©1999 Sigma-Aldrich Co.
SUPELCO
©1999 Sigma-Aldrich Co.
SUPELCO
Optimization of Extraction Conditions
and Fiber Selection for Low-Molecular
Weight Analytes and Semi-volatile
Analytes Using SPME
Robert E. Shirey and Leonard M. Sidisky
Supelco Inc., Bellefonte, PA USA

©1999 Sigma-Aldrich Co.
SUPELCO
INTRODUCTION
Developing an application using SPME can be an intimidating task
when looking at the many extraction variables and fiber coating
options. This seminar will provide a logical approach to selecting
the appropriate fiber coating and how to optimize the extraction
conditions.
To do this, two studies were investigated. One study looked at the
extraction of low-molecular weight analytes with varying
functionalities. Six different fibers were evaluated for the extraction
of these analytes. The extraction conditions such as pH of the
solution and the effects of salt were studied.
The second study looked at semi-volatile analytes using similar
parameters as in the first study. For this study nine fibers were
evaluated.
Bare fused silica
Adsorbent
Unknown
7µm Polydimethylsiloxane (PDMS)
Absorbent
Nonpolar
30µm PDMS
Absorbent
Nonpolar
100µm PDMS
Absorbent
Nonpolar
85µm Polyacrylate (PA)
Absorbent
Polar
65µm PDMS-DVB, StableFlex™
Adsorbent
Bipolar
65µm CW-DVB, StableFlex
Adsorbent
Polar
55µm/30µm DVB/Carboxen™-PDMS, StableFlex
Adsorbent
Bipolar
85µm Carboxen-PDMS, StableFlex
Adsorbent
Bipolar
Types of SPME Fibers

©1999 Sigma-Aldrich Co.
SUPELCO
The fibers can be classified by polarity or extraction type mechanism.
The polar fibers are the polyacrylate coated fibers and the Carbowax-
Divinylbenzene (CW-DVB) coated fibers. The other remaining fibers
are nonpolar or bi-polar. The nonpolar fibers have a polydimethyl-
siloxane (PDMS) coating, and the bi-polar fibers are primarily
nonpolar, but will extract some polar analytes efficiently.
The other means for classifying fibers are by extraction mechanism.
Absorbent fibers extract by partitioning into a liquid type coating. The
analytes are retained by the thickness of the coating. Adsorbent type
fibers contain porous particles suspended in a liquid phase. The
particles retain analytes in the pores or on the surface. DVB contains
primarily mesopores that extract larger analytes while Carboxen
contains more micropores which are ideal for extracting smaller
analytes. To expand the analyte range that could be extracted with one
fiber, on fiber has DVB-PDMS coated over a layer of Carboxen-
PDMS. The fibers are listed by retention strength increasing from top
to bottom. All of the adsorbent type fibers are on a StableFlex core to
reduce breakage and increase bonding strength.
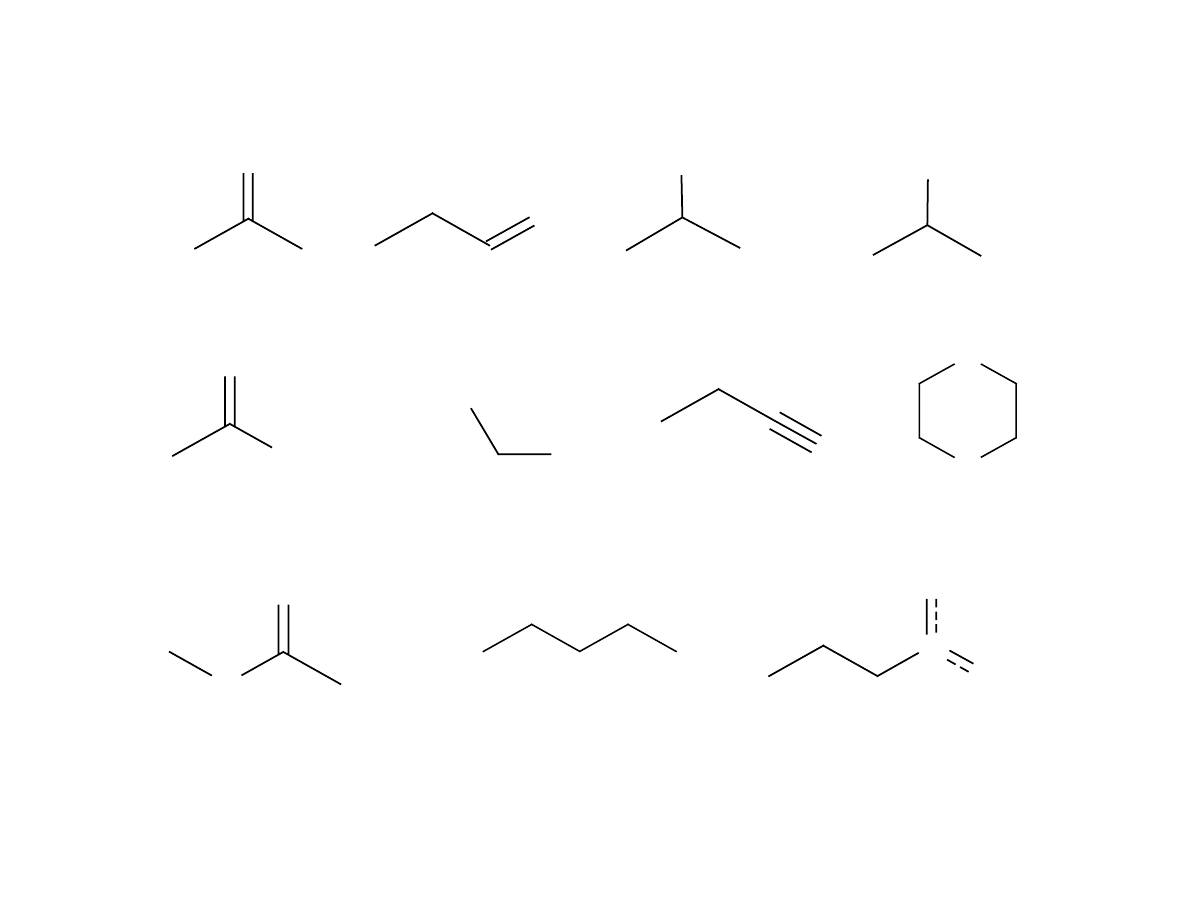
Fig 1 - Analytes in Volatile Study
Isopropanol
OH
Acetone
O
Methylacetate
Propanal
O
Dichloromethane
Cl
Cl
Acetic acid
O
OH
1,4-Dioxane
O
O
Isopropylamine
Propionitrile
Nitropropane
N
O
O
58
60
74
58
NH
2
59
84
55
60
89
88
N
Pentane
72
O
O
00-0006

©1999 Sigma-Aldrich Co.
SUPELCO
Figure 1 contains the analytes in the mixture. Most of the
analytes are similar in structure but vary by functionality. All of
the analytes have a molecular weight between 58 and 89 AMU.
There are 11 organic classes of analytes represented by this
mixture of analytes. The primary purpose of this study was to
determine the relationship between analyte polarity and fiber
coating.
Analytical Conditions for Evaluation
of
Fibers with Volatile Analytes
Sample: Water containing 25% NaCl and appropriate 0.05M
phosphate buffer, spiked with analytes to a final
concentration of 2 ppm. No NaCl in DI water samples.
Extraction: 15 min with agitation, using Varian 8200autosampler,
Heated headspace done manually at 50°C
Desorption: 2 min, temperature varies, depending on fiber
Column: 30m x 0.32mm x 4.0µm SPB™-1 SULFUR
Oven: 40°C (2 min) to 140°C at 8°C/min (1 min)
Inlet: Split/splitless, closed 0.5min, 0.75mm ID liner
Detector: FID
00-0008
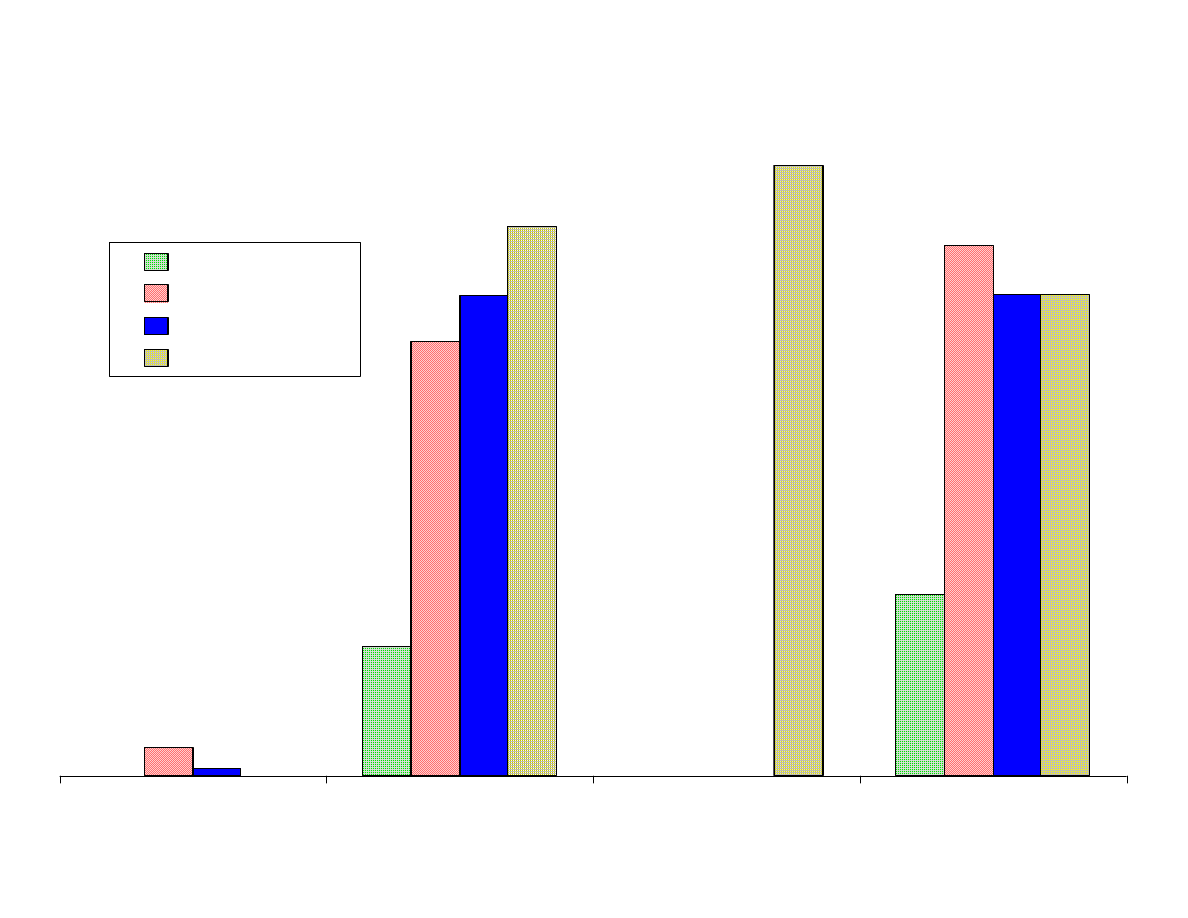
Fig 2 - Effects of pH and Ionic Strength
0
0
0
0
0
Acetic acid
Isopropanol
Isopropylamine
1,4 Dioxane
DI Water
pH=2
pH= 7
pH =11
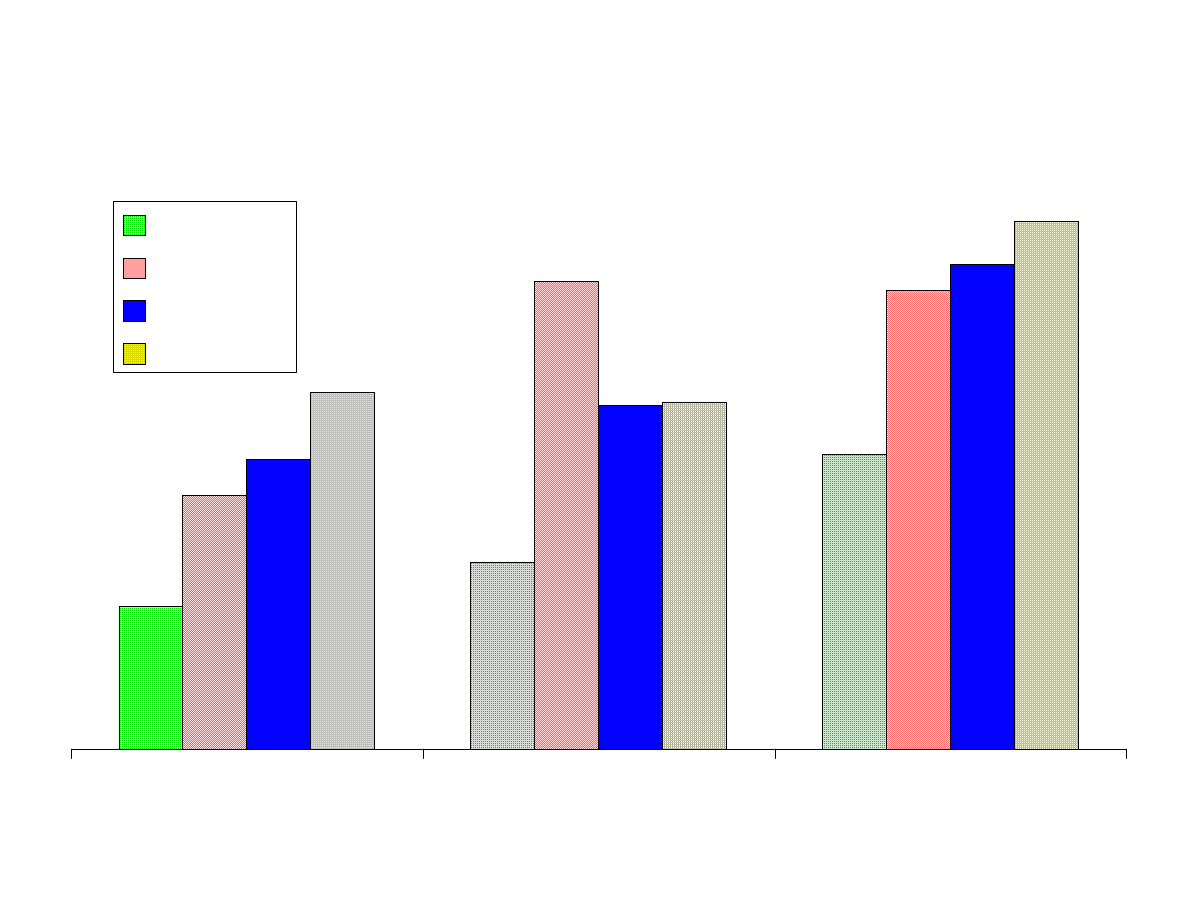
Fig 2 - Effects of pH and Ionic Strength
Cont..
