
Working with agar
What is agar?
Agar is a polysaccharide found in the cell walls of some red algae from which it's extracted by
boiling. This raw agar is then purified.
When agar is dissolved in boiling water and left to cool it will form a gel (very much like gelatin).
It can be bought in many different grades from food-grade to purified tissue culture grade.
It's main uses are in the field of microbiology, tissue culture and food preparation (as a
vegetarian substitute for gelatin).
Why is agar important?
We use agar as a gelling agent for nutrient media. By adding nutrients (agar itself does not
contain digestible compounds) a nutrient base can be made on which mushroom mycelia can
be cultivated in a flat two-dimensional way.
It can not be substituted by gelatin since gelatin is digestible and does not gel after autoclaving.
Preparing nutrient medium
There are many recipes that work well but the simplest and easiest to prepare remains:
(Smaller volumes can be prepared by simply using less ingredients in the same ratio)
MEA (Malt Extract Agar)
20 grams light malt extraxt
2 grams yeast
15-20 grams agar
1 liter water
The dry ingredients are put in a flask (erlenmeyer or similar) and water is added. Take care not
to fill the flask for more than 2/3 of it's volume or it will boil over.
When the water and dry ingredients are put in the flask the opening is stuffed with polyfill or
hydrophobic cotton. Another option is to put a piece of Tyvek over it held in place with rubber
bands. This allows for the agar to be re-melted in the microwave. A piece of aluminum foil is
put on top and the flask is swirled.
The flasks are then sterilised in the pressure cooker at 121ºC for 40 minutes.
This medium will support the growth of most saprophytic fungi. Other often used formulations
are PDA (Potato Dextrose Agar) and DFA (Dog Food Agar).

When making your own recipes keep in mind that more is not always better. When media are
too rich in nutrients mycelium will not grow or grow very poorly secreting yellowish metabolites.
Pouring dishes
When the flasks come out of the pressure cooker or autoclave the temperature of the agar will
be close to 100ºC. The medium will be liquid until the temperature drops below 40ºC. The agar
is left to cool until it has almost reached this temperaure. As a rule of thumb: If you can hold the
bottle in your bare hand for 10 seconds without real discomfort the temperature is right. Do not
let it cool too much or it will solidify in the bottle. Better to pour a little too hot than having to
reliquify the agar.
Since the lids of the dishes will be removed for a few seconds it's very important to prepare a
clean workplace. A laminar flowhood is best but an improvised transfer hood will usually give
adequate results.
Whichever is used the table top is cleaned with alcohol or lysol
(CAREFUL! THESE ARE
FLAMMABLE!)
and the dishes are taken out of the tube in which they were packaged and put
in stacks of 10 dishes.
When working in less then totally sterile environments it's important to work as fast as possible,
limiting the direct contact of agar with outside air.
The flask is swirled to mix the ingredients and the aluminum foil and polyifll are removed.
The lid of the bottom dish is lifted (in fact lifting the lid and the 9 dishes on top). Swiftly a layer
of nutient medium is poured in the dish (to 0,3-0,5 cm depth) and the lid is replaced. Quickly lift
the second lid (and the 8 remaining dishes) and work your way up in this fashion.
By letting the agar cool before pouring and by stacking the dishes condensation on the lids is
minimized. When a mug or jar with near boiling water is put on the top dish of a stack
condensation will be even less.
When the agar in the has solidified the dishes are ready to use.
Preparing agar slants
The normal way to store cultures is in so-called slants. Slants are test tubes in which the agar
was cooled on a sloped surface to give the agar a larger surface area.
Slants are prepared a little differently then petri dishes since the tubes are sterilized with the
agar in them opposed to dishes which come pre-sterilized and which are filled with sterilized
agar.

The nutrient medium is prepared by bringing the appropiate amount of water to a near boil in a
pot on the stovetop. The ingredients are added and the mixture is stirred to let them dissolve.
Agar should first be mixed with a little bit of cold water before adding or it will form lumps.
Careful, adding agar to boiling water will cause the medium to foam and boil over. Boiling agar
can cause serious burns!
Let the agar boil for a few minutes, then turn off the heat.
Tubes are filled with 5-6 ml of medium and loosely capped. A small funnel and a big syringe
are very useful here. The tubes are sterilized for 25 minutes at 121ºC.
When they come out they are left to cool on a sloped surface to increase to surface area of the
agar. When the agar has solidified the slants are ready to use.
Starting a culture from spores
To start cultures from spores one needs a sporeprint and a tool that can be sterilized to pick up
the spores (inoculation loop, scalpel or similar).
