
Abstract We examined an improved preparation method
of recycled unsaturated polyester resin from recovered
monomeric materials obtained from the depolymerization
of fi ber-reinforced plastics (FRPs). The formation of unsat-
urated polyester progressed smoothly in the presence of
catalytic amounts of Ca(OAc)
2
and Ti(OBu)
4
. The quality
of the resin was estimated by the durometer hardness test.
The strength test of FRP board prepared from recycled
resin showed suffi cient hardness for practical use (about
94% of the tensile strength of new resin). We examined the
recycled resin by using it to mold successfully an actual test
product.
Key words Chemical recycling · Recycled resins · Hardness
test · Acid catalysts · Unsaturated polyester
Introduction
Waste fi ber-reinforced plastic (FRP) has been causing
serious environmental problems. Appropriate and conve-
nient disposal of waste FRP is very diffi cult due to its
mechanical strength and chemical stability. As a result,
most waste FRP is either incinerated or disposed off in
landfi lls. FRP is made from carbon sources that are primar-
ily derived from petroleum. Therefore, from the perspec-
tive of carbon source conservation, suitable recycling of
waste FRP is strongly desired. However, waste FRP con-
tains a thermosetting resin whose material recycling is not
easy. In addition, the glass fi bers present in FRP cause
serious problems in incinerators when being thermally
treated for energy recycling. Chemical recycling is a process
in which waste FRP yields monomeric materials that are
recycled as feedstock. This is regarded as the only proce-
dure likely to improve the prospect for proper recycling of
FRP.
There have been several reports on the chemical
recycling of FRP; most of them use the glass fi bers or part
of the resin.
Recently, we reported a novel solubilization
of waste FRP under supercritical methanol conditions in the
presence of 4-dimethylaminopyridine (DMAP). In this
process, the ingredients of FRP, i.e., monomer derivative,
linker, glass fi ber, and calcium carbonate, were separated
in a simple operation.
From the recovered monomeric
material and glass fi ber, the possibility of the formation
of recycled plastic was examined.
However, further
improvements in the formation of recycled resin are neces-
sary to make our method practical and widely applicable.
This article reports an improved formation of recycled resin
as well as on the mechanical strength of an FRP test product
prepared using this resin.
Experimental
Depolymerization of FRP and preparation of recycled
monomer derivatives were performed in a 30-l pilot plant
autoclave following the previously reported procedure.
The dimethyl phthalate contents were evaluated by Gas
Chromatography analysis.
Formation of new resins in the presence of Ti(OBu)
4
– a
typical procedure. Dimethyl phthalate (100 g, 0.51 mol)
and propylene glycol (82.3 g, 1.08 mol) were poured into a
1-l separable fl ask and Ca(OAc)
2
(0.647 g, 3.6 mmol) and
Ti(OBu)
4
(0.172 g, 0.51 mmol) were then added. The
mixture was heated at 210°C for 3 h, during which about
27 g of MeOH was distilled out from the mixture. Maleic
anhydride (55.6 g, 0.57 mol) was added to the hot mixture
and the resulting reaction mixture was heated at 220°C for
an additional 3 h. The mixture was then cooled for 2 h.
Styrene monomer (145 g) was added to the mixture and the
entire resin was poured into a plastic beaker (500 ml).
Radical initiator (organic peroxide) and accelerator (cobalt
J Mater Cycles Waste Manag (2010) 12:271–274
© Springer 2010
DOI 10.1007/s10163-010-0296-7
Kazuo Yamada · Fumiaki Tomonaga · Akio Kamimura
Improved preparation of recycled polymers in chemical recycling of fi ber-
reinforced plastics and molding of test product using recycled polymers
K. Yamada · A. Kamimura (*)
Department of Applied Molecular Bioscience, Graduate School of
Medicine, Yamaguchi University, Ube 755-8611, Japan
Tel.
+
81-836-85-9231; Fax
+
81-836-85-9201
e-mail: ak10@yamaguchi-u.ac.jp
K. Yamada · F. Tomonaga
Yamaguchi Prefectural Industrial Technology Institute, Ube, Japan
Received: October 12, 2009 / Accepted: March 16, 2010
ORIGINAL ARTICLE

272
naphthenate) were added to the mixture, which yielded an
unsaturated polyester polymer plate. The quality of the
resin was estimated by the durometer hardness of the cured
product.