Fig
Acetone
Propanal
Propionitrile
DI Water
pH=2
pH= 7
pH =11
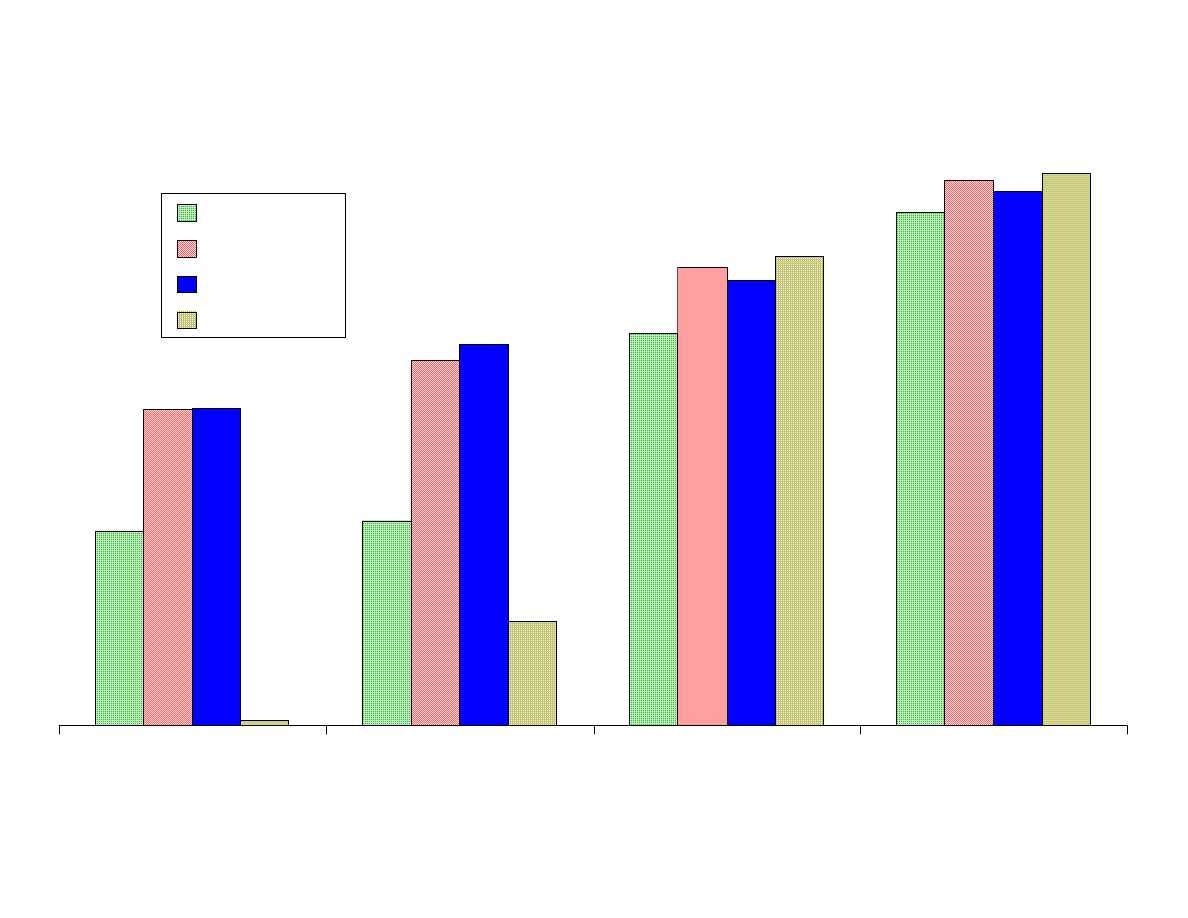
Fig 2 - Effects of pH and Ionic Strength
Cot.
Nitropropane
Methylacetate
Methylene
Chloride
Pentane
DI Water
pH=2
pH= 7
pH =11
©1999 Sigma-Aldrich Co.
SUPELCO
Figure 2 shows the comparison of the area counts from the analytes
extracted at different pH levels. To obtain the values, the area
counts from all of the fibers were averaged at each pH level.
As expected acidic analytes (acetic acid) were extracted most
efficiently from an acidic solution, pH 2, whereas bases
(isopropylamine, propionitrile) were best extracted from basic
solutions, pH 11. It was unexpected that acetone and isopropanol o
would be most efficiently extracted at pH 11. Nitropropane and
methylacetate were hydrolyzed in basic solutions which accounted
for the poor recovery at pH 11. The other analytes were not highly
affected by pH. The addition of NaCl improved the recovery of all
of the analytes especially the polar analytes.
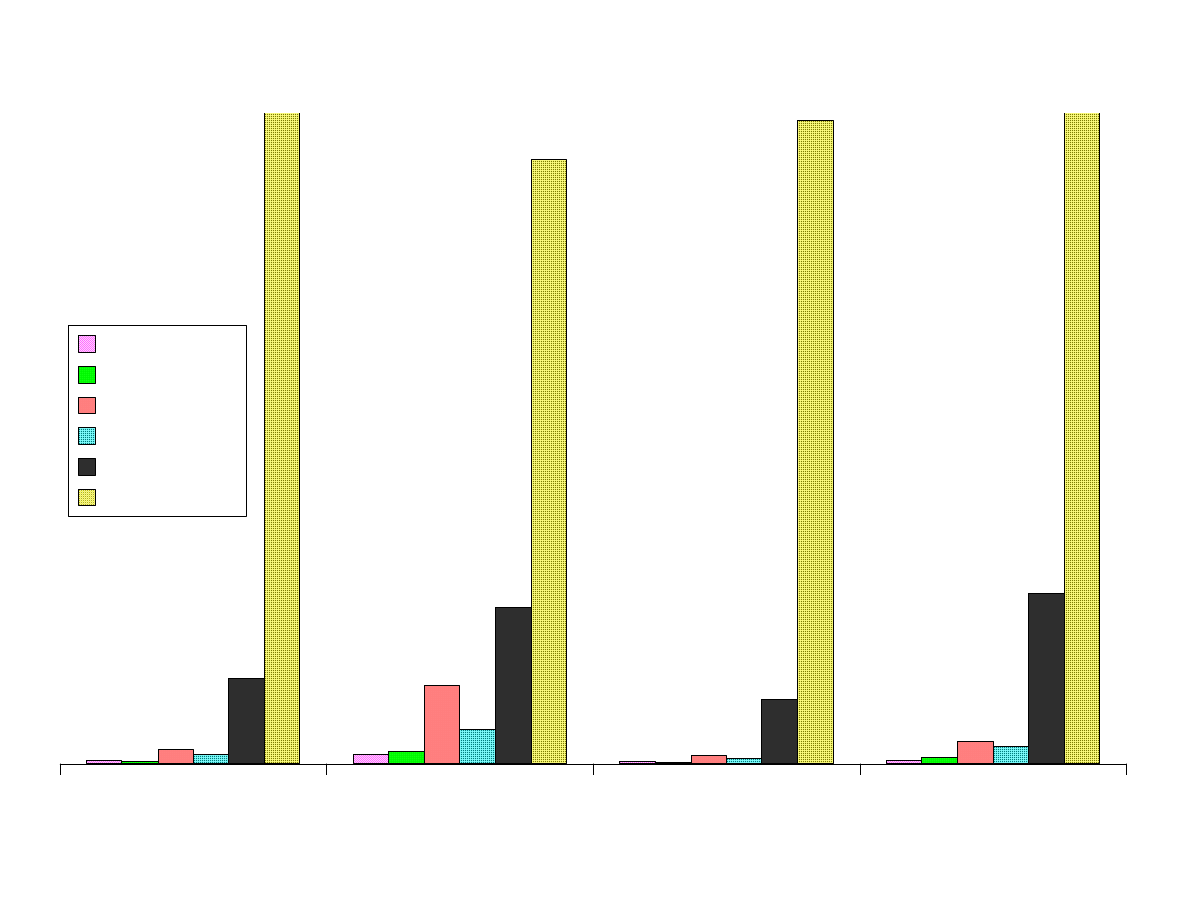
Fig. 3 - Comparison of Area Responses by Fiber Type
316
857
20
8
311
19
2
1062
121
52
8
1306
7229
758
211
9
837
32
09
499
1671
782
9
14420
5
930
1572
0
59563
55
870
6932
9
108735
Propanal
Nitropropane
Acetone
Propionitrile
PDMS
PAcrylate
PDMS-DVB
CW-DVB
DVB-CAR
Carboxen
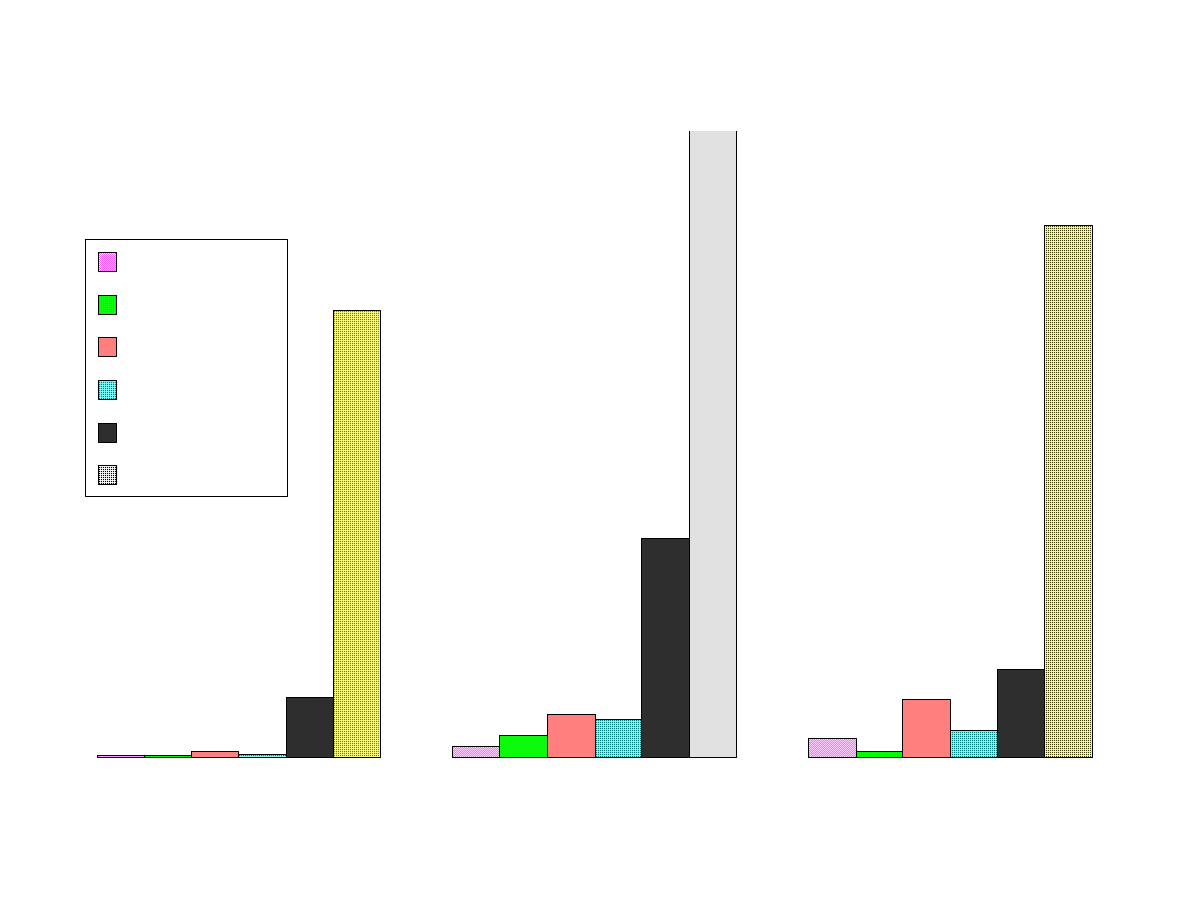
Fig 3 - Comparison of Area Responses by Fiber Type
Cont.
