
Biomaterials 23 (2002) 2499–2507
Interaction of calcium and phosphate in apatite coating on titanium
with serum albumin
Bo Feng
a,b,
*, Jiyong Chen
a
, Xingdong Zhang
a
a
Engineering Research Center in Biomaterials, Sichuan University, Chengdu 610064, China
b
Department of Material Science and Engineering, Sichuan Institute of Technology, Chengdu 610039, China
Received 4 April 2001; accepted 1 November 2001
Abstract
A Ca-deficient carbonate apatite coating on titanium was prepared by pre-calcifying titanium in a saturated Ca(OH)
2
solution
and then immersing in a supersaturated calcium phosphate solution. The interaction of the protein with the apatite coating on
titanium was investigated by scanning electron microscopy with X-ray energy dispersion spectroscopy, X-ray photoelectron
spectroscopy, X-ray diffraction and Fourier transform infrared spectroscopy. During immersion of the coating in bovine serum
albumin (BSA) solution, accompanied by an adsorption of BSA onto the coating, calcium and phosphate ions dissolved and
reprecipitated, resulting in the formation of the coating containing BSA from the surface to subsurface layers. The adsorption
modified the structure and morphology of the apatite coating on titanium and changed the protein configuration. It was also found
that the protein chemically adsorbed onto surfaces containing calcium or phosphorus, showed that both Ca and P on the apatite
coating were the binding sites with protein. The BSA adsorption onto the coating involved several elements and groups. In this
process, Ca played an essential role, and the interaction of Ca on the apatite coating with the protein stimulated the bond of the
protein at P sites. r 2002 Published by Elsevier Science Ltd.
Keywords: Apatite coating; Titanium; Bovine serum albumin; Adsorption; Interaction
1. Introduction
The bioactivity of materials is one of the important
factors that determines the success of implant materials.
For substitutes of hard tissues, the formation of bone-
like apatite at the interface between implants and tissues
is one of the markers of the bioactivity of materials. The
adsorption of proteins and the adhesion of cells on
material surfaces also relate to the bioactivity. The first
event that occurs on the surfaces of materials is the
adsorption of proteins onto the surfaces, followed by
responses of cells to the surfaces [1,2]. The presence of
the adsorbed protein layer should mediate cellular
responses to materials. On the other hand, the surface
properties and structures of the materials should play an
important role in the adsorption of proteins, while the
process of protein adsorption causes a possible change
in the surface structure and properties of materials,
including the bioactivity. Thus, the fundamental reac-
tions at the interface of biomaterials and tissue should
influence their integration and bone-bonding character-
istics [3–5].
There have been a number of studies on protein
adsorption and its effect on biomaterials and cellular
response to these materials [6–9], including calcium
phosphate and titanium. Investigations have shown that
in the presence of calcium and phosphate ions, the
adsorption of bovine serum albumin (BSA) onto
titanium powder is a function of protein concentration
and pH level, which suggest a possible conformational
change of the protein molecule [10,11]. The study on the
coprecipitation of calcium phosphate and BSA as a
coating on titanium indicated that the incorporation of
BSA significantly modified the morphology, composi-
tion, and crystallinity of the coating [12]. The pre-
adsorption of fibronectin on titanium surface strongly
inhibited the formation of calcium phosphate layer [13].
Titanium and its alloys with calcium phosphate
coatings have been increasingly used clinically, since
they permit optimization of surface properties such
as biocompatibility and bioactivity, while retaining
*Corresponding author. Fax: +86-28-5410246.
E-mail address:
fengbh@263.net (B. Feng).
0142-9612/02/$ - see front matter r 2002 Published by Elsevier Science Ltd.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 1 ) 0 0 3 8 4 - 2

favorable bulk properties including good mechanical
properties. It has been found that the initial dissolution
properties of calcium ions from plasma-sprayed apatite
coatings on titanium were dependent on the media such
as fibronectin and albumin, and the dissolution of
phosphate ions on the coatings was not significantly
affected by the presence of proteins [14]. However, the
interaction mechanism between proteins and calcium
phosphate coatings based on titanium has not been
sufficiently understood, especially, the role of calcium
and phosphate in apatite coatings.
The objective of this work was to study the interaction
mechanisms of protein with apatite coating based on
titanium through investigating the mutual reactions
between calcium, phosphate, and protein. The BSA was
chosen as a test protein. The apatite coating on titanium
was prepared by pre-calcifying titanium in a boiling
saturated Ca(OH)
2
solution and then immersing in
supersaturated solution with respect to calcium phos-
phate [15]. The surface morphology, structure and
compositions were investigated using several surface
analytical instruments.
2. Materials and methods
2.1. Materials and treatment
Commercial, pure titanium plates of 10 10 2 mm
3
in size were wet ground with 120-grid metallographic
alumina paper and then washed ultrasonically in turn
with acetone, ethyl alcohol and deionized water, and
dried at room temperature. The plates were subjected to
four kinds of pre-treatment and then were immersed in
BSA solution, which yielded samples SB, CB, PB, and
AB according to the following description:
SB: Titanium plates were immersed in BSA solution
for 1 h.
CB: Titanium plates were immersed in boiling
saturated Ca(OH)
2
solution for 30–40 min (pre-calcifica-
tion), and then in BSA solution for 1 h.
PB: Titanium plates were immersed in a pre-
phosphatization solution for 30 min at 85–951C and
then in BSA solution for 1 h. The pre-phosphatization
solution was 20% H
3
PO
4
, which was adjusted to pH
2.0–2.4 with NaOH.
