
Environ Chem Lett (2003) 1:103–106
DOI 10.1007/s10311-002-0019-y
O R I G I N A L P A P E R
V. Antoniadis · J. D. McKinley
Measuring heavy metal migration rates in a low-permeability soil
Accepted: 16 October 2002 / Published online: 13 February 2003
Springer-Verlag 2003
Abstract
Heavy metals at high concentrations are often
toxic to living organisms, and their environmental toxicity
depends on soil properties. It has long been thought that in
clay-rich, low-permeability soils, heavy metals are bound
to soil particles, and thus there are only few toxicity risks.
This study questioned this perception and tested heavy
metal mobility in such a soil, of the London Clay series,
using a benchtop centrifuge. Soil columns were placed in
the centrifuge and were infiltrated with solutions of Cu,
Ni and Zn ions, while the centrifuge was running at three
different gravity levels, at 5,280, 2,600 and 1,300 gravi-
ties. The measured rates of migration of Cu, Ni and Zn
ions were extrapolated down to 1 gravity, which repre-
sents field conditions, the conditions for which an
assessment of risk due to metal toxicity would be needed.
It was found that heavy metal movement was significant
in London Clay, Ni being the most mobile metal in the
study, followed by Zn and then Cu ions. Centrifuge
infiltration tests were proven to be a valuable tool in the
study and quantification of metal mobility in low-
permeability soil, because they were easy to run and
precise in predicting metal movement in London Clay.
Keywords
Copper · Nickel · Zinc · Centrifuge · Mobility ·
London Clay · Soils
Introduction
Heavy metal ions may have toxic effects on plants,
animals or humans, and their toxicity is linked to their
mobility in soil. The greater the mobility, the higher the
toxicity risk of these metals. Heavy metal mobility mainly
depends on soil properties (Rowell 1994). One important
process affecting heavy metal mobility in soil is sorption.
Sorption is the phenomenon in which metal ions, which
typically bear a positive charge, are attracted to solid
particles in the soil such as clay and organic matter, which
mostly bear a net negative charge. This binding is often
reversible, and metals bound onto the solids are in
equilibrium with metals in the soil water. This means that
strongly retained metal ions are removed from the soil
water and become less mobile than weakly retained ions.
It has long been thought that “heavy” soils, that is, high
clay content soils, tend to bind and immobilise heavy
metals, so that many industries feel safe when disposing
of their wastes in clayey soils (Sharma and Lewis 1994).
Yet, this simplification is not always true, since the
transport of contaminants even in clayey soils can be
significant and needs to be addressed and quantified.
There are several indices of heavy metal mobility in
soils, the retardation factor, R
d
, being a straightforward,
unitless one (Kookana et al. 1994). The natural meaning
of R
d
is that it is the ratio of the rate of movement of the
heavy metal to the rate of movement of water in which the
metal is dissolved. To give a rather simplistic example, if
Ni is dissolved in water, and the water moves 100 m in the
soil, but Ni moves 5 m, then Ni R
d
is 20 (20=100/5). It is
easy to see that the greater the metal R
d
, the higher the
metal retention by the soil, and, thus, the lower the metal
mobility.
There are two main ways to estimate heavy metal
mobility in soils. The first is adsorption experiments (or
batch tests) and the second is infiltration tests. Adsorption
experiments have been used widely for this matter, but
they have some important disadvantages, namely, they do
not represent real field conditions, they tend to underes-
timate heavy metal availability and there are at least three
J. D. McKinley
School of Civil Engineering,
Queen’s University Belfast,
David Kier Building, Stranmillis Road, Belfast, BT9 5AG, UK
V. Antoniadis (
)
)
Department of Environmental Studies,
University of the Aegean,
University Hill, 811 00 Mytilene, Greece
e-mail: vantoniadis@env.aegean.gr
Tel.: +30-2510-36265
Fax: +30-2510-36299
ways to estimate metal retardation factor R
d
. These mean
that it is difficult to draw clear conclusions from this kind
of experiments (Celorie et al. 1989), and if decision
making relies only on measurements conducted with
batch tests, heavy metal contamination can not be
properly assessed. On the other hand, infiltration tests
are more representative of natural conditions, but are
technically more challenging to run and, for clayey soils,
very time consuming, because in such soils water moves
extremely slowly. This technical difficulty concerning
clayey soils can be overcome with the use of small
laboratory centrifuges (Mitchell 1998). Centrifuges, when
spinning, apply such stresses to the soil column that has
been placed in it, that they permit the systematic
examination of heavy metal movement in the soil under
controlled and reproducible testing conditions, even in
clayey soils, without departing significantly from the real
natural soil conditions. What is more, they are simple to
run (McKinley et al. 1998).