95
6
5
445
912
4
75
6
10
60
1
265
1
304
1
2
764
7
13
83
1
831
5
1
285
2
2
883
8
105
10
4
423
14
2
147
84
20
846
327
647
255
600
Methylacetate
Methylene Chloride
Pentane
PDMS
PAcrylate
PDMS-DVB
CW-DVB
DVB-CAR
Carboxen
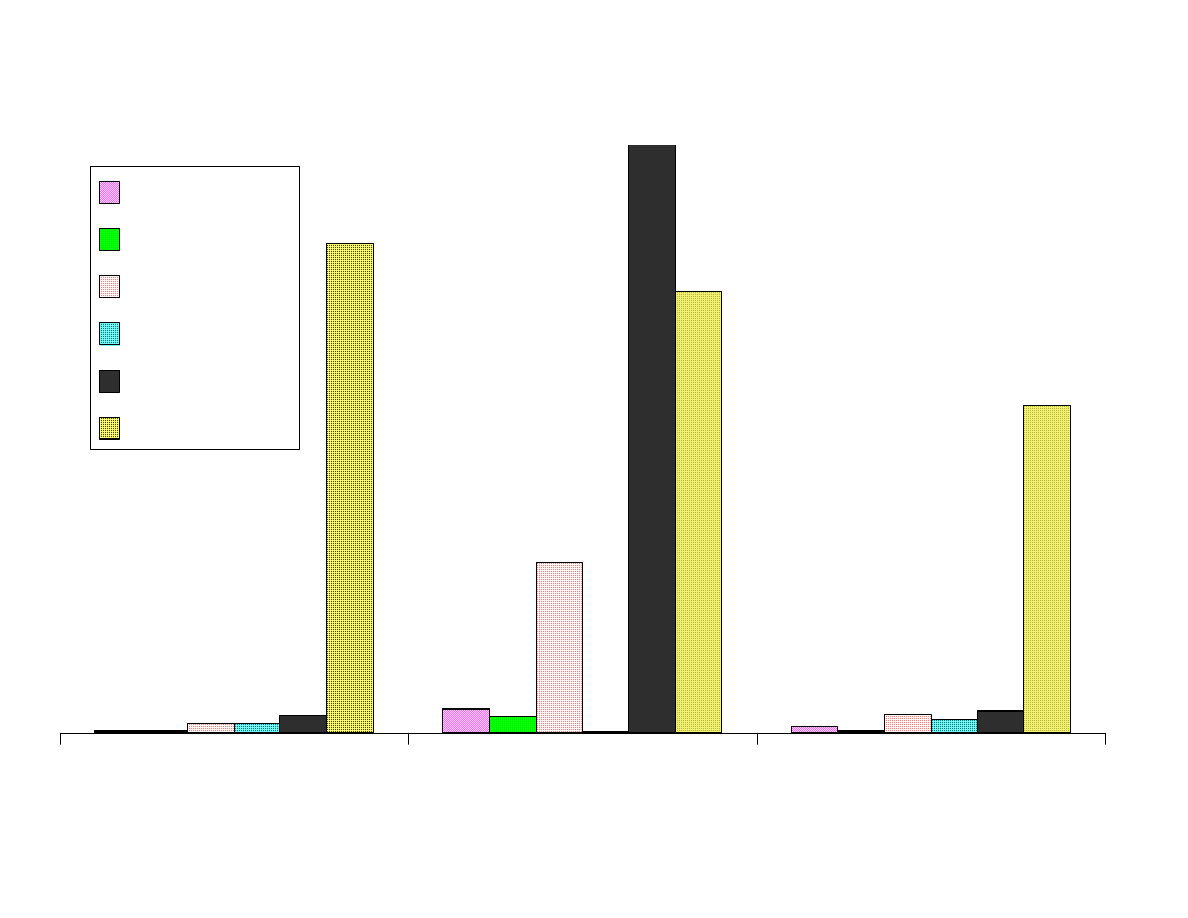
Fig. 3 - Comparison of Area Responses by Fiber Type
Cont.
144
1445
349
155
981
101
554
1105
565
50
821
1078
1317
19548
10172
35196
26365
29276
Isopropanol
Isopropylamine
1,4 Dioxane
PDMS
PAcrylate
PDMS-DVB
CW-DVB
DVB-CAR
Carboxen

©1999 Sigma-Aldrich Co.
SUPELCO
Figure 3 shows the comparison of the area responses for the analytes
extracted by the various fibers. All of the area counts recorded were
obtained from extraction at the optimum pH level for each analyte.
The Carboxen-PDMS fiber is superior to the other fibers for
extracting these low-molecular weight analytes. This fiber extracted
more than 200 times as much of the polar analytes than the 100µm
PDMS fiber. For the nonpolar analytes the advantage was not as
great, but it was still significantly better than the other SPME fibers.
Isopropylamine was the only analyte that was not most efficiently
extracted by the Carboxen-PDMS fiber. The dual layered PDMS-
DVB over Carboxen-PDMS was best for this analyte and second best
for the other analytes. The PDMS-DVB coating has a high affinity for
amines. The combination of the high affinity of PDMS-DVB for
amines coupled with the microporosity of Carboxen, makes the DVB-
Carboxen fiber the best choice for small amines.
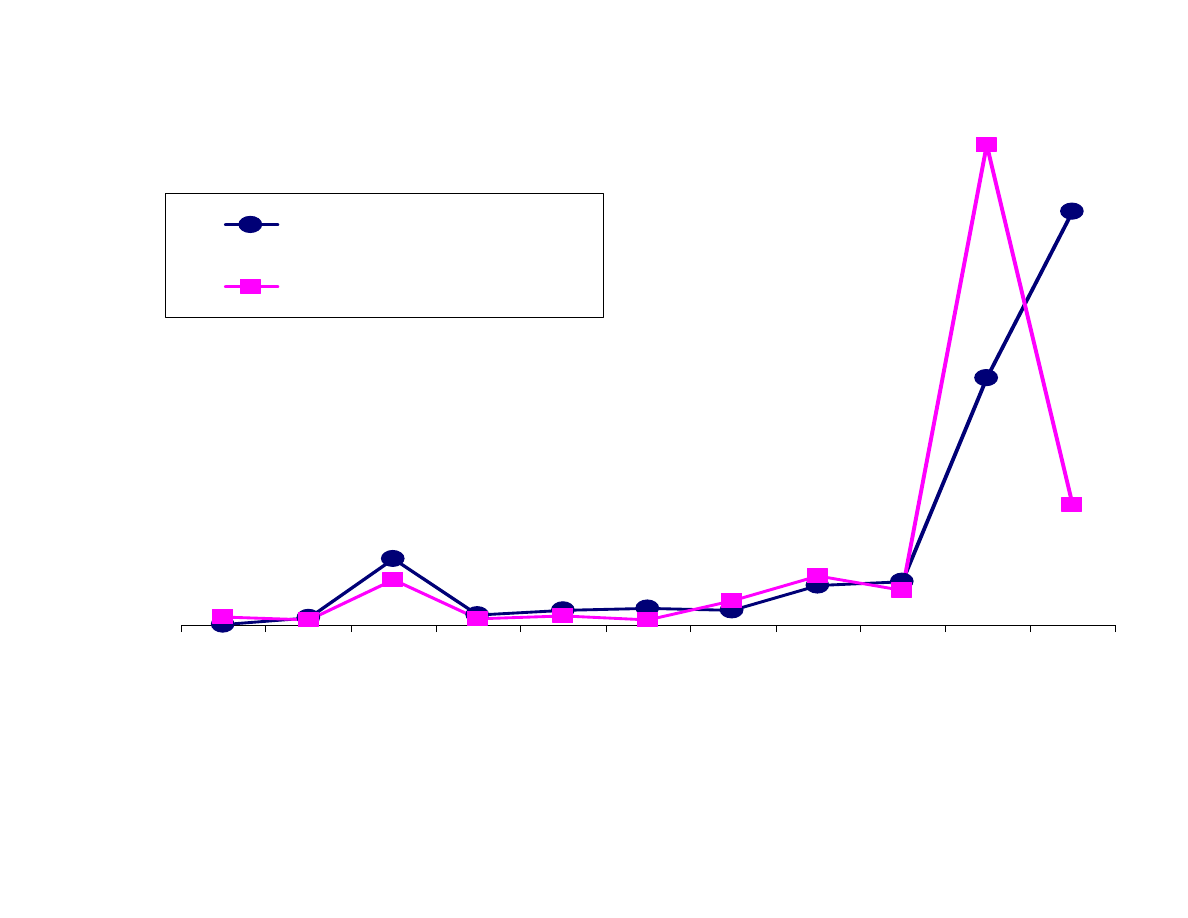
Fig 4A - Analyte Polarity vs. Area Response
A
ce
tic
a
ci
d
Is
o
p
ro
p
an
o
l
Is
o
p
ro
p
yl
am
in
e
A
cet
o
n
e
P
rop
an
al
1,
4 D
io
xan
e
P
ro
p
io
n
