (E
a
),
whereas the amount of product present at
equilibrium is determined by its stability.
Figure 16.6
How kinetic and
thermodynamic products
form
in a reaction: A → B + C
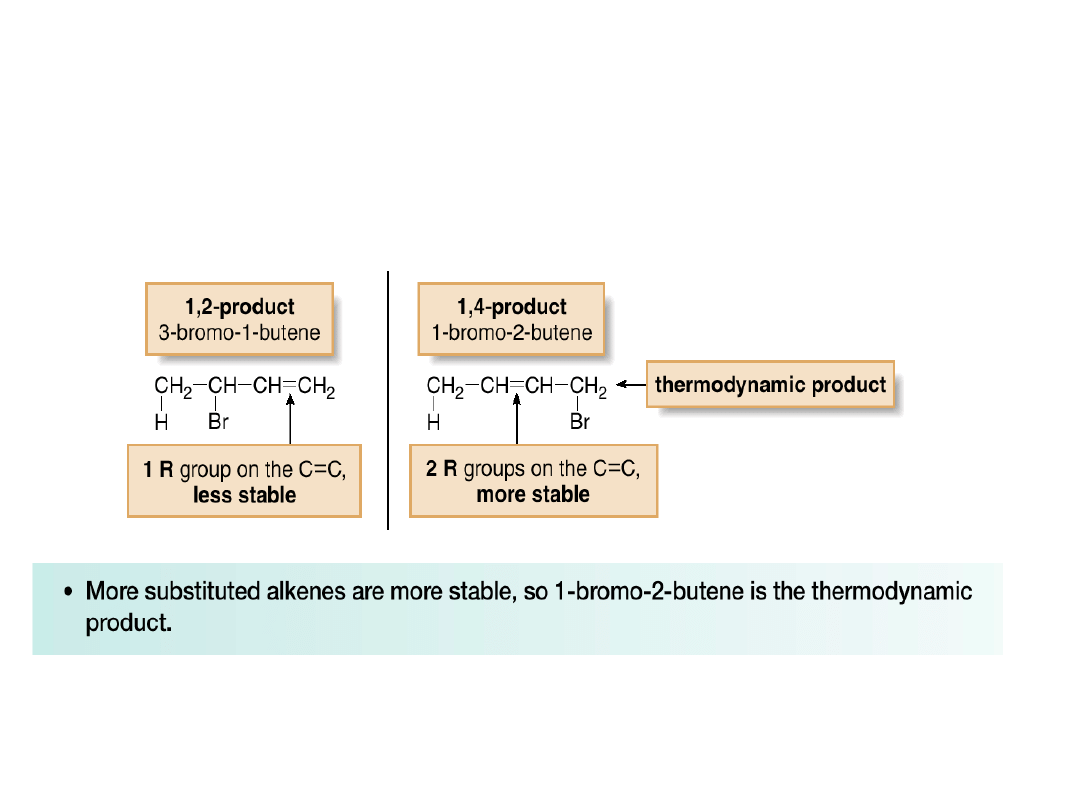
•The 1,4-product (1-bromo-2-butene) is more
stable because it has two alkyl groups bonded
to the carbon-carbon double bond, whereas the
1,2-product (3-bromo-1-butene) has only one.
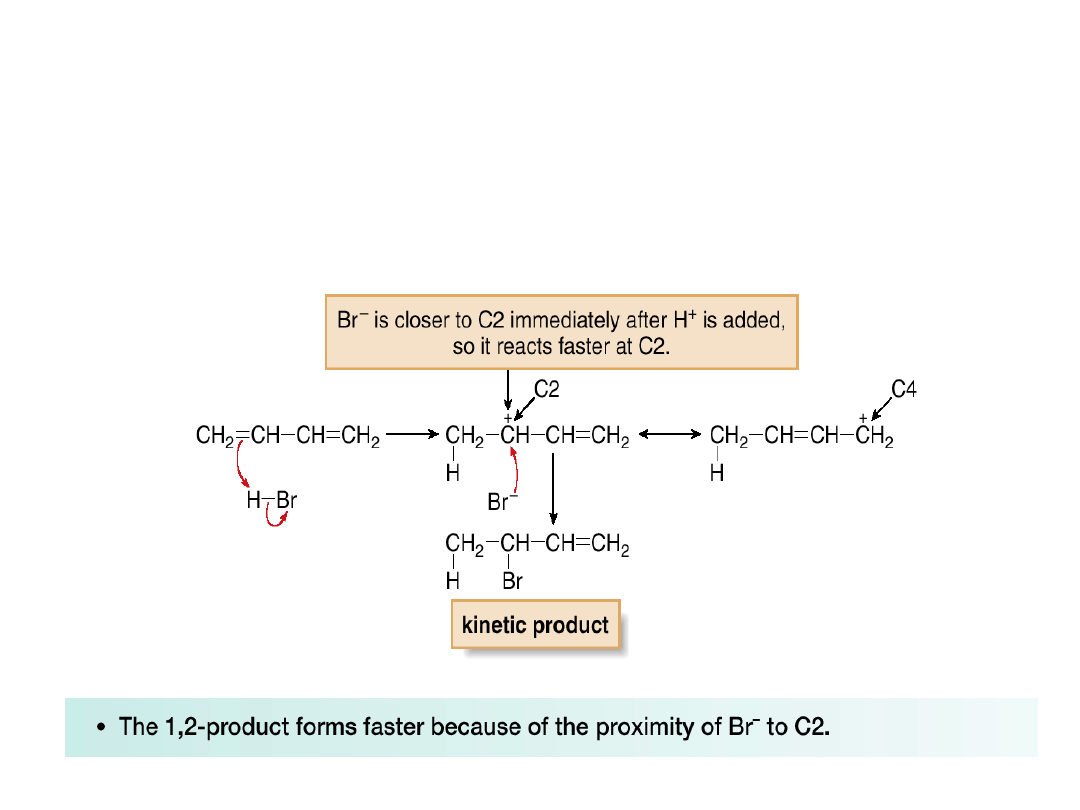
•The 1,2-product is the kinetic product because
of a proximity effect.
•The proximity effect occurs because one
species is close to another.
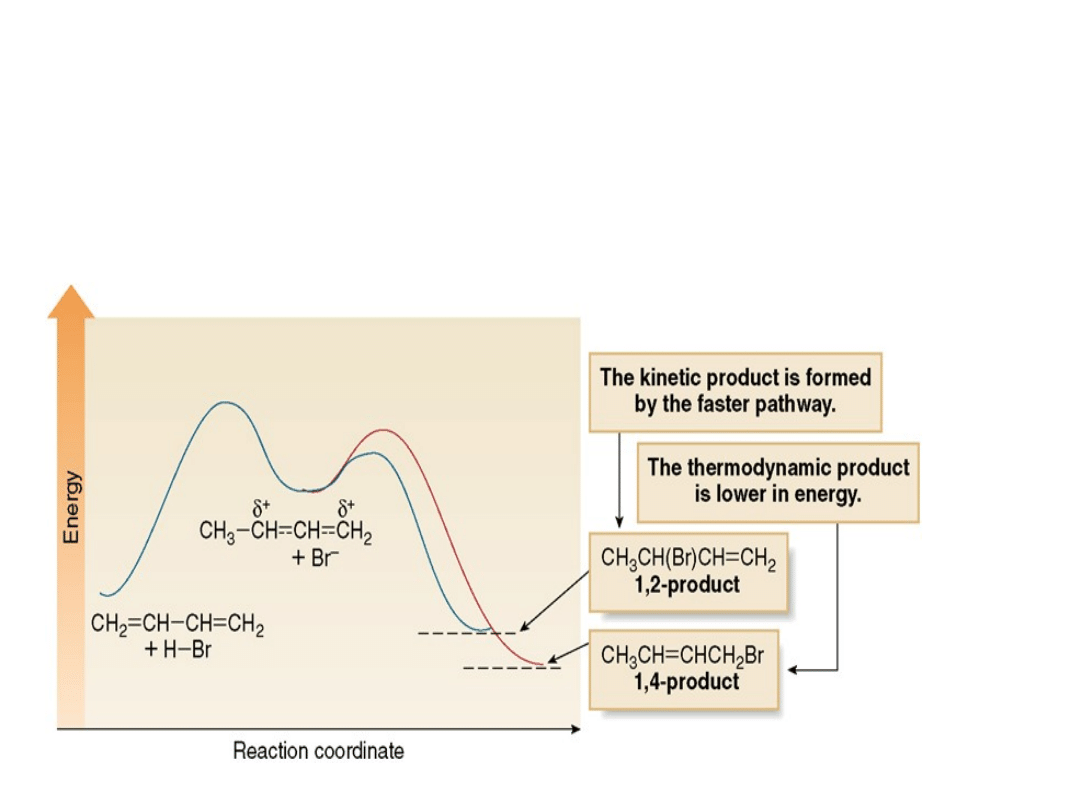
•The overall two-step mechanism for addition
of HBr to 1,3-butadiene to form both 1,2- and
1,4 addition products is illustrated in the
energy diagram below.

Why is the ratio of products temperature
dependent?
•At low temperature, the energy of activation
is the more important factor. Since most
molecules do not have enough kinetic energy
to overcome the higher energy barrier at lower
temperature, they react by the faster pathway,
forming the kinetic product.
•At higher temperature, most molecules have
enough kinetic energy to reach either
transition state. The two products are in
equilibrium with each other, and the more
stable compound, which is lower in energy,
becomes the major product.
H
3
C
HCl
Cl
+
Cl
Label each product as either the kinetic or
thermodynamic product.
Kinetic
product
Thermodyna
mic Product
The 1,2-addition product is the kinetic because ti
forms faster due to proximity. While the 1,4-
addition is the thermodynamic product and is
slower to form.
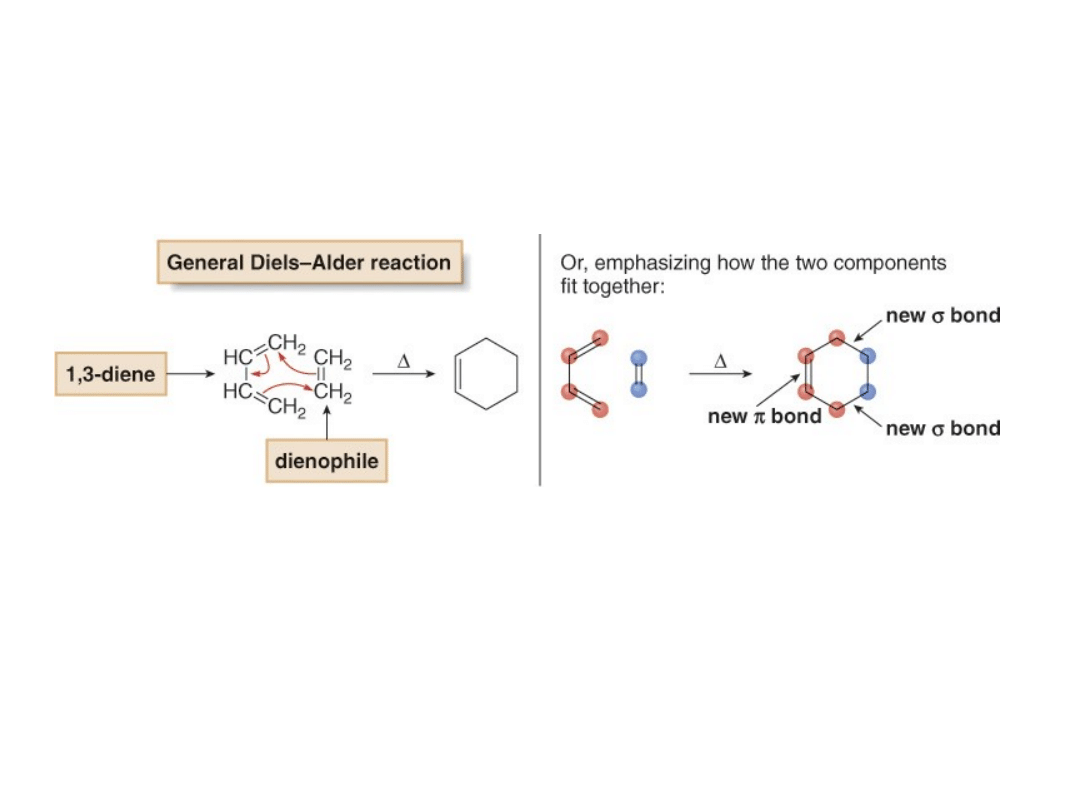
The Diels-Alder Reaction
•The
Diels-Alder reaction
is an addition reaction
between a 1,3-diene and an alkene (called a
dienophile
), to form a new six-membered ring.