AB: Titanium plates were immersed in boiling
saturated Ca(OH)
2
solution for 30–40 min and then in
20 ml supersaturated calcium phosphate solution (SCP)
in a sealed polystyrene vial at 371C for 1 week. The SCP
was refreshed every 2 days. The titanium-apatite coat-
ing, namely, CP was obtained. Finally, CP samples were
immersed in BSA solution for 1 h and 2 days; thus,
samples AB1 and AB2 were obtained, respectively. The
SCP
had
the
following
ion
concentration:
Ca
2+
F3.10 mm, HPO
4
2
F1.86 mm, Na
+
F136.8 mm,
Cl
F144.5 mm, and K
+
F3.71 mm. The solution was
buffered at pH 7.4 with tris-hydroxymethylamino-
methane and hydrochloric acid at room temperature.
BSA solution was prepared by dissolving BSA in
0.9% NaCl saline buffering at pH 7.4 with tris-
hydroxymethylaminomethane and hydrochloric acid at
room temperature, and its concentration was 1 mg/ml.
Except for pre-calcification, after each of the above
treatments, the samples were rinsed with abundant
deionized water and then dried at room temperature.
After pre-calcification, the titanium plates were super-
sonically washed.
In the immersion in Ca(OH)
2
, SCP and BSA
solutions, each of the titanium plates were hanged
vertically with a cotton thread to exclude any artifact
arising from sedimentation in the supersaturated solu-
tion. The concentration changes of SCP and BSA were
monitored with an induced couple plasma atomic
emission spectroscopy (ICP, Optima 3000XL, America).
2.2. Surface characteristics
The surface morphology of the samples was observed
by scanning electron microscopy (SEM) with X-ray
energy dispersion spectroscopy (EDS), (S-450, Hitachi,
Japan). The compositions and their binding energies on
the surface and subsurface were detected using X-ray
photoelectron spectroscopy (XPS, XSAM-8000, Eng-
land). The crystallographic features of the coatings were
determined by X-ray diffraction (XRD, D/max-IIIA
X-ray diffraction analyzer, Japan). For the analysis by
Fourier transform infrared spectroscopy (FTIR, Nico-
let-560, America), the coatings of AB were carefully
scraped, ground into powder and pressed to tablets.
3. Results
3.1. The apatite coating after BSA adsorption
3.1.1. Chemical surface characterization
It was assumed that the nitrogen signal was indicative
of the presence of protein [13,16]. Fig. 1 shows the
elemental amount of the sample surfaces. All the data
are the averages of those measured at least two samples.
The information of XPS comes from surface layer with
nanometer magnitude. That is, it was the surface result.
Fig. 1 indicates that after immersing in BSA solution
only for 1 h (AB1), the protein adsorbed onto the
surface of the apatite coating. After etching for 5 min,
there was still a considerable amount of nitrogen, that is,
within 1 h, the adsorbed protein was distributed from
the surface on to the subsurface. After 2 days, the
amount of protein adsorbed on the surface slightly
decreased (AB2) and was closer to that on the subsur-
face. The Ca/P ratio on the surface of the original
B. Feng et al. / Biomaterials 23 (2002) 2499–2507
2500
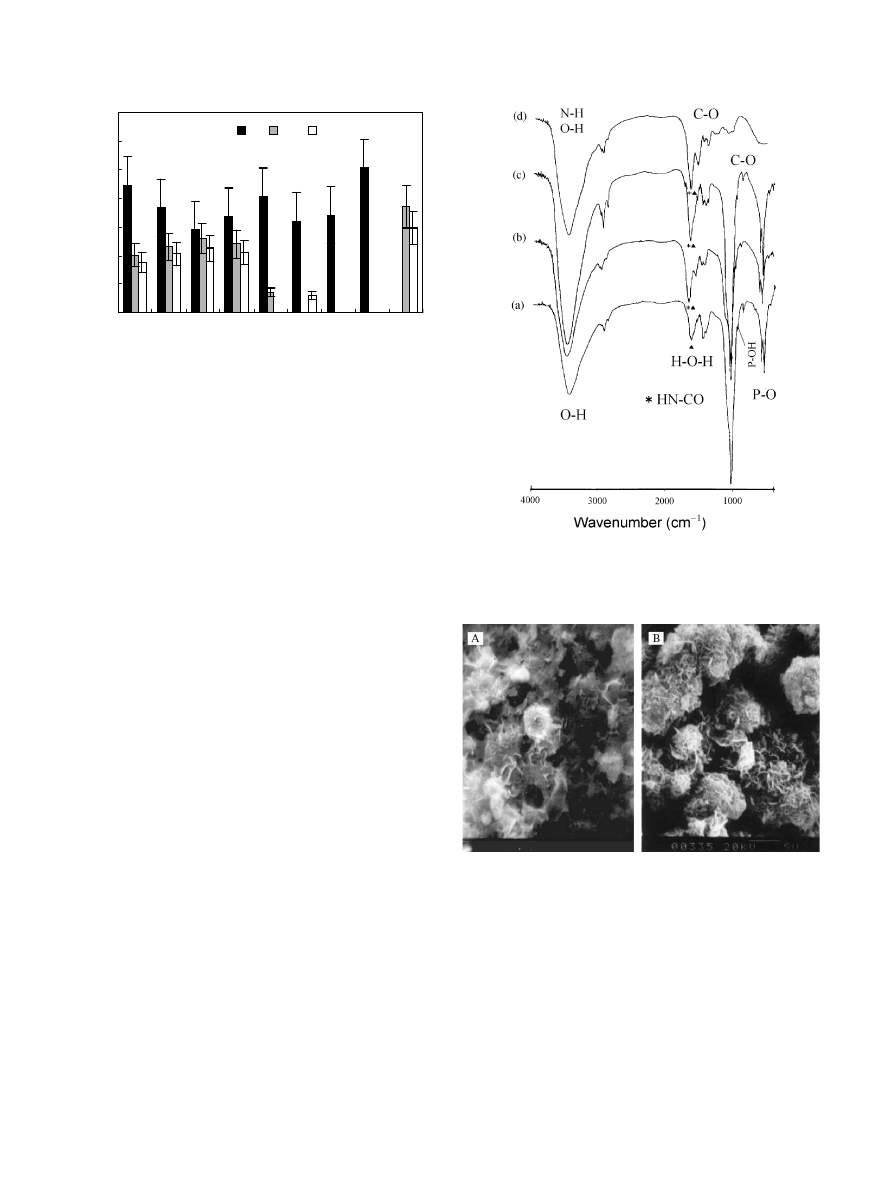
apatite coating (Fig. 1, CP) was 1.25, lower than the
datum of the apatite coating bulk obtained from EDS
analysis, 1.56, and also lower than stoichiometrical
hydroapatite, 1.67. Because the analysis depth of EDS
for coatings is of the order of micrometers, its results
should present the properties of the coating bulk. The
protein adsorption further decreased the ratio of Ca/P
on the surfaces (Fig. 1, AB), and the ratio of 1.0–1.1
agrees with other reports, 1.1–1.2 on titanium immersed
in Hank’s solution containing BSA [16]. It is noticeable
that the amount of nitrogen on CB was closer to that on
AB1.
