
Biomaterials 19 (1998) 1689 — 1694
Influence of metals on IL-6 release in vitro
G. Schmalz,* U. Schuster, H. Schweikl
Department of Operative Dentistry and Periodontology, University of Regensburg, Franz-Josef-Strau}-Allee 11, D-93053 Regensburg, Germany
Received 5 August 1997; accepted 7 March 1998
Abstract
Certain dental alloys have been claimed to cause gingival and periodontal inflammation. However, little information is available on
the molecules mediating the mechanism of such an effect. Recently, a three-dimensional cell culture system consisting of human
fibroblasts and keratinocytes has been introduced for evaluating the irritancy of cosmetic products, including the analysis of
inflammatory mediators. In the present study the influence of pure metals and a high noble dental cast alloy upon cell viability and the
synthesis of the proinflammatory mediator interleukin-6 was recorded in this in vitro skin equivalent model. The cultures were
exposed to test specimens fabricated from copper, nickel, cobalt, zinc, palladium, tin, indium, a high noble cast alloy and a dental
ceramic. Cell vitality was reduced after a 24 h exposure to copper (14—25% of untreated controls), cobalt (60%), zinc (63%), indium
(85%), nickel (87%), and the heat treated and not heat treated high noble cast alloy (87% / 90%). Dental ceramic, palladium and tin
did not influence cell viability. Increased IL-6 levels were observed in cultures exposed to copper (5—19-fold compared to untreated
controls), zinc (16-fold), cobalt (12-fold), nickel (10-fold) and palladium (4-fold). Other materials tested produced IL-6 levels
comparable to those of untreated controls. Our findings suggest that metal ions are involved in proinflammatory activity at low
toxicity and non-toxic levels as assessed by different biological endpoints.
( 1998 Published by Elsevier Science Ltd. All rights
reserved.
Keywords: Dental cast alloy; Metal biocompatibility; Proinflammatory mediators; Interleukin-6
1. Introduction
Cast alloys used in dentistry come into close and
prolonged contact with the gingiva and the oral mucosa.
Metal ions released by these alloys; e.g. nickel, have been
claimed to cause gingival and periodontal inflammation
[1, 2]. Furthermore, nickel hypersensitivity is quite com-
mon in the general population and periodontal responses
have been associated with nickel-containing crowns in
nickel-sensitive patients [3]. Cast alloys have been sub-
jected to different biological test systems; e.g. in vivo
implantation test, mucosa contact test, or various
cytotoxicity tests to evaluate the oral mucosal irritancy of
the materials. However, none of the currently available
models is optimal and until now no valid animal or in
vitro model exists to assess the irritation potential of
dental materials [4]. Accordingly, only little information
is available on the molecules mediating the mechanism of
*Corresponding author. Tel.: ##49 941 944 6024; fax: ##49 941
944 6024.
gingival and periodontal inflammation possibly caused
by dental cast alloys.
Dysregulated cytokine and immunglobulin produc-
tion at local disease sites have been considered to be
major contributors to the development of inflammatory
diseases such as lichen planus, autoimmune disorders
and some neoplastic processes [5—7]. Among the numer-
ous cytokines which are involved in the induction and
regulation of host responses in inflammation, inter-
leukin-1 (IL-1) and interleukin-6 (IL-6) seem to play
central roles in the inflammatory reaction. These
cytokines show several overlapping effects with each
other and with tumour-necrosis factor-alpha (TNF-
a)
[8]. They are commonly produced by both macrophages
and T cells, but various other cell types including fibro-
blasts and keratinocytes can produce IL-1 and IL-6. The
cytokines have been shown to enhance various immune
responses in vitro, including B lymphocyte differenti-
ation, antibody secretion, T lymphocyte proliferation
and acute phase protein synthesis. IL-1 also causes endo-
genous pyrogen-induced fever, fibroblast proliferation,
bone resorption, and collagenase and prostaglandin
E2 production by fibroblasts and chondrocytes [8, 9].
0142-9612/98/$ — See front matter
( 1998 Published by Elsevier Science Ltd. All rights reserved.