•Three curved arrows are needed to show the cyclic
movement of electron pairs because three bonds
break and two bonds and one bond form.
•Because each new bond is ~20 kcal/mol stronger
than a bond that is broken, a typical Diels-Alder
reaction releases ~40 kcal/mol of energy.
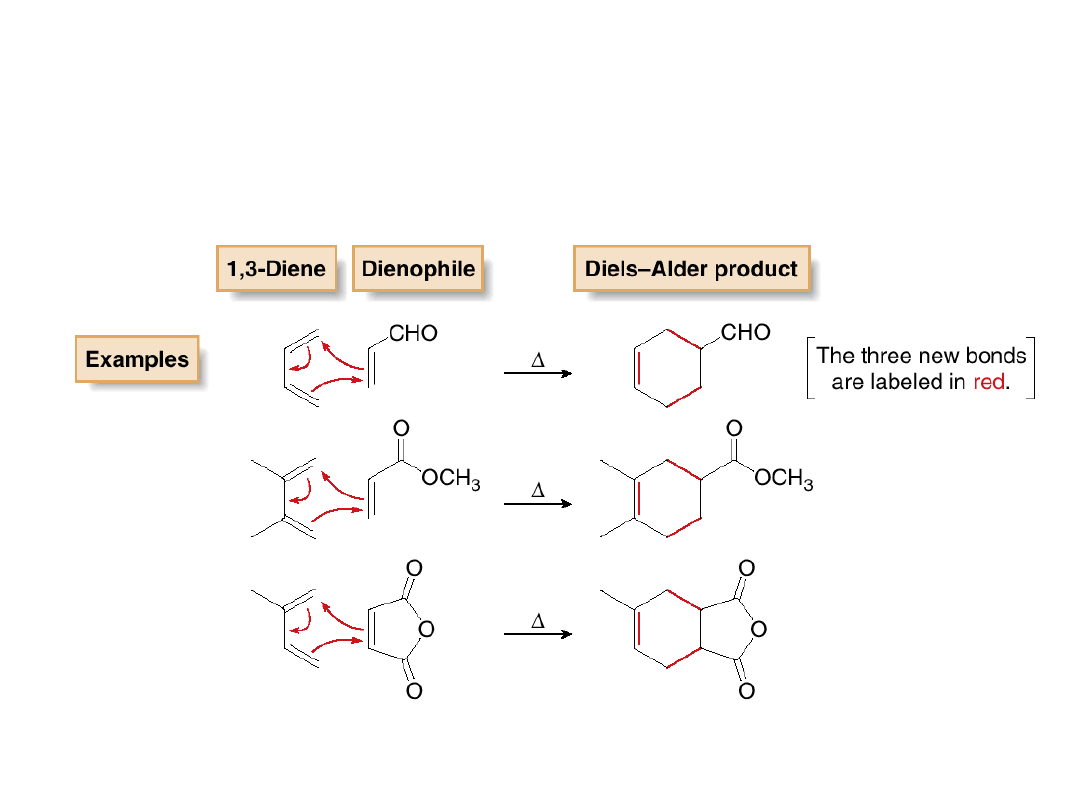
Some examples of Diels-Alder reactions are
shown below:

All Diels-Alder reactions have the following
features in common:
1. They are initiated by heat; that is, the Diels-
Alder reaction is a thermal reaction.
2. They form new six-membered rings.
3. Three bonds break, and two new C—C
bonds and one new C—C bond forms.
4. They are
concerted
; that is, all old bonds are
broken and all new bonds are formed in a
single step.
+
COOH
heat
COOH
+
CO
2
CH
3
heat
CO
2
CH
3
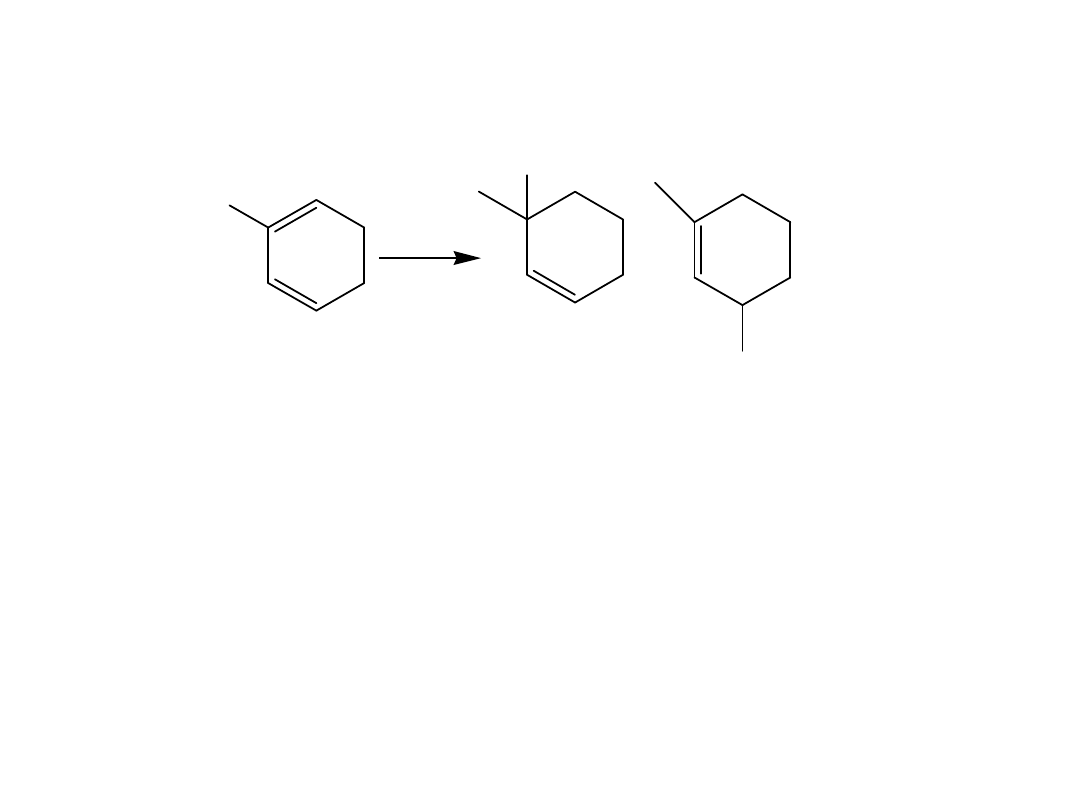
Predict the products.
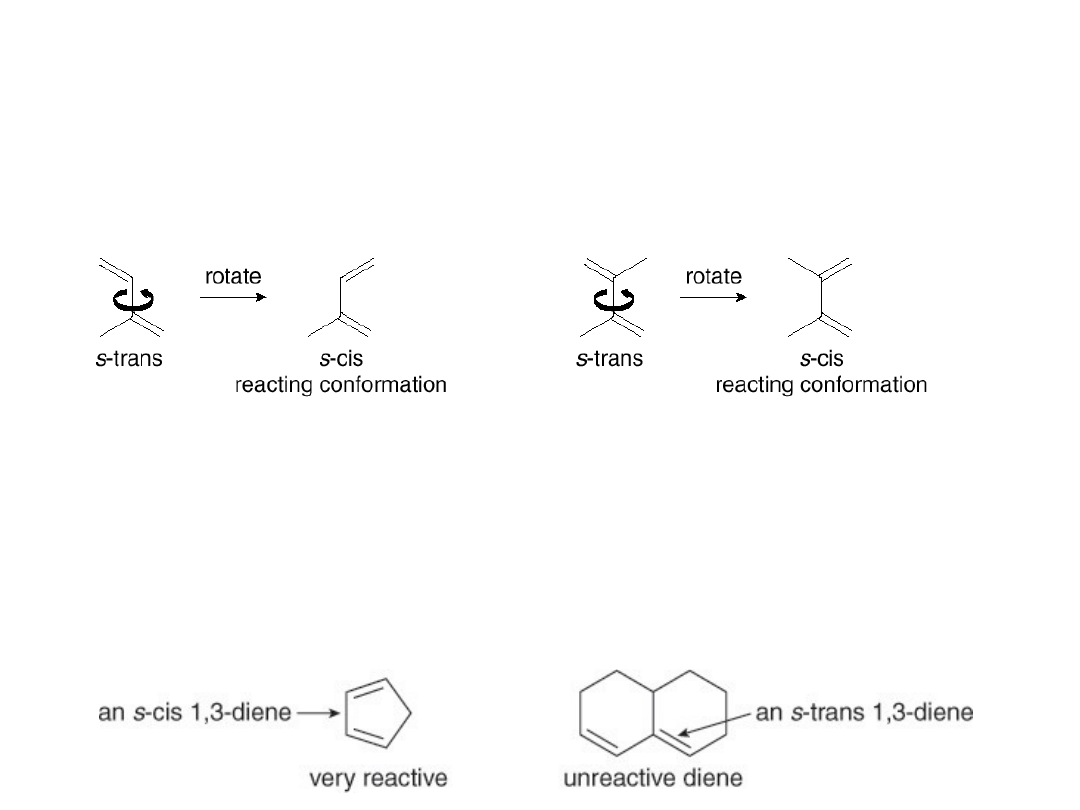
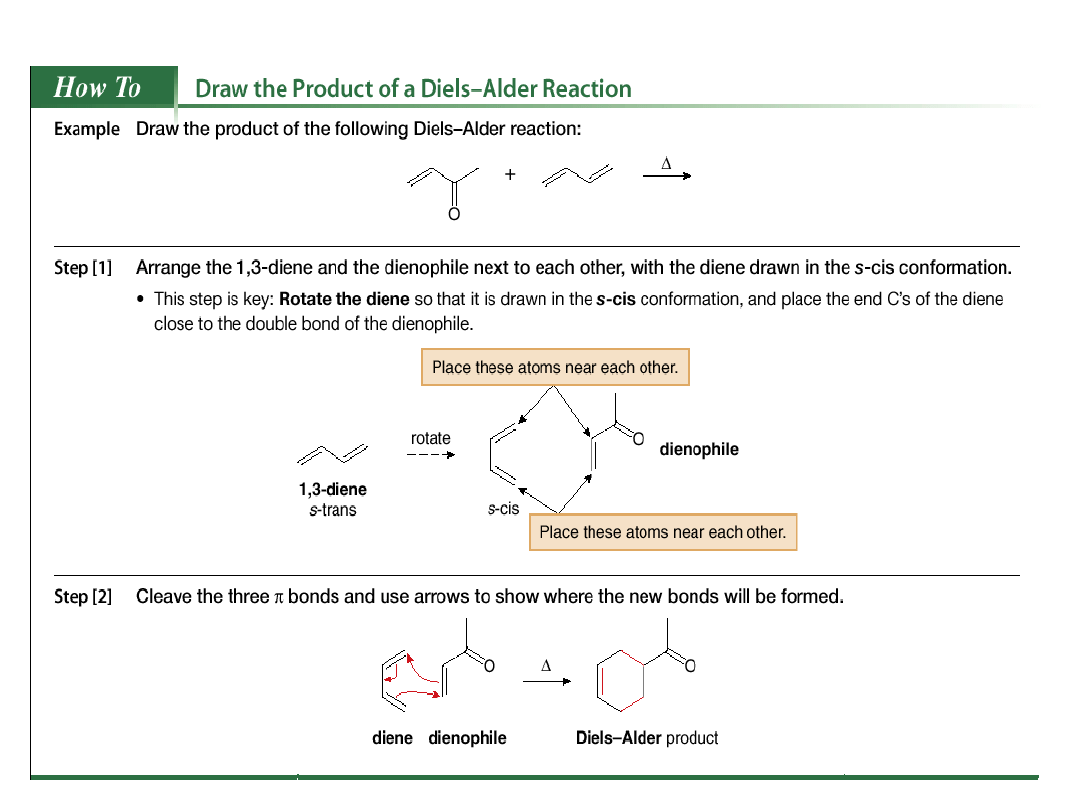
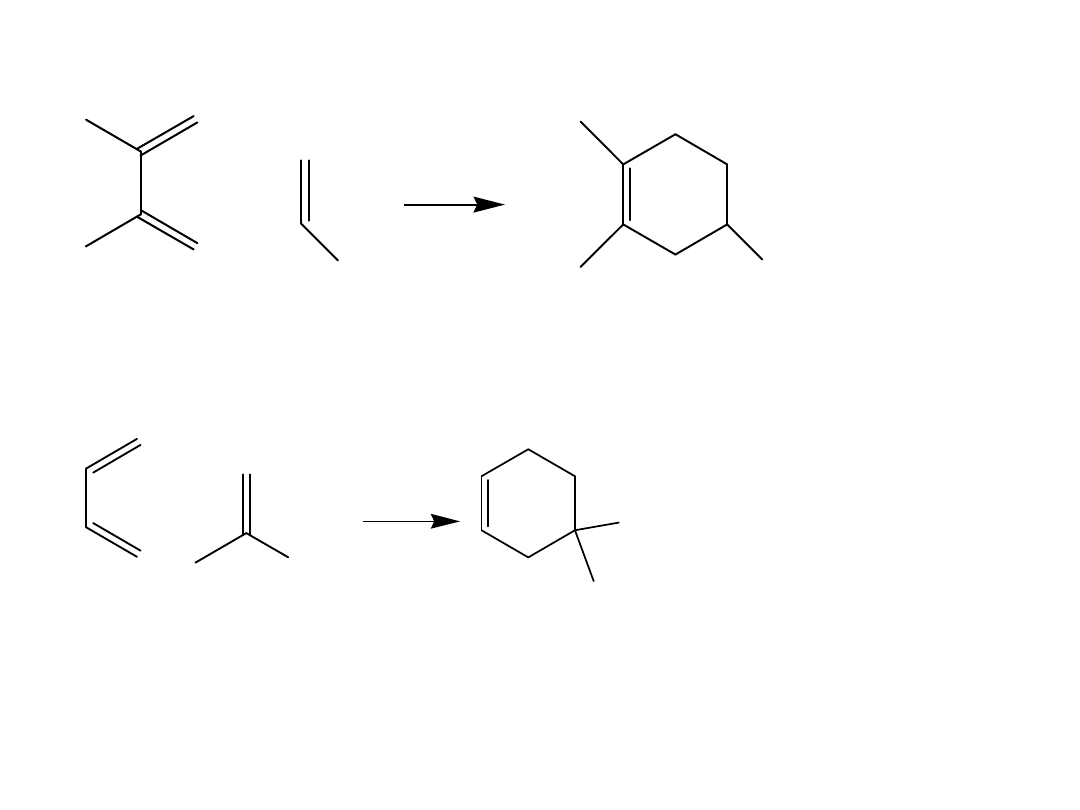
Several rules govern the course of the Diels-
Alder reaction.