EDS and XPS both indicated that the CP coating was
Ca-deficient apatite. FTIR spectra of CP, AB1 and AB2
(Fig. 2(a)–(c)) showed that the apatite coating contained
CO
3
2
, that is, they were Ca-deficient carbonate apatite.
Carbonate ions in the coating probably came from the
process in boiling saturated Ca(OH)
2
solution. During
pre-calcification, CO
2
in the air dissolved into Ca(OH)
2
solution and transformed to CO
3
2
, which coprecipitated
onto titanium with Ca
2+
and formed CaCO
3.
After the
BSA adsorption, the C–O bands probably not only
came from CO
3
2
in CP but also from COO
in the
protein adsorbed. The band at 3449 cm
1
of BSA should
include the contribution of O–H and N–H. For AB1
and AB2, the bands at
B3449 cm
1
were obviously
stronger than BSA, which possibly resulted from O–H
in the apatite coating and the protein adsorbed, as well
as N–H in the protein adsorbed. The components at
B1655 and B1549 cm
1
have been assigned for amides
I and II in BSA [17]. Because of O=CNH in the
adsorbed BSA and H–O–H in apatite coating, AB1 and
AB2 showed the stronger bands at
B1655 cm
1
than
BSA.
3.1.2. Morphology of the apatite coating
The adsorption of BSA changed the morphology of
the coating (Fig. 3). The denser and smaller flake-like
crystals aggregated to the larger crystal particles globule
like, resulting in greater porosity. The flake-like crystals
orientated more obviously perpendicularly to the
surfaces of the substrates (Fig. 3(B)).
0
2
4
6
8
10
12
14
AB1
AB2
AB1E
ABE2
CB
PB
SB
B
CP
Sample
Atomic
N
Ca
P
Fig. 1. Compositions on the surfaces of the samples obtained by XPS:
AB1
Ftitanium-apatite coating immersed in BSA solution for 1 h;
AB2
Ftitanium-apatite coating immersed in BSA solution for 2 days;
AB1E
FAB1 etched by Ar
+
for 5 min; AB2E
FAB2 etched by Ar
+
for 5 min; CB
Ftitanium pre-calcified and then immersed in BSA
solution for 1 h; PB
Ftitanium pre-phosphatized and then immersed in
BSA solution for 1 h; SB
Ftitanium immersed in BSA solution for 1 h;
B
FBSA; and CPFtitanium-apatite coating.
Fig. 2. FTIR spectra of coatings: (a) titanium-apatite coating; (b)
AB1
Ftitanium-apatite coating immersed in BSA solution for 1 h; (c)
AB2
Ftitanium-apatite coating immersed in BSA solution for 2 days;
and (d) BSA.
Fig. 3. SEM photographs of coatings on titanium: (A) titanium-
apatite coating and (B) AB2
Ftitanium-apatite coating immersed in
BSA solution for 2 days.
B. Feng et al. / Biomaterials 23 (2002) 2499–2507
2501

3.1.3. Crystallographic features of the coating
XRD analysis confirmed that the CP coating con-
sisted of apatite and a small amount of octacalcium
phosphate (OCP) and tricalcium phosphate (TCP)
(Fig. 4(a)). The lower and wider peaks of apatite in CP
coating suggest more disorientation of the grain faces or
a small quantity of amorphous in the coating. The
protein adsorption changed the crystal structure of the
coating. After protein adsorption (Fig. 4 (b) and (c)), the
intensities of (0 0 2) peak and a combined peak of (1 1 0),
(2 1 1), and (3 0 0) obviously increased. While the
intensities of (1 0 2) and (2 1 0) decreased, the low peaks
of OCP and TCP disappeared. This indicated that after
the BSA adsorption, the crystal faces of (0 0 2), (1 1 0),
(2 1 1), and (3 0 0) preferentially orientated parallel to the
surfaces of substrates. The crystallinity of the coating
increased. The greater revolution of FTIR spectra of AB
than CP also suggests that the protein adsorption caused
the increase of crystallinity (Fig. 2).