Preparation of recycled resins on a larger scale – typical
procedure. Reagent grade dimethyl phthalate (1.70 kg,
8.75 mol) and propylene glycol (1.524 kg, 20 mol) were
mixed, and to the mixture, recovered dimethyl phthalate
(57% purity, 3.2 kg, 9.4 mol), Ti(OBu)
4
(1 g), and Ca(OAc)
2
(5.7 g) were added. The mixture was heated at 200°C to
remove MeOH (545
g). Maleic anhydride (1.957
kg,
20.0 mol) was then added to the hot mixture and heated at
200°C for several hours to remove MeOH and water. This
crude polyester was cooled and transferred to an appropriate
vessel. Styrene (5.12 kg) and radical initiator were added to
start the radical polymerization. After curing, 7.73 kg of
recycled unsaturated polyester resin was obtained.
Molding of FRP board
FRP board was molded using a hand lay-up molding
method. Six 450-g/m
3
glass fi ber strand mats were set into
a stainless mold (150 mm
×
160 mm
×
5 mm cavity) that was
coated with mold lubricant. These mats were then
impregnated with the resin. The mold was pressed by a
metal plate and allowed to stand at 35°C in a thermostatically
controlled oven.
Strength test of FRP
Test pieces of the molded FRP board were examined at
25°C and 55% humidity. Tensile and bending strength were
tested according to the Japanese Industrial Standards (JIS)
K7113 and K7013. Charpy impact tests were performed
according to JIS K7061 in the fl atwise direction. Barcol
hardness was examined by Type A of JIS K7060.
Results and discussion
Waste FRP was depolymerized under standard conditions
(Scheme 1). We avoided using DMAP as a catalyst to facili-
tate the later polymerization reactions using recovered
materials, as has been discussed previously.
When resin was synthesized from the recycled monomer
under standard conditions, there was a problem with insuf-
fi cient hardness after curing. We attributed this to an insuf-
fi cient progress of the ester exchange reaction during
polyester formation. In fact, the amounts of MeOH distilled
out during the conventional esterifi cation reaction were
estimated to be less than the calculated amounts. Since the
recovered material usually contains undesirable impurities
that may negatively affect the Lewis acidity of Ca(OAc)
2
,
the rate of the ester exchange reaction decreased. To
increase the reaction rate, a stronger Lewis acid seemed
appropriate. Titanium alkoxide is known as moderately
strong Lewis acid and its acidity can be considered to be
higher than that of Ca(OAc)
2
. We examined commercially
available Ti(OBu)
4
to be used as a co-catalyst for the reac-
tion. The reaction was optimized using freshly purchased
dimethyl phthalate for these experiments (Scheme 2). As
expected, when a mixture of Ti(OBu)
4
and Ca(OAc)
2
was
added to the reaction, the amounts of MeOH that were
distilled out increased and the formation of the polyester
was accelerated. The results are summarized in Table 1.
In Table 1, certain combinations of catalysts did not
improve the transesterifi cation. We marked such entries,
where less than 70% of MeOH distilled out during the reac-
tion, with an x. In contrast, resins were successfully formed
in those reactions where more than 70% of MeOH was
distilled out. The durometer hardness of these products is
summarized in Table 1. It should be noted that most of the
resins formed under these conditions were suffi ciently hard
and of good quality because their durometer hardness
values exceeded 80. Thus, the addition of Ti(OBu)
4
enabled
acceleration of the reaction and promoted better resin for-
mation. The best hardness was observed in the resin formed
when 0.72 mol% of Ca(OAc)
2
and 0.1 mol% of Ti(OBu)
4
FRP
flakes
supercritical MeOH
275 °C
MeOH-soluble oil
insoluble inorganic residue
+
Scheme 1. Depolymerization of waste fi ber-reinforced plastic (FRP)
CO
2
Me
CO
2
Me
OH
OH
+
Ca(OAc)
2
Ti(OBu)
4
O
O
O
+
styrene
radical initiator
unsaturated
polyester
Scheme 2. Optimization of the resin formation from dimethyl phthalate and glycol
Table 1. Durometer hardness of polymer
Ca(OAc)
2
(mol% vs DMP)
Ti(OBu)
4
(mol% vs DMP)
0.00
0.05
0.10
0.15
0.20
0.25
0
×
×
0.18
×
×
0.36
×
83
86
0.54
×
86
86
86
0.72
87
0.9
×
86
87
Durometer hardness (HDD) of cured product using new resin was 88
DMP, dimethyl phthlate;
×
, transesterifi cation was not improved
273
were used. We applied these conditions to the large-scale
preparation of recycled resin as the optimum reaction
conditions.