1. The diene can react only when it adopts the s-cis
conformation.
This rotation is prevented in cyclic alkenes. When the
two double bonds are constrained to an s-cis
conformation, the diene is unusually reactive. When
the two double bonds are constrained in the s-trans
conformation, the diene is unreactive.
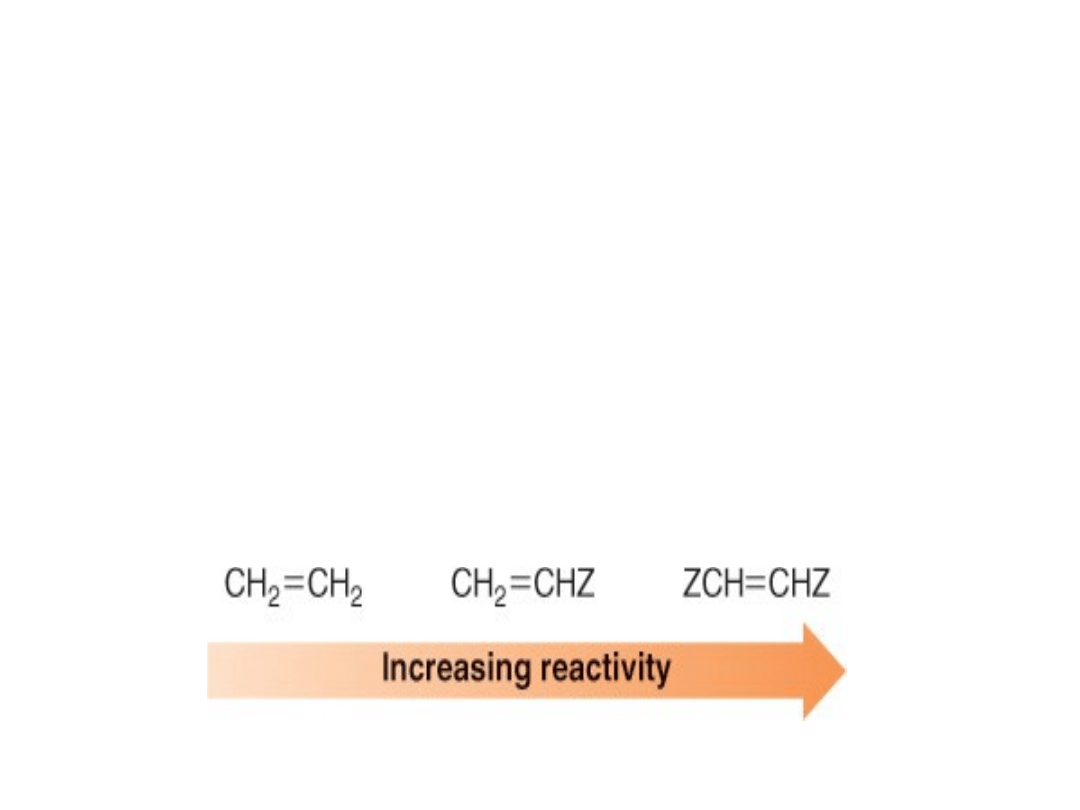
2. Electron-withdrawing substituents in the
dienophile increase the reaction rate.
•In a Diels-Alder reaction, the conjugated diene
acts as a nucleophile and the dienophile acts as
an electrophile.
•Electron-withdrawing
groups
make
the
dienophile more electrophilic (and thus more
reactive) by withdrawing electron density from
the carbon-carbon double bond.
•If Z is an electron-withdrawing group, then the
reactivity of the dienophile increases as follows:
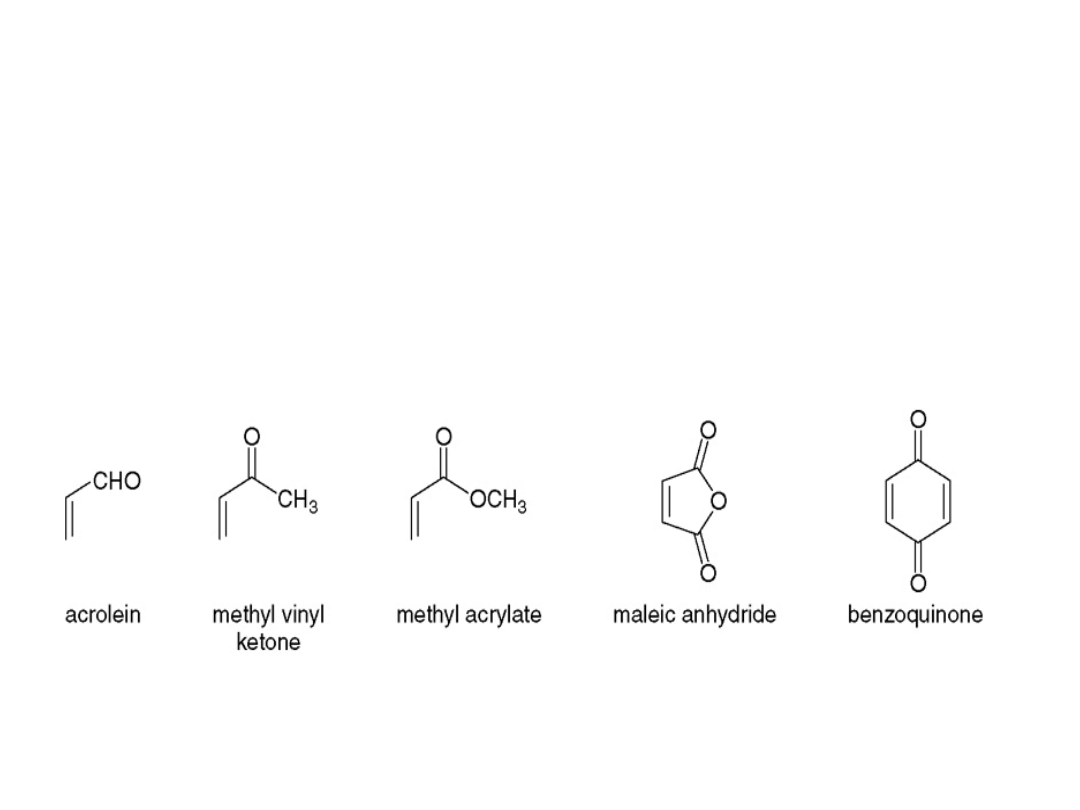
•A carbonyl group is an effective electron-
withdrawing group because it bears a partial
positive charge (+), which withdraws electron
density from the carbon—carbon double bond
of the dienophile.