3.2. Interactions of Ca
2+
and PO
4
3
with BSA
3.2.1. Change of Ca and P
The BSA adsorption on the apatite coating caused the
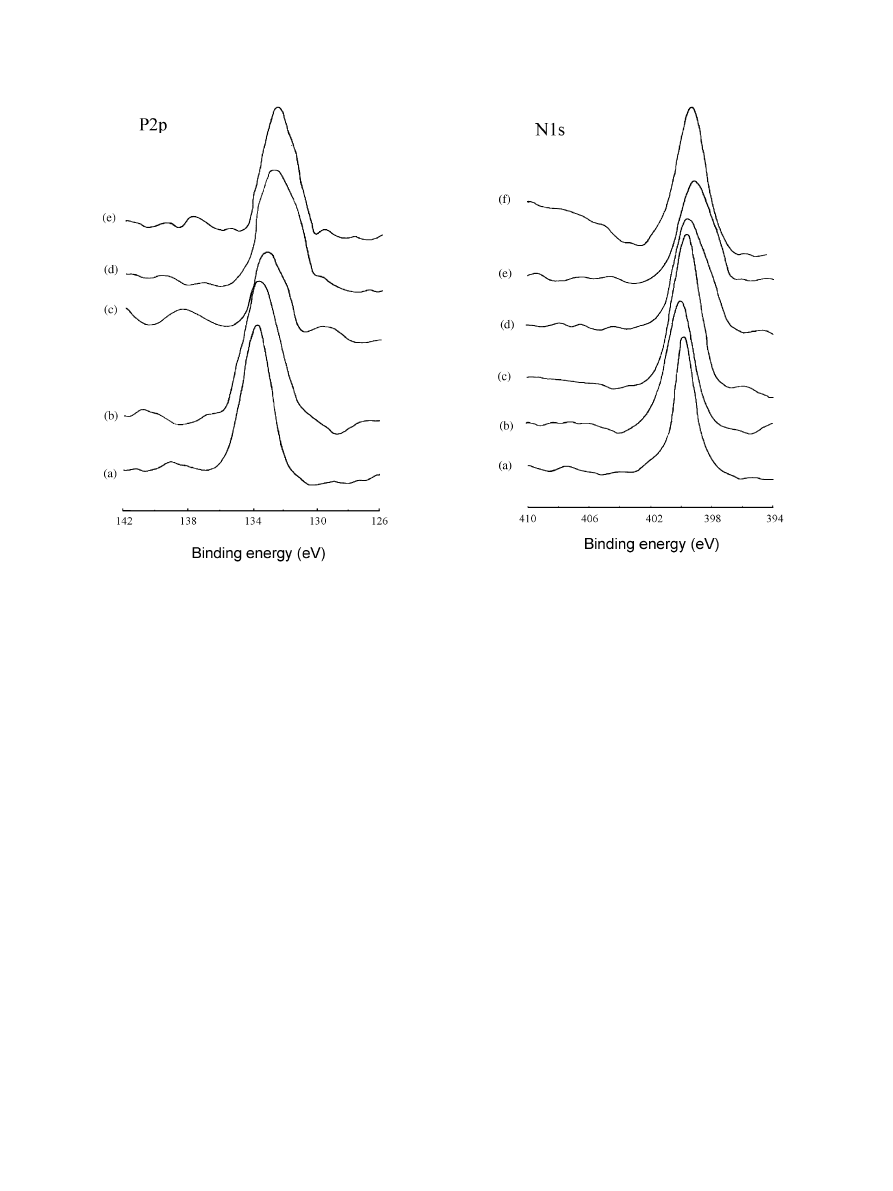
change of XPS spectra of Ca2p (Fig. 5(b), (d) and (e))
and P2p (Fig. 6(b), (d) and (e)).
In Fig. 5, the Ca2p binding energy (BE) of AB
deviated about 1 eV from CP, which is enough to
indicate the chemical interaction of Ca
2+
with BSA. The
interaction of Ca
2+
in the apatite coating with BSA only
for 1 h was remarkable and up to the extent for 2 days,
since the Ca2p BEs of AB1 and AB2 shifted to the same
level (Fig. 5(d) and (e)).
The Ca2p peak of the pre-calcified titanium (C) also
shifted to the lower BE side after the BSA adsorption
(CB) (Fig. 5(a) and (c)), and the shift value was
approximately equal to those in AB. This indicated that
Ca on the surface of the pre-calcified titanium could
interact with the protein in the absence of P. Probably,
the reaction was the same as that on CP. There have
been the reports in which the affinity of Ca to protein
was demonstrated [8,18,19].
Compared with the apatite (CP), after the protein
adsorption (AB), the P2p spectra of AB shifted to the
lower energy sides (Fig. 6(b), (d) and (e)). That is, during
BSA adsorption, PO
4
3
in the apatite coating interacted
with the protein. P2p peak of titanium pre-phosphatized
and then immersed in BSA (PB) was also located at a
lower BE level than P and CP (Fig. 6(a), (b) and (c)).
This indicated that without Ca on the surface, P could
Fig. 4. XRD patterns of coatings: (a) titanium-apatite coating; (b)
AB1
Ftitanium-apatite coating immersed in BSA solution for 1 h; and
(c) AB2
Ftitanium-apatite coating immersed in BSA solution for 2
days. The indexed peaks are HA phases. J: OCP; : TCP. The
unmarked peaks are attributed to titanium.
Fig. 5. XPS spectra for Ca2p of samples: (a) titanium pre-calcified; (b)
titanium-apatite coating; (c) CB
Ftitanium pre-calcified and then
immersed in BSA solution for 1 h; (d) AB1
Ftitanium-apatite coating
immersed in BSA solution for 1 h; and (e) AB2
Ftitanium-apatite
coating immersed in BSA solution for 2 days.
B. Feng et al. / Biomaterials 23 (2002) 2499–2507
2502
also interact solely with the protein. The significantly
smaller shift of the P2p XPS peak, compared with AB,
implies that the reaction of P with the protein is weak in
the absence of Ca.