PII S 0 1 4 2 - 9 6 1 2 ( 9 8 ) 0 0 0 2 6 - X

Interleukins also seem to play a crucial role in gingival
and periodontal inflammation. Masada et al. [10]
showed that both IL-1
a and IL-1b were produced and
released locally in periodontal disease at concentrations
sufficient to mediate tissue inflammation and bone re-
sorption. Kamagata et al. [11] found that the culture
supernatants from gingival samples biopsied from in-
flamed gingival tissues contained significantly higher
IL-1 and IL-6 activities than those from healthy ones.
Takahashi et al. [12] detected IL-6 protein mainly in
fibroblasts, endothelial cells, and macrophages of all in-
flamed gingival tissues examined, but not any in healthy
gingival tissues. Furthermore, lipopolysaccharide (LPS)
from several oral inflammatory pathogens are capable of
amplifying the local immune response and promoting
periodontal tissue inflammation and damage by stimu-
lating gingival fibroblasts and periodontal ligament cells
to secrete IL-6 [13—15].
Recently, a three-dimensional cell culture system con-
sisting of human fibroblasts and human epithelial cells
(keratinocytes) simulating cell—cell interaction between
dermis and epidermis has been introduced for evaluating
the irritancy of cosmetic products [16—21]. Further stud-
ies have demonstrated the possible suitability of this
system for toxicity testing of dental materials [4]. With
this tissue equivalent model, cell viability as well as
time-dependent release of proinflammatory mediators
following exposure to dental cast alloys can be
monitored.
Previous studies in our laboratory examined the re-
lease of prostaglandin E2, another proinflammatory
key mediator, after exposure of the cocultures to various
metals [4]. In the present study we focus on the
influence of pure metals and a high noble dental cast
alloy upon cell viability and the synthesis of the proin-
flammatory mediator IL-6 in the in vitro skin equivalent
model.
2. Materials and methods
2.1. Tissue culture
Human fibroblast—keratinocyte cocultures (Skin
2TM
model ZK1200), media and reagents were supplied by
Advanced Tissue Sciences (LaJolla, CA, USA). On arri-
val, the tissue cultures were transferred to 24-well plates
and incubated in growth medium (DMEM supple-
mented with 5% foetal calf serum) in a humidified atmo-
sphere at 37°C and 5% CO2. After 3 d growth medium
was replaced by assay medium (DMEM with 2% foetal
calf serum) for 24 h. Next, the tissues were transferred to
the surfaces of tissue culture inserts (Millicells, Advanced
Tissue Sciences) in 6-well plates containing 1 ml assay
medium per well and one test specimen was placed on
each tissue. Contamination of cell cultures was excluded
by visual control of the cultures under the light micro-
scope. After 0.5 min, 1, 2, 3, 5, 7, 10 and 24 h of exposure,
respectively, the tissue culture inserts were transferred to
new wells containing fresh assay medium. Each material
was tested in triplicate; untreated fibroblast—keratinocyte
cocultures were used as negative controls. Specimens of
copper were included in each experiment as a positive
reference material; results of cell survival and IL-6 pro-
duction, respectively, are plotted to the left of results
found from exposure to other materials tested in the
same experiment (Figs. 1 and 3).
2.2. Sample preparation
The specifications of the test materials are given in
Table 1. The surfaces of the test specimens (10 mm
]
10 mm
]1 mm) were treated as previously described [4].
Briefly, they were first abraded (1200 sand paper), then
cleaned with 70% ethanol and sterile water, and dried.
Half of the high noble cast alloy specimens were heat
treated by exposure to 800°C for 10 min in order to
simulate the ceramic firing process [22]. Specimens of
a dental ceramic (In-Ceram), an aluminium oxide ce-
ramic system, were prepared according to the manufac-
turer’s instructions (VITA, Bad Sa¨ckingen, Germany).
2.3. Cell viability assay and quantification
of IL-6 secretion
Cell viability of exposed cell cultures was determined
by mitochondrial dehydrogenase activity (MTT-assay)
after 24 h [23]. Survival rates of the negative control
tissues were set to represent 100% viability. Results were
expressed as a percentage of the untreated control.
IL-6 release from treated and untreated cocultures was
investigated using an ELISA test system according to the
manufacturer’s instructions (Advanced Tissue Sciences).
100
ll aliquots were taken from exposed media and the
amount of cytokine release was quantified against a stan-
dard curve of purified human IL-6. IL-6 secretion of the
negative control tissues was set to 100%. Results of
the other test materials were expressed as a percentage
of the untreated control to yield comparable data.
