
Biomaterials 23 (2002) 569–576
Increasing hydroxyapatite incorporation into
poly(methylmethacrylate) cement increases osteoblast
adhesion and response
M.J. Dalby
a,
*
,1
, L.Di Silvio
a
, E.J. Harper
b
, W.Bonfield
b,2
a
IRC in Biomedical Materials, Institute of Orthopaedics, Brockley Hill, Stanmore, Middlesex HA7 4LP, UK
b
IRC in Biomedical Materials, Queen Mary College, University of London, Mile End Road, London E1 4NS, UK
Received 20 November 2000; accepted 3 April 2001
Abstract
Poly(methylmethacrylate) (PMMA) is the current standard for cement held prostheses.It forms a strong bond with the implant,
but the bond between the cement and the bone is considered to be weak, with fibroblastic cells observed at the implant site, rather
than direct bone contact, a contributing factor leading to implant failure.Incorporation of hydroxyapatite (HA) increases the
biological response to the cement from tissue around the implant site, thus giving increased bone apposition.In this study, PMMA
discs with 0, 4.6 and 8.8 vol%. HA were examined. Primary human osteoblast-like cells (HOBs) were used for the biological
evaluation of the response to the cements in vitro.Morphology was observed using scanning electron microscopy (SEM) and
confocal laser scanning microscopy (CLSM).Measurement of tritiated thymidine (
3
H-TdR) incorporation and alkaline phosphatase
(ALP) activity were used to assess proliferation and differentiation.A synergy between increasing focal contact formation,
cytoskeletal organisation, cell proliferation and expression of phenotype was observed with increasing HA volume.Preferential
anchorage of HOBs to HA rather than PMMA was a prominent observation. r 2001 Elsevier Science Ltd.All rights reserved.
Keywords:
Bone cement; Osteoblasts; Hydroxyapatite
1. Introduction
Poly(methylmethacrylate) (PMMA) is a self-curing
acrylic polymer with no adhesive properties.The cement
was developed in the early 1960s by Charnley and Smith
[1].PMMA’s ability to conform to the shape of its
surroundings, allows even distribution of the load
caused by the implant, and forms a strong mechanical
bond with the implant.Although PMMA is still the
current standard for cement held prostheses, it is an
inert material with fibroblastic cells observed at the
bone/cement interface [1,2].The bone/cement interface
is considered to be the weak link in cement-held
prostheses providing a barrier to direct fracture
healing.The poor tissue/cement interaction is attri-
buted to many factors: high polymerisation exotherm
[3],
leaching
of
toxic
unreacted
methylmetha-
crylate (MMA) monomer [4], polymerisation shrinkage,
mismatching of bone/cement modulus leading to
micromotion upon loading [5], cement wear particles
evoking inflammatory reactions, and the incorporation
of radiopacifiers (radio-opaque markers) into the
cement [6].
Mechanical characteristics have also historically been
a problem with PMMA cements; polymers produced by
mixing of the cement phases are brittle, and have a poor
fatigue life.Fracture of cements has been reported to
lead to aseptic loosening and tissue necrosis [7].
Although joint replacements, in general, are successful
with approximately 90% lasting ten years, failures are
common.In 1995, 18% of the 40,000 hip replacement
operations performed in the UK were revisions [8].The
most common reason for failure was aseptic loosening,
with failure of the cement mantle being a contributing
factor [9].
*Corresponding author.Tel.
: +44-0141-3302931; fax: +44-0141-
3303730.
E-mail address:
m.dalby@bio.gla.ac.uk (M.J. Dalby).
1
Now at: Centre for Cell Engineering, Institute of Biomedical and
Life Sciences, University of Glasgow, Glasgow G12 8QQ, Scotland,
UK.
2
Now at: Department of Materials Science and Metallurgy,
University of Cambridge, Pembroke Street, Cambridge, CB2 3QZ,
UK.
0142-9612/02/$ - see front matter r 2001 Elsevier Science Ltd.All rights reserved.
PII: S 0 1 4 2 - 9 6 1 2 ( 0 1 ) 0 0 1 3 9 - 9

The addition of low volume fractions of particulate
materials (
o10 vol%), such as hydroxyapatite (HA),
offer the possibility of strengthening the material with-
out a severe detrimental effect upon stress distribution,
or causing flow problems.It is noted that whilst highly
bioactive, the mechanical properties of HA are not ideal
[10], with a tensile strength of 50–70 MPa, Young’s
modulus of 35–120 GPa, and fracture toughness of 0.5–
1.5 MN m
3/2
.The addition of up to 40 wt% HA
(approx.13 vol%) to PMMA cement has been shown
to increase the fracture toughness [11], and the addition
of up to 15 wt% HA (approx.5 vol%) led to an increase
in flexural modulus [12].The tensile and compressive
strengths have been observed to remain constant with
up to 15 wt% HA added to PMMA [12,13].
The static and fatigue properties of a modified
PMMA cement including 17.5 wt% HA (approx.