it
ri
le
N
it
ro
p
ro
p
an
e
M
et
h
yl
acet
at
e
M
eth
yl
en
e C
h
lo
ri
d
e
P
en
ta
n
e
100µm PDMS
Polyacrylate
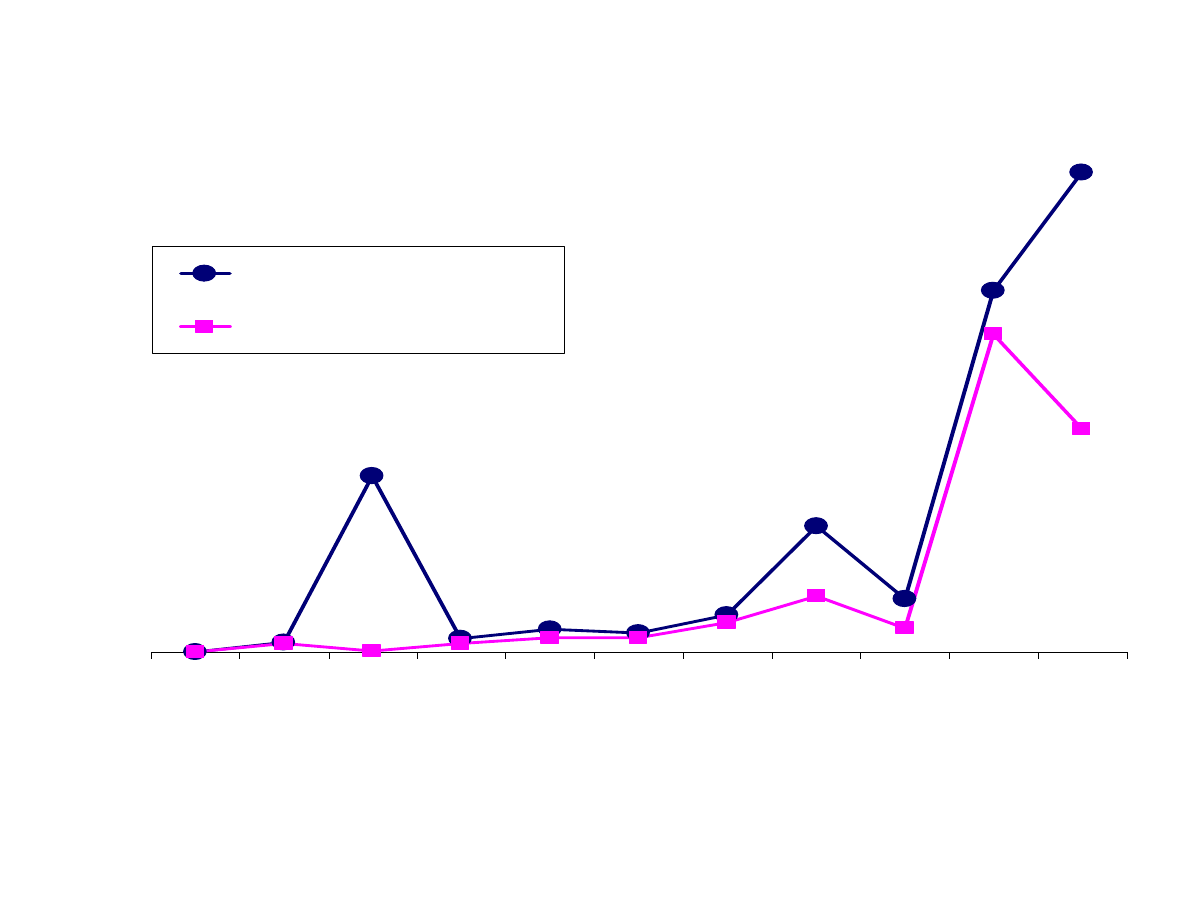
Fig 4B - Fiber Polarity vs. Area Response
A
c
et
ic
a
ci
d
Is
o
p
ro
p
a
no
l
Is
o
p
ro
p
yl
a
m
in
e
A
ce
to
n
e
P
ro
p
a
n
a
l
1
,4
D
io
x
an
e
P
ro
p
io
n
it
ri
le
N
it
ro
p
ro
p
an
e
M
et
h
yl
ac
et
at
e
M
et
h
y
le
n
e
C
h
lo
ri
d
e
P
en
ta
n
e
PDMS-DVB
Carbowax-DVB

©1999 Sigma-Aldrich Co.
SUPELCO
Figure 4 shows analyte polarity decreasing from left to right with
respect to fiber polarity. Figure 4A contains a polar (polyacrylate)
and nonpolar (100µm PDMS) absorbent type fibers, whereas,
Figure 4B contains the adsorbent type fibers, the polar CW-DVB
and the less polar PDMS-DVB. For both types of fibers, the more
polar fibers did not extract the polar analytes better than the nonpolar
analytes. Because of the small size of these analytes, fiber polarity
had little or no influence on the extraction of the polar analytes. In
both cases, the less polar fibers were better for the extraction of the
more polar analytes. The only relationship that was seen between
fiber and analyte polarity was that the polar fibers extracted less of
the nonpolar analytes. This would provide some selectivity for the
polar fibers.
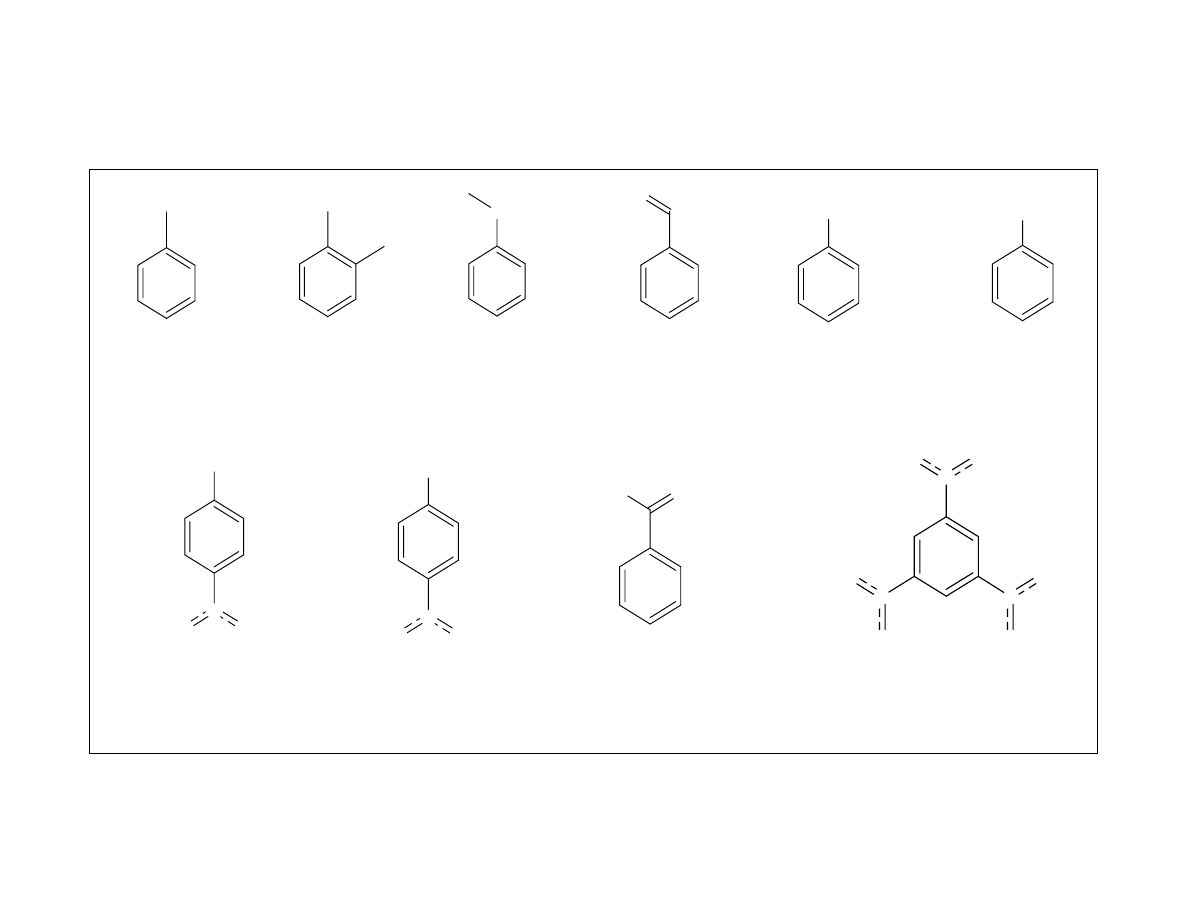
Toluene
92
o-Xylene
106
Anisole
O
108
Benzaldehyde
O
106
Phenol
OH
94
Aniline
NH
2
93
Benzoic acid
O
HO
p-Nitrophenol
OH
N
O
O
139
p-Nitroaniline
NH
2
N
O
O
1,3,5-Trinitrobenzene
N
O
O
N
O
O
N
O
O
138
122
213
Fig.5
- Semi-volatile Analytes Used in Study
G00129
8
00-0014
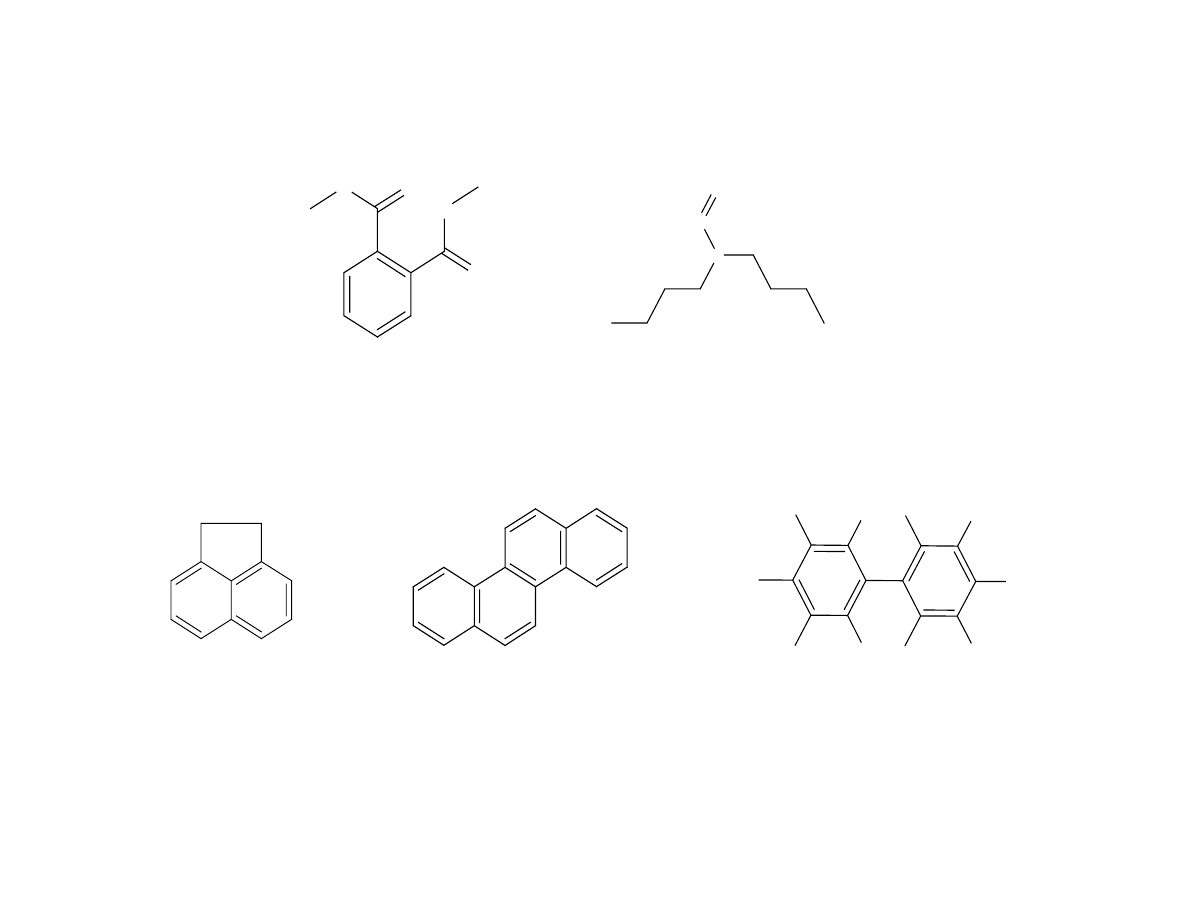
Fig. 5 - Semi-volatile Analytes Used in Study
Cont.