We next performed strength tests on recycled FRP pre-
pared from the recovered materials. The outline of the pro-
cedure is summarized in Scheme 3. Three types of FRPs
were formed by our method; the molding conditions used
are summarized in Table 2. Type A FRP was made with
new resin and was used as a control; types B and C were
recycled resins prepared from recovered materials. Type C
was made from 100% recycled material and type B was
made from an equal (50:50) blend of new and recovered
materials. We performed several tests with the three types
of FRPs and the results are shown in Figs. 1–3.
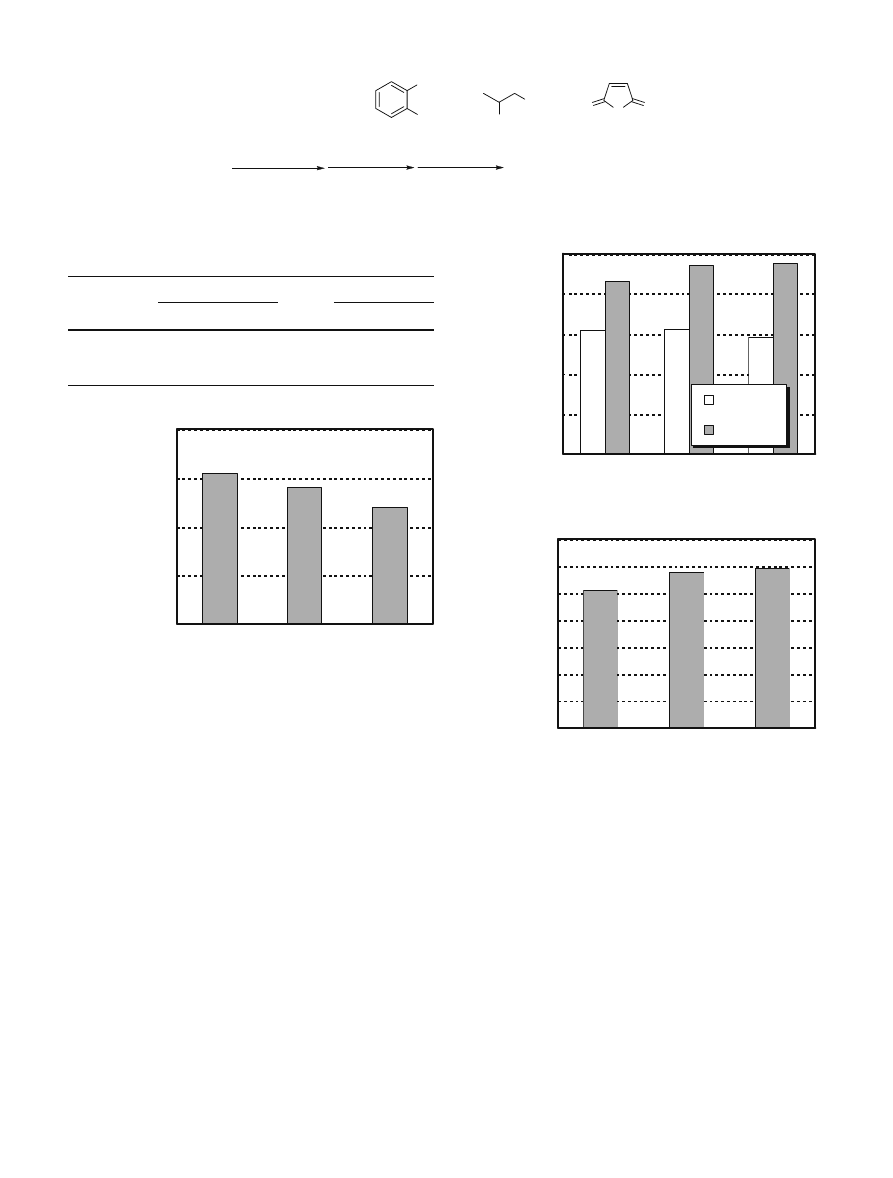
Figure 1 represents the results of the Barcol hardness
tests on the FRPs. As the contents of recovered materials
increased, the hardness decreased slightly. However, the
decrease in hardness was only about 2% and so this may
not be considered as a serious defect. The mechanical
strengths of the FRPs were then determined (Fig. 2). As
shown in Fig. 2, the tensile strengths were approximately
the same for both the new and recycled resins. It is interest-
ing to note that the bending strengths for the recycled resin
were slightly higher than those for the new resin.