Statistical analysis was performed applying the non-
parametric Mann—Whitney pairwise test. Each test ma-
terial was tested versus the untreated control as well as
versus the matching copper.
3. Results
Three-dimensional human fibroblast—keratinocyte co-
cultures were exposed to pure metals frequently found in
dental cast alloys, a high noble alloy, and a dental ce-
ramic. Cell viability was monitored by mitochondrial
1690
G. Schmalz et al. / Biomaterials 19 (1998) 1689—1694
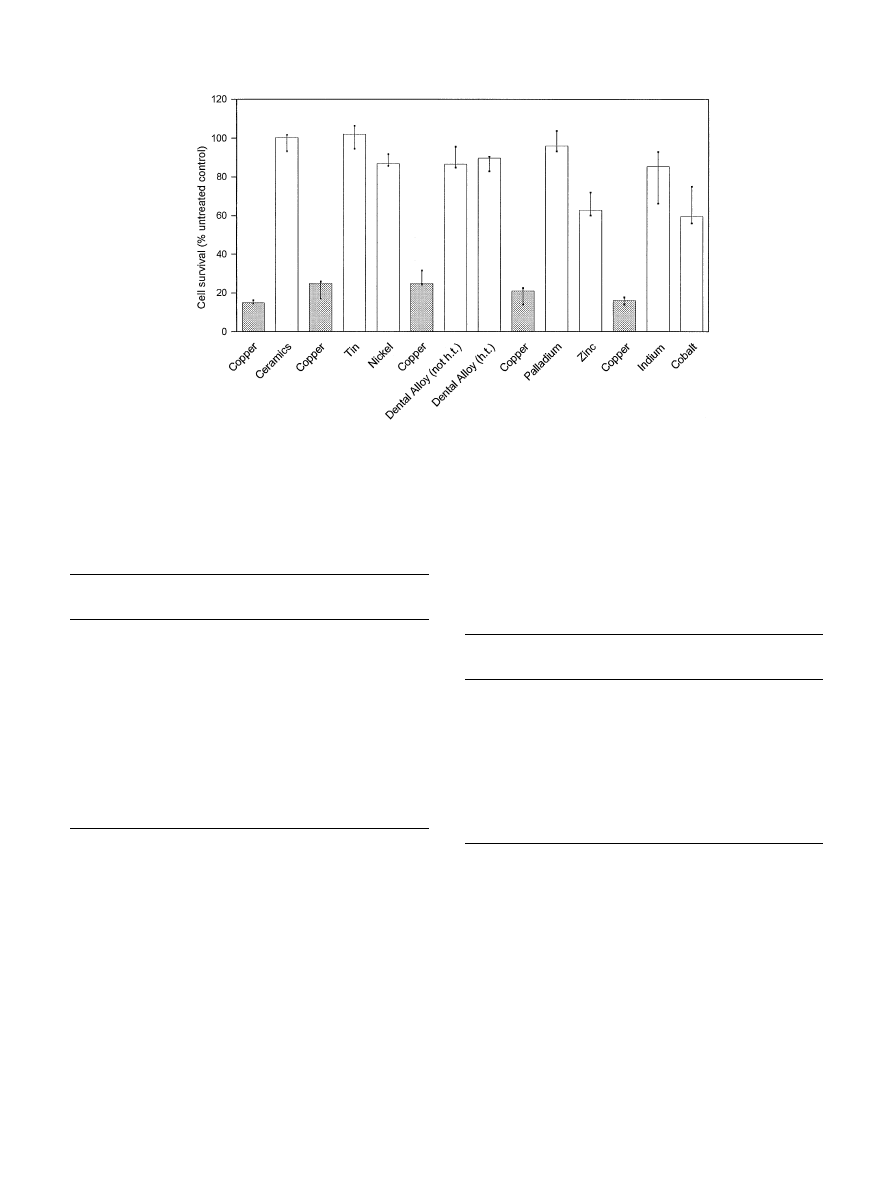
Fig. 1. Summary of cell survival (MTT assay). Human fibroblast—keratinocyte co-cultures were exposed to various metals and one high noble cast
alloy (h.t."heat treated; not h.t."not heat treated) for 24 h. Values are medians, minima and maxima from triplicates; the data are expressed as
percentage of untreated control cultures. Cell survival rates after exposure to copper specimens, included as a reference material in each experiment, are
presented to the left of cell survival rates from exposure to other test materials.