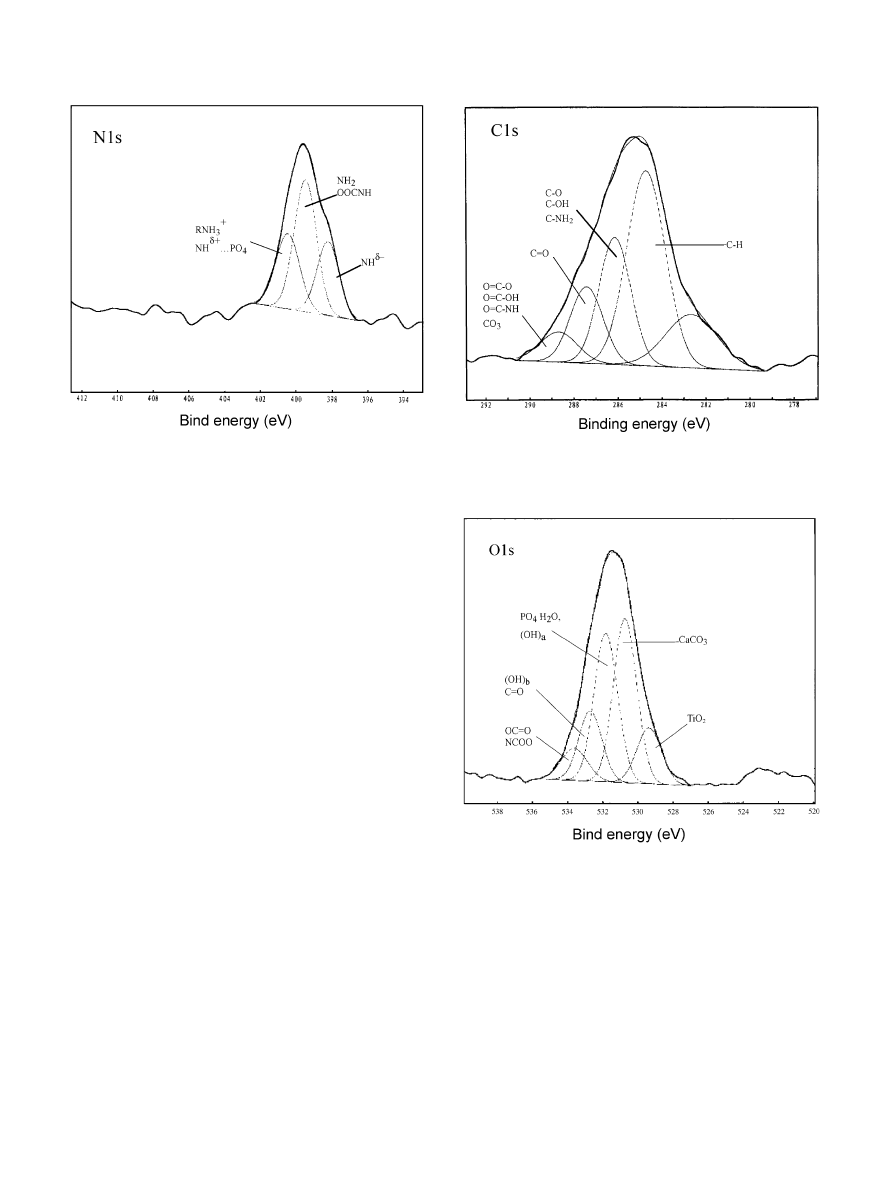
3.2.2. Change of N
Though the changes of N1s spectra were not so
obvious as Ca2p and P2p, after immersion in BSA
solution for 1 h, N1s BE levels of the pre-phosphatized
titanium, the pre-calcified titanium and the apatite
coating deviated from that of BSA, and shifted to the
higher BE sides (Fig. 7(b)–(d), and (f)). The N1s BE of
CB was located almost at the same position as AB1,
similar to the shift of Ca2p in AB and CB. But, N1s of
PB appeared to be different from AB1.
Since the BSA adsorption resulted in the same
changes of Ca2p and N1s spectra of the pre-calcified
titanium and the apatite coating, it might be inferred
that phosphate ions in apatite did not affect the
interaction of Ca with the protein. But after BSA was
adsorbed, the P2p and N1s spectra of pre-phosphatized
titanium differed from those of apatite, implying that Ca
mediated the interaction of phosphate ions in apatite
with the protein.
In addition, the N1s BE level of AB1 was higher than
that of BSA (Fig. 7(d) and (f)). After immersion for 2
days, N1s peak of AB2 was closer to that of BSA
(Fig. 7(e) and (f)), which implied that with time,
chemical combination between apatite and N-groups
in BSA was weakened, since the physical adsorption,
i.e., the overlayer adsorption of BSA increased. After 2
days, more amide groups were far from the interface of
the coating and the solution, and the conformational
change of the protein decreased. This supports a
hypothesis that the extent of the conformational change
of the protein decreases with the increase in the amount
absorbed [20,21].
In Fig. 7(d) and (e), the wide N1s peaks of AB1and
AB2 suggest that N in BSA adsorbed onto the apatite
coating had more than one chemical state. As an
example, Fig. 8 gives a possible deconvolution of N1s
spectrum of AB1, according to the Gauss curve-fitting
routine.
It should be pointed out, that N1s spectrum of
titanium-adsorbed protein (SB) was different from the
Fig. 6. XPS spectra for P2p of samples: (a) titanium pre-phosphatized;
(b) CP
Ftitanium-apatite coating; (c) PBFtitanium pre-phosphatized
and then immersed in BSA solution for 1 h; (d) AB1
Ftitanium-apatite
coating immersed in BSA solution for 1 h; and (e) AB2
Ftitanium-
apatite coating immersed in BSA solution for 2 days.
Fig. 7. XPS spectra for N1s of samples: (a) SB
Ftitanium immersed in
BSA solution for 1 h; (b) PB
Ftitanium pre-phosphatized and then
immersed in BSA solution for 1 h; (c) CB
Ftitanium pre-calcified and
then immersed in BSA solution for 1 h; (d) AB1
Ftitanium-apatite
coating immersed in BSA solution for 1 h; and (e) AB2
Ftitanium-
apatite coating immersed in BSA solution for 2 days; and (f) BSA.