G00129
9
00-0015
Acenaphthene
N,N-Nitrosodibutylamine
N
N
O
158
194
Chrysene
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
Cl
154
228
Decachlorobiphenyl
499
Dimethyl phthalate
O
O
O
O

©1999 Sigma-Aldrich Co.
SUPELCO
Figure 5 shows the analytes chosen for the semi-volatile study.
Most of the analytes selected contained aromatic rings with
varying functionalities. Some had one functional group while
others had two or three functional groups to vary the polarity of
the analytes. The effects of molecular size of the analytes was a
desired input for this study. To vary the size, two PAHs and
decaclorobiphenyl were added to the mixture.
Analytical Conditions for Evaluation of
Fibers with Semi-volatile Analytes
Sample: Water containing 25% NaCl and appropriate 0.05M
phosphate buffer, spiked with analytes to a final
concentration of 75 ppb
Extraction: Directly immersed for 30 min with agitation
Heated headspace, 65°C for 30 min with agitation
Desorption: 3 min, temperature varies, depending on fiber
Column: 30m x 0.25mm x 0.25µm PTE™-5
Oven: 45°C (2 min) to 210°C at 10°C/min, then to 320°C at
20°C/min (10 min)
Inlet: Split/splitless, closed 1 min, 0.75mm ID liner
Detector: MS ion trap, m/z = 50-515 at 0.6 sec/scan
00-0017
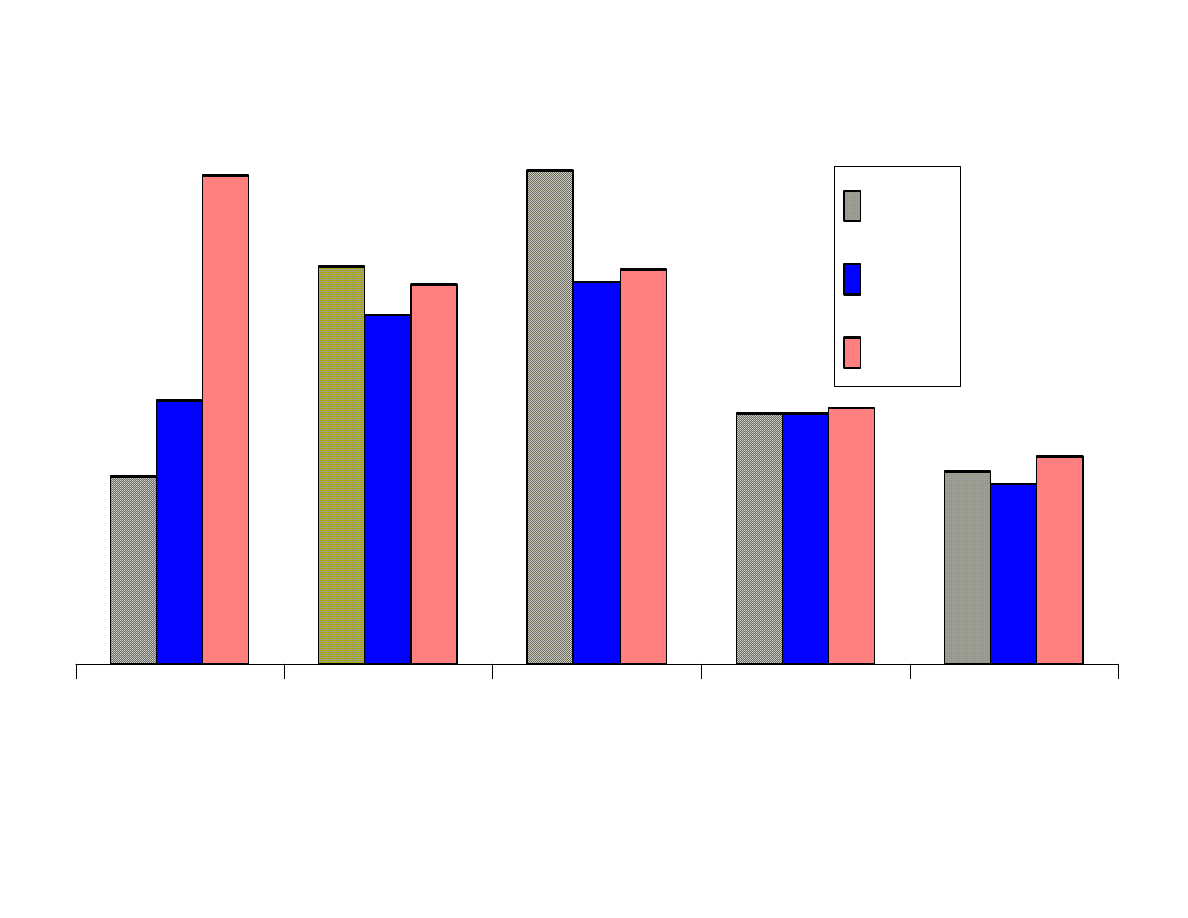
Fig. 6 - Effects of pH
Dibutyl
nitrosoamine
Acenaphthene Decachloro
biphenyl
o-Xylene
Toluene
pH=2
pH=7
pH=11
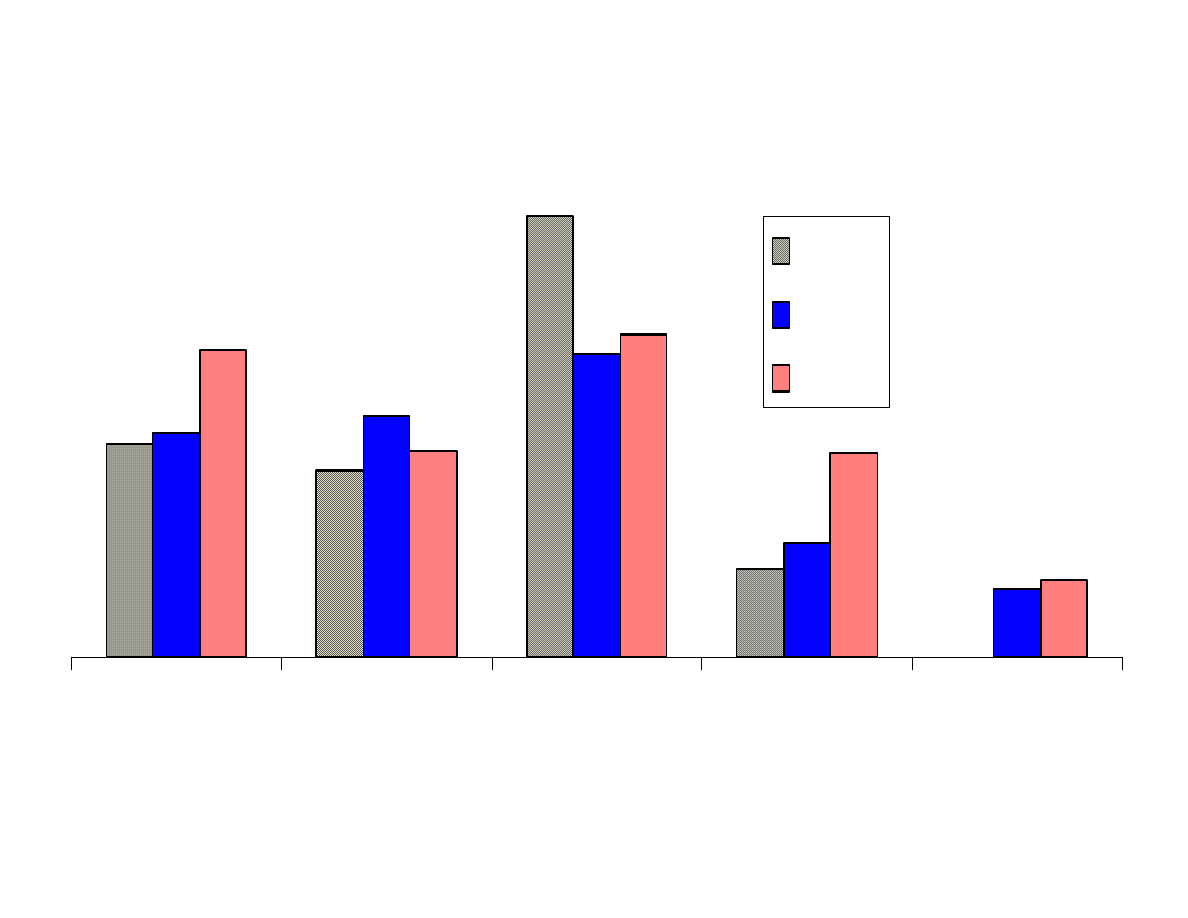
Fig. 6 - Effects of pH (cont.)
Anisole
Benzaldehdye
Chrysene
Dimethyl
phthalate
Aniline
pH=2
pH=7
pH=11
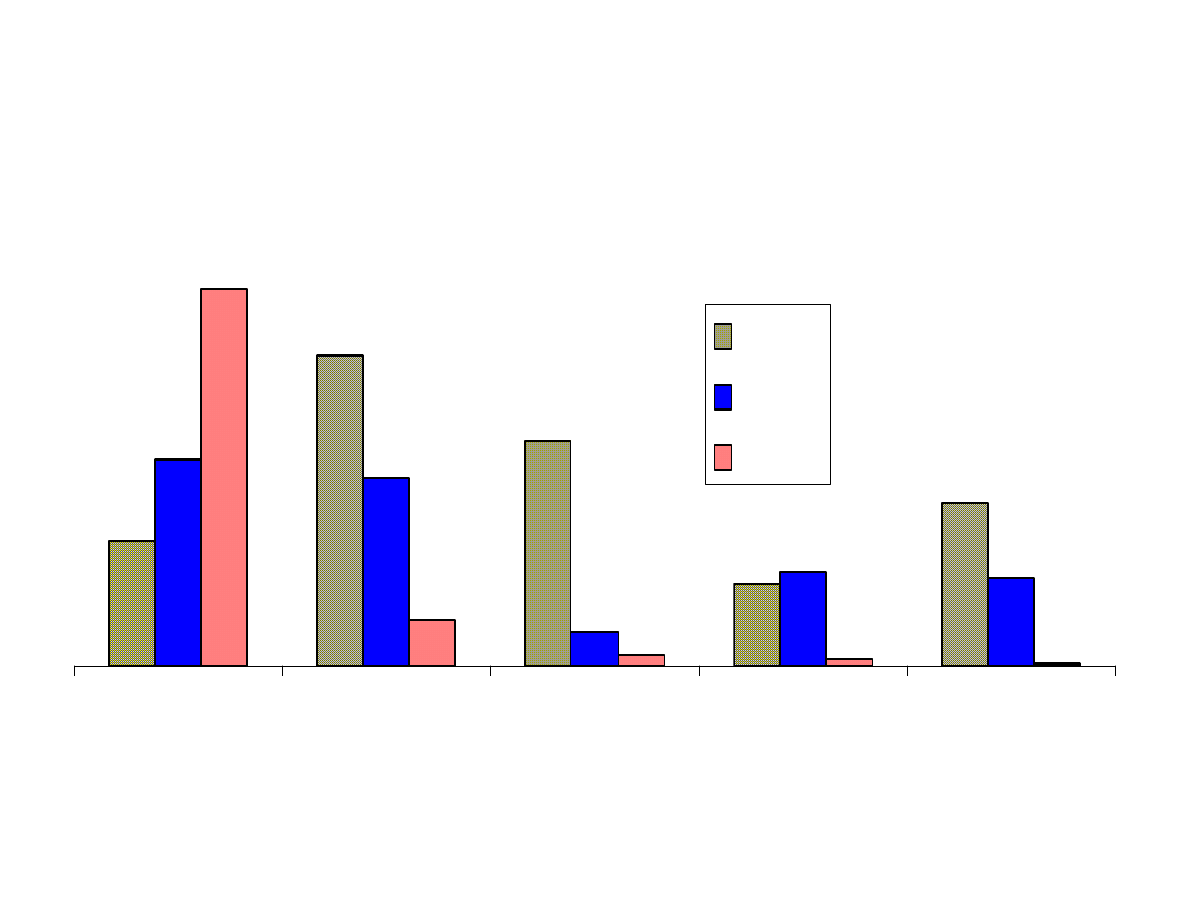
Fig. 6 - Effects of pH (cont.)
p-Nitroaniline
1,3,5-
Trinitrobenzene
Benzoic acid
Phenol
p-Nitrophenol
pH=2
pH=7
pH=11

©1999 Sigma-Aldrich Co.