6 vol%) were measured as part of a multi-centre
European Community (EC) project [10,14].The char-
acteristics were assessed after both vacuum and open
bowl mixing.The tensile, compressive and flexural
strengths were found to decrease by 6–9% after both
methods of cement mixing.However, when tested in
fatigue, the physiological loading regime, the addition of
the HA caused a significant decrease in fatigue life after
hand mixing but when tested after vacuum mixing there
was no detrimental influence seen for HA incorporation.
Therefore, these results demonstrate that a relatively
small volume of HA may be added before significant
mechanical deterioration is observed.The poor reinfor-
cing properties of HA are mainly due to poor interfacial
bonding between the cement and HA, as seen on
fracture surfaces via scanning electron microscopy
(SEM) [10,14].The addition of HA to PMMA cement
has been shown to have the advantage of acting as a
heat sink.
HA is a synthetic calcium phosphate that resembles
bone mineral.Its surface is highly reactive producing
favourable attachment and bioactivity.In addition, it
has
osseoconductive
and
osseoinductive
effects
[9,15–17].
The objective of this study was to investigate
potentially bioactive cements capable of strengthening
the mechanical retention of the implant by allowing
direct bone apposition.An in vitro tissue culture model
was used to evaluate the biological response, on
conventional PMMA, PMMA/4.6 vol% and PMMA/
8.8 vol% HA. In vitro systems allow the study of tissue
response to a material without the complexities asso-
ciated with in vivo models [18,19].Primary human
osteoblast-like (HOB) cells were used as they are
representative of a cell type that can be obtained from
living bone, and have the characteristics of osteoblasts
of living bone in the body [20].
A previous in vitro study [21] has shown that
osteoblast-like cells had an enhanced proliferation on
PMMA/HA cement.This study specifically examines
the attachment of HOBs to the cements using visualisa-
tion of the actin cytoskeleton, and observation of
vinculin at sites of focal contact using confocal laser
scanning microscopy (CLSM).SEM has been used to
observe cell morphology, and proliferation and pheno-
type have been quantified biochemically.
2. Materials and methods
2.1. Materials
PMMA cement discs (Coripharm GmbH, Germany),
1.2 cm in diameter, with 0, 4.6 and 8.8 vol% HA
powder, were prepared by addition of the MMA
monomer to the PMMA polymer.The mixture was
stirred
under
controlled
temperature
conditions
(22
721C), until the mixture became wet enough to
spatula into moulds.Prepared discs were sterilised by
gamma irradiation at a dose of 2.5 Mrad (Swann
Morton, UK) alongside medical equipment.
2.2. Cell isolation and in vitro cell culture
HOB cells were isolated from the femoral head of a
patient undergoing total joint replacement.Trabecular
bone fragments were dissected from the femoral head
and washed several times in phosphate buffered saline
(PBS), followed by a final wash in complete medium
(Dulbecco’s modified Eagle’s medium (DMEM), sup-
plemented with 10% foetal calf serum (FCS), 1% non-
essential amino acids, l-ascorbic acid (150 g ml
1
),
0.02 m l-glutamine, 0.01 m HEPES, 100 units ml
1
peni-
cillin and 100 mg ml
1
streptomycin).The bone chips
were further chopped with scalpel blades, and incubated
in complete medium at 371C, 5% CO
2
in a humid
atmosphere.Once an osteoid seam of cells transfering
from the fragments to the culture plastic was observed,
the chips were transferred to a collagenase (100 U ml
1
)
and trypsin (300 U ml
1
) in PBS (0.01 m Hepes buffered)
solution.The bone was digested on a roller at 371C for
20 min.The supernatant was centrifuged (200 rpm,
181C, 5 min) and a cell pellet was obtained.The pellet
was resuspended in fresh medium (5 ml) and plated into
a 25 ml tissue culture flask.The HOBs were charac-
terised by measurement of alkaline phosphatase (ALP)
(biochemical and histochemical), osteocalcin, procolla-
gen type 1, and response to parathyroid hormone
(measurement of cAMP) [20].HOB cells were cultured
on the materials and control Thermanox discs (TMX,
Life Technologies, Paisley, UK) at 2 10
6
cells ml
1
for
1, 3, 7, 14, and 28 days under conditions described in a
previous study [22], briefly cells were incubated at 371C
in humidified air with 5% CO
2
(the culture medium was
changed at selected time intervals).
M.J. Dalby et al. / Biomaterials 23 (2002) 569–576
570

2.3. Cell growth and differentiation
Cell growth and proliferation were assessed using
measurement of total DNA and [
3
H]-TdR incorpora-
tion, while measurement of ALP activity was used to
confirm osteoblast phenotype.These methods have been
described in detail in a previous study [22].In brief, a
Hoechst 33285 (DNA specific fluorescent dye) was
reacted with cell lysates and DNA standards of
concentrations 0, 0.31, 0.62, 1.25, 2.5, 5, 10, and
20 mg ml
1
, in saline sodium citrate buffer (pH 7.0).