With a sterilised inoculation loop some spores are scraped from the spore print and the loop is
streaked across the surface of the agar in an 'S' pattern. The inoculation loop is resterilised
before using it for other inoculation.
Often spore germination is visible within a few days but it may take as long as four weeks
before visible germination takes place. If no germination takes places it may be necessary to
rehydrate the spores in some sterile water for 24 hours before streaking them on agar.
If contaminants are encountered one can choose to discard the whole dish or one can transfer
the mycelium away from the contamination to a new dish. When bacterial contamination is
present this can take place in a flowhood. If sporulating molds are present isolation should take
place in a still-air environment to prevent the release of mold spores.
The germinating spores will form a mycelium that by itself is not capable of fruiting called a
'monokaryon'. When the mycelia of two spores fuse they will form a new type of mycelium that
IS capable of fruiting, a 'dikaryon'. This process will happen by itself and one dish with
germinated spores will often contain dozens of these combinations.
A dish with germinated spores will often look a little bit messy, with monokaryons and
dikaryons competing with each other for nutrient sources. Here one has two options: use this
mixture of substrains (a 'multispore') or select a pure substrain. Each substrain has it's own
properties but generally resembles it's parents. Generally speaking flushes from pure
substrains are more uniform and better yeilding than a mixture of substrains.
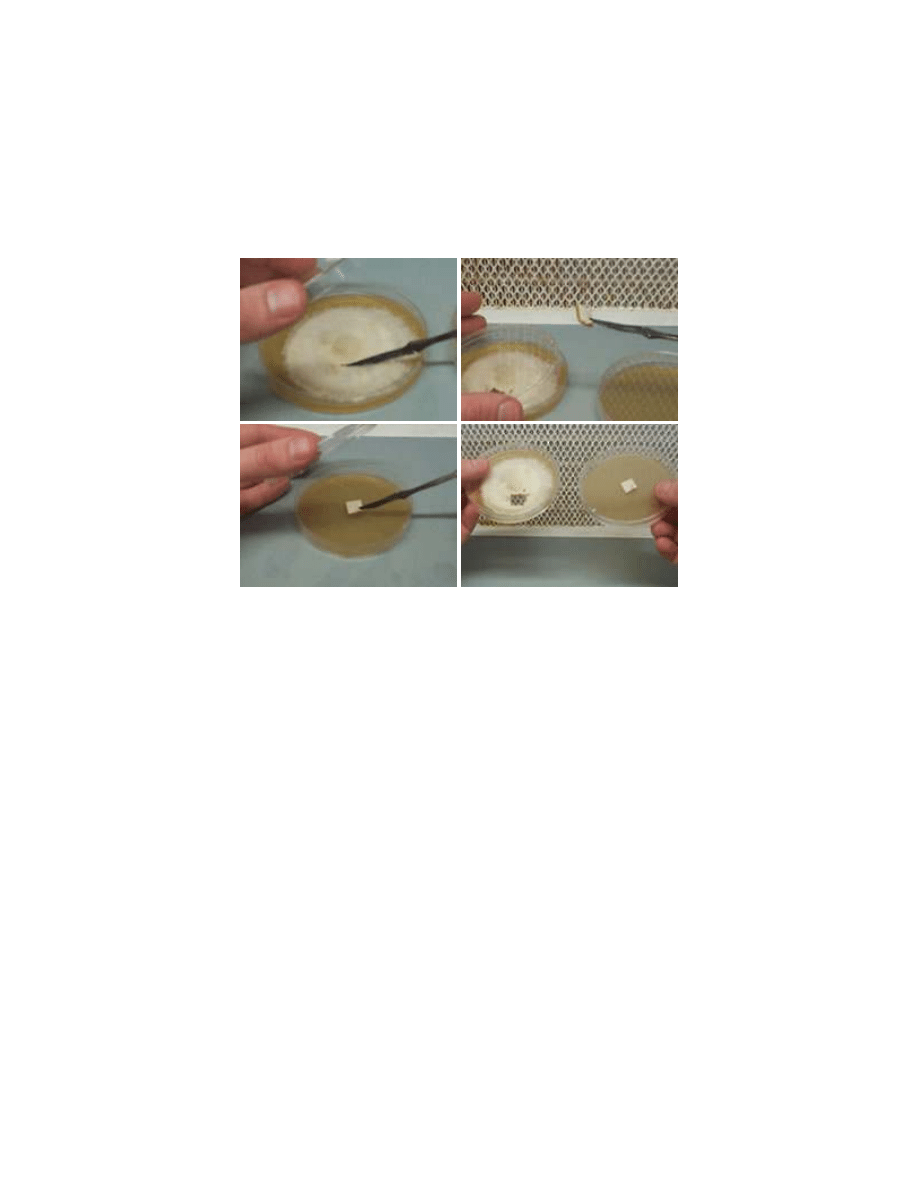
To select pure substrains small agar squares from a multispore germination are transferred to
fresh media. Growth from these squares will still be pretty messy but often some sectors will
develop that show healthy growth. These healthy sectors should be transferred to new dishes
again. This process is repeated until the growth in a dish is uniform and non-sectoring. This is a
pure substrain.