Figure 3 represents the results for the Charpy impact test
on the resins. It was again observed that FRPs B and C
molded using recovered materials were stronger than the
newly formed FRP A. These unexpectedly superior quali-
ties of recycled FRP are probably due to their increased
softness as compared to the newly formed FRP. Thus, the
impurities in the recovered materials made the recycled
resins softer than the newly formed resins. This was due to
incomplete polymerization. The softness provided slightly
better results for the bending test and impact strength. In
the bending test, tensile stress occurs on the outside of the
bent section, and when transformation of the stretched
resin exceeds the limit, failure occurs. Since the soft resin
followed this transformation more closely, its bending
Recovered material
+
CO
2
Me
CO
2
Me
OH
OH
+
O
O
O
+
Ca(OAc)
2
Ti(OBu)
4
styrene
radical initiator
recycled FRP
glass fiber mat
Scheme 3. The formation of recycled FRP
Table 2. Conditions for the formation of recycled fi ber-reinforced
plastic (FRP)
TYPE
Resin used
Glass fi ber content
(wt% vs total amount)
(wt% vs FRP)
A
New resin 100
38
B
New 50
+
recycled 50
39
C
Recycled resin 100
39
40
42
44
46
48
A
B
C
Barcol hardness (HBI-A)
Fig. 1. Barcol hardness of recycled fi ber-reinforced plastic (FRP)
0
50
100
150
200
250
A
B
C
Tensile
Bending
Strength(MPa)
Fig. 2. Mechanical strength of recycled FRP
0
20
40
60
80
100
120
140
A
B
C
Charpy impact strength (kJ/m2)
Fig. 3. Charpy impact test on recycled FRP

274
strength became stronger. In the impact test, the resin trans-
formation in a larger area caused absorption of more
energy. Hence, the quality of the recycled FRP was deemed
satisfactory.
Finally, we performed resin production on a large scale
and used it to prepare a test product. We chose the lid of a
composter in which food garbage is recycled into compost
as the test product. A total of 20 wt% of recycled resin was
used for the production of the material. The production
went smoothly, and the test product was prepared success-
fully. Figure 4 shows the product. The test product exhib-
ited suffi cient strength and had no problems when an adult
(about 70 kg) stepped on the product. This product was
used in the fi eld for 8 months without any problems.
In conclusion, we developed an improved method for the
preparation of unsaturated polyester, with which we suc-
cessfully prepared recycled plastics on a large scale. Use of
Ti(OBu)
4
effectively catalyzed the resin formation and
shortened the reaction time. The obtained recycled plastic
was of better quality compared to new plastics. We con-
cluded that the successful formation of a recycled test
product will open a new avenue for chemical recycling of
FRP.
References
1. Research Association of Feedstock Recycling of Plastics, Japan
(ed) (2005) Technology for feedstock recycling of plastic wastes.
CMC, Tokyo
2. Nomaguchi K, Nakagawa T (2009) FRP recycling in EU, NA, and
the Pacifi c Rim area – challenging development in chemical recy-
cling by subcritical water process. Rev Automot Eng 30:3–10
3. Nomaguchi K, Shibata K (2008) FRP recycling. Promotion toward
commercialization of next-generation chemical recycling technol-
ogy (in Japanese). Purasuchikkusu Eji 54:73–75
4. Ohsawa I, Takahashi J, Uzawa K, Ashida T, Shibata K (2008) Re-
molding and the fl exural, compressive and interlaminar shear
evaluation of unidirectional-CF/epoxy using recycling carbon fi ber
(in Japanese). Kyoka Purasuchikkusu 54:285–291
5. Nakagawa T (2008) Chemical recycling of FRP using subcritical
water: enhanced and horizontal recycling of thermosetting resin
waste (in Japanese). Fain Kemikaru 37:14–24
6. Shibata K (2007) FRP recycling technology (in Japanese). Net-
towaku Porima 28:247–256
7. Nomaguchi K, Nakagawa T (2007) Latest trends in FRP recycling.
Proliferation of cement kiln processes, thriving development of
next-generation chemical recycling technology, and the challenges
of environmentally compatible design (in Japanese). Purasuchik-
kusu Eji 53:88–91
8. Sasaki M, Iwaya T, Goto M (2007) Subcritical fl uid fractionation
of fi ber-reinforced plastics and chemicals recovery (in Japanese).