Fluorescence was measured on a Fluoroscan fluorimeter
(Ascent, Life Science International.Excitation wave-
length of 355 nm, emission wavelength of 450 nm), and
the sample DNA content calculated from the standard
curve.
3
H-TdR was measured on days 1 and 7 on both the
materials and the control TMX.The cells were
incubated with 1 mCi ml
1 3
H-TdR (Amersham Interna-
tional, UK) for 24 h before lysis.Trichloroacetic acid
(TCA) precipitation of the lysates was used to measure
the thymidine incorporation.The precipitate was filtered
onto a membrane using a Millipore filtration system
(Millipore Multiscreen), and any unbound radionucleo-
tide was washed away by filtering 10% TCA through the
membrane.The precipitate was dissolved in 0.01 m KOH
solution, and the
3
H-TdR incorporation measured by
scintillation counting.
Osteoblastic phenotype was determined biochemically
by measuring ALP production from the HOB cells.ALP
activity was determined using a COBAS-BIO (Roche,
UK) centrifugal analyser. p-Nitrophenol phosphate in a
diethanolamine buffer (Merck, UK) was used as a
substrate for ALP.The reaction product, p-Nitrophenol
is yellow at alkaline pH (9.8), and can be quantified at a
wavelength of 405 nm.
2.4. Cell morphology
Materials were seeded with HOB cells at a density of
1.5 10
4
cells ml
1
, and incubated at 371C in humidified
air and 5% CO
2
for 1 day.Cells were fixed with 1.5%
gluteraldehyde buffered in 0.1 m sodium cacodylate,
after 1 h fixation period, the cells were washed in 0.2 m
sodium cacodylate overnight.Cells were post-fixed in
1% osmium tetroxide and 1% tannic acid, then
dehydrated through a series of alcohol concentrations
(20%, 30%, 40%, 50%, 60%, 70%), stained in 0.5%
uranyl acetate (in 70% alcohol), then dehydrated further
(90%, 96%, 100% alcohol).The final dehydration was
in hexamethyl–disilazane, followed by air-drying.Once
dry, the samples were sputter coated before examination
under a JEOL 6300 SEM.
2.5. Focal contact formation
HOB
cells
were
seeded
onto
the
materials
(1 10
4
cells ml
1
) and cultured for 3 days.At this
point the cells were fixed in 4% formaldehyde/phos-
phate buffered saline (PBS).The samples were washed
with PBS after fixation, and permeabilised using a
permeabilising buffer (10.3 g sucrose, 0.292 g NaCl,
0.06 g MgCl
2
, 0.476 g Hepes buffer, 0.5 ml Triton X, in
100 ml water, pH 7.2) at 41C.The samples were then
incubated at 371C for 5 min in 1% BSA/PBS, followed
by the addition of either anti-vinculin primary antibody
(anti-human raised in mouse, hVIN-1, Sigma, Poole,
UK) for 1 h (371C).The samples were washed in PBS/
Tween 20.A secondary biotin conjugated rabbit anti-
mouse antibody (DAKO, UK) was added (1 : 50) for 1 h
(371C).A further wash followed, with final incubation in
strepdavadin-texas red (Vector, UK, 1 : 100) for 30 min
at 41C.After a final wash, the samples were viewed on a
CLSM (Noran).
2.6. Cytoskeletal organisation
HOB cells were seeded onto the materials (1
10
4
cells ml
1
) and cultured for 3 days.At each time
point the cells were fixed in 4% PBS.The samples were
washed after fixation with PBS, and permeabilised using
a permeabilising buffer (10.3 g sucrose, 0.292 g NaCl,
0.06 g MgCl
2
, 0.476 g Hepes buffer, 0.5 ml Triton X, in
100 ml water, pH 7.2) at 41C.The samples were then
incubated at 371C for 5 min in 1% BSA/PBS, followed
by the addition of Phalloidin–FITC probe (Sigma,
Poole, UK) for 1 h (371C).The samples were washed
in PBS/Tween 20 (3 5 min rinses) and viewed by
CLSM (Noran).
2.7. Statistics
All statistics were performed using SPSS Statware
software which ran a Tukey test, one way ANOVA, for
non-parametric data.
3. Results
Morphological investigation by SEM showed prefer-
ential anchorage to HA compared to the cement
polymer by the cell filopodia during HOB attachment
(Fig.1). The cells were also seen to anchor to other
surrounding cells in preference to the PMMA polymer.
Normal, flattened, osteoblast morphology (Fig.1a) was
noted on all test materials.