One can also use the multispore cultures to directly inoculate spawn. Since mushrooms are
composed of mycelial threads (of one substrain, not of a mixture) one can always isolate pure
strains from mushrooms as soon as they emerge from this multispore culture.
Starting a culture from a fruitbody
Since mushrooms are composed of mycelial threads (of one substrain, not of a mixture) , as
mentioned before, one can use tissue from a mushroom to start new cultures. However there is
no advantage to cloning a mushroom that is grown from a pure strain. Cloning mushrooms is
useful when one starts with multispore inoculations or for cloning wild specimens.
We like to isolate pure lines by letting multispore cultures fruit and consequently isolating
mycelia from the healthiest looking fruitbodies. This saves time and ensures that the obtained
strain is indeed one that is willing to fruit on the selected substrate under the same
environmental conditions.
Most mushroom species produce fruitbodies that are sterile on the inside. The practical
approach to isolating some tissue is to split open the mushroom and to cut a piece of tissue
from the inside. This mushroom fragment is put on agar. Care is taken to not let the scalpel or
the fragment come in contact with the outside of the mushroom. This will surely lead to
bacterial contamination.
To increase the chance of succes at least 10 dishes are inoculated taking care not to cause
cross-contamination (re-sterilize the scalpel in between isolations).

Often within a few days the fragment will spring to life. Becoming fuzzy and showing new
growth on the agar surface. If contaminants are visible one can try to isolate the mycelium
away from the contaminants.
Even though a pure (sub)strain should be growing on the agar sectoring may be visible, often
healthy zones mixed with cottony or fuzzy ones. The reason for this is not known. Some strains
have a tendency to show this phenomenon more than others. The healthy mycelium is then
transferred to a new dish until a healthy pure culture is established.
Taping dishes and tubes
To prevent moisture loss and the introduction of contaminants dishes and tubes are taped. For
this purpose small rolls of clingfilm are cut from a big roll with a sharp serrated knife. The
clingfilm is strechted out a bit and wrapped around the dish, effectively taping the dish and lid
together. Polyethylene clingfilm allows for slow gas exchange.

To store cultures slants are inoculated with small agar squares. The cap is put on loosely and
the tube neck is taped with polyethylene clingfilm. Once the agar is fully colonized the slants
are put in the fridge where they can be kept for at least a year. It's wise to check the viability of
cultures each year by inoculating some dishes with the culture.
Incubating cultures
Cultures should be incubated at the right temperature. If the temperature is too high mycelia
will often start to sweat, sickening the culture. If the temperature is too low mycelia will grow
slowly. In general too low is better than too high.
It's also very important not to let the temperature fluctuate too much as this causes
condensation to form on the lids. The temperature should be kept as constant as possible.
Dishes are incubated upside down so even if condensation forms it will not disturb mycelial
growth.
Storing cultures
Wyszukiwarka
Podobne podstrony:
AMACOM, A Survival Guide for Working With Bad Bosses Dealing With Bullies, Idiots, Back stabber
GWT Working with the Google Web Toolkit (2006 05 31)
18 Lesson18 Working With Templates
(Ebook English) Crafts Beading Working With Metal Clay
Benefits of Working with a Coach
GT1 Working With Manual
Working with Dryads
Working with Sources
#0936 Working With the IT Department
Working with Oneness by Llewellyn Vaughan Lee
MAPS Vol11 No2 Working with Difficult Psychedelic Experiences
Handbook for Working with Defendants and Offenders with Mental Disorders Third Edition
FIDE Trainers Surveys 2017 05 19 Miguel Illescas Working with Computers
AMACOM, A Survival Guide for Working With Bad Bosses Dealing With Bullies, Idiots, Back stabbers, A
ASP NET Module 1 Working with Microsoft ASP NET
Greg Webb The All Magic Reader Working With Stage Thread
#0752 – Working With Unreliable People
więcej podobnych podstron