B. Feng et al. / Biomaterials 23 (2002) 2499–2507
2503
above samples, suggesting that it is unlikely that
titanium and TiO
2
on the surfaces of CB and PB affect
the protein adsorption.
3.2.3. Change of C and O
After protein adsorption, C1s and O1s XPS spectra of
AB, CB, and PB were all different from those of BSA
and the apatite coating (not shown). As mentioned
above, it could be excluded that titanium and TiO
2
on
the surfaces of CB and PB influenced upon the changes
of carbon and oxygen. A remarkable change was
widening of the C1s and O1s spectra due to the protein
adsorption. It is suggested that oxygen and carbon
species increased. By the Gauss curve-fitting routine,
O1s and C1s XPS spectra of the samples can be
deconvoluted into a few subpeaks. For example, Figs. 9
and 10 illustrate, respectively, the possible deconvolu-
tions of C1s and O1s of AB1. However, since there are
many kinds of O- and C-groups in the protein and the
apatite coating, that complicate chemical reactions
between BSA and apatite, it is very difficult to confirm
those groups that contributed to the C1s and O1s XPS
spectra, respectively. At least, there were the possibilities
that O1s spectra included the contribution of OH, H
2
O,
CO
3
2
, –COO , PO
4
3
, C=O, O–C=O, and –NCOO ,
and C1s included CH, CH
2
, and CH
3
(from contami-
nant organic compounds and BSA), and C–OH, C–
NH
2
, –COO , HNCOO
and CO
3
2
, while N1s could
relate to =NH, HNCOO , –NH
3
+
, N
d+
-O
d
, and
NH
d+
-PO
4
(3+d)
. Among them, the existence of
CO
3
2
, PO
4
3
, and HNCOO
was consistent with FTIR
analyses.
4. Discussion
Of the results from EDS and FTIR analyses, the
coating prepared by pre-calcification and then immer-
sion in SCP is a Ca-deficient carbonate apatite. After
BSA adsorption, besides the presence of groups contain-
ing nitrogen, the components of the coating were almost
unchanged and it was still Ca-deficient carbonate
apatite. However, XPS data (Fig. 1) show that the Ca/
P ratio on the surface of the coating with BSA was lower
Fig. 8. Representative deconvolution of XPS envelope for N1s on
titanium pre-calcified and then immersed in BSA solution for 1 h.
Fig. 9. Representative deconvolution of XPS envelope for C1s on
titanium pre-calcified and then immersed in BSA solution for 1 h.
Fig. 10. Representative deconvolution of XPS envelope for O1s on
titanium pre-calcified and then immersed in BSA solution for 1 h.
B. Feng et al. / Biomaterials 23 (2002) 2499–2507
2504

than that before adsorption. As the other studies have
shown [14], Ca-deficient or the so-called P-rich surfaces
are easy to adsorb the protein.
It is surprising that the amount of the adsorbed
protein on the coating after the immersion in BSA
for 2 days (AB2) was lower than that for 1 h (AB1).
After etching for 5 min (Fig. 1, AB1E), the N on
the subsurface was still detected, in an amount lower
than on the surface. This indicates that the protein
not only existed at the coating surfaces, but also
distributed in the some depth in the coating. In the
FTIR, lower P–O band intensity of AB1 than AB2
and CP suggest the dissolution–reprecipitation of PO
4
3
.
At the early stage of immersion in the BSA solution,
PO
4
3
and Ca
2+
on the coating rapidly dissolved into
the solution since their concentrations in the solution
were very low. The protein had a higher concentration
in the solution than on the coating surface and adsorbed
onto the coating surface. At the same time, Ca
2+
and
PO
4
3
dissolved in the solution and the protein adsorbed
onto the coating surface; Ca
2+
and PO
4
3
concentrations
in solution increased gradually with time, and both ions
reprecipitated onto the coating at a greater speed.
The ICP analyses confirmed the concentration changes
of Ca and P in the solution. This behavior was
responsible for the change of the morphology and
the crystal structure of the coatings. Other investigators
also observed the high initial dissolution of PO
4
3
in
the biological fluid [22,23]. As a result, the protein
distributed itself from the surfaces to the subsurface
of the coatings. The larger adsorption quantity of
BSA at the initial period, i.e., for 1 h, was attributable
to its faster initial adsorption speed. The lower
amount
of nitrogen on the subsurface of AB1
suggested that the amount of adsorbed protein at
the very early stage had not reached the maximum. On
the surface and subsurface of AB2, since the equilibrium
of the adsorption–desorption of protein and the
dissolution–reprecipitation of calcium and phosphate
ions was built within 2 days, the amounts of nitrogen are
similar.
Proteins have an influence in inducing crystal growth
on calcium phosphate surfaces [23]. Here, the protein
adsorption modified the morphology and crystal struc-
ture of the coating. The OCP and TCP phases, calcium
phosphate at crystal defects and carbonate species often
easily dissolve. The crystal faces with high density of
atoms and low interplanar distance have high surface
energy and low thermodynamic stability. The dissolu-
tion of Ca–P and the adsorption of protein easily occur
via these faces or sites, while the orientation of crystal
grains increased. This might lead to the increase of the
intensities of the crystal faces (0 0 2), (1 1 0), (2 1 1), and
(3 0 0) in XRD pattern and the decrease of the faces
(1 0 2) and (2 1 0). It was suggested that the adsorption of
proteins and the recrystallization of calcium phosphate
might also affect the kinetics of phase transformations
in vivo [24].