Table 1
Composition of test materials
Material
Manufacturer
Purity/
composition
Ceramic (In-Ceram) VITA, Bad Sa¨ckingen, Germany
Al2O3
Tin
Aldrich, Steinheim, Germany
99.999%
Palladium
Aldrich, Steinheim, Germany
99.999%
Nickel
Aldrich, Steinheim, Germany
99.98%
Zinc
Aldrich, Steinheim, Germany
99.999%
Copper
Aldrich, Steinheim, Germany
99.98%
High noble alloy
(Stabilor 7404)
Degussa, Hanau, Germany
Au 58%,
Ag 25%,
Pd 13%,
Zn 4%
Indium
Aldrich, Steinheim, Germany
99.999%
Cobalt
Aldrich, Steinheim, Germany
99.99%
dehydrogenase activity (MTT-assay) after a 24 h expo-
sure period (Fig. 1). Copper was the most toxic material
tested. Exposure to copper caused a time-dependent de-
crease in cell viability to levels of 14—25% compared to
untreated control tissues in repeated experiments. Cell
survival rates were significantly different from those
caused by exposure to all other test materials (p40,05;
Table 2). Therefore, copper was included as a positive
control material in all experiments. Zinc and cobalt re-
duced cell survival rates to about 60% of control cul-
tures. Nickel, indium and the high noble cast alloy (heat
treated as well as not heat treated specimens) were only
Table 2
Statistical analysis of survival rates of human fibroblast—keratinocyte
cocultures after exposure to test materials. Statistical analysis was
performed applying the Mann—Whitney pairwise test; significant
differences (p40.05) are indicated by #, the absence of significance
is indicated by n.s.
Test material
Versus untreated
Versus Copper
control
Ceramic (In-Ceram)
n.s.
#
Tin
n.s.
#
Palladium
n.s.
#
Copper
#
Nickel
#
#
Dental alloy (not heat treated)
#
#
Dental alloy (heat treated)
#
#
Zinc
#
#
Indium
#
#
Cobalt
#
#
weakly toxic with cell survival rates of about 90% of
untreated control groups. A dental ceramic (In-Ceram),
and the metals palladium and tin did not influence cell
viability after 24 h. With the exception of tin, palladium,
and the dental ceramic, all materials tested induced cell
viability rates which were significantly different from
those of untreated control tissues (p40,05; Table 2).
In parallel to the determination of cell viability rates,
IL-6 levels released from untreated cultures and cultures
exposed to test materials were measured by ELISA.
Fig. 2 demonstrates the time course of IL-6 release from