•Some common dienophiles are shown below:
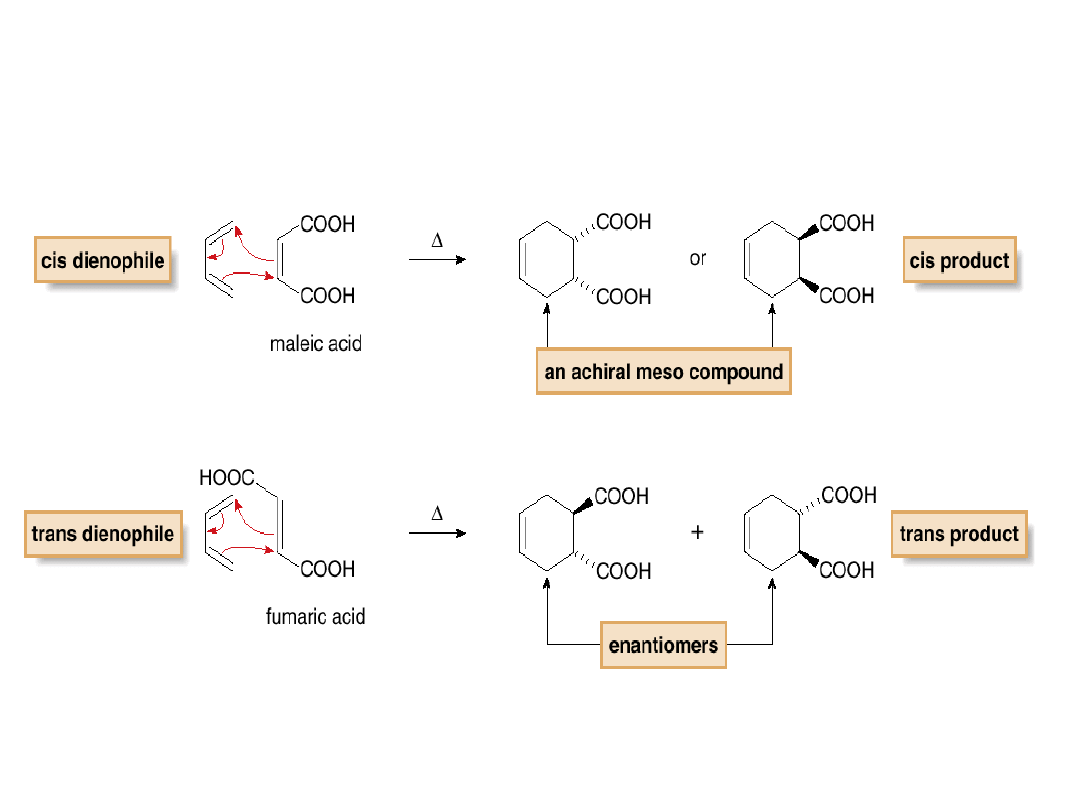
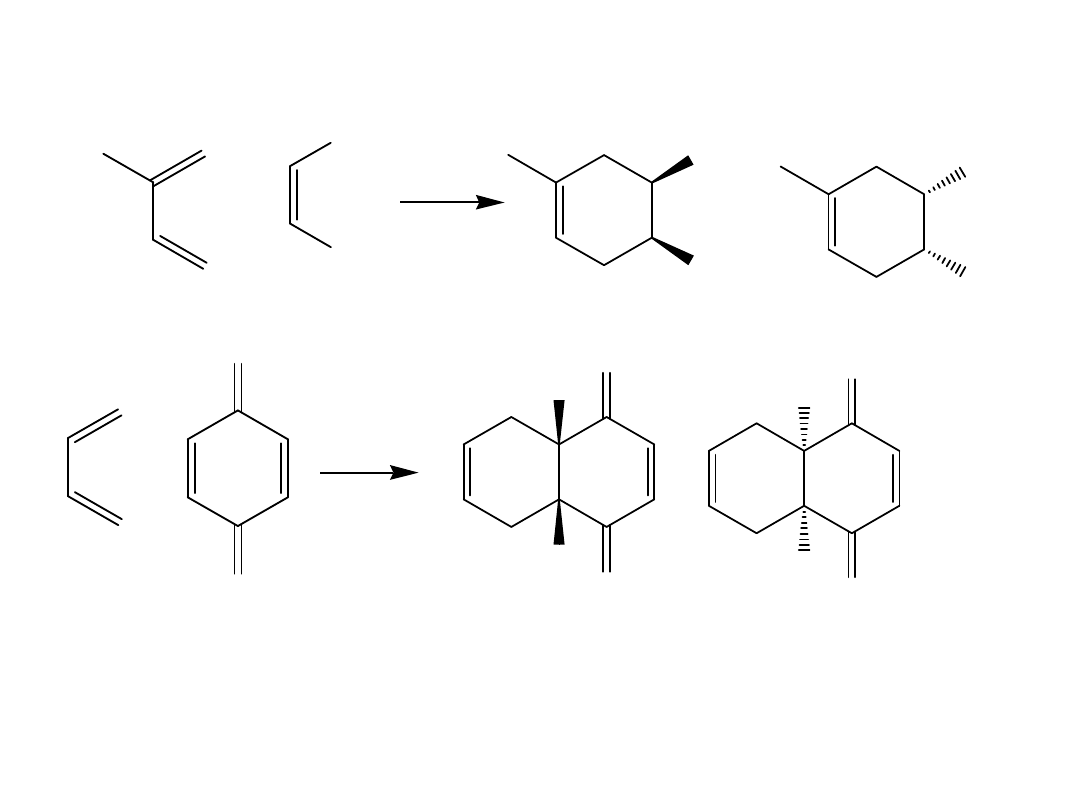
3.
The stereochemistry of the dienophile is
retained.
+
CO
2
CH
3
CO
2
CH
3
heat
CO
2
CH
3
CO
2
CH
3
+
CO
2
CH
3
CO
2
CH
3
+
O
O
heat
O
O
H
H
+
O
O
H
H
Predict the products.
These two cis products are identical.
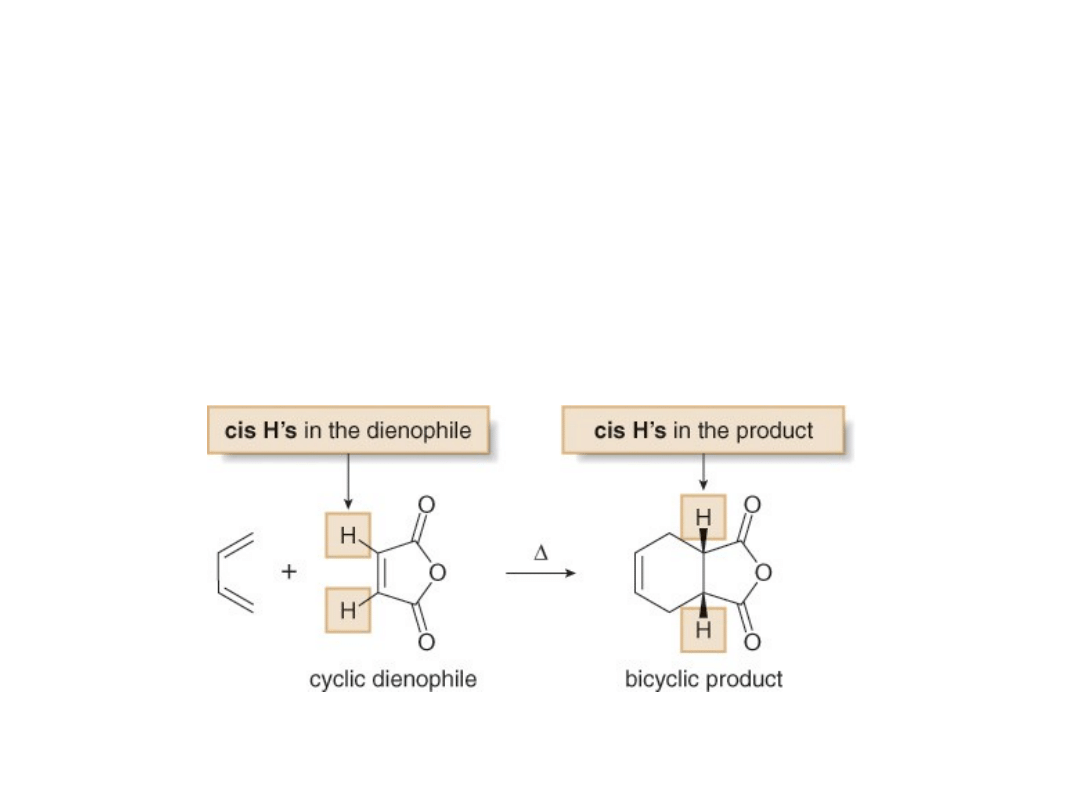
•A cyclic dienophile forms a
bicyclic product
.
•A bicyclic system in which two rings share a
common C—C bond is called a fused ring
system. The two H atoms of the ring fusion
must be cis, because they were cis in the
starting dienophile
•A bicyclic system of this sort is said to be
cis-
fused
.
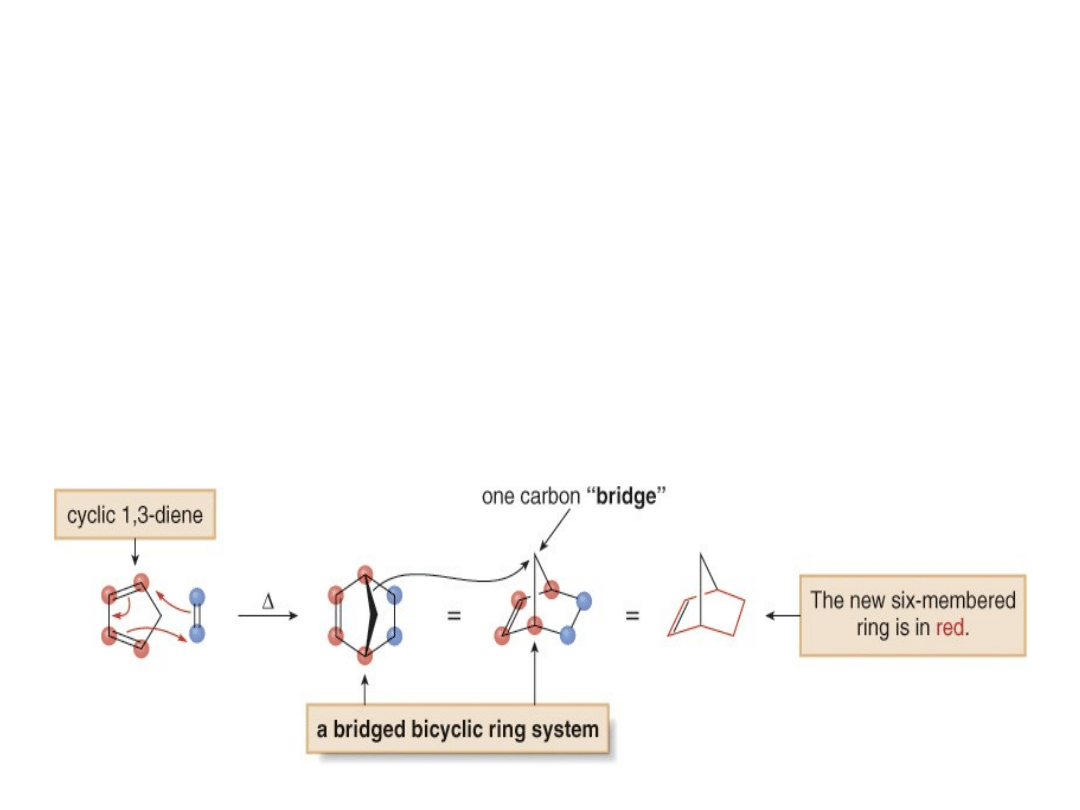
4.
When endo and exo products are possible,
the endo product is preferred.