Thus, the scope of this study was to examine the
mobility of heavy metals in a clayey soil, using centrifuge
infiltration tests, in order to assess whether there are risks
for metal movement. The metal ions studied were Cu
2+
,
Ni
2+
and Zn
2+
, because they are among the most
important in their group and represent a variety of
behaviours in the soil. They are important because they
are all toxic at high concentrations to living organisms,
and they represent a variety of soil behaviours, because
Cu is typically strongly bound onto soil solids, while Ni
and Zn are typically more mobile in the soil environment
(Antoniadis and Alloway 2001).
Experimental
London Clay was chosen for use in this study. London
Clay is a stiff to very stiff, fissured clay with a hydraulic
conductivity between 10
10
and 10
12
m s
1
(Dewhurst et
al. 1998). Blocks of the soil were recovered from
tunnelling work below the Thames in Westminster,
central London. X-ray diffraction analysis indicated that
the principal minerals in the recovered samples were
quartz, kaolinite, montmorillonite and muscovite. Of
these, the montmorillonite will have the biggest influence
on the sorption of heavy metals from solution, because it
has a high surface area and a high cation exchange
capacity. The blocks of clay were broken up, allowed to
air-dry and crushed to a fine powder.
Nine infiltration tests on compacted London Clay have
been conducted, using three different solutions containing
500 mg L
-1
Cu, as Cu(NO
3
)
2
, 500 mg L
-1
Ni, as Ni(NO
3
)
2
,
and 500 mg L
-1
Zn, as Zn(NO
3
)
2
. The cells were
centrifuged at three different gravity levels: 5,280, 2,600
and 1,300 gravities, thus giving 9 tests: 3 metals 3
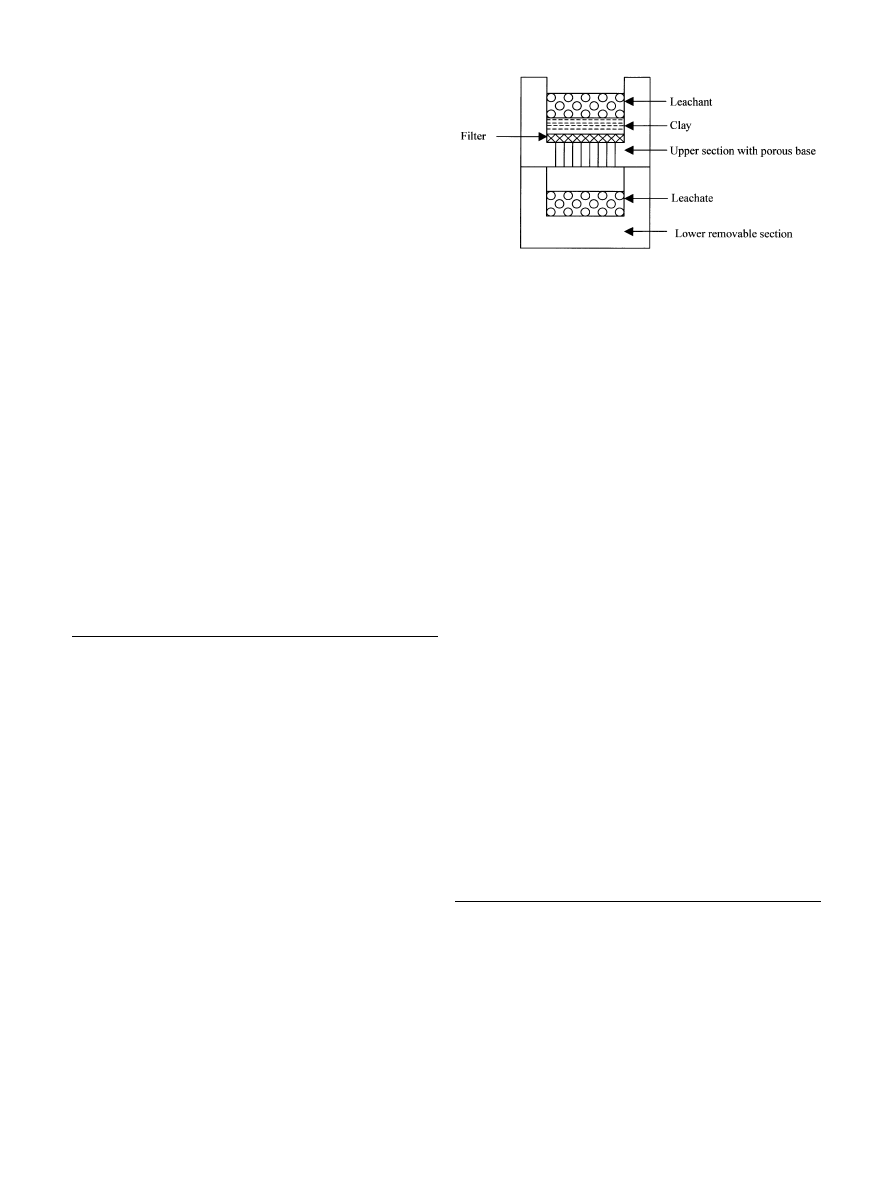
gravity levels = 9. Figure 1 shows the centrifuge
infiltration cell used for this study. The cell has an upper
section with a porous base, which retains the soil, and a