SUPELCO
Figure 6 shows that the results from extraction of the analytes at 3
pH levels were for the most part as expected. The more basic
analytes such as aniline, p-nitroaniline and nitrosodibutylamine
were best extracted at pH 11. The acidic analytes, p-nitrophenol
and benzoic acid were best extracted at pH 2. Moderately acidic
phenol, was better extracted at a neutral pH than at pH 2. This has
been shown in previous studies. Trinitrobenzene was best extracted
at pH 2. This analyte is not stable in highly basic solutions.
Dimethylphthalate extracted most efficiently from basic solutions.
This result was not expected. It appears that anisole and chrysene
are best extracted at pH 2, but these results are probably not
significant to make that conclusion. The remaining neutral analytes
were not affected by pH.
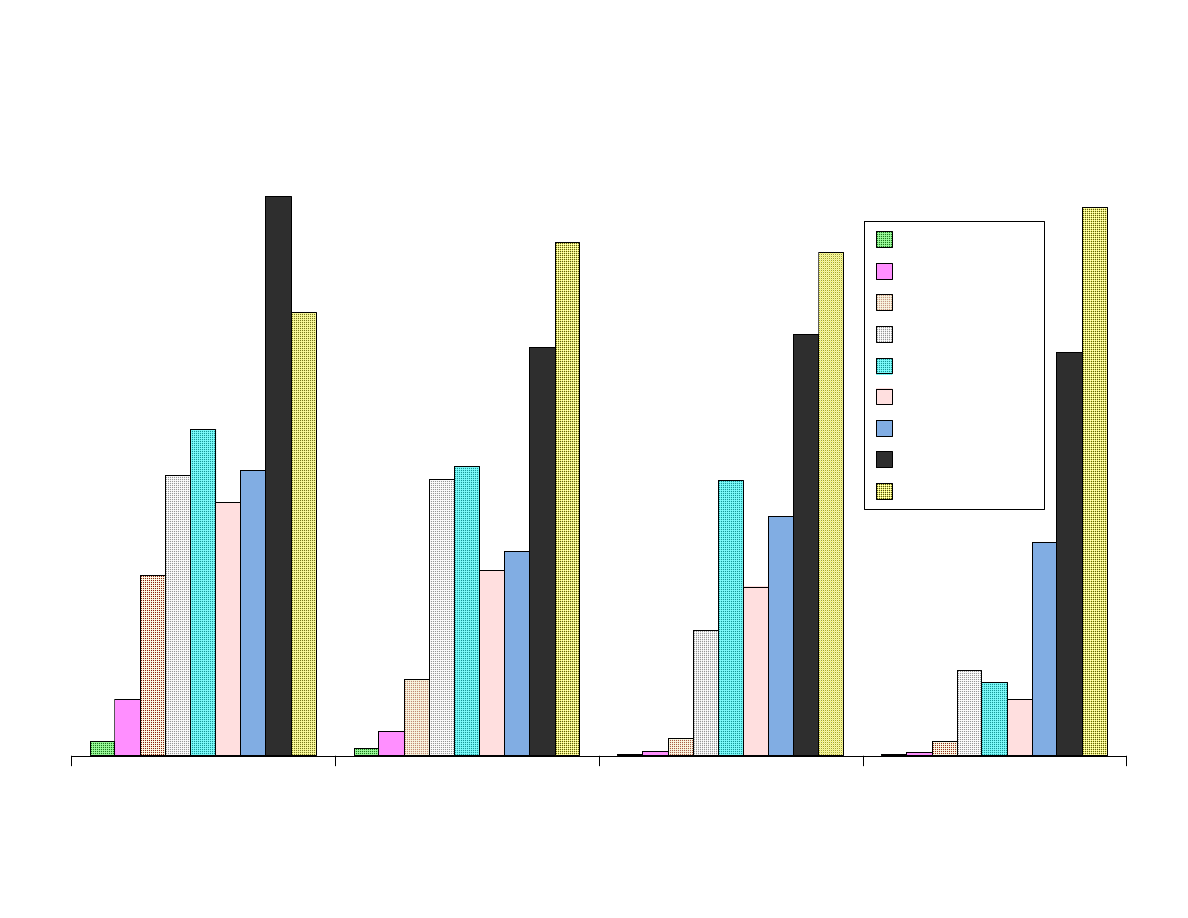
Fig 7A - Comparison of Area Responses by Fiber Type
1.
3
7
E
+
05
7
.83
E
+
04
1.
8
2
E
+
04
1.
6
6
E
+
04
5.08E+06
5.43E+06
5.54E+06
4.98E+06
o-Xylene
Toluene
Anisole
Benzaldehdye
Bare FS
7µm
30µm
100µm
Pacrylate
PDMS-DVB
CW-DVB
DVB-CAR
Carboxen
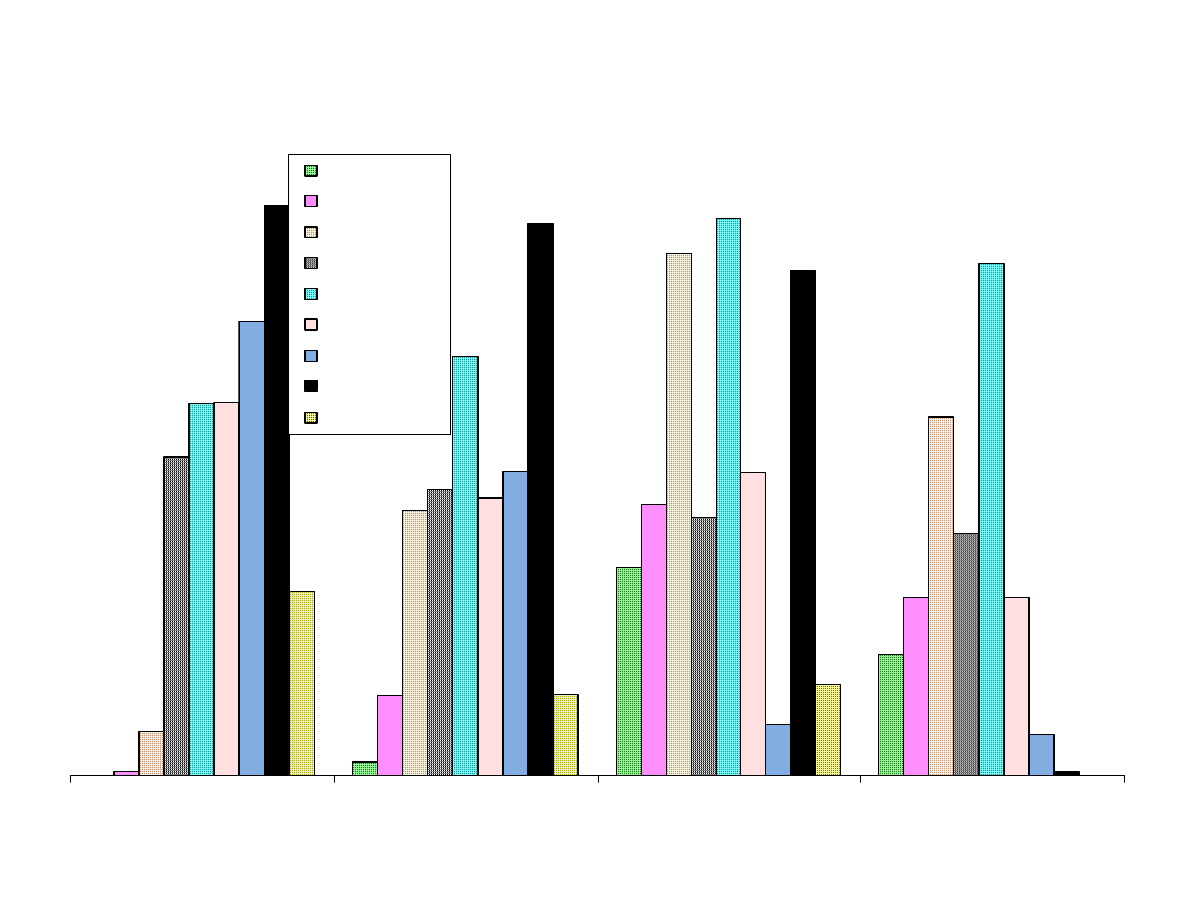
Fig 7B - Area Response vs.
Fiber Type
2.
29E
+
0
5
4.
50E
+
0
3
9.94E+06
9.14E+06
9.
04
E
+
0
5
1.02E+07
9.85E+06
4.
88E
+
0
3
Dibutylnitrosoamine
Acenaphthene
Decachlorobiphenyl
Chrysene
Bare FS
7µm
30µm
100µm
Pacrylate
PDMS-DVB
CW-DVB
DVB-CAR
Carboxen
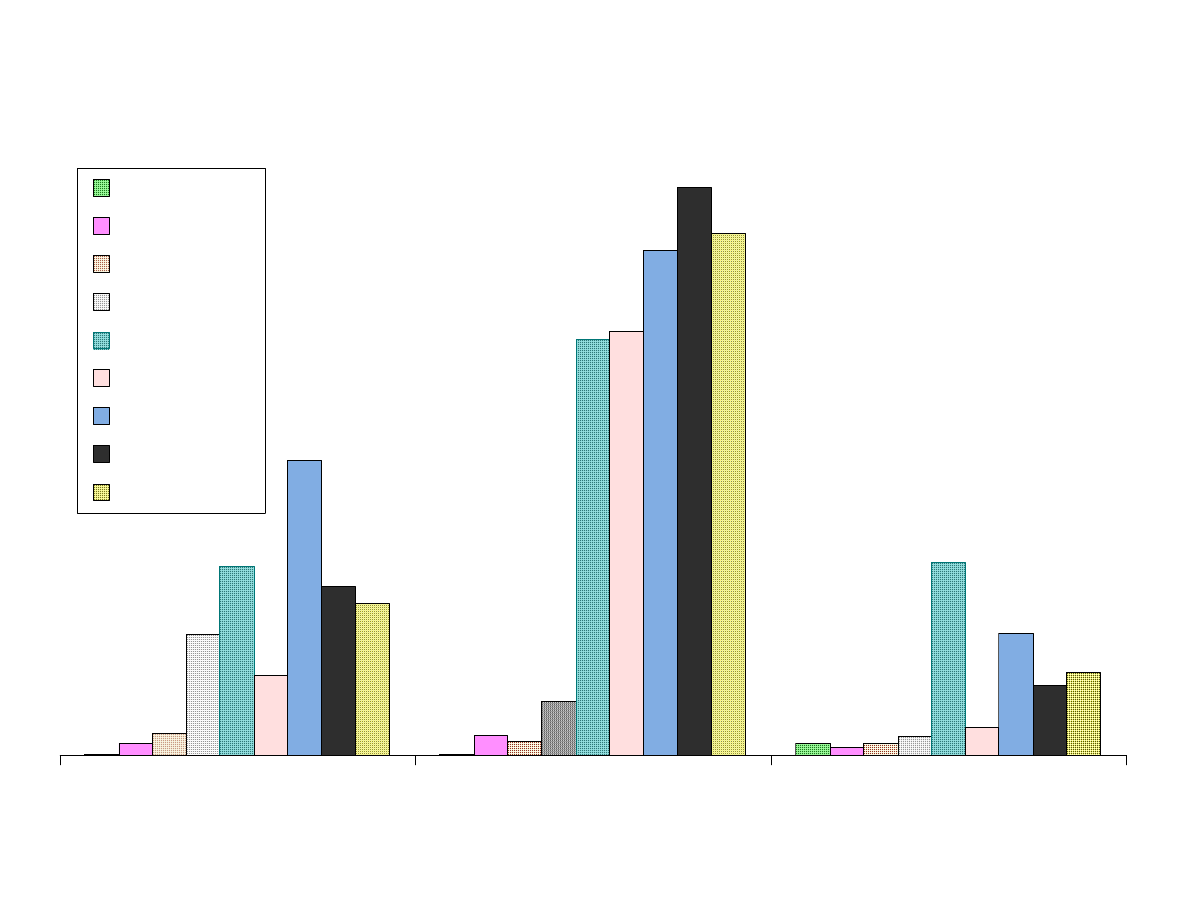
Fig. 7C - Area Response vs. Fiber Type
5.