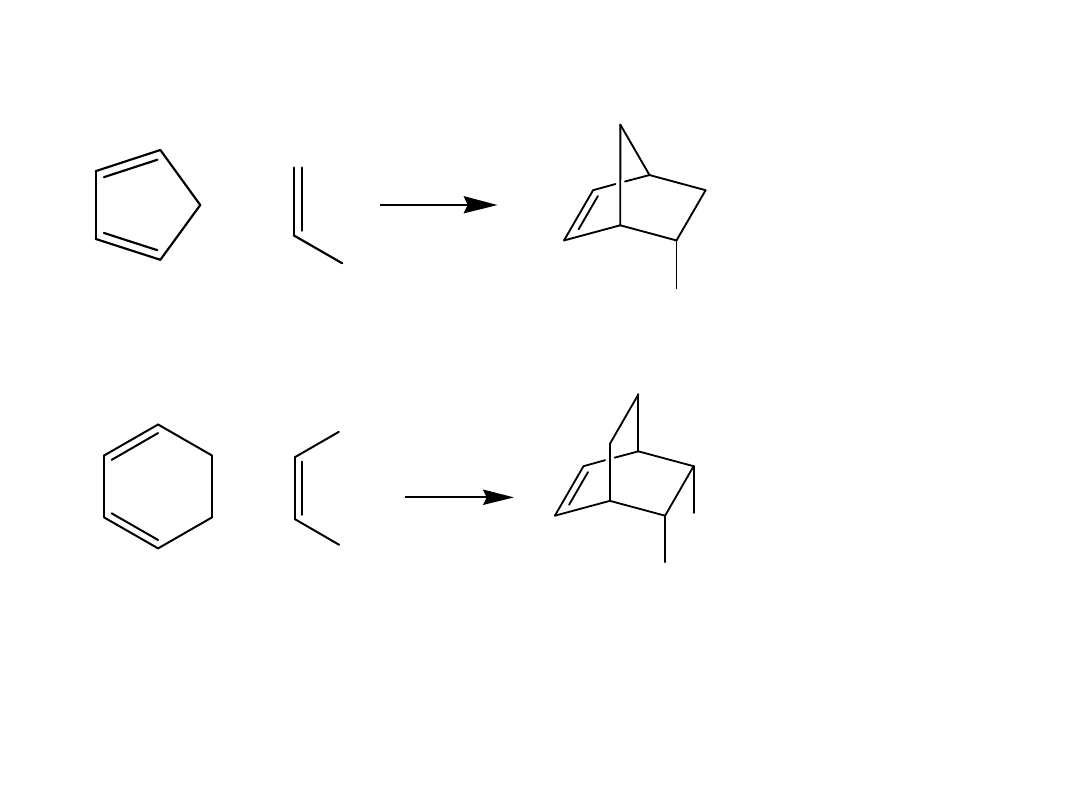
•Consider the reaction of 1,3-cyclopentadiene with
ethylene. A new six-membered ring forms and above
the ring there is a one atom “bridge.”
•Thus, the product is bicyclic, but the carbon atoms
shared by both rings are non-adjacent.
•A bicyclic ring system in which the two rings share
non-adjacent carbon atoms is called a
bridged ring
system
.
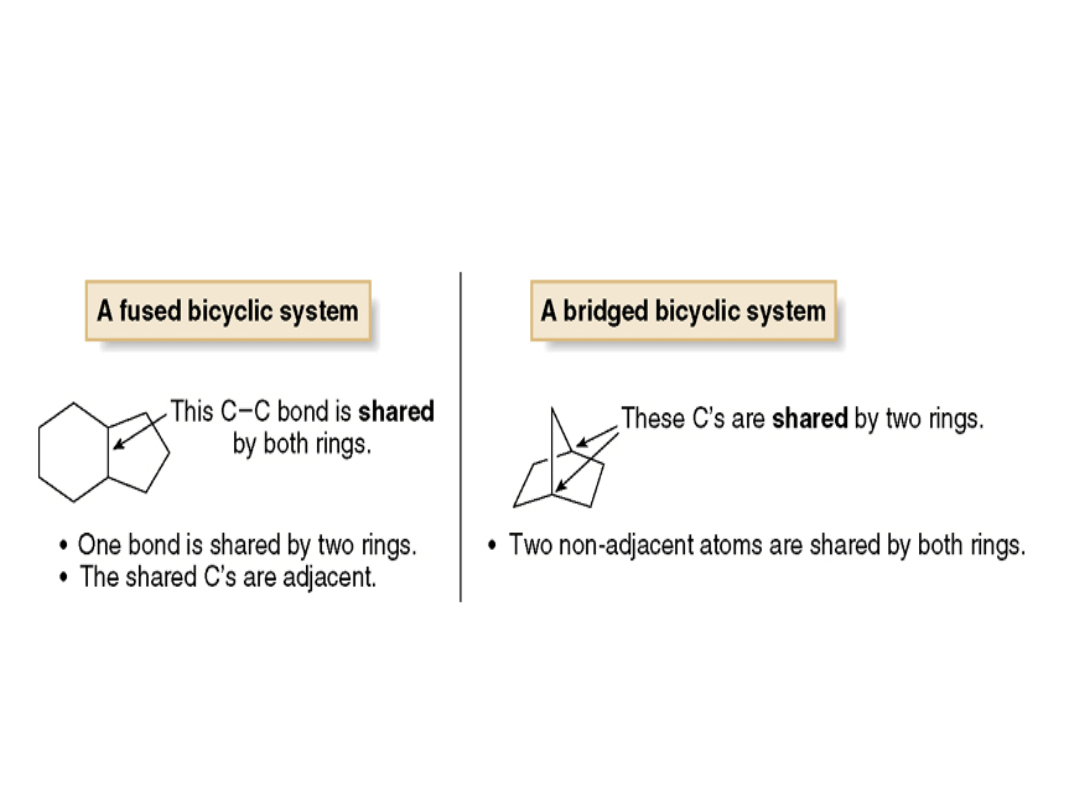
Figure 16.10
Fused and bridged bicyclic
ring systems compared
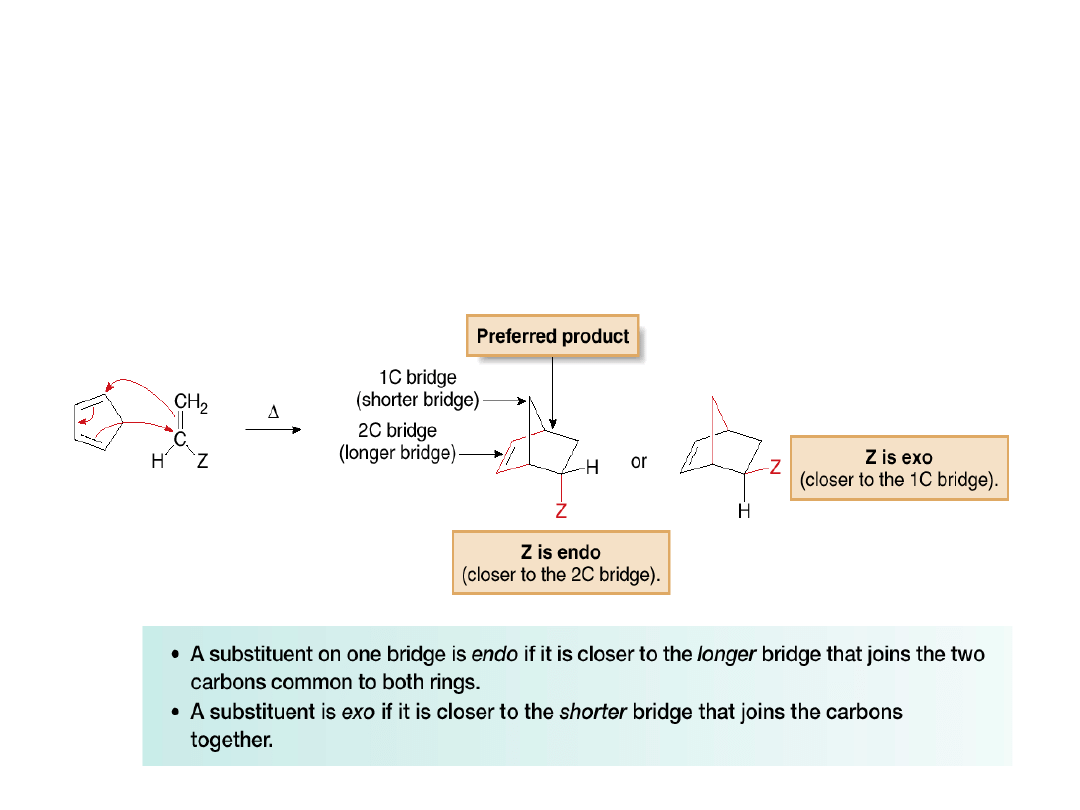
•When
cyclopentadiene
reacts
with
a
substituted alkene as the dienophile (CH
2
=CHZ),
the substituent Z can be oriented in one of two
ways in the product.
•The terms
endo
and
exo
are used to indicate
the position of Z.
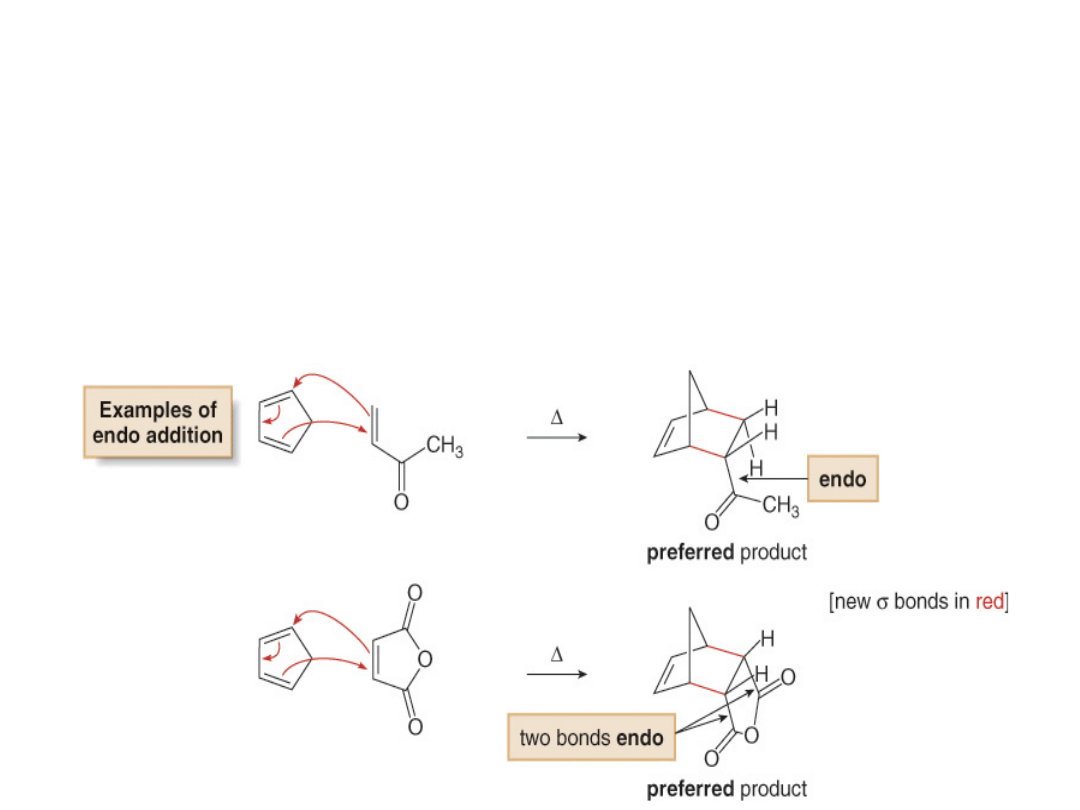
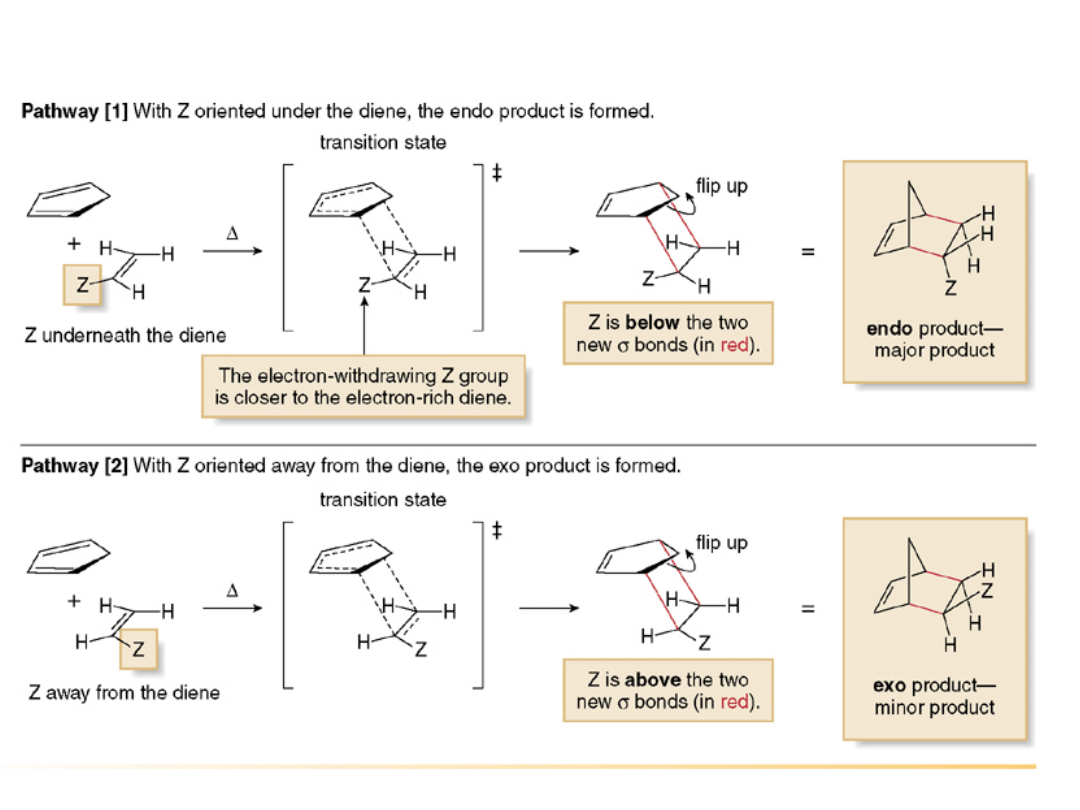
•In a Diels-Alder reaction, the endo product is
preferred, as shown in the two examples
below. The transition state leading to the endo
product allows more interaction between the
electron
rich
diene
and
the
electron-
withdrawing substituent Z on the dienophile,
an energetically favorable arrangement.