Though FTIR spectra indicate that the BSA adsorp-
tion did not significantly change the bulk components in
the coatings, XPS shows that the chemical states of
some elements on the surfaces and subsurfaces of the
coatings with BSA were different from the original
coatings (CP). It is interpreted that the protein
chemically bonds with rather than physically adsorb
onto the coating. Albumin and fibronectin have been
shown to bind the plasma-sprayed HA-coated Ti in
ionic manner, which was proved by the dependence of
the protein adsorption on pH level and the ability of
EDTA, a calcium chelator, to release bound proteins
into solution [14,25].
There has not been a consistent view on whether
Ca
2+
or PO
4
3
ion is the site binding proteins to apatite.
It is mostly accepted that proteins combine with apatite
through Ca
2+
[11,19,26–28], but PO
4
3
as the binding site
has also been put forward [2], as others who believed
that both Ca
2+
and PO
4
3
provide the major driving
force for protein adsorption [19]. Figs. 5 and 6 show that
the BE levels of Ca2p and P2p of AB were different
from CP. It is implied that both Ca and P are probable
binding sites between BSA and apatite. For adsorption
of acidic proteins including albumin, only the Ca site
(Ca-bridging) was thought to be its binding site
[11,18,19,25]. A possible cause was supposed to be the
point of zero charge of BSA, 4.7–4.8 [1,25]. In neutral
biological fluid, BSA would be negatively charged and
would combine with Ca
2+
on apatite by electrostatic
attractive forces.
In this work, the XPS spectra showed that the protein
adsorption led to the changes of BE levels of Ca2p on
the pre-calcified surface and P2p on the pre-phospha-
tized surface, supporting the view that both Ca and P
can become binding-sites for proteins, including acidic
proteins. Moreover, combining the adsorbed N amount
on the PB, and the difference between N1s BE levels of
PB and of BSA in the XPS spectra, and the difference
between P2p BE levels of P and PB, the inference for the
existence of the P binding site should be reasonable.
Because PO
4
3
ions are negatively charged, electrostatic
effects would not be the dominant factor in the process
of BSA binding P, so that BSA possibly adsorbed on to
the apatite through lateral interaction in a covalent
bond, instead of an ion bond as at the Ca sites.
Moreover, Ca2p peak in CB was located at approxi-
mately the same position as that in AB, which indicated
that Ca occurred solely or coexisted with P all react
strongly with the protein to the same extent. For the
surface pre-phosphatized and then adsorbed by the
protein (PB), compared with the surface pre-calcified
and then adsorbed by the protein (CB), the P2p shift
was obviously smaller than the Ca2p shift, which
indicated that at the pre-phosphatized titanium surface,
B. Feng et al. / Biomaterials 23 (2002) 2499–2507
2505

P reacted with protein weaker than that of Ca at the pre-
calcified surface. In the coexistence of Ca and P (AB),
P2p shift was larger. In addition, CB and AB1 had
approximately equal N1s BE. The interaction of Ca
2+
in apatite with BSA was probably unrelated to PO
4
3
,
and Ca
2+
obviously affected the interaction between
PO
4
3
in apatite and BSA. It could be inferred that the
combination of the protein to the apatite surface mainly
depended on Ca
2+
.
The deconvolutions of C1s, O1s and N1s spectra after
BSA adsorption suggest that C, O and N probably
produce some complicated changes. The adsorption of
the protein onto the apatite coating should include a
series of synergistic functions, involving many kinds of
physical and chemical interactions between Ca, P, C, O
and N elements or their groups. Among them, the
interaction of Ca with the protein was the most
important and influenced the other reactions, including
the binding of the protein with the apatite at the P sites.
5. Conclusions
1. When the apatite coating on titanium was immersed
in the bovine serum albumin solution, dissolution
and reprecipitation of Ca and P on the apatite
coating accompanied the protein adsorption, result-
ing in the distribution of the protein in some depth
beneath the surface layer of the coating.
2. The protein chemically bonded to the apatite coating.
Both Ca and P at the coating were the binding sites at
which the BSA adsorbed onto the apatite. The
protein adsorption onto the apatite coating was a
synergistic process involving several elements and
groups. In this process, Ca played an essential role,
and the interaction of Ca at the apatite coating with
the protein stimulated the bond of the protein at P
binding sites.
3. The adsorption of BSA onto the apatite coating
modified the surface compositions and structure of
the coating.
References
[1] Dion I, Baquey C, Monties J-R, Havlik P. Haemocompatibility of
Ti6Al4V alloy. Biomaterials 1993;14:122–6.
[2] Healy KE, Ducheyne P. Hydration and preferential molecular
adsorption on titanium in vitro. Biomaterials 1992;13:553–61.
[3] Albrektsson T, Hansson HA, Ivarsson B. Interface analysis of
titanium and zirconium bone implants. Biomaterials 1985;6:97–101.
[4] Albrektsson T, Arnebrant T, Larsson K, Nylander T, Sennerby L.
Effect of a glycoprotein monomolecular layer on the integration
of titanium implants in bone. In: Christel P, Meunier A, Lee AJC,
editors. Biological and biomechanical performance of biomate-
rials. Amsterdam: Elsevier, 1986. p. 349–54.
[5] Walton AG, Koltisko B. Protein structure and the kinetics of
interaction with surfaces. In: Cooper SL, Peppas NA, editors.
Biomaterials: interfacial phenomena and applications. Washing-
ton, DC: American Chemical Society, 1982. p. 245–63.
[6] Veerman ECI, Suppers RJF, Klein CPAT, deGroot K, Nieuw
Amerongen AV. SDS-PAGE analysis of the protein layers
adsorbing in vivo and in vitro to bone substituting materials.
Biomaterials 1987;8:442–8.
[7] Sodek J, Zhang Q, Glodberg HA, et al. Non-collagenous bone
proteins and their role in substrate-induced bioactivity. In: Davies
JE, editor. The bone–biomaterial interface. Toronto: University
of Toronto Press, 1991. p. 97–110.