A higher number of focal adhesion plaques, viewed by
vinculin staining, was observed as HA incorporation
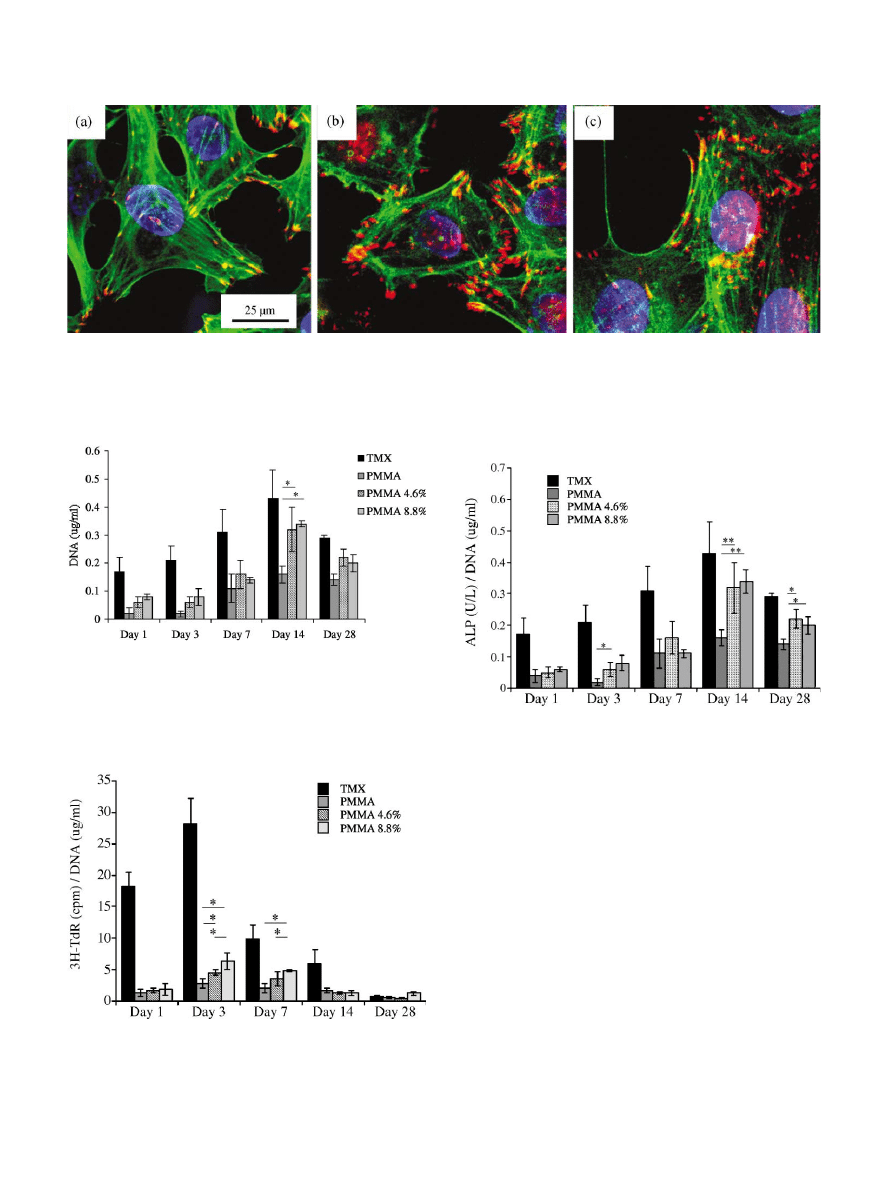
into the cements increased (Fig.2). Actin cytoskeleton
organisation was observed to increase with adhesion
M.J. Dalby et al. / Biomaterials 23 (2002) 569–576
571

plaque expression, hence increasing with volume of HA
incorporated (Fig.2).Cells on the plain PMMA samples
showed very diffuse actin.On 4.
6 vol% HA incorpo-
rated cements the cytoskeletons were seen to be more
clearly organised, linking to many more adhesion
plaques.On the 8.8 vol% HA samples, actin was clearly
organised with many stress fibres apparent.With all
samples the relationship between the adhesion plaques
and the actin microfilaments was seen.
Cell growth was seen to increase from day 1 to day 28
from the total DNA results (Fig.3). HA incorporation
was seen to increase total DNA content on the materials
compared to plain PMMA, but no differences were seen
from 4.6% to 8.8% HA/PMMA.
Proliferation on the cements and TMX control was
seen to peak at day 3, with basal levels of cell turnover
observed at, and after, day 14.At days 1, 3, and 7,
proliferation was seen to increase with HA incorpora-
tion into the PMMA, with significant differences
between plain PMMA and PMMA with 8.8 vol% HA
incorporation (Fig.4).
ALP activity was seen to increase up to day 14 on the
cements and TMX, with enzyme activity increasing with
volume of HA incorporation at this time point (Fig.5).
Highly significant differences were observed from plain
PMMA to cements incorporating PMMA, although no
statistical differences were observed between 4.6 and
8.8 vol% HA cements.