+
CO
2
CH
3
heat
CO
2
CH
3
+
CO
2
CH
3
CO
2
CH
3
heat
CO
2
CH
3
CO
2
CH
3
Predict the products.

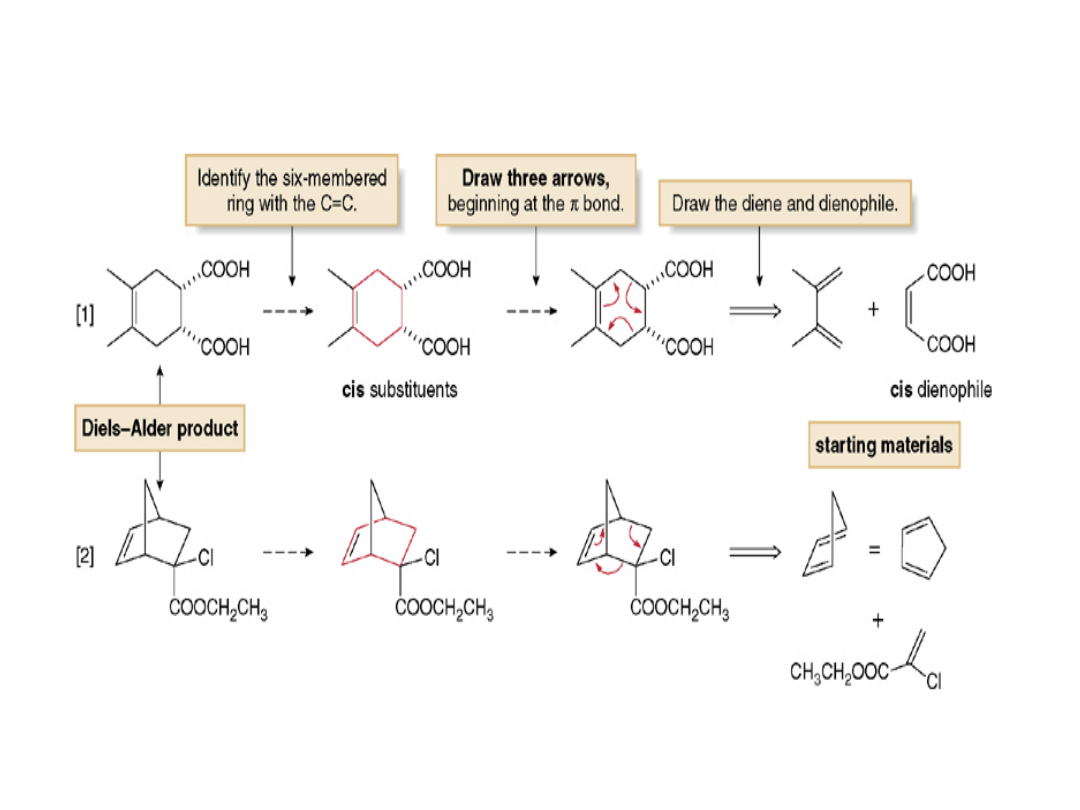
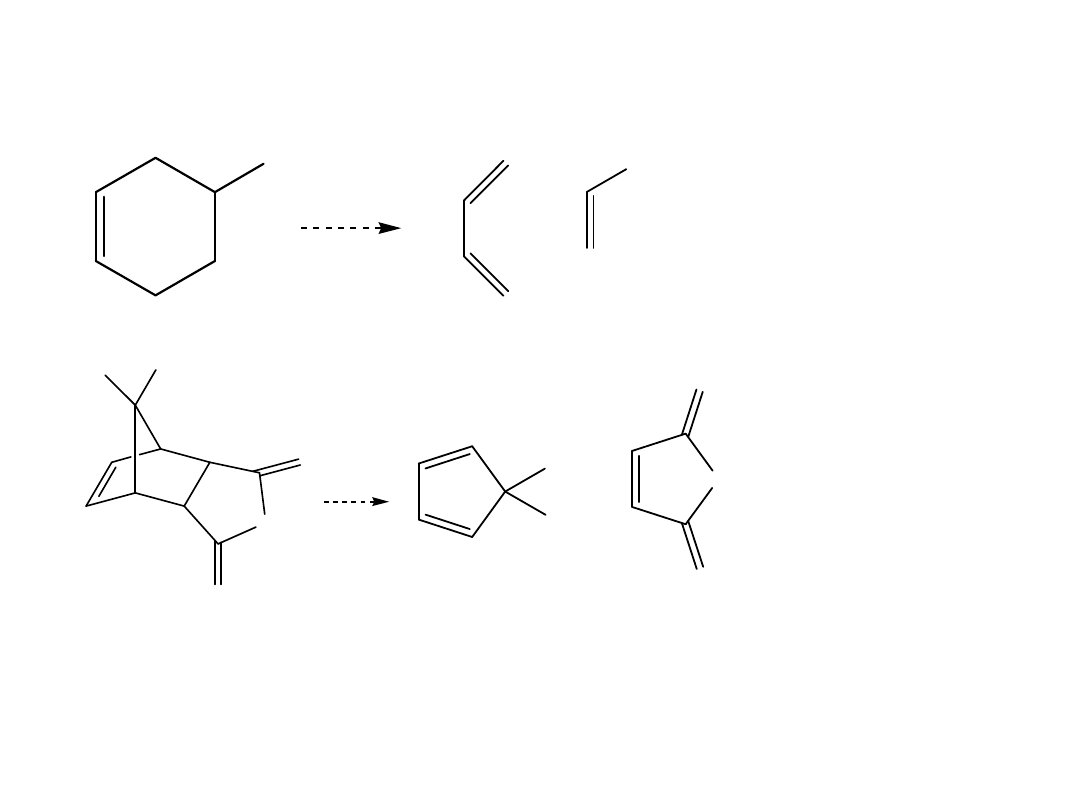
The Diels-Alder Reaction—Retrosynthetic
Analysis
To draw the starting materials from a given
Diels-Alder adduct:
•Locate the six-membered ring that contains
the C=C.
•Draw three arrows around the cyclohexane
ring, beginning with the bond and two
bonds, and forming three bonds.
•Retain the stereochemistry of substituents on
the C=C of the dienophile; cis substituents on
the six-membered ring give a cis dienophile.
CO
2
CH
2
CH
3
+
CO
2
CH
2
CH
3
O
Cl
Cl
O
O
Cl
Cl
+
O
O
O
Predict the starting
materials.
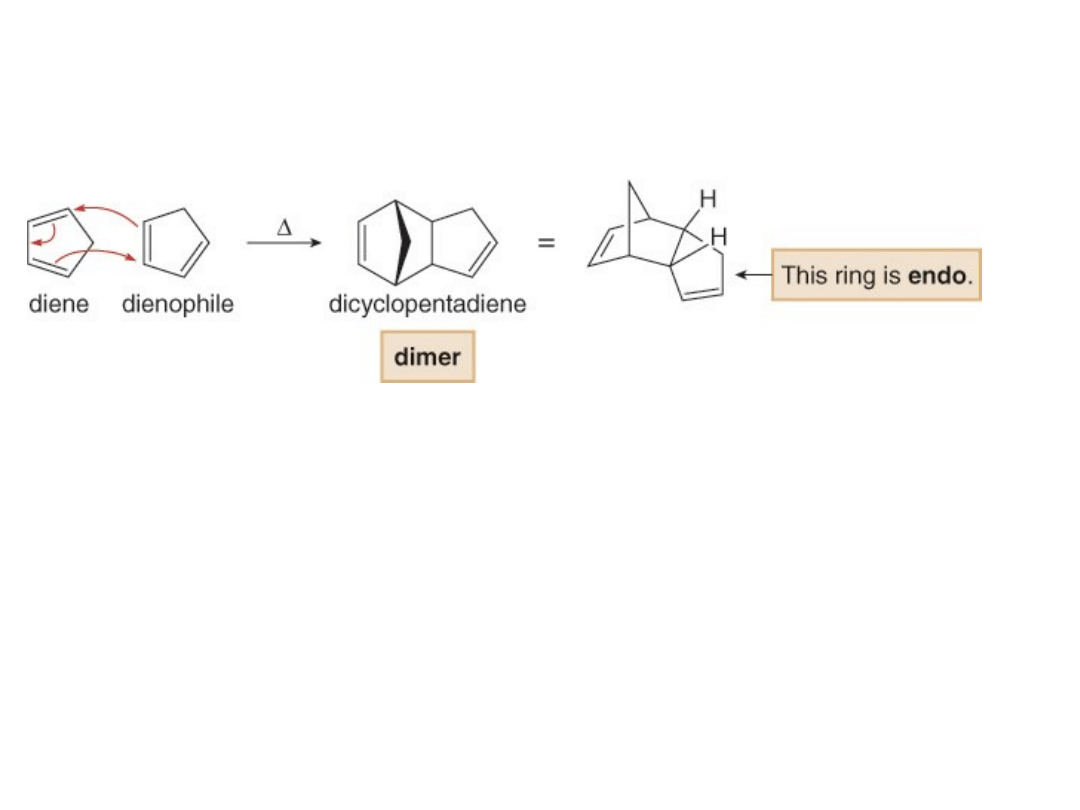
The Retro Diels-Alder Reaction
•The formation of dicyclopentadiene is so
rapid that it takes only a few hours at room
temperature
for
cyclopentadiene
to
completely dimerize.