lower section within which the leachate collects. In use,
the cell resembles a falling head permeameter. The
centrifuge equipment permitted four such infiltration cells
to be used at once. Dried clay was slurried with deionised
water, left overnight and then placed in the infiltration
cell. Initially, the cells ran until one pore volume was
collected, producing a layer of compacted clay on the
porous filter. This took approximately 2 h, typically, for
the high speed, and 3 h for the medium and low speeds.
The centrifuge was then stopped, the supernatant fluid
was replaced with leachant solutions and infiltration tests
were performed at the three gravity levels at 21 C.
Periodically, the centrifuge was stopped and fresh
leachant was added to the top of the cell to replace fluid
passing through the clay, the collected leachate being
removed at the same time. This leachate was acidified
with 5% nitric acid for storage, and the heavy metal
content was determined by inductively coupled plasma-
optical emission spectroscopy (ICP-OES).
In order to account for the natural soil variability each
test was repeated. The number of pore volumes required
for the leachate concentration to rise to half of the
leachant concentration is a measure of the mobility of the
contaminant, being approximately equal to the retardation
factor, R
d
(Shackelford and Redmond 1995). For these
tests, this level corresponds to 250 mg L
-1
. A second
estimate for R
d
was also calculated by curve fitting to the
breakthrough curve using the CXTFIT (Toride et al.
1995). The two methods showed a remarkable agreement,
but in the Results and Discussion section, the R
d
derived
from CXTFIT was preferred. The statistical significance
in differences between the data points in Fig. 3 is
indicated by the error bars, which represent the standard
error for each data point.