4. Discussion
SEM observation of preferential anchorage to HA in
composite materials has been previously observed, with
PMMA cement [21], and HAPEX
TM
[18,19].The
surface of implant materials presented to cells can be
considered as a foreign chemical species with reactive
sites.The end groups of polymer chains may interact
with reactive groups such as extracellular matrix (ECM)
proteins or carbohydrate molecules in serum.When a
material is implanted in vivo, it is immediately covered
with a thin layer of extracellular fluid, and it is through
this layer that the cells interact with the implant material
[23–25].ECM proteins form the most important
components of this surface layer for cellular attachment;
and include collagen, fibronectin, osteopontin, throm-
bin, thrombospondin, laminin, sialoprotein, fibrinogen,
anchorin, tenascin C, laminin, and vitronectin [26–28].
It could be postulated that HA presents a correct
scaffold for attachment of ECM adhesion proteins,
compared to PMMA.Thus, cell filopodia ‘probing’ the
material surface could be encouraging integrin mediated
cell adhesion to ECM components [29].Integrin
proteins are located within cell adhesion plaques (focal
contacts), and are thus involved in cellular adhesion in
the response to material surfaces.They are transmem-
brane receptors that bind to specific ECM components
and the cell cytoskeleton, characterised by combinations
of a and b subunits such that different subunit
combinations produce receptors with different ligand
specificities.Integrins have specificities for bone ECM
adhesion proteins as mentioned above [30,31].
Fig.1. Scanning electron micrographs for HOBs on the test cements
after 24 h.HOBs on 0 vol% HA cement showed very few filopodia.(a)
Preferential anchorage of HOB cell filopodia to HA exposed on the
surface of 4.6 vol% HA/PMMA. (b) 8.8 vol% HA/PMMA (c),
indicating the physiological chemistry of HA compared to PMMA
polymer.
M.J. Dalby et al. / Biomaterials 23 (2002) 569–576
572
Integrin mediated cell adhesion to substrate materials
influences subsequent cell responses, including spread-
ing, proliferation and differentiation [32].This is
brought about by signal transduction from integrins
located at adhesion plaques to the cell nucleus via the
cytoskeleton [33].
Formation of focal contacts is the start point for
normal animal cell function.Anchorage dependent cells
rarely proliferate in suspension, and remain rounded.
Cells require anchorage to undergo the G1 phase of the
cell cycle, but loosen their contacts and round up for the
M phase of division.This cycle of attachment and
detachment allows cells to rearrange their contacts to
accommodate daughter cells [34].Prolonged suspension
results
in
anoikis
(apoptosis
resulting
from
‘homelessness’) [35,36].
Fig.2. Confocal laser scanning micrographs (vinculin adhesion plaques (red/orange, yellow crossover), actin (green), nucleus (blue) 0 vol% HA/
PMMA (a) 4.6 vol% HA/PMMA (b), and 8.8 vol% HA/PMMA (c), after 72 h. Increasing cytoskeletal organisation was noted from 0 to 4.6 to
8.8 vol% HA in PMMA.
Fig.3. Total DNA (mg/ml) on control TMX, and PMMA with 0, 4.6
and 8.8 vol% HA incorporation. Cell growth was seen to steadily
increase from day 1 to day 28.Differences were seen from plain
PMMA to PMMA with HA (results are the mean
7SD, n ¼ 5; t-test;
*p
o0:05).
Fig.4.
3
H-TdR incorporation (cpm)/DNA (mg/ml) on control TMX,
and PMMA with 0, 4.6 and 8.8 vol% HA incorporation. Proliferation
was seen to be highest on day 3 with statistically significant differences
between HA volumes (results are the mean
7SD, n ¼ 5; t-test;
*p
o0:05).
Fig.5. ALP activity (U/l)/DNA (mg/ml) on control TMX, and
PMMA with 0, 4.6 and 8.8 vol% HA incorporation. ALP activity
was seen to be highest on day 14 with highest activity observed on HA
filled samples (results are the mean
7SD, n ¼ 5; t-test; *po0:05;
**p
o0:01).
M.J. Dalby et al. / Biomaterials 23 (2002) 569–576
573

These statements are substantiated by the vinculin
immunolocalisation results showing greater numbers of
adhesion plaques to be present with increasing HA.As
integrin proteins are located within cell adhesion
plaques, it can be assumed that with the increasing
occurrence of vinculin focal contacts, there are higher
levels of integrin/ECM interaction.Focal contacts have
been described as transmembrane junctions from the
ECM to the cytoskeleton and cytoplasm, and are said to
be transducers of extracellular signals [37].
Integrin cytoplasmic domains act as sites of nuclea-
tion foci for cytoskeletal assembly [33], and it is via these
interactions that integrins initiate signal transduction,
thus suggesting roles in proliferation, differentiation,
morphogenesis, and wound healing [38].Indeed, with
increasing expression of focal contacts, increased
organisation of actin cytoskeleton was observed.The
actin microfilament cytoskeleton is involved in the
formation of cell processes, cell shape, and cell attach-
ment.As the cell adheres to a substrate material
filopodia are formed and moved into place by actin
acting upon the plasma membrane.The actin is
observed in the filopodia as directed tight parallel
bundles.Contractile stress fibres are seen once the
filopodia are attached [39].