untreated control tissues and IL-6 secretion triggered by
G. Schmalz et al. / Biomaterials 19 (1998) 1689—1694
1691
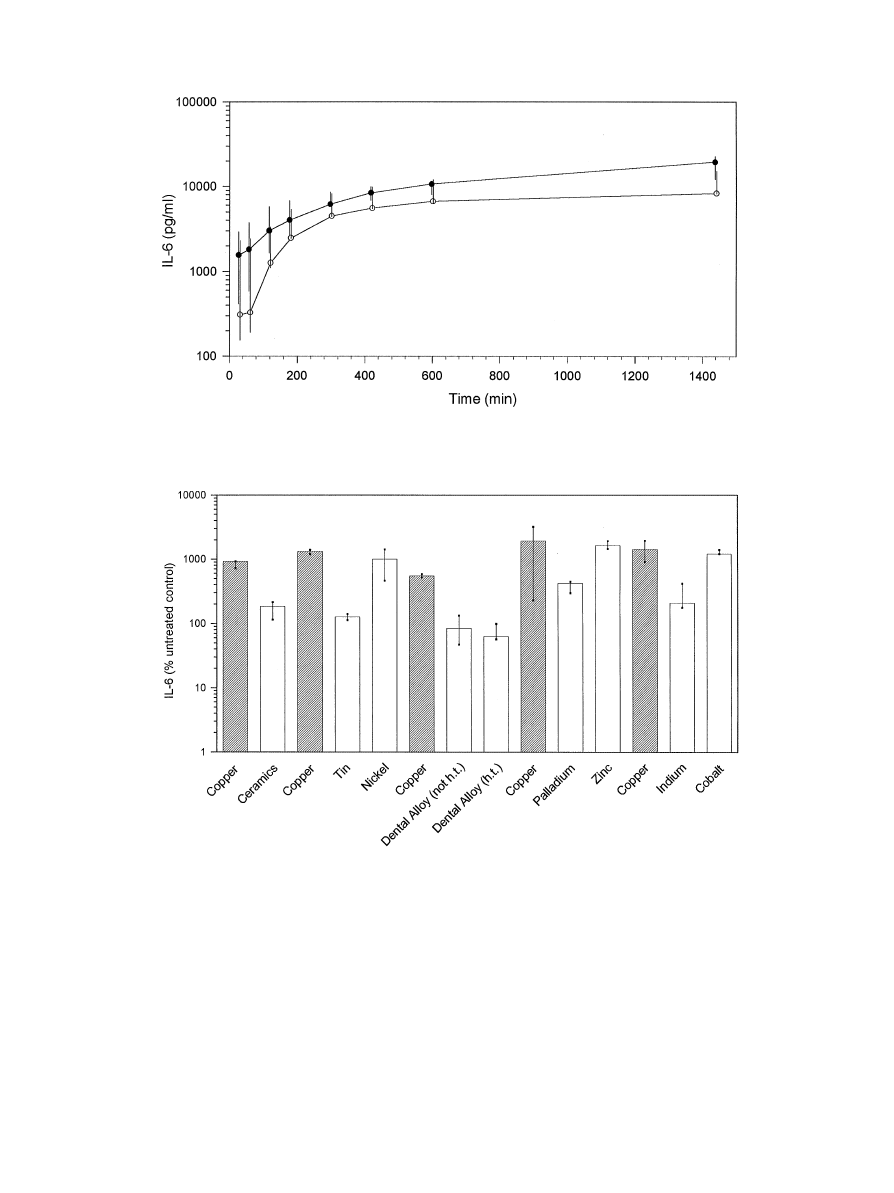
Fig. 2. Time course of total IL-6 release from human fibroblast—keratinocyte cocultures. IL-6 release from untreated cell cultures is indicated by open
circles, IL-6 release from cell cultures exposed to ceramic is indicated by filled circles. Values are medians, minima and maxima from triplicates.
Fig. 3. Summary of IL-6 release from human fibroblast—keratinocyte cocultures. Cell cultures were exposed to various metals and one high noble cast
alloy (h.t."heat treated; not h.t."not heat treated) for 24 h. The indicated values are medians, minima and maxima from triplicates; the data are
expressed as percentage of untreated control cultures. IL-6 release after exposure to copper specimens, included as a reference material in each
experiment, is presented to the left of IL-6 amounts found from exposure to other test materials.
specimens of dental ceramic, respectively. Small amounts
of IL-6 were continuously released from untreated con-
trol tissues during a 24 h observation period. Likewise,
a 24 h exposure to specimens of the non-toxic dental
ceramic had no significant influence on IL-6 secretion
(Fig. 2). This indicated that handling and the weight of
test specimens had only a small but not significant influ-
ence on the levels of IL-6 release.
A clear induction of IL-6 release was triggered to
various degrees by test materials of various cytotoxic
potencies (Fig. 3). The highest amounts of IL-6 in each
experiment were determined in cell cultures exposed to
copper, but the difference from some materials (pallad-
ium, nickel, zinc, cobalt) was not significant (p50,05;
Table 3). After a 24 h exposure to copper 5—19 times
higher values were obtained compared to untreated
1692
G. Schmalz et al. / Biomaterials 19 (1998) 1689—1694

Table 3
Statistical analysis of IL-6 release by fibroblast-keratinocyte cocultures
after exposure to test materials. Statistical analysis was performed
applying the Mann—Whitney pairwise test; significant differences
(p40.05) are indicated by #, the absence of significance is indicated
by n.s.
Test material
Versus untreated
Versus Copper
control
Ceramic (In-Ceram)
n.s.
#
Tin
n.s.
#
Palladium
#
n.s.