Chorinkai Saishin Gijutsu 9:27–31
9. Shibata K (2006) FRP recycling using depolymerization of unsatu-
rated polyester under ordinary pressure (in Japanese). Fain Kemi-
karu 35:14–20
10. Nakagawa T, Urabe T, Hidaka M, Maekawa T, Okumoto S,
Yoshida H (2006) FRP recycling technology using subcritical water
hydrolysis (in Japanese). Nettowaku Porima 27:88–95
11. Yoshikai K, Sakamoto J (2004) Research on chemical recycling of
mannequins made of FRP using glycol decomposition (in Japa-
nese). Kyoka Purasuchikkusu 50:398–402
12. Shibata K (2003) Trend of chemical recycling of thermosets (in
Japanese). Nippon Setchaku Gakkaishi 39:226–230
13. Kubota S, Maeda T, Mori H (2003) Feedstock recycling of polyes-
ter resin wastes by decomposition with hydroxy carboxylic acid (in
Japanese). Nippon Setchaku Gakkaishi 39:240–247
14. Okajima I, Yamada K, Sugata T, Sako T (2002) Decomposition of
epoxy resin and recycling of CFRP with sub- and supercritical
water (in Japanese). Kougakukai Ronbunshu 28:553–558
15. Kamimura A, Yamada K, Kuratani T, Oishi Y, Watanabe T,
Yoshida T, Tomonaga F (2008) DMAP as an effective catalyst to
accelerate suffi cient solubilization of waste FRP: a new method for
recycling waste plastics. ChemSusChem 1:845–850
16. Kamimura A, Konno E, Yamamoto S, Watanabe T, Yamada K,
Tomonaga F (2009) Formation of recycled plastics from depoly-
merized monomers derived from waste fi ber reinforced plastics. J
Mater Cycles Waste Manag 11:38–41
17. Kamimura A, Konno E, Yamamoto S, Watanabe T, Yamada K,
Tomonaga F (2009) Improved method for the formation of recy-
cled resins from depolymerized products of waste fi ber-reinforced
plastics (FRP): simple and effective purifi cation of recovered
monomers by washing with water. J Mater Cycles Waste Manag
11:133–137
18. Kamimura A, Tomonaga F, Yamada K (2008) Novel chemical
recycling of waste FRP using supercritical alcohol and organocata-
lysts (in Japanese). Kagakukougyo 59:560–568
Fig. 4. Image of test product made using 20% recycled FRP
Wyszukiwarka
Podobne podstrony:
w3 recykling tworzyw sztucznych
w3 recykling tworzyw sztucznych
36 Zbiórki i recyklingu tworzyw sztucznych w rur odpadów budowlanych i rozbiórkowych strumienia
30 Recykling tworzyw sztucznych ognioodpornych z WEEE, problemów technicznych i środowiskowych
19 Recykling tworzyw sztucznych Wgląd metod oceny wpływu cyklu życia
24 Reakcje polimerów w nadkrytycznym płynów do chemicznego recyklingu odpadów z tworzyw sztucznych
C1 Recykling chemiczny PMMA, PET RECYKLING, Przetwórstwo tworzyw sztucznych
23 Recyklingu polimerów, materiałów opakowaniowych z tworzyw sztucznych, stosując technikę rozpuszcz
Recykling odpadowych tworzyw sztucznych
RECYKLING ODPADÓW SZTUCZNYCH
2013 03 26 W 7 TRM Przygotowanie obiektu i budowyid 28270 ppt
cygan,podstawy ochrony środowiska, recykling odpadów sztucznych
Recykling odpadów sztucznych. sprawko, Mechatronika, Recykling
Instrumenty pochodne (26 stron), Rynek instrumentów pochodnych w Polsce znajduje się dopiero w bardz
Fermentacja 1 kolo lepsze, uniwersytet warmińsko-mazurski, inżynieria chemiczna i procesowa, rok III
Podstawy procesu uplastyczniania., PET RECYKLING, Przetwórstwo tworzyw sztucznych
Tworzywa sztuczne i metale, Klub Miłośników Przyrody - kółko przyrodnicze klasa 1, Recykling, Inform
więcej podobnych podstron