26
E+
03
4.
24
E+
03
5.
51
E+
04
8.
95
E
+
04
8.87E+05
1.35E+06
2.61E+06
Aniline
Dimethylphthalate
Benzoic acid
Bare FS
7µm
30µm
100µm
Pacrylate
PDMS-DVB
CW-DVB
DVB-CAR
Carboxen
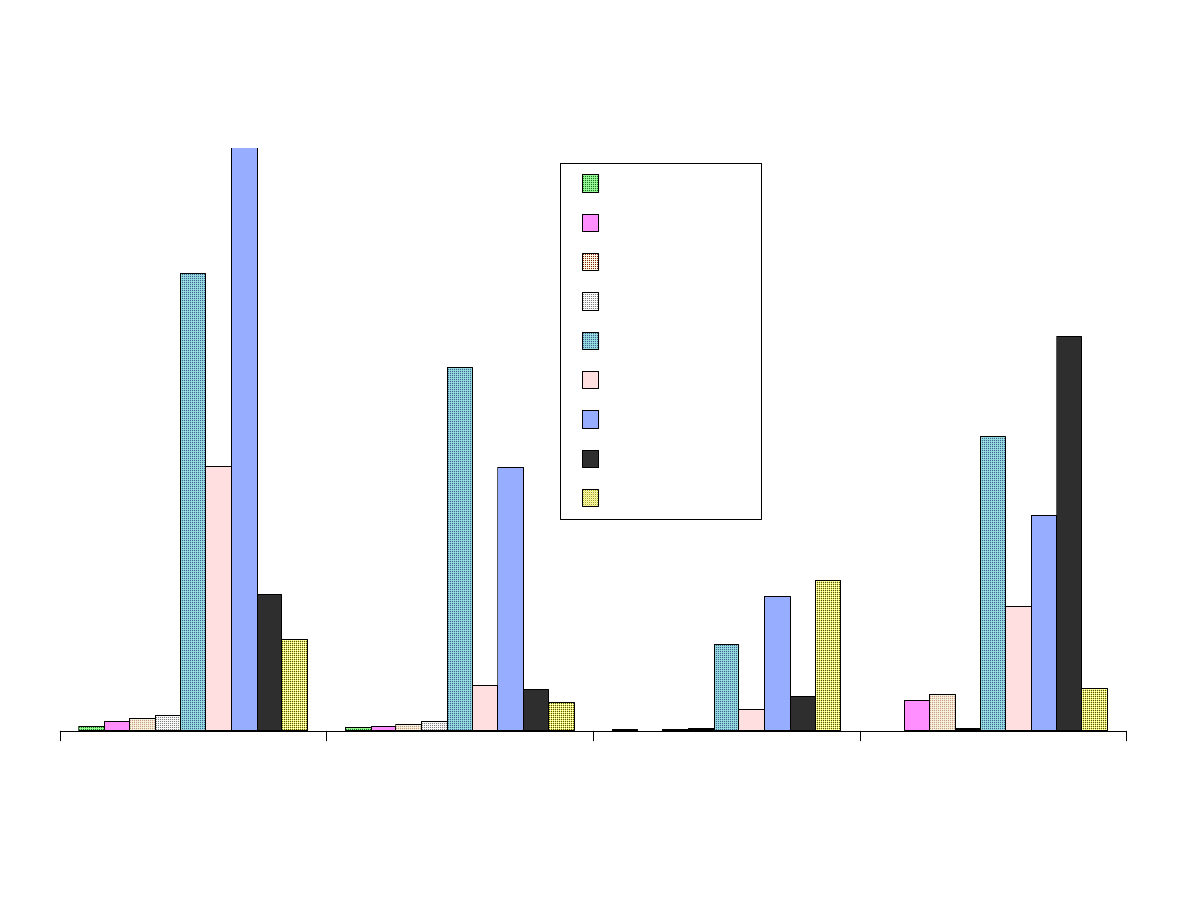
Fig. 7D - Area Response vs. Fiber Type
3.
81
E
+
0
3
2.
99
E
+
0
3
0
4.
7
3
E
+
03
7
.04
E
+
03
6.27E+05
1.34E+06
6.80E+05
2.58E+05
p-Nitroaniline
p-Nitrophenol
Phenol
1,3,5-
Trinitrobenzene
Bare FS
7µm
30µm
100µm
Pacrylate
PDMS-DVB
CW-DVB
DVB-CAR
Carboxen
00-0021

©1999 Sigma-Aldrich Co.
SUPELCO
Figure 7 shows the results from the extraction of the analytes with the
nine SPME fibers.
The smaller, nonpolar analytes shown in Figure 7A are extracted best
with the Carboxen containing fibers. The porosity of Carboxen
enables it to retain these smaller analytes.
Figure 7B contains the larger nonpolar analytes and
nitrosodibutylamine. Chrysene and decachlorbiphenyl are poorly
extracted by Carboxen. These larger analytes are efficiently extracted
by PDMS fibers and polyacrylate. Bare fused silica can also extract
these analytes but not reproducibly.
Figure 7C contains more polar analytes that are best extracted with the
polar fibers CW-DVB and polyacrylate. The affect of fiber polarity is
more significant with larger analytes. Dimethylphthalate is extracted
most efficiently by the adsorbent containing fibers. It was not
extracted well by PDMS fibers.
Figure 7D contains polar analytes that are best extracted with polar
fibers. The effect of fiber polarity is significant. TNB was best
extracted by DVB-Carboxen fiber.
Fig. 8A - Analyte Polarity vs. Area Response
0.E+00
1.E+06
2.E+06
3.E+06
4.E+06
5.E+06
6.E+06
7.E+06
8.E+06
9.E+06
C
hry
sen
e
Decach
lo
ro
bi
ph
en
yl
Ac
en
ap
ht
he
ne
o-
Xy
le
ne
Tol
ue
ne
A
nis
ol
e
B
en
zal
de
hd
ye
D
im
et
hy
lp
ht
hal
at
e
D
ib
uty
ln
itr
os
oam
in
e
A
nilin
e
p-
Ni
tr
oa
nilin
e
1,
3,
5-T
ri
ni
tro
be
nz
en
e
p-
N
itro
phe
no
l
Phe
nol
B
en
zoi
c aci
d
100µm PDMS
PAcrylate
©1999 Sigma-Aldrich Co.
SUPELCO
Fig. 8B - Analyte Polarity vs. Area Response
0.E+00
1.E+06
2.E+06
3.E+06
4.E+06
5.E+06
6.E+06
7.E+06
8.E+06
9.E+06
C
hr
ysen
e
D
ecach
lo
ro
bi
phe
ny
l
A
cen
ap
ht
he
ne
o-
X
yl
ene
T
ol
ue
ne
A
ni
so
le
Be
nza
ld
eh
dy
e
D
im
et
hy
lp
ht
hal
ate
n-
Di
but
yl
ni
tr
os
oa
m
ine
A
nili
ne
p-
Ni
tr
oa
ni
line
1,
3,
5-
T
ri
ni
tr
ob
en
zen
e
p-
Ni
tr
op
he
nol
Ph
en
ol
B
en
zoic
a
ci
d
PDMS-DVB
CW-DVB
00-0023

©1999 Sigma-Aldrich Co.
SUPELCO
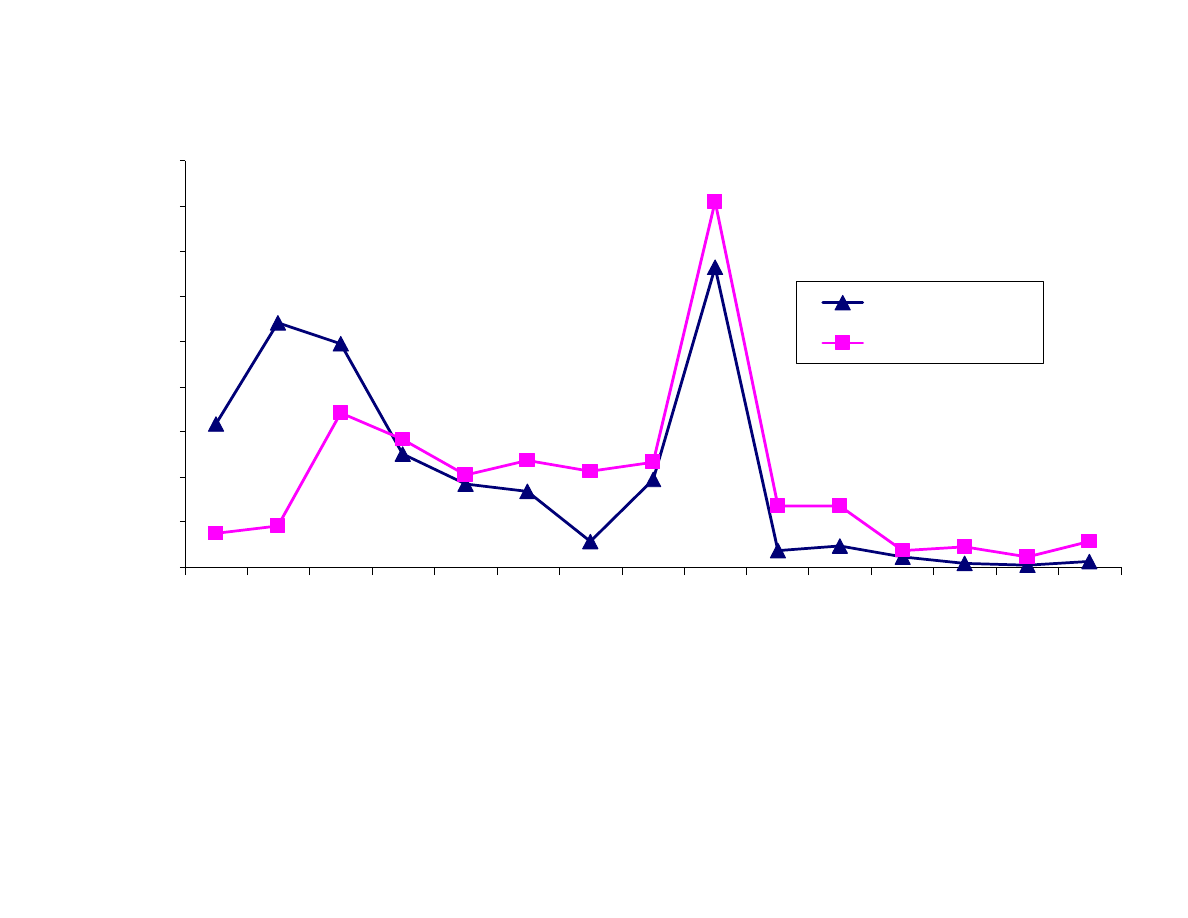
Figure 8 contains plots of analyte polarity, increasing from left to
right vs. fiber polarity.