When a cell adheres to ECM via integrins, the
integrins
are
coupled
to
actin
via
focal
adhesion proteins.At this initial binding stage, actin is
under no tension, as myosin is in the inactive
conformation.Rho activation promotes myosin light
chain phosphorylation resulting in conformational
change.This, in turn, causes actin alignment putting
tension on the integrins.The tension applied results in
the clustering of integrins within an adhesion plaque.
Integrin clustering, and integrin ECM ligand binding
produces colonisation of ECM proteins to the adhesion
plaques.
Integrin clustering induces signal transduction path-
ways from focal adhesion kinase (FAK) and activation
of Rho stimulating various kinases, including Rho-
kinase and phosphatidylinositol phosphate-5 kinase
(PIP 5-kinase) [40].
The findings in this study show that increased HA
incorporation into the cements leads to increased
cellular proliferation and expression of phenotype from
an increase in expression of focal contacts.The results
for thymidine incorporation and ALP show normal
osteoblastic trends with an initial high level of prolifera-
tion, followed by subsequent increase in ALP activity
[41,42].
The therapeutic value of any bone biomaterial is to
induce the rapid deposition of collagenous bone matrix
followed by matrix mineralisation.It has been reported
that bone formation is mainly dependent upon the
number, rather than the activity of osteoblastic cells [43],
and cell number is largely dependent upon cell
adherence and proliferation [27].Thus, the initial
proliferation and cell recruitment on the material
surface is of great importance to terminal differentiation
of cells in contact with a material.
ALP activity is associated with bone formation, and it
is produced in high levels during the bone formation
phase, thus making it a good indicator of bone
formation activity [44,45].
Robinson proposed as early as 1923 that ALP may
have a role in elevating calcium and phosphate levels to
the point of spontaneous precipitation [46].The enzyme
has roles in hydrolysis of pyrophosphate and ATP, that
are inhibitors of calcification, and is involved in
hydrolysing organic phosphoesters (e.g. ATP, ADP,
AMP, etc.) to orthophosphate (PO
4
), which is used to
form the nascent CaPO
4
mineral [47–50].
Integrins only form a part of the signalling pathways
employed by cells in vitro.As well as signalling from the
material via absorbed ECM, there are many autocrine
transductive pathways producing ‘community’ effects on
the cells, hence organising differentiated tissue forma-
tion.Cell receptors include ion-channel linked (e.
g.
Ca
2+
), G-protein linked (e.g. Ras), and enzyme-linked
(e.g. tyrosine kinases) [51–54].
There appears to be a flow from the formation of
focal contact to the activity of ALP in relation to
changing PMMA/HA composition, indicative of signal
transduction.Increasing biological activity in response
to increasing HA content has been observed.When
developing a bioactive material both mechanical and
biological characteristics must be considered, and a
balance made.This cement has shown some loss of
mechanical integrity with HA addition during testing,
with decrease of flexural and compressive strength,
tensile strength, and fatigue strength [14], but an
increase in bioactivity has been observed.The correct
HA/PMMA combination must be sought to optimise
the cement.
Once the balance has been found, loading of HA into
cements may be the way forward in producing cements
with the flow properties that surgeons require, and the
biological properties that benefit the patient.
Acknowledgements
We thank EPSRC for IRC funding.We also thank
Mrs.C.Clifford, Dr.M.M.Knight, Dr.Z.Luklinska
and Mr.R.Whitenstall.
References
[1] Lautenschlager EP, Stupp SI, Keller JC.Structure and properties
of acrylic bone cement.In: Ducheyne P, Hastings GW, editors.
M.J. Dalby et al. / Biomaterials 23 (2002) 569–576
574

Functional behaviour of orthopaedic biomaterials.USA: Frank-
lin Books, 1984.p.87–117.
[2] Downes S, Kayser MV, Blunn G, Ali SY.An electron
microscopal study of the interaction of bone with growth
hormone loaded bone cement.Cells Mater 1991;1:171–6.
[3] Amstutz HC, Gruen T.Clinical application of polymethylmetha-
crylate for total joint replacement.In: Ahstrom JP, editor.
Current practice of orthopaedic surgery.St.Louis: CV Mosby
Company, 1973.p.158–82.
[4] Vasquez B, Elvira C, Levenfeld B, et al.Application of tertiary
amines with reduced toxicity to the curing process of acrylic bone
cements.J Biomed Mater Res 1997;34:129–36.
[5] Oonishi H.Mechanical and chemical bonding of artificial joints.
Clin Mater 1990;5:217–33.
[6] Gomez-Barrena E, Chang JD, Rimnac CM, Salvati EA.The role
of polyethylene properties in osteolysis after total HIP replace-
ment.Insruct Course Lect 1996;45:187–97.
[7] Downes S.Methods of improving drug release from poly(methyl-
methacrylate) bone cement.Clin Mater 1991;7:227–31.