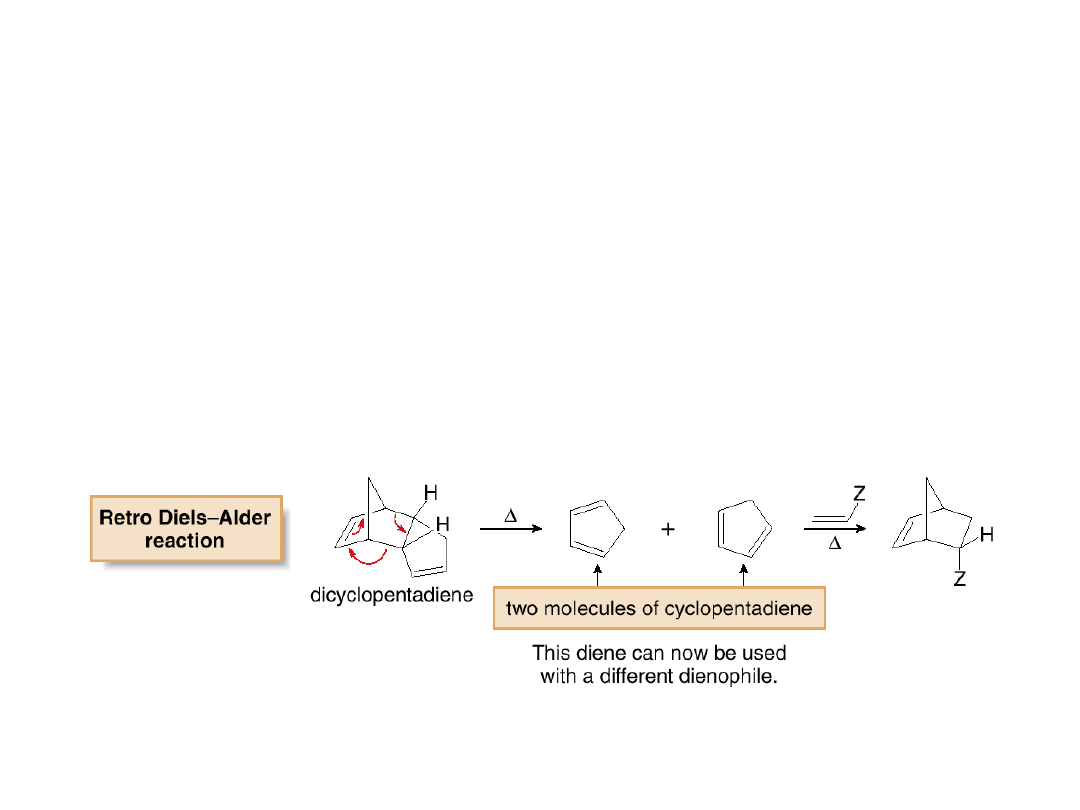
•When heated, dicyclopentadiene undergoes a
retro
Diels-Alder
reaction
,
and
two
molecules
of
cyclopentadiene are re-formed.
•If
the
newly
produced
cyclopentadiene
is
immediately treated with a different dienophile, it
reacts to form a new Diels-Alder adduct with this
dienophile.
•This is how cyclopentadiene used in Diels-Alder
reactions is produced.
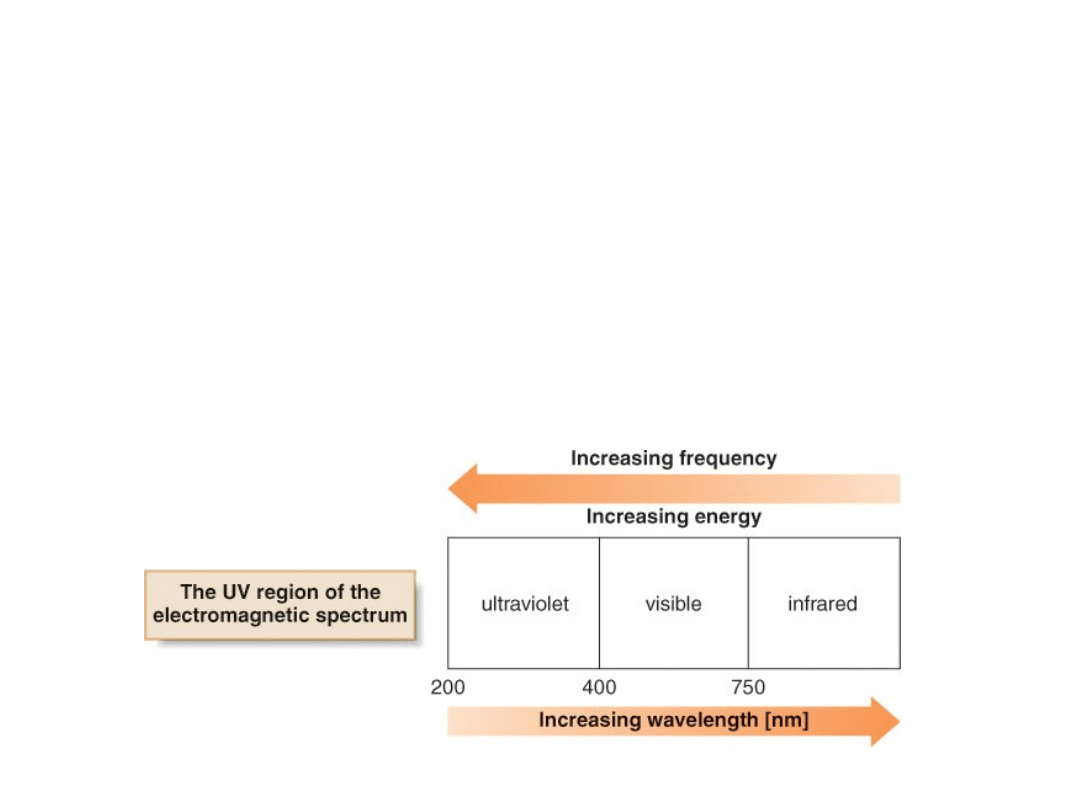
Conjugated Dienes and Ultraviolet Light
•The absorption of ultraviolet (UV) light by a
molecule can promote an electron from a lower
electronic state to a higher one.
•Ultraviolet light has a slightly shorter
wavelength (and thus higher frequency) than
visible light.
•The most useful region of UV light for this
purpose is 200-400 nm.
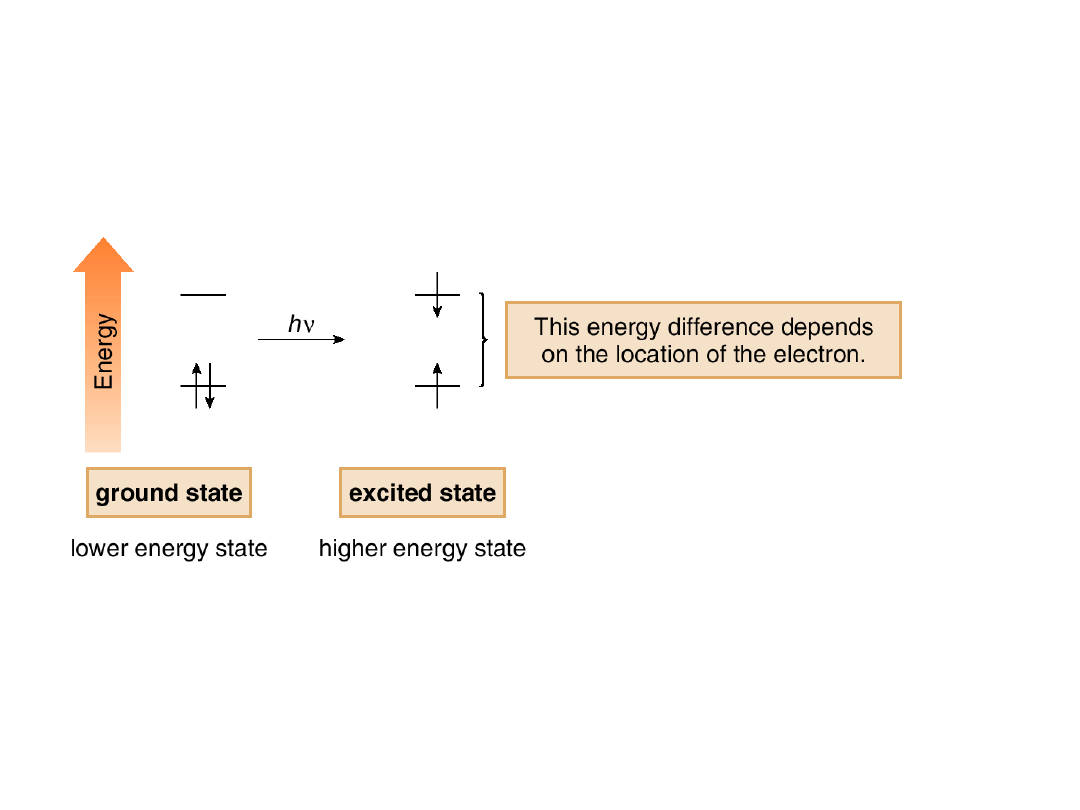
•When electrons in a lower energy state (the
ground state) absorb light having the
appropriate energy, an electron is promoted to
a higher electronic state (excited state).
•The energy difference between the two
states depends on the location of the
electron.
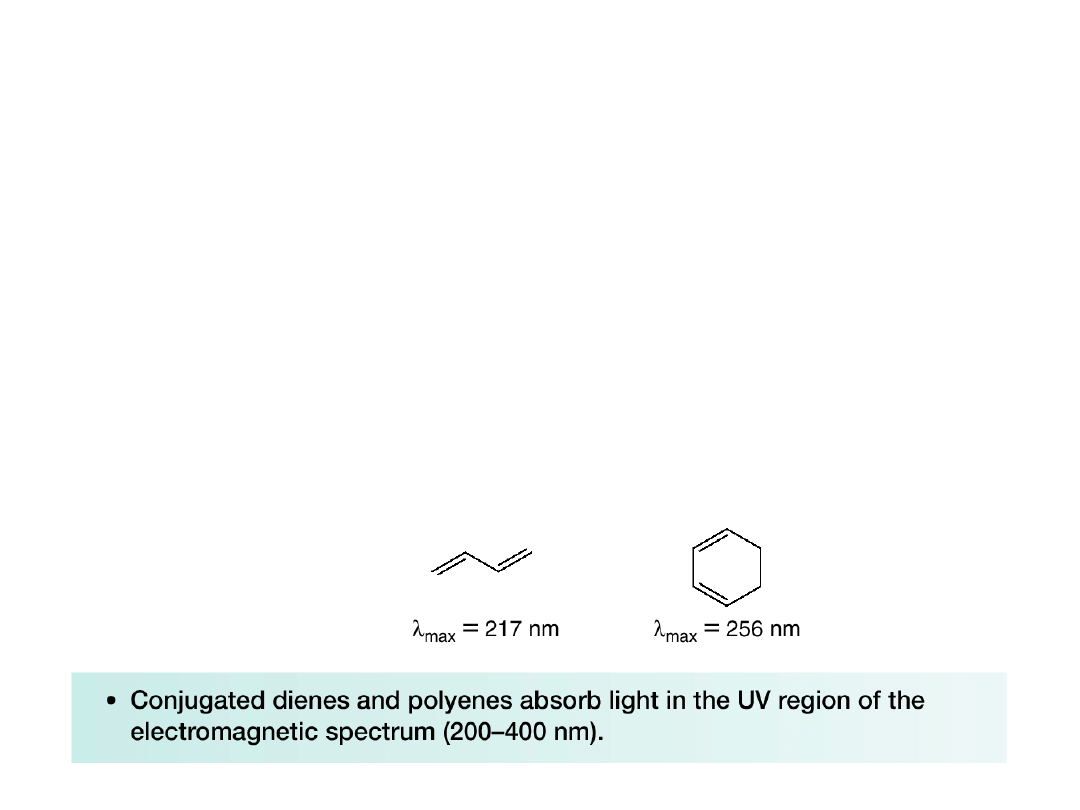
•The promotion of electrons in bonds and
unconjugated bonds requires light having a
wavelength of < 200 nm; that is, a shorter
wavelength and higher energy than light in the
UV region of the electromagnetic spectrum.