Figure 8A shows the absorbent fibers,100µm PDMS and the 85µm
polyacrylate. The polar polyacrylate fiber not only extracts the polar
analytes better, in some cases 3 orders of magnitude better, but it
also extracts the nonpolar analytes better. However, the response is
only 1.5-2 times greater. The affinity that polyacrylate has for
aromatic compounds is the reason for the high extraction affinity for
these nonpolar analytes.
Figure 8B shows the adsorption type fibers. In this case the less
polar PDMS-DVB fiber extracted the nonpolar analytes better than
the more polar CW-DVB fiber. The CW-DVB fiber was more
efficient at extracting the more polar analytes than the PDMS-DVB
fiber. These results were as expected
.
©1999 Sigma-Aldrich Co.
SUPELCO
SUPELCO
Fig 9
-
Effects of Coating Thickness on Analyte
Recovery
00-0024
Tolu
ene
o-X
ylen
e
Ace
nap
hth
ene
Chr
yse
ne
Dec
ach
loro
biph
eny
l
Bare FS
7µm
30µm
100µm
PDMS Fibers
30 Min. Ext.

©1999 Sigma-Aldrich Co.
SUPELCO
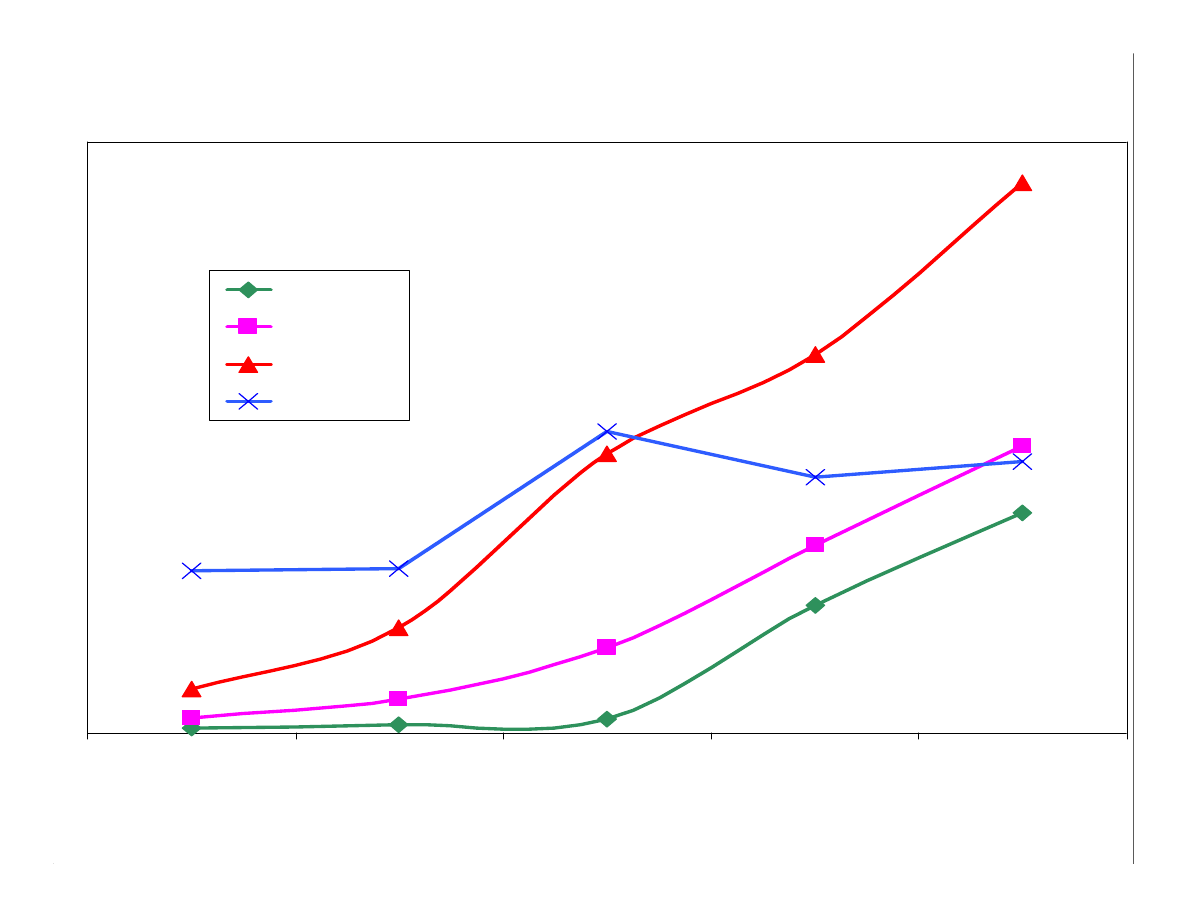
Figure 9 shows the results from a 30 min. extraction of the
analytes
with the 3 PDMS and bare fused silica fibers. The 100µm PDMS
fiber extracts the lower molecular weight analytes efficiently, but the
efficiency of the larger analytes is not as good. An extraction time of
30 min.is not a sufficient time to allow the larger analytes to migrate
into the coating. The amount of analyte extracted would increase
with a longer extraction time.
The 30µm PDMS is a suitable fiber for extracting both lower and
higher molecular weight analytes within a reasonable amount of
time. This is a good fiber choice for PAHs and PCBs.
The 7µm PDMS has less capacity and poorly extracts the lower
molecular weight analytes, but it is suitable for higher molecular
weight analytes. Bare fused silica and the 7µm produced parallel
lines indicating the the extraction mechanism is similar. Most likely
the 7µm PDMS fiber extracts by both adsorption and absorption.
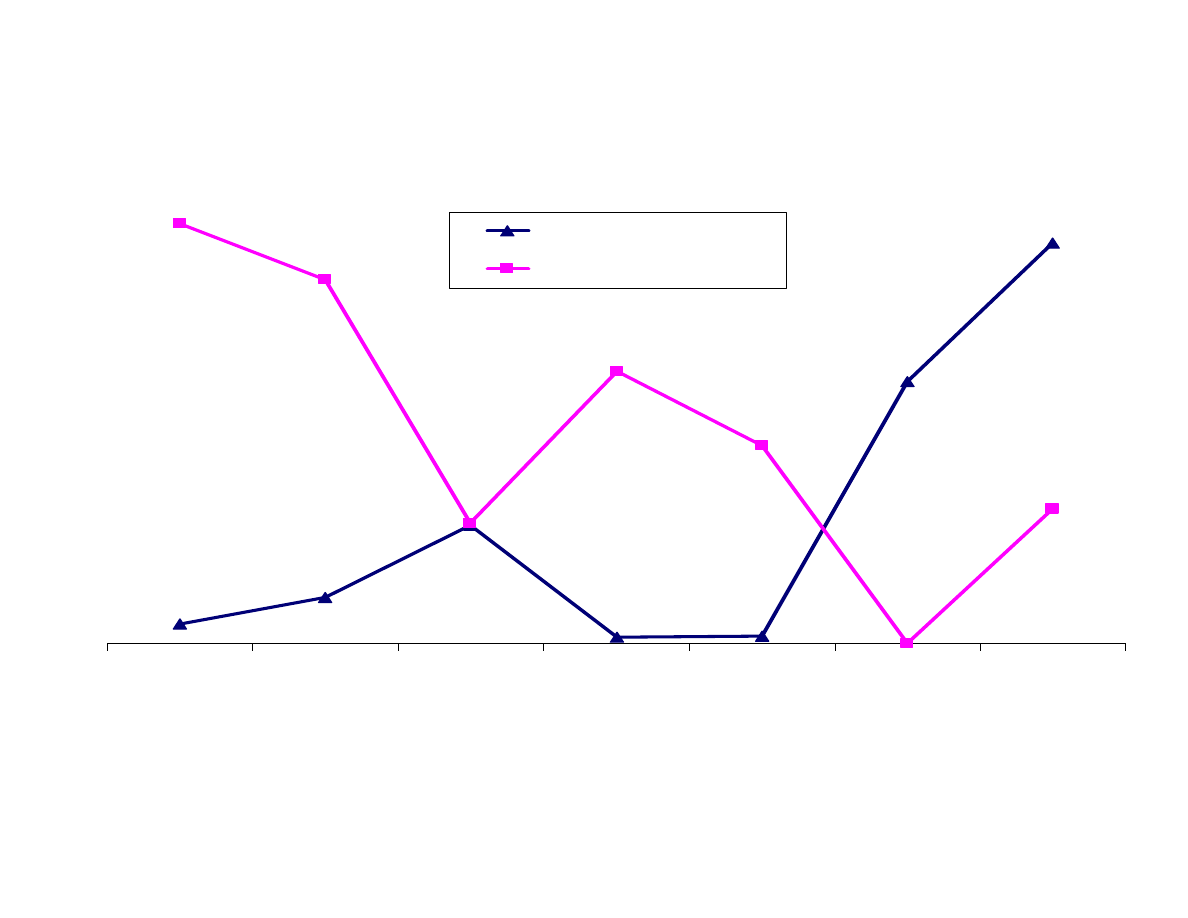
Fig. 10 - Analyte Size vs. Area Response
T
o
lu
en
e
o
-X
y
len
e
A
cen
ap
h
th
en
e
n
-D
ib
uty
ln
it
ro
soa
m
in
e
Di
m
et
h
yl
p
h
th
al
at
e
C
h
ryse
n
e
D
eca
ch
lo
ro
b
ip
h
e
n
yl
7µm PDMS
Carboxen-PDMS
©1999 Sigma-Aldrich Co.
SUPELCO
Figure 10 compares the extraction of analytes with the Carboxen-
PDMS fiber and the 7µm PDMS fiber. As expected, as the
molecular weight of the analyte increases, the response drops when
using the Carboxen-PDMS fiber. This is particularly true for PAHs.
Either the analytes are not being desorbed off the fiber or are too
large to be extracted. It is most likely the former, but it could be a
combination.
The responses for the analytes extracted with the 7µm PDMS fiber
increase as the molecular weight increases. The more polar analytes
are not efficiently extracted by the PDMS fiber.
©1999 Sigma-Aldrich Co.
SUPELCO
CONCLUSIONS
•
The pH of the extraction solution affects recovery of polar
analytes.
•
The addition of 25% NaCl improves analyte recovery
•
Analytes with a molecular weight of < 90 AMU are most
efficiently extracted by the Carboxen-PDMS fiber.
•
analyte polarity has little impact on fiber polarity for low
molecular weight analytes.
•
Analyte polarity and fiber polarity are directly related for analytes
with molecular weights over 90AMU.
•
Larger analytes >150 AMU and PAHs are poorly extracted by
Carboxen- PDMS fibers. Layering DVB over Carboxen expands
the range.
•
Thin absorbent type fibers are ideal for extracting nonpolar, high
molecular weight analytes
Wyszukiwarka
Podobne podstrony:
Multi objective thermodynamic optimization of combined Brayton and inverse Brayton cycles using gene
Influence of extraction parameters and medium on efficiency
Optimization of Intake System and Filter of an Automobile Using CFD Analysis
Functional Origins of Religious Concepts Ontological and Strategic Selection in Evolved Minds
Antioxidant and antimicrobial activity of extracts
Production and Characterisation of extracts
Maps of the Ancient World Ortelius A Selection of 30 Maps from The Osher and Smith Collections
Guide for solubilization of membrane proteins and selecting tools for detergent removal
Composition and Distribution of Extracellular Polymeric Substances in Aerobic Flocs and Granular Slu
Aerobic granules with inhibitory strains and role of extracellular polymeric substances
Design and performance optimization of GPU 3 Stirling engines
Functional Origins of Religious Concepts Ontological and Strategic Selection in Evolved Minds
development of models of affinity and selectivity for indole ligands of cannabinoid CB1 and CB2 rece
Eurocode 6 Part 2 1996 2006 Design of Masonry Structures Design Considerations, Selection of Mat
Effect of vacuum microwave drying on selected mechanical and rheological properties of carrot
1 1 William Blake Songs of Innocence and Experience (Selected poems)
Calendar of Backyard Gardening Operations for Selected Temperate Fruit and Nut Trees
fairclough semiotic condition of social reproduction and transformation
więcej podobnych podstron