Copper
#
Nickel
#
n.s.
Dental alloy (not heat treated)
n.s.
#
Dental alloy (heat treated)
n.s.
#
Zinc
#
n.s.
Indium
#
#
Cobalt
#
n.s.
control groups; the differences of IL-6 levels between cell
cultures treated with copper and untreated cultures were
statistically significant (p40.05).
Significantly increased IL-6 levels were also observed
in cultures exposed to zinc (16-fold compared to un-
treated controls, p40.05) and cobalt (12 fold, p40.05).
Nickel which was only mildly toxic in the MTT-assay
induced IL-6 levels about 10-fold higher than those de-
termined in control cultures (p40.05). Similarly, pallad-
ium, which was non-toxic in the MTT-assay, caused
a 4-fold increase of IL-6 release compared to negative
controls (p40.05). Indium-induced IL-6 levels, which
were significantly higher than those of untreated control
tissues (2,6 fold, p40.05), but significantly lower than
those determined after exposure to copper (p40.05). The
other materials tested produced IL-6 levels comparable
to those of untreated control tissues. Statistical analysis
of IL-6 release by fibroblast—keratinocyte cocultures
after exposure to test materials is shown in Table 3.
4. Discussion
Cast alloys used in dentistry come into close and
prolonged contact with the gingiva and the oral mucosa.
Certain alloys have been claimed to cause inflammation
of gingival and periodontal tissues [1, 2]. Here, a three-
dimensional coculture model consisting of human
keratinocytes and fibroblasts was used as a skin tissue
equivalent model to simulate in vivo conditions and to
isolate mediators of tissue inflammation in vitro. Three-
dimensional cell cultures has been used to test the
cytotoxicity of dental alloys and dental filling materials
as well as to monitor the time-dependent release of
the proinflammatory mediator Prostaglandin E2 [4, 24].
It is well established that cytokine and immunglobulin
production at local disease sites are major molecules in
the transduction of inflammation. Interleukin-6 plays
a central role in these reactions.
The toxicity of the test materials was mainly low after
a 24 h exposure period except for copper, which was the
most toxic material tested in this study. After a 24 h
exposure to copper, cell survival rates of 14—25% com-
pared to untreated control cultures were obtained. Iden-
tical survival rates using the same tissue cultures were
measured in a previous study [4]. Exposure to copper
increased IL-6 secretion about 5—19-fold in comparison
to negative controls. It was observed that, unlike cell
survival rates, the amount of IL-6 released from cultures
exposed to copper varied considerably in repeated ex-
periments employing different batches of cocultures.
Therefore, we carried a negative, untreated control with
each experiment. Results of test materials were nor-
malized to this control, which represents 100% IL-6
release. In addition, other studies also describe consider-
able variations of cytokine measurements [6, 10], imply-
ing that variations of cytokine levels are a common
observation with the quantification of proinflammatory
mediators. The increase of IL-6 amount triggered by
copper was even underestimated when simply compared
to the spontanous IL-6 release from untreated cells with
cell survival rates of 100%, because copper reduced cell
survival to at least 25%. Therefore, the induction of IL-6
release from human keratinocytes and fibroblast cell
cultures by copper cannot be separated from the
cytotoxicity of the metal. Identical oberservations were
made in a previous study which investigated the release
of
the
proinflammatory
mediator
Prostaglandin
E2 (PGE2) from the same tissues. Copper also induced
significantly higher PGE2 levels (6—25-fold) compared to
negative controls [4]. These findings are in accordance
with in vivo data from Iijima [1]. He found that copper
powders, applied in cavities of maxillary root surfaces in
rats, cause extensive damage to the gingival tissues and
are associated with chronic inflammatory cells.
Cytotoxicity of cobalt and zinc ions was also shown
recently [25]. In parallel to their moderate cytotoxic
potential these metals also induced markedly increased
levels of IL-6 protein compared to untreated control
tissues. IL-6 amounts were not significantly different
from those caused by exposure to copper specimens.
Therefore, the cytotoxic effects of zinc and cobalt cannot
be separated from the induction of IL-6 release from
human keratinocytes and fibroblast cell cultures, as is the
case for copper.