•With conjugated dienes, the energy difference
between the ground and excited states
decreases, so longer wavelengths of light can
be used to promote electrons.
•The wavelength of UV light absorbed by a
compound is often referred to as its
max
.
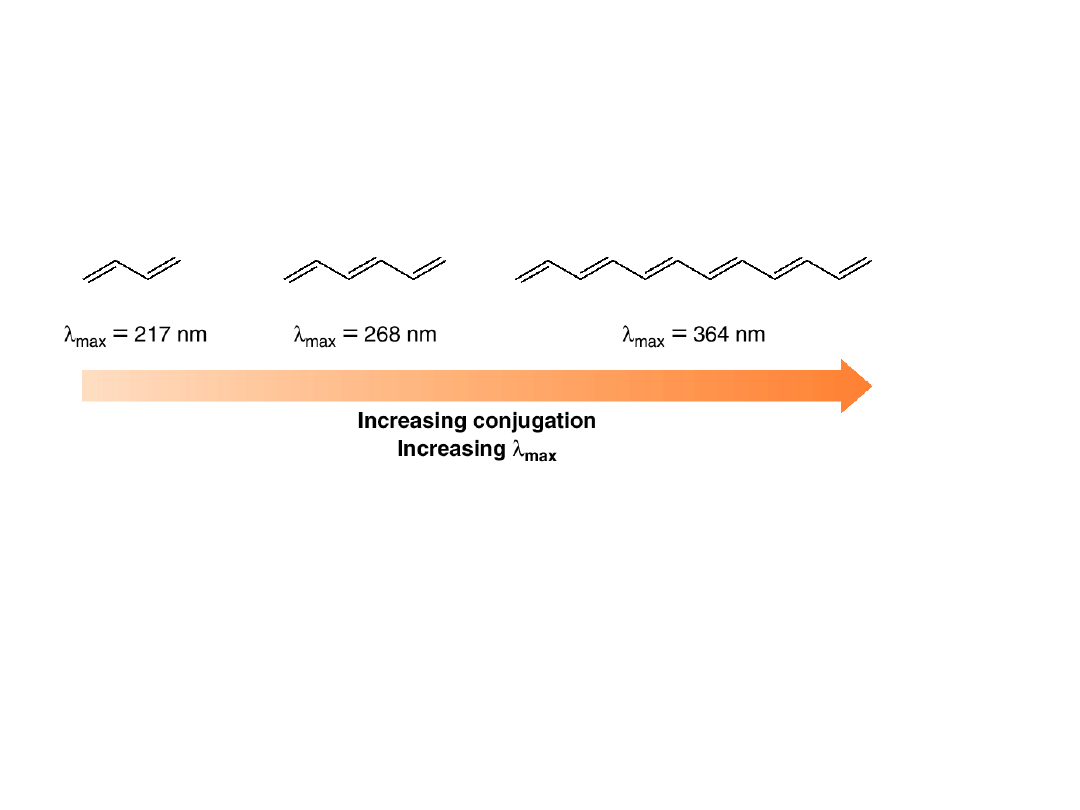
•As the number of conjugated bonds
increases, the energy difference between the
ground and excited state decreases, shifting
the absorption to longer wavelengths.
•With molecules having eight or more
conjugated bonds, the absorption shifts
from the UV to the visible region, and the
compound takes on the color of the light it
does not absorb.
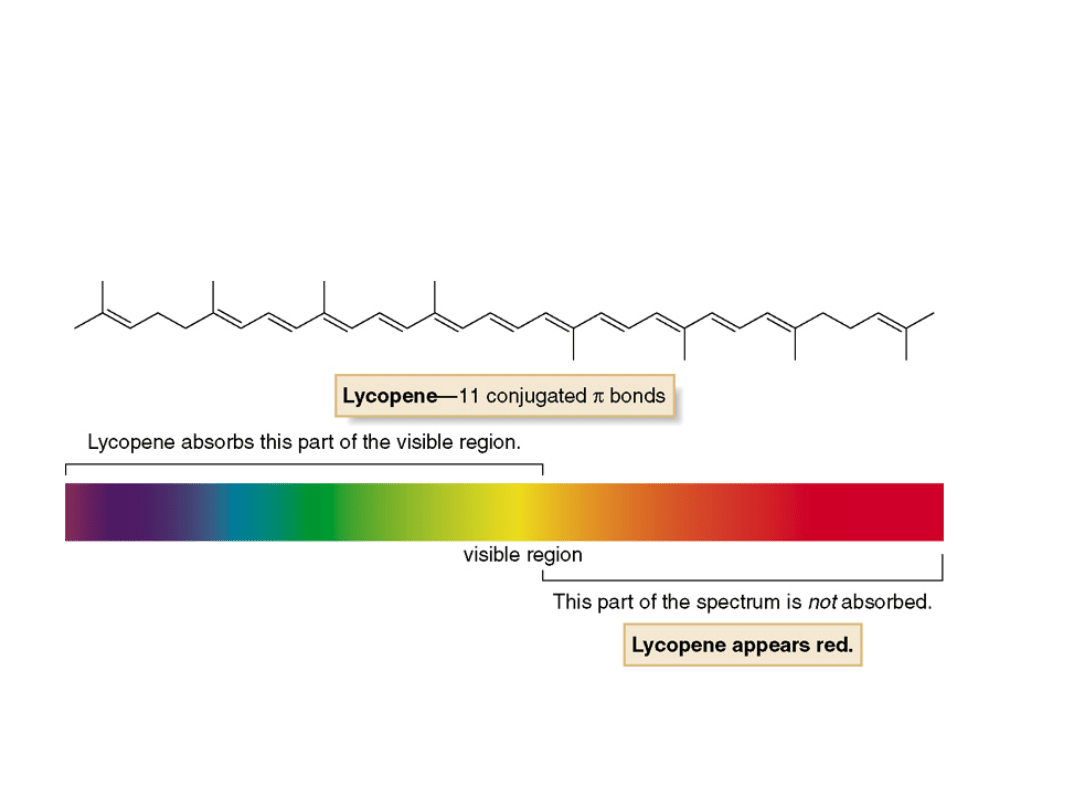
•Lycopene
absorbs visible light at
max
= 470
nm, in the blue-green region of the visible
spectrum. Because it does not absorb light in
the red region, lycopene appears bright red.
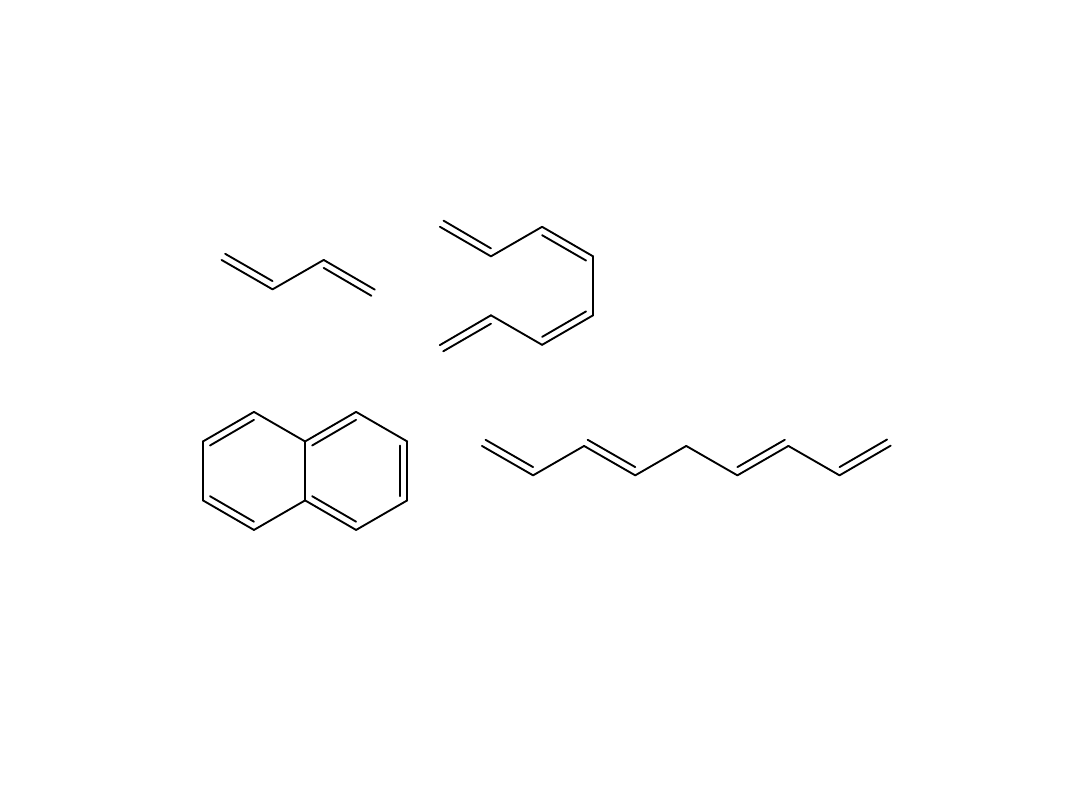
A
B
C
D
Which compound absorbs the longest wavelength of
radiation?
Document Outline
- PowerPoint Presentation
- Slide 2
- Slide 3
- Slide 4
- Slide 5
- Slide 6
- Slide 7
- Slide 8
- Slide 9
- Slide 10
- Slide 11
- Slide 12
- Slide 13
- Slide 14
- Slide 15
- Slide 16
- Slide 17
- Slide 18
- Slide 19
- Slide 20
- Slide 21
- Slide 22
- Slide 23
- Slide 24
- Slide 25
- Slide 26
- Slide 27
- Slide 28
- Slide 29
- Slide 30
- Slide 31
- Slide 32
- Slide 33
- Slide 34
- Slide 35
- Slide 36
- Slide 37
- Slide 38
- Slide 39
- Slide 40
- Slide 41
- Slide 42
- Slide 43
- Slide 44
- Slide 45
- Slide 46
- Slide 47
- Slide 48
- Slide 49
- Slide 50
- Slide 51
- Slide 52
- Slide 53
- Slide 54
Wyszukiwarka
Podobne podstrony:
Conjugation Part 1
odmiana czasownikow nieregularnych conjugar es facil part 3
odmiana czasownikow nieregularnych conjugar es facil part 1
odmiana czasownikow nieregularnych conjugar es facil part 2
odmiana czasownikow nieregularnych conjugar es facil part 2
odmiana czasownikow nieregularnych conjugar es facil part 1
odmiana czasownikow nieregularnych conjugar es facil part 3
GbpUsd analysis for July 06 Part 1
~$Production Of Speech Part 2
20 H16 POST TRANSFUSION COMPLICATIONS KD 1st part PL
Discussions A Z Intermediate handout part 1
part 20
Eurocode 3 Part 1 11 Pren 1993 1 11 (Eng)
Part 2
C102723 D W0064 CIR PART
hejka, IV rok, IV rok CM UMK, Kardiologia, giełdy, part I
Mechanizmy działania antybiotyków, materiały farmacja, Materiały 4 rok, farmacja 4 rok part 2, farma
tpl zal, materiały farmacja, Materiały 4 rok, farmacja 4 rok part 1, TPPL
Mąka part 2, Archeologia, II Rok
więcej podobnych podstron