[8] Ellingsen JE. A study on the mechanism of protein adsorption to
TiO
2
. Biomaterials 1991;12:593–6.
[9] El-Ghannam A, Ducheyne P, Shapiro IM. Serum protein adsorp-
tion on bioactive ceramics and glass and the effect on osteoblast
adhesion. Trans 21st Annual Meeting of Society for Biomaterials.
Society of Biomaterials, MN. San Francisco. 1995. p. 46.
[10] Zeng H, Chittur KK, Lacefield WR. Analysis of bovine serum
albumin adsorption on calcium phosphate and titanium surfaces.
Biomaterials 1999;20:377–84.
[11] Diana T, Hughes W, Graham E. Adsorption of bovine serum
albumin on to titanium powder. Biomaterials 1996;17:859–64.
[12] Wen HB, de Wijn JR, van Blitternwijk CA, de Groot K.
Incorporation of bovine serum albumin in calcium phosphate
coating on titanium. J Biomed Mater Res 1999;46:245–52.
[13] Serro AP, Fernandes AC, Saramago B. Calcium phosphate
deposition on titanium in the presence of fibronectin. J Biomed
Mater Res 2000;49:345–52.
[14] Bender SA, Bumgardner JD, Roach MD, Bessho K, Ong JL.
Effect of protein on dissolution of HA coatings. Biomaterials
2000;21:299–305.
[15] Feng B, Chen JY, Zhang XD. Bioactivation of titanium by
calcification and effect of surface roughness. Proceedings of the
Sixth World Biomaterials Congress Transactions, vol. III, Society
for Biomaterials, USA, 2000. p.1515.
[16] Serro AP, Fernandes AC, Saramago B, Lina J, Barbosa MA.
Apatite deposition on titanium surfaces
Fthe role of albumin
adsorption. Biomaterials 1997;18:963–8.
[17] Ong JL, Chittur KK, Lucas LC. Dissolution/reprecipitation and
protein adsorption studies of calcium phosphate coatings by FT-
IR/ATR techniques. J Biomed Mater Res 1994;28:1337–46.
[18] Bernardi G, Giro MG, Gaillard C. Chromatography of polypep-
tides and proteins on hydroxyapatite columns: some new
developments. Biochim Biophys Acta 1972;278:409–20.
[19] Hay DI, Moreno EC. Differential adsorption and chemical
affinities of proteins for apatite surfaces. J Dent Res Special
Issue B 1979;58(B):930–40.
[20] Kondo A, Murakami F, Higashitani K. Circular dichroism
studies on conformational changes in protein molecules upon
adsorption on ultrafine polystyrene particles Ii32. Biotech Bioeng
1992;40:889–94.
[21] Norde W, Lyklema J. The adsorption of human plasma
albumin and bovine pacreas ribonuclease at negatively charged
polystyrene surfaces. I. Adsorption isotherms. Effect of charge,
ionic
strength
and
temperature.
J
Colloid
Interface
Sci
1978;66:257–65.
[22] Ong JL, Lucas LC. Auger electron spectroscopy and its use for
the characterization of titanium and hydroxyapatite surfaces.
Biomaterials 1998;19:455–65.
[23] Margolis HC, Moreno EC. Kinetics of hydroxyapatite dissolution
in acetic, lactic, and phosphoric acid solutions. Calcif Tissue Int
1992;50:137–43.
[24] Johnson MS-A, Paschalis E, Nancollas GH. Kinetics of miner-
alization, demineralization and transformation of calcium phos-
phate at mineral and protein surfaces. In: Davis JE, editor. The
bone–biomaterial interface. Toronto: University of Toronto
Press, 1991. p. 68–75.
B. Feng et al. / Biomaterials 23 (2002) 2499–2507
2506

[25] Hlady V, Furedi-Milhofer H. Adsorption of human albumin on
precipitated hydroapatite. J Colloid Interface Sci 1979;69:460–8.
[26] Sterinberg D, Klinger A, Kohavi D, Sela MN. Adsorption of
human salivary proteins to titanium powder. I. Adsorption of
human salivary albumin. Biomaterials 1995;16:1339–43.
[27] Bernadi G, Kawasali T. Chromatography of polypeptides and
proteins on hydroxyapatite columns. Biochim Biophys Acta
1968;160:301–10.
[28] Vasin SL, Rosanova IB, Sevastianov VI. J Biomed Mater Res
1998;39:491–7.
B. Feng et al. / Biomaterials 23 (2002) 2499–2507
2507
Document Outline
- Interaction of calcium and phosphate in apatite coating on titanium with serum albumin
Wyszukiwarka
Podobne podstrony:
05 DFC 4 1 Sequence and Interation of Key QMS Processes Rev 3 1 03
05 DFC 4 1 Sequence and Interation of Key QMS Processes Rev 3 1 03
A D Seergev Nonlinear interaction of the pantograph of electric rolling stock and the overhead cate
Evaluating interface strength of calcium phosphate sol
cicourel, a v the interaction of discourse, cognition and culture
Interaction of fraternal birth order and handedness in the
Wojczulanis Jakubas, Katarzyna i inni Who bullies whom at a garden feeder Interspecific agonistic i
part3 18 Some Interactions of Pragmatics and Grammar
Interaction of hydroxyapatite
Formation and growth of calcium phosphate on the surface of
Effect of calcium ion
Interactions of fibroblasts with soldered and laser
The effect of the interaction of various spawn grains with different culture medium on carpophore
Interaction of orthopaedic
Dyson, Rebecca M i inni Interactions of the Gasotransmitters Contribute to Microvascular Tone (Dys)
Comparison of theoretical and experimental free vibrations of high industrial chimney interacting
Interacting With Folks on Different Levels of the Poker Food Chain
Comparison of theoretical and experimental free vibrations of high industrial chimney interacting
więcej podobnych podstron