Nickel and palladium showed moderate and no
cytotoxicity, respectively. But in spite of the slight toxic-
ity, it is a well-known fact that nickel and palladium can
induce hypersensitivities [26, 27]. Both metals do not
increase PGE2 release from cell cultures as described
previously [4]. Interestingly, nickel as well as palladium
induce increased IL-6 secretion compared to untreated
G. Schmalz et al. / Biomaterials 19 (1998) 1689—1694
1693

control groups, indicating that the production of IL-6
might be a specific effect for the induction of hypersensi-
tivity or inflammation by sub-toxic concentrations of
nickel and palladium. The findings are in accordance
with other studies demonstrating the production of IL-6
after exposure of alveolar macrophages to non-toxic con-
centrations of nickel hydroxy carbonate [28]. The
authors concluded that the release of IL-6 might be
responsible, at least partly, for inflammation and pneu-
motoxicity associated with nickel exposure.
In summary, our findings suggest that metal ions are
involved in proinflammatory activity at low toxicity and
non-toxic levels. IL-6 and PGE2 seem to be two possible
mediators of gingival and periodontal inflammation. It is
obvious that various test parameters (MTT, interleukins,
prostaglandins) provide a better characterization of the
test compound’s toxic potency than a single endpoint.
Acknowledgements
The authors thank Dr LJ Nunez (Memphis, TN, USA)
for a critical reading of the manuscript. The skilled tech-
nical assistance of U Zorn is gratefully acknowledged.
References
[1] Iijima S. Histopathological study of the effect of pure metals to the
periodontal tissues. Nippon Shishubyo Gakkai Kaishi 1989;
31:997—1020.
[2] Wirz J. Scha¨digung des Parodontes durch zahna¨rztliche Wer-
kstoffe. Zahna
( rztl Welt/Reform 1993;102:146—62.
[3] Bruce GJ. Nickel-hypersensitivity — related periodontitis. Com-
pend Contin Educ Dent 1994;16(178):180—4.
[4] Schmalz G, Arenholt-Bindslev D, Hiller K-A, Schweikl H. Epi-
thelium-fibroblast co-culture for assessing mucosal irritancy of
metals used in dentistry. Eur J Oral Sci 1997;105:86—91.
[5] Fucikova T, Tesar V, Masek J, Bartunkova J, Rychlik I. Adhesion
molecules and cytokines in vasculitides. Bratisl Lek Listy 1995;
96:523—7.
[6] Karagouni EE, Dotsika EN, Sklavounou A. Alteration in peri-
pheral blood mononuclear cell function and serum cytokines in
oral lichen planus. J Oral Pathol Med 1994;23:28—35.
[7] Okuyama S. Evolution of Hodgkin’s disease. Med Hypotheses
1996;47:199—213.
[8] Feliciani C, Gupta AK, Sauder DN. Keratinocytes and
cytokine/growth factors. Crit Rev Oral Biol Med 1996;7:300—18.
[9] Akira S, Kishimoto T. IL-6 and NF-IL6 in acute-phase response
and viral infection. Immunological Reviews 1992;127:25—50.
[10] Masada MP, Persson R, Kenney JS, Lee SW, Page RC, Allison
AC. Measurement of interleukin-1 beta in gingival crevicular
fluid: implications for pathogenesis of periodontal disease. J Peri-
odontal Res 1990;25:156—63.
[11] Kamagata Y, Miyasaka N, Inoue H, Hashimoto J, Iida M.
Cytokine production in inflamed human gingival tissues—inter-
leukin-6. Nippon Shishubyo Gakkai Kaishi 1989;31:1081—7.
[12] Takahashi K, et al. Assessment of interleukin-6 in the pathogen-
esis of periodontal disease. J Periodontol 1994;65:147—153.
[13] Dongari-Bagtzoglou AI, Ebersole JL. Production of inflammat-
ory mediators and cytokines by human gingival fibroblasts fol-
lowing bacterial challenge. J Periodontal Res 1996;31:90—8.