[8] Working Party of the Institute of Materials.Materials Technol-
ogy Foresight in Biomaterials.Institute of Materials, 1995.
[9] Guan JL, Chen HC.Signal transduction in cell–matrix interac-
tions.Int Rev Cytol 1996;168:82–121.
[10] Harper EJ.Bioactive bone cements.Proc Instn Mech Engrs
1998;212:113–20.
[11] Perek J, Pilliar RM.Fracture toughness of composite acrylic bone
cement.J Mater Sci: Mater Med 1992;3:334.
[12] Vallo CI, Montemartini MA, Fanovich MA, Porto Lopez JM,
Caudrado TR.Polymethylmethacrylate based bone cement mod-
ified with hydroxyapatite.J Biomed Mater Res 1999;48:150–8.
[13] Sogal A, Hulbert SF.Mechanical properties of a composite bone
cement: polymethylmethacrylate and hydroxyapatite.Bioceramics
1992;5:213–24.
[14] Harper EJ, Cauich-Rodriguez J, Dingeldein E, Bonfield W.
Mechanical characterisation of optimised PMMA based bone
cement with HA reinforcement.European Report Bibochem,
1997.
[15] Posner AS, Betts F.Synthetic amorphous calcium phosphate and
its relation to bone mineral structure.Accounts Chem Res
1975;8:273–81.
[16] Stephenson PK, Freeman MA, Revell PA, Germain J, Tuke M,
Pirie CJ.The effect of hydroxyapatite coating on ingrowth of
bone into cavities of an implant.J Arthroplasty 1991;6:51–8.
[17] Ohgushi H, Goldberg VM, Caplan AI.Hetrotopic osteogenesis in
porous ceramics induced by marrow cells.J Orthop Res
1989;7:568–78.
[18] Huang J, Wang M, Tanner KE, Bonfield W, Di Silvio L.In vitro
mechanical and biological assesment of hydroxyapatite-reinforced
polyethylene composite.J Mater Sci: Mater Med 1997;8:1–5.
[19] Huang J, Di Silvio L, Wang M, Tanner KE, Bonfield W.In vitro
assesment of hydroxyapatite- and bioglass-reinforced polyethy-
lene composites.Bioceramics 1997;10:519–22.
[20] Di Silvio L.A novel application of two biomaterials for the
delivery of growth hormone and its effect on osteoblasts.PhD
thesis, University of London, 1995.
[21] Dalby MJ, Di Silvio L, Harper EJ, Bonfield W.In vitro
evaluation of a new PMMA cement reinforced with hydroxyapa-
tite.J Mater Sci: Mater Med 1999;10:793–6.
[22] Di Silvio L, Dalby MJ, Bonfield W.In vitro response of
osteoblasts to hydroxyapatite-reinforced polyethylene compo-
sites.J Mater Sci: Mater Med 1998;9:845–8.
[23] Kasemo B, Lausmaa J.Material–tissue interfaces: the role of
surface properties and processes.Environ Health Prospects
1994;102:41–5.
[24] Kasemo B, Lausmaa J.Surface science aspects on inorganic
biomaterials.Crit Rev Biocompat 1986;2:335–80.
[25] Takahiro S, Yamamoto T, Toriyama M, et al.Surface instability
of calcium phosphate ceramics in tissue culture medium and the
effect on adhesion and growth of anchorage dependent animal
cells.J Biomed Mater Res 1997;34:507–17.
[26] Gronowicz G, McCarthy MB.Response of human osteoblasts to
implant materials: integrin-mediated adhesion.J Orthop Res
1996;14:878–87.
[27] El-Ghannam A, Ducheyne P, Shapiro IM.Bioactive material
template for in vitro synthesis of bone.J Biomed Mater Res
1995;29:359–70.
[28] Cowles EA, DeRome ME, Pastizzo G, Brailey LL, Gronowicz
GA.Mineralization and the expression of matrix proteins during
in vitro bone development.Calcif Tissue Int 1998;62:74–82.
[29] Dalby MJ, Di Silvio L, Harper EJ, Bonfield W.Initial interaction
of osteoblasts with the surface of a hydroxyapatite polymethyl-
methacrylate cement.Biomaterials 2000, in press.
[30] Clover J, Dodds RA, Gowen M.Integrin subunit expression by
human osteoblasts and osteoclasts in situ and in culture.J Cell Sci
1992;103:267–71.
[31] Degasne I, Basle MF, Demais V, et al.Effects of roughness,
fibronectin, and vitronectin on attachment, spreading, and
proliferation of human-osteoblast like cells (Saos-2) on titanium
implants.Calcif Tissue Int 1999;64:499–507.
[32] Sinha R, Morris F, Suken A, Shah S, Tuan R.Surface
composition of orthopaedic implant metals regulates cell
attachment, spreading, cytoskeletal organisation of primary
human
osteoblasts
in
vitro.Clin
Orthop
Relat
Res
1994;305:258–72.