Results and discussion
Relative mobility of metal ions of Cu, Ni and Zn
In order to assess the mobility of Cu, Ni and Zn in a low
permeability soil, infiltration tests using a benchtop
centrifuge were performed at three different gravity
levels, namely 1,300, 2,600 and 5,280 gravities. Figure 2
shows the breakthrough curves for the infiltration of
500 mg L
-1
Cu and 500 mg L
-1
Ni, respectively, through
Fig. 1
Centrifuge infiltration cell used to study metal ion mobility
in London Clay
104
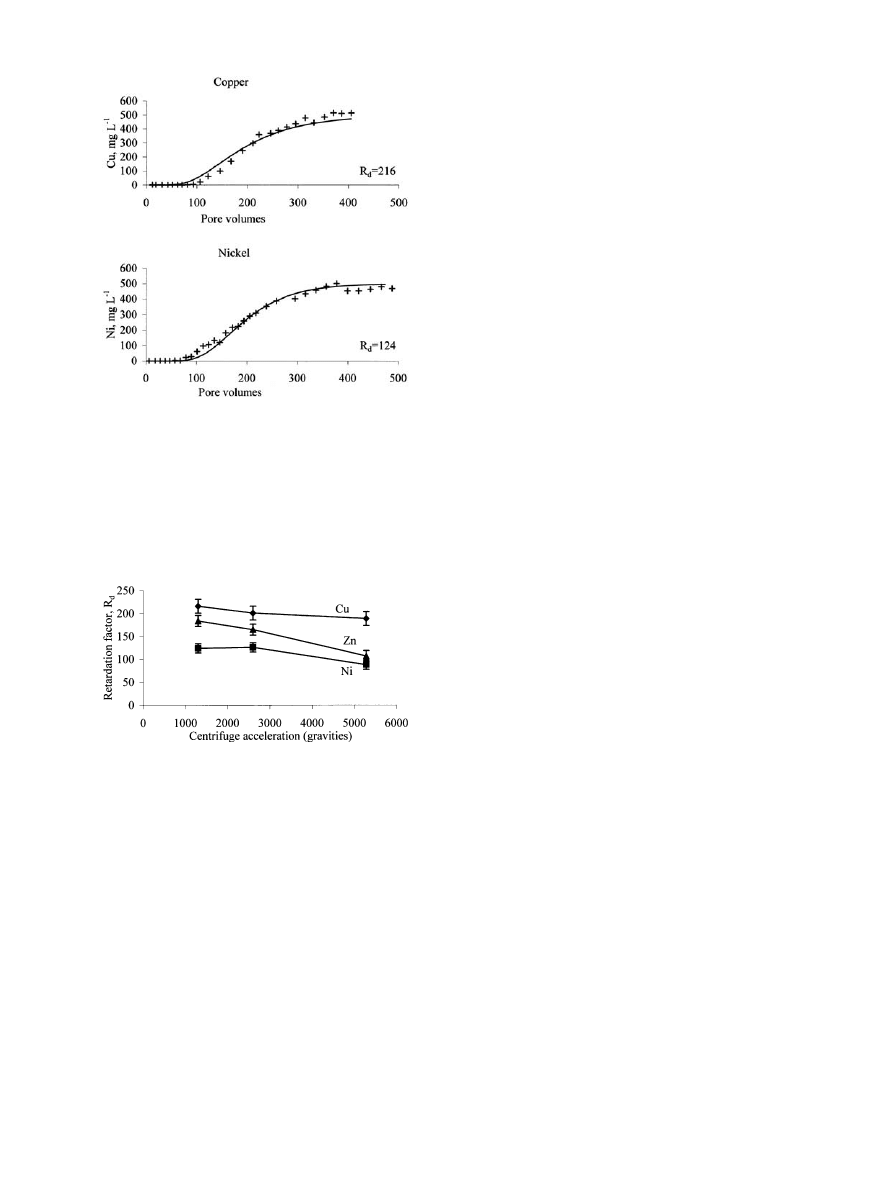
London Clay. For simplicity, only the 1,300 gravities run
for Cu and Ni are presented in Fig. 2. The breakthrough
curves for the infiltration of 500 mg L
-1
Zn are presented
in Antoniadis and McKinley (2000). It was found that at
all three levels of centrifuge acceleration Cu mobility was
significantly lower than that of Ni and Zn. The retardation
factor R
d
for Cu at 5,280 gravities was 189€1, while for
Ni it was 88€2 and for Zn 107€9. At 1,300 gravities the
R
d
values for Cu, Ni and Zn were 216€15, 124€6 and
184€12, respectively (Fig. 3). This is in agreement with
Alloway (1995), who stated in his review that Cu is
sorbed rather preferentially over Ni and Zn onto soil
solids, especially onto the soil organic groups. This shows
that centrifuge infiltration tests can predict successfully
the fate and mobility of heavy metals, even in a “difficult”
clay material. However, it is evident that the differences
between R
d
values of Cu and Ni and of Cu and Zn
decreased as gravity levels decreased. The difference in
R
d
values between Cu and Ni was 101 at 5,280 gravities
and decreased to 92 at 1,300 gravities, while the
difference between Cu and Zn was 82 at 5,280 gravities
and decreased to 32 at 1,300 gravities.