[14] Ogura N, et al. Stimulation of interleukin-6 production of peri-
odontal
ligament
cells
by
Porphyromonas
endodontalis
lipopolysaccharide. Biochem Med Metab Biol 1994;53:130— 6
[15] Rossano F, Rizzo A, Sanges MR, Cipollaro de L’Ero G, Tufano
MA. Human monocytes and gingival fibroblasts release tumor
necrosis factor-alpha, interleukin-1 alpha and interleukin-6 in
response to particulate and soluble fractions of Prevotella mela-
ninogenica and Fusobacterium nucleatum. Int J Clin Lab Res
1993;23:165—8
[16] Contard P, Bartel RL, Jacobs L II, Perlish JS, MacDonald ED.
Culturing keratinocytes and fibroblasts in a three-dimensional
mesh results in epidermal differentiation and formation of a basal
lamina-anchoring zone. J Invest Dermatol 1993;100:35—9.
[17] De Wever B, Rheins LA. Skin 2TM. An in vitro human skin
analog. In: Rougier A, Goldberg AM, Maibach HI, editors. In
vitro skin toxicology. Irritation, phototoxicity, sensitization. Al-
ternative models in toxicology. New York: Mary Ann Liebert
Publisher, New York 1994;10:121—31.
[18] Fleischmajer R, Contard P, Schwartz E, MacDonald ED II,
Jacobs LI, Sakai LY. Elastin-associated microfibrils (10 nm) in
a three-dimensional fibroblast culture. J Invest Dermatol
1991;97:638—43.
[19] Osborne R, Perkins MA. An approach for development of alter-
native test methods based on mechanisms of skin irritation. Food
Chem Toxicol 1994;32:133—42.
[20] Slivka SR, Landeen LK, Zeigler F, Zimber MP, Bartel RL.
Characterization, barrier function, and drug metabolism of an
in vitro skin model. J Invest Dermatol 1993;100:40—6.
[21] Whalen E, Donnelly TA, Naughton G, Rheins LA. The develop-
ment of three-dimensional in vitro human tissue models. Hum
Exp Toxicol 1994;13:853—9.
[22] Council on Dental Materials, Instruments and Equipment. Clas-
sification system for cast alloys. J Am Dent Assoc 1984;109:766.
[23] Mosmann T. Rapid colorimetric assay for cellular growth and
survival: Application to proliferation and cytotoxicity assays.
J Immun Meth 1983;65:55—63.
[24] Schmalz G, Nu¨tzel K, Schuster U, Schweikl H. An in vitro pulp
chamber with three-dimensional cell cultures. J Dent Res
1997;76:78.
[25] Schedle A, et al. Response of L-929 fibroblasts, human gingival
fibroblasts, and human tissue mast cells to various metal cations.
J Dent Res 1995;74:1513—20.
[26] Aberer W, Holub H, Strohal R, Slavicek-R. Palladium in dental
alloys—the dermatologists’ responsibility to warn?. Contact
Dermatitis 1993;28:163—5.
[27] Sauder DN. Epidermal cytokines in allergic contact dermatitis.
J Am Acad Dermatol 1995;33:786—800.
[28] Arsalane K, Gosset P, Hildebrand HF, Voisin C, Tonnel AB,
Wallaert B. Nickel hydroxy carbonate increases tumor necrosis
factor alpha and interleukin-6 secretion by alveolar macrophages.
J Appl Toxicol 1994;14:375—9.
1694
G. Schmalz et al. / Biomaterials 19 (1998) 1689—1694
Wyszukiwarka
Podobne podstrony:
The Influence of` Minutes
20 255 268 Influence of Nitrogen Alloying on Galling Properties of PM Tool Steels
3 The influence of intelligence on students' success
72 1031 1039 Influence of Thin Coatings Deposited by PECVD on Wear and Corrosion Resistance
Influence Of Magnetic Field On Two Phase Flow Convective Boiling Of Some Refrigerant Mixtures
Zen in the Influence of the Sword
Influence of drying methods on drying of bell pepper (Tunde Akintunde, Afolabi, Akintunde)
84 1199 1208 The Influence of Steel Grade and Steel Hardness on Tool Life When Milling
The influence of Aristotle on Alfarabi
Język angielski The influence of the media on the society
network memory the influence of past and current networks on performance
6 63 76 Influence of Surface Heat Treatment on Thermal Fatique Behaviour
influence of parents on thier childrens sexual orientation
3 27 37 Influence of the Temperature on Toughness of DIEVAR
83 1183 1198 Influence of Non Metallic Inclusions in Super Finish Wire Cutting
więcej podobnych podstron