[33] Juliano RL, Haskill S.Signal transduction from the extracellular
matrix.J Cell Biol 1993;120:577–85.
[34] Alberts B, Bray D, Lewis J, Raff M, Watson J.Molecular biology
of the cell.New York: Garland Publishing Inc., 1994.
[35] Meredith JE, Fazeli B, Schwartz MA.The extracellular matrix as
a cell survial factor.Mol Biol Cell 1993;4:953–61.
[36] Frisch SM, Francis H.Disruption of endothelial cell–matrix
interactions induces apoptosis.J Cell Biol 1994;124:619–26.
[37] Schneider GB, Whitson SW, Cooper LF.Restricted and
coordinated expression of beta3-integrin and bone sialoprotein
during cultured osteoblast differentiation.Bone 1999;24:321–7.
[38] Amos LA, Amos WB.Molecules of the cytoskeleton.London:
Macmillian Education Ltd., 1991. p. 253.
[39] Burridge K, Chrzanowska-Wodnick M.Focal adhesions, con-
tractility, and signaling.Ann Rev Cell Dev Biol 1996;12:
463–519p.
[40] Stein G, Lian J.Molecular mechanisms mediating developmental
and hormone-regulated expression of genes in osteoblasts.In:
Node M, editor.Cellular and molecular biology of bone.New
York: Academic Press, 1993.p.47–91.
[41] Vrouwenvwelder W, Groot C, de Groot K.Behaviour of fetal rat
osteoblasts cultured in vitro on bioactive glass and nonreactive
glasses.Biomaterials 1992;13:382–92.
[42] Marie PJ.Human endosteal osteoblastic cells: relationship with
bone formation.Calcif Tissue Int 1995;56:S13–6.
[43] Christenson R.Biochemical markers of bone metabolism: an
overview.Clin Biochem 1997;30:573–93.
[44] Sabokbar A, Millett P, Myer B, Rushton N.A rapid, quantitative
assay for measuring alkaline phosphatase activity in osteoblastic
cells in vitro.Bone and Mineral 1994;27:57–67.
[45] Robinson R.The possible significance of hexosephosphoric esters
in ossification.J Biochem 1923;17:286–93.
[46] Ali SY.Mechanism of calcification.In: Owen R, Goodfellow J,
Bullough P, editors.Scientific foundations of orthopaedics and
traumatology.London, UK: Heineman, 1980.p.175–84.
[47] Ali SY.Matrix formation and mineralisation in bone.In:
Whitehead CC, editor.Bone biology and skeletal disorders.
Abingdon, UK: Carfax Publishing Company, 1992.p.19–38.
M.J. Dalby et al. / Biomaterials 23 (2002) 569–576
575

[48] Anderson HC.Matrix vesicles of cartilage bone.In: Bourne GH,
editor.The biochemistry and physiology of bone.New York,
USA: Academic Press, 1976.p.135–57.
[49] Akisaka T, Gay CV.Ultrastructural demonstration of p-
nitrophenyl phosphatase (p-NPPase) activity in the epiphyseal
growth plate.Acta Histochem Cytochem 1986;19–21.
[50] Berridge MJ, Bootman MD, Lipp P.Calcium
Fa life and death
signal.Nature 1998;395:645–8.
[51] Bootman MD, Berridge MJ.The elementary principles of calcium
signaling.Cell 1995;83:675–8.
[52] Scaife RM, Langdon WY.c-Cbl localizes to actin lamellae and
regulates lamellipodia formation and cell morphology.J Cell Sci
2000;113:215–26.
[53] Spaargaren M, Bos JL.Rab5 induces rac dependant lamellipodia
formation and cell migration.Mol Biol Cell 1999;10:3239–50.
[54] Sumi T, Matsumoto K, Takai Y, Nakamura T.Cofilin
phosphorylation and actin cytoskeletal dynamics regulated by
Rho and Cdc42 activated LIM-Kinase 2.J Cell Biol 1999;
147:1519–32.
M.J. Dalby et al. / Biomaterials 23 (2002) 569–576
576
Wyszukiwarka
Podobne podstrony:
68 979 990 Increasing of Lifetime of Aluminium and Magnesium Pressure Die Casting Moulds by Arc Ion
Sodium hydroxide
Increase Creativity instructions
How To Naturally Increase your Height
potassium hydroxide 18 crown 6 eros rp230
Increase Creativity cover
Increase SA briefing
Hydroxyzinum iv
hydroxylamine eros rh057
Increase blue, Fan Fiction, Dir en Gray
Increase in pre shock pause caused by drug administration before defibrillation
Hydroxyzinum tabl
Poly(3 hydroxyalkanoates)
Hydroxypropyl?llulose
Hydroxyethyl?llulose
68 979 990 Increasing of Lifetime of Aluminium and Magnesium Pressure Die Casting Moulds by Arc Ion
It was getting increasingly difficult
Increased diversity of food in the first year of life may help protect against allergies (EUFIC)
więcej podobnych podstron