The effect of centrifuge acceleration in metal mobility
It was also found that the mobility of Cu, Ni and Zn
increased with an increasing acceleration level. As noted
above, the retardation factor R
d
for Cu increased signif-
icantly with decreasing centrifuge acceleration. This was
probably due to the metal having less time to interact with
the soil, which in turn seems to have reduced the
adsorption of Cu onto the soil, and thus its mobility was
enhanced at 5,280 gravities. The reduced mobility of Cu
indicates that there is a scaling effect, which affects Cu
mobility significantly. By extrapolating the data series to
unity gravity (the gravity level at field conditions) there is
a characteristic R
d
value that can be obtained. The
extrapolated value provides a valid indication of the metal
mobility through the London Clay in the field. This
scaling effect was clear for Zn as well, as R
d
values for Zn
increased significantly with reduced centrifuge accelera-
tion. The same scaling effect was indicated for Ni as well,
but this time not so clearly as for Cu and Zn. That means
that for Ni the scaling effect was not important down to
1,300 gravities, because the R
d
values at 1,300 and 2,600
did not show significant differences.
It should be noted that in all three elements we
observed the anticipated scaling effect: as the acceleration
levels decreased the differences between the R
d
values
obtained for each metal also decreased. In other words, it
was anticipated that for each metal ion there is a gravity
level, at which metal mobility reaches equilibrium with
the soil matrix as gravity approaches unity. At this gravity
level, the metal R
d
value becomes identical to that at unity
gravity.
The data suggest that Ni reached this equilibrium at
1,300 gravities, while for Cu and Zn probably more steps
of reduced centrifuge acceleration should be taken. This
means that it is rather safer to conclude that Ni mobility in
London Clay under real field conditions is very likely to
be of R
d
124 (that is, the same as that of 1,300 and
2,600 gravities). As for Cu and Zn, an approximation of
the unity gravity R
d
value, after extrapolating the
measured R
d
values, could be 221 and 212, respectively.
It is important to note that at unit gravity the differences
in R
d
values between Cu and Ni and between Cu and Zn
are less significant than those at 5,280 gravities. This is in
agreement with the previously found trend of reduced
differences between the R
d
values of Cu, Ni and Zn as
acceleration was reduced. This indicates that the data
Fig. 2
Infiltration of 500 mg L
-1
of copper and nickel ions through
London Clay: breakthrough curves for Cu
2+
and Ni
2+
at centrifuge
acceleration of 1,300 gravities. Crosses represent the actual data
points, while solid lines represent the CXTFIT curve fit model of
the first run. The second run was similar, thus it was not included.
Note that Cu needs a greater number of pore volumes to reach half
of the initial metal concentration (that is 250 mg L
-1
), and this
signifies the fact that Cu is less mobile than Ni. The greater R
d
value for Cu, computed by the CXTFIT model using these data also
gives evidence of the same fact
Fig. 3
Retardation factor R
d
for Cu
2+
, Ni
2+
and Zn
2+
obtained from
their breakthrough curves at centrifuge accelerations of 1,300,
2,600 and 5,280 gravities through columns of London Clay. R
d
values for Ni seem to be reaching a plateau with reducing
centrifuge acceleration. It is safer to point to a R
d
value for Ni at
unity gravity (at real field conditions) than for Cu and Zn
105

extrapolation for Cu and Zn, although not as firm as for
Ni, can be a good approximation for Cu and Zn mobility
under field conditions.
Conclusion
The rate of migration of Cu, Ni and Zn through
consolidated London Clay has been successfully mea-
sured using a permeameter-style infiltration column cell
in a laboratory centrifuge. This method is easy to run,
gives reproducible results and overcomes the difficulties
of handling a clayey soil and changing irreversibly the
soil natural conditions, problems typically encountered by
infiltration column and batch adsorption experiments,
respectively.
The tests predicted successfully the mobility of Cu, Ni
and Zn, indicating that Ni is significantly more mobile
than Cu and Zn, and that Zn is more mobile than Cu.
It was found that there was a scaling effect for Cu, Ni
and Zn, showing that the R
d
values for both metals can
safely be extrapolated to unity gravity, which is the real
field conditions. However, the data suggested that Ni
reached an equilibrium in this particular soil matrix and,
thus, its R
d
value at unity gravity can be indicated more
confidently than the R
d
for Cu and Zn.
Acknowledgements
This work was sponsored by the Engineering
and Physical Sciences Research Council, grant no. GR/M27067.
This support is gratefully acknowledged.
References
Alloway BJ (1995) Soil processes and the behaviour of metals. In:
Alloway BJ (ed) Heavy metals in soils. Blackie Academic and
Professional, London, pp 38–57
Antoniadis V, Alloway BJ (2001) Availability of Cd, Ni and Zn to
ryegrass in sewage sludge-treated soils at different tempera-
tures. Water, Air Soil Pollut 132:201–214
Antoniadis V, McKinley JD (2000) Leaching tests in a laboratory
centrifuge on zinc migration in London Clay. In: Garnier J,
Thorel L, Haza E (eds) Physical modelling and testing in
environmental geotechnics. NECER, Paris, pp 53–60
Celorie JA, Vinson TS, Woods SL, Istok JD (1989) A comparison
of sorption equilibrium distribution coefficients using batch and
centrifugation methods. J Environ Quality 18:307–313
Dewhurst DN, Aplin AC, Sarda JP, Yang Y (1998) Compaction-
driven evolution of porosity and permeability in natural
mudstones an experimental study. J Geophys Res Solid Earth
103 (B1):651661
Kookana, RS, Naidu R, Tiller KG (1994) Sorption non-equilibrium
during cadmium transport through soils. Aust J Soil Res
32:635–651
McKinley JD, Price BA, Lynch RJ, Schofield AN (1998) Centri-
fuge modelling of the transport of a pulse of two contaminants
through a clay layer. Gotechnique 48:421429
Mitchell RJ (1998) Centrifugation in geoenvironmental practice
and design. Can Geotech J 35:630640
Rowell DL (1994) Soil science: methods and applications. Long-
man, Harlow
Shackelford CD, Redmond PL (1995) Solute breakthrough curves
for processed kaolin at low flow rates. ASCE J Geotech Eng
121:1732
Sharma HD, Lewis SP (1994) Waste containment systems, waste
stabilization, and landfills. Wiley, New York
Toride N, Leji FJ, van Genuchten MT (1995) The CXTFIT code for
estimating transport parameters from laboratory or field tracer
experiments, Version 2.1: Research report No. 137. US salinity
laboratory, Agricultural research service, US Department of
Agriculture, Riverside, California
106
Wyszukiwarka
Podobne podstrony:
Simulation Of Heavy Metals Migration In Peat Deposits
Heavy metal toxicity,effect on plant growth and metal uptake
Does Heavy Metal Corrupt Youth
Quantitative dilatometric analysis of intercritical annealing in a low silicon TRIP steel
Cyna metal laski?,85 do?lów laborat
Trzy zapałki chords, Hard Rock & Heavy Metal
Cyna metal proszek?,85 do?lów laborat
AJA Results of the NPL Study into Comparative Room Acoustic Measurement Techniques Part 1, Reverber
Barron Measured Early Lateral Energy Fractions In Concert Halls And Opera Houses
[Friedrich Schneider] Size and Measurement of the Informal Economy in 110 Countries Around the Worl
64 919 934 New Trends in Thin Coatings for Sheet Metal Forming Tools
Kundalini Is it Metal in the Meridians and Body by TM Molian (2011)
Low Temperature Differential Stirling Engines(Lots Of Good References In The End)Bushendorf
M Kernis Measuring Self Esteem in Context
Environmental Law Enforcement measures and?fectiveness in
Cognitive Processes Underlying Context Effects in Attitude Measurement
więcej podobnych podstron